Abstract
Triglyceride-rich lipoproteins (TRLs) are macromolecular complexes consisting of core lipids, most commonly cholesteryl esters and triglycerides, enveloped by a single layer of phospholipids, apolipoproteins, and variable amounts of free cholesterol. The most common TRLs are very-low-density lipoproteins (VLDLs) and chylomicrons. They are considered as biomarkers of increased atherosclerotic cardiovascular disease (ASCVD) risk and can be measured using a specific equation to identify abnormal levels. Elevated TRLs are proatherogenic and have been linked to adverse cardiovascular disease (CVD) outcomes. Therapies aimed at reducing TRL levels are focused on reducing serum triglycerides through lifestyle (e.g., diet, physical activity) and pharmacological therapies (e.g., fibrates, omega-3 (OM3) fatty acids, statins). This chapter reviews the biochemical mechanisms of TRLs and how they may contribute to atherosclerosis, landmark trials of pharmacological agents developed for the management of hypertriglyceridemia (HTG), and novel therapies in development.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hypertriglyceridemia
- Triglyceride-rich lipoproteins
- Risk enhancers
- Cardiovascular disease
- Dyslipidemia
- Atherosclerosis
Introduction
Epidemiology of Hypertriglyceridemia
Elevated plasma triglycerides (TGs) are among the most common lipid abnormalities encountered in clinical practice. As elaborated upon in the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) and the American College of Cardiology (ACC)/American Heart Association (AHA) guidelines, hypertriglyceridemia (HTG) is classified as borderline high at TG levels of 150–199 mg/dL, mild to moderately high at TG levels of 200–499 mg/dL, and very/severely high at TG levels greater than 500 mg/dL (Grundy et al. 2004, 2019). The prevalence of HTG is approximately 10% of the adult population in Europe (Laufs et al. 2020; Hegele et al. 2014). However, a study of the National Health and Nutrition Examination Survey (NHANES) conducted between 2007 and 2014 estimated an overall prevalence of HTG in the United States to be considerably higher (25.9%); this includes 12.3 million statin-treated patients with TGs ≥ 150 mg/dL (Fan et al. 2020). Of these, 6.4 million had concomitant type 2 diabetes mellitus (T2DM) or atherosclerotic cardiovascular disease (ASCVD). Whilst mild to moderately high TG levels are common, very high HTG (TGs ≥ 500 mg/dL) is rare, representing only 1.6% of the US population (Christian et al. 2011).
Hypertriglyceridemia as a Risk Enhancer
It is well established that HTG is associated with an increased risk of developing ASCVD (Miller et al. 2011; Hulley et al. 1980). For many years, the extent to which HTG promoted coronary atherosclerosis was difficult to reconcile because TGs per se are not taken up by vascular wall macrophages (Peng et al. 2017; Thomsen et al. 2014; Jorgensen et al. 2013). Rather, the lipoprotein complex containing TGs, or triglyceride-rich lipoproteins (TRLs), become atherogenic following hydrolysis by lipoprotein lipase (LPL), due to formation of cholesterol-enriched by-products; these smaller particles, referred to as remnants (Do et al. 2013), are easily transported across the endothelium (Fogelstrand and Boren 2012). TRL remnants are highly atherogenic because they carry more cholesterol per molecule than LDL and thus do not need to be modified for uptake by macrophages (in contrast to LDL) (Nordestgaard and Varbo 2014), thereby facilitating foam cell formation and atherosclerotic plaque deposition (Zilversmit 1979). In addition, TG hydrolysis facilitates free fatty acid (FFA) release; in the vascular endothelium, this may result in local inflammation and injury (Saraswathi and Hasty 2006). Taken together, HTG is a marker for elevated concentrations of atherogenic cholesterol-enriched remnant particles that perpetuate low-grade inflammation, foam cell formation, and atherosclerotic plaques that contribute to elevated risk of CVD (Nordestgaard and Varbo 2014).
Observational studies have examined HTG as an independent risk factor for coronary atherosclerosis (Miller et al. 2002). A meta-analysis of 21 studies involving 57,077 patients across multiple countries demonstrated the consistency of an association between elevated TG levels and risk of CVD (Austin et al. 1998). In univariate analysis, each 1-mmol increase in TGs was associated with a relative risk (RR) of 1.32 (95% confidence interval (CI), 1.26–1.39) and 1.76 (95% CI, 1.50–2.02) in men and women, respectively, after adjustment for high-density lipoprotein cholesterol (HDL-C) (Austin et al. 1998). A subsequent meta-analysis of 68 studies from the Emerging Risk Factors Collaboration evaluated 302,430 individuals without known vascular disease at baseline. Their observation noted a gradual association between elevated TGs and ischemic stroke and CVD; however, following adjustment for HDL-C and non-HDL-C, the association was no longer statistically significant (Emerging Risk Factors C et al. 2009). Furthermore, Tirosh et al. followed 13,953 healthy, untreated, young men (aged 25–34 years) with TG levels <300 mg/dL for 10.5 years to assess the association between changes over time in fasting TG and CVD risk (Tirosh et al. 2007). At baseline, TG levels in the top quintile were associated with a fourfold increase of CVD compared to those in the lowest TG quintile, even after adjustment for HDL-C and other CVD risk factors (Tirosh et al. 2007). These findings support HTG as a biomarker of elevated CVD risk.
