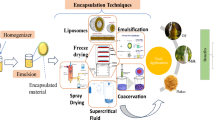
Abstract
The food industry has long recognized nanoparticle delivery for uses ranging from slow-release vitamin and mineral formulations to nutritional supplement. Small particles that can be easily dissolved are used to create a bioavailable formula or add vitamins and minerals to a “clear” food beverage thus helping fat-based food products taste better. The colloidal delivery strategy (primarily dealing with micro and nanoencapsulations) has been deemed a priceless resource for resolving many issues involved in food fortification. Encapsulated minerals can be applied and integrated into the formulation of food and medicine to provide unique benefits for the development of physically and chemically improved functional products. In this chapter, we explain different nano and microencapsulations of vitamins and minerals and how food fortification can be used as a low-cost solution to vitamins and minerals deficiency.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Nanoparticles delivery approach has obtained constant recognition from the food industry in the last few decades for applications that range from the slow-release vitamin and mineral formulations and micronutrients encapsulated and stabilized for nutritional supplement utilization. Because of the current growth and prominence of “niche” subgroups of useful ingredients, the colloidal delivery approach (dealing mainly with the micro and nanoencapsulations) has been considered as a useful resource for resolving multiple difficulties associated with food fortification. Encapsulation can be defined as either layering a droplet, gas, or solid particle center with a solid polymeric matrix or integrating active components into polymer matrices to reduce the interaction of the encapsulated substances with the surrounding environment [1]. Colors, flavors, preservatives, and other bioactive components are added to packaged foods to improve their aesthetic and shelf life. Furthermore, fortified and value-added items frequently require specific health-promoting bioactives, such as micronutrients and nutraceuticals. There are numerous challenges in food fortification; among which, durability, matrix compatibility, and organoleptic changes are common difficulties [2]. Fortification is a difficult process due to difficulties in product processing and issues related to in vivo bioavailability, controlled release, and bio accessibility. Nano- and microencapsulation can address some or all of these problems. The gastro-intestinal (GI) tract stability of the encapsulated materials could also be improved to enable controlled drug release at suitable GI targets [3]. The present chapter discusses a variety of colloidal systems (micro and nano) and their applications in vitamin and mineral encapsulation and delivery.
4.2 Foods and Nutraceuticals Vitamins
4.2.1 Vitamins: Dietary and Biological Needs
Probiotics should include vitamins necessary for human growth and development (like niacin and choline). The advantages of nutraceuticals are more than simply preventing illness. Oil and water-soluble vitamins function as antioxidants and hormone regulators. Vitamin B-complex is beneficial for vision, immunity, bones, teeth, and skin. Vitamin E improves cognitive function.
4.2.2 Vitamins: Stability and Formulation Concerns
Vitamins encounter a number of challenges, including oxidative stability. Other additives may also impede water-soluble vitamins, impacting texture, appearance, and storage. Vitamin A (retinoids): When exposed to air and heat, trace metal ions Fe2+ and Cu2+ increase the oxidation sensitivity of vitamin A.
Thiamine, a heat and UV-sensitive vitamin B, is a potent Maillard reactive due to its chemistry. Baking, pasteurizing, or boiling thiamine-fortified meals can reduce their content by up to 50%. B2 vitamin: Riboflavin is a brilliant yellow pigment soluble in water. Visible spectrum light below 500–520 nm can degrade riboflavin. Vitamin B3 : Niacin is highly stable when exposed to heat, light, air, and alkalis. Niacin can be destabilized by other micronutrients, including minerals.
Vitamin B5 : Pantothenic acid decomposes when heated under slightly acidic to neutral conditions but stays stable within the pH range of 5–7. It is used as calcium and sodium salts to enhance stability in the gastrointestinal pH. Vitamin B6 : Pyridoxine is heat stable, but not pyridoxal or pyridoxamine. Vitamin B6 is degraded by oxidation, UV radiation, and alkaline conditions. Vitamin B7 appears to be reasonably stable; however, heat treatment causes relatively minor losses. It is stable in air at neutral and acidic PH, but susceptible to alkaline pH. Vitamin B9 : Folic acid is a reddish, tasteless, and odorless vitamin, which is water soluble at 12.5 mg/mL. Light, air, acid, and alkali deactivate it. Vitamin B12 : In addition to being heat stable, light, oxygen, acid, and alkali cause vitamin B12 to lose its activity. Vitamin C : Ascorbic acid is easily degraded by metals such as copper and iron during production and storage. In neutral or alkaline conditions, it is oxidized by air. Metals, particularly copper and iron, and enzymes expedite vitamin C breakdown (particularly enzymes containing copper or iron, such as ascorbic acid oxidase). Vitamin D : D2 and D3 are crystalline compounds white to yellow in color. They are insoluble in vegetable oils and water but can be dissolved in organic solvents. Light, oxygen, and acid quickly dissociate both these vitamins, especially in solution and powder forms. However, they tend to isomerize in oily solutions. The crystalline compounds have good thermal stability. Vitamin E : Food processing and storage reduce vitamin E levels due to light, oxygen, and heat. Some foods can lose half their flavor after just 2 weeks. Vegetable oils are destroyed by frying. During storage, tocopherol esters (acetate and succinate of tocopherol) resist oxidation, which makes them useful in nutritional supplements. Vitamin K : Light and alkaline substances decompose K1 into a yellow-oily liquid. Vitamin K2 is a crystalline substance that is resistant to heat and reducing agents but susceptible to acid, alkali, light, and oxidizing agents.
4.3 The Encapsulation of Vitamins (Nano and Micro) Colloidal Form
4.3.1 Dispersions of Solids in Liquids
4.3.1.1 Microparticles
In recent years, microencapsulation has become more popular in pharmaceuticals. It has been found that functional ingredients can be delivered in microstructures of size between 1 and 1000 μm. In contrast to microspheres, the microcapsule core is enclosed in a well-formed polymer shell. Encapsulated vitamins are protected from the external environment. Vitamin C is a well-studied model vitamin for encapsulation. Vitamin C powder is stable but not in the solution form as light and oxygen readily destroy vitamin C in the solution. Trace metal ions and formulation can also affect vitamin C stability. Thus, processing can easily degrade vitamin C, causing discoloration. Vitamin C can also interact with other substances, changing the product’s flavor or color. Microencapsulation has been widely used for the stabilization and processing of vitamin C in food items. A wall-forming material, ethyl cellulose, and carnauba wax were used to study the characteristics of ascorbic acid and four different encapsulation methods. Carnauba wax releases slower than ethyl cellulose. Core-shell microcapsules encapsulating vitamin C (2–5 μm) was prepared using a new interfacial/emulsion reaction. Folic acid was encapsulated in alginate and pectin microparticles [4]. Depending on the experiment, the microparticles were of 300–650 nm in size. Less trapping efficiency means less folic acid release. Microencapsulation has also been utilized for light-sensitive vitamins like A and D. Gelatin-acacia coacervates contain vitamin A palmitate . Acacia was mixed with vitamin A palmitate in maize oil, and the pH was adjusted to make microcapsules. This research focused on the effects of hydrocolloid mixing ratio, hardening agent concentration, and drying technique. An average diameter of 25 μm and encapsulation efficiency of 83.43% were achieved. Alginate and chitosan were used by Albertini et al. to create a double-layer microcapsule containing vitamin A palmitate [10]. Encapsulation increased vitamin A palmitate storage stability compared to the pure form. A novel sort of microcapsule was recently reported by solidifying heated emulsion droplets. Tocopherol, a liposoluble vitamin, was effectively encapsulated into microcapsules. Sunflower oil and water were emulsified at 85 °C, and then diluted in cold water to create micro-capsule-size particles. The sudden change in temperature led the oil droplets to freeze. Making microcapsules with a core-shell structure is as simple as adding calcium chloride to the cold water phase and stirring. Firstly, α-tocopherol was dissolved in the oil phase, and then encapsulation was accomplished as previously described. The antioxidant tests showed that encapsulation has no influence on α-tocopherol antioxidant activity [5].
