Abstract
Fractionated radiotherapy and stereotactic radiosurgery are commonly used for cranial base tumors. Typically, small volume tumors respond very well with limited complications and good tumor control to both radiation modalities. Tumor control is much better for benign WHO grade 1 tumors than grade 2 and 3 tumors. Radiosurgery is successfully implemented as part of a comprehensive treatment strategy with combination of micro- and radiosurgery. Radiosurgery is also increasingly adopted as a tool for long-term repeated treatment in management of tumors that have become chronic with repeated need for treatment of progression or recurrences. Radiation of larger volumes is an accepted part of management of locally aggressive tumors although better prospective data are needed to define the benefit in relation to natural history, recurrences, and alternative management strategies. Radiation treatment of larger tumors requires fractionation. Traceable data and prospective trials are lacking but treatment of larger volumes appears less successful.
Small tumors can usually be treated with stereotactic single-dose regimens and low morbidity, while larger, fractionated volumes are associated with more radiation toxicity and complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Radiation therapies are frequently used in the management of meningioma, schwannoma, chordoma, and chondrosarcoma. Radiation may either be administered to cure, “control” a visible tumor volume or to prevent regrowth after gross total surgical removal. In modern skull base era, combined functional preservation surgery and radiation therapy strategy has replaced aggressive morbid surgery. In some complex anatomical locations such as cavernous sinus, radiation therapies have become a safer primary treatment modality for several benign pathologies. Efficiency and complications are different for different treatment modalities and selection of management is dependent also on patient parameters and values. The management strategy is therefore best optimized with shared decision-making.
The commonest radiation tools comprise Gamma Knife radiosurgery and linear accelerator applications. The commonest strategies are single-dose radiosurgery (SRS) and fractionated radiation therapy (FRT); lately fractionation is increasingly used with stereotactic techniques for targeting.
Linear accelerators and the Gamma Knife provide gamma radiation: photons. Other treatments utilize neutrons, protons, or carbon ions. Radiotherapy for head- and neck malignances or pituitary tumors (Chap. 19) is not covered in this chapter.
History
Lars Leksell visited pioneer functional surgeon Henry Wycis in 1947 to learn stereotactic surgery and subsequently developed a new stereotactic apparatus based on an arc-principle. He soon decided to treat pain or movement disorders with radiation and the stereotactic technique to make lesion without the need of a craniotomy. The first design employed x-rays [16] and was followed by a proton-beam [19]. Leksell found the proton beam-technology too complex, while proton beam therapy was developed for medical application at the Harvard Cyclotrone Laboratory in collaboration with Drs. William Sweet and Raymond Kjellberg of the Massachusetts General Hospital [15]. For Lars Leksell, the Gamma Knife, where parallel photon-beams from cobalt-60 sources were focused to one target and allowed all rays to reach the focus simultaneously without a need for repositioning, became the most practical solution [17]. The design was adapted for spherical targets and applied for treatment of AVMs [34] and vestibular schwannomas [18]. Lars Leksell considered the Gamma Knife to be an exclusive tool with very limited indications and prognosticated that three Gamma Knives would cover the global need of radiosurgery. The indications have proven to be wider than expected by the inventor, but the meticulous effort to select an instrument is equally relevant for all neurosurgeons today: “Tools used by the surgeon must be adapted to the task and where the human brain is concerned, no tool can be too refined.”
Gamma Knife Radiosurgery (GKRS) and Single-Dose Radiosurgery (SRS)
GKRS is typically used with a curative intent for well-defined tumor volumes <10 mL, preferably benign tumors with WHO grade 1. Initially, the treatment goals for benign tumors were tumor shrinkage. The goal was then shifted from “shrinkage” to “control” to decrease cranial nerve morbidity.