Metabolism and Atherogenic Potential of Triglyceride-Rich Lipoproteins
Biochemical/Regulatory Pathways of TGs and Lipoproteins
Triglyceride-rich lipoproteins are macromolecular complexes consisting of core lipids, most commonly cholesteryl esters and triglycerides, enveloped by a single layer of phospholipids, apolipoproteins, and variable amounts of free cholesterol (Ginsberg 2002). Circulating TRLs consist of very-low-density lipoproteins (VLDLs), VLDL remnants, chylomicrons, and intermediate-density lipoproteins (IDLs). The lipoprotein core is composed of hydrophobic TGs and cholesterol esters (CEs), whereas the hydrophilic surface consists of phospholipids, free cholesterol, and apolipoproteins (apos) that play a key role in plasma lipid regulation (Miller et al. 2011). Chylomicrons are the largest TRLs obtained from dietary fat and consist of numerous apos (A-I, A-II, A-IV, A-V, B-48, C-II, E) (Feingold and Grunfeld 2000a) with apolipoprotein B48 (ApoB-48) viewed as an essential protein vital for secretion into the lymphatic system prior to release into the systemic circulation (Feingold and Grunfeld 2000a).
VLDLs are composed of apolipoprotein B100 (ApoB-100) and triglycerides. They are synthesized by hepatocytes and secreted into the systemic circulation whereupon LPL-mediated hydrolysis results in the release of FFAs that are utilized as an energy source by the peripheral muscle or stored in adipose tissue reserves for subsequent utilization (Miller et al. 2011; Feingold and Grunfeld 2000a; Dallinga-Thie et al. 2010).
Metabolic Consequences and Impact of TRLs on ASCVD
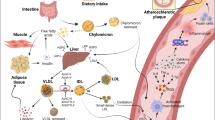
Hypertriglyceridemia ensues from increased production or decreased catabolism of TRLs. This, in turn, impacts the metabolism of LDL and HDL (Miller et al. 2011). Hepatic overproduction of VLDL activates cholesterol ester transfer protein (CETP) to facilitate the transfer of TG from VLDL to LDL (and HDL) in exchange for cholesteryl ester. The resulting by-products, TG-enriched LDL particles, are avidly hydrolyzed by hepatic triglyceride lipase (HTGL) (Fig. 12.1).
Metabolic implications resulting from high triglycerides. Apo A-1 apolipoprotein A-1, Apo B-100 apolipoprotein B-100, CE cholesteryl ester, CETP cholesteryl ester transfer protein, DGAT diacylglycerol acyltransferase, FFA free fatty acid, HDL high-density lipoprotein, HTGL hepatic triglyceride lipase, LDL low-density lipoprotein, TG triglyceride, VLDL very low density lipoprotein. (Adapted from Miller et al. 2011)
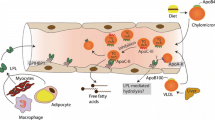
These small, dense LDL particles traverse the endothelium where they do not bind as well to high-affinity LDL receptors compared to normal-sized LDL particles, are more susceptible to oxidation, and exhibit preferential and unregulated uptake by macrophages (Fig. 12.2) (Laufs et al. 2020; Miller et al. 2011; Chait and Eckel 2019; Mudd et al. 2007).
Mechanisms of enhanced atherogenesis of small, dense LDL. LDL-R low-density lipoprotein receptor, TXA2 thromboxane A2, PAI-1 plasminogen activator inhibitor-1. (Reproduced from Elsevier as Open Access Content from Mudd et al. 2007)
Increased VLDL production as a result of excess insulin and fatty acid secretion is also observed in HTG states where increased concentration of ApoC-III, an inhibitor of LPL, may upregulate proinflammatory signaling pathways that also contribute to atherosclerosis (Stahel et al. 2018; Xiao et al. 2016).
In clinical studies, TRLs are consistently associated with elevated ASCVD risk, independent of coexisting metabolic derangements (Nordestgaard and Varbo 2014; Ganda et al. 2018; Jepsen et al. 2016; Varbo et al. 2013, 2015). For example, The Copenhagen General Population Study examined 58,547 individuals initially free of ASCVD, diabetes, and statin use. They found that statin-noneligible individuals with TGs > 264 mg/dL demonstrated similar risk of ASCVD compared with statin-eligible patients with lower TGs (Madsen et al. 2018). Fasting TG levels were also found to predict long- and short-term cardiovascular risks after acute coronary syndrome (ACS) in the dal-OUTCOMES study. Specifically, subjects with the lowest TG levels (~100 mg/dL) at baseline also experienced the lowest likelihood of CVD events (Schwartz et al. 2015, 2012), consistent with previous observations made in the PROVEIT-TIMI 22 trial (Miller et al. 2008).
Landmark Clinical Trials of TRLs and ASCVD
Based on accumulating data in support of HTG as a biomarker of CVD risk, studies have been conducted in recent years to evaluate TG-lowering therapies with respect to (1) efficacy in reducing TGs without raising LDL and (2) extent of ASCVD reduction. In part, this reflects data from prior studies that demonstrated TG-lowering therapies to raise LDL-C levels in patients with very high TGs (greater than 500 mg/dL) or not to have evaluated CVD risk in an exclusive HTG cohort (Skulas-Ray et al. 2019). To address these issues, three clinical trials were designed. The TG-lowering therapies tested were (1) omega-3 fatty acids containing eicosapentaenoic acid (EPA) with or without docosahexaenoic acid (DHA) and (2) fibrates.
