Abstract
Historically, peptide drug discovery has been very successful in the development of receptor-targeted medicines such as related to insulin and glucagon-like peptide 1 agonists as well as many other examples with respect to many receptors and other extracellular targets. In recent years, there has been significant progress to advance new peptide modalities focused especially on macrocyclic design and leveraging lead molecules from biological and/or chemical diversity approaches, including mRNA-display libraries, phage-display libraries, DNA-encoded synthetic libraries, and one-bead-one-compound synthetic libraries. Such work builds upon existing peptidomimetic and peptide analog optimization strategies involving a native cognate peptide (or protein fragment) and iterative structure-based design. Likewise, there has been incredible progress in structural biology and computational modeling that is contributing to peptide drug modalities, including linear and macrocyclic peptides as well as peptidomimetic analogs thereof. Collectively, this armamentarium of peptide modalities has contributed to the acceleration of breakthrough preclinical molecules. A greater understanding of drug-like properties to tackle an increasing number of intracellular targets (e.g., enzymes and protein–protein interactions) as well as deeper insights related to cell uptake mechanisms, including passive transport and both cationic and lipophilic partitioning models, is being achieved. This chapter exemplifies a few specific cases of intracellular targets and varying peptide drug modalities which illustrate success toward a new wave of novel peptide therapeutics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cell-penetrating peptides
- Macrocyclic peptides
- Peptidomimetics
- Intracellular targets
- Protease
- Proteasome
- Phosphatase
- Farnesyl transferase
- GTPase
- Src SH2
- XIAP
- MCL-1
- HIF-1α
- NEMO
- CFTR
- MDM2
- MDM4
- Protein
- protein interactions
8.1 Introduction
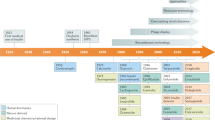
One of the most extraordinary adventures for peptide drug discovery since the beginning of the twenty-first century has been the pioneering efforts across academia, biotech, and pharma to advance the generation, optimization, and development of breakthrough peptide therapeutics for intracellular targets. In this chapter, it is my intention to reflect upon key scientific concepts and innovative technologies that have contributed to some very hopeful emerging peptide modalities for intracellular target space. I have been blessed to have seen this phenomenal story take place from the time of the discovery of cyclosporine A (CsA) and my graduate studies in the field of peptide science at the University of Arizona which began in the mid-1970s. Although my earliest foray into what may now be defined as peptide drug discovery was actually focused on G protein-coupled receptors, I acquired a multidisciplinary mindset from my mentor, Professor Victor Hruby, which enabled me to do “the deep dive” into the abyss of intracellular space to tackle many different types of therapeutic targets over my career, including proteases (e.g., HIV-1 protease, interleukin-converting enzyme), phosphatases (e.g., PTP1b), transferases (e.g., Ras farnesyl transferase), kinases (e.g., Src), GTPases (e.g., K-Ras), apoptotic modulatory proteins (e.g., Mcl-1), and transcription/translation factors (e.g., p53, β-catenin, eIF4E, and Myc). This work has integrated and leveraged chemistry, biology, structural biology, biophysical chemistry, computational chemistry, cell biology, and pharmacology in rather fascinating ways to develop both tools and rules to design novel peptides having cell permeability, stability, and in vivo efficacy that may be further advanced as clinical candidates.
Relative to the varying intracellular targets which I have abovementioned, the diversity of peptide modalities that have been advanced as key tools or preclinical and/or clinical development candidates include peptidomimetics (including de novo designed nonpeptides), macrocyclic peptides (incorporating α-helical, reverse-turn, and β-strand motifs), and both classic and highly modified linear peptides (incorporating unusual amino acids, backbone surrogates, and/or non-amino acid building blocks) and, with increasing focus, conjugates thereof with other therapeutic modalities. Several examples of such diversity will be shared in this chapter. Of course, CsA well-illustrates a macrocyclic peptide incorporating unusual amino acids (e.g., non-canonical side chains and a D-isomer) and multiple N-methylation of the peptide backbone. In fact, CsA has inspired many academic, biotech, and pharma efforts to leverage macrocyclic peptides for intracellular targets with respect to seeking CsA-like passive permeability properties. Such endeavors have led to a deeper understanding of biophysical, conformation, and structural principles correlating with cellular uptake for such macrocyclic peptides. It will be the overarching goal of this chapter to highlight key learnings from peptide drug discovery efforts that have been especially exploited by innovative macrocyclization design concepts and platforms.
8.2 Intracellularly Targeted Peptides: Some Historical Milestones
In retrospect, drug discovery efforts to advance intracellularly targeted peptides have gone through many different pathways, both conceptually and experimentally, in terms of how to traverse the cell membrane (vide infra). Consequently, the design of peptides having cell permeability properties and the ability to modulate intracellular therapeutic targets has required in most cases a tour de force to successfully advance peptides, including peptidomimetics and de novo designed nonpeptides.
Cyclosporine A (CsA)
The macrocyclic peptide, natural product CsA (Fig. 8.1) has several chemical attributes to understand its structure-activity and structure-permeability relationships. The N-to-C backbone cyclized structure of CsA includes several N-methylated amino acids and one D-amino acid (Stahelin 1996). It exhibits passive permeability, despite having >500 molecular weight, and such has been attributed to its conformational flexibility and ability to exhibit intramolecular H-bonding favorable to lipid membrane interaction as well as intermolecular H-bonding with water. The impact of CsA on the field of intracellularly targeted peptide drug discovery has been extraordinary, especially with respect to its intrinsic cell permeability properties. In fact, CsA is a key benchmark macrocyclic peptide for passive transport (e.g., cell permeability and/or oral bioavailability) for macrocyclic peptide drug discovery efforts (Nielsen et al. 2017; Naylor et al. 2017; Pye et al. 2017; Chatterjee et al. 2012; Kelly et al. 2021; Naylor et al. 2018). Mechanistically, CsA binds to the cytosolic protein cyclophilin (also known as immunophilin) within lymphocytes (e.g., T cells). The CsA–cyclophilin complex then inhibits calcineurin and subsequent calcineurin-dependent production of interleukin-2 (Azzi et al. 2013).
Src SH2 Antagonist, AP22408
In the early 1990s, unique noncatalytic domains were identified with the first being the tyrosine kinase and Sr and then for many intracellular proteins, including kinases, phosphatases, and adapter proteins such as Grb-2 (Sawyer et al. 2002). Historically, the biotech company Ariad Pharmaceuticals was founded to explore the signal transduction role of such Src homology (SH) domains, including both SH2 and SH3. In the specific case of SH2 domains, the cognate ligand was determined to be phosphotyrosine (pTyr) containing proteins and with sequence specificity about the pTyr residue, particularly those immediately C-terminal to it (Sawyer et al. 2002). Noteworthy was a series of novel peptidomimetic and de novo nonpeptide designs that ultimately led to the first potent, in vivo active Src SH2 antagonist AP22408 (Fig. 8.1) (Bohacek et al. 2001; Shakespeare et al. 2000). Based on a co-crystal structure of the noncatalytic Src homology 2 (SH2) domain of Src complexed with citrate in the phosphotyrosine (pTyr) binding pocket, the design of a novel 3′,4′-diphosphonophenylalanine (Dpp) as a pTyr mimic was achieved. AP22408 also incorporates a bicyclic nonpeptide template to replace the tripeptide sequence C-terminal to the pTyr.