4.3.1.2 Polymeric Nanoparticles
The size of colloidal particles is in the sub-micron range. This category comprises polymer and solid lipid nanoparticulate systems . Adding vitamins and minerals to a “clear” food beverage that makes fat-based foods taste better without changing their overall taste, or creating a bioavailable formula relies on tiny particles that are easily dissolved. Vitamins encapsulated in colloidal particles can be used in products like fortified milk, juices, and cooking oils. Liposoluble actives can be encapsulated by antisolvents, or nanoprecipitation, and co-precipitating them with hydrophilic polymers makes it convenient to load them in colloidal particles [6]. This approach has been used to encapsulate liposoluble vitamins like A, D, E, and K. Vitamin E (tocopherol) was co-precipitated with wheat gluten vegetable protein fractions (gliadins). They were formed as colloidal particles with an encapsulation effectiveness of 77% or higher and dual release features. The anti-solvent technique works well with zein, corn protein rich in proline. Zein interacts with particles composed of charged biopolymers , such as caseinate and chitosan. Submicron nanoparticles were synthesized using the ionic zein and α-tocopherol with hydrophobic interactions [7]. These particles were poly dispersible and ranged from 300–1000 nm in size. The zein-chitosan combination generated substantially smaller and homogenous sized particles (400–500 nm). When chitosan was added to the preparation process, it improved the control of the encapsulated vitamin’s release. A liposoluble vitamin, cholecalciferol, was coated with zein-carboxymethyl chitosan nanoparticles [8].
Phase separation was used to produce monodispersed nanoparticles as small as 120 nm. The controlled release of vitamin D3 from nanoparticle increased its photostability. These solid lipid nanoparticles (SLNs) have been employed to deliver lipophilic bioactives for food applications. The initial stage of making an oil-soluble vitamin loaded SLNs is dispersion in the lipid phase. For high melting lipid carriers, dispersion of vitamins in lipid carriers takes place at high temperature (about 80 °C). Aqueous surfactant solution is subsequently mixed with the melt, resulting in the spontaneous production of SLNs [9]. SLNs are made from glyceryl behenate and tripalmite encapsulated vitamin A and its palmitate. Factors affecting size and shape were assessed, such as manufacturing process, surfactant system, and lipid type, which have an influence on experimental outcomes. The interplay between these variables affects the internal and external architecture of SLNs, and therefore the distribution of vitamins inside SLNs (in the lipid matrix or in the outer shell).
4.3.2 Liquid-in-Liquid Dispersions
4.3.2.1 Emulsions, Microemulsions and Nanoemulsions
In food science, emulsions are the most explored colloidal system, forming the basis of a wide range of edible goods, like ice creams , sauces, spreads, whipped creams, etc.
A mixture is made up of dispersed and continuous liquids. The use of surface active amphiphilic emulsifiers stabilizes the internal phase. Emulsions oil/water (o/w) or water/oil (w/o) are commonly used in food science and technology. For margarines and butter, a continuous phase of solid fat gives the finished product a structural appearance. This is because the water continuous phase is less viscous. However, changing the internal phase can affect emulsion rheology in general, it is either gelled or increases the proportion of internal phase (or both). Double or multiple emulsions generate an o/w emulsion, which is further emulsified into an oil phase or water phase [10]. Size and stability of droplets can also be considered when classifying emulsions. Emulsions having droplet sizes greater than 1 μm are termed as macroemulsions. The droplets of size 1 μm/1000 nm or smaller fall. When homogenization is performed, high-pressure or high-shear homogenizers are used, which might produce nanoemulsions or mini emulsions. The droplet size of microemulsions is typically smaller than 100 nanometers and they are formed spontaneously within liquids. Surfactant and co-surfactant synergy reduces interfacial tension, resulting in spontaneous production [11].
Despite their popularity, macroscale emulsions are thermodynamically unstable and tend to cream and coalesce with time. Emulsions are frequently employed to encapsulate and supply both liposoluble and water-soluble vitamins. This enhances water dispersibility and bioavailability while preventing oil-soluble vitamins from deterioration in the product condition and the human body condition. Numerous studies have used the most active form of vitamin E that is α-tocopherol emulsion, which is food-grade [12]. It was determined that the distribution of α-tocopherol in macroemulsion systems may be enhanced by emulsions of α-tocopherol using a pseudo phase model for thermodynamically stable microemulsions. As a result of α-tocopherol’s surfactant-like properties (polar phenolic headgroup with hydrocarbon tail), they identified it mostly in the interfacial region. High pressure homogenization encapsulated α-tocopherol in o/w nanoemulsions with droplet sizes typically smaller than 500 nm [13]. The protein coating the tocopherol-filled fat droplets protected them from oxidation. Vitamin E-loaded nanoemulsions were recently described. This novel nanoemulsion contains vitamin E with >99% efficiency and particles are on average 78 nm in size. Microemulsion consisting of water, vitamin E, ethyl butyrate, EL-35, and ethanol was also studied. A regulated vitamin E released from microemulsions was shown having droplet size of 20 nm. Vitamins A and D can also be encapsulated in emulsions. Encapsulated trans retinoic acid/retinol is required in a variety of emulsion processes [14]. Besides high loading, encapsulation of vitamin A must address retention of loaded vitamins. Researchers employed an oil gelling agent, and coated oil droplets with solid fat as an alternative to oil to enhance vitamin retention in the oil phase thus encapsulating the vitamin. By means of a high- pressure homogenizer, 10% oil/90% water emulsion was created with an average particle size of 220 nm.
4.3.3 Self-Assembled Colloidal Dispersions
An organized structure or pattern is produced when pre-existing, disordered components interact. In an aqueous medium, an amphiphilic compound generates self-assembled colloids. The transport of hydrophobic and hydrophilic bioactive can be achieved by microparticles, procolloidals, lipid bilayers, liquid crystal phases, and mesophases [15].
4.3.3.1 Micelles
Molecules containing lipids are micelles that form spheres in aqueous solutions. Fatty acids have an amphipathic nature, which contain both water-loving (polar head groups) and water-hating sites, causing micelle formation (the long hydrophobic chain). A micelle’s polar head group creates a surface, and the nonpolar hydrophobic tail group is located inside and away from water. Single hydrocarbon chains make micelle-forming fatty acids round, reducing steric hindrance. Micelles are too bulky for the two hydrophobic chains in the fatty acids found in glycolipids and phospholipids and they prefer “lipid bilayers” [16]. Micelles arise spontaneously due to hydrophobic interactions between molecules. From simple casein micelles in milk to sophisticated digesting dietary mixed micelles, fat-soluble vitamins (A, D, E, and K) enter the bloodstream via GIT. Micelles also act as mechanisms for transporting fat-soluble vitamin from the digestive tract to the blood. Externally manufactured micelles include lipid-soluble vitamins [17]. In order to supply lipid-soluble vitamin D2, casein micelles from milk were used. Nano delivery systems such as casein micelles are found in milk to stabilize and transport minerals like calcium and vitamin D2 which are encapsulated in 155 nm sized particles .In addition to stabilizing the encapsulated vitamins, the assembled micelles also protect them from photochemical destruction. In a recent study, an ultra-high-pressure homogenization was employed to encapsulate vitamin D3 into reassembled casein micelles. A double-blind placebo-controlled clinical research in 87 human volunteers reported that vitamin D3 infused in reassembled casein micelles was highly bioavailable. The vitamin-encapsulated micelles formed large aggregates after they interacted with bile. Unlike empty micelles, the encapsulated vitamins influenced aggregation. As polymeric micelles cannot afford vitamin K absorption, the researchers concluded that free bile acid is essential for bioavailability. Micellar systems have also been used to analyze that vitamin C is water-soluble, in addition to liposoluble vitamins. In a surfactant solution, the degree of ascorbic acid oxidation depends on the concentration of surfactant. Critical micellar concentration (CMC) is the concentration at which ascorbic acid oxidation is at its peak, which is then declined by the surfactant concentration in both SDS and CTAB.
4.3.3.2 Liposomes
Liposomes in aqueous solutions are self-assembled vesicles made up of lipids and lipid-like molecules. Liposomes may contain both hydrophilic and hydrophobic actives. Their internal pH may be altered, allowing them to contain otherwise unstable chemicals. Liposomes can be made in many forms and sizes. Phospholipids can be organized to produce numerous or single vesicles. The dairy industry uses vitamins A, D, and E so that vitamins are stabilized and delivered to enhance nutritional quality. Banville et al. [18] found that liposome-encapsulated vitamin D recovered 62% more vitamin D than vitamin D produced commercially (43%) and cream contains 41% solubilized vitamin D. Vitamins were protected from degradation by liposomal encapsulation. The liposome approach protects water-soluble vitamins and has been studied showing beneficial effects on water-soluble vitamins (vitamin C). Soy phosphatidylcholine liposomes protected heat-labile vitamin C [19]. They were also evaluated with orange juice. Some researchers have found that poly phosphatidyl choline (60:40) liposomes can improve the storage stability of vitamin C. Encapsulated water-soluble and liposoluble vitamins are shown in Fig. 4.1.