Prior to launching the Reduction of Cardiovascular Events with Icosapent Ethyl – Intervention Trial (REDUCE-IT), icosapent ethyl (IPE), a highly purified formulation of eicosapentaenoic acid (EPA), patients with moderate HTG (baseline levels, 200–499 mg/dL) and severe HTG (baseline levels, 500–2000 mg/dL) were evaluated. Not only was there a significant reduction in median TGs (22% and 33%, respectively) but there was also no rise in LDL-C in either study (Bays et al. 2011; Ballantyne et al. 2012). Previously, the Japan EPA Lipid Intervention Study (JELIS) assessed the role of purified EPA (1.8 g) administered daily to patients with hypercholesterolemia (total cholesterol >6.5 mmol/L or 250 mg/dL) but without HTG (median baseline TGs ~150 mg/dL), who were also receiving low-dose pravastatin or simvastatin (Yokoyama et al. 2007). Overall, there was an 18% reduction in CVD events in the group who received purified EPA. However, a post hoc analysis of the subgroup with baseline TGs ≥150 mg/dL demonstrated a more robust reduction by 53% in CVD events in subjects assigned to EPA (Saito et al. 2008). This observation builds upon prior data from fibrate studies (Sacks et al. 2010) that found that patients with dyslipidemia, defined by HTG (≥204 mg/dL) and low HDL-C (≤34 mg/dL), exhibited benefits compared to subjects without dyslipidemia.
Thus, these trials paved the way for testing the hypothesis as to whether patients with HTG would benefit from these therapies with respect to CVD outcomes.
REDUCE-IT
REDUCE-IT was a phase III double-blind, randomized, placebo-controlled trial to evaluate CVD outcomes in 8179 patients with established CVD or in high-risk primary prevention patients aged 50 years and older with T2DM and at least 1 additional risk factor, fasting TGs (135–499 mg/dL) and LDL-C (41–100 mg/dL on statin therapy) (Bhatt et al. 2019a). Enrolled patients were randomized to either IPE 4 g/day or mineral oil placebo and were followed up for a median of 4.9 years. At the end of 1 year of IPE treatment, serum TGs and LDL-C were reduced by 19.7% and 6.6%, respectively, compared to placebo treatment (p < 0.001 for both). Additionally, patients receiving IPE experienced a significant reduction of 39.9% in baseline high-sensitivity C-reactive protein (hsCRP) (p < 0.0001) (Bhatt et al. 2019a; Bazarbashi and Miller 2020a). Primary endpoints such as CVD death, nonfatal myocardial infarction (MI), nonfatal stroke, unstable angina, and coronary revascularization occurred in 23% of patients receiving IPE versus 28.3% in the placebo arm (hazard ratio (HR) 0.75 (0.68–0.83), p < 0.001), with a number needed to treat (NNT) of 21 patients over the study duration to prevent 1 event (95% CI, 15–33) (Bhatt et al. 2019a) (Fig. 12.3).
The REDUCE-IT trial primary endpoint. HR hazard ratio, RRR relative risk reduction, ARR absolute risk reduction, NNT number needed to treat. (From Bhatt et al. 2019a. Copyright © (2019) Massachusetts Medical Society. Reprinted with permission)
In addition to the primary endpoint, prespecified hierarchical testing revealed significant improvement in the key secondary composite endpoint (CVD death, nonfatal MI, stroke), with individual endpoints including CVD death and the composite of total mortality, nonfatal MI, or nonfatal stroke. Finally , there was a 13% reduction in all-cause mortality that trended toward, but did not attain, statistical significance (Fig. 12.4).
The REDUCE-IT trial prespecified hierarchical endpoint. RRR relative risk reduction, CI confidence interval. (From Bhatt et al. 2019a. Copyright © (2019) Massachusetts Medical Society. Reprinted with permission)
Subgroup analysis of the trial (REDUCE-IT REVASC) examined total on-trial coronary revascularization procedures as well as recurrent revascularization procedures and subtypes. Patients allocated to IPE experienced a 34% reduction in initial coronary revascularization compared to that of placebo (p < 0.0001; NNT, 24), with similar results observed for recurrent revascularization intervention (Peterson et al. 2021). Overall, initial as well as repeat (second, third, and fourth) CVD events were reduced, yielding a 31% reduction in total events (Fig. 12.5) (Bhatt et al. 2019b).
The REDUCE-IT trial first and subsequent events. RR relative risk, HR hazard ratio, CI confidence interval. (Reproduced from Elsevier as Open Access Content from Bhatt et al. 2019b)
Notably, while on treatment, TGs accounted for only a small proportion of the benefits observed (Miller 2019) and EPA levels were a robust predictor of multiple CVD endpoints in the REDUCE-IT trial (Bhatt et al. 2020). Taken together, a high daily intake of purified EPA improved CVD risk in patients with HTG at an increased risk of CVD.
STRENGTH
The STRENGTH (Statin Residual Risk Reduction with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia) trial was a double-blinded, randomized, multicenter trial of 13,078 participants designed to examine omega-3 carboxylic acids (CAs), EPA, and DHA, in statin-treated patients at high CVD risk (defined as 1) the presence of established ASCVD in coronary, peripheral, carotid, or aortic regions, (2) T1DM or T2DM aged 40 or older for men or aged 50 and older for woman with at least one risk factor, including smoking, hypertension, hsCRP 2 mg/dL or higher, or high albuminuria, with HTG (200–500 mg/dL) and low HDL-C. Enrolled patients were randomized to receive 4 g/d of omega-3 CAs or corn oil and followed up for a median period of 42 months. The study was terminated on January 8, 2020 after a prespecified interim analysis reported study futility, despite favorable reductions in TGs, non-HDL, and hsCRP (−19%, −6.1%, and −20%, respectively, p < 0.001 compared to placebo) (Nicholls et al. 2020). Additionally, the levels of apolipoprotein C-III were decreased in the omega-3 CA arm but not in placebo (−7% vs +5.9%, p < 0.001). Unfortunately, no differences were observed in the primary endpoint of CV death, MI, stroke, coronary revascularization, or unstable angina requiring hospitalization (12% on omega-3 CAs vs 12.2% on placebo (HR 0.99 (95% CI, 0.90–1.09, p = 0.84))). Similarly, there were no statistically significant differences in the secondary endpoint (CV death, stroke, or MI) or in all-cause mortality (Nicholls et al. 2020).