Ras Farnesyl Transferase Inhibitor, L-744,832
The highly coveted cancer target family of Ras proteins requires lipid modification by farnesyl isoprenoid by the farnesyltransferase (FTase) as a primary pathway and by an alternative process involving geranylgeranyltransferase (GGTase). Both enzymes are capable of effecting prenylation of Ras proteins as a so-called CAAX motif in an irreversible manner at the tetrapeptide’s cysteine sulfhydryl group (Appels et al. 2005). Albeit the rational design of FTase inhibitors has successfully generated many potent molecules, including clinical candidates, this strategy has not shown efficacy against KRAS-driven cancers in humans. Hence, the pursuit of dual-specific inhibitors of both FTase and GGTase became a new strategy for next-generation clinical candidates, assuming they may overcome toxicity limitations (Appels et al. 2005). Exemplary of designed peptidomimetic CAAX-based FTase inhibitors is L-744,832 (Fig. 8.2) which was shown to be effective in combination with taxane-induced mitotic arrest and apoptosis in breast cancer cell lines (Lobell et al. 2002).
Cell-Penetrating Peptide (CPP) Progenitor, HIV-1 TAT49–57
Investigations on HIV-1 showed that the TAT protein contained a cell membrane transduction motif enabling permeability which might be exploited as a carrier modality if conjugated to drug payloads (Vives 2003). The HIV-1 Tat49–57 peptide RKKRRQRRR (Fig. 8.1) was the progenitor of what is now a superfamily (>100) of CPPs and the first of major subclass that is structurally characterized as having arginine-rich sequences. Other noteworthy CPPs discovered subsequently included antennapedia homeodomain protein43–58, viral protein VP22267–300, and nuclear localization signal sequences (Pooga and Langel 2015; Milletti 2012; Koren and Torchilin 2012). Furthermore, beyond the more widely used term CPPs, other names are found in the literature protein transduction domains (PTDs) and membrane translocating sequences (MTSs). All may be classified into three groups: (i) basic peptides such as Tat peptide, (ii) basic/amphiphilic peptides such as Antp, and (iii) hydrophobic peptides such as MTS (Futaki et al. 2003). More recently, a new class of hybrid macrocyclic CPPs has been developed (Appiah Kubi and Pei 2020) to further expand the potential therapeutic utility of this modality (vide infra).
HIV-1 Protease Inhibitors, U-81749 and Saquinavir
One of the most promising targets that were first identified as critical to HIV-1 cellular infection and processing to enable replication of the retrovirus was an aspartyl protease, namely HIV-1 protease (Debouck et al. 1987). Noteworthy, HIV-1 protease was unique in that it was a C-2 symmetric homodimer with its two catalytic aspartyl residues being part of an active site created upon homodimerization of the two relatively small-sized (99 amino acids) monomers. HIV-1 protease inhibitor drug discovery became a worldwide effort throughout the 1990s (Debouck 1992). The first reported synthetic peptidomimetic inhibitor of HIV-1 protease exhibiting cellular activity was U-81749 (Fig. 8.1) (McQuade et al. 1990). It was essentially a tripeptide template incorporating a nonhydrolyzable amide isostere (i.e., CH(OH)CH2) that was exemplary of diverse amide bond surrogates that were designed to advance highly potent peptidomimetic as well as novel nonpeptide inhibitors of HIV-1 protease (Ghosh et al. 2016; Roberts et al. 1990). The peptidomimetic saquinavir was the first HIV-1 protease inhibitor that was FDA-approved for the treatment of HIV infection in AIDS patients.
Bortezomid, the Proteasome Inhibitor
A rather unique intracellular protease is the proteasome, and by way of the well-known ubiquitin-proteasome pathway, there exists targeted destruction of cellular proteins. With respect to cell cycle regulation and both cell proliferation and survival, especially in cancer cells, the proteasome was first recognized as a compelling therapeutic target for cancer cell therapy (Fogli et al. 2021). The first proteasome inhibitor that was advanced into clinical trials was bortezomib (Fig. 8.1), a boronic acid-containing peptidomimetic that was designed to effectively inhibit the serine protease active site of the proteasome (Adams 2002, 2004). Bortezomib was highly potent (Ki <1 nM) and effective across a broad range of cancer cell lines.
HCV Protease Inhibitor, Grazoprevir
A breakthrough for the treatment of chronic hepatitis C was achieved by treatment by a combination of NS3/4A protease inhibitors and NS5A inhibitors such as exemplified by grazoprevir (Fig. 8.1) and elbasvir, respectively (Matthew et al. 2020). The structure-based design of grazoprevir illustrates drug design focus on the substrate-binding active sites of NS3/4A to achieve optimal molecular recognition for both increased potency and decreased resistance (Harper et al. 2012).
8.3 Expanding Intracellular Target Space: Emerging Peptide Modalities
In the beginning, the most compelling therapeutic targets for peptide drug discovery were receptors (e.g., G protein-coupled receptors). However, as advancements in molecular and cell biology were being achieved during the latter half of the twentieth century, it was increasingly obvious that a universe of intracellular targets existed for the discovery of peptide modalities. Varying approaches, including the generation of synthetic or biological peptide libraries, target reporter cellular screening, and structural biology (X-ray, NMR, HDX-MS, and, more recently, cryo-EM) are each significantly contributing to an expanding treasury of intracellular therapeutic targets. Several peptide modalities have been advanced to interrogate intracellular target space. Such efforts generally first identify high-affinity binding leads that may then be tested in cellular assays to initiate the early lead optimization process. Unquestionably, this is where the proverbial “rubber hits the road” in terms of translating intracellular target druggability from binding to cellular efficacy as well as cellular permeability and cellular metabolic stability.
The most powerful approaches that are enabling such lead identification efforts include super-diverse macrocyclic peptide library screening derived from synthetic (e.g., one-bead, one-compound) or biologic (e.g., phage-, mRNA-, or DNA-display) technologies (Qian et al. 2015; Chen and Heinis 2015; Bashiruddin and Suga 2015; Zhu et al. 2018; Appiah Kubi et al. 2019). Exemplary peptide and peptidomimetic modalities, including macrocyclic and CPP-conjugate peptides, are described (vide infra) relative to several intracellular targets (e.g., PTP1B, XIAP-BIR3, Mcl-1 BH3, p53-MDM2/4, Ras GTPase–Raf, NEMO, CTFR-CAL, and HIF-1α).