Tumor Control
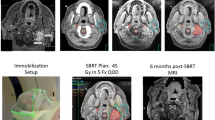
Control is defined as absence of growth compared to pretreatment scanning after 1–2 years. The 2-year limit compensates for the occurrence of temporary, reversible tumor swelling which is particularly common in treatment of schwannomas. Subsequently, the radiation doses were decreased without detectable loss of tumor control while radiation complications were minimized. Treatment has excellent 10-year tumor control for grade-1 meningiomas, schwannomas, and low-grade chondrosarcomas that can be treated with a prescription dose of >13 Gy. Figure 11.1 shows examples of radiosurgery results. Approximately 7% of meningiomas may need further treatment due to continued growth either within the targeted high-dose volume or 15% outside: “out of field recurrence” [21]. The former represent failure to control tumor with the intended dose, while the latter represent failure to identify a valid treatment target.
Out-of-field recurrences after Gamma Knife radiosurgery of a meningioma initially originating from the clivus. Example for a long-term response and local tumor control within the radiosurgically treated target. A recurrence outside the initial radiation field developed from the tumor’s “dural tail,” which is generally not included in the radiosurgical treatment field. The patient was retreated with Gamma Knife resulting in tumor regression even in the recurring/progressive parts but developed a further “out-of-field recurrence” within the right cavernous sinus 54 months after the initial treatment
Neurological Function Preservation
GKRS is not feasible for lesions of larger volume, since a single effective dose >13 Gy to a volume exceeding 10 mL will deliver inappropriate and potentially harmful radiation to surrounding parenchyma or nerves. The optic nerves and the brain stem are particularly sensitive and should not be exposed to more than 8 Gy. A shorter distance than 3 mm between the radiosurgical target and visual pathways does not allow safe radiosurgery. Complications depend on target and surrounding structures. Following SRS, general radiation morbidity such as fatigue, headaches, and vertigo is very rare, while cranial nerve complications are possible. The risk of trigeminal or facial nerve complications is 4–6% after SRS for vestibular schwannoma. Despite the highly focused radiation, exposure occurs outside the target and provides a small risk for radiation induced tumors [30]. It has also been suggested that radiation can induce malignant degeneration and recurrence of very aggressive tumors after radiosurgery is a rare but possible complication [6].
The choice between radio- and microsurgery as single therapy for small skull base meningiomas depends on location, differential diagnoses, and expected survival. For a treatment decision, expected benefit of radiosurgery should be compared to expected natural history without treatment. Indications to treat asymptomatic tumors that are not known to grow are weak. Treatment is indicated for growing lesions or lesions that are symptomatic. At best, radiosurgery reaches long-term control comparable to Simpson grade 2–3, so microsurgery is preferable if grade 1 removal without cranial nerve damage is possible and defendable. Treatment of small tumors that compress or irritate cranial nerves depends on location. Optic nerves must be decompressed microsurgically, while compression of nerves inside the cavernous sinus is better treated with radiation. Symptoms from nerves V to VIII are sometimes alleviated by radiosurgery but microsurgical decompression is frequently indicated to ameliorate symptoms. One must also consider the possibility that a radiological diagnosis is incorrect and evaluate the potential risk of inadvertently radiating an inflammatory or infectious process. A biopsy may be indicated for safe management.
GKRS in Combined Micro- and Radiosurgical Treatment for Meningiomas
GKRS provides 10-year control rates similar to Simpson grade 2 surgery for smaller meningiomas. Subsequently, a tailored subtotal removal (Simpson grade 4) followed by radiosurgery of the residual was hypothesized to provide similar tumor control as Simpson grade 2 (Fig. 11.2). Combined treatment, named “Simpson grade 4 gamma” [23], was clinically implemented and evaluated. Immediate neurological complications decreased, and retrospective case series supported hypotheses of 80–95% tumor control of complex cranial base meningiomas, which is equivalent to Simpson grade 1 surgery of such tumors.
Combined micro- and radiosurgery, the Simpson grade 4 gamma concept, is implemented as comprehensive treatment with microsurgery followed by radiosurgery within 3–6 months before additional follow-up. The rationale is that no part of the tumor is left untreated and it is expected that tumor control may be better if a tumor is treated before it has grown outside previous resection margins or undergone selection of aggressive tumor clones. Others argue that up-front radiosurgery adds risk while many meningioma residuals may not grow fast enough to require treatment. Hence, an alternative strategy is maximal safe removal with subsequent radiological follow-up and decision to offer radiosurgery if the residual grows. The limited available evidence supports better total long-term tumor control with comprehensive up-front treatment [23, 24]. Still, individual conditions vary and either strategy may be preferable depending on age, risk, and other options to treat potential recurrences. The latter situation also arises if a tumor thought to be cured recurs during follow-up. Literature suggests that recurrent meningiomas may have lower control rates than tumors that did not fail previous surgery or radiation [24].