Why Were Results of REDUCE-IT and STRENGTH Discrepant?
Despite similar reductions in triglyceride levels in the two studies, REDUCE-IT exhibited higher circulating levels of EPA compared to STRENGTH (89.6 vs 144 micrograms/mL), and this finding may have contributed to the benefits observed in REDUCE-IT but not in STRENGTH. Alternatively, DHA may have blunted the CVD benefits in STRENGTH. Another study, the Omega-3 Fatty Acids in Elderly Patients with Myocardial Infarction (OMEMI) trial, did not show clinical benefits on CVD outcomes (nonfatal MI, unscheduled revascularization, stroke, hospitalization for HF, or all-cause mortality) in post-MI seniors (70 years and older) assigned to 1.8 g/day of EPA/DHA vs placebo over a 2-year period (Kalstad et al. 2020).
In addition to the favorable results obtained in two clinical trials (JELIS and REDUCE-IT), experimental evidence has also demonstrated a beneficial role for EPA in endothelial function, cellular inflammation, oxidative stress, and platelet aggregation (Borow et al. 2015). Moreover, EPA improves HDL functionality by upregulating cholesterol efflux and inhibiting cytokine-mediated adhesion molecule expression (Tanaka et al. 2018). Finally, EPA reduces the expressions of proinflammatory genes and microRNAs that influence atherogenic metabolic signaling pathways (Mason et al. 2020; Bazarbashi and Miller 2020b). In contrast, DHA increases membrane fluidity and promotes lipid domain changes and disordering effects (Mason et al. 2020) that may partially temper the benefits of EPA.
PROMINENT
The PROMINENT (Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes) is an ongoing randomized, double-blind, placebo-controlled multicenter trial evaluating the selective peroxisome proliferator-activated receptor alpha (PPARα) modulator, pemafibrate (K-877), in high-risk patients (i.e., T2DM with or without preexisting CVD) with mild to moderate HTG (200–499 mg/dL) and low HDL-C (≤40 mg/dL) receiving statins and other standard-of-care therapies (NCT03071692) (Pradhan et al. 2018). The study is fully enrolled (n = 10,391) with patients randomized to either pemafibrate 0.2 mg twice a day or placebo. The mean follow-up duration is 4 years with an estimated completion date in 2022. The primary outcome measure is time to first occurrence of a composite of the following endpoints: MI, ischemic stroke, unstable angina requiring unplanned coronary revascularization, and cardiovascular death. Secondary outcomes include all-cause mortality, hospitalization for heart failure, any coronary revascularization, and new or worsening peripheral arterial disease (PAD) (Pradhan et al. 2018).
Current Treatments for HTG
Lifestyle Modifications
Because HTG may result from unhealthy dietary habits associated with visceral obesity and metabolic syndrome, the primary strategy with mild to moderate HTG (200–499 mg/dL) is lifestyle intervention. In patients with very high TGs (fasting levels equal to or greater than 500 mg/dL), pharmacological therapy is combined with lifestyle intervention (Miller et al. 2011).
The ACC/AHA and the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines address the management of lifestyle factors that promote physical activity and weight loss as critical components for the management of HTG (Grundy et al. 2019; Mach et al. 2020). Physical activity as recommended by the ACC/AHA (COR 1, LOE B) consists of 150 mins per week or more of moderate intensity (e.g., brisk walking at a rate of 3–4 miles per hour) or 75 mins per week of more vigorous intensity. For each kilogram of weight loss achieved, there is an approximate 8 mg/dL decrease in TGs (Arnett et al. 2019). Dietary recommendations include vegetables, fruits, legumes, nuts, whole grains, and fish as is the custom of the Mediterranean diet that is associated with 10–15% reduction in TGs and decreased ASCVD risk (COR 1, LOE B) (Miller et al. 2011; Arnett et al. 2019). Replacing saturated fats with dietary monounsaturated and polyunsaturated fats may also contribute to TG and ASCVD reduction (COR IIa, LOE B) (Arnett et al. 2019).
Traditional TG-Lowering Therapies
Statins remain a treatment of choice in high-risk patients (e.g., CVD, T2DM) with mild to moderate HTG. On average, statins reduce TG levels by 10–30%, depending upon the statin used and the associated baseline TGs (Miller et al. 2011; Stein et al. 1998). Niacin inhibits hepatic diacylglycerol acetyltransferase 2 (DGAT2) and VLDL synthesis, resulting in 20% or more decreases in plasma TG levels (Feingold and Grunfeld 2000b; Kamanna et al. 2013; Birjmohun et al. 2005). However, niacin is rarely used due to its unfavorable side effect profile and failure to reduce CVD events in clinical trials (Group HTC 2013). Fibrates are the most potent TG-lowering therapies currently available with ~20–50% reductions via PPARα-mediated activation of LPL (Group AS et al. 2010). Results from the Action to Control Cardiovascular Risk in Diabetes (ACCORD), a large randomized controlled trial (RCT) comparing fenofibrate and statin therapy to statin monotherapy, did not demonstrate clinical benefits in patients with T2DM (Group AS et al. 2010). However, a prespecified analysis in patients with TGs >200 mg/dL and HDL <35 mg/dL did show a trend toward statistical significance (p = 0.06) (Elam et al. 2011). As illustrated in Fig. 12.3, other fibrate trials have suggested clinical benefits of fibrates in patients with HTG and low HDL-C. Consequently, the results of PROMINENT are expected to provide more conclusive data as to whether fibrate therapy may play an important role in CVD risk reduction for high-risk patients with HTG. Fibrate therapy is generally well tolerated, although the combination of gemfibrozil and statins is not recommended due to the increased risk of myopathy and caution should be exercised when combining fenofibrate with statins (Kamanna et al. 2013; Zhao et al. 2016).
