Abstract
In spite of the significant technical and technological progress in neurosurgery and the continuous discoveries by the basic research, adamantinomatous craniopharyngioma remains a significant clinical challenge. Actually, the huge size of the tumor, its multiple cystic components, the encasement of Willis’ circle and optic pathways, and the invasion of the hypothalamus often prevent its safe surgical resection. Moreover, the local aggressiveness of the tumor accounts for a high risk of recurrence even after a gross total resection. For these reasons, more and more efforts are being dedicated to enhance the knowledge about AC and improve the tools for its treatment.
This paper is dedicated to the most recent advances concerning the AC management. Promising, new insights come for the basic research, thanks to the updates on the role of the WNT-β-catenin pathway (important for the tumor genesis and progression, not yet developed enough for a safe target therapy in children but useful for determining the prognosis) and the inflammatory mediators (widely overexpressed, especially by the cyst of the tumor, and for which target therapies are being developed). Moreover, further factors and pathways are under investigation.
Also the development of new treatment strategies accounts for the improvement of the prognosis and the quality of life of AC patients. The enhancement of the experience with the endoscopic techniques (both transsphenoidal and transventricular approaches) actually allows to perform a less invasive but effective surgery that can be coupled with new modalities of radiation therapy aiming at obtaining a reliable control of the disease and protecting the endocrinological, ophthalmological, and neurological functions. A special mention is finally deserved by the techniques specifically designed for the intracystic therapy (as cyst fenestration alone or in combination with administration of radionuclides or bleomycin or interferon-α) that are here analyzed together with the aforementioned advances.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Adamantinomatous craniopharyngioma (AC) is a rare brain tumor arising from remnants of the craniopharyngeal duct epithelium. It accounts for 1.53–2.92/100,000 cases/year in children under 15 years and for 5–10% of sellar tumors in the pediatric population [1, 2]. AC is largely most common than the papillary variant in children and, in about 90% of the cases, it shows a cystic or multicystic component with a variable size [3].
Since the first experience with this tumor by Harvey Cushing [4], AP still continues to represent a significant challenge for the clinicians for several reasons:
-
1.
The cystic components may reach a huge volume, determining acute hydrocephalus as well as causing compression even in intracranial districts far from the site of origin (Figs. 4.1 and 4.2). Cases of gigantic AC even reaching the posterior fossa and the cerebello-pontine angle are not rare in the clinical practice [5];
-
2.
The site of origin together with the progressive growth in the cisternal spaces accounts for both the frequent encasement of eloquent or vital structures (optic pathways, pituitary gland and stalk, hypothalamus, Willis’ circle) and the possible endocrinological, visual, and neurological deficits at diagnosis and after the treatment (Figs. 4.1 and 4.2);
-
3.
The aforementioned aspects make the surgical management of AC challenging. The gross total resection (GTR) of this tumor, which is still the most effective treatment option whenever possible to ensure the longest disease and progression free survival, is hard to be obtained [3, 6];
-
4.
In spite of the apparently benign biological behavior, the risk of tumor recurrence is high even after GTR [3]. Moreover, AC can show an aggressive course with recurrence even after an appropriate surgical and adjuvant (radiation therapy) management [7];
-
5.
A solid multidisciplinary team is needed for a proper management of the patients affected by AC, either in childhood and adulthood, namely because of the possibly permanent posttreatment deficits, which range from the hormonal to the visual, neurological, and cognitive ones. Moreover, successful surgery can be complicated also by unusual events, such as vasospasm in case of huge cyst removal [8].
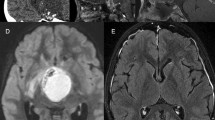
Typical MRI appearance of AC in a 9-year-old boy. Note the large, multiple, and not homogeneous cysts (a–f), invading also the retroclival space of the posterior fossa (b). The solid portion is extended from the sellar/suprasellar region to the third ventricle, making the hypothalamus not recognizable (a) and the third ventricle visible only in its posterior part (f). An encasement of the Willis’ circle is present (c). After gadolinium administration, an irregular enhancement of the solid portion and a thin enhancement of the wall of the major cyst can be appreciated (e, f). Because of the mass effect, especially due to the greater cyst (d), a biventricular hydrocephalus results (b, c)
CT scan of the same case in Fig. 4.1. Note the irregular appearance of the cysts and the gross calcifications in the solid portion (a) and along the wall of the major cyst (b)
In this chapter, the latest advances in the knowledge and the management of cystic AC are addressed, focusing onto the pertinent literature as well as on the authors’ experience. The first part of the article is dedicated to the most important new insights from the base research. Actually, to improve the knowledge of such a complex tumor is mandatory to enhance its treatment, looking also for possible target therapies. Under a biologically and molecular point of view, indeed, AC is a surprisingly rich tumor. The second part encompasses the progresses in the management strategies.
4.2 Basic Research
4.2.1 CTNNB1-WNT–β-Catenin
The dysregulation of WNT/β-catenin pathway is typical of AC, being observed in 57–96% of cases [9]. The WNT pathway is necessary for the organ formation during the embryogenesis and for maintaining the stem cells in adulthood, while the β-catenin is a key protein among the WNT pathway encoded by CTNNB1 gene [10, 11]. More in details, β-catenin is a cytoplasmic protein whose role is to control gene transcription and cell adhesion and migration. Somatic mutations in exon3 of CTNNB1 gene are hallmarks leading to lack of regulatory residues of the β-catenin protein stability. Following such modifications, β-catenin tends to accumulate inside the cells as a result of lacking β-catenin destruction complex formation (via alterations of serine and threonine residues at phosphorylation sites of GSK-3β) [12, 13]. Cytoplasmatic increase in β-catenin levels leads to its translocation into the nucleus where interaction with transcription factors, as lymphoid enhancer–binding factor 1/T-cell-specific transcription factor, takes place. Those interactions stimulate cell proliferation and migration through the expression of the actin bundling protein fascin-1 and activation of WNT pathway [9]. Such a condition leads to a growth factor signaling disruption and eventually to an increased expression in epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), growth hormone (GH) receptor, and insulin-like growth factor (IGF)-1 receptor (IGF-1R), thus explaining the invasion properties of AC cells [14, 15].
The β-catenin accumulation has been shown also by immunohistochemical studies demonstrating how, though tumor cells show normal membranous expression of β-catenin (despite carrying CTNNB1 mutations), not all tumoral cells but, rather, only clusters of them present the β-catenin accumulation [3, 13, 15,16,17]. Only this small population of cells would be responsible for AC growth and proliferation [18].
Animal studies depicted the role of β-catenin-accumulating cell clusters as a control tower for AC cells. In these experiments on murine models, a degradation-resistant (activated) form of β-catenin is expressed in either Rathke’s pouch derivatives (Hesx1Cre/+/Ctnnb1lox(ex3)/+ mouse line; AC embryonic model) or Sox2-expressing adult pituitary stem cells (Sox2CreERT2/+/Ctnnb1lox(ex3)/+ mouse line; AC inducible model) [19]. Clusters of this β-catenin would act in a paracrine manner by secreting promoting factors or inflammatory modulators as SHH, FGFs, BMPs, TGFB1, IL1, IL6, and other chemokines. The described paracrine tumorigenesis mechanism is believed to be determined by β-catenin cluster cells’ positivity for cellular senescence markers, such as viable but nonproliferative expression of cell-cycle inhibitors, increased lysosomal compartment, presence of DNA damage, and activation of a DNA damage response [3, 20]. These cluster cells are, thus, able to promote proliferation and invasion of the nearer AC cells improving at the same time their pro-tumorigenic microenvironment. Further corroboration of the importance of senescence in AC came from the upcoming evidence of efficacy of ‘senolytics’ drugs in reducing tumorigenic potential [3, 21].
Despite the centrality of the WNT/β-catenin signaling in AC development, no current targeted treatment options are available yet. In adults, non-CNS tumors’ inhibition via reagents such as XNW7201, CGX1321, and RXC004 is reported in ongoing trials, but there are no data on children yet also because of the concerns on the possible off-target effects [9]. However, the CTNNB1 mutation remains an important prognostic factor, pointing a higher risk of recurrence [22]. The recent study by Zhu et al. actually confirmed that the cyst components in AC with CCTNB1 mutation show more aggressive radiological characteristics than in the wild type [23]. These radiological characteristics are: presence of multiple cysts (vs. single cyst), irregular shape of the cyst (vs. regular shape), hypointense signal of the interior cyst on T1-MRI (vs. hyperintense), enhancement of the cyst wall after gadolinium (vs. missing enhancement), and adherence to the optic chiasm (vs. no compression on the optic chiasm).
4.2.2 Inflammatory Mediators
Differently from the WNT/β-catenin pathway, which is crucial to understand the ontogenesis of AC but still with a limited role for its treatment, the inflammatory response that AC can generate plays a promising role not only to explain the tumor development and progression but also to find possible therapeutic targets. Both AC cystic and solid components, indeed, express various cytokines, chemokines, and inflammatory mediators, which can be involved in various cancer cells activities [24]. Therefore, the link between immune response and AC raises a great interest in the scientific community just because of the potentially wide field of therapeutic options that it opens [9, 14, 19, 25].
According to immunohistochemical and proteomic studies, the composition of cystic fluid appears to be characterized by a rich spectrum of well-known cytokines. Actually, some studies highlighted the presence of high levels of cyst fluid IL-6, IL-8, CXCL1, and IL-10, while other studies reported on high levels of tumor necrosis factor as well as inflammasome-like patterns triggered by the cholesterol crystals typically present in AC [19, 26]. The aforementioned studies also pointed out an overexpression of immunosuppressive factors (IL-10, indoleamine-pyrrole 2,3-dioxygenase and galectin-1) as well as programmed death ligand 1 (PD-L1) [14, 15, 19, 21, 26, 27].
The proteomic analysis, performed onto AC cystic fluid with high-performance liquid chromatography and mass spectrometry, usually shows high levels of inflammatory proteins [28]. In the authors’ group experience, α-defensins 1–3 were the first of such proteins to be detected [29]. Human α-defensins are contained in the azurophilic granules of neutrophils, working as antibacterial and antiviral factors. Increased levels can be observed in the saliva of patients with oral squamous cells carcinoma and in the plasma of patients with sepsis. The high levels observed in AC cystic fluid would suggest no blood barrier disruption for the cyst formation, since the serum levels of defensins are very low. The preoperative high levels of α-defensins we found would therefore indicate a role of inflammation in stimulating the secretion of cyst fluid by the epithelial cells of the cyst wall, while the decrease of defensins in the postoperative period would account for the reduced fluid production and the cyst shrinkage [29]. As further support to this hypothesis, the same study demonstrated that a treatment with interferon-α (an antiviral protein produced by peripheral leukocytes) is able to reduce the defensins levels through an antitumoral effect on the squamous epithelial cells, an immunomodulatory action on the recruitment of inflammatory cells, and an antiangiogenic activity. Moreover, Jokonoya et al. reported on the antibacterial properties of AC cyst fluid mediated by α-defensins [30]. Their study started from the evidence of a low rate of infection in exposed Ommaya reservoir after skin breakdown in patients with AC. The α-defensins’ immune response of AC cyst fluid was demonstrated against Gram positives, but not against Gram negatives. A possible explanation for this discrepancy is that some components of the cystic fluid, like magnesium, alkaline phosphatase, blood urea nitrogen, glucose, urea, and creatinine, are more likely to interfere with Gram-positive bacteria than Gram-negative ones. Finally, other studies depicted how interferon-α treatment leads to a decrease not only in the cyst fluid concentrations of α-defensins, but also in the cyst volume (tumor shrinkage). Such a fluid volume reduction appears to be mediated by the activation of the Fas apoptotic pathway [31, 32].