PTP1B Phosphatase
The tyrosine phosphatase PTP1B is ubiquitously expressed, including in tissues such as the liver, muscles, and fat that are responsive to insulin (Zhang and Zhang 2007; Zinker et al. 2002). There exists a great deal of biochemical, genetic, and pharmacological evidence implicating PTP1B as a negative regulator in insulin signaling. Specifically, PTP1B can interact with and dephosphorylate activated insulin receptor or insulin receptor substrates. Aberrant expression of PTP1B can contribute to diabetes and obesity in humans. Antisense-based oligonucleotides targeting PTP1B have advanced to clinical trials and have shown efficacy in type 2 diabetes (Elchebly et al. 1999). A highly potent peptidomimetic inhibitor (Fig. 8.2) of PTP1B has been discovered (Shen et al. 2001) which leveraged a nonhydrolyzable pTyr moiety and optimized interactions at adjacent binding pockets to the active site of PTP1B. Noteworthy is the potency and high selectivity of this peptidomimetic for PTP1B versus other phosphatases. Such PTP1B inhibitors may enhance insulin signaling and augment insulin-stimulated glucose uptake.
XIAP-BIR3
The X-chromosome-linked inhibitor of apoptosis (XIAP) and cytosolic inhibitor of apoptosis (cIAP) represent a family of proteins that act as inhibitors of caspases by direct interaction through their baculoviral IAP repeat (BIR) domains with caspases. Peptide, peptidomimetic, and small-molecule drug discovery has been focused on the BIR domains to identify inhibitors that would displace caspase-bound XIAP proteins (Abbas and Larisch 2020). TL32711 (Birinapant) is a novel bivalent peptidomimetic inhibitor (Fig. 8.2) that shows preferential binding to cIAP1 versus cIAP2 and XIAP, and it has advanced to clinical trials (Seigal et al. 2015).
MCL-1
The BH3 domain of proapoptotic intracellular protein BIM can bind to the BH3 hydrophobic groove of BCL-2 antiapoptotic proteins and directly activate the apoptotic effector proteins BAK and BAX. The hydrocarbon-stapled α-helical peptide BIM-SAHBA (Fig. 8.2) was designed relative to replacing a key salt bridge in an i to i + 4 manner to incorporate alpha-methylated amino acids having terminal alkene moieties on each of the two amino acid side chains that would undergo ring-closing metathesis (Edwards et al. 2015). BIM-SAHBA was found to primarily bind the intracellular antiapoptotic BCL-2 family protein MCL-1. Specificity studies showed MCL-1 knockout mouse embryonic fibroblasts were resistant to apoptosis induced by BIM-SAHBA (Hadji et al. 2019).
HIF-1α
Cells express a family of hypoxia-inducible transcription factors (HIFs) when under a state of reduced oxygen levels. HIFs are heterodimeric basic helix–loop–helix proteins composed of a regulatory α (HIF1α) and a constitutively expressed β (HIF1β; also termed ARNT) subunit. Furthermore, the C-terminal transactivation domain (CTAD) of HIF1α interacts with coactivator protein p300 (or its ortholog CREB binding protein [CBP]) (Semenza 2012). It is the HIF/p300 complex which then mediates transactivation of hypoxia-inducible genes that play major roles in cancer (e.g., angiogenesis, invasion, and altered energy metabolism (REF)). Relative to the discovery of inhibitors of the protein–protein interaction between HIF and p300, the structure-based design of novel oxopiperazine helical mimetic (OHM) HIF inhibitor OHM-1 (Fig. 8.2) was found to exhibit high binding affinity to p300 and both cellular and in vivo efficacy to reduce tumor burden in a triple-negative breast cancer xenograft mouse model (Lao et al. 2014). Noteworthy is the chiral OHM peptidomimetic template which bridges peptide backbone NH groups via ethylene to conformationally constrain OHM-1 into α-helix.
K-Ras GTPase
Another sought-after cancer target is that of Ras mutations, such as those occurring in K-Ras, N-Ras, and H-Ras (Khan et al. 2020). Recently, a small-molecule drug, sotorasib, which covalently forms a covalent bond to Cys-12 of mutated K-Ras, has been FDA approved for K-Ras G12C-mutated lung cancer (Skoulidis et al. 2021). A more desirable drug modality that may be able to target a greater range of mutated Ras in tumors remains a focus of intense research worldwide. A compelling macrocyclic peptide, KRpep-2d, that was first discovered from phage-display libraries was found to be the first selective inhibitor of K-Ras G12D, a predominant K-Ras mutation (Sakamoto et al. 2017). Subsequent lead optimization studies led to the bicyclic peptide, KS-58 (Fig. 8.2), that was shown to be active in vivo against K-Ras G12D-driven human pancreatic tumor xenografts (Sakamoto et al. 2020). It was also shown that a combination of KS-58 with gemcitabine resulted in enhanced antitumor activity. KS-58 incorporates N-to-C cyclization and a dithioether bridge between two Cys residues to create the conformationally constrained bicyclic peptide inhibitor.
NEMO
Nuclear factor kappa B (NF-κB) represents a family of transcription factors involved in the regulation of immune response, inflammation, cell differentiation, and cell survival (Zhang et al. 2017). Two different signaling pathways, one canonical and one canonical, lead to the NF-κB activation. With respect to canonical NF-κB signaling, cellular receptor activation results in an active inhibitor of κB (IκB) kinase (IKK) complex which consists of IKKα, IKKβ, and NEMO (a.k.a. IKKγ). Relative to IKK, inhibition of NEMO interaction is viewed compelling for anti-inflammatory and anticancer strategies as it may not modify basal NF-κB activity required for normal B- and T-cell function (Baima et al. 2010). Exploiting the concept that macrocyclic peptides may bind challenging protein–protein interactions, the design of a bicyclic NEMO-targeted peptide (Fig. 8.2) simultaneously incorporating cell-permeability properties was shown to effectively inhibit NEMO–IKKβ interaction as well as exhibit inhibition of canonical NF-κB signaling in mammalian cells and the proliferation of cisplatin-resistant ovarian cancer cells (Rhodes et al. 2018). The NEMO inhibitor incorporated a 1,3,5-tricarboxy-benzene moiety to provide a cross-linking bicyclic structure, and its cell permeability properties correlated with its cationic substructure (i.e., Arg residues) as well as its hydrophobicity and conformational constraints to ultimately confer partitioning into lipid membranes and triggering of endocytosis to drive cellular uptake.