4.3.3.3 Procolloidal System
Incorporating an aqueous phase dilutes a procolloidal system. Water-soluble colloids are formed when these concentrated systems are diluted with liquid crystal phases that form microphases when diluted. A fine oil-in-water emulsion is formed when oil, a surfactant, a co-surfactant, and a co-solvent are gently mixed together. Spontaneously produced aqueous microemulsions (50–1000 nm droplet size) are developed in the GI tract after ingestion of soft or firm gelatin capsules. When a surfactant interacts with a co-surfactant, spontaneous dispersion is produced on the interface, and as a result of co-surfactant assistance, the surfactant film formed has a low interfacial energy and a low bending stress. The incorporation and encapsulation of vitamin E in procolloidal systems, especially those that self-emulsify, have been extensively studied, due to its powerful nutraceutical and pharmaceutical properties [20]. Self-emulsifying and self-micro emulsifying vitamin E systems notably enhance bioavailability. Self-emulsifying systems increased tocotrienol bioavailability by two to three times in six healthy individuals who participated in the study. Food products have been extensively studied for water- and liposoluble vitamins delivered through liposomes. However, mass-production of these systems in a reproducible manner remains unsolved. The use of proliposomes is one of the methods for scaling up liposome production. Proliposomes are phospholipid particles formulated with functional ingredients, which are soluble in water and when hydrated above the transition temperature and agitated form liposomes. The addition of beta-carotene to a proliposomal formulation containing hydrogenated soy phosphatidylcholine improved chemical stability. The shelf life was reported to be 60 days when refrigerated [21]. Moreover, proliposomal formulations of glyceryl dioleate-PEG12 and glyceryl dipalmitate-PEG23 at 35% and 20% weight, respectively, were effectively combined with Vitamins D and E.
4.3.4 Dry Matrix Encapsulation
Industrial feasibility and scale-up include spray-drying, extruding, coating with pans, and using fluidized beds. These methods can easily produce microcapsules with a consistent particle size distribution.
4.3.4.1 Spray-Drying
The food industry’s most popular encapsulation method is spray-drying. The process is cheap, easy, and produces consistent particles. Spray-drying is thought to be cheaper than most encapsulation processes. There are usually two steps in spray-drying: Firstly, a coating or core material solution is prepared, and then the solvent is evaporated, which is termed as dispersion. Secondly, a layer of coating material is applied over the core material or a coating of polymer matrix containing a core material is distributed. Food manufacturers have used spray-drying encapsulation since the late 1950s to protect flavor oils from deterioration and to better handle liquids. However, one drawback of spray-drying is the requirement of good water solubility of the coating material. The availability of coating materials is limited due to their water-based nature; therefore, several biopolymers have been used to coat water-soluble vitamins (vitamin C) such as gum Arabic, pea protein, and chitosan [22]. A microcapsule of less than 10 nanometers in size was produced using pea protein, chitosan, and gum Arabic, while rice starch was used to produce microcapsules of 5–40 μm. We found that microencapsulating vitamin C microcapsules containing gum Arabic and starch improved its oxidative stability, and that microcapsules of vitamin C prepared using pea protein and chitosan showed controlled release . The same group used pea protein concentrate liposoluble vitamins (α-tocopherol) for encapsulation [23].
4.3.4.2 Extrusion (in a Glassy Matrix)
Extrusion-based microencapsulation is a relatively newer method of drying in contrast to spray-drying, which is performed by forcing a series of dies, molten polymer and core material into a dehydrating liquid. Discrete microcapsules, drying the material, and pulverizing have also been accomplished with other materials such as gelatin, sodium alginate, carrageenan, gum acacia, fats and fatty acids, waxes, etc.; however, with this method, the coating material completely encapsulates the core material, providing excellent stability against oxidative and other chemical degradation. Maltodextrin and gelatin were encapsulated in a glassy matrix containing tocopherol in a 3:1:1 ratio [24]. The glassy matrix also protected the encapsulated α-tocopherol from oxidation, while encapsulating carotene in amorphous trehalose delayed degradation time.
4.3.5 Vitamins Encapsulation in Cyclodextrins
Cyclodextrins are cyclic oligomers formed by enzyme conversion of starch. The d-glucopyranose unit has six, seven, or eight (-CD) units joined together by α-(1,4) bonds. Their structure allows them to form molecular complexes with many hydrophobic molecules. Encapsulation of hydrophobic drugs protects volatile aromatic compounds from degradation. The delivery of functional ingredients and bioactives, including vitamins, is the major advantage of CDs [25]. A CD’s structure resembles a cage with a hydrophobic cavity. Hydrophobic compound inclusion complex is formed when less polar guest molecules replace the polar hydrophobic cavity, which is lined with water molecules. Though the exact cause of the inclusion complex formation is unknown, the following mechanism is thought to be involved: A polar interaction between a guest molecule and the hydrophobic cavity releases molecules of cavity i.e., water, and bulk water replaces the energetically disfavored polar interactions between the water molecules in the cavity and the included water. True inclusion complexes can only be formed by lipid soluble vitamins in cyclodextrins. Thus, the creation of inclusion complexes to encapsulate liposoluble vitamins has been studied extensively. The retinoid-CD complexes were made in aqueous alcohol at ambient temperature. Many studies have shown that hydroxyl propyl-CD and -CD inclusion complexes increase the solubility of vitamin A. Other studies have shown that CD complexation stabilizes vitamin A by inhibiting photoisomerization and photodegradation of retinoids [26]. Beyond retinoids-CD complexes, most studied vitamin is vitamin D as CD complex. The low water solubility of vitamin D affects its biological activity. Vitamin D and CD inclusion complexes can be made using ethanol as a solvent. A study on the stability of vitamin D after cyclodextrin complexation has also been reported. Menadione is another liposoluble vitamin (or vitamin K3). Benzene is incorporated into the cavity of a CD , while the quinine moiety is inserted outside the cavity, thus improving its aqueous solubility [27].
4.4 The Food Industry and Encapsulated Minerals
Encapsulated minerals can be integrated into the formulations of food and medicine to provide unique benefits and sensory features to the physically and chemically improved functional products. Encapsulated mineral salts have advantages such as reduced discoloration, reduced taste and smell by masking-off flavor, production and storage processes that control the release of mineral components, and improved taste and smell. Encapsulation of minerals into dairy products and table salt has been studied extensively during the last two decades. C4H8FeN2O4, NH4Fe(SO4)2, and C6H10FeO6 are some examples of electrolytic-Fe (Table 4.1) [28]. Soy-yogurt and soy milk are fortified with encapsulated tricalcium phosphate (Ca3(PO4)2) and calcium citrate (Ca3(C6H5O7)2) salts. KI and KIO3 are the most common encapsulated iodine fortification salts, while C4H2FeO4 and FeSO4 are the most common encapsulated Fe fortification salts.
4.4.1 Dairy Products Fortified with Encapsulated Minerals
4.4.1.1 Milk
Despite being one of the world’s most vital foods, milk is low in Fe. Thus, fortifying milk with encapsulated iron might be beneficial. Increasing Fe intake, for example, FeSO4 microencapsulated in milk and its bioavailability was studied using a lecithin liposome method. The fortified milk’s Fe bioavailability did not change after 6 months of storage and heat treatment. A similar bioavailability rate of Fe to high-bioavailable FeSO4 absorption was also recorded. Coatings made from PGMS were used to microencapsulate Fe to fortify milk. While just 3% of Fe was released in vitro (pH 6.0), increasing pH from 5.0 to 8.0 dramatically boosted the rate of Fe release from 12.3 to 95.7% .The sensory study found no significant variations in most sensory features between the control and microencapsulated-Fe samples after 3 days of storage [39]. Moreover, the unencapsulated Fe sample had greater TBA than the encapsulated Fe sample. It was discovered that free Fe-fortified milk had a higher TBA value. Supplemented TBA of milk with high microencapsulated-Fe loadings was not altered. Microencapsulation slowed down lipid oxidation by 60%, while masking milk’s metallic taste. However, comparing astringency and bitterness to control milk, microencapsulated Fe milk produced similar results. The fortification ability of Fe microcapsules in milk and the factors that affect the physical, chemical, and sensory properties of the final product were investigated. Fortification of low-level milk with Fe microcapsules (0.1 0.3% w/v) did not increase TBA levels. It was found that 0.1% (w/v) Fe-microcapsule powder was optimal for producing fortified milk. Researchers observed that microencapsulated FeSO4 in a powdered milk diet had better Fe bioavailability than the control diet. One study selected three Fe microcapsules fortified with EE from four distinct techniques (62.97% 74.85%) [40]. To compare the organoleptic ratings of Fe-fortified milk with the control milk, they used modified starch and sodium alginate microcapsules (10 mg/L Fe). GA, MD, and modified starch were mixed and vaporized to create microcapsules of Fe with an average size of 15.54 μm [41]. When compared to fortified milk containing Fe microcapsules, panelists preferred salt-fortified milk over microcapsule-fortified milk. In addition, the microcapsules of Fe in fortified milk had better bioavailability than those in control and Fe salt enriched milk. The sensory qualities of microcapsules of Fe from three Fe salts were also studied. All treatments had the same appearance and flavor. The flavor of the control and C6H10FeO6 enriched milks did not differ significantly. This product was the finest option for fortifying pasteurized liquid milk. In comparison to cow milk (120 mg/100 g), soy milk contains a lower level of calcium (12 mg/100 g) [42]. As a result, many researchers have tried to improve the nutritional value of soy milk by adding calcium. It was discovered that adding Ca salts (Ca3(PO4)2 and Ca3(C6H5O7)2) to soy beverage was ineffective due to unfavorable Ca protein interactions, coagulation, and precipitation. Using a lecithin liposome structure could fortify soy milk in a better way. Researchers added Ca to soy milk (110 mg Ca/100 g soy milk) to match the level to that in cow’s milk. The fortified soy milk was stable and had high Ca bioavailability for 1 week at 4 °C. Ca3(PO4)2 in the soy milk was 2000 mg per liter, and to prevent Ca protein interaction and enhance the stability of soy milk , researchers added 30 g/L potassium citrate to samples. However, combining Ca3(PO4)2 and C6H5K3O7 reduced soy milk stability.