SRS is increasingly employed as a complementary tool to microsurgery during long-term management of recurrent meningiomas. Such meningiomas have become viewed as a chronic disease with multiple recurrences that need repeated treatments rather than a disease that is operated and treated with adjuvant radiation.
GKRS for Schwannoma
GKRS is well established for small to moderately sized vestibular schwannomas and has, by analogy, been used for other schwannomas.
Tumor Control
A tumor <10 mL treated with a 13Gy margin dose had a control rate of 97% with after a mean follow-up of 6 years [4], 92% at a mean follow-up of 9 years [39], and 92% with a 10-year minimum (12.5-year mean) follow-up [11]. These high control-rates may have to be adjusted to compensate for the fact that many small tumors would not have grown during follow-up even without treatment and an adjusted control rate of 78–87% was suggested by Miller et al. [27]. In contrast, Wangerid et al. found no difference in tumor control between tumors with or without demonstrated growth, suggesting that control of schwannoma growth is independent of demonstrated growth. The radiation response in relation to tumor growth may thus differ between meningiomas and schwannomas. Fractionated radiation of 50 Gy in fractions of 1.8–2.0 Gy has been described as a successful treatment for vestibular schwannomas, but selection of cases and limited follow-up makes conclusions regarding long-term efficacy difficult. It appears that long-term control is also >95%, but cranial nerve complications are commoner with fractionated therapies than SRS [31]. It is technically possible to treat larger schwannomas with FRT than SRS, but literature data are mostly based on small tumor for both treatment modalities.
Neurological Function Preservation
Large tumors would require high radiation doses with ensuing risks of radiation damage to normal tissue. Instead, a combination of subtotal tailored microsurgery followed by immediate (within 3 months) or delayed SRS is employed to secure long-term control while minimizing risks of cranial nerve damage [26]. After subtotal surgery, approximately 30% of residual schwannomas grow within 10 years and SRS, either before demonstrated growth or after, provides 85–100% long-term tumor control [26]. For small Koos grade 1&2 vestibular schwannomas, hearing preservation is principally different between micro and radiosurgery. Analysis of time-based reporting shows a clear trend of gradually declined hearing during 10-year follow-up after radiation treatments, while patients who preserve hearing after microsurgical removal tend to maintain hearing [7]. Thus, young patients with vestibular schwannoma and excellent hearing may benefit from complete microsurgical removal of the tumor, provided hearing is preserved (Chap. 38).
Nonvestibular schwannomas are biologically similar to vestibular schwannomas and also appear to respond to radiosurgery [33].
GKRS for Other Skull Base Tumors
Aggressive tumors are treated with higher radiation prescription doses than benign tumors, typically >16 Gy. Recurrence rates are higher than for slowly growing tumors. Aggressive and atypical meningiomas had a 45% 5-year control rate [12], chordoma had a 50% 5-year control rate [8], and chondrosarcoma >65% [9]. GKRS is a tool for long-term management including retreatments of these tumors. Many patients are cured or controlled during long-term management. The most aggressive tumors within each group will become intractable from recurrences, regrowth, and invasion.
Linear Accelerator Therapies
Fractionated Radiation (FRT), Intensity Modulated Radiotherapy (IMRT), and Stereotactic Radiation (CyberKnife)
Linear accelerators are used for single-dose radiosurgery with CyberKnife or for fractionated radiation (FRT) [1]. Typically, modern applications attempt to combine the benefits of fractionation that allows a higher total radiation dose to normal structures, with precise stereotactic targeting that increases dose gradients between tumor and normal tissue in resemblance with SRS. Intensity modulated radiotherapy (IMRT) is one such application that allows superior design of the radiation field compared to traditional FRT.