The extensive proteomic analysis on AC cystic fluid, obtained by integrating bottom-up and the top-down approach, allowed us to find several other inflammatory or similar proteins, thus leading to the hypothesis that AC is an “inflammatory” tumor [28, 33,34,35]. The most important among them are: (1) Apolipoproteins, possibly involved in the cyst fluid formation and characterization. Apolipoprotein C-I (synthetized in the liver and secreted in the plasma but expressed also in the brain) is actually necessary for the cholesterol and triglycerides’ removal from the tissues. Apolipoprotein A-I, on the other hand, is involved in the inverse cholesterol transport and cholesterol esterification and is characterized by anti-inflammatory and antioxidant properties; (2) α1-antichymotrypsin, possibly involved in the production of the cyst fluid. Secreted glycoprotein of the serpin family, this protein acts as inhibitor of chymotrypsin-like serine proteases and mast cell chymasesm, thus having a role in inducing the acute phase of inflammation (it is upregulated in CSF of patients with glioblastoma); (3) α2-HS-glycoprotein (or fetuin A), possibly involved in the cyst fluid formation and characterization. It is a serum glycosylated heterodimer involved in the tissue mineralization and in the processes of pathological mineralization. By binding of small clusters of calcium and phosphate, it can act as pro-inflammatory, pro-calcification, and signaling protein; (4) β-thymosins, possibly involved in the progression of AC. β-Thymosins are a family of 16 peptides first isolated from the calf thymus. The isoform β4 and β10 are abundant in extracellular fluid because of a cell damage or a secretory process. In cystic AC, the isoform β4 (Tβ4) is 10 times more expressed than β10. Tβ4 is a major G-actin-sequestering molecule in mammals, regulating the organization of the cytoskeleton and thus influencing the cell differentiation, migration, and morphogenesis. In addition, it promotes angiogenesis, tissue repair, and tumor growth. The upregulation of Tβ4 would be able to promote the motility and invasion of AC cells by activating the matrix metalloproteinase 7 and by activating the WNT/β-catenin pathway [36, 37].
The key role of inflammation in the genesis/progression of AC has been confirmed by several other studies demonstrating an overexpression of inflammatory mediators both in the solid and in the cystic component [19, 25,26,27, 38,39,40,41]. In summary, these studies show that: (1) The cyst fluid has a high concentration of cytokines and chemokines (namely, IL-6, IL-8, IL-10, CXCL1) that correspond to the transcriptomic analysis of the solid counterpart; (2) IL-6, in particular, seems involved in inducing the inflammatory reaction surrounding the tumor. AC is able to produce IL-6 and its receptor (IL-6R) and glycoprotein 130 which are useful also for the AC cells migration; the block of IL-6 with monoclonal antibody (tocilizumab) significantly decreases AC cells migration; (3) The transcriptomic analysis, on the other hand, demonstrates high levels of several inflammatory factors (FGF, TGFB, and BMP families) as evidenced by immunostaining against the phosphorylated proteins pERK1/2, pSMAD3, and pSMAD1/5/9. The inhibition of the MAPK/ERK pathway with trametinib (a MEK inhibitor) reduces the proliferation and increases the apoptosis in AC cultures; (4) On these grounds, given this inflammatory role in AC genesis and progression, several targets for inflammation blockade are under investigation. As mentioned, one of the more interesting targets is IL-6, whose blockage was proven efficient in systemic illnesses such as juvenile idiopathic arthritis, multicentric Castleman disease, and CAR (chimeric antigen receptor) T-cell-induced cytokine release syndrome. Tocilizumab and siltuximab are indeed human monoclonal antibodies binding both IL-6 receptor and IL-6, thus hindering IL-6 from exerting its proinflammatory effects. In addition, antagonists of pro-inflammatory cytokines such as IL-8 and CXCL1 have also shown therapeutic potential in preclinical human cancer models. Among the potential drugs, BX-IL8, an antibody that inhibits IL-8 function, is supposed to be useful in countering tumor growth in various preclinical studies, even though no definite data are available yet, thus preventing clinical application at the moment [42, 43].
4.2.3 Other Factors and Pathways
4.2.3.1 Programmed Cell Death Protein 1/Programmed Death-Ligand 1 (PD-1/PD-L1)
AC has been demonstrated to show tumor cell-intrinsic PD-1 expression in whorled epithelial cells with nuclear-localized β-catenin and to express PD-L1 by tumor cells comprising the cyst lining [44]. PD-1 and PD-L1 could be potential targets since the epithelial cells exhibit elevated target for rapamycin (mTOR) and mitogen-activated protein kinase (MAPK) signaling.
4.2.3.2 Sonic Hedgehog Pathways (SHH)
SHH was considered a promising source for target therapy because it plays a role in the regulation of cell differentiation and proliferation, and because it is involved in the development of the Rathke’s pouch [45]. Actually, an upregulation of SHH signaling has been found in AC [46]. Nevertheless, the studies on animal models discourage the use of SHH inhibitors for the treatment of AC. Indeed, visomodegib, a SHH pathway inhibitor, produces a significant reduction of the median survival in murine models and increases the tumor cells proliferation in AC human cultures [47].
4.2.3.3 BRAF
BRAF mutations, although peculiar of papillary craniopharyngioma (PC), can be encountered also in AC. BRAF gene encodes for B-Raf, a cytosolic kinase in the mitogen-activated protein kinase (MAPK) pathway. The mutation on V600E point raises great interest about the possible therapeutic implications. Indeed, in tumors like melanoma, targeted therapies against this variant of mutated BRAF showed a significant efficacy, modifying completely the prognosis and the treatment protocols [3, 15]. An interesting report on BRAF role in AC has been provided by Petralia et al. who made a thorough proteomic analysis [48]. Accordingly, even though the BRAFV600E mutation is rare in AC in comparison with PC, the proteomic changes in non-mutated AC still resemble those of BRAFV600E low grade glioma tumors. Such a finding would suggest a hypothetical benefit of chemotherapy against BRAF mutation, despite the presence of the gene alteration.
4.2.3.4 Retinoic Acid Receptors (RARs)
As confirmation of the relatively high number of molecular alterations that it can show, AC also presents immunoreactivity to RARs, namely RARa, RARb, and RARg, the RARg/RARb immunoreactivity ratio being strictly related to the AC recurrence risk. RARs’ role is to drive cell maturation and differentiation; therefore, alterations in their pathways might result in tumorigenesis. RAR isotypes’ expression seems to be associated with a higher recurrence rate. Such a finding was related to the expression of specific cathepsins, which are proteases necessary for cell turnover. Cathepsins D, B, and K seem to be most important cathepsins involved, which are already known to have a role in neurodegenerative diseases [14, 15, 49,50,51].
4.2.3.5 BMP and MMPs
Bone morphogenetic protein (BMP) and matrix metalloproteinases (MMPs) pathways are involved in the aforementioned paracrine growth-related signaling [15, 18]. Those proteins are downstream effectors of the WNT/β-catenin-mediated transcription, thus being abnormal in function in CTNNB1 mutations. AC cells highly express both BMP2 and BMP4, which result in the formation of the intracystic calcic components. Alterations in MMPs encoding, on the other hand, lead to a modification in the extracellular environment resulting in an enhanced tumor invasive capacity.
4.2.3.6 VEGF and HIF-1α
Vascular endothelial growth factor (VEGF) is a key regulator in angiogenesis being involved in many tumor growths, while hypoxia inducible factor 1α (HIF1α) is a transcription factor often altered affected in cancer cells where the cellular response to hypoxia is downregulated. With regard to AC, there are conflicting results about the role these factors could play in the tumor recurrence. Actually, Liu et al. documented their high levels in recurrent AC, while Xu and colleagues did not experience this phenomenon [52, 53].
4.2.3.7 Survivin
Survivin, which is an antiapoptotic protein, seems to be involved in AC tumorigenesis. In fact, this protein was found to be selectively increased in AC compared to normal brain [54]. In addition, survivin is present in higher levels in recurrent ACs compared with nonrecurrent ones [14].
4.2.3.8 Ep-CAM
Epithelial cell adhesion molecule (Ep-CAM) is a cell-to-cell adhesion molecule whose altered expression can be found in several various systemic tumors as well as in the AC stellate reticulum cells and whorl-like arrays. This finding is considered to be associated with higher risk of AC recurrence [14, 55].
4.2.3.9 Osteonectin
Osteonectin is a protein that can be seldom involved in metastatic cancers. Some studies suggested its role in organizing the stromal tissue surrounding AC cells facilitating their diffusion [14, 56].
4.2.3.10 P53 Protein
p53 is a well-known cell-cycle regulatory protein and tumor suppressor most often involved in tumorigenesis [57]. Its role in AC was largely debated, being initially denied and, afterwards, reconsidered based on new evidences that seem to correlate p53 level increase with the AC recurrence risk: the higher the p53 levels, the higher the chance to have a more aggressive AC [14, 58].
4.2.3.11 Ki-67
Ki-67 is a nuclear protein commonly used as a proliferation marker [59]. Similarly, to what happen with p53, increased Ki-67 expression appears to be common in aggressive AC. Prieto et al. suggested that high Ki-67 expression can be considered as a reliable marker in predicting aggressive behavior and recurrence risk of AC [14, 60].
4.3 Treatment Options
4.3.1 Evolution of the Surgical Management
Being deep-seated tumors, embracing vital neural and vascular structures, AC has been representing one of the major challenges for neurosurgery over the last century. Nonetheless, progress and diffusion of microsurgical techniques made these lesions increasingly approachable, leading to a progressive adoption of aggressive resective strategies. This was motivated by the traditional belief that the “benign” histology of craniopharyngioma made it a curable disease, so that it was worth taking the risks of radical excision. This trend, culminating in the 1990s, was reinforced by the increased availability of hormonal substitutes, which permitted to balance the postoperative endocrinological and electrolyte disturbances that were one of the dreaded consequences of aggressive surgical approaches [61, 62]. On the other hand, however, thanks to the accumulating evidence gathered through large follow-up series, the long-term outcome of aggressively treated patients (especially those with hypothalamic involvement) was often showed to be disappointing because of the relatively high rate of recurrence (in at least 17.6% of patients after microsurgical gross total resection) and the inveterate consequences of multi-hormone imbalances (with up to 70% of patients developing diabetes insipidus and 30% obesity or hyperphagia) [61, 63,64,65]. Therapeutic approaches thus started to shift towards the experimentation of less invasive strategies that could provide a good balance between disease control and perioperative and long-term morbidity.
Owing to its peculiar characteristics, AC has especially benefited from this trend, with the introduction of dedicated treatment options that promise to reach such a balance [61, 62, 66, 67]. Indeed, the search for an equilibrium is favored, on one hand, by high recurrence rates of AC (up to 47%) and, on the other hand, by the significant decompression that can be obtained with less efforts by employing treatment strategies that are less invasive and well-tolerated [66, 68]. The second phase in the history of AC treatment has thus seen the diffusion of a less radical surgical philosophy, with the affirmation of maximal safe resection followed by adjuvant radiosurgery; in parallel, tumors with predominant cystic components started to be treated with minimally invasive catheter drainage (with or without the intracystic administration of therapeutic substances), eventually followed by irradiation of the remaining solid tumor components.
In the last years, the accumulating data on long-term outcome of patients treated with minimally invasive strategies, and especially on the long-term consequence of radiation treatment in children [69], have promoted a resurgence of interest towards surgery, with a special look towards novel approaches that could allow for the benefits of substantial/complete resection in face of lower complication rates when compared to traditional microsurgical approaches.