4.4.1.2 Yogurt
The main consumers of yogurt are children
, menstruating, pregnant or nursing women, and teenagers. FeSO4 and a microencapsulated compound of Fe whey protein were added to yogurt for 7 days at −70 °C to assess lipid oxidation and sensory properties. The three oxidation rates (TBA and peroxide) did not change significantly. Iron-enriched yogurt tasted rusted and metallic. The sensory panel favored the Fe whey protein complex microencapsulated yogurt since the overall flavor and quality were better [43]. In recent years, researchers have enriched probiotic yogurt with Lactobacillus acidophilus
and iron microcapsules derived from whey protein complexes. Compared to unfortified yogurt, fortified yogurt had less oxidized flavors. TBA levels were elevated due to interactions among iron and casein proteins, prooxidant activity of oxygen species, and stimulated lipid oxidation. In oxidative conditions, free fatty acids get accumulated. Three commercial Fe-salt microcapsules were added to yogurt to improve its organoleptic quality. Both the control and FeSO4 microcapsule samples passed the sensory test after 7 days of storage. C4H8FeN2O4 and C6H10FeO6 yogurts were clearly distinguished from the control. TBA and peroxide levels were the highest in C4H8FeN2O4 yogurt, whereas the control and C6H10FeO6 supplemented samples showed the lowest TBA and peroxide level. Microcapsules of FeSO4 were found to be useful and ideal for fortifying yogurt. On 0, 1, 2, and 3 weeks, the starter bacteria count did not differ between the control and Fe-fortification samples. The quantity of these bacteria decreased with extended storage in both control and Fe-fortified yogurt samples. The added Fe had no impact on yogurt bacterial viability. Free Fe enriched yogurts had lower TBA values than encapsulated Fe enriched yogurt [44]. After 21 days, there were significant variations in astringent, oxidized, and overall acceptability assessments. To improve Fe-bioavailability, it was advised to add 80 mg Fe-WP/L to yogurt without affecting its appearance or organoleptic properties. This yogurt drink had Fe microencapsulated in it. Unencapsulated Fe was added, pH was decreased and titratable acidity was increased, while the microbial count of the yogurt was found to be unaffected by unencapsulated Fe. TBA kinetics in encapsulated therapies was also noted. Unencapsulated Fe changed the astringency and bitterness of yogurt drink
, whereas encapsulated Fe concealed Fe taste and flavor. FeSO4 7H2O, 12 mg Fe/L, and calcium (Ca3C6H5O7)2, 600 mg Ca/L, enhanced the stability of soy yogurt. The investigation was conducted for 28 days at 10 °C. Although the fortified sample had low viscosity, extending the storage period had no significant effect on this parameter. Moreover, there were no significant variations in all sensory characteristics as well as acidity levels during the storage. Fe-fortified yogurt stored for 14 days exhibited both physicochemical and sensory qualities. The yogurts were fortified with Cold-set WPI gel powder encapsulating Fe-encapsulated in FeSO4 solution (20–60 mg Fe/kg). Compared to the unfortified control sample, WPI-Fe particles fortified yogurt (up to 60 mg of Fe/kg) exhibited identical qualitative characteristics (color and flavor). A physicochemical and sensory evaluation demonstrated that samples fortified with WPI-Fe may retain quality criteria for 2 weeks regardless of the Fe concentration employed [45]. A Fe-enriched usual yogurt (B225 g) was likewise reported to give up to 60% of a woman’s daily Fe requirement, with no evident color or flavor differences from the control yogurt. This feat would be unachievable with even minimal levels of Fe fortification in yogurt. To maximize the effects of three independent factors on EE and Hunter Lab color features, an application of a second-order polynomial model using the RSM-central composite design was used. The optimal pH, WPI content, and Fe concentration for pre-paring WPI-Fe particles were 7.0, 6.8%, and 18.8 mM, respectively. This is because the particles were released into the intestinal environment at a high rate (95%), WPI-Fe particles were generated under optimal conditions, and a site-specific network was an ideal delivery option of Fe (Pancreatin was added, pH 7.5). However, it was found that iron encapsulated in only 28% of powders could be released in the stomach environment
(pepsin, pH 1.2).
4.4.1.3 Cheese
Cheese , like milk, is low in Fe. Most research on Fe encapsulation in cheese has been focused on Cheddar. According to IDFA (2015), 28.5% of all cheese consumed in the US was cheddar. Researchers reported that FeCl3, Fe casein, FIP WP, and Fe WP complex constitute the Fe content of cheddar cheese. The study used FeSO4 as a Fe source to fortify cheese, which showed a somewhat higher TBA level than unfortified cheese. TBA value and oxidized off-taste had no correlation with Fe level. Another link was between off-taste or flavor of the oxidized cheese and TBA value. Aged cheese had no effect on TBA levels, oxidized off-taste, or cheese flavor scores. The greatest bioavailability of Fe was 85% for FeCl3, 71% for Fe casein, 73% for FIP-WP, and 72% for Fe WP. Feeding equivalent fortified Fe for 10 and 14 days showed similar basal Fe level, while the bioavailability sources were 5%, 4%, 6%, and 3% (P ≤ 0.05) [46]. Nonetheless, FeSO4 had 85% maximum and 5% basal bioavailability, and it accelerated lipid oxidation. In addition to WP FeCl3, researchers employed the same fortification method as before. FeSO4 produced oxidation and off flavors in aged cheddar. Sensory perception was better in samples having Fe WP complexes. The chemical and sensory qualities of cheddar cheese were examined with encapsulated-Fe. Encapsulated cheese ripened slower than unencapsulated cheese. During ripening, compounds with short-chain FFAs and neutral volatiles were generated insignificantly. The samples with maximum encapsulated Fe had the most bitterness, astringency, and sourness. However, it did not influence the quality of cheddar cheese, whether LMFS (large microencapsulated FeSO4, 700, 1000 m) or SMFS (small microencapsulated FeSO4, 220, 422 m) enriched cheddar cheese [47]. After 90 days, LMFS (66%) and SMFS (91%) recovered Fe. No significant differences in lipid oxidation rate or chemical makeup (for example, dry matter, fat, ash, magnesium, zinc, and calcium) were observed. Microencapsulating FeSO4 in cheddar cheese did not overpower the flavor, odor, or color of Fe. Less SMFS-fortified cheddar cheese was found to have better Fe retention and sensory attributes. Mozzarella cheese was fortified with ferric chloride, WP, and casein sodium iron salts (25–50 mg Fe/kg). Mozzarella cheese with 50 mg Fe/kg exhibited an off-odor, metallic or oxidized flavor, and more unattractive qualities. Consumers despised all mozzarella due to metallic and off flavors. Cheddar cheese was fortified with FeCl3 and Fe protein components, indicating that it is not possible to generally apply Fe fortification to every other cheese. To plain feta cheese, 80 mg/kg of compounds containing iron (FeSO4, FeCl3, and microencapsulated FeSO4) was added. The chemistry and FFAs of the control and Fe or vitamin C samples were not different. Fe-supplemented cheese compared to unsupplemented cheese revealed a significant difference in Fe TBA and organoleptic ratings, which were lowest in feta cheese containing microencapsulated iron (80 mg) and vitamin C (150 mg) [48].