Still, the indications and treatment strategies differ between SRS and FRT. In SRS, dose conformity to the tumor is a primary goal, while FRT applications typically add a 2–5 mm margin to the treatment volume to ensure that the tumor and possible surrounding tumor transition zone receive enough radiation. Even the CyberKnife, which is designed for SRS, provides an inferior conformity but better homogeneity compared to the Gamma Knife [29]. Fractionated therapies are not limited by target volume and proximity to optic nerves as much as single-dose therapies. They are superior to treat small volume tumors such as optic sheath meningiomas, where extremely good long-term results can be expected with long-term tumor control in all patients and improved vision in >50% [32].
Curative Intent
It is difficult to evaluate long-term outcomes after FRT and compare to SRS from literature reports. For small optic-sheath meningiomas long-term outcomes were comparable regarding control and superiority for nerve function. Other series of larger and more heterogenous meningiomas quote good short-term but worse long-term results. Astradsson et al. report good short-term but only 64% 10-year control of “anterior skull base meningiomas” with a mean volume of 21 cc (0.33–152) in a prospective cohort with mean follow-up of 65 months [3]. Combs et al. reported an 88% 10-year control but only 53% in “high-risk” tumors with a mean follow-up of 107 months [5]. The control rate for larger tumors that comprise residuals of operated symptomatic tumors or growing recurrent meningiomas would be important to analyze but is not clearly available in the literature on FRT for meningiomas.
Radiation toxicity is a potential problem for long-term survivors after cranial FRT [2]. FRT carries a higher risk of focal and general radiation toxicity than SRS. Late fatigue, headaches, and vertigo were found in 40% and acute toxicity comprising vertigo, alopecia, and fatigue in 60%, while >10% needed corticosteroid therapy for focal reactions [14]. The indications for FRT and expected benefits must be strong. In a previous retrospective evaluation of “cost-benefit” based on complications and tumor control suggested that only 7% of 44 patients with FRT for meningioma benefitted from FRT [25].
Adjuvant Treatment
FRT is usually recommended by different professional bodies and guidelines for adjunctive therapy after surgery of more aggressive tumors including high-grade meningiomas [10], chordomas, and malignant tumors. The supporting data comprise retrospective studies where progression-free survival has been longer in radiated patients than controls. Treatment bias is likely in such cohorts and, moreover, overall survival would be a better parameter to optimize, since aggressive tumors are bound to recur and need to be retreated if possible. SRS can usually be repeated, while FRT to 60 Gy limits future radiation therapies and may also make subsequent microsurgery difficult or impossible. The scientific analyses and support for FRT of aggressive tumors is weak and should probably be individualized to a high extent.
Hadron Therapy
Protons and carbon ion comprise hadrons. They are the commonest heavy charged particle therapies for brain tumors and employed for better targeted dose distribution than photon therapies, particularly for larger volume targets. In contrast to photons, protons and carbon ion beams slowly increase energy deposit as they penetrate the tissue. Toward the end of penetration depth, the energy deposit rises sharply and forms a peak: a “Bragg-peak” [20]. Subsequently, target coverage integrates multiple beams with the benefit of the Bragg-peak. The target volume is less sharply and accurately defined than a GKRS or CyberKnife target volume and employed for larger volumes with limited needs of millimeter-precision. The commonest application is radiation of the surgical field after chordoma surgery. Most treatment guidelines for chordoma recommend gross total removal (realistically oncologic resection) followed by proton beam surgery. Reported 5-year local control rates were 50–70% and 50–75% 10-year overall survival [36].
For chondrosarcomas, control rates were 75–99% [36] and 85% for meningiomas [37]. Skull base meningiomas too large for GKRS are thus a possible indication. Reported data suggest a high degree of tumor control. Yet, comparison to natural history and other treatment strategies have not been done and the reported series contain biologically different tumors of different sizes and locations. It is thus difficult to use published data for personalized management and selection of which patients would benefit most from radiation with minimal complications. Compared to traditional photon therapies, hadron technologies with a potential for SRS-like targeting have been advocated to minimize radiation toxicities. In contrast, the limited precision of a large radiated volume can cause complications and serious sequelae. Proton-beam therapy after chordoma surgery can cause brain stem necrosis and the total dose delivered can cause other radiation-associated adverse events. As of today, available evidence does not support superiority of hadron therapies over photons, nor are studies underway to resolve the issue [13].
