Abstract
Most autoimmune inflammatory rheumatologic disorders are multisystem and heterogeneous with several shared features, especially at the early stages. Early diagnosis is usually difficult, as physicians have to discriminate from non-rheumatic disorders as well as among autoimmune diseases with overlapping manifestations. Moreover, the serum markers are shared across these diseases, such as an anti-nuclear antibody or rheumatoid factor, that contribute to making a diagnosis uncertain at times. When there is no gold standard in support of diagnosis or classification, a substantial group of patients remains undiagnosed for years. Precision or personalized medicine, a concept that has been pioneered in oncology, posits that that individual genetic risk and molecular pathogenesis can contribute to accurate diagnosis and offer the most appropriate therapy options for a given patient. Recent advances in technology and analytical tools that assess big data now present the possibility of more accurate classification of diseases and pave the way to precision medicine in rheumatology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The concept of diagnosis, or the art or act of identifying a condition from its signs and symptoms, has been discussed for nearly 5000 years. In fact, the concept of diagnosis antedated that of medicine. It was well developed before there was any relationship to treatment and was, in fact, originally more related to prognosis. Since the development of scientific-based medicine following the Flexner Report of 1910, diagnosis has been the centerpiece of medicine, and we recognize many great physicians because of their diagnostic skills [1, 2].
Of all medical disciplines, rheumatology especially prided itself as the bastion of great diagnosticians. We were often the last resort of patients with an array of hard-to-explain signs and symptoms and can often affix a label to a patient, even if the label is only a restatement of the array of signs and symptoms without much in the way of physiologic or therapeutic implication. In addition, sometimes when a diagnosis could not be made, frustration may have resulted in discounting or even stigmatizing the patient. Over the past few decades, diagnosis has become more complex with an array of molecular and imaging technologies that have often changed medical nosology and affected the approach to diagnosis. In addition, the advent of newer and more effective targeted therapies has increased the importance of accurate diagnosis. As a result of all of these changes, diagnosis has evolved from a concept in medicine to one that has numerous other implications, including psychological, sociological, and also political and economic. Herein, we will discuss the concept of diagnosis and future directions in systemic lupus erythematosus (SLE), a most challenging disease in rheumatology.
SLE
SLE is a complex, prototypic autoimmune disease characterized by loss of tolerance and sustained autoantibody production. Strong genetic influences have been demonstrated in family and twin studies [3,4,5]. Moreover, data shows that ancestry affects not only incidence and prevalence as well as renal involvement, but also molecular pathways and autoantibody profiles, that results in differential response to treatments in SLE [6,7,8,9].
The vastly diverse nature of the disease presents immense challenges to physicians for diagnosis and treatment. Despite improved prognosis over the last 50 years, the chance of being dead at the age of 35 for a patient diagnosed with SLE at the age of 20 is still one in seven. In a recent study, SLE ranked tenth in the leading cause of death in women between 15 and 24 years of age and is the only chronic inflammatory disorder, ranking higher than diabetes mellitus or HIV [1, 2]. Therefore, early diagnosis and introducing the best suitable treatment for the patients are of utmost importance to improve prognosis further.
Diagnosis in SLE relies heavily on the physician’s clinical judgment based on a combination of clinical signs/symptoms and available clinical tests (most frequently ANAs). Physicians’ experiences are important in solving the problem of lupus diagnosis. However, diagnostic delays and misdiagnosis are inevitable in a disease with such heterogeneity, as several conditions mimic SLE. A survey of more than 2500 UK lupus patients in 2014 showed that the mean time between patients’ first awareness of SLE symptoms and actual diagnosis was 6.4 years and half of the patients reported that they had been misdiagnosed initially [10].