Omega-3 Fatty Acids
Both EPA and DHA reduce TG levels to a similar extent (~5–10% per gram), although differential effects have been observed on other lipoprotein lipids and metabolic biomarkers (Borow et al. 2015; Mori et al. 2000; Sahebkar et al. 2018). As noted above, IPE is an ultra-purified prescription form of EPA (>96% purity) and was initially approved as an add-on therapy in patients with very high TGs (≥500 mg/dL). Other prescription OM3s (e.g., omega-3 acid ethyl esters) that contain EPA and DHA have also been approved for very high TGs.
Based upon the results of the REDUCE-IT trial, the Food and Drug Administration (FDA) has recently approved IPE as an adjunctive therapy for the management of patients with TGs (150–499 mg/dL) and CVD or DM and at least one additional CVD risk factor (Bazarbashi and Miller 2020a; FDA approves use of drug to reduce risk of cardiovascular events in certain adult patient groups 2019; Orringer et al. 2019; VASCEPA 2019). Concurrently, the National Lipid Association, the European Society of Cardiology, the American Association of Clinical Endocrinologists/American College of Endocrinology, and the American Diabetes Association also released updates to their standard-of-medical-care guidelines and now recommend IPE to prevent CVD in high-risk patients with elevated TGs (135–500 mg/dL) (Mach et al. 2020; Orringer et al. 2019; American Diabetes A 2019).
Novel and Future Therapies
Apo-CIII Inhibition
While apolipoprotein C-III is known to inhibit LPL and function as a regulator of TG metabolism, several therapies aimed at regulating ApoC-III concentrations have emerged. Small antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and monoclonal antibodies are among the therapies developed to specifically inhibit ApoC-III (Taskinen et al. 2019). Volanesorsen is an anti-ApoC-III antisense oligonucleotide administered subcutaneously every 2 weeks. In the APPROACH (A Study of Volanesorsen in Patients with Familial Chylomicronemia Syndrome) trial, volanesorsen reduced TGs by 77% in patients with familial chylomicronemia syndrome (FCS) (Witztum et al. 2019). However, a major and unanticipated adverse event, thrombocytopenia, halted its approval by the FDA for FCS. By contrast, the European Medicine Agency granted volanesorsen an indication within the orphan drug designation. A second-generation ASO directed against ApoC-III may be more promising as thrombocytopenia has not occurred in early-phase studies. Further testing of AKCEA-APOCIII-LR, an N-acetylgalactosamine (GalNac)-conjugated anti-ApoC-III ASO, is anticipated in high-risk patients with HTG.
Angiopoietin-Like Protein 3 (ANGPTL3) Inhibition
Angiopoietin-like protein 3 (ANGPTL3) is a circulating protein synthesized and secreted by the liver (Koishi et al. 2002). ANGPTL3 has been shown to play an integral role in the regulation of lipid and glucose metabolism, in part via inhibition of lipoprotein lipase (Mattijssen and Kersten 2012). Inhibition of ANGPTL3 has been demonstrated pharmacologically using the monoclonal antibody evinacumab. In healthy volunteers with mild to moderate elevation in TGs (150–450 mg/dL) or LDL-C (100 mg/dL or greater), evinacumab (administered subcutaneously or intravenously) reduced TGs and LDL-C by 76% and 23%, respectively (Dewey et al. 2017). ANGPTL3 can also be inhibited by gene-targeted inactivation of messenger RNA (mRNA) via antisense oligonucleotides (ASOs). In a study of 43 participants randomized to multiple doses of the IONIS-ANGPTL3-LRx ASO, decreases in TGs (33.2%–63.1%), LDL-C (1.3%–32.9%), VLDL-C (27.9%– 60%), non-HDL-C (10%–35.6%), and apoB (3.4%–25.7%) were observed compared to placebo (Graham et al. 2017). Both medications were well tolerated without any major serious adverse events reported during early testing.
Gemcabene
Gemcabene is a dialkyl ether dicarboxylic acid lipid-regulating compound that enhances the clearance of VLDLs via reduction of hepatic ApoC-III messenger RNA (mRNA), thereby playing a potential therapeutic role in reducing TGs at levels of 200 mg/dL or higher (Bays et al. 2003; Stein et al. 2016). Gemcabene was licensed from Pfizer Inc. by Gemphire Therapeutics Inc. in 2011 for the treatment of patients with hypercholesterolemia or HTG who were otherwise unable to effectively lower LDL or TGs or were intolerant to standard therapies. In 2015, a new IND (Investigational New Drug) application for gemcabene was filed. In 2016, gemcabene was studied in COBALT-1, an open-label trial of patients with homozygous familial hypercholesterolemia (HoFH), and demonstrated a dose-dependent change in the mean percentage and absolute changes in LDL (Gaudet et al. 2019). Most recently, results of a 12-week study to assess the efficacy, safety, and tolerability of gemcabene in subjects with severe hypertriglyceridemia (INDIGO-1) (NCT02944383) have demonstrated a 47% reduction in TGs for patients taking gemcabene 600 mg daily when compared with placebo (27%). This compound has not yet received FDA approval.