CFTR-CAL
Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes for a chloride ion channel, is causative of cystic fibrosis (Dechecchi et al. 2018). Membrane expression of CFTR is negatively regulated by CFTR-associated ligand (CAL). Therefore, designing an inhibitor of CAL may rescue mutant CFTR function. Recently, the macrocyclic peptide PGD97 (Fig. 8.2) incorporating a disulfide capable of intracellular reduction was found (Dougherty et al. 2020) to have potent (low nM) and selective binding to CAL, good stability in serum, and efficacy in mutant F508del homozygous cells to increase short circuit currents as well as potential therapeutic effects of small-molecule correctors (e.g., VX-661). Therefore, PGD97 exemplifies a promising lead for the treatment of cystic fibrosis. This work provides incentive to further design strategies to create macrocyclic peptide “prodrugs” exploiting intracellular S-S ring opening to enable target binding properties.
p53–MDM2/4
The human transcription factor protein p53, and so-called guardian of the genome, is well known to induce cell cycle arrest and apoptosis in response to DNA damage and cellular stress and therefore has a critical role in protecting cells from malignant transformation (Eskandari et al. 2021; Ng et al. 2018; Carvajal et al. 2018). Inactivation of p53 by deletion or mutation or through overexpression of inhibitory proteins is most common in human cancers. Furthermore, cancers that overexpress the suppressor proteins MDM2 and MDMX, but have wild-type p53, provide the opportunity to restore p53-dependent cell cycle arrest and apoptosis if the MDM2 and MDM4 may be effectively blocked by inhibitors. Both aberrant MDM2 overexpression and gene amplification as well as that of MDM4 exist in many tumors. The first potent and selective small-molecule inhibitors of MDM2, the so-called Nutlins (Vassilev 2005), provided proof of concept that restoration of p53 activity is a promising approach to cancer therapy. Nevertheless, these and other small-molecule efforts were limited to only MDM2 specificity, and essentially all were inactive against MDM4. In contrast, p53 mimicry via the design of the potent and in vivo effective stapled α-helical peptide ATSP-7041 (Chang et al. 2013) (Fig. 8.2) exemplified a key benchmark for this peptide modality as related to its collective structural features (e.g., α-methyl-amino acids and cyclization by hydrocarbon stapling), cell uptake properties (e.g., lipophilic partitioning and translocation from membrane to the cytosolic compartment), metabolic stability, and pharmacokinetic properties (Sawyer et al. 2018).
8.4 Unlocking the Secrets of Cell Permeability: Exploiting Innovative Tools
Of no surprise, over the past two decades there has been a profound focus on peptide modalities for intracellular targets in terms of drug design strategies that are becoming increasingly sophisticated in terms of exploiting innovative tools to tackle cell permeability and, with yet greater aspiration, oral bioavailability (Rosania and Thurber 2021; Hochman et al. 2021; Peier et al. 2021; Peraro et al. 2018; Furukawa et al. 2016, 2020; Sahni et al. 2020; Qian et al. 2014; Dougherty et al. 2019; Schwochert et al. 2016; Ahlbach et al. 2015; Bockus et al. 2015; Hewitt et al. 2015; Goetz et al. 2014; Wang et al. 2015; Aubry et al. 2010; Gordon et al. 2016). Such efforts include an increasing number of macrocyclic peptides being advanced with academia, biotech, and pharma focused on intracellularly targeted drug discovery and, along with it, oral bioavailability (Nielsen et al. 2015; Rafi et al. 2012; Guimaraes et al. 2012; Herce et al. 2014; Rezgui et al. 2016; LaRochelle et al. 2015). It is anticipated that accumulation of data from this work will enable QSAR models and predictive design in the future to exploit peptide modalities as novel therapeutics. Key physicochemical and biophysical properties of structurally diverse peptide and peptidomimetics can be systematically analyzed to support lead optimization (Table 8.1). Some properties that are being woven into QSAR models and predictive design strategies include molecular weight (e.g., 500–2000 dalton range), lipophilicity (experimental and/or calculated LogP and typically in the 2–5 range), H-bond donor and acceptors (typically seeking less H-bond donors to solvent via intramolecular H-bonding and/or masking by N-methylation), and polar surface area ([PSA], typically seeking lower PSA) (Holm et al. 2011). In this regard, benchmark peptides such as CsA and/or other well-characterized macrocyclic peptides having cell permeability properties by varying mechanisms would be highly recommended.
As illustrated in this chapter, the design of peptides and peptidomimetics is one of the most intriguing opportunities nowadays because of the opportunistic availability of chemically diverse amino acid building blocks as well as novel conformational constraints by backbone modifications and/or macrocyclization. Such unnatural amino acids include D-enantiomers, Nα-methylated amino acids, cyclic amino acids, Cα-methylated amino acids, β-amino acids, and a host of novel side chain modifications for many of these building blocks. Indeed, conformational diversity is deemed especially critical to such multifaceted design strategies for both target binding, cellular permeability, and metabolic stability as related to macrocyclic peptides of varying ring size, bicyclization, and related innovative chemistries for cyclization that may impact their overall physicochemical and biophysical properties.
As a relatively simplistic model to map the likely multiple ways by which peptides and peptidomimetics may achieve cell uptake, it is apparent that three major mechanisms for cell permeability include passive transport, lipophilic partitioning, and cationic partitioning (Fig. 8.3). First, in the case of CsA and an emerging constellation of CsA-like macrocyclic peptides that are achieving cell permeability via passive transport (Furukawa et al. 2016, 2020; Schwochert et al. 2016; Ahlbach et al. 2015; Bockus et al. 2015; Hewitt et al. 2015), there is great promise for this peptide modality to tackle targets which have been deemed virtually undruggable with small molecules. Likewise, highly modified peptidomimetics such as previously exemplified by those targeting HIV-1 protease, proteasome, HCV protease, and HIF/p300 (vide supra) provide framework for designing such molecules. Second, in the case of ATSP-7041 (vide supra) and other stapled α-helical peptides targeting intracellular protein–protein interactions (Levin 2015; Guerlavais and Sawyer 2014; Sawyer et al. 2018; Chang et al. 2013; Peier et al. 2021), the design of amphipathic molecules having a high propensity to partition into cell membranes and then undergo translocation to the cytosol is being recognized as another mechanism of cell uptake that has similar attributes to passive transport albeit yet different. In contrast to what may be considered somewhat counter-intuitive in terms of its physicochemical and biophysical properties, ATSP-7041 is both a lipophilic and quite soluble peptide of which the latter may be attributed to a single Glu within is sequence. Third, in the case of Arg-rich linear and macrocyclic CPPs (Appiah Kubi and Pei 2020; Rhodes et al. 2018; Dougherty et al. 2019, 2020; Sahni et al. 2020; Qian et al. 2014; Herce et al. 2014; LaRochelle et al. 2015; Holm et al. 2011), this peptide modality is well recognized to leverage cationic partitioning into cell membranes and undergo delivery into the cytosolic compartment via endocytic mechanisms. Obviously, the requirement for endosomal escape is critical to the optimization of such CPPs, and as exemplified by the macrocyclic CPP inhibitors of NEMO and CFTR-CAL (vide supra), there is significant promise to both intracellular targeted design and to create novel conjugates with other modalities (e.g., peptide, protein, and oligonucleotide) as is being pursued by Entrada Therapeutics.