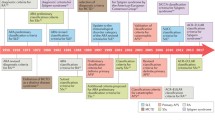
Given the considerable heterogeneity in SLE, most efforts have been directed toward developing more precise classification criteria that aim to assemble cohorts that are representative of the majority with disease for clinical research. Scientifically, classification criteria target high specificity to classify accurately even if it means a trade-off of lower sensitivity, which is more important for diagnosis. The 1997 revised version of the 1982 ACR criteria is highly specific (96%) for classification when at least 4 of 11 criteria are positive [11]. However, sensitivity was remarkably lower compared to the criteria of the Systemic Lupus International Collaborating Clinics (SLICC) (83% vs. 97%), but the specificity dropped off to 84% in the latter [12]. The development process of the recent ACR/EULAR SLE classification aimed to improve sensitivity compared to ACR 1997 criteria as well as applicability to early or new onset lupus without compromising specificity and focusing on true autoimmune disease. The 2019 European League against Rheumatism (EULAR)/American College of Rheumatology (ACR) SLE classification criteria reached the combination of high sensitivity and specificity of 96.1% and 93.4%, respectively, with comparable sensitivity to SLICC criteria in capturing early disease (Table 8.1). However, it is important to emphasize that these are classification criteria used for research purposes and not diagnostic criteria employed in clinical practice. If employed as diagnostic criteria, they will identify only the most stereotypical patients, leaving many with fewer features of lupus undiagnosed and often frustrated.
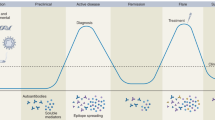
The repositioning of anti-nuclear antibody (ANA) as an obligatory entry criterion for the 2019 SLE classification criteria has spurred vigorous debates [6,7,8]. Targeting individuals with true autoimmunity was the impetus behind the proposal and acceptance of ANA as a key entry criterion. An ANA titer of 1:80 has been shown to have a sensitivity of 98%, and although rare at onset, ANA-negative SLE cases may exist, which may cause a small subset of SLE patients to be unclassified [13, 14]. If the classification criteria were used for diagnosis, this would be a problem, but it is less of an issue for classification for research purposes [15, 16]. Of note, physicians should understand the assay used to detect ANA since these techniques have intrinsic differences and may provide disparate results. For example, the results of an indirect immunofluorescence (IIF) assay using human epithelial type 2 cells (HEp-2- IIFA) may vary in different laboratories because of its dependence on visual reading of antibody patterns [17]. In addition, an IIF assay may be disadvantageous because of its low specificity at low antibody titers [18]. The disease duration and treatment may also affect ANA seroconversion [19]. Interestingly, the recent phase II belimumab trial showed that 29.5% of established SLE patients were found to be negative for ANA at enrollment, raising the possibility that ANA positivity may reflect an immunologically active state and patients with positive ANA may respond to some therapies differently [20, 21].
Although data have shown that autoantibodies, such as ANA, appear in the blood as early as 9.4 years (mean 3.3 years) before the clinical onset of SLE, given its low specificity, and that it is also positive in up to 20% of healthy individuals [22], screening ANA-positive patients with nonspecific symptoms is usually ineffective. Some autoantibodies are highly specific for SLE diagnosis, such as anti-double-stranded DNA (anti-ds DNA) , anti-Sm, and anti-ribosomal P, but they are less sensitive. For example, anti-dsDNA is an important biomarker for SLE diagnosis and disease activity, and its prevalence ranges between 50% and 75% depending on the assay, disease state, and ethnicity [6, 23, 24]. Indirect assays to measure complement proteins or their cell-bound activation products have proven to be informative and reliable [25]. The assay detecting cell-bound complement activation products (Exagen, Vista, CA, USA), which outperforms anti-dsDNA by up to 48% in terms of sensitivity, may be helpful in supporting the diagnosis of SLE [25].
Can Genomics and Transcriptomics Be Used to Diagnose SLE?
The advent of high-throughput genotyping, coupled with contemporary bioinformatics approaches and modeling, has significantly improved the understanding of the pathophysiology of several multigenic complex diseases, including SLE [26,27,28]. So far, approximately 100 genetic susceptibility loci at genome-wide significance have been identified in SLE, some of which are shared with other autoimmune disorders. The individual gene effects, however, are somewhat small (relative risk <2), and unlikely to assist in diagnosis or predict outcome when utilized individually [29, 30].
Genetic risk scores (GRS) are numeric scores that combine a large number of disease-associated genetic variants that are weighted by SLE risk odds ratios and reflect the disease-associated genetic load in an individual patient [31]. Therefore, the idea of utilizing GRS as a tool for predicting disease susceptibility and outcome has become a tantalizing approach that has been explored by several groups in SLE. However, some reports had limitations because of sample size or were restricted to limited sets of single nucleotide polymorphisms (SNP) based on immunochip analysis [32,33,34,35].