In the next paragraphs, an outline of the different treatment options, which have been developed or enhanced in the last years to offer new strategies to deal with this complex disease, is provided. An important limit of the literature concerning AC is the difficulty in making comparisons among the different studies. First of all, the definition of disease progression varies widely, with different authors attributing different meanings to cyst regrowth [70]. A second bias is related to the fact that the data are mainly referred to patients treated in an elective setting, even if also after an emergent treatment (e.g., AC associated with acute hydrocephalus) there is room for planning the surgical steps in order to obtain a good balance between immediate treatment efficacy and long-term outcome. A further limitation is generated by the still existing discussion about the use of microsurgical (craniotomic) versus endoscopic (transsphenoidal) approaches. Microsurgery offers a wider exposure of the surgical field and a large space for the surgical maneuvers, while endoscopy grants a minimally invasive approach with a better visualization of hidden angles. Therefore, it is about two different techniques to be used for different types of patients. Since the goal of the present chapter is the management of cystic AC, the advances in the endoscopic approaches are reported because they have resulted particularly useful in this subset of patients also due to the double option provided by the transsphenoidal and the transventricular option.
4.3.2 Endoscopic Approaches
Compared with microsurgery, whose evolution started much earlier, the endoscopic approaches have showed huge progresses in the last two decades and are gaining more and more consensus in the management of AC [71]. The success of endoscopic techniques is favored by the possibility to obtain a significant shrinkage of the (often huge) cystic component of the tumor with a poorly invasive approach, which is easily used even in emergency. Should tumor remnants be left behind or the tumor regrowth, the treatment can be completed with adjuvant therapies. Indeed, endoscopic techniques can be used alone and in combination with each other or with other treatment modalities. A recent development of endoscopic surgery, designed to decrease complications and enhance effectiveness, is represented just by the combination of endonasal and transventricular endoscopy for the management of very large AC. Deopujari et al. reported on 18 cases (16 children and 2 young adults) out of a series of 125 cases who were treated first with a trans-ventricular endoscopic procedure to decompress the cystic component, followed by EES 2–6 days later [72]. This strategy allowed the patients to improve after the cyst decompression (often also because of relief of hydrocephalus) and to undergo the remaining tumor removal in an optimal setting. GTR was actually obtained in 84% of these patients, with only two cases of permanent diabetes insipidus and no cases of morbid obesity.
4.3.2.1 Endonasal Transsphenoidal Surgery
The use of endonasal endoscopic surgery (EES) in the pediatric population has significantly expanded over the last decade, as documented by the parallel increase in the number of published data on this technique; more than one third of the papers including the keywords “transsphenoidal” and “children” have been published in the last 5 years. This growing interest has also been reflected in the field of craniopharyngioma treatment, motivated, on one side, by the resurgence of surgery (as opposed to conservative treatments) and, on the other, by the need to find alternative approaches to the traditional microsurgical treatment.
Some peculiarities of pediatric patients were traditionally deemed as potential obstacles to EES (i.e., small nostrils and nasal cavities, insufficient pneumatization of the sphenoid bone); through the accumulation of experience (and thanks to the refinement in endoscopic instrumentation), it has been shown that these drawbacks can be easily overcome [71, 73]. In fact, endonasal endoscopic approaches have been shown to be a feasible and safe alternative for the treatment of pediatric craniopharyngiomas. In 2011, Elliott and colleagues published a systematic review comparing EES and traditional trans-frontal surgery (TFS), gathering data on 2773 microsurgically treated patients vs. 373 EES cases, with a special focus on pediatric patients [63]. In spite of the obvious limitations of such a study (retrospective analysis, inherent selection bias), the evidence showed that EES was associated with good postoperative outcome and lower rates of complications with respect to traditional surgical approaches. Indeed, a 72.1% vs. 60.9% GTR, 8.0% vs. 17.6% recurrence after GTR, 85.5% vs. 47.7% vision improvement, 2.3% vs. 13% vision deterioration, 3.1% vs. 9.4% neurological morbidity, and 23.9% vs. 69.1% postoperative diabetes insipidus were reported; the rate of postoperative obesity/hyperphagia was the same for both types of treatment (32%). These observations have been confirmed by other large-scale studies, as the national retrospective series published by Lin and colleagues in 2017, outlining the outcome of the 314 craniopharyngioma patients treated in the USA in 2003, 2006, and 2009 [74]. When compared to TFS, EES was associated with lower rates of diabetes insipidus (38% vs. 69%), lower rates of panhypopituitarism (5% vs. 8%), cranial nerve deficits (1% vs. 6%), postoperative stroke (2% vs. 5%), seizures (0 vs. 12%), and death (0% vs. 1%). As expected, there was a statistically significant association between EES and shorter hospital length-of-stay (LOS), EES patients having a 6.6-days mean LOS (median 4 days), while TFS patients a 12.3-days mean LOS (median 10 days). However, cerebrospinal fluid (CSF) leak affected 19% EES versus 4% TFS resections, thus confirming this complication as the weakest aspect of this kind of surgery.
The view offered by EES is advantageous over TFS especially when dealing with AC with sellar extension and/or origin from the pituitary stalk (as often seen in children), allowing a good direct visualization of pituitary gland and stalk, optic pathways, and related feeding vessels. For this reason, EES was originally reserved to predominantly intrasellar craniopharyngiomas. In recent years, however, the alternative viewpoint on suprasellar structures (and hypothalamus itself) provided by EES has been increasingly exploited to approach lesions with suprasellar extension or even purely extrasellar location [72, 75,76,77,78,79] and recurrent tumors [78]. Reported outcomes of these more extensive approaches are generally good, and even if they appear less optimal when compared to the mentioned general data on EES, they still compare favorably with TFS. GTR rates actually range from 45 to 85% (similar to TFS) [78, 79], and complication rates as follows: new onset diabetes insipidus from 31 to 63%, new anterior pituitary dysfunction between 40% and 80%, visual deterioration between 0 and 26%, and new onset neurologic deficits between 0 and 10% [75, 78, 79]. The number of published cases is still limited, and the heterogeneity of data suggests that there is still room for improvement with increasing expertise. Indeed, the complication rates are still too high in these instances. It is interesting to note that, according to the blind post-hoc survey performed by Jeswani and colleagues among the series of extensive EES approaches, all their EES and TFS cases could have been approached by both routes according to the preference of the different surgeons, somewhat attenuating the selection bias but also suggesting the surgeons’ preference and expertise as a way to reduce complications [79].
If the transcranial microsurgical route still maintains a remarkable value in the AC management, the microsurgical sublabial access (SA) is almost abandoned in favor of EES. Especially in children, the authors’ personal experience on 51 children (34 undergoing EES and 17 undergoing SA) demonstrated several statistically significant advantages of EES over SA that can be summarized as follows [73, 80]: (1) shorter mean LOS (4 vs. 5 days); (2) lower transfusion rate (20% vs. 60%); (3) lower need of nasal packing (20% vs. 100%); (4) better quality of the early postoperative course as measured by a 0–8 pain scale (2.7 vs. 4.2 mean value). The 2 nostrils-4 hands technique provided also a significantly better intraoperative view other than a poorer mucosal trauma and, in particular, a minor disruption of naso-facial bone structures that is important for the facial growth in children.
4.3.2.2 Transventricular Endoscopy (TE)
In approaching AC extending to the third ventricle, Hollon and colleagues developed a score to help in selecting patients who are candidates to transventricular endoscopic treatment [81]. According to their algorithm, lesions without hypothalamic involvement are candidate to surgical resection (EES or TFS), aiming at radicality whenever possible; lesions with hypothalamic involvement are treated with TE if predominantly cystic in nature or in absence of chiasmopathy. If chiasmopathy is present, lesions with hypothalamic involvement are preferably candidate to surgical debulking. Aside from the predominantly cystic nature of tumors, the main criterion dictating the choice of an endoscopic versus open microsurgical approach was hypothalamic involvement, due to the high risk of endocrine disruption associated with open resection in these cases [64]. According to the TE technique, the tumor cyst lesion is approached and fenestrated superiorly through the foramen of Monro and then inferiorly (at the level of the floor of the third ventricle) by “passing through” the cyst and creating a third-ventriculostomy. The authors report good postoperative outcomes, with good cyst volume reduction and no patients developing postoperative hormonal imbalances or acute visual deficits; residual disease was managed with stereotactic radiosurgery (unfortunately, data on long-term follow-up are limited). Takano and colleagues have reported good long-term results in terms of disease control and endocrinologic function with TE cyst fenestration followed by radiosurgery for residual tumor tissue: the reported 5-year recurrence rate for the 9 treated patients was 14.6%; no endocrinologic or visual complications were observed [68].
One of the main risks of endoscopic cyst fenestration is represented by potential spillover of cystic fluid in the ventricular system, resulting in aseptic meningitis, even if it is difficult to quantify the real impact of this phenomenon on surgical practice [68, 82,83,84]. It is worth noting that the spillage of the fluid outside the cyst did not produce any ill effects both in the personal series and in that of other authors [85]. However, to drain the cyst content through an endoscopically guided catheter before fenestrating the cyst has been proposed as an easy and effective way to prevent this event [83].
As mentioned, TE can also be indicated as a first approach in patients developing symptomatic hydrocephalus due to third ventricular involvement. An endoscopic procedure, while less immediately available than the placement of an external CSF drainage, allows for both a rapid and reliable treatment of hydrocephalus while easing the way for an elective surgical step [68, 72].
4.3.2.3 Keyhole Endoscopic-Assisted Surgical Approaches
Strategies to deal with giant cystic craniopharyngiomas, which pose significant challenges to surgeons, have been repeatedly described in the literature [5, 86,87,88]. In such cases, an intracystic endoscopic approach can provide significant advantages, allowing to safely access impervious anatomical locations by “navigating through” the cyst. After strategical placement of an expanded burr hole (or mini-craniotomy), the dura is opened, surgical route is explored and prepared microsurgically, and then an endoscope is introduced to access the lesion. There are reports of huge cases that have been successfully managed with an endoscopic approach alone by entering the cyst and promoting its collapse while removing its solid components [86]. It is interesting to compare such reports with almost identical cases managed through complex skull base approaches, e.g., trans-petrous route, with good results [5]. Both endoscopic-assisted and craniotomic experiences on such complex cases, however, are often based on case reports with missing data on long-term follow-up.
In cases that cannot be managed satisfactorily through endoscopic approaches alone, endoscopy can anyway be helpful in decompressing the cyst and allowing for a safer and easier microsurgical resection [87]. Moreover, as described below, transventricular endoscopy can be useful to optimally guide the positioning of an intracystic catheter, in order to obtain a good communication between the cyst cavity and the ventricular system and thus promoting both cyst collapse and continuous washout by CSF circulation. This can be followed by other treatments (such as radiosurgery).
4.3.3 Radiation Therapy (RT)
As previously mentioned, there is a solid experience with the use of RT for the adjuvant treatment of AC. The progressive adoption of radiosurgical techniques to treat tumor residue (or recurrence) has paralleled the trend towards a less aggressive surgical philosophy, encouraged by data supporting the non-inferiority of subtotal resection plus adjuvant radiation treatment versus gross total resection in terms of disease control [89,90,91]. In particular, according to the systematic review published by Yang and colleagues in 2010, the long-term outcome of GTR and STR + RT did not differ significantly, the 5-year PFS being 67% after GTR and 69% after STR + SR; and the 10-year OS being 98% after GTR and 95% after STR + RT [92]. Both groups had comparable mean tumor sizes and epidemiological data. The benefits of adjuvant radiation treatment after complete or incomplete surgical resection have also been confirmed by later reviews, which emphasized the low rates of postoperative endocrinological and visual complications [90]. Complication rate appears to be lower for patients treated by an adjuvant setting than in those treated for recurrence [89]. Table 4.1 summarizes the main case series published to date covering different radiation treatments [89, 93,94,95,96,97].
