Abstract
Immune checkpoint inhibitors (ICIs) represent one of the most exciting therapeutic advancements in the field of oncology. While myocarditis is one of the rarest side effects of immune checkpoint inhibitor (ICI) therapies with an incidence of 1%, it ranks as one of the deadliest with a mortality rate approaching 25–50% [1]. The nuances involved in diagnosing ICI-associated myocarditis and a wide range of symptoms characterizing the clinical presentation create a challenge in identifying with great specificity the disease in clinical trials, in clinical case series, and, ultimately, in patients [2]. The keys to diagnosis and treatment of ICI-associated myocarditis lie with a circumspect understanding of the various cardiac biomarkers, noninvasive imaging modalities, and invasive methods that are combined in conjunction with the patient’s clinical presentation to establish the diagnosis and treatment plan. While ICI-associated myocarditis is a clinically distinct entity, the emerging diagnosis and treatment strategies for this disease are founded on the diagnostic and treatment principles established for cardiotoxic chemotherapy agents, viral myocarditis, and cardiac allograft rejection. However, ICI-associated myocarditis remains unique in that there remains much work in not only seeking a uniform definition for the disease process but also in discovering increasingly specific biomarkers and novel imaging techniques to further aid in diagnosis. Furthermore, while high-dose steroids are acknowledged as a mainstay treatment for the disease, the discovery of second-line agents that may successfully control disease progression is still underway, in addition to identifying the patient characteristics for those at highest risk of failing frontline therapies.
Chapter debriefing: Power point video presentation is provided at the end of the chapter.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Immune checkpoint inhibitors
- Immunotherapy
- Immune-related adverse events
- Myocarditis
- Cardiotoxicity
- Cardio-oncology
Available Immune Checkpoint Inhibitors
As of 2021, the immune checkpoint inhibitor (ICI) therapies available in the United States are cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) inhibitor (ipilimumab), three PD-1 inhibitors (pembrolizumab, nivolumab, and cemiplimab), and three PD-L1 inhibitors (atezolizumab, avelumab, and durvalumab) [3]. In cases of untreated or metastatic melanoma, ipilimumab and nivolumab monotherapy have individually improved survival in these patients [4, 5]. Later studies showed improved survival and antitumor activity using combination ICI therapy (nivolumab and ipilimumab) for untreated melanoma [6]. Since then, the use of ICI has been expanded to several malignancies including many genitourinary cancers and lung cancers [7, 8]. With the increased use of ICI as both first-line cancer therapy and combination therapy, clinicians must be aware of the potential for myocarditis and be vigilant to diagnose and treat the potentially fatal cardiotoxicity.
Cardiac Side Effect Profile of Immune Checkpoint Inhibitors
The side effects of ICI are called immune-related adverse events (irAEs) for which several grading scales exist. In oncology clinical trials, the Common Terminology Criteria for Adverse Events (CTCAE) are often used; however, due to limitations in these criteria for irAEs, the American Society of Clinical Oncology (ASCO) released clinical practice guidelines in 2018 for grading the severity of irAEs specifically [9]. Differences between the CTCAE grading of myocarditis and the ASCO guidelines can be seen in Table 2.1. In addition, the ASCO guidelines provide expert consensus recommendations for treatment of different grades of myocarditis. It should also be noted that myocarditis is not the only potential cardiac irAE from ICI therapy. The ASCO guidelines group the grading of irAE to include all cardiac irAEs which include myocarditis, pericarditis, arrhythmias, impaired ventricular function with heart failure, and vasculitis. In addition to pericarditis, recurrent pericardial effusion requiring pericardiocentesis has been recognized with ICI therapy [10]. Also, a recent study by Drobni et al. showed that patients on ICI had increased risk of atherosclerotic events (HR 3.3, 95% CI 2.0–5.5, p < 0.001) compared to controls [11]. The role of inflammation in atherosclerosis is well established, and more studies are needed to see if ICIs lead to increased atherosclerotic events. Due to the limited literature regarding other cardiac adverse events and the high mortality of myocarditis, this chapter will focus only on diagnosing and treating ICI-associated myocarditis.
Mechanisms of Myocardial Toxicity
While the precise mechanism of ICI-associated myocarditis is unknown, work has been done to elucidate it. Researchers at the Vanderbilt-Ingram Cancer Center performed histopathological analyses of the hearts of two patients with metastatic melanoma who had fatal reactions to one infusion of the ipilimumab-nivolumab combination [12]. The histopathology in one patient demonstrated patches of highly concentrated lymphocytic infiltrate within the myocardium, sinus node, and atrioventricular node. Isolated myocytes within the skeletal muscle were targeted for destruction by lymphocytes [12]. The histopathology of the second patient similarly showed evidence of lymphocytic myocarditis and myositis. To further characterize the nature of the destructive lymphocytes, the researchers performed next-generation sequencing of the CDR3 region and the antigen-binding portion of the T-cell receptor beta chain [12]. They found specific T-cell receptor sequences in infiltrates from the cardiac muscle, skeletal muscle, and tumors, suggesting that epitopes from each of these three tissues were recognized by the same T-cell clones [12]. Given that only striated muscle (cardiac and skeletal) was affected by the lymphocytes, it is also possible that the same T-cell receptor may be targeting a tumor antigen and a different but homologous muscle antigen. Finally, a third mechanism is that different T-cell receptors are targeting different antigens [12]. Further molecular studies with larger cohorts would be required to elucidate the exact mechanism of action.
ICI-Associated Myocarditis
The diagnosis of ICI-associated myocarditis can be problematic in the clinical setting because of the lack of a uniform definition and lack of specificity of many noninvasive imaging modalities. When studies report ICI-associated myocarditis, the incidence varies greatly and is likely due to lack of a widely accepted uniform approach to concretely establishing the diagnosis [1]. Decades before immune checkpoint inhibitors were in clinical use, the 1986 Dallas Criteria attempted to provide a histopathological designation for defining viral myocarditis, which requires an inflammatory infiltrate of the myocardial tissue and associated myocyte necrosis or damage that cannot be attributed to an ischemic event [13]. Endomyocardial biopsy for a tissue diagnosis is still considered the gold standard for myocarditis diagnosis [14, 15]; however, due to the invasive nature of this procedure and the need for a specialized center with pathologists experienced in interpreting cardiac pathology, its use is limited in the general clinical setting. For this reason, many reports of ICI-associated myocarditis rely on a combination of the clinical presentation, cardiac biomarker analysis, and noninvasive cardiac imaging (electrocardiogram, echocardiogram, cardiac magnetic resonance imaging) to diagnose myocarditis [2].