Not testing results across ancestries is another caveat of most studies. Chen L et al. performed a GRS analysis for SLE across Chinese and European populations. Utilizing three European and two Chinese GWAS datasets and training on a dataset for one population, they tried to predict SLE in the other dataset [32]. Perhaps not surprisingly, they found the most SLE predicted SNPs were enriched in patients with kidney involvement, indicating that most SLE-associated variants also confer risk for lupus nephritis. Another takeaway from this study was the correlation between GRS and age of onset in lupus, which corroborated in both European and Chinese populations, albeit it was independent of renal involvement. Notably, another large independent European GWAS also showed that higher GRS is associated with renal disease and SLE onset at a younger age [35]. This group also demonstrated that a high GRS had the potential of predicting patient outcomes.
In summary, these studies may render an example of incorporating GRS information into the clinical diagnosis of an SLE patient and may assist in diagnosis of lupus nephritis early. However, the GRS studies are still in their early stages and their role in diagnosis of generalized lupus remains to be determined.
Finally, the question remains whether a genetic diagnosis will equate with a clinical diagnosis and how both false positives and false negatives will affect the perception of the utility of the GRS approach. Since there remains debate about whether the genetic tendency will establish the diagnosis of lupus, the utility of the GRS will require considerable debate and eventually a consensus to be accepted.
As autoimmunity precedes overt clinical disease [22], signs and symptoms can be relatively nonspecific in the early stages of SLE. Thereby, early diagnosis can be challenging. A longitudinal study of more than 9000 SLE patients showed that early diagnosis (<6 months) and early adequate treatment result in fewer numbers of flares, low hospitalization rate, and low lupus-related medical costs compared to the matched SLE patients who were diagnosed later than 6 months [36]. This study underlines again the importance of early diagnosis and intervention to prevent damage and eventually mortality. Genetic risk factors are widely known for many diseases; however, their translation into clinical practice is still in its infancy. Knevel et al. have developed a GRS (G-PROB) using genome-wide significant variants (p ≤ 5×108) from previously published genome-wide association studies (GWAS) and tested its potential as a diagnostic tool in a set of patients with inflammatory arthritis (rheumatoid arthritis, systemic lupus erythematosus, spondyloarthropathy, psoriatic arthritis, and gout) [37]. Coupled with good discriminatory capacity (area under the curve (AUC), 0.69–0.84), it could single out a likely diagnosis for 45% of patients with a positive predictive value of 0.64 that could be further improved with the addition of serologic data. Despite only being tested in Caucasian cohorts, the results of this study demonstrate the potential clinical utility of the GRS for diagnosis, especially when incorporating serologic findings, such as ANA, and, possibly, clinical manifestations.
Transcriptomic analysis might also contribute to diagnosis. Based on a meta-analysis of 40 independent publicly available gene expression studies containing 7471 transcriptomic profiles, Haynes et al. identified a core gene set (93-gene signature, SLE MetaSignature) that is dysregulated in patients with SLE and distinguishes SLE from other relevant rheumatic disorders and infections [38]. They further validated the SLE MetaSignature in a prospective study comprising patients with juvenile-onset SLE, juvenile idiopathic arthritis, and healthy subjects. This study demonstrates the potential value of the integration of gene expression studies into the clinic as a means to improve the diagnosis of SLE.
Despite these promising advances in genetic and molecular pathogenesis, lupus remains a clinical diagnosis. Regardless of the elegant genetic and molecular advances, it remains uncertain how this information will be integrated into the process of assigning a diagnosis to an individual patient.
Gene Expression Studies and Organ Involvement
In addition to molecular diagnosis of SLE, several groups have attempted to utilize transcriptomic data from blood, purified T and B cells, myeloid cells, and, although less common, cells from tissue in order to stratify patients based on their molecular signatures or predict disease activity. Grouping patients based on molecular signatures could also be a successful strategy for clinical trials, which may pave the way for personalized precision medicine.