With respect to older radiosurgical techniques, which allowed for the targeting of spherical volumes (e.g., gamma knife), more recent techniques (such as proton beam therapy, or intensity-modulated radiation treatment) are able to precisely mold the irradiation target on tumor conformation, potentially allowing for an even lower rate of normal tissue injury (with reported visual morbidity rate 0–6% for cyber knife) [70, 90]. At the same time, the progressive adoption of frameless technologies has allowed for a better flexibility in dose fractionation [7, 89, 90]. A well-known limit of these treatments is the restricted accessibility due to high costs and the complex technological apparatuses required. Moreover, no significant differences on PFS have been demonstrated with different radiosurgical techniques [70]. For example, in the comparative case series dealing with proton beam therapy vs. intensity-modulated radiation treatment published by Bishop and colleagues, no significant differences between groups were identified in 3-year overall survival rate, cyst progression, and nodular progression [89]. It is worth noting, however, that the follow-up period was too short to formulate definitive comparisons.
In spite of these technological advances, AC continues to represent a special challenge also when dealing with RT. In contrast with solid craniopharyngiomas, cystic tumors show an unpredictable response to RT, and the behavior of the solid and cystic components of mixed tumors during treatment appears to be independent from each other [7, 91, 98]. Indeed, the shrinkage of the solid part can often be accompanied by stability or increase of the cyst volume (in up to 40% of patients) [89, 99,100,101]. This causes both a modification of target conformation over the course of treatment and an increase in the radiation dose required to cover the cyst volume, which besides is relatively insensitive to radiation [89]. The integrated management of the cyst by means of a catheter with reservoir (see below) would be then useful to make radiation treatment more effective by reducing the volume of the cystic component [99, 101]. There could be also room for the development of protocols combining RT and intracystic therapies. This would allow to effectively target all components of the tumor.
The management of patients showing recurrence after RT is complex. Considering the potential number of surgical procedures that patients may undergo after the first recurrence, it has been proposed that, at least in this context, GTR should remain a goal [102]. This supports the reemerging tendency towards radical surgical approaches in selected cases, as described above. Additional treatment strategies with subcutaneously administered interferon have also been proposed to slow the course of the disease in these patients, in order to delay the surgical intervention (see below).
4.3.4 Specific Management Options for the Cyst
4.3.4.1 Intracystic Catheter
One of the major changes in the treatment of AC has been the larger use of approaches specifically focused on the management of the cystic component. The most direct among them is the cyst drainage through an intracystic catheter connected to a subcutaneous reservoir (Rickham or, more frequently, Ommaya reservoir). This simple tool allows a rapid cyst decompression with symptom relief and repeated percutaneous cyst aspirations in case of intracystic fluid re-accumulation. Such an approach is considered as a transient measure in (quickly) growing AC or in patients in poorly clinical condition to gain time for a more “radical” treatment. However, quite surprisingly, good results in purely cystic AC have been reported after the stereotactic placement of the catheter alone. More in details, up to 70% of patients did not require further treatments after a follow-up longer than 7 years [103] and the progression free survival did not differ significantly from that of microsurgically treated patients [66]. Two thirds of the patients in the series by Moussa and colleagues were younger than 16 years, thus pointing out the need for even longer observation to confirm the good published results [103], while the patients treated stereotactically in the series by Rachinger and colleagues were considerably older (median age: 54 years) [66]. Similar results have been obtained also with different approaches to implant the catheter for the cyst aspiration, e.g., with endoscopic guidance [104].
A proper placement of the catheter is mandatory to achieve good results. A key point is represented by the position of the catheter holes, which should span across the cyst wall, thus ensuring a continuous drainage of cystic fluid into CSF spaces (cisterns and/or ventricles). To enhance this effect, some authors proposed to modify the catheter by placing additional holes [105] and to pierce through the cyst from wall to wall, creating a double communication with both the ventricular system and the basal cisterns [66].
When dealing with mixed or anatomically complex AC, the “catheter plus reservoir” option can be utilized as part of a more elaborate surgical strategy. For example, preoperative stereotactic reservoir system placement and staged cyst aspiration have been used in patients with mixed lesions candidate to microsurgical resection, facilitating surgery and reportedly obtaining lower rates of postoperative hormonal and electrolyte imbalances with respect to microsurgical resection alone [88]. Placement of reservoir systems has also been presented as a valuable adjunct to radiosurgical treatment [99, 101]. As mentioned, cystic tumors are challenging radiosurgical targets, partly due to the inhomogeneous radiation sensitivity of their components and partly because of the often-huge size reached by the tumor. Therefore, preemptive stereotactic cyst drainage facilitates radiosurgery and allows an optimization of conformational and dosimetric parameters by removing the least radiosensitive part of the mass and by reducing the irradiation field [101]. On the contrary, catheter drainage for cyst recurrence after radiosurgery has shown disappointing results, with most patients eventually requiring further treatments [102].
Catheters can be also left in place after transventricular endoscopic resection of lesions abutting the ventricular system [105, 106]. With respect to stereotactic guidance (neuronavigation), catheter placement under direct visualization provides the theoretical advantage of a tailored targeting, thus offering a more effective and persistent drainage of the cyst fluid [104, 106,107,108]. This approach would be especially useful for granting an optimal communication of the catheter with both the cyst and ventricular system, as already mentioned. For this reason, it has also been proposed for tumors without direct involvement of the third ventricle or without associated hydrocephalus [109]. Recently, robotic guidance of stereotactic procedures has been used as another means to improve targeting accuracy compared with standard neuronavigation [99, 101]. In these reports, frameless navigation with laser-assisted registration of anatomical landmarks provided guidance to a robotic instrument holder that was precisely brought to the desired trajectory.
On the other hand, at least according to past studies, a significant difference in terms of complication rates among the different techniques for catheter placement has not been observed [110]. In fact, a rate of complications (misplacement or contrast leakage) of 16.3% has been reported regardless the surgical modality. Such a result was explained considering variations in resistance and elasticity of the cyst wall as the decisive factor affecting the surgical outcome. Independently from the surgical technique, actually, the increased resistance of the cyst wall could determine the sliding of the catheter over the cyst rather than its actual perforation [110].
4.3.4.2 Beta-Emitting Radionuclides
Intracystic injection with β-emitting sources (such as Yttrium90, Rhenium186, Aurum198, or Phosphorous32) has been used as another means to directly target neoplastic tissue with sparing of the surrounding neural and vascular structures. The 3–4 mm penetration width of beta-radiation makes it especially effective in damaging the cyst wall, while solid tumor nodules are less affected. Their use still raises some controversies because of the feared side effects and, in particular, the safety principles required for their transport, handle and disposal, and the poor availability of the radioisotopes, which reduce their application.
Phosphorous32 (P32) is generally preferred over Yttrium90 and other radionuclides due to its longer half-life, lower required dose, and lower half-value tissue penetrance (0.8–1.1 mm vs. 1.1–2.2 mm) [111, 112]. Brachytherapy with intracystic injection of P32 has been proposed as a relatively safe means of providing selective radiation damage to tumoral tissue. When used alone, however, this treatment has shown a partial effectiveness, with short-term induction of cyst shrinkage in 70–80% of patients but significant rates of recurrence, especially due to the limited efficacy of P32 on the solid part of the tumor and on the collateral cysts [113,114,115]. Also, case series reported good results (75% rate of disease control), but mainly after a short follow-up period (mean follow-up: 48.6 months) [116].
The observed endocrinological and visual sequelae after intracystic irradiation vary widely, even if the global incidence of side effects appears to be generally as low as <5% [85, 114,115,116]. Long-term collateral radiation damage to vascular and neural structures cannot be excluded [85, 111]. According to Kickingereder et al., who reported on one of the largest series (53 patients), a permanent neurological side effect was observed in 3.9% of cases, while the rate of deterioration in hormonal functions was 2% [112]. Therefore, the complication risk seems to be competitive with other treatments.
With regard to another feared risk of radioactive drugs, which is represented by the spillover of the radioactive agent itself into the cerebrospinal fluid, it is difficult to quantify the incidence of this phenomenon (which anyway appears to be anecdotical) and to evaluate its real toxic impact [117]. In order to improve the management of the treated patients, some authors have presented experimental biochemical and physical means of monitoring radioactivity diffusion through the evaluation of radioactivity in biological patient samples and the use of gamma cameras [117, 118].
The future in this field could be represented by the identification of the responders to radionuclides, in order to propose such a limited resource only to the best candidates. This way has been followed by Hu et al. who recently reported on an interesting study on P32 interstitial therapy for recurrent craniopharyngiomas (both AC e PC) [119]. In details, the authors investigated 32 patients with recurrent craniopharyngioma treated by P32 colloid interstitial radiotherapy. The tumor imaging features of the patients were classified into 4 types according to the thickness of the cyst wall: I-purely cystic with thin wall; II-mainly cystic (solid part <25%) with one or more cyst, thin wall; III-as above but with thick wall; IV-partially cystic, multiple cysts, thick wall. The expression of vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor-2 (VEGFR-2) was evaluated with immunohistochemistry before radiotherapy. Only VEGFR-2 expression was associated with the imaging features of tumors. As a final result, craniopharyngiomas with thin cysts (type I and II) and expression of VEGFR-2 (clues of high radiosensitivity) showed a good response to P32 treatment, while types III and IV (no VEGFR-2 expression) did not.
A further means to improve the control of the disease and to limit the radiation exposure is to identify other therapeutic substances for intracystic injection. The crucial point advancing the search for nonsurgical and non-radiating therapeutic methods is the need, namely in younger children, to delay the time of surgical and/or radiation treatment and the related potential adverse effects on growth, hormonal and electrolyte balance, and neurologic function. The main experience has been accumulated around bleomycin and, more recently, around interferon-alpha.
4.3.4.3 Bleomycin
After the studies conducted in vitro by Kubo and colleagues in 1974 [120], who demonstrated the toxicity of bleomycin on craniopharyngioma cell cultures, several authors have presented the results of in vivo intracystic injection of this drug. In spite of the long experience with this treatment and the relative abundance of gathered data, the available evidence is highly heterogeneous. Actually, even three sequential Cochrane reviews failed in providing any recommendation about intracystic bleomycin use in the clinical practice, suggesting the need of randomized trials conducted on larger and more homogeneous patient samples [121,122,123]. However, the missing randomized trials, justified by the rarity of AC and the small niche represented by the intracystic treatments, do not cancel the impact of a 30-year experience and about 300 articles on the use of bleomycin [124].
Bleomycin is a glycopeptide antibiotic secreted by Streptomyces verticillus acting on inhibition of DNA and RNA synthesis. It is very effective in squamous cell carcinomas and, for this reason, it has been used also in AC (with presently the same epithelial characteristics). Bleomycin is instilled in the tumor cyst through an Ommaya reservoir. The dose varies according to the Center and the size of the cyst (usually 2–5 mg for each administration; 3 doses per week (daily administration in some Centers); meanly for 5 weeks).