4.4.2 Fortification of Salt by Encapsulating Iron and Iodine
4.4.2.1 Dual Fortified Salt
Since Fe compounds have a greater stability than iodine compounds, encapsulating iodine to provide a protective partition was explored. C4H2FeO4 or FeSO4 (1 g/kg Fe) was blended with FBC to make DFS from iodine (KI and KIO3) microcapsules. Later, the stability of several fortifiers was studied in relation to temperature and relative humidity during storage. A good result was obtained for encapsulating iodine into dextrin, indicating a wall-to-core ratio of 1:200. After 30 days of storage at 40 °C and 60% relative humidity, DFS was enhanced with FeSO4 and KI lost 100% of its iodine concentration, whereas KIO3 lost 93% at 40 °C and 60% relative humidity; DFS retained 79.7% iodine and KIO3 retained 80.9% iodine. C4H2FeO4 and KI microcapsules demonstrated the greatest iodine retention (98.4%, 101.9%) at temperature of 40 °C and a relative humidity of 60% for 12 months. Despite improvements in iodine chemical stability, fine microcapsules generated by the technique prevented encapsulation [49]. Also, iodizing salt with KIO3 is expensive. Condensing Fe-compounds in salt formations, iodine and Fe DFS were created and tested for storage stability in highland (Nairobi) and coastal (Mombasa) cities in Kenya. Nairobi retained 87.3% Fe and only 92% iodine in encapsulated DFS with a KI and C4H2FeO4 premix, whereas Mombasa retained 92% Fe and 90% iodine. DFS encapsulated in C4H2FeO4 resisted color fluctuation and increased bioavailability. It was found that encapsulating Fe in DFS reduced unpleasant sensory characteristics and iodine loss, while maintaining considerable bioavailability. Researchers studied 19 Fe-containing compounds (e.g., fumarate, sulfate, pyrophosphate, and elemental Fe) bound to indigenous Moroccan and Cote d’Ivoire salts. After 6 months of storage, samples were examined for iodine concentration and color. According to the findings, encapsulation did not prevent most chemicals from ionic losses and undesired organoleptic alterations. Iron oxide (B2.5 and 0.5 m) particles were used to enhance salt. In the presence of Fe (C4H2FeO4) and iodine (KI and KIO3), the stability of table DFS was measured in the mountains and coasts of Kenya. Also, high iodine (90%) was preserved after 3 months of storage. Encapsulated C4H2FeO4 was combined with salt-encapsulated-KI or iodate salt to make DFS . Because of the significant retention of Fe21, a little quantity of Fe21 was oxidized to Fe31 (17%). During distribution and retail, both iodine and Fe21 were preserved, making DFS with iodine and encapsulated C4H2FeO4 extremely stable for 3 months. In vitro and in vivo studies have been focused on Fe bioavailability in iodine DFS and encapsulating C4H2FeO4; initially, researchers made a C4H2FeO4 premix for salt integration by agglomerating C4H2FeO4 into salt-size particles and then encapsulation was performed [50]. The in vitro bioavailability of various DFS and encapsulated C4H2FeO4 premixes generated by numerous techniques was then examined. The materials and techniques used in encapsulating C4H2FeO4 marginally affected the in vitro Fe bioavailability. The researchers discovered that adding TiO2 to the coating materials as a masking agent effectively concealed the reddish-brown color of C4H2FeO4 and improved overall sensory acceptance and iodine stability. Bioavailability of C4H2FeO4 encapsulated in a lipid structure was determined indicating that FeSO4 was 95% bioavailable compared to FeSO4 in rats. Extrusion-based encapsulation of Fe (C4H2FeO4) enhanced the iodine stability in iodized salt. The optimal formulation contained C4H2FeO4 (10%), binders (30%), TiO2 (25%), and Methocel, a water-soluble polymer (10%). Uncoated Fe particles, on the other hand, lost 50% of their DFS iodine content during extrusion. The usual salt distribution mechanism will be used to supply iodine and iron to DFS. Li et al. [51] investigated whether it was possible to encapsulate C4H2FeO4 in DFS using the cold-forming extrusion process. Extrudable dough containing substantial levels of C4H2FeO4 was thus possible using grain flours [51]. Additionally, all extruded Fe particles (300,700 nm) were analyzed indicating high in vitro Fe digestibility. Yadava et al. [52] enhanced masking colors and coating polymers phases utilizing extrusion agglomeration to prepare a fortification of salt with Fe premix. After mixing iodine and Fe21, stability was tested at 35 °C and 60% relative humidity for 90 days after Fe premix with iodized salt. Water-soluble polymeric coatings showed a good efficacy to retain micronutrients at 10% encapsulation capacity. They also had great bioavailability and pleasing sensory characteristics. To add encapsulated Fe (C4H2FeO4) to coarse iodized salt, Romita et al. [53] used spray drying. A 10% w/v suspension was employed, and 1.2% of the suspension was dissolved using biopolymers for 6 months. DFS was monitored in iodine stability at room temperature (20% RH) and under harsh conditions (40 °C and 60% RH). Sixty percentage of unencapsulated samples exhibiting reduced iodine stability was not surprising.
4.4.2.2 Triple Fortified Salt
A formulation containing iron, iodine, and vitamin A , three essential micronutrients in a single food product is a low-cost method of fortifying foods. Strong metabolic interactions among these components can treble salt fortification. A mixture of micronized Fe4O21P6, KIO3, and retinyl palmitate, was incorporated into HPF to create TFS with 30 g of I, 2 mg of iron, and 60 g of vitamin A in each gram of salt. After 6 months of storage, the color stable TFS lost iodine and vitamin A (B12 15%) [54]. At 10 months, the TFS group had much less vitamin A and Fe anemia deficit than the iodized salt group. In addition to Fe4O21P6, KI, and retinyl palmitate (vitamin A), HPF nano capsules were also used to formulate TFS. TFS’s color changes during storage were acceptable, as was the loss of iodine, which was similar to that in iodized salt. Retinyl palmitate was found to be highly stable, with only 12% loss after 6 months. The overall sensory tolerability of TFS and iodized salt was not found to be changed.