One of the key features in SLE is the prominent expression of interferon (IFN)-inducible genes, an interferon gene signature (IGS) regardless of disease activity [39, 40]. An IGS may be induced by both type I and type II IFNs, in which type I IFNs with 13 IFN-alpha genes (A1, A2, A4, A5, A6, A7, A8, A10, A13, A14, A16, A17, and A21) and IFNB1, IFNW1, and IFNE are likely the major contributors. Type II IFN, IFNG, also induces an IGS through its distinct receptor, but its role in the SLE pathogenesis has been largely deduced from in vitro studies [40,41,42]. Using the weighted gene expression network analysis (WGCNA), a bioinformatics approach to derive gene modules in the dataset based on co-expression [43], IGS has been investigated in detail by looking at differences in various blood cells from patients with SLE and compared with the results of patients with other autoimmune rheumatic diseases and healthy volunteers [44]. The results demonstrate that a T-cell-specific module is exclusively expressed in SLE, whereas monocyte and neutrophils may be present similarly in other diseases and controls [44, 45]. Together with the hypomethylation of type I IFNs in naïve CD4 + T cells, these results suggest that type I IFN T-cell signaling may contribute to SLE disease pathogenesis [46].
In order to understand how disease activity affects gene expression and if it helps to group patients or disease manifestations, longitudinal gene expression studies are needed. In this context, Banchereau et al. performed a blood transcriptome profile of a longitudinal cohort of pediatric SLE patients [47]. They were able to group SLE patients into seven subsets, where each group was associated with a specific gene module. Of those, the identified neutrophil transcripts were enriched in patients with active lupus nephritis. They also found a robust plasmablast signature that was associated with disease activity, and the signal was stronger in African ancestry patients [47]. Although easier to obtain, blood transcriptome analyses provide a more general picture, especially when cell-specific transcriptome differences are targeted. Moreover, access to matched transcriptome data in the whole blood and tissue would provide a better understanding of how to interpret the differences or changes in cell populations as well as signaling pathways detected in many SLE studies. Labonte et al. developed an in-house tool based on differentially expressed T-cell receptor genes (TCR), immunoglobulin genes, and HLA genes in most SLE studies then created a Biologically Informed Gene Clustering (BIG-C) platform utilizing more than 40 SLE and control microarray datasets [48]. Gene set variation analysis employing the IFNA2, IFNB1, IFW1, IFNG, TNF, IL12, and the IFN core signature genes demonstrated prominent expression of IFNB1 and IFNW1 signatures differentially associated with organ involvement. The researchers found strong IFNB1 enrichment in skin and synovium in comparison to those in the kidneys in SLE patients [40]. This result is particularly interesting as several case reports show that drug-induced SLE arises with positive dsDNA after treatment with IFNB1 in multiple sclerosis [49, 50]. Besides proposing IFNB1 as an intriguing treatment target of SLE, the results of this study also demonstrated that IGS is less likely to correlate with the disease activity because of prolonged expression of IGS in monocytes.
Lupus nephritis is a leading cause of morbidity and mortality of SLE. Although advances have been made through immunologic discoveries and genetic association studies in SLE, the outlook for patients with LN has not improved dramatically over the years, as ~10% still progress to end-stage renal disease (ESRD) [51, 52]. Renal biopsy is the gold standard for treatment decisions; however, the International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification is limited by reliance on only histologic findings by mostly light microscopy without integrating recent molecular insights. Conventionally used biomarkers such as proteinuria or serologic markers have a limited ability to predict renal prognosis adequately, and persistent proteinuria can be secondary to residual activity, chronic damage, or comorbid conditions. Nevertheless , no robust markers have been identified yet to replace kidney biopsy, despite the extensive search for blood or urinary biomarkers.
Single-cell RNA sequencing (sc-RNA-seq) is a powerful unbiased approach to overcome the aforementioned limitations of the bulk analysis, such as defining cell types that link to observed gene expressions as well as provide new insights into the diverse mechanisms involved in the pathogenesis of tissue injury in kidneys. Recent studies show the potential of transcriptomic profiling of skin biopsies as a biomarker of lupus nephritis by performing sc-RNA-seq in lupus kidney and skin tissues [53, 54]. These studies also demonstrate that residential cells, such as kidney epithelial cells, in addition to infiltrating cells contribute to the LN disease progression.