Fibroblast Growth Factor 21 (FGF21)
Fibroblast growth factor 21 is a cytokine with biological pleiotropic properties including, but not limited to, regulating cell growth, differentiation, and metabolism. FGF21 is mainly regulated by PPARα in the liver and PPARζ in adipocytes. Therapy with FGF21 analogues alleviate dyslipidemia and increase adiponectin levels. Four different FGF21 therapies have emerged (LY2405319, PF-05231023, AMG876/AKR-001, pegbelfermin) with demonstrated reductions in serum TG levels in humans (Geng et al. 2020). To date, none of the FGF21 analogues have been approved by the FDA and the majority are currently in preclinical animal models. However, pegbelfermin, an FGF21 analogue, was recently studied in a 16-week randomized, double-blinded, phase 2a clinical trial in human patients with nonalcoholic steatohepatitis. The results showed a significant decrease in absolute hepatic fat fraction in the group receiving 10 mg pegbelfermin daily (−6.8% vs −1.3%; p = 0.0004) compared with placebo (Sanyal et al. 2019). Most recently, AKR-001, a long-acting human immunoglobulin 1 (IgG1) Fc–FGF21 fusion protein, was studied in patients with type 2 diabetes over 4 weeks of treatment. Markers of lipid metabolism were analyzed and demonstrated a trend toward improvement in the lipoprotein profile. A maximal reduction in fasting TGs of 69% and 55% in 1- and 2-week dosing, respectively, was observed (Kaufman et al. 2020). Other FGF21 candidates (e.g., BIO89-100) are currently under evaluation for patients with severe HTG (equal to or greater than 500 mg/dL) (NCT04541186).
Current Recommendations
The 2018 ACC/AHA guideline on the management of blood cholesterol places very little emphasis on HTG (Grundy et al. 2019). They define HTG as fasting or nonfasting levels between 175 and 499 mg/dL and recommend lifestyle therapy as the cornerstone of management, similar to recommendations based on the 2011 AHA Statement (Miller et al. 2011). In patients with HTG and a high estimated 10-year risk of ASCVD (7.5% likelihood of an ASCVD event over 10 years), the recommendation is to initiate or intensify statin therapy (Fig. 12.6) (Grundy et al. 2019). It remains to be determined whether IPE therapy will be prioritized for treatment of mild to moderate HTG in future guidelines, though as noted earlier, multiple professional guidelines endorse the use of high-dose IPE for high-risk patients with elevated TG (135–500 mg/dL).
2018 ACC/AHA guideline on the management of hypertriglyceridemia. (Reproduced from Elsevier as PMC Open Access Content from the 2018 ACC/AHA Guideline (Grundy et al. 2019)
Summary
While TRLs contribute to elevated CVD risk in patients with HTG, only recently has evidence emerged that lowering TGs may translate into reduced CVD risk. While we await the results of the soon-to-be completed clinical trials (e.g., PROMINENT) and continue to investigate novel therapies, it is clear that the persistently elevated risk in this group despite statin therapy may be amenable to effective therapies (e.g., IPE) that help mitigate this risk.
Abbreviations
- ACCORD:
-
Action to Control Cardiovascular Risk in Diabetes
- ANCHOR:
-
Effect of AMR101 (Ethyl Icosapentate) on Triglyceride Levels in Patients on Statins with High TG Levels (>200 and <500 mg/dL)
- ANGPTL3:
-
Angiopoietin-Like Protein 3
- Apo:
-
Apolipoprotein
- APPROACH:
-
A Study of Volanesorsen in Patients with Familial Chylomicronemia Syndrome
- ASCVD:
-
Atherosclerotic Cardiovascular Disease
- CVD:
-
Cardiovascular Disease
- DHA:
-
Docosahexaenoic Acid
- EPA:
-
Eicosapentaenoic Acid
- FFA:
-
Free Fatty Acid
- HDL-C:
-
High-Density Lipoprotein Cholesterol
- HF:
-
Heart Failure
- HTG:
-
Hypertriglyceridemia
- IPE:
-
Icosapent Ethyl
- JELIS:
-
Japan Eicosapentaenoic Acid Lipid Intervention Study
- LDL-C:
-
Low-Density Lipoprotein Cholesterol
- LPL:
-
Lipoprotein Lipase
- MARINE:
-
Efficacy and Safety of AMR101 (Ethyl Icosapentate) in Patients with Fasting Triglyceride (TG) Levels >500 and <2000 mg/dL
- OM3FA:
-
Omega-3 Fatty Acids
- OMEMI:
-
Omega-3 Fatty Acids in Elderly Patients with Myocardial Infarction
- PPARα:
-
Peroxisome Proliferator-Activated Receptor Alpha
- PROMINENT:
-
Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes
- REDUCE-IT:
-
Reduction of Cardiovascular Events with Icosapent Ethyl – Intervention Trial
- RRR:
-
Relative Risk Reduction
- STRENGTH:
-
STatin Residual Risk Reduction with EpaNova in HiGh CV Risk PatienTs with Hypertriglyceridemia
- T2DM:
-
Type 2 Diabetes Mellitus
- TG:
-
Triglyceride
- TRL:
-
Triglyceride-Rich Lipoprotein
- VLDL:
-
Very-Low-Density Lipoprotein
References
American Diabetes A. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S103–S23.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. 2019;74(10):e177–232.
Austin MA, Hokanson JE, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B.
Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110(7):984–92.
Bays HE, McKenney JM, Dujovne CA, Schrott HG, Zema MJ, Nyberg J, et al. Effectiveness and tolerability of a new lipid-altering agent, gemcabene, in patients with low levels of high-density lipoprotein cholesterol. Am J Cardiol. 2003;92(5):538–43.
Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the multi-center, plAcebo-controlled, randomized, double-blINd, 12-week study with an open-label extension [MARINE] trial). Am J Cardiol. 2011;108(5):682–90.