Drug delivery remains integral to the future development of intracellularly targeted peptide and peptidomimetic therapeutics (Lemmer and Hamman 2013; Maher et al. 2016; Danielsen 2021; Brayden et al. 2020; Di 2015; Rader et al. 2018; Zizzari et al. 2021). Opportunities here include varying routes of administration, such as intravenous, subcutaneous, oral, and nasal. Collectively, biophysical, pharmacokinetic (PK), and absorption-distribution-metabolism-excretion properties remain extremely important for translating preclinical lead molecules to clinical candidates. Such properties may generally be “target agnostic” and have more to do with optimizing solubility, permeability, stability, and exposure levels in vivo to enable possible correlation between pharmacological efficacy and PK/ADME properties. Ultimately, the oral bioavailability potential to exploit to the high diversity of peptide modalities may also leverage formulations with permeability enhancers for either transcellular or paracellular transport.
8.5 Future Intracellularly Targeted Peptide Drugs: Clinical Trials and Beyond
Several intracellularly targeted peptidomimetics and peptides have been successfully advanced into clinical trials and/or FDA-approved. They include many FDA-approved peptidomimetics targeting HIV-1 protease (e.g., saquinavir by Hoffmann-La Roche, ritonavir by AbbVie, indinavir by Merck & Co., nelfinavir by Hoffman-La Roche, amprenavir by GlaxoSmithKline, lopinavir by AbbVie, atazanavir by Bristol-Myers Squibb, fosamprenavir by GlaxoSmithKline, tipranavir by Boehringer Ingelheim, and darunavir by Janssen Therapeutics), proteasome (e.g., bortezomib by Millennium Pharmaceuticals, carfilzomib by Onyx Pharmaceuticals, and ixazomib by Takeda), and HCV protease (e.g., grazoprevir by Merck, telaprevir by Vertex Pharmaceuticals and Johnson & Johnson, glecaprevir by AbbVie, and paritaprevir by Abbvie). Noteworthy is the Phase 2 clinical development of the stapled α-helical peptide ALRN-6924. Furthermore, numerous other macrocyclic peptides are undergoing intense preclinical development or are entering clinical trials for various intracellularly targets, including both enzymes and protein–protein interactions as described in this chapter and others (e.g., transcription factors β-catenin and Myc/Max). Lastly, albeit not intracellularly targeted in the classic sense, but cell membrane targeted in terms of known mechanisms of action, are the FDA-approved antibiotic peptides (e.g., Orbactiv by the Med Company, Dalvance by Vicuron, and Cubicin by Cubist/MSD).
Beyond the realm of specific preclinical and clinical development of promising novel peptide and peptidomimetic therapeutics for intracellular targets, it is of the utmost importance to also highlight the fact that many biotech companies have contributed significantly in terms of innovative platforms to advance such peptide modality-inspired medicines. Examples of these companies include (A–Z listing) Aileron Therapeutics, Aplomex, Circle Pharma, Entrada Therapeutics, FAKnostics, Fog Pharma, IDP Pharma, Nimble Therapeutics, Orbit Discovery, PeptiDream, Polyphor, Promakhos Therapeutics, ProteXase Therapeutics, Ra Pharma (now UCB), SyntheX, Spotlight Therapeutics, and Unnatural Products.
References
Abbas R, Larisch S. Targeting XIAP for promoting cancer cell death-the story of ARTS and SMAC. Cells. 2020;9:663.
Adams J. Development of the proteasome inhibitor PS-341. Oncologist. 2002;7:9–16.
Adams J. The development of proteasome inhibitors as anticancer drugs. Cancer Cell. 2004;5:417–21.
Ahlbach CL, Lexa KW, Bockus AT, Chen V, Crews P, Jacobson MP, Lokey RS. Beyond cyclosporine A: conformation-dependent passive membrane permeabilities of cyclic peptide natural products. Future Med Chem. 2015;7:2121–30.
Appels NM, Beijnen JH, Schellens JH. Development of farnesyl transferase inhibitors: a review. Oncologist. 2005;10:565–78.
Appiah Kubi G, Pei D. Cell-penetrating and mitochondrion-targeting molecules. Methods Enzymol. 2020;641:311–28.
Appiah Kubi G, Dougherty PG, Pei D. Designing cell-permeable macrocyclic peptides. Methods Mol Biol. 2019;2001:41–59.
Aubry S, Aussedat B, Delaroche D, Jiao CY, Bolbach G, Lavielle S, Chassaing G, Sagan S, Burlina F. MALDI-TOF mass spectrometry: a powerful tool to study the internalization of cell-penetrating peptides. Biochim Biophys Acta. 2010;1798:2182–9.
Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191:5785–91.
Baima ET, Guzova JA, Mathialagan S, Nagiec EE, Hardy MM, Song LR, Bonar SL, Weinberg RA, Selness SR, Woodard SS, Chrencik J, Hood WF, Schindler JF, Kishore N, Mbalaviele G. Novel insights into the cellular mechanisms of the anti-inflammatory effects of NF-kappaB essential modulator binding domain peptides. J Biol Chem. 2010;285:13498–506.
Bashiruddin NK, Suga H. Construction and screening of vast libraries of natural product-like macrocyclic peptides using in vitro display technologies. Curr Opin Chem Biol. 2015;24:131–8.
Bockus AT, Lexa KW, Pye CR, Kalgutkar AS, Gardner JW, Hund KC, Hewitt WM, Schwochert JA, Glassey E, Price DA, Mathiowetz AM, Liras S, Jacobson MP, Lokey RS. Probing the physicochemical boundaries of cell permeability and Oral bioavailability in lipophilic macrocycles inspired by natural products. J Med Chem. 2015;58:4581–9.
Bohacek RS, Dalgarno DC, Hatada M, Jacobsen VA, Lynch BA, Macek KJ, Merry T, Metcalf CA 3rd, Narula SS, Sawyer TK, Shakespeare WC, Violette SM, Weigele M. X-ray structure of citrate bound to Src SH2 leads to a high-affinity, bone-targeted Src SH2 inhibitor. J Med Chem. 2001;44:660–3.
Brayden DJ, Hill TA, Fairlie DP, Maher S, Mrsny RJ. Systemic delivery of peptides by the oral route: formulation and medicinal chemistry approaches. Adv Drug Deliv Rev. 2020;157:2–36.
Carvajal LA, Neriah DB, Senecal A, Benard L, Thiruthuvanathan V, Yatsenko T, Narayanagari SR, Wheat JC, Todorova TI, Mitchell K, Kenworthy C, Guerlavais V, Annis DA, Bartholdy B, Will B, Anampa JD, Mantzaris I, Aivado M, Singer RH, Coleman RA, Verma A, Steidl U. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci Transl Med. 2018;10:eaao3003.