Given that several inflammatory autoimmune diseases share common nonspecific symptoms early in the disease stage, comparing gene expression profiles of these conditions might be informative in clinical practice to segregate and treat early [55,56,57,58]. In a recent study, the gene expression profiles of SLE synovium were interrogated by using knee synovia samples from SLE, rheumatoid arthritis (RA), and osteoarthritis (OA) patients. Bioinformatic analyses revealed a myeloid-cell-mediated inflammation that governs the immunopathogenesis of lupus arthritis [59]. Upregulated differentially expressed genes in RA, on the other hand, indicated T cells, B cells, NK, NKT cells, and the other lymphocytes. In another study, 91% of IFN-inducible genes were differentially expressed in systemic sclerosis (SSc) as in SLE within the same platform compared to healthy individuals [60]. A subset of SSc patients who were also grouped as “lupus-like” phenotype showed type I IFN and plasma cell signatures. They also found a correlation between the type I IFN signature and the presence of lymphopenia, anti-topoisomerase, and anti-U1RNP antibodies.
In summary, the recent state-of-the-art technologies have advanced the understanding of the underlying molecular heterogeneity in SLE. Simultaneously, analyzing data from multiple sources including various cells and tissues at the cellular, molecular, and protein level will be important in the future to stratify diseases into clinically relevant groups. Leveraging these advances provides the chance to devise molecular tools that improve SLE diagnosis and help to predict early organ involvement.
Summary and Conclusions
Currently, the diagnosis of SLE involves the use of clinical tools that have not changed in many years. Because of their imprecision and a lack of consensus on what constitutes lupus in the clinic, many patients remain undiagnosed for protracted periods of time. Recently, genetic and molecular tools have been developed that afford the possibility of improving the precision of lupus diagnosis. Whether these will evolve to the point of clinical utility and whether they will be embraced by the clinical community remain major challenges for the field and patients living with recognized or undiagnosed lupus.
References
Ludmerer KM. Commentary: understanding the Flexner report. Acad Med. 2010;85(2):193–6.
Duffy TP. The Flexner report–100 years later. Yale J Biol Med. 2011;84(3):269–76.
Shai R, Quismorio FP, Li L, Kwon OJ, Morrison J, Wallace DJ, et al. Genome-wide screen for systemic lupus erythematosus susceptibility genes in multiplex families. Hum Mol Genet. 1999;8(4):639–44.
Kuo CF, Grainge MJ, Valdes AM, See LC, Luo SF, Yu KH, et al. Familial aggregation of systemic lupus erythematosus and Coaggregation of autoimmune diseases in affected families. JAMA Intern Med. 2015;175(9):1518–26.
Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, et al. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992;35(3):311–8.
Catalina MD, Bachali P, Yeo AE, Geraci NS, Petri MA, Grammer AC, et al. Patient ancestry significantly contributes to molecular heterogeneity of systemic lupus erythematosus. JCI Insight. 2020;5(15).
Feldman CH, Hiraki LT, Liu J, Fischer MA, Solomon DH, Alarcón GS, et al. Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with Medicaid coverage, 2000–2004. Arthritis Rheum. 2013;65(3):753–63.
Peschken CA, Katz SJ, Silverman E, Pope JE, Fortin PR, Pineau C, et al. The 1000 Canadian faces of lupus: determinants of disease outcome in a large multiethnic cohort. J Rheumatol. 2009;36(6):1200–8.
Pons-Estel BA, Catoggio LJ, Cardiel MH, Soriano ER, Gentiletti S, Villa AR, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore). 2004;83(1):1–17.
Morgan C, Bland AR, Maker C, Dunnage J, Bruce IN. Individuals living with lupus: findings from the LUPUS UK Members Survey 2014. Lupus. 2018;27(4):681–7.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725.
Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677–86.
Leuchten N, Hoyer A, Brinks R, Schoels M, Schneider M, Smolen J, et al. Performance of antinuclear antibodies for classifying systemic lupus erythematosus: a systematic literature review and meta-regression of diagnostic data. Arthritis Care Res (Hoboken). 2018;70(3):428–38.
Choi MY, Clarke AE, St Pierre Y, Hanly JG, Urowitz MB, Romero-Diaz J, et al. Antinuclear antibody-negative systemic lupus erythematosus in an international inception cohort. Arthritis Care Res (Hoboken). 2019;71(7):893–902.