Overall, ICI-associated myocarditis is among the rarest but most fatal irAEs [16]. Surveying the literature, ICI-associated myocarditis has a reported incidence ranging from 0.04% to 1.14% with an associated mortality of 25–50% [3, 17]. For relative comparison with the incidence of other irAEs, Wang et al. conducted a query of the World Health Organization (WHO) pharmacovigilance database (VigiBase-VigiLyze) and performed a meta-analysis of published trials to establish the incidence of ICI-associated toxic effects [16]. For example, in anti-CTLA-4 deaths, 70% were usually from colitis [16]. Anti-PD-1/PD-L1-related fatalities were often from pneumonitis (35%), hepatitis (22%), and neurotoxic effects (15%) [16]. Combination PD-1/CTLA-4 deaths were frequently from colitis (37%) and myocarditis (25%) [16]. In a fatality rate analysis of the irAEs, myocarditis composed 39.7% of cases, whereas endocrine had 2%, colitis had 5% reported fatalities, and other organ system toxic effects ranged from 10% to 17% of reported fatal outcomes [16]. Additionally, in a retrospective review of 3545 patients treated with ICIs from 7 academic centers, the overall fatality rate from ICI-related events was 0.6%, and cardiac and neurologic events together composed 43% of those [16]. Initial pharmacovigilance studies published early in the acknowledgment of ICI-associated myocarditis showed that myocarditis only occurred in 0.27% of patients treated with a combination of ipilimumab and nivolumab [12]. However, as awareness of recognition of myocarditis has improved, more contemporary studies report a prevalence of myocarditis around 1% that is generally accepted [16, 17]. Other ICI-associated cardiotoxicities including pericardial tamponade, myocardial infarction, stroke, cardiac failure, and cardiorespiratory arrest approach a similar individual incidence rates ranging from 0.7% to 2.0%, according to a meta-analysis of 22 clinical trials of PD-1 and PD-L1 inhibitors for lung cancer [18].
The clinical presentation of ICI-associated myocarditis can range from the asymptomatic patient with slightly elevated troponin to the patient in cardiogenic shock on multiple pressors with advanced atrioventricular block and ventricular arrhythmias (Table 2.2) [1, 3]. Few diagnoses in medicine carry such a heterogeneous repertoire of presentations. The clinical presentation of myocarditis often mimics other common acute cardiac disorders such as acute coronary syndrome or heart failure with common symptoms of chest pain/pressure, dyspnea, orthopnea, and lower extremity edema [3] (Table 2.2).
Timing Onset of Myocarditis
Based on small patient cohorts, the first 2–3 months are high risk for onset of myocarditis, and patients need only one to two ICI doses before being at risk for myocarditis [3, 17]. This timing of onset is comparable to the overall onset of fatal toxic effects seen for all irAEs, which typically occurred within the first 1–3 months of therapy initiation for combination therapy, anti-PD-1, and ipilimumab monotherapy (median 14.5, 40, and 40 days, respectively) [16]. A breakdown of the timing of onset of irAEs per organ system seen with combination therapy shows that the renal, hepatic, endocrine, pulmonary, gastrointestinal, and dermatologic organ systems are affected at 3.75, 2.62, 2.16, 1.93, 1.63, and 0.71 months, respectively [19]. The median time to onset of myocarditis after ICI therapy is initiated is 34 days (range 21–75 days as recorded in 35 patients described by Mahmood et al. [17]). In another cohort of 30 patients analyzed by Escudier et al., patients were diagnosed with myocarditis at a median of 65 days (range 2–454 days) after an average of 3 infusions of the medicine [20]. In an analysis of 33 patients with ICI-associated myocarditis from VigiBase, the World Health Organization (WHO) database of individual safety case reports, the median onset to diagnosis was 27 days (range 5–155 days), with 76% occurring in the first 6 weeks. Of these patients, 64% had only received one or two doses of ICI [21]. In a Bristol-Myers Squibb corporate safety database of 20,594 patients, 18 drug-related severe adverse events of myocarditis were reported (0.09%). In patients receiving ipilimumab and nivolumab combination therapy, myocarditis occurred at a median of 17 days (range 13–64 days) after one dose of treatment [12]. Another observation worth noting is that combination ICI therapy tends to lead to increased observance of severe myocarditis events in association with severe myositis (grades 3–4) compared with single-agent use only (0.24% vs. 0.15%) [12]. Patients can present with multiple irAEs or overlap syndromes, and myocarditis most commonly overlaps with myositis and myasthenia gravis irAEs [16].
Diagnostic Testing Considerations in ICI-Associated Myocarditis
Given that the presenting symptoms of myocarditis have such a wide range of differential diagnoses including the spectrum of acute coronary syndrome, heart failure, pericardial effusion, and side effects of other irAEs, the diagnostic schema to begin an investigation into myocarditis should necessarily include testing for these other disease processes as well (Fig. 2.1) [1,2,2].
Troponin
Prior to patients receiving monotherapy or combination immunotherapy agents, there is general agreement among several authors in the cardio-oncology field; their proposed treatment algorithm is that these patients have a documented baseline troponin and electrocardiogram (ECG) [1, 2]. In particular, troponin I is preferred over troponin T due to its better specificity for myocardial injury, though it still can be elevated in other non-myocardial situations including chronic kidney disease and pulmonary embolus. Troponin T is additionally not preferred as a marker for ICI-associated myocarditis because in cases where the patient also has myositis, there are already elevated levels of creatine kinase and its isoforms as well as troponin T, which is a protein integral to the contraction of both skeletal and heart muscles [2]. Troponin I has a greater specificity for myocyte injury in patients with clinically suspected myocarditis than creatine kinase levels, and while superior to other markers, they are still non-specific and when normal do not exclude myocarditis [15]. The other value of troponin is it has both diagnostic and prognostic values with some studies showing a fourfold increase in major adverse cardiac events with higher troponin levels [17]. Some literatures based on expert opinion have recommended consideration of troponin surveillance early after ICI initiation; however, two small prospective single-arm studies have been limited by the low incidence of myocarditis with the majority of troponin elevations not being related to myocarditis but rather other etiologies [22, 23]. Early assessment to rule out myocarditis is essential to limit interruptions in ICI therapy if a surveillance strategy is used.