4.5 Iron Encapsulation in Cereals and Bakery Products
FeSO4 was incorporated into mixers that mix baking flour, and a fine-sized white powder with freely flowing characteristics was obtained which could be stored without quality loss for 6 months [55]. Noodles encapsulated with Fe (B5 mg Fe per serving) got comparable sensory scores to those containing inorganic fortification agents such as ferric sodium ethylenediamine tetraacetate (NaFeEDTA) and other ingredients. A three-month storage at ambient temperature did not influence physical (color) or oxidative (peroxide value) parameters. Biofortified wheat flours were made by Biebinger et al. with HPF wall material [56]. The prepared microcapsules may be able to overcome undesirable sensory changes and Fe shortage in biscuits made with wheat flour. Average iodine loss during spray-cooling was B25%, but no measurable iodine losses occurred during baking. Fortified wheat-based biscuits were compared with non-fortified controls in a feeding trial randomized with Kuwaiti young women. Biscuits that were fortified with Fe microcapsules and those that were unfortified were the same in taste and color. However, the fortified ones lowered the prevalence of Fe-deficiency by 50%. The fortified samples group had higher serum ferritin and urine iodine levels. The encapsulated FeSO4 Fe absorption was measured. Iron deficiency in Thai women was overcome by consuming foods based on wheat reinforced with unencapsulated FeSO4, electrolytic Fe, or elemental Fe reduced by hydrogen. After 20 weeks, about 11% of the prescribed Fe dosage was absorbed. Encapsulation could not impact Fe bioavailability. Low-extraction wheat flours can be supplemented with FeSO4 or C4H2FeO4 encapsulated (less than 0.8% ash) at the same amounts as without encapsulation [57]. In terms of quality parameters, Fe (Fe4O21P6, FeSO4, NaFeEDTA, and decreased Fe) and gluten have significant effects. Both elements also influenced the sensory attributes such as odor and the color of wheat bread. The color, stiffness of the crust, cell count, odor, and metallic taste of the crust were found to significantly differ in unfortified gluten-free loaves than Fe-fortified loaves. The encapsulation also failed to protect Fe from oxidation, since the color, taste, and texture of breads fortified with FeSO4 varied from those of unfortified breads. Children in Sao Paulo childcare centers previously rated bread supplements encapsulated with FeSO4 as acceptable as reported by Souto et al. [58]. Breads that were fortified with Fe were less acceptable to children than those that were not. In young children, fortified bread may offer some protection against Fe deficiency anemia. Using a paste made from rice flour, iron compounds (0.5 and 1.0 g Fe/100 g), and 25% water, Moretti et al. [59] produced a replica of rice grains. In order to achieve a final Fe content of 5 mg per 100 g of rice, Fe components (1:100 or 1:200) were combined with rice grains. For this purpose, dispersible micronized Fe4O21P6 (B0.5 m) was cold extruded, and reagent-grade Fe4O21P6, Fe4O21P6 (B2.5 and B20 m), FeSO4 encapsulated in a liposome and HPF, as well as rice grains containing FeSO4 (positive control) were utilized [60]. The rice seeds were simulated and dried overnight to a 10% water content. Uncooked and cooked rice grains had very different colors due to all Fe components except for micronized Fe4O21P6 (0.5 20 m). The enriched rice seeds with Fe4O21P6 and elemental Fe had the lowest Fe loss)2%) during the 30 min pre-washing at 30 °C. Researchers in India hope to develop Rice seeds fortified with iron and outstanding sensory properties and enhanced bioavailability rates using micronized Fe4O21P6. Li and colleagues created Ultra Rice with C4H2FeO4, mixtures of micronized Fe41O21P6, FeNaEDTA, thiamine, and other antioxidants. Fe was not bioavailable or stable when added to Ultra Rice. Rice seeds that were encapsulated with Fe4O21P6 exhibited an acceptable creamy-yellow color, but 50% less thiamine [61]. Unencapsulated C4H2FeO4 exhibited a reddish-brown tint. By combining FeNaEDTA with encapsulated C4H2FeO4, in vitro bioavailability was improved. Using colloids or particles of Fe4O21P6 could reduce thiamine losses while maintaining physical and organoleptic properties for 32 weeks at 60% RH and 40 °C. Dietary thiamine loss was minimal; however, oxidative rancidity was observed. BHA, BHT, hydrophilic citric acid, and sodium hexa meta phosphate were found to be the most effective storage forms of uncoated and encapsulated Fe containing free-radical scavenging constituents. Women who consumed cooked Ultra rice containing micronized Fe4O21P6 daily reduced their anemia by 80% and Fe deficiency by 29%. Beinner, Velasquez-Melendez, Pessoa, and Greiner also reported that the consumption of Ultra Rice encapsulated with micronized Fe4O21P6 (3.14 μm) reduced Fe deficiency and anemia in Brazilian youngsters (6–24 months) [62].
4.6 Minerals Encapsulation in Other Foods Fortification
The effects of injecting L-Ca with EPC into rabbits before slaughter were studied to determine its impact on meat aging. An infusion of L-Ca into rabbit meat could lower meat’s ageing without contaminating it or causing any physical trauma. Water in oil mulsion was prepared using an emulsifier such as Tween 60 to make a water-in-corn-oil emulsion, indicating that the TBARS generation was low, while the Fe-EE (99.75%) was high [63]. To assess the effect of Fe on fish oil stability, fish oil-based emulsion was created and combined with droplets of the initial emulsion. TBA values increased when Fe interacted with fish oil and activated oxidation processes. A spray drying process was conducted to encapsulate natural green pigments from Pandan leaves with three coatings (GA, MS, and MD). For powders encapsulated using Ms. wall material, SEM micro-graphs showed spherical and smooth particles, while powders were generated by the encapsulants. MD and GA surface shrinking Greenness, total chlorophyll, and antioxidants were determined to be best in Zn chlorophyll microcapsules, which comprised of 30% Ms. The produced powder has a predicted half-life (462 days) longer than GA (330 days) and MD (330 days) powders. Fe microparticles were studied in black beans in 2011, which contains 5–10 mg encapsulated Fe per spoon [64]. Using samples supplemented with FeSO4 micro-encapsules could help prevent or control anemia. Peach and apple juice were used to determine Fe status. Fe4O21P6 was the core and lecithin was the wall of spray-dried Fe microcapsules.
4.7 Application of Nanoparticles in Minerals
In recent years, scientists have become interested in reducing mineral inclusion levels and enhancing mineral absorption through nanoparticles. Mineral NPs are generally spherical and under 100 nm in size. They are frequently employed in agriculture, food, and pharmaceutical industries. Natural mineral nanoparticles (NMNs) have numerous industrial and medical applications. Se-NPs are the most commonly used NPs in the food industry to inhibit bacterial growth and biofilm formation. Melatonin, PEG, adenosine triphosphate, polysaccharides, and epigallocatechin-3-gallate are employed to make Se-NPs with minimal toxicity [65]. A nutritional supplement containing Se-NPs may help improve the body’s function and bio accessibility, as Se offers several benefits for the body’s health, including enhancing the immune system and reducing cancer cell proliferation. Meal supplementation with Se-NPs lowered bioavailability in Se-deficient mice. We are also aware of the antibacterial properties of inorganic nanometal oxides such magnesia, zirconium, and calcium oxide (CaO) NPs. In order to kill bacteria, metal oxide nanoparticles produce reactive oxygen species. Surface superoxide and H2O2 can cause intracellular leakage and cell death. In vitro studies of MgO-NPs conducted on human liver (HepG2), kidney (NRK-52E), gut (Caco-2), and lung (A549) cell lines indicated oxidative stress, DNA damage, and cell death, according to the USDA (21CFR184.1431) [66]. Thus, their use in consumer goods should be monitored for safety reasons. Several studies have shown the efficacy of MgO-NPs in treating various ailments. In U87 human astrocytoma, MgO-NPs were less cytotoxic than ZnO and TiO2 NPs. Boubeta et al. [67] employed MRI agents made of Fe/MgO nanoshells. Iron/MgO-NPs mediated magnetic hyperthermia in cancer therapy. Nano cryosurgery for cancers and Iron oxide (Fe2O3) nanoparticles are biocompatible and less toxic than other metals [67]. A scientist reported the use of Fe2O3-NPs as mineral supplements, controlled release medicinal and nutraceutical biomaterials, and colorants in cosmetics. The USDA (21CFR182.8991) also considers ZnO, one of the five Zn compounds, to be safe. By adding antimicrobial ZnO-NPs to active packaging films, especially for meat formulas, we investigated ways to prevent food-borne infections [68]. It was discovered that these NPs are not only antimicrobial but also a Zn source for numerous meals. Although they possess antibacterial properties, Ca3(PO4)2-, CaCO3-, and Ca3(C6H5O7)2-NPs have also shown promise in agriculture, poultry, and food processing. In addition to improving serum Ca levels and bone mineral density, Ca-based nanoparticles are highly bioavailable in the body.
4.8 Conclusion
In summary, the information provided herein can help in the encapsulation of difficult-to-formulate liposoluble vitamins, reactive water-soluble vitamins, and chemically sensitive water-soluble and liposoluble vitamins. Inventors select encapsulation techniques and micro/nanostructures based on resource availability, material qualities, process economics, and end-use applications. These techniques should also be safe to use, have a long shelf life, and require no dangerous chemicals or toxic solvents in their production. Recent research has focused on how to provide active substances like micronutrients in foods. In this perspective, most food fortification and nutraceutical R&D should concentrate on nano- and microencapsulation. To avoid bottlenecks, the hunt for food-grade protective coatings, shorter development times, better understanding of encapsulating techniques, and better understanding of in vivo behavior of encapsulates should be continued.
Abbreviations
- AAO:
-
Aryl-alcohol oxidase
- BHA:
-
Butylated hydroxyanisole
- BHT:
-
Butylated hydroxytoluene
- CD:
-
Cyclodextrin
- CMC:
-
Critical micellar concentration
- CPO:
-
Chloroperoxidase
- CTAB:
-
Cationic surfactant cetyl trimethyl ammonium bromide
- DFS:
-
Dual fortified salt
- EPC:
-
Egg phosphatidylcholine
- FeCl3:
-
Ferric chloride
- FeSO4:
-
Ferrous sulphate
- FFAs:
-
Free fatty acids
- FIP WP:
-
Ferri polyphosphate whey protein
- GA:
-
Gum Arabic
- GIT:
-
Gastrointestinal tract
- IDFA:
-
International Dairy Foods Association
- L-Ca:
-
Encapsulated calcium in liposome
- LMFS:
-
Large microencapsulated FeSO4
- MD:
-
Maltodextrin
- Μm:
-
Micrometer
- MRI:
-
Magnetic resonance imaging
- nm :
-
Nanometer
- SDS:
-
Sodium dodecyl sulfate
- SLNs :
-
Solid-liquid nanoparticles
- SMFS:
-
Small microencapsulated FeSO4
- TBA:
-
Thiobarbituric acid
- TBARS:
-
TBA reactive substance
- TFS:
-
Triple fortified salt
- USDA:
-
United State Department of Agriculture
- WPI:
-
Whey protein isolate
References
Desai KGH, Park HJ. Encapsulation of vitamin C in tripolyphosphate cross-linked chitosan microspheres by spray drying. J Microencapsul [Internet]. 2005;22(2):179–92. https://doi.org/10.1080/02652040400026533
Fang Z, Bhandari B. Encapsulation techniques for food ingredient systems. In: Food materials science and engineering. Oxford: Wiley-Blackwell; 2012. p. 320–48.