Chang YS, Graves B, Guerlavais V, Tovar C, Packman K, To KH, Olson KA, Kesavan K, Gangurde P, Mukherjee A, Baker T, Darlak K, Elkin C, Filipovic Z, Qureshi FZ, Cai H, Berry P, Feyfant E, Shi XE, Horstick J, Annis DA, Manning AM, Fotouhi N, Nash H, Vassilev LT, Sawyer TK. Stapled alpha-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc Natl Acad Sci U S A. 2013;110:E3445–54.
Chatterjee J, Laufer B, Kessler H. Synthesis of N-methylated cyclic peptides. Nat Protoc. 2012;7:432–44.
Chen S, Heinis C. Phage selection of bicyclic peptides based on two disulfide bridges. Methods Mol Biol. 2015;1248:119–37.
Danielsen EM. Intestinal permeation enhancers: lessons learned from studies using an organ culture model. Biochim Biophys Acta Biomembr. 2021;1863:183474.
Debouck C. The HIV-1 protease as a therapeutic target for AIDS. AIDS Res Hum Retrovir. 1992;8:153–64.
Debouck C, Gorniak JG, Strickler JE, Meek TD, Metcalf BW, Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987;84:8903–6.
Dechecchi MC, Tamanini A, Cabrini G. Molecular basis of cystic fibrosis: from bench to bedside. Ann Transl Med. 2018;6:334.
Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17:134–43.
Dougherty PG, Sahni A, Pei D. Understanding cell penetration of cyclic peptides. Chem Rev. 2019;119:10241–87.
Dougherty PG, Wellmerling JH, Koley A, Lukowski JK, Hummon AB, Cormet-Boyaka E, Pei D. Cyclic peptidyl inhibitors against CAL/CFTR interaction for treatment of cystic fibrosis. J Med Chem. 2020;63:15773–84.
Edwards AL, Wachter F, Lammert M, Huhn AJ, Luccarelli J, Bird GH, Walensky LD. Cellular uptake and ultrastructural localization underlie the pro-apoptotic activity of a hydrocarbon-stapled BIM BH3 peptide. ACS Chem Biol. 2015;10:2149–57.
Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–8.
Eskandari M, Shi Y, Liu J, Albanese J, Goel S, Verma A, Wang Y. The expression of MDM2, MDM4, p53 and p21 in myeloid neoplasms and the effect of MDM2/MDM4 dual inhibitor. Leuk Lymphoma. 2021;62:167–75.
Fogli S, Galimberti S, Gori V, Del Re M, Danesi R. Pharmacology differences among proteasome inhibitors: implications for their use in clinical practice. Pharmacol Res. 2021;167:105537.
Furukawa A, Townsend CE, Schwochert J, Pye CR, Bednarek MA, Lokey RS. Passive membrane permeability in cyclic peptomer scaffolds is robust to extensive variation in side chain functionality and backbone geometry. J Med Chem. 2016;59:9503–12.
Furukawa A, Schwochert J, Pye CR, Asano D, Edmondson QD, Turmon AC, Klein VG, Ono S, Okada O, Lokey RS. Drug-like properties in macrocycles above MW 1000: backbone rigidity versus side-chain lipophilicity. Angew Chem Int Ed Engl. 2020;59:21571–7.
Futaki S, Goto S, Suzuki T, Nakase I, Sugiura Y. Structural variety of membrane permeable peptides. Curr Protein Pept Sci. 2003;4:87–96.
Ghosh AK, Osswald HL, Prato G. Recent progress in the development of HIV-1 protease inhibitors for the treatment of HIV/AIDS. J Med Chem. 2016;59:5172–208.
Goetz GH, Philippe L, Shapiro MJ. EPSA: a novel supercritical fluid chromatography technique enabling the design of permeable cyclic peptides. ACS Med Chem Lett. 2014;5:1167–72.
Gordon LJ, Allen M, Artursson P, Hann MM, Leavens BJ, Mateus A, Readshaw S, Valko K, Wayne GJ, West A. Direct measurement of intracellular compound concentration by RapidFire Mass Spectrometry offers insights into cell permeability. J Biomol Screen. 2016;21:156–64.
Guerlavais V, Sawyer TK. Advancements in stapled peptide drug discovery & development. Annu Rep Med Chem. 2014;49:331–45.
Guimaraes CR, Mathiowetz AM, Shalaeva M, Goetz G, Liras S. Use of 3D properties to characterize beyond rule-of-5 property space for passive permeation. J Chem Inf Model. 2012;52:882–90.
Hadji A, Schmitt GK, Schnorenberg MR, Roach L, Hickey CM, Leak LB, Tirrell MV, LaBelle JL. Preferential targeting of MCL-1 by a hydrocarbon-stapled BIM BH3 peptide. Oncotarget. 2019;10:6219–33.
Harper S, McCauley JA, Rudd MT, Ferrara M, DiFilippo M, Crescenzi B, Koch U, Petrocchi A, Holloway MK, Butcher JW, Romano JJ, Bush KJ, Gilbert KF, McIntyre CJ, Nguyen KT, Nizi E, Carroll SS, Ludmerer SW, Burlein C, DiMuzio JM, Graham DJ, McHale CM, Stahlhut MW, Olsen DB, Monteagudo E, Cianetti S, Giuliano C, Pucci V, Trainor N, Fandozzi CM, Rowley M, Coleman PJ, Vacca JP, Summa V, Liverton NJ. Discovery of MK-5172, a macrocyclic hepatitis C virus NS3/4a protease inhibitor. ACS Med Chem Lett. 2012;3:332–6.
Herce HD, Garcia AE, Cardoso MC. Fundamental molecular mechanism for the cellular uptake of guanidinium-rich molecules. J Am Chem Soc. 2014;136:17459–67.
Hewitt WM, Leung SS, Pye CR, Ponkey AR, Bednarek M, Jacobson MP, Lokey RS. Cell-permeable cyclic peptides from synthetic libraries inspired by natural products. J Am Chem Soc. 2015;137:715–21.
Hochman J, Sawyer T, Duggal R. Overcoming cellular and systemic barriers to design the next wave of peptide therapeutics. In: Quantitative analysis of cellular drug transport, disposition, and delivery. New York: Springer; 2021.
Holm T, Andaloussi SE, Langel U. Comparison of CPP uptake methods. Methods Mol Biol. 2011;683:207–17.
Kelly CN, Townsend CE, Jain AN, Naylor MR, Pye CR, Schwochert J, Lokey RS. Geometrically diverse lariat peptide scaffolds reveal an untapped chemical space of high membrane permeability. J Am Chem Soc. 2021;143:705–14.