Natriuretic Peptide
The natriuretic peptides (B-type natriuretic peptide and NT-proBNP [N-terminal pro-B-type natriuretic peptide]) have been considered for the diagnosis of myocarditis, but they are not specific enough for this purpose. They generally indicate the degree of stress on the ventricles and thus are elevated in patients with heart failure exacerbations or severe left ventricular dysfunction. They may also be elevated in the setting of inflammation [2]. Since not all patients with myocarditis present in heart failure, normal natriuretic peptides should not exclude the diagnosis. A recent study evaluating the association between NT-proBNP levels and grade of myocarditis showed an association with troponin T levels but not with proBNP [24].
Electrocardiogram (ECG)
Electrocardiographic changes frequently accompany ICI-associated myocarditis. However, a patient may have a completely normal ECG and still have the diagnosis [25, 26]. While there is no specific sign on an ECG that determines whether a patient has ICI-associated myocarditis, there are some non-specific changes that can suggest myocarditis in the right clinical setting [3]. Patients admitted to the hospital with suspected myocarditis should be monitored on telemetry for early detection of arrhythmias and other electrical changes [3].
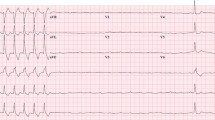
Complete atrioventricular block is the most common electrical complication of ICI-associated myocarditis (Fig. 2.2) [27]. ICI-associated myocarditis can present with conduction disturbances ranging from bundle branch block to complete heart block. Other inflammation-associated arrhythmias include sinus tachycardia, atrial fibrillation, ventricular tachycardia and fibrillation, and frequent supraventricular and ventricular premature beats and non-specific ECG changes such as Q wave formation, ST depression, and diffuse T wave inversions [3, 20, 25]. While some patients may have PR interval prolongation induced by ICI-associated myocarditis that resolves with treatment of the inflammation [3], one registry showed that overall patients with myocarditis did not have a prolonged PR interval [28].
Electrocardiograms of a patient who developed immune checkpoint inhibitor-associated myocarditis. (a) Baseline electrocardiogram 3 months prior to presentation with myocarditis. The electrocardiogram shows normal sinus rhythm with Q waves in V1 and V2 consistent with the patient’s history of a previous anteroseptal myocardial infarction. (b) Initial electrocardiogram upon presentation to the emergency room with dyspnea and fatigue after which patient was diagnosed with myocarditis by endomyocardial biopsy. The electrocardiogram shows complete heart block with a ventricular escape rhythm. (c) Electrocardiogram after one dose of 1000 mg methylprednisolone showing sinus rhythm with recovery of atrioventricular conduction; however, a left bundle branch block persisted
In an international registry comparing QRS duration and QTc interval between 140 myocarditis cases and 179 controls across multiple time points, it was found that the QRS duration (representing ventricular depolarization) prolonged with myocarditis, but the QTc interval (corrected using the Fridericia formula) remained unchanged [28]. The sensitivity for myocarditis with a QRS duration of >110 ms was determined to be 48.6% with a specificity of 87%, and a QRS duration of >130 ms yielded a sensitivity of 16.4% and a specificity of 92.6% [28]. In fact, an increase in the QRS duration of 10 milliseconds at the time of diagnosis of myocarditis conferred a 1.30-fold increase in the odds of major adverse cardiac events (MACE) including cardiovascular death, cardiac arrest, cardiogenic shock, and hemodynamically significant complete heart block [28].
Echocardiography
The echocardiogram is another noninvasive imaging modality to aid the clinician in the diagnosis of ICI-associated myocarditis [3, 29]. Echocardiography is fast, readily available, and relatively lower cost when compared to other imaging modalities, such as cardiac magnetic resonance (CMR) imaging. The ease of use of echocardiography makes it ideal for serial evaluations of the heart to investigate changes in clinical condition [30]. With increased use of point-of-care ultrasound, also known as bedside echocardiograms, patients presenting in the emergency room can be quickly examined to look for the presence of a new pericardial effusion, which would raise suspicion for myocarditis [3]. Escudier et al. reported that three-fourth of patients diagnosed with ICI-associated myocarditis developed left ventricular dysfunction on echocardiography [20]. However, Mahmood et al. reported that ejection fraction was within normal limits in more than half of the patients who suffered ICI-associated myocarditis [17]. A normal ejection fraction in ICI-associated myocarditis is not necessarily a sign of a benign course, which is why it is important especially in these persons to proceed to tests such as cardiac magnetic resonance imaging (MRI) to find evidence of myocardial inflammation and fibrosis [26].
Overall, the clinical guidelines are not unanimous regarding obtaining baseline echocardiograms prior to initiating ICI therapy. ASCO guidelines regarding managing irAEs in patients on ICI therapy are neither in favor of nor against obtaining one prior to therapy initiation [9], likely reflecting the aforementioned results of studies showing that a normal ejection fraction will not necessarily identify nor predict course in a patient with ICI-associated myocarditis. In contrast, ASCO guidelines regarding general cardiotoxicity of any cancer therapeutic recommend obtaining an echocardiogram prior to starting any of the potentially cardiotoxic standard chemotherapies such as anthracyclines [31]. With how uncommon myocarditis is, there may not be a benefit for performing echocardiogram at baseline in all patients, and once better risk factors for myocarditis are found, it may be useful in a higher risk subset.