Boron Neutron Capture Therapy (BNCT)
Neutrons of moderate energy interact very weakly with organic tissues and are largely harmless, while neutrons that interact with boron cause a nuclear fission with a resulting Li-isotope, a photon and alpha particle. The latter damages its adjacent tissue to the distance of 7 mu, the size of a cell, before annihilation. BNCT capitalized on this property by delivering boron to a target tissue followed by neutron radiation. In available applications, boron-phenylalanine or sodium borocaptate, which is enriched in tumors such as meningiomas, is administered intravenously followed by radiation with epithermal neutrons from an accelerator or reactor. The technology is not largely available, and a remaining challenge is the targeting of boron to tumor. BNCT has mostly been used for “intractable” tumors and has subsequently not demonstrated to be curative. Still, BNCT can be used despite pervious FRT and appears to delay growth of aggressive meningiomas [35].
Radiopeptide Therapy for Meningiomas
Radiopeptide therapy is an unsealed radiation therapy, which uses photon-emitting radionucleotides bound to peptides that target somatostatin receptors which are abundantly expressed by meningioma cells. “Intractable” meningiomas have been treated with DOTATOC or DOTATATE labeled with Lutetium 177 or Yttrium 90. Efficacy is difficult to establish in a meningioma cohort with a poor prognosis. Yet the prognosis seemed to be beneficially influenced [28] by treatment. It is probable that treatment of patients with a minimal tumor volume or with less grave prognoses can show better benefit.
Brachytherapy
In brachytherapy, a sealed radiation source is placed in a tumor requiring therapy. Brachytherapy is feasible despite previous radiation therapy. Iodine-125, with a half-life of 59 days, has been used after surgical resection as an “ultimum refugium” for “intractable” meningiomas. Jaaskelainens group found an actuarial 24-month survival of 62% with some tumor response in most treated patients (17/22). Preexisting cranial nerve deficits were ameliorated in 47% and new cranial nerve deficits developed in 36% of treated patients in similar proportions of patients [38]. In a different study, a 25-year series of 42 patients, median progression free survival was reported to be 11 months and overall survival 3 years [22].
Conclusion
Radiation is well established and considered indicated for several groups of patients with skull base tumors. Yet, systematic data to evaluate or support its long-term benefit are very weak and largely based on uncontrolled and retrospective patient series. Taken together, literature provides a picture of good long-term control of smaller tumors with benign phenotypes and stereotactic technology to achieve a good dose gradient between tumor and normal structures. Both single-dose and fractionated regimens appear to be effective. The benefit of SRS and Gamma Knife are better conformity while fractionation allows treatment plans that include sensitive nervous structures. In contrast, radiation of larger volumes or more aggressive tumors has lower long-term control. The scientific literature provides evidence in the form of longer recurrence free survival in cohorts subjected to radiation than nonradiated patients. The retrospective studies may suffer from biases and benefits are often difficult to evaluate. Moreover, strategies where patients with slowly growing or benign skull base tumors are considered to suffer from a chronic condition and handled with individualized therapeutic interventions as needed have not been evaluated and compared to an approach with upfront guidelines for all patients.
References
Alfredo C, Carolin S, Güliz A, Anne K, Antonio P, Alberto C, Stefano P, Antonino G, Harun B, Markus K, Franziska M, Phuong N, Franziska L, Peter V, Volker B, David K. Normofractionated stereotactic radiotherapy versus CyberKnife-based hypofractionation in skull base meningioma: a German and Italian pooled cohort analysis. Radiat Oncol. 2019;14(1):201. https://doi.org/10.1186/s13014-019-1397-7. Erratum in: Radiat Oncol. 2020 Dec 14;15(1):279. PMID: 31718650; PMCID: PMC6852939.