Bazarbashi N, Miller M. Icosapent ethyl: drug profile and evidence of reduced residual cardiovascular risk in patients with statin-managed LDL-C cholesterol. Expert Rev Cardiovasc Ther. 2020a;18(4):175–80.
Bazarbashi N, Miller M. Icosapent ethyl: niche drug or for the masses? Curr Cardiol Rep. 2020b;22(10):104.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with Icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019a;380(1):11–22.
Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019b;73(22):2791–802.
Bhatt DL, Miller M, Steg G, et al. on Behalf of the REDUCE-IT Investigators. , editor EPA levels and cardiovascular outcomes in the reduction of cardiovascular events with icosapent ethyl–intervention trial. American College of Cardiology Virtual Annual Scientific Session Together with World Congress of Cardiology (ACC 2020/WCC); 2020 March 30, 2020; Chicago, United States. Online: American College of Cardiology; 2020.
Birjmohun RS, Hutten BA, Kastelein JJ, Stroes ES. Efficacy and safety of high-density lipoprotein cholesterol-increasing compounds: a meta-analysis of randomized controlled trials. J Am Coll Cardiol. 2005;45(2):185–97.
Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242(1):357–66.
Chait A, Eckel RH. The chylomicronemia syndrome is most often multifactorial: a narrative review of causes and treatment. Ann Intern Med. 2019;170(9):626–34.
Christian JB, Bourgeois N, Snipes R, Lowe KA. Prevalence of severe (500 to 2,000 mg/dl) hypertriglyceridemia in United States adults. Am J Cardiol. 2011;107(6):891–7.
Dallinga-Thie GM, Franssen R, Mooij HL, Visser ME, Hassing HC, Peelman F, et al. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis. 2010;211(1):1–8.
Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med. 2017;377(3):211–21.
Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet. 2013;45(11):1345–52.
Elam M, Lovato LC, Ginsberg H. Role of fibrates in cardiovascular disease prevention, the ACCORD-lipid perspective. Curr Opin Lipidol. 2011;22(1):55–61.
Emerging Risk Factors C, Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000.
Fan W, Philip S, Granowitz C, Toth PP, Wong ND. Prevalence of US adults with triglycerides >/= 150 mg/dl: NHANES 2007-2014. Cardiol Ther. 2020;9(1):207–13.
FDA approves use of drug to reduce risk of cardiovascular events in certain adult patient groups [press release]. U.S Food and Drug Administration Website, 2019.
Feingold KR, Grunfeld C. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A, et al., editors. Triglyceride lowering drugs. South Dartmouth: Endotext; 2000a.
Feingold KR, Grunfeld C. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, et al., editors. Introduction to lipids and lipoproteins. South Dartmouth: Endotext; 2000b.
Fogelstrand P, Boren J. Retention of atherogenic lipoproteins in the artery wall and its role in atherogenesis. Nutr Metab Cardiovasc Dis. 2012;22(1):1–7.
Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72(3):330–43.
Gaudet D, Durst R, Lepor N, Bakker-Arkema R, Bisgaier C, Masson L, et al. Usefulness of gemcabene in homozygous familial hypercholesterolemia (from COBALT-1). Am J Cardiol. 2019;124(12):1876–80.
Geng L, Lam KSL, Xu A. The therapeutic potential of FGF21 in metabolic diseases: from bench to clinic. Nat Rev Endocrinol. 2020;16(11):654–67.
Ginsberg HN. New perspectives on atherogenesis: role of abnormal triglyceride-rich lipoprotein metabolism. Circulation. 2002;106(16):2137–42.
Graham MJ, Lee RG, Brandt TA, Tai LJ, Fu W, Peralta R, et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med. 2017;377(3):222–32.
Group AS, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–74.
Group HTC. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2013;34(17):1279–91.
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. J Am Coll Cardiol. 2004;44(3):720–32.
Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25):e1082–e143.
Hegele RA, Ginsberg HN, Chapman MJ, Nordestgaard BG, Kuivenhoven JA, Averna M, et al. The polygenic nature of hypertriglyceridaemia: implications for definition, diagnosis, and management. Lancet Diabetes Endocrinol. 2014;2(8):655–66.
Hulley SB, Rosenman RH, Bawol RD, Brand RJ. Epidemiology as a guide to clinical decisions. The association between triglyceride and coronary heart disease. N Engl J Med. 1980;302(25):1383–9.
Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem. 2016;62(4):593–604.
Jorgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjaerg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J. 2013;34(24):1826–33.
Kalstad AA, Myhre PL, Laake K, Tveit SH, Schmidt EB, Smith P, et al. Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized controlled trial. Circulation. 2020;143(6):528–39.
Kamanna VS, Ganji SH, Kashyap ML. Recent advances in niacin and lipid metabolism. Curr Opin Lipidol. 2013;24(3):239–45.
Kaufman A, Abuqayyas L, Denney WS, Tillman EJ, Rolph T. AKR-001, an Fc-FGF21 Analog, showed sustained pharmacodynamic effects on insulin sensitivity and lipid metabolism in type 2 diabetes patients. Cell Rep Med. 2020;1(4):100057.
Koishi R, Ando Y, Ono M, Shimamura M, Yasumo H, Fujiwara T, et al. Angptl3 regulates lipid metabolism in mice. Nat Genet. 2002;30(2):151–7.
Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J. 2020;41(1):99–109c.
Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111–88.
Madsen CM, Varbo A, Nordestgaard BG. Unmet need for primary prevention in individuals with hypertriglyceridaemia not eligible for statin therapy according to European Society of Cardiology/European Atherosclerosis Society guidelines: a contemporary population-based study. Eur Heart J. 2018;39(7):610–9.
Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2020;40(5):1135–47.
Mattijssen F, Kersten S. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochim Biophys Acta. 2012;1821(5):782–9.