Much work is being made in the field of echocardiography to utilize more sensitive methods in detecting ICI-associated myocarditis before symptoms manifest [32]. In fact, one methodology, two-dimensional speckle tracking echocardiography (2D-STE)-derived strain and strain rate, can detect changes in myocardial mechanics before changes in LVEF occur, so it aims to find preclinical signs of ventricular dysfunction [30]. To do this, 2D-STE uses software to assemble a global assessment of LV myocardial mechanics using three spatial dimensions of cardiac deformation – longitudinal, circumferential, and radial strain and strain rate [30]. In studies of patients treated with anthracyclines, taxanes, and trastuzumab, 2D-STE has shown early decreases in global longitudinal, circumferential, and radial strain or systolic or early diastolic strain rate [33,34,35,36,37]. Hypothesizing that global longitudinal strain (GLS) will be decreased in patients receiving ICIs just like those receiving standard cardiotoxic chemotherapy, Awadalla et al. retrospectively compared echocardiographic GLS by speckle tracking in cases of ICI-associated myocarditis (n = 101) from a large international multicenter registry with controls (n = 99) [38]. The summary of findings was that GLS decreases in patients with ICI-associated myocarditis and, furthermore, lower GLS was strongly associated with major adverse cardiac events (MACE) [38].
Cardiac Magnetic Resonance (CMR) Imaging
Cardiac magnetic resonance (CMR) imaging is the gold standard diagnostic imaging tool for myocarditis in the noninvasive arsenal of available tests [25]. CMR is not often used as a frontline screening tool due to its expense, lack of availability in certain hospital settings, incompatibility with other patient-worn devices including implantable cardioverter-defibrillators and pacemaker leads, longer test completion length, and intra-hospital transportation considerations in patients who are critically ill requiring intensive care unit stays and multiple complex life support machines [32].
The 2018 Lake Louise criteria, as put forth by the Journal of the American College of Cardiology Scientific Expert Panel, detail the different parametric mapping techniques that may be used to diagnose myocardial inflammation in patients in whom myocardial inflammation is likely active [39]. The original Lake Louise criteria were published in 2009, centering on three diagnostic characteristics of myocardial tissue which are (1) edema, (2) hyperemia, and (3) necrosis/scar, as seen on validated CMR imaging techniques such as T2-weighted imaging, early gadolinium enhancement (EGE), and late gadolinium enhancement (LGE) [39]. Using the 2009 criteria, if two out of three of the criteria were met, then there was a high likelihood of the presence of acute myocarditis (inflammation) [40].
The updated 2018 Lake Louise criteria have been validated by multiple studies as having a high diagnostic accuracy, with one meta-analysis reporting both a high sensitivity and high specificity at 80% and 87%, respectively [41]. The criteria are founded on the following principle: evidence of inflammatory myocardial injury is seen based on at least one T2-based criterion (global or regional increase of myocardial T2 relaxation time or an increased signal intensity in T2-weighted CMR images) and at least one T1-based criterion (increased myocardial T1, extracellular volume, or LGE). Thus, the current Lake Louise criteria are as follows:
-
1.
Main criteria (2 of 2): CMR highly suggests myocarditis with great specificity if both myocardial edema and nonischemic myocardial injury are identified. However, if only one of these two is identified, myocarditis may still be identified under the appropriate clinical circumstances.
-
(a)
Myocardial edema identified with abnormal findings on T2 mapping or T2-weighted images
-
(b)
Nonischemic myocardial injury identified with abnormal findings on T1 mapping, LGE, or extracellular volume fraction
-
(a)
-
2.
Supportive criteria (suggestive, but not diagnostic): These criteria support a diagnosis of myocarditis in a clinical setting that lacks the 2 of 2 main criteria.
-
(a)
Pericarditis
-
(i)
Evidence of pericardial effusion or abnormal LGE/T2 or T1 findings in pericardium
-
(i)
-
(b)
Left ventricular systolic dysfunction
-
(i)
Regional or global wall motion abnormalities
-
(i)
-
(a)
While CMR is less sensitive than endomyocardial biopsy at diagnosing myocarditis [9], it can provide more certainty in the clinician’s diagnosis of a patient with suspect ICI-associated myocarditis and obviate the need for invasive procedures [42].
When it comes to applying the CMR utility in the population of patients with ICI-associated myocarditis, there are some notable downfalls. In one series, 26% (8/31) patients diagnosed with ICI-associated myocarditis did not have LGE on CMR [17]. In another study, 77% (10/13) patients with the diagnosis of ICI-associated myocarditis who underwent CMR did not have LGE on CMR [20]. Using an international registry, Zhang et al. analyzed 103 ICI-associated myocarditis patients who also had a CMR and found that LGE on CMR was present in 48% overall, and elevated T2-weighted short tau inversion recovery (STIR) was present in 28% overall [43]. Delayed CMR imaging was noted to increase in sensitivity for detecting ICI-associated myocarditis as the presence of LGE was 21.6% when CMR was performed within 4 days of admission and increased to 72.0% when CMR was performed on day 4 of admission or later [43]. This data supports a possible explanation for patients with a negative LGE myocarditis, which is the scans were performed too early in the disease process to detect nonischemic myocardial injury. Fifty-six patients of the 103 registry patients in the Zhang et al. study had cardiac histopathology obtained, and LGE was present in 35% of patients with pathological fibrosis, and elevated T2-weighted STIR signal was present in 26% with a lymphocytic infiltration [43].
Endomyocardial Biopsy (EMB)
While CMR is the noninvasive diagnostic gold standard, endomyocardial biopsy (EMB) is the de facto gold standard test for the diagnosis of ICI-associated myocarditis [15, 32]. Historically, the histopathological diagnosis of myocarditis is based on the Dallas Classification System, devised in 1987 by eight cardiac pathologists known as the Dallas panel [13, 14]. The histopathological diagnosis of myocarditis is defined by myocyte necrosis and/or degeneration with adjacent inflammatory infiltrates [14, 44]. In ICI-associated myocarditis, immunohistochemical staining typically shows CD8+ T-cell infiltration intermixed with subsets of CD4+ T cells and CD68+ monocyte/macrophage lineages [24]. In addition, prominent expression of programmed death ligand 1 on immunohistochemical staining has been observed in areas of inflammatory infiltrate as displayed in Fig. 2.3 [24].