Generally, good immediate or early results on the cyst volume reduction are reported in the literature, with a variable but significant percentage of patients achieving a > 90% cyst reduction, compared with disappointing data on long-term disease control [85, 125]. A single randomized controlled trial, conducted on a small patient sample (7 patients; mean age 9.6 years), compared intracystic bleomycin, intracystic P32, and the combination of both [126]. The results suggested that combination treatment (bleomycin+P32) is more effective than the different treatments alone; nevertheless, two patients in this series (22%) reported severe adverse events (bilateral thalamic infarction). Indeed, more than the short-term effect, the main limit of intracystic bleomycin therapy seems to be the adverse reactions associated with the treatment and the possible extracystic drug diffusion. Commonly, patients experience fever (up to 70% of cases), headache, and nausea just after bleomycin administration; moreover, some patients show delayed toxic effects due to documented damage to nearby neural and vascular structures [122, 127]. These range from steroid-responsive neurological deficits, to severe manifestations such as ischemic vasculopathy, irreversible optic pathway damage, hypothalamic damage, and diabetes insipidus [127,128,129,130]. Unfortunately, a negative leakage test does not eliminate the risk of such reactions [128, 129].
For these reasons, the use of bleomycin as an intracystic agent is progressively falling out of favor, while research has been directed towards the experimentation of less toxic agents, such as interferon alpha and, more recently, target therapies [125].
4.3.4.4 Interferon Alpha
Interferon-α (IA) is a cytokine produced by leucocytes belonging to the family of interferons (natural signaling proteins with antiviral activities) together with interferon-β (fibroblasts) and interferon-γ (lymphocytes) [131, 132]. IA is secreted by macrophages and lymphocytes as response to viral infection or tumor progression. It is codified by 15 genes of the chromosome 9. Its antitumoral activity is realized through three different mechanisms: (1) differentiation of T-lymphocytes into T-helper 1 lymphocytes (response against a specific target) and inhibition of other lymphocytes; (2) stimulation of the proliferation on natural killer cells and macrophages, and stimulation of their production interleukine-1 and IA itself; (3) increased identification of the tumor cells through the expression of the major histocompatibility complex class 1 and expression of surface antigens.
The rationale for the use of IA for the treatment of AC originates from the experience with skin tumors sharing the same embryological origin (i.e., squamous and basal cell carcinomas), where subcutaneously administered IA has a well-documented efficacy [133]. This rationale was further supported by the favorable combination between the characteristics of AC cyst (inflammatory genesis) and the immunomodulatory, proapoptotic, and antimicrobial effects of IA that can effectively act against the cyst reaccumulation or growth [9, 30, 32, 35, 41]. In the late 199’s, Jakacki and colleagues conducted the first exploratory phase II trial by administering subcutaneous IA to patients with craniopharyngioma, demonstrating a significant radiologic response in 25% of treated subjects [133]. On this basis, other groups started to investigate the use of intra-tumoral interferon injection, with the potential advantages of having both less systemic side effects and higher local drug activity and persistence. Cavalheiro and colleagues realized the first clinical studies on the IA intracystic injection and reported a complete response in about two thirds of patients with cystic tumors in the short term (mean follow-up: 1.7 years), with only mild adverse events (mainly self-limiting headaches, fever, fatigue) [31]. Therefore, the authors prepared an administration protocol, which is shared by all the Centers using IA (with small variants), emphasizing the possibility to repeat the treatment several times and the absence of treatment discontinuation [6]. The dose scheme for IA intracystic administration is three million International Units (IU) of interferon-α-2a every other day for a total of 12 administrations per cycle (36 million IU). The cycle can be repeated 1 month after the end of the previous one for 3 or more times. IA is administered through an Ommaya reservoir and an intracystic catheter, previously placed by a microsurgical approach (almost abandoned), by neuronavigation alone, or by neuroendoscopic control (especially if hydrocephalus has to be managed at the same time).
The results on the clinical studies with IA published so far have been summarized on Table 4.2. Such a cumulative experience shows a response of AC cyst in the majority of cases, with a complete cyst effacement in about a half of the patients (as a result both cysts collapse due to the aspiration and effects of IA) and a partial response or cyst/stabilization in the remaining cases (Fig. 4.3). Such a result is variably maintained during the follow-up (meanly lower than 5-years), about one third of cases requiring further treatments during this period. The largest study published to date is an international multicenter cooperative study provided in 2017 by Kilday and colleagues [136]. Overall, 56 children affected by AC received intracystic IA according to the Toronto protocol (borrowed from the original Cavalheiro et al. one), that is: (1) permeability study, conducted at least 2 weeks after catheter implantation; (2) aspiration of cystic fluid and instillation of three million IU every other day for 12 days (totaling 36 million IU); (3) cycle repeated if deemed appropriate and tolerated. All patients were under 18 years of age (median age: 6.3 years): 23% received intracystic IA as a first treatment, while the others had previously received other treatments. Median follow-up was of 5.1 years (median follow-up after IA administration: 2.7 years). Seventy-five percent of patients showed clinical or radiological progression during observation, but 33% of them did not require treatment for this progression at last follow-up. The mean time to surgery or radiotherapy for progression was of 5.8 years. Forty-one percent of patients in the cohort did not experience any adverse event; the most common side effects of treatment were influenza-like malaise (29%), headaches (18%), fatigue (13%), transient hyponatremia (2%), appetite loss (2%), and weight loss (2%). None of these symptoms were severe (maximum grade II). However, there were two cases of brain toxicity (optic pathway damage with radiologic evidence of edema and brain atrophy with hydrocephalus) due to suspected extravasation of IA in CSF spaces.
Pretreatment MRI of a large AC in a 5-year-old girl: axial (a, b), sagittal (c), and coronal views (d) after gadolinium show a small suprasellar solid component and a large suprasellar cyst, effacing the third ventricle and causing biventricular hydrocephalus. MRI of the same case performed 8 months later (after endoscopic insertion of intracystic catheter, intraoperative cyst reduction by aspiration and one cycle of IA): note the significant reduction of the cyst and the re-expansion of the third ventricle with resolution of the hydrocephalus (e–g). The solid portion of AC is roughly unchanged
A possible advantage of IA over other intracystic drugs is its safety. Indeed, IA was generally believed to show a low potential for toxicity in case of extracystic diffusion [6, 31, 134, 135]. Also in the personal experience, based on only one case of brain IA diffusion, this event did not produce any symptoms. However, along with the progressive accumulation of clinical data, there have been reports of suspected IA neurotoxicity [136, 138, 139]. Although, in some instances, the toxicity shows a spontaneous resolution, this event raises the problem of reliable leak tests before administration (Fig. 4.4).
CT-scan-based leak test. First, a basal CT scan is performed to verify the correct placement of the catheter and size and volume of the cyst (a, b). Afterwards, the iodate contrast medium is injected into the cyst through the subcutaneous reservoir looking for a possible spillage of the contrast. The quantity of contrast medium to be administered is calculated according to the quantity of cyst fluid taken out before the injection. The test is negative if the cyst is «designed» by the contrast medium without its diffusion into the brain (c–f)
Aside from studies about intracystic IA, there has been some continuing interest around subcutaneously administered pegylated IA (PIA), which has been used by Yeung and colleagues [135], who treated recurrent AC with 1–3 μg/kg/week of PIA for 2 years, and recently by Goldman and colleagues [137], who treated recurrent or unresectable AC in children and young adults with or up to 18 courses (108 weeks) of PIA (Table 4.2). In spite of the limited number of patients (overall: 23 cases) and the short follow-up in both series, these studies provide some promising data in terms of disease stabilization. Yeung et al. actually had all their 5 patients stabilized or better after a 43-month mean follow-up [135]. According to Goldman et al., no significant radiological response was evident (especially in patients who previously received RT), but an improvement of the median PFS was detected (19.5 months) [137]. As foreseeable, systemic toxicities were more frequent and severe than those reported with intracystic therapy alone. However, only grade 2 and 3 hematologic toxicities occurred (no grade 4 or 5). Further studies are needed to better assess this treatment modality.
In summary, therapy with intracystic IA offers a favorable risk/benefit ratio and a safe and relatively easy means to control disease progression. However, it has to be considered mainly as a transient option to delay more aggressive treatments. Due to the relative rarity of AC, it is hard to evaluate its implications in a randomized setting and on homogeneous patient samples. Further studies shall better explore the implications of its usage in clinical practice, both alone and in combination with other treatments.
4.3.4.5 Future Directions
In the era of target therapies, the results of the aforementioned basic researches are expected to allow for the development of therapeutic agents (both intracystic and systemic) acting on the molecular pathways guiding tumor growth and cyst accumulation and maintenance [15, 41]. One example is the IL-6 pathway, for which target drugs are already available and have been tried in craniopharyngioma patients with initial but promising data [25]. Tocilizumab (an anti-IL6 monoclonal antibody) was systemically administered to two patients as a compassionate treatment after recurrence. The first patient (age: 7 years) was treated for recurrence after catheter cyst drainage and radiosurgery; he completed a 7-month cycle obtaining tumor volume reduction (and stability during subsequent follow-up) and no side effects. Another patient (age: 3 years) had the first cycle interrupted after 8 months due to disease progression; therapy was then started again with a combination of tocilizumab and bevacizumab (an antiangiogenetic, anti-VEGF monoclonal antibody) obtaining disease control. During this second cycle with combination therapy, the patient developed transient grade 3 neutropenia [25].
Application of similar treatments for intracystic administration could allow for a greater local effect with less adverse events. This could allow for a long-lasting tumor (and symptoms) control, both as a means to delay surgery and/or RT, to make them less invasive and to prevent recurrence afterwards.
4.4 Placement of Intracystic Catheter
The Ommaya placement is a crucial option in the current management of AC, either to perform cyst aspiration and to deliver selected drugs into the cyst, being intracystic chemotherapy one of the major advances in AC treatment. Some authors use a direct microsurgical placement via subfrontal craniotomic approach (mainly in the past) [31, 140], while others utilize a stereotactic navigated or robotized insertion [104, 141]. Although both the previous options maintain their effectiveness, the placement of the intracystic catheter by a navigated endoscopic transventricular approach is the most commonly used technique in the current clinical practice. This type of approach, indeed, conjugates the mini-invasiveness of neuroendoscopy with the reliability of neuronavigation and offers the possibility of a direct visual control of the position of the catheter across the cyst and the opportunity to perform additional surgical maneuvers, if needed (e.g., tumor biopsy, cyst aspiration, third ventriculostomy, septostomy). As expected, several differences can be observed among the different authors with regard to technical details, as head positioning, instrumentation, number of burr holes, width in the opening of the cyst wall, and so on. On the other hand, there is quite a general agreement in considering neuronavigation as a key feature in performing this kind of surgery.
Head position: As a basic principle of neuroendoscopy, the patient’s head is placed in neutral position with a degree of flexion sufficient to have the burr hole in the higher point of the surgical field to minimize CSF leak and bubbles formation. Many authors, namely pediatric neurosurgeons, do not utilize head holders in this procedure [104,105,106,107,108,109]. On the other hand, authors mainly dealing with adults reported the use of the Mayfield head holder as a key feature of their ventriculoscopic technique [81].
Instrumentation: The choice of the ventriculoscope represents the main difference alongside the reported technical notes, as result of the experience and the resources of the different centers. The endoscopes most commonly used were: (1) 7-mm rigid endoscope with a 30° optic and associated trocar (Karl Storz, Tuttlingen, Germany) [105, 108]; (2) 4 mm rigid endoscope with a 0° optic and associated trocar (Karl Storz, Tuttlingen, Germany) [81, 104]; (3) Oi-Samii neuroendoscopic system (Oi-Samii Handi Pro; Karl Storz, Germany) introduced through a 14-F peel-away sheath [107]; (3) videoscope (Olympus Corporation, Tokyo, Japan) passed by an endoscopic sheath (NeuroportTM; Olympus Corp., Tokyo, Japan) [109]; (4) Gaab with 0° optic telescope (Karl Storz, Tuttlingen, Germany) with its trocar [106].