Aggarwal R, Ringold S, Khanna D, Neogi T, Johnson SR, Miller A, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res (Hoboken). 2015;67(7):891–7.
Aringer M, Johnson SR. Classifying and diagnosing systemic lupus erythematosus in the 21st century. Rheumatology (Oxford). 2020;59(Suppl 5):v4–v11.
Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol. 2020;16(10):565–79.
Mahler M, Meroni PL, Bossuyt X, Fritzler MJ. Current concepts and future directions for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. J Immunol Res. 2014;2014:315179.
Acosta-Mérida A, Isenberg DA. Antinuclear antibodies seroconversion in 100 patients with lupus. Clin Exp Rheumatol. 2013;31(4):656.
Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61(9):1168–78.
Putterman C, Pisetsky DS, Petri M, Caricchio R, Wu AHB, Sanz I, et al. The SLE-key test serological signature: new insights into the course of lupus. Rheumatology (Oxford). 2018;57(9):1632–40.
Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526–33.
Pisetsky DS, Spencer DM, Rovin B, Lipsky PE. Role of ANA testing in the classification of patients with systemic lupus erythematosus. Ann Rheum Dis. 2019; https://doi.org/10.1136/annrheumdis-2019-216259.
Aggarwal A. Role of autoantibody testing. Best Pract Res Clin Rheumatol. 2014;28(6):907–20.
Putterman C, Furie R, Ramsey-Goldman R, Askanase A, Buyon J, Kalunian K, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med. 2014;1(1):e000056.
Vasquez-Canizares N, Wahezi D, Putterman C. Diagnostic and prognostic tests in systemic lupus erythematosus. Best Pract Res Clin Rheumatol. 2017;31(3):351–63.
Giannopoulou EG, Elemento O, Ivashkiv LB. Use of RNA sequencing to evaluate rheumatic disease patients. Arthritis Res Ther. 2015;17:167.
Taylor KE, Chung SA, Graham RR, Ortmann WA, Lee AT, Langefeld CD, et al. Risk alleles for systemic lupus erythematosus in a large case-control collection and associations with clinical subphenotypes. PLoS Genet. 2011;7(2):e1001311.
Langefeld CD, Ainsworth HC, Cunninghame Graham DS, Kelly JA, Comeau ME, Marion MC, et al. Transancestral mapping and genetic load in systemic lupus erythematosus. Nat Commun. 2017;8:16021.
Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12(12):716–30.
Brown MA, Aletaha D. Genetic risk scores in inflammatory arthritis: a new era? Nat Rev Rheumatol. 2020;16(10):545–6.
Chen L, Wang YF, Liu L, Bielowka A, Ahmed R, Zhang H, et al. Genome-wide assessment of genetic risk for systemic lupus erythematosus and disease severity. Hum Mol Genet. 2020;29(10):1745–56.
Webb R, Kelly JA, Somers EC, Hughes T, Kaufman KM, Sanchez E, et al. Early disease onset is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype in lupus patients. Ann Rheum Dis. 2011;70(1):151–6.
Gianfrancesco MA, Balzer L, Taylor KE, Trupin L, Nititham J, Seldin MF, et al. Genetic risk and longitudinal disease activity in systemic lupus erythematosus using targeted maximum likelihood estimation. Genes Immun. 2016;17(6):358–62.
Reid S, Alexsson A, Frodlund M, Morris D, Sandling JK, Bolin K, et al. High genetic risk score is associated with early disease onset, damage accrual and decreased survival in systemic lupus erythematosus. Ann Rheum Dis. 2020;79(3):363–9.
Oglesby A, Korves C, Laliberté F, Dennis G, Rao S, Suthoff ED, et al. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl Health Econ Health Policy. 2014;12(2):179–90.
Knevel R, le Cessie S, Terao CC, Slowikowski K, Cui J, Huizinga TWJ, et al. Using genetics to prioritize diagnoses for rheumatology outpatients with inflammatory arthritis. Sci Transl Med. 2020;12(545).
Haynes WA, Haddon DJ, Diep VK, Khatri A, Bongen E, Yiu G, et al. Integrated, multicohort analysis reveals unified signature of systemic lupus erythematosus. JCI Insight. 2020;5(4).