Endomyocardial biopsy of a patient with immune checkpoint inhibitor-associated myocarditis. (a) Hematoxylin and eosin stain at 20× power lens showing inflammatory infiltrate and myocyte loss consistent with myocarditis. (b) Programmed cell death ligand 1 immunohistochemistry stain showing diffuse uptake in corresponding areas of inflammatory infiltrate
Biopsy comes with several technical considerations. Myocarditis often affects the myocardium focally with patchy immune cell infiltration, and thus sampling error can occur if biopsies are not obtained from areas of myocarditis [14]. Obtaining samples from the affected areas is critical, or there is a risk of false negatives [14, 24]. At least five different samples help increase the yield [45]. However, despite current practice to optimize yield with 4–6 biopsies and attempts at targeting biopsy location via triangulation with CMR, one postmortem analysis of myocarditis cases determined that more than 17 samples were actually needed in order to accurately diagnose myocarditis in >80% of cases [46]. This highlights the limitations in the sensitivity of the EMB, which is overall 70% in pure myocarditis cases [46]. Furthermore, 17 biopsies are neither feasible nor safe in clinical practice, and the number of biopsies obtained to achieve diagnostic answers must be balanced with the risks of EMB. The most concerning of risks is perforation, which is reported in <1% by experienced operators. Overall, an endomyocardial biopsy is typically performed in less than 15% of myocarditis cases due to the above limitations and effectiveness of studies earlier in the diagnostic algorithm at providing reasonable evidence of ICI-associated myocarditis [28, 47].
Management
Currently there are no studies or randomized controlled trials evaluating treatment options for ICI-associated myocarditis [1, 3]. Treatments will vary by institution and local expertise. Current recommendations borrow from treatment of other irAEs and cardiac allograft rejection treatment strategies. Treatment of ICI-associated myocarditis consists of a dual-pronged approach including cessation of the culprit immunotherapy agent and early initiation of immunosuppression consisting of glucocorticoids in the form of oral prednisone and intravenous methylprednisolone [3, 17, 48]. There are data to suggest that patients receiving higher doses of corticosteroids (1–2 mg/kg/day) early in their disease onset exhibit recovery of left ventricular function and experience less MACE [17, 20, 49]. In clinical practice, the average time from admission to administration of steroids in the retrospective series by Mahmood et al. was 21.4 ± 16 hours (range 1–60 hours) [17]. From this retrospective series, the suggested treatment is 1000 mg of methylprednisolone daily for 3 days as a standard starting dose, with 1 mg/kg daily of either oral or intravenous steroids thereafter and a rapid taper over 4–6 weeks or until symptoms improve to grade 1 [17, 50]. ASCO clinical practice guidelines support this regimen for treatment of irAEs, suggesting initiation of methylprednisolone 1–2 mg/kg with tapering over also at least 4–6 weeks, with allowance for re-escalation as clinically needed [9]. In patients receiving glucocorticoid doses equivalent to greater than or equal to 20 mg of prednisone daily for 1 month or longer, it is important to remember to prescribe concomitant pneumocystis pneumonia prophylaxis [1]. Trimethoprim sulfamethoxazole can be given as one double-strength tablet daily (or three times per week) or as one single-strength tablet daily in patients with normal kidney function [51].
There is no consensus on the standard duration of treatment length [9]. Down-trending levels of troponin, improvements in LVEF, and resolution of conduction abnormalities are markers that can indicate that the treatment is effective, but these goals can take more than 6–12 weeks to accomplish [1]. In fact, trials for viral myocarditis treatment include steroid durations of at least 12 weeks and up to a year [3]. For ICI-associated myocarditis, isolated case reports track troponin levels to assess steroid response and increase the taper doses and extend the treatment duration if the levels increase [3]. If this strategy is not effective, immunomodulators are the next medications in the treatment arsenal for ICI-associated myocarditis (Fig. 2.4) [3].
In patients who do not improve on high-dose steroids, there are second-line options as detailed in various case reports or case series [3]. Various institutions have used different immunologic medications in these cases including intravenous immunoglobulin (pleiotropic immunomodulating actions) [52,53,54], anti-thymocyte globulin (depletes T lymphocytes) [55], mycophenolate (powerful inhibitor of lymphocyte proliferation) [53], infliximab (monoclonal antitumor necrosis factor alpha antibody) [24], plasmapheresis [24, 56], alemtuzumab (CD52 monoclonal antibody) [57], abatacept (a CTLA-4 agonist which blocks CD86/CD80-CD28 interaction) [58], and belatacept (a second-generation form of abatacept with higher binding affinity to CD86/CD80) [1, 3]. The overall effectiveness of these agents is unclear, as their use has been documented in a limited number of cases. Additionally, patients whose disease course progresses on high-dose steroids are typically very ill, requiring intensive care unit level of care [53]. Infliximab should be used with caution in these critically ill patients as it can worsen heart failure in patients with acute decompensated heart failure, though this risk appears dose-dependent [59]. Given that preclinical studies have shown that serum levels of tumor necrosis factor alpha are elevated in patients with heart failure and that severity of disease corresponds with higher levels, Chung et al. did a preliminary investigation asking the question of whether infliximab, the antibody to this inflammatory cytokine, would be helpful in patients with moderate to severe heart failure [59]. The authors found that neither low-dose (5 mg/kg) nor high-dose (10 mg/kg) infliximab improved the patient’s clinical heart failure symptoms despite effective suppression of cytokine levels, though the 5 mg/kg dose did confer a modest increase in ejection fraction [59]. Patients receiving 10 mg/kg infliximab had increased risk of death from any cause or increased hospitalization from heart failure that persisted for up to 5 months after the cessation of therapy, suggesting the dose-dependent nature of this therapy [59]. However, in diametric opposition to the infliximab data just presented and in spite of the black box warning of infliximab on its use in heart failure, Zhang et al. showed that in four of their patients with ICI-associated myocarditis who failed high-dose steroids and were in intensive care unit settings for acute decompensated heart failure or high-risk arrhythmia sequelae of their disease, a single dose of infliximab 5 mg/kg has been effective and safe [60]. All four patients survived their initial infliximab dosing without worsening heart failure [60]. Given the low numbers of patients involved in these cohorts or case reports secondary to low incidence of ICI-associated myocarditis, it is expected for future studies to often contradict each other, and once again, meta-analyses of more of these studies could help determine more definitive standards with regard to this second-line therapeutic.
