Abstract
An alarming propensity of various psychological and physiological disease states are associated with stress and trauma-related illnesses such as PTSD. This underscores the necessity to adopt a comprehensive and integrative perspective. Therefore, this chapter elucidates an integrative biopsychological view derived from interdisciplinary fields of research: clinical psychology, neuroscience, genetics and epigenetics, psychoneuroimmunology, mitochondria, and gut microbiota. The first section summarises the effect of traumatic stress, traumatic load, the role of the fear/trauma network linked with PTSD. The next section reviews latest findings on brain and cognitive alterations in PTSD and proposes a model that aims to provide a novel perspective on different treatment approaches. The third section outlines the prospective role of genetics and epigenetic alterations in PTSD. The fourth section provides pivotal insights from biomolecular studies on the role of altered mitochondrial functioning, oxidative stress, and immune regulation in psychological/traumatic stress and PTSD. Gut microbiota research is gaining momentum, hence the final section outlines the implications of gut microbiota in stress and PTSD. Overall, we have endeavoured to provide a state-of-the-art integrative view on the biopsychology of PTSD and its comorbidities.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
An alarming propensity of various psychological and physiological disease states are associated with stress and trauma-related illnesses such as PTSD. This underscores the necessity to adopt a comprehensive and integrative perspective. Therefore, this chapter elucidates an integrative biopsychological view derived from interdisciplinary fields of research: clinical psychology, neuroscience, genetics and epigenetics, psychoneuroimmunology, mitochondria, and gut microbiota. The first section summarises the effect of traumatic stress, traumatic load, the role of the fear/trauma network linked with PTSD. The next section reviews latest findings on brain and cognitive alterations in PTSD and proposes a model that aims to provide a novel perspective on different treatment approaches. The third section outlines the prospective role of genetics and epigenetic alterations in PTSD. The fourth section provides pivotal insights from biomolecular studies on the role of altered mitochondrial functioning, oxidative stress, and immune regulation in psychological/traumatic stress and PTSD. Gut microbiota research is gaining momentum, hence the final section outlines the implications of gut microbiota in stress and PTSD. Overall, we have endeavoured to provide a state-of-the-art integrative view on the biopsychology of PTSD and its comorbidities.
2 Role of Cumulative Trauma Exposure, Traumatic Stress, and Trauma Load in PTSD Risk
2.1 Trauma Load and the Dose-Response Effect on PTSD
Traumatic stress refers to experiences which elicit feelings of helplessness, fear, or horror, along with an alarm response triggering acute release of stress hormones (Kolassa et al. 2010a). The higher the number of different trauma event types experienced, the higher the traumatic load of an individual (Schneider et al. 2020). Higher traumatic load leads to a higher risk for lifetime PTSD in a dose–response relationship (Kolassa et al. 2010b), which seems to be similarly present in both biological sexes (Wilker et al. n.d.). This dose–response relationship is also termed as building-block effect (Neuner et al. 2004). And for instance, this effect is reflected in individuals with a history of childhood maltreatment (CM) as trauma exposure in childhood sensitises individuals to the detrimental consequences of trauma even in later stages of their life (i.e. increasing the risk for PTSD, depression, and somatic symptoms) (Behnke et al. 2020).
Furthermore, PTSD prevalence can reach 100% due to extreme levels of trauma load (see Fig. 4.1) (Kolassa et al. 2010c), i.e. there is no ultimate resilience for PTSD and upon extreme trauma exposure any individual could develop PTSD. A study conducted by Kolassa et al. (2010c) among refugees (n = 444) who survived the Rwandan genocide (1994) showed that higher traumatic load increases current as well as lifetime PTSD risk and severity, along with curtailing gradual spontaneous remission from PTSD. This study proposed traumatic load as a root cause of both chronicity and symptom severity of PTSD (Kolassa et al. 2010a).