Rönnblom L, Alm GV, Eloranta ML. The type I interferon system in the development of lupus. Semin Immunol. 2011;23(2):113–21.
Catalina MD, Bachali P, Geraci NS, Grammer AC, Lipsky PE. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Commun Biol. 2019;2:140.
Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016;75(11):2014–21.
Jackson SW, Jacobs HM, Arkatkar T, Dam EM, Scharping NE, Kolhatkar NS, et al. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med. 2016;213(5):733–50.
Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, et al. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29(1):150–64.
Flint SM, Jovanovic V, Teo BW, Mak A, Thumboo J, McKinney EF, et al. Leucocyte subset-specific type 1 interferon signatures in SLE and other immune-mediated diseases. RMD Open. 2016;2(1):e000183.
Alarcón-Riquelme ME. New attempts to define and clarify lupus. Curr Rheumatol Rep. 2019;21(4):11.
Coit P, Jeffries M, Altorok N, Dozmorov MG, Koelsch KA, Wren JD, et al. Genome-wide DNA methylation study suggests epigenetic accessibility and transcriptional poising of interferon-regulated genes in naïve CD4+ T cells from lupus patients. J Autoimmun. 2013;43:78–84.
Banchereau R, Hong S, Cantarel B, Baldwin N, Baisch J, Edens M, et al. Personalized Immunomonitoring uncovers molecular networks that stratify lupus patients. Cell. 2016;165(6):1548–50.
Labonte AC, Kegerreis B, Geraci NS, Bachali P, Madamanchi S, Robl R, et al. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS One. 2018;13(12):e0208132.
Bonaci-Nikolic B, Jeremic I, Andrejevic S, Sefik-Bukilica M, Stojsavljevic N, Drulovic J. Anti-double stranded DNA and lupus syndrome induced by interferon-beta therapy in a patient with multiple sclerosis. Lupus. 2009;18(1):78–80.
Sladkova V, Mares J, Lubenova B, Hlustik P, Kanovsky P. Drug-induced systemic lupus erythematosus in interferon beta-1b therapy. Neuro Endocrinol Lett. 2011;32(1):4–6.
Davidson A, Aranow C, Mackay M. Lupus nephritis: challenges and progress. Curr Opin Rheumatol. 2019;
Yu F, Haas M, Glassock R, Zhao MH. Redefining lupus nephritis: clinical implications of pathophysiologic subtypes. Nat Rev Nephrol. 2017;13(8):483–95.
Der E, Suryawanshi H, Morozov P, Kustagi M, Goilav B, Ranabothu S, et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat Immunol. 2019;20(7):915–27.
Der E, Ranabothu S, Suryawanshi H, Akat KM, Clancy R, Morozov P, et al. Single cell RNA sequencing to dissect the molecular heterogeneity in lupus nephritis. JCI Insight. 2017;2(9).
Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1771–4.
Finckh A, Liang MH, van Herckenrode CM, de Pablo P. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum. 2006;55(6):864–72.
Maas K, Chan S, Parker J, Slater A, Moore J, Olsen N, et al. Cutting edge: molecular portrait of human autoimmune disease. J Immunol. 2002;169(1):5–9.
Olsen N, Sokka T, Seehorn CL, Kraft B, Maas K, Moore J, et al. A gene expression signature for recent onset rheumatoid arthritis in peripheral blood mononuclear cells. Ann Rheum Dis. 2004;63(11):1387–92.
Hubbard EL, Catalina MD, Heuer S, Bachali P, Robl R, Geraci NS, et al. Analysis of gene expression from systemic lupus erythematosus synovium reveals myeloid cell-driven pathogenesis of lupus arthritis. Sci Rep. 2020;10(1):17361.
Assassi S, Mayes MD, Arnett FC, Gourh P, Agarwal SK, McNearney TA, et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 2010;62(2):589–98.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Yavuz, S., Lipsky, P.E. (2022). Diagnosis of Systemic Lupus Erythematosus in the Age of Precision Medicine. In: Lockshin, M.D., Crow, M.K., Barbhaiya, M. (eds) Diagnoses Without Names. Springer, Cham. https://doi.org/10.1007/978-3-031-04935-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-04935-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04934-7
Online ISBN: 978-3-031-04935-4
eBook Packages: MedicineMedicine (R0)