Miller M. Icosapent ethyl for hypertriglyceridemia: insights from the REDUCE-IT trial. Futur Cardiol. 2019;15(6):391–4.
Miller M, Cosgrove B, Havas S. Update on the role of triglycerides as a risk factor for coronary heart disease. Curr Atheroscler Rep. 2002;4(6):414–8.
Miller M, Cannon CP, Murphy SA, Qin J, Ray KK, Braunwald E, et al. Impact of triglyceride levels beyond low-density lipoprotein cholesterol after acute coronary syndrome in the PROVE IT-TIMI 22 trial. J Am Coll Cardiol. 2008;51(7):724–30.
Miller M, Stone NJ, Ballantyne C, Bittner V, Criqui MH, Ginsberg HN, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–333.
Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, et al. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr. 2000;71(5):1085–94.
Mudd JO, Borlaug BA, Johnston PV, Kral BG, Rouf R, Blumenthal RS, et al. Beyond low-density lipoprotein cholesterol: defining the role of low-density lipoprotein heterogeneity in coronary artery disease. J Am Coll Cardiol. 2007;50(18):1735–41.
Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268–80.
Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35.
Orringer CE, Jacobson TA, Maki KC. National Lipid Association Scientific Statement on the use of icosapent ethyl in statin-treated patients with elevated triglycerides and high or very-high ASCVD risk. J Clin Lipidol. 2019;13(6):860–72.
Peng J, Luo F, Ruan G, Peng R, Li X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017;16(1):233.
Peterson BE, Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, et al. Reduction in revascularization with icosapent ethyl: insights from REDUCE-IT revascularization analyses. Circulation. 2021;143(1):33–44.
Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, et al. Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J. 2018;206:80–93.
Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363(7):692–4; author reply 4-5.
Sahebkar A, Simental-Mendia LE, Mikhailidis DP, Pirro M, Banach M, Sirtori CR, et al. Effect of omega-3 supplements on plasma apolipoprotein C-III concentrations: a systematic review and meta-analysis of randomized controlled trials. Ann Med. 2018;50(7):565–75.
Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, et al. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200(1):135–40.
Sanyal A, Charles ED, Neuschwander-Tetri BA, Loomba R, Harrison SA, Abdelmalek MF, et al. Pegbelfermin (BMS-986036), a PEGylated fibroblast growth factor 21 analogue, in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 2a trial. Lancet. 2019;392(10165):2705–17.
Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. J Lipid Res. 2006;47(7):1406–15.
Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367(22):2089–99.
Schwartz GG, Abt M, Bao W, DeMicco D, Kallend D, Miller M, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol. 2015;65(21):2267–75.
Skulas-Ray AC, Wilson PWF, Harris WS, Brinton EA, Kris-Etherton PM, Richter CK, et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association. Circulation. 2019;140(12):e673–e91.
Stahel P, Xiao C, Hegele RA, Lewis GF. The atherogenic dyslipidemia complex and novel approaches to cardiovascular disease prevention in diabetes. Can J Cardiol. 2018;34(5):595–604.
Stein EA, Lane M, Laskarzewski P. Comparison of statins in hypertriglyceridemia. Am J Cardiol. 1998;81(4A):66B–9B.
Stein E, Bays H, Koren M, Bakker-Arkema R, Bisgaier C. Efficacy and safety of gemcabene as add-on to stable statin therapy in hypercholesterolemic patients. J Clin Lipidol. 2016;10(5):1212–22.
Tanaka N, Irino Y, Shinohara M, Tsuda S, Mori T, Nagao M, et al. Eicosapentaenoic acid-enriched high-density lipoproteins exhibit anti-atherogenic properties. Circ J. 2018;82(2):596–601.
Taskinen MR, Packard CJ, Boren J. Emerging evidence that ApoC-III inhibitors provide novel options to reduce the residual CVD. Curr Atheroscler Rep. 2019;21(8):27.
Thomsen M, Varbo A, Tybjaerg-Hansen A, Nordestgaard BG. Low nonfasting triglycerides and reduced all-cause mortality: a mendelian randomization study. Clin Chem. 2014;60(5):737–46.
Tirosh A, Rudich A, Shochat T, Tekes-Manova D, Israeli E, Henkin Y, et al. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Intern Med. 2007;147(6):377–85.
Varbo A, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation. 2013;128(12):1298–309.
Varbo A, Freiberg JJ, Nordestgaard BG. Extreme nonfasting remnant cholesterol vs extreme LDL cholesterol as contributors to cardiovascular disease and all-cause mortality in 90000 individuals from the general population. Clin Chem. 2015;61(3):533–43.
VASCEPA. VASCEPA label 2019. Available from: https://www.vascepa.com/assets/pdf/Vascepa_PI.pdf.
Witztum JL, Gaudet D, Freedman SD, Alexander VJ, Digenio A, Williams KR, et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med. 2019;381(6):531–42.
Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the atherogenic dyslipidemia complex: the next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes. 2016;65(7):1767–78.
Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–8.
Zhao S, Wang F, Dai Y, Lin L, Tong Q, Liao Y, et al. Efficacy and safety of fenofibrate as an add-on in patients with elevated triglyceride despite receiving statin treatment. Int J Cardiol. 2016;221:832–6.
Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60(3):473–85.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bazarbashi, N., Miller, M. (2022). Triglyceride-Rich Lipoproteins. In: Shapiro, M.D. (eds) Cardiovascular Risk Assessment in Primary Prevention. Contemporary Cardiology. Humana, Cham. https://doi.org/10.1007/978-3-030-98824-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-98824-1_12
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-98823-4
Online ISBN: 978-3-030-98824-1
eBook Packages: MedicineMedicine (R0)