Al-Mefty O, Kersh JE, Routh A, Smith RR. The long-term side effects of radiation therapy for benign brain tumors in adults. J Neurosurg. 1990;73(4):502–12. https://doi.org/10.3171/jns.1990.73.4.0502. PMID: 2204689.
Astradsson A, Wiencke AK, MunckafRosenschold P, Engelholm SA, Ohlhues L, Roed H, Juhler M. Visual outcome after fractionated stereotactic radiation therapy of benign anterior skull base tumors. J Neuro-Oncol. 2014;118(1):101–8. https://doi.org/10.1007/s11060-014-1399-0. Epub 2014 Feb 15. PMID: 24532196; PMCID: PMC4023078.
Boari N, Bailo M, Gagliardi F, Franzin A, Gemma M, del Vecchio A, Bolognesi A, Picozzi P, Mortini P. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg. 2014;121(Suppl):123–42.
Combs SE, Adeberg S, Dittmar JO, Welzel T, Rieken S, Habermehl D, Huber PE, Debus J. Skull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT). Radiother Oncol. 2013;106(2):186–91. https://doi.org/10.1016/j.radonc.2012.07.008. Epub 2012 Aug 18. PMID: 22906549.
Couldwell WT, Cole CD, Al-Mefty O. Patterns of skull base meningioma progression after failed radiosurgery. J Neurosurg. 2007;106(1):30–5. https://doi.org/10.3171/jns.2007.106.1.30. PMID: 17236485.
Coughlin AR, Willman TJ, Gubbels SP. Systematic review of hearing preservation after radiotherapy for vestibular Schwannoma. Otol Neurotol. 2018;39(3):273–83. https://doi.org/10.1097/MAO.0000000000001672. PMID: 29342035; PMCID: PMC5807198.
Förander P, Bartek J Jr, Fagerlund M, Benmaklouf H, Dodoo E, Shamikh A, Stjärne P, Mathiesen T. Multidisciplinary management of clival chordomas; long-term clinical outcome in a single-institution consecutive series. Acta Neurochir. 2017;159(10):1857–68. https://doi.org/10.1007/s00701-017-3266-1. Epub 2017 Jul 22. PMID: 28735379; PMCID: PMC5590026.
Förander P, Rähn T, Kihlström L, Ulfarsson E, Mathiesen T. Combination of microsurgery and Gamma Knife surgery for the treatment of intracranial chondrosarcomas. J Neurosurg. 2006;105(Suppl):18–25. https://doi.org/10.3171/sup.2006.105.7.18. PMID: 18503325.
Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, von Deimling A, Stavrinou P, Lefranc F, Lund-Johansen M, Moyal EC, Brandsma D, Henriksson R, Soffietti R, Weller M. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–91. https://doi.org/10.1016/S1470-2045(16)30321-7. Epub 2016 Aug 30. PMID: 27599143.
Hasegawa T, Kida Y, Kato T, Iizuka H, Kuramitsu S, Yamamoto T. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than 10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013;118(3):557–65.
Helis CA, Hughes RT, Cramer CK, Tatter SB, Laxton AW, Bourland JD, Munley MT, Chan MD. Stereotactic radiosurgery for atypical and anaplastic Meningiomas. World Neurosurg. 2020;144:e53–61. https://doi.org/10.1016/j.wneu.2020.07.211. Epub 2020 Aug 3. PMID: 32758657.
Jefferson T, Formoso G, Venturelli F, Vicentini M, Chiarolla E, Ballini L. Hadrontherapy for cancer. An overview of HTA reports and ongoing studies. Recenti Prog Med. 2019;110(12):566–586. English. https://doi.org/10.1701/3278.32516. PMID: 31909760.
Kaul D, Budach V, Misch M, Wiener E, Exner S, Badakhshi H. Meningioma of the skull base: long-term outcome after image-guided stereotactic radiotherapy. Cancer Radiother. 2014;18(8):730–5. https://doi.org/10.1016/j.canrad.2014.07.159. Epub 2014 Oct 11. PMID: 25307475.
Kjellberg RN, Koehler AM, Preston WM, Sweet WH. Stereotaxic instrument for use with the Bragg peak of a proton beam. Confin Neurol. 1962;22:183–9. https://doi.org/10.1159/000104360. PMID: 14033248.
Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316–9. PMID: 14914373.
Leksell L. Cerebral radiosurgery. I. Gammathalamotomy in two cases of intractable pain. Acta Chir Scand. 1968;134(8):585–95. PMID: 5713443.
Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand. 1971;137(8):763–5. PMID: 4948233.
Leksell L, Larsson B, Andersson B, Rexed B, Sourander P, Mair W. Lesions in the depth of the brain produced by a beam of high energy protons. Acta Radiol. 1960;54:251–64. https://doi.org/10.3109/00016926009172547. PMID: 13760648.
Lesueur P, Calugaru V, Nauraye C, Stefan D, Cao K, Emery E, Reznik Y, Habrand JL, Tessonnier T, Chaikh A, Balosso J, Thariat J. Proton therapy for treatment of intracranial benign tumors in adults: a systematic review. Cancer Treat Rev. 2019;72:56–64. https://doi.org/10.1016/j.ctrv.2018.11.004. Epub 2018 Dec 1. PMID: 30530009.
Lippitz BE, Bartek J Jr, Mathiesen T, Förander P. Ten-year follow-up after Gamma Knife radiosurgery of meningioma and review of the literature. Acta Neurochir. 2020;162(9):2183–96. https://doi.org/10.1007/s00701-020-04350-5. Epub 2020 Jun 26. PMID: 32591948; PMCID: PMC7415024.
Magill ST, Lau D, Raleigh DR, Sneed PK, Fogh SE, McDermott MW. Surgical resection and interstitial Iodine-125 brachytherapy for high-grade Meningiomas: a 25-year series. Neurosurgery. 2017;80(3):409–16. https://doi.org/10.1227/NEU.0000000000001262. PMID: 27258768.
Mathiesen T, Gerlich A, Kihlström L, Svensson M, Bagger-Sjöbäck D. Effects of using combined transpetrosal surgical approaches to treat petroclival meningiomas. Neurosurgery. 2007;60(6):982–91; discussion 991-2. https://doi.org/10.1227/01.NEU.0000255476.06247.F1. PMID: 17538371.
Mathiesen T. Combined microsurgery and Gamma Knife radiosurgery in skull base meningioma. In: Misra B, Kaye A, Laws E, editors. Current progress in neurosurgery, vol. 1. Vancouver: Tree Life Media; 2015. ISBN 978–93-83989.
Mathiesen T, Kihlström L, Karlsson B, Lindquist C. Potential complications following radiotherapy for meningiomas. Surg Neurol. 2003;60(3):193–8; discussion 199-200. https://doi.org/10.1016/s0090-3019(03)00377-x. PMID: 12922028.
Mathiesen T, Förander P, Pettersson D. Schwannomas. In: Kirollos R, Helmy A, Thomson S, Hutchinson P, editors. Oxford textbook of neurological surgery. Oxford University Press; 2019. ISBN: 9780198746706.
Miller T, Lau T, Vasan R, Danner C, Youssef AS, van Loveren H, Agazzi S. Reporting success rates in the treatment of vestibular schwannomas: are we accounting for the natural history? J Clin Neurosci. 2014;21(6):914–8.
Mirian C, Duun-Henriksen AK, Maier AD, Pedersen MM, Jensen LR, Bashir A, Graillon T, Hrachova M, Bota D, van Essen M, Spanjol P, Kreis C, Law I, Broholm H, Poulsgaard L, Fugleholm K, Ziebell M, Munch T, Walter A, Mathiesen T. Somatostatin receptor-targeted radiopeptide therapy in treatment-refractory meningioma: an individual patient data meta-analysis. J Nucl Med. 2020:jnumed.120.249607. https://doi.org/10.2967/jnumed.120.249607. Epub ahead of print. PMID: 32859705.