Khan I, Rhett JM, O’Bryan JP. Therapeutic targeting of RAS: new hope for drugging the “undruggable”. Biochim Biophys Acta, Mol Cell Res. 2020;1867:118570.
Koren E, Torchilin VP. Cell-penetrating peptides: breaking through to the other side. Trends Mol Med. 2012;18:385–93.
Lao BB, Grishagin I, Mesallati H, Brewer TF, Olenyuk BZ, Arora PS. In vivo modulation of hypoxia-inducible signaling by topographical helix mimetics. Proc Natl Acad Sci U S A. 2014;111:7531–6.
LaRochelle JR, Cobb GB, Steinauer A, Rhoades E, Schepartz A. Fluorescence correlation spectroscopy reveals highly efficient cytosolic delivery of certain penta-arg proteins and stapled peptides. J Am Chem Soc. 2015;137:2536–41.
Lemmer HJ, Hamman JH. Paracellular drug absorption enhancement through tight junction modulation. Expert Opin Drug Deliv. 2013;10:103–14.
Levin JI. Macrocycles in drug discovery. Cambridge: Royal Society of Chemistry; 2015.
Lobell RB, Liu D, Buser CA, Davide JP, DePuy E, Hamilton K, Koblan KS, Lee Y, Mosser S, Motzel SL, Abbruzzese JL, Fuchs CS, Rowinsky EK, Rubin EH, Sharma S, Deutsch PJ, Mazina KE, Morrison BW, Wildonger L, Yao SL, Kohl NE. Preclinical and clinical pharmacodynamic assessment of L-778,123, a dual inhibitor of farnesyl:protein transferase and geranylgeranyl:protein transferase type-I. Mol Cancer Ther. 2002;1:747–58.
Maher S, Mrsny RJ, Brayden DJ. Intestinal permeation enhancers for oral peptide delivery. Adv Drug Deliv Rev. 2016;106:277–319.
Matthew AN, Zephyr J, Nageswara Rao D, Henes M, Kamran W, Kosovrasti K, Hedger AK, Lockbaum GJ, Timm J, Ali A, Kurt Yilmaz N, Schiffer CA. Avoiding drug resistance by substrate envelope-guided design: toward potent and robust HCV NS3/4A protease inhibitors. mBio. 2020;11:e00172–20.
McQuade TJ, Tomasselli AG, Liu L, Karacostas V, Moss B, Sawyer TK, Heinrikson RL, Tarpley WG. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science. 1990;247:454–6.
Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–60.
Naylor MR, Bockus AT, Blanco MJ, Lokey RS. Cyclic peptide natural products chart the frontier of oral bioavailability in the pursuit of undruggable targets. Curr Opin Chem Biol. 2017;38:141–7.
Naylor MR, Ly AM, Handford MJ, Ramos DP, Pye CR, Furukawa A, Klein VG, Noland RP, Edmondson Q, Turmon AC, Hewitt WM, Schwochert J, Townsend CE, Kelly CN, Blanco MJ, Lokey RS. Lipophilic permeability efficiency reconciles the opposing roles of lipophilicity in membrane permeability and aqueous solubility. J Med Chem. 2018;61:11169–82.
Ng SY, Yoshida N, Christie AL, Ghandi M, Dharia NV, Dempster J, Murakami M, Shigemori K, Morrow SN, Van Scoyk A, Cordero NA, Stevenson KE, Puligandla M, Haas B, Lo C, Meyers R, Gao G, Cherniack A, Louissaint A Jr, Nardi V, Thorner AR, Long H, Qiu X, Morgan EA, Dorfman DM, Fiore D, Jang J, Epstein AL, Dogan A, Zhang Y, Horwitz SM, Jacobsen ED, Santiago S, Ren JG, Guerlavais V, Annis DA, Aivado M, Saleh MN, Mehta A, Tsherniak A, Root D, Vazquez F, Hahn WC, Inghirami G, Aster JC, Weinstock DM, Koch R. Targetable vulnerabilities in T- and NK-cell lymphomas identified through preclinical models. Nat Commun. 2018;9:2024.
Nielsen DS, Lohman RJ, Hoang HN, Hill TA, Jones A, Lucke AJ, Fairlie DP. Flexibility versus rigidity for orally bioavailable cyclic hexapeptides. Chembiochem. 2015;16:2289–93.
Nielsen DS, Shepherd NE, Xu W, Lucke AJ, Stoermer MJ, Fairlie DP. Orally absorbed cyclic peptides. Chem Rev. 2017;117:8094–128.
Peier A, Ge L, Boyer N, Frost J, Duggal R, Biswas K, Edmondson S, Hermes JD, Yan L, Zimprich C, Sadruddin A, Kristal Kaan HY, Chandramohan A, Brown CJ, Thean D, Lee XE, Yuen TY, Ferrer-Gago FJ, Johannes CW, Lane DP, Sherborne B, Corona C, Robers MB, Sawyer TK, Partridge AW. NanoClick: a high throughput, target-agnostic peptide cell permeability assay. ACS Chem Biol. 2021;16:293–309.
Peraro L, Deprey KL, Moser MK, Zou Z, Ball HL, Levine B, Kritzer JA. Cell penetration profiling using the chloroalkane penetration assay. J Am Chem Soc. 2018;140:11360–9.
Pooga M, Langel U. Classes of cell-penetrating peptides. Methods Mol Biol. 2015;1324:3–28.
Pye CR, Hewitt WM, Schwochert J, Haddad TD, Townsend CE, Etienne L, Lao Y, Limberakis C, Furukawa A, Mathiowetz AM, Price DA, Liras S, Lokey RS. Nonclassical size dependence of permeation defines bounds for passive adsorption of large drug molecules. J Med Chem. 2017;60:1665–72.
Qian Z, LaRochelle JR, Jiang B, Lian W, Hard RL, Selner NG, Luechapanichkul R, Barrios AM, Pei D. Early endosomal escape of a cyclic cell-penetrating peptide allows effective cytosolic cargo delivery. Biochemistry. 2014;53:4034–46.
Qian Z, Upadhyaya P, Pei D. Synthesis and screening of one-bead-one-compound cyclic peptide libraries. Methods Mol Biol. 2015;1248:39–53.
Rader AFB, Weinmuller M, Reichart F, Schumacher-Klinger A, Merzbach S, Gilon C, Hoffman A, Kessler H. Orally active peptides: is there a magic bullet? Angew Chem Int Ed Engl. 2018;57:14414–38.
Rafi SB, Hearn BR, Vedantham P, Jacobson MP, Renslo AR. Predicting and improving the membrane permeability of peptidic small molecules. J Med Chem. 2012;55:3163–9.
Rezgui R, Blumer K, Yeoh-Tan G, Trexler AJ, Magzoub M. Precise quantification of cellular uptake of cell-penetrating peptides using fluorescence-activated cell sorting and fluorescence correlation spectroscopy. Biochim Biophys Acta. 2016;1858:1499–506.