Champagne CP, Fustier P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr Opin Biotechnol [Internet]. 2007;18(2):184–90. https://doi.org/10.1016/j.copbio.2007.03.001.
Madziva H, Kailasapathy K, Phillips M. Alginate pectin microcapsules as a potential for folic acid delivery in foods. J Microencapsul. 2005;22:343–51.
Patel AR, Remijn C, Heussen PCM, Adel R, Velikov KP. Novel low-molecular-weight-gelator-based microcapsules with controllable morphology and temperature responsiveness. ChemPhysChem. 2013;14:305–10.
Velikov KP, Pelan E. Colloidal delivery systems for micronutrients and nutraceuticals. Soft Matter [Internet]. 2008;4(10):1964. https://doi.org/10.1039/b804863k.
Duclairoir C, Orecchioni AM, Depraetere P, Andnakache E. Alpha-tocopherol encapsulation and in vitro release from wheat gliadin nanoparticles. J Microencapsul. 2002;19:53–60.
Luo Y, Teng Z, Wang Q. Development of zein nanoparticles coated with carboxymethyl chitosan for encapsulation and controlled release of vitamin D3. J Agric Food Chem [Internet]. 2012;60(3):836–43. https://doi.org/10.1021/jf204194z.
Gonnet M, Lethuaut L, Boury F. New trends in encapsulation of liposoluble vitamins. J Control Release [Internet]. 2010;146(3):276–90. https://doi.org/10.1016/j.jconrel.2010.01.037.
Tcholakova S, Vankova N, Denkov ND, Danner T. (2007). Emulsification in turbulent flow: 3. Daughter drop-size distribution. Journal of Colloid and Interface Science. 310(2), 570–589. http://dx.doi.org/10.1016/j.jcis.2007.01.097
Ziani K, Fang Y, Mcclements DJ. Encapsulation of functional lipophilic components in surfactant-based colloidal delivery systems: vitamin E, vitamin D, and lemon oil. Food Chem. 2012;134:1106–12.
Feng JL, Wang ZW, Zhang J, Wang ZN, Liu F. Study on food-grade vitamin E microemulsions based on nonionic emulsifiers. Colloids Surf A Physicochem Eng Asp. 2009;339:1–6.
Hukat R, Relkin P. Lipid nanoparticles as vitamin matrix carriers in liquid food systems: on the role of high-pressure homogenisation, droplet size and adsorbed materials. Colloids Surf B: Biointerfaces. 2011;86:119–24.
Eskandar NG, Simovic S, Prestidge CA. Chemical stability and phase distribution of all-trans-retinol in nanoparticle-coated emulsions. Int J Pharm [Internet]. 2009;376(1–2):186–94. https://doi.org/10.1016/j.ijpharm.2009.04.036.
Patel AR, Velikov KP. Biopolymeric colloidal particles loaded with polyphenolic antioxidants. In: Antioxidant polymers. Hoboken, NJ: Wiley. 2012; 427–58.
Semo E, Kesselman E, Danino D, Livney Y. Casein micelle as a natural nano-capsular vehicle for nutraceuticals. Food Hydrocoll [Internet]. 2007;21(5–6):936–42. https://doi.org/10.1016/j.foodhyd.2006.09.006.
Haham M, Ish-Shalom S, Nodelman M, Duek I, Segal E, Kustanovich M, et al. Stability and bioavailability of vitamin D nanoencapsulated in casein micelles. Food Funct [Internet]. 2012;3(7):737–44. https://doi.org/10.1039/c2fo10249h.
Banville C, Vuillemard JC, Lacroix C. Comparison of different methods for fortifying cheddar cheese with vitamin D. Int Dairy J. 2000;10:375–82.
Marsanasco M, Márquez AL, Wagner JR, del V. Alonso S, Chiaramoni NS. Liposomes as vehicles for vitamins E and C: an alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res Int [Internet]. 2011;44(9):3039–46. https://doi.org/10.1016/j.foodres.2011.07.025.
Alayoubi A, Satyanarayanajois SD, Sylvester PW, Nazzal S. Molecular modelling and multi simplex optimization of tocotrienol-rich self-emulsified drug delivery systems. Int J Pharm. 2012;426:153–61.
Moraes M, Carvalho JMP, Silva CR, Cho S, Sola MR, Pinho SC. Liposomes encapsulating beta-carotene produced by the proliposomes method: characterisation and shelf life of powders and phospholipid vesicles. Int J Food Sci Technol [Internet]. 2013;48(2):274–82. https://doi.org/10.1111/j.1365-2621.2012.03184.x.
Pierucci APTR, Andrade LR, Baptista EB, Volpato NM, Rocha-Leão MHM. New microencapsulation system for ascorbic acid using pea protein concentrate as coat protector. J Microencapsul [Internet]. 2006;23(6):654–62. https://doi.org/10.1080/02652040600776523.
Pierucci AP, Andrade LR, Farina M, Pedrosa C, Rocha-Leuo MH. Comparison of α-tocopherol microparticles produced with different wall materials: pea protein a new interesting alternative. J Microencapsul. 2007;24:201–13.
Farias MC, Moura ML, Andrade L, Leão MHMR. Encapsulation of the alpha-tocopherol in a glassy food model matrix. Mater Res [Internet]. 2007;10(1):57–62. https://doi.org/10.1590/s1516-14392007000100013.
Patel AR, Vavia PR. Effect of hydrophilic polymer on solubilization of fenofibrate by cyclodextrin complexation. J Incl Phenom Macrocycl Chem. 2006;56:247–51.
Yap KL, Liu X, Thenmozhiyal JC, Ho PC. Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexes by phase solubility, photostability, physicochemical and computational analysis. Eur J Pharm Sci [Internet]. 2005;25(1):49–56. https://doi.org/10.1016/j.ejps.2005.01.021.
Zielenkiewicz W, Terekhova IV, Koźbiał M, Poznanski J, Kumeev RS. Inclusion of menadione with cyclodextrins studied by calorimetry and spectroscopic methods. J Phys Org Chem [Internet]. 2007;20(9):656–61. https://doi.org/10.1002/poc.1223.
Majeed H, Jamshaid Qazi H, Safdar W, Fang Z. Microencapsulation can be a novel tool in wheat flour with micronutrients fortification: current trends and future applications—a review. Czech J Food Sci. 2013;31:527.
Khiralla GM, El-Deeb BA. Antimicrobial and antibiofilm effects of selenium nano-particles on some foodborne pathogens. LWT—Food Sci Technol. 2015;63(1001):1007.
Zhang J, Taylor EW, Wan X, Peng D. Impact of heat treatment on size, structure, and bioactivity of elemental selenium nanoparticles. Int J Nanomed [Internet]. 2012;7:815–25. https://doi.org/10.2147/IJN.S28538.
Wang D, Taylor EW, Wang Y, Wan X, Zhang J. Encapsulated nanoepigallocatechin-3-gallate and elemental selenium nanoparticles as paradigms for nano-chemoprevention. Int J Nanomed. 2012;7:1711–21.
Ungvári É, Monori I, Megyeri A, Csiki Z, Prokisch J, Sztrik A, et al. Protective effects of meat from lambs on selenium nanoparticle supplemented diet in a mouse model of polycyclic aromatic hydrocarbon-induced immunotoxicity. Food Chem Toxicol [Internet]. 2014;(64):298–306. https://doi.org/10.1016/j.fct.2013.12.004.
Sarkar A, Sil PC. Iron oxide nanoparticles mediated cytotoxicity via PI3K/AKT pathway: role of quercetin. Food Chem Toxicol [Internet]. 2014;(71):106–15. https://doi.org/10.1016/j.fct.2014.06.003.