Nakazawa H, Mori Y, Komori M, Tsugawa T, Shibamoto Y, Kobayashi T, Hashizume C, Uchiyama Y, Hagiwara M. Simulational study of a dosimetric comparison between a Gamma Knife treatment plan and an intensity-modulated radiotherapy plan for skull base tumors. J Radiat Res. 2014;55(3):518–26. https://doi.org/10.1093/jrr/rrt136. Epub 2013 Dec 17. PMID: 24351459; PMCID: PMC4014159.
Paddick I, Cameron A, Dimitriadis A. Extracranial dose and the risk of radiation-induced malignancy after intracranial stereotactic radiosurgery: is it time to establish a therapeutic reference level? Acta Neurochir. 2020; https://doi.org/10.1007/s00701-020-04664-4. Epub ahead of print. PMID: 33325003.
Persson O, Bartek J Jr, Shalom NB, Wangerid T, Jakola AS, Förander P. Stereotactic radiosurgery vs. fractionated radiotherapy for tumor control in vestibular schwannoma patients: a systematic review. Acta Neurochir. 2017;159(6):1013–21. https://doi.org/10.1007/s00701-017-3164-6. Epub 2017 Apr 13. PMID: 28409393; PMCID: PMC5425507.
Pintea B, Boström A, Katsigiannis S, Gousias K, Pintea R, Baumert B, Boström J. Prognostic factors for functional outcome of patients with optic nerve sheath Meningiomas treated with stereotactic radiotherapy-evaluation of own and meta-analysis of published data. Cancers (Basel). 2021;13(3):522. https://doi.org/10.3390/cancers13030522. PMID: 33572990; PMCID: PMC7866383.
Pollock BE, Foote RL, Stafford SL. Stereotactic radiosurgery: the preferred treatment for patients with non-vestibular schwannomas? Int J Radiat Oncol Biol Phys. 2002;52:1002–7.
Steiner L, Leksell L, Greitz T, Forster DM, Backlund EO. Stereotaxic radiosurgery for cerebral arteriovenous malformations. Report of a case. Acta Chir Scand. 1972;138(5):459–64. PMID: 4560250.
Takeuchi K, Kawabata S, Hiramatsu R, Matsushita Y, Tanaka H, Sakurai Y, Suzuki M, Ono K, Miyatake SI, Kuroiwa T. Boron neutron capture therapy for high-grade skull-base meningioma. J Neurol Surg B Skull Base. 2018;79(Suppl 4):S322–7. https://doi.org/10.1055/s-0038-1666837. Epub 2018 Jul 3. PMID: 30210985; PMCID: PMC6133692.
Uhl M, Herfarth K, Debus J. Comparing the use of protons and carbon ions for treatment. Cancer J. 2014;20(6):433–9. https://doi.org/10.1097/PPO.0000000000000078. PMID: 25415691.
Vlachogiannis P, Gudjonsson O, Montelius A, Grusell E, Isacsson U, Nilsson K, Blomquist E. Hypofractionated high-energy proton-beam irradiation is an alternative treatment for WHO grade I meningiomas. Acta Neurochir. 2017;159(12):2391–400. https://doi.org/10.1007/s00701-017-3352-4. Epub 2017 Oct 24. PMID: 29064038; PMCID: PMC5686253.
Vuorinen V, Heikkonen J, Brander A, Setälä K, Sane T, Randell T, Paetau A, Pohjola J, Mäntylä M, Jääskeläinen J. Interstitial radiotherapy of 25 parasellar/clival meningiomas and 19 meningiomas in the elderly. Analysis of short-term tolerance and responses. Acta Neurochir. 1996;138(5):495–508. https://doi.org/10.1007/BF01411167. PMID: 8800323.
Wangerid T, Bartek J Jr, Svensson M, Förander P. Long-term quality of life and tumour control following gamma knife radiosurgery for vestibular schwannoma. Acta Neurochir. 2014;156(2):389–96. https://doi.org/10.1007/s00701-013-1924-5. Epub 2013 Nov 6. PMID: 24193890.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Mathiesen, T. (2022). Role of Radiotherapy in Modern Skull Base Surgery. In: Youssef, A.S. (eds) Contemporary Skull Base Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-99321-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-99321-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99320-7
Online ISBN: 978-3-030-99321-4
eBook Packages: MedicineMedicine (R0)