Abatacept and belatacept, both classes of CTLA-4 agonists, work by inhibiting CD28-B7-mediated T-cell co-stimulation thus leading to rapid global T-cell deactivation, enacting a specific targeted reversal of the pathways that are activated by immune checkpoint inhibition [58]. Salem et al. used abatacept in a patient with metastatic lung cancer who had received three doses of nivolumab and subsequently developed myocarditis, with disease progression including troponin rise and high-burden ventricular ectopy even on high-dose intravenous methylprednisolone and plasmapheresis [58]. The introduction of abatacept reduced the troponin levels, premature ventricular contractions, and myositis, allowing her to be discharged 2 months later [58]. Alemtuzumab is an antibody to CD52, which is present on the surface of mature immune cells and leads to complement-mediated destruction of these peripheral immune cells [57]. Esfahani et al. reported a woman with stage IV melanoma who developed ICI-associated myocarditis after receiving pembrolizumab with disease progression in the form of life-threatening arrhythmias despite high-dose steroids, mycophenolate mofetil, plasmapheresis, and rituximab [57]. Initiation of a single cycle of alemtuzumab rapidly depleted T cells and resulted in termination of the arrhythmia, normalization of inflammatory markers, and recovery from the intensive care unit setting [57]. While these single cases show signs of promise in these immunosuppressive agents, larger randomized controlled trials would be needed to determine efficacy and dosing of these second-line therapies for ICI-associated myocarditis [3].
In cases of ICI-associated myocarditis, the ICI should be held during any signs of toxicity, even mild ones, because of the high mortality tied with ICI-associated myocarditis [3]. This is in contrast with management of other organ system irAEs, in which the ICI can be continued in cases of grade 1 toxicity [9]. Based on very limited data, restarting an ICI is not recommended after occurrence of ICI-associated myocarditis [9], but this view is controversial, and there are studies that indicate that the risk of recurrence is not as high as first thought [1]. One case report supporting holding ICI therapy indefinitely due to the risks details a man with metastatic melanoma who developed nivolumab-induced myocarditis after ten infusions and recovered on high-dose steroids opted to proceed again despite risks of fatality with single-agent pembrolizumab and subsequently died from recurrence of ICI-associated myocarditis and its complications after only one infusion [61]. In defense of attempting a second round of ICIs, re-trial of an ICI was conducted in 4 out of 30 patients in the Escudier et al. cohort without incidence of repeat ICI-associated myocarditis [20]. With limited data to guide decisions on restarting an ICI after ICI-associated myocarditis, the decision is typically made on an individualized basis in a multidisciplinary discussion taking into account cancer status and prognosis, prior responses and cardiotoxicity to immunotherapy, availability of alternatives, and patient preference after an informed discussion [68 Ganatra]. In patients experiencing severe (grade 3) or life-threatening (grade 4) toxicities, permanent discontinuation of ICI therapy is recommended as risks far outweigh benefits [9].
Advanced Management
Patients with critical acute decompensated heart failure requiring advanced support due to ICI-associated myocarditis should be under care in the intensive care unit and managed according to the American College of Cardiology/American Heart Failure guidelines [62]. In addition to diuretic drips, pressors, inotropes, mechanical circulatory support, and life-threatening arrhythmia management such as pacemakers (temporary or permanent), patients like these should be considered for second-line immunosuppressive therapies as described earlier on an individualized basis [3]. The general treatment principle for institutions regarding ICI-associated myocarditis is to treat as aggressively as possible as there are several cases of reversibility [60]. Goals of care discussions and palliative measures may also be appropriate depending on the clinical situation and multidisciplinary discussions with heart failure and cardiothoracic surgery and oncology [60].
Conclusion
With cancer surpassing cardiovascular disease as the major cause of mortality in some countries, treating cancer patients with immune checkpoint inhibitors is going to become more common [63]. ICI-associated myocarditis will become increasingly more relevant in the future as currently approximately 50% of the cancer population is eligible for immune checkpoint inhibitors [64]. The low frequency of ICI-associated myocarditis would almost be negligible in the consideration of giving ICI therapy were it not for its potential lethality [64]. Existing study outcomes vacillate with regard to positive or negative findings of certain treatments of ICI-associated myocarditis, as should be expected given the overall relatively low incidence of this irAE compared with others, making randomized controlled trials difficult to conduct. Future directions include gathering more extensive clinical data to guide standardization of diagnostic and therapeutic protocols versus institution- or experience-based protocols [64]. Benchwork and clinical translational laboratories involving biological samples from patients will play a large role in driving further illumination of the pathogenesis of ICI-associated myocarditis at the molecular and cellular levels, which will help guide the clinician’s methodology of diagnosis and treatment of this important disease [64].
Abbreviations
- ASCO:
-
American Society of Clinical Oncology
- CMR:
-
Cardiac magnetic resonance
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- ECG:
-
Electrocardiogram
- ICIs:
-
Immune checkpoint inhibitors
- irAE:
-
Immune-related adverse event
- MACE:
-
Major adverse cardiac events
References
Zhang L, Reynolds KL, Lyon AR, Palaskas N, Neilan TG. The evolving immunotherapy landscape and the epidemiology, diagnosis, and management of cardiotoxicity. JACC CardioOncol. 2021;3(1):35–47. https://doi.org/10.1016/j.jaccao.2020.11.012.
Bonaca MP, Olenchock BA, Salem JE, Wiviott SD, Ederhy S, Cohen A, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140(2):80–91. https://doi.org/10.1161/circulationaha.118.034497.