Mirhosseini M, Afzali M. Investigation into the antibacterial behavior of suspensions of magnesium oxide nanoparticles in combination with nisin and heat against Escherichia coli and Staphylococcus aureus in milk. Food Control. 2016;68(208):215.
Akbar A, Anal AK. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control [Internet]. 2014;38:88–95. https://doi.org/10.1016/j.foodcont.2013.09.065.
Rossi L, Velikov KP, Philipse AP. Colloidal iron(III) pyrophosphate particles. Food Chem [Internet]. 2014;151:243–7. https://doi.org/10.1016/j.foodchem.2013.11.050.
Vijayakumar MP, Balakrishnan V. Evaluating the bioavailability of calcium phos- phate nanoparticles as mineral supplement in broiler chicken. Indian J Sci Technol. 2014;7(1475):1480.
Ataee RA, Derakhshanpour J, Mehrabi Tavana A, Eydi A. Antibacterial effect of calcium carbonate nanoparticles on Agrobacterium tumefaciens. Iran J Mil Med. 2011;13(65):70.
Kwak HS, Yang KM, Ahn J. Microencapsulated iron for milk fortification. J Agric Food Chem [Internet]. 2003;51(26):7770–4. https://doi.org/10.1021/jf030199.
Gupta C, Chawla P, Arora S, Tomar SK, Singh AK. Iron microencapsulation with blend of gum arabic, maltodextrin and modified starch using modified solvent evapora- tion method-Milk fortification. Food Hydrocoll. 2015;43:622–8.
Chang YH, Lee SY, Kwak H-S. Physicochemical and sensory properties of milk fortified with iron microcapsules prepared with water-in-oil-in-water emulsion during storage. Int J Dairy Technol. 2016;69(3):452–9.
Saeidy S, Keramat J, Nasirpour A. Physicochemical properties of calcium- fortified soymilk with microencapsulated and chelated calcium salt. Eur Food Res Technol. 2014;238(105):112.
Jayalalitha V, Balasundaram B, Palanidorai A, Naresh KC. Fortification of encapsulated iron in probiotic yoghurt. Int J Agric Res Rev. 2012;2(2):80–4.
Subash R, Elango A. Microencapsulated iron for fortification in yoghurt. Food Sci Res J [Internet]. 2015;6(2):258–62. https://doi.org/10.15740/has/fsrj/6.2/258-262.
Cavallini DCU, Rossi EA. Soy yogurt fortified with iron and calcium: stability during the storage. Aliment Nutr. 2009;20(7):13.
Kwak HS, Ju YS, Ahn HJ, Ahn J, Lee S. Microencapsulated iron fortification and flavor development in cheddar cheese. Asian Australas J Anim Sci. 2003;16(1205):1211.
Arce A, Sc AM. Effect of micro-encapsulated ferrous sulfate on Cheddar cheese composition, divalent cation balance and acceptability. East Lansing: Michigan State University; 2016.
Jalili M. Chemical composition and sensory characteristics of Feta cheese fortified with iron and ascorbic acid. Dairy Sci Technol. 2016;96(4):579–89.
Diosady LL, Alberti JO, Venkatesh Mannar MG. Microencapsulation for iodine stability in salt fortified with ferrous fumarate and potassium iodide. Food Res Int [Internet]. 2002;35(7):635–42. https://doi.org/10.1016/s0963-9969(01)00166-1.
Diosady L, Yusufali R, Oshinowo T, Laleye L. A study of storage and distribution of double fortified salts in Kenya. J Food Eng. 2006;76:547–56.
Li YO, Diosady LL, Wesley A. Iron in vitro bioavailability and iodine storage stability in double fortified salt (DFS). Food Nutr Bull. 2009;30(327):335.
Yadava D, Olive Li Y, Diosady LL, Wesley AS. Optimisation of polymer coating process for microencapsulating ferrous fumarate for salt double fortification with iodine and iron. J Microencapsul [Internet]. 2012;29(8):729–38. https://doi.org/10.3109/02652048.2011.651493.
Romita D, Cheng Y-L, Diosady LL. Microencapsulation of ferrous fumarate for the production of salt double fortified with iron and iodine. Int J Food Eng [Internet]. 2011;7(3):5. https://doi.org/10.2202/1556-3758.2122.
Wegmüller R, Zimmermann MB, Bühr VG, Windhab EJ, Hurrell RF. Development, stability, and sensory testing of microcapsules containing iron, iodine, and vitamin A for use in food fortification. J Food Sci. 2006;71(2):S181–7.
Kongkachuichai R, Kounhawej A, Chavasit V, Charoensiri R. Effects of various iron fortificants on sensory acceptability and shelf-life stability of instant noodles. Food Nutr Bull. 2007;28(2):165–72.
Biebinger R, Zimmermann MB, Al-Hooti SN, Al-Hamed N, Al-Salem E, Zafar T, et al. Efficacy of wheat-based biscuits fortified with microcapsules containing ferrous sulfate and potassium iodate or a new hydrogen-reduced elemental iron: a randomised, double-blind, controlled trial in Kuwaiti women. Br J Nutr. 2009;102:1362–9.
Kiskini A, Kapsokefalou M, Yanniotis S, Mandala I. Effect of different iron compounds on wheat and gluten-free breads. J Sci Food Agric. 2010;90:1136–45.
Souto TS, Brasil ALD, Taddei JADC. Acceptability of bread fortified with microencapsulated iron by children of daycare centers in the south and east regions of Sao Paulo City, Brazil. Rev Nutr. 2008;21:647–57.
Moretti D, Lee T-C, Zimmermann MB, Nuessli J, Hurrell RF. Development and evaluation of iron-fortified extruded rice grains. J Food Sci. 2005;70(5):S330–6.
Hotz C, Porcayo M, Onofre G, García-Guerra A, Elliott T, Jankowski S, et al. Efficacy of iron-fortified Ultra Rice in improving the iron status of women in Mexico. Food Nutr Bull. 2008;29(2):140–9.
Li Y, Diosady LL, Jankowski S. Stability of vitamin B1in Ultra Rice® in the presence of encapsulated ferrous fumarate. Int J Food Sci Nutr [Internet]. 2008;59(1):24–33. https://doi.org/10.1080/09637480701554103.
Beinner MA, Velasquez-Meléndez G, Pessoa MC, Greiner T. Iron-fortified rice is as efficacious as supplemental iron drops in infants and young children. J Nutr [Internet]. 2010;140(1):49–53. https://doi.org/10.3945/jn.109.112623.
Choi SJ, Decker EA, McClements DJ. Impact of iron encapsulation within the interior aqueous phase of water-in-oil-in-water emulsions on lipid oxidation. Food Chem [Internet]. 2009;116(1):271–6. https://doi.org/10.1016/j.foodchem.2009.02.045.
Ferreira BS, Cardoso BT, Pereira HVR, Pierucci AP, Pedrosa C, Citelli M. Acceptability of black beans (Phaseolus vulgaris L.) fortified with iron microparticles. Rev Ceres. 2011;58:548–53.
Zhang Y, Li X, Huang Z, Zheng W, Fan C, Chen T. Enhancement of cell permeabilization apoptosis-inducing activity of selenium nanoparticles by ATP surface decoration. Nanomedicine [Internet]. 2013;9(1):74–84. https://doi.org/10.1016/j.nano.2012.04.002.
Mahmoud A, Ezgi Ö, Merve A, Özhan G. In vitro toxicological assessment of magnesium oxide nanoparticle exposure in several mammalian cell types. Int J Toxicol [Internet]. 2016;35(4):429–37. https://doi.org/10.1177/1091581816648624.
Boubeta CM, Balcells L, Cristofol R, Sanfeliu C, Rodrıguez E, Weissleder R, et al. Self-assembled multifunctional Fe/MgO nanospheres for magnetic resonance imaging and hyperthermia. Nanomedicine. 2010;6(2):362–70.
Akbar A, Anal AK. Zinc oxide nanoparticles loaded active packaging, a challenge study against Salmonella typhimurium and Staphylococcus aureus in ready-to-eat poultry meat. Food Control. 2014;38:88–95.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Al-Duhaidahawi, D. (2022). Nano and Microencapsulation of Foods, Vitamins and Minerals. In: Egbuna, C., Jeevanandam, J., C. Patrick-Iwuanyanwu, K., N. Onyeike, E. (eds) Application of Nanotechnology in Food Science, Processing and Packaging . Springer, Cham. https://doi.org/10.1007/978-3-030-98820-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-98820-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-98819-7
Online ISBN: 978-3-030-98820-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)