Rhodes CA, Dougherty PG, Cooper JK, Qian Z, Lindert S, Wang QE, Pei D. Cell-permeable bicyclic peptidyl inhibitors against NEMO-IkappaB kinase interaction directly from a combinatorial library. J Am Chem Soc. 2018;140:12102–10.
Roberts NA, Martin JA, Kinchington D, Broadhurst AV, Craig JC, Duncan IB, Galpin SA, Handa BK, Kay J, Krohn A, et al. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990;248:358–61.
Rosania GR, Thurber GM. Quantitative analysis of cellular drug transport, disposition, and delivery. New York: Springer; 2021.
Sahni A, Qian Z, Pei D. Cell-penetrating peptides escape the endosome by inducing vesicle budding and collapse. ACS Chem Biol. 2020;15:2485–92.
Sakamoto K, Kamada Y, Sameshima T, Yaguchi M, Niida A, Sasaki S, Miwa M, Ohkubo S, Sakamoto JI, Kamaura M, Cho N, Tani A. K-Ras(G12D)-selective inhibitory peptides generated by random peptide T7 phage display technology. Biochem Biophys Res Commun. 2017;484:605–11.
Sakamoto K, Masutani T, Hirokawa T. Generation of KS-58 as the first K-Ras(G12D)-inhibitory peptide presenting anti-cancer activity in vivo. Sci Rep. 2020;10:21671.
Sawyer TK. Renaissance in peptide drug discovery: the third wave. In: Peptide-based drug discovery: challenges and new therapeutics. Cambridge: Royal Society of Chemistry; 2017.
Sawyer TK, Bohacek RS, Dalgarno DC, Eyermann CJ, Kawahata N, Metcalf CA 3rd, Shakespeare WC, Sundaramoorthi R, Wang Y, Yang MG. SRC homology-2 inhibitors: peptidomimetic and nonpeptide. Mini Rev Med Chem. 2002;2:475–88.
Sawyer TK, Partridge AW, Kaan HYK, Juang YC, Lim S, Johannes C, Yuen TY, Verma C, Kannan S, Aronica P, Tan YS, Sherborne B, Ha S, Hochman J, Chen S, Surdi L, Peier A, Sauvagnat B, Dandliker PJ, Brown CJ, Ng S, Ferrer F, Lane DP. Macrocyclic alpha helical peptide therapeutic modality: a perspective of learnings and challenges. Bioorg Med Chem. 2018;26:2807–15.
Schwochert J, Lao Y, Pye CR, Naylor MR, Desai PV, Gonzalez Valcarcel IC, Barrett JA, Sawada G, Blanco MJ, Lokey RS. Stereochemistry balances cell permeability and solubility in the naturally derived phepropeptin cyclic peptides. ACS Med Chem Lett. 2016;7:757–61.
Seigal BA, Connors WH, Fraley A, Borzilleri RM, Carter PH, Emanuel SL, Fargnoli J, Kim K, Lei M, Naglich JG, Pokross ME, Posy SL, Shen H, Surti N, Talbott R, Zhang Y, Terrett NK. The discovery of macrocyclic XIAP antagonists from a DNA-programmed chemistry library, and their optimization to give lead compounds with in vivo antitumor activity. J Med Chem. 2015;58:2855–61.
Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–14.
Shakespeare W, Yang M, Bohacek R, Cerasoli F, Stebbins K, Sundaramoorthi R, Azimioara M, Chi V, Pradeepan S, Metcalf C. Structure-based design of an osteoclast-selective, nonpeptide src homology 2 inhibitor with in vivo antiresorptive activity. Proc Natl Acad Sci. 2000;97:9373–8.
Shen K, Keng YF, Wu L, Guo XL, Lawrence DS, Zhang ZY. Acquisition of a specific and potent PTP1B inhibitor from a novel combinatorial library and screening procedure. J Biol Chem. 2001;276:47311–9.
Skoulidis F, Li BT, Dy GK, Price TJ, Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F, Kato T, Curioni-Fontecedro A, Sacher A, Spira A, Ramalingam SS, Takahashi T, Besse B, Anderson A, Ang A, Tran Q, Mather O, Henary H, Ngarmchamnanrith G, Friberg G, Velcheti V, Govindan R. Sotorasib for lung cancers with KRAS p.G12C mutation. N Engl J Med. 2021;384:2371–81.
Stahelin HF. The history of cyclosporin A (Sandimmune) revisited: another point of view. Experientia. 1996;52:5–13.
Vassilev LT. p53 activation by small molecules: application in oncology. J Med Chem. 2005;48:4491–9.
Vives E. Cellular uptake [correction of utake] of the Tat peptide: an endocytosis mechanism following ionic interactions. J Mol Recognit. 2003;16:265–71.
Wang CK, Northfield SE, Swedberg JE, Colless B, Chaousis S, Price DA, Liras S, Craik DJ. Exploring experimental and computational markers of cyclic peptides: charting islands of permeability. Eur J Med Chem. 2015;97:202–13.
Zhang S, Zhang ZY. PTP1B as a drug target: recent developments in PTP1B inhibitor discovery. Drug Discov Today. 2007;12:373–81.
Zhang Q, Lenardo MJ, Baltimore D. 30 years of NF-kappaB: a blossoming of relevance to human pathobiology. Cell. 2017;168:37–57.
Zhu Z, Shaginian A, Grady LC, O’Keeffe T, Shi XE, Davie CP, Simpson GL, Messer JA, Evindar G, Bream RN, Thansandote PP, Prentice NR, Mason AM, Pal S. Design and application of a DNA-encoded macrocyclic peptide library. ACS Chem Biol. 2018;13:53–9.
Zinker BA, Rondinone CM, Trevillyan JM, Gum RJ, Clampit JE, Waring JF, Xie N, Wilcox D, Jacobson P, Frost L, Kroeger PE, Reilly RM, Koterski S, Opgenorth TJ, Ulrich RG, Crosby S, Butler M, Murray SF, McKay RA, Bhanot S, Monia BP, Jirousek MR. PTP1B antisense oligonucleotide lowers PTP1B protein, normalizes blood glucose, and improves insulin sensitivity in diabetic mice. Proc Natl Acad Sci U S A. 2002;99:11357–62.
Zizzari AT, Pliatsika D, Gall FM, Fischer T, Riedl R. New perspectives in oral peptide delivery. Drug Discov Today. 2021;26:1097–105.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sawyer, T.K. (2022). Emerging Peptide Drug Modalities for Intracellular Target Space. In: Jois, S.D. (eds) Peptide Therapeutics. AAPS Advances in the Pharmaceutical Sciences Series, vol 47. Springer, Cham. https://doi.org/10.1007/978-3-031-04544-8_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-04544-8_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04543-1
Online ISBN: 978-3-031-04544-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)