Palaskas N, Lopez-Mattei J, Durand JB, Iliescu C, Deswal A. Immune checkpoint inhibitor myocarditis: pathophysiological characteristics, diagnosis, and treatment. J Am Heart Assoc. 2020;9(2):e013757. https://doi.org/10.1161/jaha.119.013757.
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. https://doi.org/10.1056/NEJMoa1003466.
Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. https://doi.org/10.1056/NEJMoa1412082.
Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. https://doi.org/10.1056/NEJMoa1414428.
Thana M, Wood L. Immune checkpoint inhibitors in genitourinary malignancies. Curr Oncol. 2020;27(Suppl 2):S69–s77. https://doi.org/10.3747/co.27.5121.
Onoi K, Chihara Y, Uchino J, Shimamoto T, Morimoto Y, Iwasaku M, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):1362. https://doi.org/10.3390/jcm9051362.
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36(17):1714–68. https://doi.org/10.1200/jco.2017.77.6385.
Palaskas N, Morgan J, Daigle T, Banchs J, Durand JB, Hong D, et al. Targeted cancer therapies with pericardial effusions requiring pericardiocentesis focusing on immune checkpoint inhibitors. Am J Cardiol. 2019;123(8):1351–7. https://doi.org/10.1016/j.amjcard.2019.01.013.
Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK, et al. Association between immune checkpoint inhibitors with cardiovascular events and atherosclerotic plaque. Circulation. 2020;142(24):2299–311. https://doi.org/10.1161/CIRCULATIONAHA.120.049981.
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–55. https://doi.org/10.1056/NEJMoa1609214.
Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987;1(1):3–14.
Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol. 1987;18(6):619–24. https://doi.org/10.1016/s0046-8177(87)80363-5.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48, 48a–48d. https://doi.org/10.1093/eurheartj/eht210.
Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–8. https://doi.org/10.1001/jamaoncol.2018.3923.
Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71(16):1755–64. https://doi.org/10.1016/j.jacc.2018.02.037.
Hu YB, Zhang Q, Li HJ, Michot JM, Liu HB, Zhan P, et al. Evaluation of rare but severe immune related adverse effects in PD-1 and PD-L1 inhibitors in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2017;6(Suppl 1):S8–s20. https://doi.org/10.21037/tlcr.2017.12.10.
Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol. 2017;35(34):3815–22. https://doi.org/10.1200/jco.2016.72.1167.
Escudier M, Cautela J, Malissen N, Ancedy Y, Orabona M, Pinto J, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136(21):2085–7. https://doi.org/10.1161/circulationaha.117.030571.
Moslehi JJ, Salem J-E, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391(10124):933. https://doi.org/10.1016/S0140-6736(18)30533-6.
Waliany S, Neal Joel W, Reddy S, Wakelee H, Shah Sumit A, Srinivas S, et al. Myocarditis surveillance with high-sensitivity troponin I during cancer treatment with immune checkpoint inhibitors. JACC CardioOncol. 2021;3(1):137–9. https://doi.org/10.1016/j.jaccao.2021.01.004.
Lee Chuy K, Oikonomou EK, Postow MA, Callahan MK, Chapman PB, Shoushtari AN, et al. Myocarditis surveillance in patients with advanced melanoma on combination immune checkpoint inhibitor therapy: the Memorial Sloan Kettering Cancer Center experience. Oncologist. 2019;24(5):e196–e7. https://doi.org/10.1634/theoncologist.2019-0040.
Palaskas NL, Segura A, Lelenwa L, Siddiqui BA, Subudhi SK, Lopez-Mattei J, et al. (2021) Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail. https://doi.org/10.1002/ejhf.2265.
Sławiński G, Wrona A, Dąbrowska-Kugacka A, Raczak G, Lewicka E. Immune checkpoint inhibitors and cardiac toxicity in patients treated for non-small lung cancer: a review. Int J Mol Sci. 2020;21(19):7195.
Chen DY, Huang WK, Chien-Chia WV, Chang WC, Chen JS, Chuang CK, et al. Cardiovascular toxicity of immune checkpoint inhibitors in cancer patients: a review when cardiology meets immuno-oncology. J Formos Med Assoc. 2020;119(10):1461–75. https://doi.org/10.1016/j.jfma.2019.07.025.
Atallah-Yunes SA, Kadado AJ, Kaufman GP, Hernandez-Montfort J. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol. 2019;145(6):1527–57. https://doi.org/10.1007/s00432-019-02927-x.
Zlotoff DA, Hassan MZO, Zafar A, Alvi RM, Awadalla M, Mahmood SS, et al. Electrocardiographic features of immune checkpoint inhibitor associated myocarditis. J Immunother Cancer. 2021;9(3):e002007. https://doi.org/10.1136/jitc-2020-002007.
Gürdoğan M, Yalta K. Myocarditis associated with immune checkpoint inhibitors: practical considerations in diagnosis and management. Anatol J Cardiol. 2020;24(2):68–75. https://doi.org/10.14744/AnatolJCardiol.2020.79584.
Villarraga HR, Herrmann J, Nkomo VT. Cardio-oncology: role of echocardiography. Prog Cardiovasc Dis. 2014;57(1):10–8. https://doi.org/10.1016/j.pcad.2014.05.002.
Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35(8):893–911. https://doi.org/10.1200/jco.2016.70.5400.
Abraham Theodore P, Aras MA. Echo-strain to check up on checkpoint inhibitors∗. J Am Coll Cardiol. 2020;75(5):479–81. https://doi.org/10.1016/j.jacc.2019.11.048.
Fallah-Rad N, Walker JR, Wassef A, Lytwyn M, Bohonis S, Fang T, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57(22):2263–70. https://doi.org/10.1016/j.jacc.2010.11.063.
Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25(7):733–40. https://doi.org/10.1016/j.echo.2012.04.007.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107(9):1375–80. https://doi.org/10.1016/j.amjcard.2011.01.006.
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Tan TC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5(5):596–603. https://doi.org/10.1161/CIRCIMAGING.112.973321.
Stoodley PW, Richards DA, Hui R, Boyd A, Harnett PR, Meikle SR, et al. Two-dimensional myocardial strain imaging detects changes in left ventricular systolic function immediately after anthracycline chemotherapy. Eur J Echocardiogr. 2011;12(12):945–52. https://doi.org/10.1093/ejechocard/jer187.