Number of burr holes: The number of burr holes also varies according to the author’s experience. All papers report the pre-coronal area as the chosen location for the burr hole(s). Some authors suggest to use a single burr hole to insert both the endoscope and the catheter [81, 104,105,106, 109], while other authors prefer to use two accesses to optimize control of the instruments [107]. In our institution, we prefer to adapt this choice tailoring it on the patient’s characteristics, selecting the one-hole approach in cases with large ventricles and the two-hole approach in case of small ventricles. Such a strategy represents the evolution of the technique reported in 2009 [108].
Width in the opening of the cyst wall: A further difference found in the literature is the size of cyst wall opening. In fact, some authors suggest to make a small opening in the cyst wall to avoid excessive leakage of the intracystic fluid as well as to reduce the chance of dislocation of the catheter [107, 108], while other authors propose to widely open the cyst to aspirate the fluid and to shrink the cyst before inserting the catheter [81, 104,105,106, 109].
Further steps: The most commonly reported extra procedures are ETV [109] and tumor biopsy [106]. A general agreement exists on to carefully reconstruct the galea and to suture the skin in multiple layers to reduce CSF leak and to adequately protect the subcutaneous reservoir.
Figures 4.5 and 4.6 summarize the personal procedure for intracystic catheter placement.
The patient is supine with the head slightly flexed. A L-shaped skin flap is planned according to the trajectory selected by navigation (a). Two pre-coronal burr holes are then placed: the posterior one is used to introduce the endoscope, while the anterior one is utilized to introduce the catheter under both navigation and endoscopic views (b, c). The endoscope is kept in place until the end of the procedure (in particular, once the subcutaneous reservoir is connected) to rule out possible displacement of the catheter (d). Finally, the skin is sutured (e)
Intraoperative endoscopic view of the case in Fig. 4.5. The procedure is performed under neuronavigation, either because of the small ventricles and because of the need to follow the right trajectory (a). The AC cyst is approached through the right foramen of Monroe and fenestrated by Thulium-laser (b). The catheter is ready to be inserted into the cyst (asterisk) (b). A small fenestration is done to ensure a quick closure of the cyst wall around the catheter and to avoid possible dislocation of the catheter rather than to limit the spillage of the cyst fluid (c). Finally, the catheter is introduced into the cyst for the length previously calculated according to the MRI (d). Postoperative MRI showing the correct position of the catheter (e)
References
Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst. 2005;21(8–9):622–7.
Wang K-C, Hong SH, Kim S-K, Cho B-K. Origin of craniopharyngiomas: implication on the growth pattern. Childs Nerv Syst. 2005;21(8–9):628–34.
Müller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera J-P, Puget S. Craniopharyngioma. Nat Rev Dis Primers. 2019;5(1):75.
Cushing H. Intracranial tumours. J Nerv Ment Dis. 1932;76(5):539.
Kiran NAS, Suri A, Kasliwal MK, Garg A, Ahmad FU, Mahapatra AK. Gross total excision of pediatric giant cystic craniopharyngioma with huge retroclival extension to the level of foramen magnum by anterior trans petrous approach: report of two cases and review of literature. Childs Nerv Syst. 2008;24(3):385–91.
Dastoli PA, Nicácio JM, Silva NS, Capellano AM, Toledo SRC, Ierardi D, Cavalheiro S. Cystic craniopharyngioma: intratumoral chemotherapy with alpha interferon. Arq Neuropsiquiatr. 2011;69(1):50–5.
Moorthy RK, Backianathan S, Rebekah G, Rajshekhar V. Utility of interval imaging during focused radiation therapy for residual cystic craniopharyngiomas. World Neurosurg. 2020;141:e615–24.
Yu Y, Dong Z, Chen D, Chen F. Pediatric giant craniopharyngioma: surgical field soak in diluted nimodipine solution reduces cerebral vasospasm. World Neurosurg. 2020;141:113.
Hengartner AC, Prince E, Vijmasi T, Hankinson TC. Adamantinomatous craniopharyngioma: moving toward targeted therapies. Neurosurg Focus. 2020;48(1):E7.
Cani CMG, Matushita H, Carvalho LRS, Soares IC, Brito LP, Almeida MQ, Mendonça BB. PROP1 and CTNNB1 expression in adamantinomatous craniopharyngiomas with or without β-catenin mutations. Clinics (Sao Paulo). 2011;66(11):1849–54.
Hölsken A, Stache C, Schlaffer SM, Flitsch J, Fahlbusch R, Buchfelder M, Buslei R. Adamantinomatous craniopharyngiomas express tumor stem cell markers in cells with activated Wnt signaling: further evidence for the existence of a tumor stem cell niche? Pituitary. 2014;17(6):546–56.
Kato K, Nakatani Y, Kanno H, et al. Possible linkage between specific histological structures and aberrant reactivation of the Wnt pathway in adamantinomatous craniopharyngioma. J Pathol. 2004;203(3):814–21.
Sekine S, Shibata T, Kokubu A, Morishita Y, Noguchi M, Nakanishi Y, Sakamoto M, Hirohashi S. Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am J Pathol. 2002;161(6):1997–2001.
Coury JR, Davis BN, Koumas CP, Manzano GS, Dehdashti AR. Histopathological and molecular predictors of growth patterns and recurrence in craniopharyngiomas: a systematic review. Neurosurg Rev. 2020;43(1):41–8.
Gupta S, Bi WL, Giantini Larsen A, Al-Abdulmohsen S, Abedalthagafi M, Dunn IF. Craniopharyngioma: a roadmap for scientific translation. Neurosurg Focus. 2018;44(6):E12.
Buslei R, Hölsken A, Hofmann B, Kreutzer J, Siebzehnrubl F, Hans V, Oppel F, Buchfelder M, Fahlbusch R, Blümcke I. Nuclear beta-catenin accumulation associates with epithelial morphogenesis in craniopharyngiomas. Acta Neuropathol (Berl). 2007;113(5):585–90.
Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. 2011;108(28):11482–7.
Wang C-H, Qi S-T, Fan J, Pan J, Peng J-X, Nie J, Bao Y, Liu Y-W, Zhang X, Liu Y. Identification of tumor stem-like cells in admanatimomatous craniopharyngioma and determination of these cells’ pathological significance. J Neurosurg. 2019;133(3):664–74.
Apps JR, Carreno G, Gonzalez-Meljem JM, et al. Tumour compartment transcriptomics demonstrates the activation of inflammatory and odontogenic programmes in human adamantinomatous craniopharyngioma and identifies the MAPK/ERK pathway as a novel therapeutic target. Acta Neuropathol (Berl). 2018;135(5):757–77.
Gonzalez-Meljem JM, Martinez-Barbera JP. Senescence drives non-cell autonomous tumorigenesis in the pituitary gland. Mol Cell Oncol. 2018;5(3):e1435180.
Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58.
Hara T, Akutsu H, Takano S, et al. Clinical and biological significance of adamantinomatous craniopharyngioma with CTNNB1 mutation. J Neurosurg. 2018;131(1):217–26.
Zhu W, Tang T, Yuan S, Chang B, Li S, Chen M. Prediction of CTNNB1 mutation status in pediatric cystic adamantinomatous craniopharyngioma by using preoperative magnetic resonance imaging manifestation. Clin Neurol Neurosurg. 2021;200:106347.
Martelli C, Serra R, Inserra I, et al. Investigating the protein signature of adamantinomatous craniopharyngioma pediatric brain tumor tissue: towards the comprehension of its aggressive behavior. Dis Markers. 2019;2019:3609789.
Grob S, Mirsky DM, Donson AM, Dahl N, Foreman NK, Hoffman LM, Hankinson TC, Mulcahy Levy JM. Targeting IL-6 is a potential treatment for primary cystic craniopharyngioma. Front Oncol. 2019;9:791.
Donson AM, Apps J, Griesinger AM, et al. Molecular analyses reveal inflammatory mediators in the solid component and cyst fluid of human adamantinomatous craniopharyngioma. J Neuropathol Exp Neurol. 2017;76(9):779–88.
Whelan R, Prince E, Gilani A, Hankinson T. The inflammatory milieu of adamantinomatous craniopharyngioma and its implications for treatment. J Clin Med. 2020;9(2):519. https://doi.org/10.3390/jcm9020519.
Martelli C, Iavarone F, Vincenzoni F, et al. Proteomic characterization of pediatric craniopharyngioma intracystic fluid by LC-MS top-down/bottom-up integrated approaches. Electrophoresis. 2014;35(15):2172–83.
Pettorini BL, Inzitari R, Massimi L, Tamburrini G, Caldarelli M, Fanali C, Cabras T, Messana I, Castagnola M, Di Rocco C. The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs Nerv Syst. 2010;26(12):1779–84.
Jokonya L, Reid T, Kasambala M, Mduluza-Jokonya TL, Fieggen G, Mduluza T, Kalangu KKN, Naicker T. Antimicrobial effects of craniopharyngioma cystic fluid. Childs Nerv Syst. 2020;36(11):2641–6.
Cavalheiro S, Dastoli PA, Silva NS, Toledo S, Lederman H, da Silva MC. Use of interferon alpha in intratumoral chemotherapy for cystic craniopharyngioma. Childs Nerv Syst. 2005;21(8–9):719–24.
Ierardi DF, Fernandes MJS, Silva IR, Thomazini-Gouveia J, Silva NS, Dastoli P, Toledo SRC, Cavalheiro S. Apoptosis in alpha interferon (IFN-alpha) intratumoral chemotherapy for cystic craniopharyngiomas. Childs Nerv Syst. 2007;23(9):1041–6.
Desiderio C, Martelli C, Rossetti DV, et al. Identification of thymosins β4 and β10 in paediatric craniopharyngioma cystic fluid. Childs Nerv Syst. 2013;29(6):951–60.
Desiderio C, Rossetti DV, Castagnola M, Massimi L, Tamburrini G. Adamantinomatous craniopharyngioma: advances in proteomic research. Childs Nerv Syst. 2021;37(3):789–97.
Massimi L, Martelli C, Caldarelli M, Castagnola M, Desiderio C. Proteomics in pediatric cystic craniopharyngioma. Brain Pathol. 2017;27(3):370–6.
Andoniadou CL, Gaston-Massuet C, Reddy R, Schneider RP, Blasco MA, Le Tissier P, Jacques TS, Pevny LH, Dattani MT, Martinez-Barbera JP. Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol (Berl). 2012;124(2):259–71.
Nemolato S, Restivo A, Cabras T, et al. Thymosin β4 in colorectal cancer is localized predominantly at the invasion front in tumor cells undergoing epithelial mesenchymal transition. Cancer Biol Ther. 2012;13(4):191–7.
Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69(1):88–96.
Markham A, Patel T. Siltuximab: first global approval. Drugs. 2014;74(10):1147–52.
Todd CM, Salter BM, Murphy DM, Watson RM, Howie KJ, Milot J, Sadeh J, Boulet L-P, O’Byrne PM, Gauvreau GM. The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulm Pharmacol Ther. 2016;41:34–9.
Zhou J, Zhang C, Pan J, Chen L, Qi S-T. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human adamantinomatous craniopharyngioma cells and promotes tumor cell migration. Mol Med Rep. 2017;15(6):4123–31.
Huang S, Mills L, Mian B, Tellez C, McCarty M, Yang X-D, Gudas JM, Bar-Eli M. Fully humanized neutralizing antibodies to interleukin-8 (ABX-IL8) inhibit angiogenesis, tumor growth, and metastasis of human melanoma. Am J Pathol. 2002;161(1):125–34.
Mian BM, Dinney CPN, Bermejo CE, Sweeney P, Tellez C, Yang XD, Gudas JM, McConkey DJ, Bar-Eli M. Fully human anti-interleukin 8 antibody inhibits tumor growth in orthotopic bladder cancer xenografts via down-regulation of matrix metalloproteases and nuclear factor-kappaB. Clin Cancer Res. 2003;9(8):3167–75.
Coy S, Rashid R, Lin J-R, et al. Multiplexed immunofluorescence reveals potential PD-1/PD-L1 pathway vulnerabilities in craniopharyngioma. Neuro-Oncol. 2018;20(8):1101–12.
Rimkus TK, Carpenter RL, Qasem S, Chan M, Lo H-W. Targeting the sonic hedgehog signaling pathway: review of smoothened and GLI inhibitors. Cancer. 2016;8(2):22. https://doi.org/10.3390/cancers8020022.
Gump JM, Donson AM, Birks DK, et al. Identification of targets for rational pharmacological therapy in childhood craniopharyngioma. Acta Neuropathol Commun. 2015;3:30.
Carreno G, Boult JKR, Apps J, et al. SHH pathway inhibition is protumourigenic in adamantinomatous craniopharyngioma. Endocr Relat Cancer. 2019;26(3):355–66.
Petralia F, Tignor N, Reva B, et al. Integrated proteogenomic characterization across major histological types of pediatric brain cancer. Cell. 2020;183(7):1962–85.
Cermak S, Kosicek M, Mladenovic-Djordjevic A, Smiljanic K, Kanazir S, Hecimovic S. Loss of cathepsin B and L leads to lysosomal dysfunction, NPC-like cholesterol sequestration and accumulation of the key Alzheimer’s proteins. PLoS One. 2016;11(11):e0167428.
Lefranc F, Chevalier C, Vinchon M, et al. Characterization of the levels of expression of retinoic acid receptors, galectin-3, macrophage migration inhibiting factor, and p53 in 51 adamantinomatous craniopharyngiomas. J Neurosurg. 2003;98(1):145–53.
Lubansu A, Ruchoux M-M, Brotchi J, Salmon I, Kiss R, Lefranc F. Cathepsin B, D and K expression in adamantinomatous craniopharyngiomas relates to their levels of differentiation as determined by the patterns of retinoic acid receptor expression. Histopathology. 2003;43(6):563–72.
Liu H, Liu Z, Li J, Li Q, You C, Xu J. Relative quantitative expression of hypoxia-inducible factor 1α messenger ribonucleic acid in recurrent craniopharyngiomas. Neurol India. 2014;62(1):53–6.
Xu J, Zhang S, You C, Wang X, Zhou Q. Microvascular density and vascular endothelial growth factor have little correlation with prognosis of craniopharyngioma. Surg Neurol. 2006;66(Suppl 1):S30–4.
Zhu J, You C. Craniopharyngioma: survivin expression and ultrastructure. Oncol Lett. 2015;9(1):75–80.
Tena-Suck ML, Ortiz-Plata A, Galán F, Sánchez A. Expression of epithelial cell adhesion molecule and pituitary tumor transforming gene in adamantinomatous craniopharyngioma and its correlation with recurrence of the tumor. Ann Diagn Pathol. 2009;13(2):82–8.
Ebrahimi A, Honegger J, Schluesener H, Schittenhelm J. Osteonectin expression in surrounding stroma of craniopharyngiomas: association with recurrence rate and brain infiltration. Int J Surg Pathol. 2013;21(6):591–8.
Kim S, An SSA. Role of p53 isoforms and aggregations in cancer. Medicine (Baltimore). 2016;95(26):e3993.
Tena-Suck ML, Salinas-Lara C, Arce-Arellano RI, Rembao-Bojórquez D, Morales-Espinosa D, Sotelo J, Arrieta O. Clinico-pathological and immunohistochemical characteristics associated to recurrence/regrowth of craniopharyngiomas. Clin Neurol Neurosurg. 2006;108(7):661–9.
Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–22.
Prieto R, Pascual JM, Subhi-Issa I, Jorquera M, Yus M, Martínez R. Predictive factors for craniopharyngioma recurrence: a systematic review and illustrative case report of a rapid recurrence. World Neurosurg. 2013;79(5–6):733–49.
Flitsch J, Aberle J, Burkhardt T. Surgery for pediatric craniopharyngiomas: is less more? J Pediatr Endocrinol Metab. 2015;28(1–2):27–33.
Sainte-Rose C, Puget S, Wray A, Zerah M, Grill J, Brauner R, Boddaert N, Pierre-Kahn A. Craniopharyngioma: the pendulum of surgical management. Childs Nerv Syst. 2005;21(8–9):691–5.
Elliott RE, Jane JA, Wisoff JH. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery. 2011;69(3):630–43.
Enayet AER, Atteya MME, Taha H, Zaghloul MS, Refaat A, Maher E, Abdelaziz A, El Beltagy MA. Management of pediatric craniopharyngioma: 10-year experience from high-flow center. Childs Nerv Syst. 2021;37(2):391–401.
Puget S, Garnett M, Wray A, et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106(1 Suppl):3–12.
Rachinger W, Oehlschlaegel F, Kunz M, Fuetsch M, Schichor C, Thurau S, Schopohl J, Seelos K, Tonn J-C, Kreth F-W. Cystic craniopharyngiomas: microsurgical or stereotactic treatment? Neurosurgery. 2017;80(5):733–43.
Schubert T, Trippel M, Tacke U, van Velthoven V, Gumpp V, Bartelt S, Ostertag C, Nikkhah G. Neurosurgical treatment strategies in childhood craniopharyngiomas: is less more? Childs Nerv Syst. 2009;25(11):1419–27.
Takano S, Akutsu H, Mizumoto M, Yamamoto T, Tsuboi K, Matsumura A. Neuroendoscopy followed by radiotherapy in cystic craniopharyngiomas—a long-term follow-up. World Neurosurg. 2015;84(5):1305–15.
Burman P, van Beek AP, Biller BMK, Camacho-Hübner C, Mattsson AF. Radiotherapy, especially at young age, increases the risk for de novo brain tumors in patients treated for pituitary/sellar lesions. J Clin Endocrinol Metab. 2017;102(3):1051–8.
Greenfield BJ, Okcu MF, Baxter PA, Chintagumpala M, Teh BS, Dauser RC, Su J, Desai SS, Paulino AC. Long-term disease control and toxicity outcomes following surgery and intensity modulated radiation therapy (IMRT) in pediatric craniopharyngioma. Radiother Oncol. 2015;114(2):224–9.
Locatelli D, Massimi L, Rigante M, Custodi V, Paludetti G, Castelnuovo P, Di Rocco C. Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. Int J Pediatr Otorhinolaryngol. 2010;74(11):1298–302.
Deopujari CE, Karmarkar VS, Shah N, Vashu R, Patil R, Mohanty C, Shaikh S. Combined endoscopic approach in the management of suprasellar craniopharyngioma. Childs Nerv Syst. 2018;34(5):871–6.
Massimi L, Rigante M, D’Angelo L, Paternoster G, Leonardi P, Paludetti G, Di Rocco C. Quality of postoperative course in children: endoscopic endonasal surgery versus sublabial microsurgery. Acta Neurochir. 2011;153(4):843–9.
Lin Y, Hansen D, Sayama CM, Pan I-W, Lam S. Transfrontal and transsphenoidal approaches to pediatric craniopharyngioma: a national perspective. Pediatr Neurosurg. 2017;52(3):155–60.
Alalade AF, Ogando-Rivas E, Boatey J, Souweidane MM, Anand VK, Greenfield JP, Schwartz TH. Suprasellar and recurrent pediatric craniopharyngiomas: expanding indications for the extended endoscopic transsphenoidal approach. J Neurosurg Pediatr. 2018;21(1):72–80.
Ali ZS, Lang S-S, Kamat AR, Adappa ND, Palmer JN, Storm PB, Lee JYK. Suprasellar pediatric craniopharyngioma resection via endonasal endoscopic approach. Childs Nerv Syst. 2013;29(11):2065–70.
Almeida JP, Suppiah S, Karekezi C, Marigil-Sanchez M, Wong JS, Vescan A, Gentili F, Zadeh G. Extended endoscopic approach for resection of craniopharyngiomas. J Neurol Surg Part B Skull Base. 2018;79(2):S201–2.
Bal E, Öge K, Berker M. Endoscopic endonasal transsphenoidal surgery, a reliable method for treating primary and recurrent/residual craniopharyngiomas: nine years of experience. World Neurosurg. 2016;94:375–85.
Jeswani S, Nuño M, Wu A, Bonert V, Carmichael JD, Black KL, Chu R, King W, Mamelak AN. Comparative analysis of outcomes following craniotomy and expanded endoscopic endonasal transsphenoidal resection of craniopharyngioma and related tumors: a single-institution study. J Neurosurg. 2016;124(3):627–38.
Rigante M, Massimi L, Parrilla C, Galli J, Caldarelli M, Di Rocco C, Paludetti G. Endoscopic transsphenoidal approach versus microscopic approach in children. Int J Pediatr Otorhinolaryngol. 2011;75(9):1132–6.
Hollon TC, Savastano LE, Altshuler D, Barkan AL, Sullivan SE. Ventriculoscopic surgery for cystic retrochiasmatic craniopharyngiomas: indications, surgical technique, and short-term patient outcomes. Oper Neurosurg. 2018;15(2):109–19.
Chen JX, Alkire BC, Lam AC, Curry WT, Holbrook EH. Aseptic meningitis with craniopharyngioma resection: consideration after endoscopic surgery. J Neurol Surg Rep. 2016;77(4):e151–5.
Halliday J, Cudlip S. A new technique of endoscopic decompression of suprasellar craniopharyngioma cyst. Acta Neurochir. 2019;161(11):2285–8.
Satoh H, Uozumi T, Arita K, Kurisu K, Hotta T, Kiya K, Ikawa F, Goishi J, Sogabe T. Spontaneous rupture of craniopharyngioma cysts. A report of five cases and review of the literature. Surg Neurol. 1993;40(5):414–9.
Steinbok P, Hukin J. Intracystic treatments for craniopharyngioma. Neurosurg Focus. 2010;28(4):E13.
Chen A, Zhou R, Yao X, Ai M, Sun T. Neuroendoscopic treatment of giant cystic craniopharyngioma in the foramen magnum: report of two cases. Childs Nerv Syst. 2020;37(7):2387–90. https://doi.org/10.1007/s00381-020-04965-0.
Gangemi M, Seneca V, Mariniello G, Colella G, Magro F. Combined endoscopic and microsurgical removal of a giant cystic craniopharyngioma in a six-year-old boy. Clin Neurol Neurosurg. 2009;111(5):472–6.
Zhu W, Li X, He J, Sun T, Li C, Gong J. A reformed surgical treatment modality for children with giant cystic craniopharyngioma. Childs Nerv Syst. 2017;33(9):1491–500.
Bishop AJ, Greenfield B, Mahajan A, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys. 2014;90(2):354–61.
Conti A, Pontoriero A, Ghetti I, Senger C, Vajkoczy P, Pergolizzi S, Germanò A. Benefits of image-guided stereotactic hypofractionated radiation therapy as adjuvant treatment of craniopharyngiomas. A review. Childs Nerv Syst. 2019;35(1):53–61.
Lee C-C, Yang H-C, Chen C-J, Hung Y-C, Wu H-M, Shiau C-Y, Guo W-Y, Pan DH-C, Chung W-Y, Liu K-D. Gamma knife surgery for craniopharyngioma: report on a 20-year experience. J Neurosurg. 2014;121(Suppl):167–78.
Yang I, Sughrue ME, Rutkowski MJ, Kaur R, Ivan ME, Aranda D, Barani IJ, Parsa AT. Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus. 2010;28(4):E5.