Awadalla M, Mahmood SS, Groarke JD, Hassan MZO, Nohria A, Rokicki A, et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis. J Am Coll Cardiol. 2020;75(5):467–78. https://doi.org/10.1016/j.jacc.2019.11.049.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–76. https://doi.org/10.1016/j.jacc.2018.09.072.
Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis: a JACC White Paper. J Am Coll Cardiol. 2009;53(17):1475–87. https://doi.org/10.1016/j.jacc.2009.02.007.
Lagan J, Schmitt M, Miller CA. Clinical applications of multi-parametric CMR in myocarditis and systemic inflammatory diseases. Int J Cardiovasc Imaging. 2018;34(1):35–54. https://doi.org/10.1007/s10554-017-1063-9.
Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2018;11(11):1583–90. https://doi.org/10.1016/j.jcmg.2017.12.008.
Zhang L, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, Thuny F, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. 2020;41(18):1733–43. https://doi.org/10.1093/eurheartj/ehaa051.
Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113(6):876–90. https://doi.org/10.1161/circulationaha.105.584532.
Spiegelhalter DJ, Stovin PG. An analysis of repeated biopsies following cardiac transplantation. Stat Med. 1983;2(1):33–40. https://doi.org/10.1002/sim.4780020105.
Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64(10):1235–45. https://doi.org/10.1016/s0025-6196(12)61286-5.
Ammirati E, Cipriani M, Moro C, Raineri C, Pini D, Sormani P, et al. Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: Multicenter Lombardy Registry. Circulation. 2018;138(11):1088–99. https://doi.org/10.1161/circulationaha.118.035319.
Pirozzi F, Poto R, Aran L, Cuomo A, Galdiero MR, Spadaro G, et al. (2021) Cardiovascular toxicity of immune checkpoint inhibitors: clinical risk factors. Curr Oncol Rep 23(2):13–. https://doi.org/10.1007/s11912-020-01002-w.
Zhang L, Zlotoff DA, Awadalla M, Mahmood SS, Nohria A, Hassan MZO, et al. Major adverse cardiovascular events and the timing and dose of corticosteroids in immune checkpoint inhibitor–associated myocarditis. Circulation. 2020;141(24):2031–4. https://doi.org/10.1161/CIRCULATIONAHA.119.044703.
Puzanov I, Diab A, Abdallah K, Bingham CO 3rd, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. https://doi.org/10.1186/s40425-017-0300-z.
Stern A, Green H, Paul M, Vidal L, Leibovici L. Prophylaxis for pneumocystis pneumonia (PCP) in non-HIV immunocompromised patients. Cochrane Database Syst Rev. 2014;2014(10):CD005590. https://doi.org/10.1002/14651858.CD005590.pub3.
Norwood TG, Westbrook BC, Johnson DB, Litovsky SH, Terry NL, McKee SB, et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer. 2017;5(1):91. https://doi.org/10.1186/s40425-017-0296-4.
Arangalage D, Delyon J, Lermuzeaux M, Ekpe K, Ederhy S, Pages C, et al. Survival after fulminant myocarditis induced by immune-checkpoint inhibitors. Ann Intern Med. 2017;167(9):683–4. https://doi.org/10.7326/l17-0396.
Balanescu DV, Donisan T, Palaskas N, Lopez-Mattei J, Kim PY, Buja LM, et al. Immunomodulatory treatment of immune checkpoint inhibitor-induced myocarditis: pathway toward precision-based therapy. Cardiovasc Pathol. 2020;47:107211. https://doi.org/10.1016/j.carpath.2020.107211.
Tay RY, Blackley E, McLean C, Moore M, Bergin P, Gill S, et al. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer. 2017;117(7):921–4. https://doi.org/10.1038/bjc.2017.253.
Frigeri M, Meyer P, Banfi C, Giraud R, Hachulla A-L, Spoerl D, et al. Immune checkpoint inhibitor-associated myocarditis: a new challenge for cardiologists. Can J Cardiol. 2018;34(1):92.e1–3. https://doi.org/10.1016/j.cjca.2017.09.025.
Esfahani K, Buhlaiga N, Thébault P, Lapointe R, Johnson NA, Miller WH Jr. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380(24):2375–6. https://doi.org/10.1056/NEJMc1903064.
Salem JE, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380(24):2377–9. https://doi.org/10.1056/NEJMc1901677.
Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure. Circulation. 2003;107(25):3133–40. https://doi.org/10.1161/01.CIR.0000077913.60364.D2.
Zhang RS, Padegimas A, Murphy KM, Evans PT, Peters CJ, Domenico CM, et al. Treatment of corticosteroid refractory immune checkpoint inhibitor myocarditis with infliximab: a case series. Cardio-Oncology. 2021;7(1):13. https://doi.org/10.1186/s40959-021-00095-x.
Tajmir-Riahi A, Bergmann T, Schmid M, Agaimy A, Schuler G, Heinzerling L. Life-threatening autoimmune cardiomyopathy reproducibly induced in a patient by checkpoint inhibitor therapy. J Immunother. 2018;41(1):35–8. https://doi.org/10.1097/cji.0000000000000190.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23(8):628–51. https://doi.org/10.1016/j.cardfail.2017.04.014.
Dagenais GR, Leong DP, Rangarajan S, Lanas F, Lopez-Jaramillo P, Gupta R, et al. Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2020;395(10226):785–94. https://doi.org/10.1016/s0140-6736(19)32007-0.
Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L, Kitsis RN (2021) Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest 131(5). https://doi.org/10.1172/jci145186.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Electronic Supplementary Material
Data 2.1
(PPTX 76190 kb)
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kaczmarek, J.V., Palaskas, N.L. (2022). Cardiology (Heart). In: Wang, Y. (eds) Managing Immunotherapy Related Organ Toxicities. Springer, Cham. https://doi.org/10.1007/978-3-031-00241-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-00241-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-00240-3
Online ISBN: 978-3-031-00241-0
eBook Packages: MedicineMedicine (R0)