Harrabi SB, Adeberg S, Welzel T, Rieken S, Habermehl D, Debus J, Combs SE. Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: maximal tumor control with minimal side effects. Radiat Oncol Lond Engl. 2014;9:203.
Iwata H, Tatewaki K, Inoue M, Yokota N, Baba Y, Nomura R, Shibamoto Y, Sato K. Single and hypofractionated stereotactic radiotherapy with CyberKnife for craniopharyngioma. J Neurooncol. 2012;106(3):571–7.
Kobayashi T, Kida Y, Mori Y, Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103(6 Suppl):482–8.
Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, Marsh H, Brada M. Craniopharyngioma—a long-term results following limited surgery and radiotherapy. Radiother Oncol. 1993;26(1):1–10.
Tsugawa T, Kobayashi T, Hasegawa T, et al. Gamma knife surgery for residual or recurrent craniopharyngioma after surgical resection: a multi-institutional retrospective study in Japan. Cureus. 2020;12(2):e6973.
Niranjan A, Lunsford LD. The role of Leksell radiosurgery in the management of craniopharyngiomas. Prog Neurol Surg. 2019;34:166–72.
Jarebi M, Coutte A, Bartier F, Khormi Y, Peltier J, Lefranc M. A novel, hybrid, stereotactic approach (radiosurgery and dual Ommaya reservoirs) for the treatment of mixed (polycystic and solid) craniopharyngioma. Stereotact Funct Neurosurg. 2019;97(4):266–71.
Lamiman K, Wong KK, Tamrazi B, Nosrati JD, Olch A, Chang EL, Kiehna EN. A quantitative analysis of craniopharyngioma cyst expansion during and after radiation therapy and surgical implications. Neurosurg Focus. 2016;41(6):E15.
Laville A, Coutte A, Capel C, Maroote J, Lefranc M. Dosimetric and volumetric outcomes of combining cyst puncture through an Ommaya reservoir with index-optimized hypofractionated stereotactic radiotherapy in the treatment of craniopharyngioma. Clin Transl Radiat Oncol. 2020;23:66–71.
Klimo P, Venable GT, Boop FA, Merchant TE. Recurrent craniopharyngioma after conformal radiation in children and the burden of treatment. J Neurosurg Pediatr. 2015;15(5):499–505.
Moussa AH, Kerasha AA, Mahmoud ME. Surprising outcome of Ommaya reservoir in treating cystic craniopharyngioma: a retrospective study. Br J Neurosurg. 2013;27(3):370–3.
Frio F, Solari D, Cavallo LM, Cappabianca P, Raverot G, Jouanneau E. Ommaya reservoir system for the treatment of cystic craniopharyngiomas: surgical results in a series of 11 adult patients and review of the literature. World Neurosurg. 2019;132:e869–77.
Lauretti L, Legninda Sop FY, Pallini R, Fernandez E, D’Alessandris QG. Neuroendoscopic treatment of cystic craniopharyngiomas: a case series with systematic review of the literature. World Neurosurg. 2018;110:e367–73.
Shukla D. Transcortical transventricular endoscopic approach and Ommaya reservoir placement for cystic craniopharyngioma. Pediatr Neurosurg. 2015;50(5):291–4.
Joki T, Oi S, Babapour B, Kaito N, Ohashi K, Ebara M, Kato M, Abe T. Neuroendoscopic placement of Ommaya reservoir into a cystic craniopharyngioma. Childs Nerv Syst. 2002;18(11):629–33.
Pettorini BL, Tamburrini G, Massimi L, Caldarelli M, Di Rocco C. Endoscopic transventricular positioning of intracystic catheter for treatment of craniopharyngioma. J Neurosurg Pediatr. 2009;4(3):245–8.
Ndukuba K, Ogiwara T, Nakamura T, Abe D, Ichinose S, Horiuchi T, Ohaegbulam S, Hongo K. Cyst fenestration and Ommaya reservoir placement in endoscopic transcortical transventricular approach for recurrent suprasellar cystic craniopharyngioma without ventriculomegaly. J Clin Neurosci. 2020;72:425–8.
Zanon N, Cavalheiro S, da Silva MC. Does the choice of surgical approach to insert an intratumoral catheter influence the results of intratumoral cystic treatment? Surg Neurol. 2008;70(1):66–9.
Julow JV. Intracystic irradiation for craniopharyngiomas. Pituitary. 2013;16(1):34–45.
Kickingereder P, Maarouf M, El Majdoub F, et al. Intracavitary brachytherapy using stereotactically applied phosphorus-32 colloid for treatment of cystic craniopharyngiomas in 53 patients. J Neurooncol. 2012;109(2):365–74.
Ansari SF, Moore RJ, Boaz JC, Fulkerson DH. Efficacy of phosphorus-32 brachytherapy without external-beam radiation for long-term tumor control in patients with craniopharyngioma. J Neurosurg Pediatr. 2016;17(4):439–45.
Barriger RB, Chang A, Lo SS, Timmerman RD, DesRosiers C, Boaz JC, Fakiris AJ. Phosphorus-32 therapy for cystic craniopharyngiomas. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2011;98(2):207–12.
Maarouf M, El Majdoub F, Fuetsch M, Hoevels M, Lehrke R, Berthold F, Voges J, Sturm V. Stereotactic intracavitary brachytherapy with P-32 for cystic craniopharyngiomas in children. Strahlenther Onkol Organ Dtsch Rontgengesellschaft Al. 2016;192(3):157–65.
Yu X, Zhang J, Liu R, Wang Y, Wang H, Wang P, Chen J, Liu S. Interstitial radiotherapy using phosphorus-32 for giant posterior fossa cystic craniopharyngiomas. J Neurosurg Pediatr. 2015;15(5):510–8.
Chang H, Zhang J, Cao W, Wang Y, Zhao H, Liu R, Guo S. Drug distribution and clinical safety in treating cystic craniopharyngiomas using intracavitary radiotherapy with phosphorus-32 colloid. Oncol Lett. 2018;15(4):4997–5003.
Babaei M, Ghahramani-Asl R, Sadoughi H-R, Sardari D, Shahzadi S. Evaluation of bremsstrahlung radiation dose in stereotactically radiocolloid therapy of cystic craniopharyngioma tumors with 32P radio-colloid. Australas Phys Eng Sci Med. 2018;41(3):697–711.
Hu C, Chen J, Meng Y, Zhang J, Wang Y, Liu R, Yu X. Phosphorus-32 interstitial radiotherapy for recurrent craniopharyngioma: expressions of vascular endothelial growth factor and its receptor-2 and imaging features of tumors are associated with tumor radiosensitivity. Medicine (Baltimore). 2018;97(26):e11136.
Kubo O, Takakura K, Miki Y, Okino T, Kitamura K. Intracystic therapy of bleomycin for craniopharyngioma—effect of bleomycin for cultured craniopharyngioma cells and intracystic concentration of bleomycin (author’s transl). No Shinkei Geka. 1974;2(10):683–8.
Liu W, Fang Y, Cai B, Xu J, You C, Zhang H. Intracystic bleomycin for cystic craniopharyngiomas in children (abridged republication of cochrane systematic review). Neurosurgery. 2012;71(5):909–15.
Zhang S, Fang Y, Cai BW, Xu JG, You C. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database Syst Rev. 2016;7:CD008890.
Zheng J, Fang Y, Cai BW, Zhang H, Liu W, Wu B, Xu JG, You C. Intracystic bleomycin for cystic craniopharyngiomas in children. Cochrane Database Syst Rev. 2014;9:CD008890.
Wisoff JH. Commentary: Intracystic bleomycin for cystic craniopharyngiomas in children (abridged republication of Cochrane systematic review). Neurosurgery. 2012;71(5):E1063–4.
Mrowczynski OD, Langan ST, Rizk EB. Craniopharyngiomas: a systematic review and evaluation of the current intratumoral treatment landscape. Clin Neurol Neurosurg. 2018;166:124–30.
Jiang R, Liu Z, Zhu C. Preliminary exploration of the clinical effect of bleomycin on craniopharyngiomas. Stereotact Funct Neurosurg. 2002;78(2):84–94.
Lafay-Cousin L, Bartels U, Raybaud C, Kulkarni AV, Guger S, Huang A, Bouffet E. Neuroradiological findings of bleomycin leakage in cystic craniopharyngioma: report of three cases. J Neurosurg. 2007;107(4 Suppl):318–23.
Belen D, Er U, Yigitkanli K, Bolay H. Delayed neurotoxic complication of intracavitary bleomycin therapy for craniopharyngioma in a child who had previously undergone radiosurgery: case report. J Neurosurg. 2007;106(5 Suppl):391–3.
Cho W-S, Kim S-K, Wang K-C, Phi JH, Cho B-K. Vasculopathy after intracystic bleomycin administration for a recurrent cystic craniopharyngioma: case report. J Neurosurg Pediatr. 2012;9(4):394–9.
Hukin J, Steinbok P, Lafay-Cousin L, Hendson G, Strother D, Mercier C, Samson Y, Howes W, Bouffet E. Intracystic bleomycin therapy for craniopharyngioma in children: the Canadian experience. Cancer. 2007;109(10):2124–31.
Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6(1):34–55.
Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–55.
Jakacki RI, Cohen BH, Jamison C, et al. Phase II evaluation of interferon-alpha-2a for progressive or recurrent craniopharyngiomas. J Neurosurg. 2000;92(2):255–60.
Cavalheiro S, Di Rocco C, Valenzuela S, et al. Craniopharyngiomas: intratumoral chemotherapy with interferon-alpha: a multicenter preliminary study with 60 cases. Neurosurg Focus. 2010;28(4):E12.
Yeung JT, Pollack IF, Panigrahy A, Jakacki RI. Pegylated interferon-α-2b for children with recurrent craniopharyngioma. J Neurosurg Pediatr. 2012;10(6):498–503.
Kilday J-P, Caldarelli M, Massimi L, et al. Intracystic interferon-alpha in pediatric craniopharyngioma patients: an international multicenter assessment on behalf of SIOPE and ISPN. Neuro-Oncol. 2017;19(10):1398–407.
Goldman S, Pollack IF, Jakacki RI, et al. Phase II study of peginterferon alpha-2b for patients with unresectable or recurrent craniopharyngiomas: a pediatric brain tumor consortium report. Neuro-Oncol. 2020;22(11):1696–704.
Sharma J, Bonfield CM, Singhal A, Hukin J, Steinbok P. Intracystic interferon-α treatment leads to neurotoxicity in craniopharyngioma: case report. J Neurosurg Pediatr. 2015;16(3):301–4.
Tiedemann LM, Manley P, Smith ER, Dagi LR. Visual field loss in a case of recurrent cystic craniopharyngioma during concomitant treatment with pegylated interferon α-2b. J Pediatr Hematol Oncol. 2016;38(1):e26–8.
Cavalheiro S, Sparapani FV, Franco JO, da Silva MC, Braga FM. Use of bleomycin in intratumoral chemotherapy for cystic craniopharyngioma: case report. J Neurosurg. 1996;84(1):124–6.
Alén JF, Boto GR, Lagares A, de la Lama A, Gómez PA, Lobato RD. Intratumoural bleomycin as a treatment for recurrent cystic craniopharyngioma. Case report and review of the literature. Neurocir Astur Spain. 2002;13(6):479–85; discussion 485.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bianchi, F., Benato, A., Massimi, L. (2022). Treatment of Cystic Craniopharyngiomas: An Update. In: Di Rocco, C. (eds) Advances and Technical Standards in Neurosurgery. Advances and Technical Standards in Neurosurgery, vol 45. Springer, Cham. https://doi.org/10.1007/978-3-030-99166-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-99166-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99165-4
Online ISBN: 978-3-030-99166-1
eBook Packages: MedicineMedicine (R0)