The increasing number of traumatic event types experienced (i.e. traumatic load) also increases the probability to fulfil the PTSD criteria at least once in a lifetime, P (Lifetime PTSD). In the case of extreme trauma load, the probability of lifetime PTSD approaches 100% (Figure adapted from Kolassa et al. 2010c)
Notably, higher traumatic load is also associated with higher levels of appetitive aggression, i.e. an individual’s disposition to perpetrate violence along with deriving pleasure from inflicting violence. In formerly abducted rebel-war survivors (n = 1166) from Northern Uganda, appetitive aggression and the rate of perpetrated violence were found to be specifically elevated among those individuals who were abducted at a young age and experienced high traumatic load and combat events (Zeller et al. 2020).
2.2 Fear/Trauma Network Model: Role of “Cold” and “Hot” Memories in PTSD
The Fear/trauma network model of PTSD conceptualised by Elbert and colleagues postulates that the traumatic stress actuated intense fear/traumatic memories are stored in propositional networks, which can be shaped by new experiences through principles of associative learning and neuroplasticity (Wilker et al. 2014a; Elbert et al. 2015). As a core feature of PTSD includes fragmented memories, this model differentiates between “hot” and “cold” memories based on the terminology proposed by Metcalfe and Jacob (Metcalfe and Jacobs 1996). On the one hand, cold memories characterise autobiographical contextual information of specific events such as time, space, knowledge about period of life, dates, external circumstances, verbally accessible memories. On the other hand, “hot” memories encompass the stored information such as sensory and perceptual (e.g. hearing screams, smelling blood, burning houses); emotional or affective (e.g. fear, horror, disgust, sadness); cognitive (e.g. “I can’t do anything”, “I will die”); introspective or physiological (feeling of physiological reactions such as strong heartbeat, fast breathing sweating) (Elbert et al. 2015; Schauer et al. 2005). Due to its associative nature any trauma-related stimulus can trigger the entire fear network (Wilker and Kolassa 2013). Therefore, any further exposure to traumatic stress and increased trauma load could eventually lead to a loss of the connection between “cold” and “hot” memories, whereas “hot” memories connect with increased excitatory power, thus fortifying fear/trauma network in PTSD (Neuner et al. 2020).
3 Brain and Cognitive Alterations in PTSD
Individuals with PTSD show global brain atrophy and cognitive impairment in contrast to healthy controls with and without traumatic experiences (Bromis et al. 2018; Scott et al. 2015). Consistent with these findings, individuals with PTSD show approximately a 1.5-fold risk for developing dementia in comparison with healthy controls (Günak et al. 2020). In addition to global alterations, specific brain regions with pronounced abnormalities have been found. These include regions of the limbic and paralimbic system such as hippocampus, amygdala, and insula, as well as regions of the prefrontal cortex such as the anterior cingulate and orbitofrontal cortex (Bromis et al. 2018). In the following, we depict how these brain alterations are linked with specific PTSD symptoms.
3.1 Connection of Brain Alterations with PTSD Symptoms
The phenomenon of fragmented memories in PTSD characterised by impaired episodic memory (“cold” memories) with overactive implicit memory (“hot” memories) could be explained by hippocampal and amygdala alterations. Impaired episodic memory seems to be reflected by atrophy and hypoactivity of the hippocampus (Logue et al. 2018). Reduced inhibition of the limbic system through the medial prefrontal cortex may account for intrusion symptoms in PTSD (hot memories) (Fenster et al. 2018). In response to trauma-related stimuli and imaginations, PTSD patients demonstrated hyperactivity of the amygdala (involved in fear regulation) and insula (involved in bodily awareness) along with hypoactivity of the ventromedial prefrontal cortex (involved in top-down inhibition of the limbic system (Hayes et al. 2012; Hopper et al. 2007; Rauch et al. 2006).
Symptoms of poor concentration seem to be reflected in impaired working memory and processing speed (Scott et al. 2015). Altered prefrontal processing could be a brain-related correlate of these cognitive impairments (Moores et al. 2008).
Symptoms of persistent negative affect and anhedonia could be explained by a downregulated reward system (Nawijn et al. 2015). The striatum depicted reduced activation in anticipation of rewards (wanting) and after receiving the reward (liking).
Symptoms of altered arousal and reactivity could be connected to an upregulation of the salience network (involved in stimuli-driven, bottom-up attentional processes), along with a downregulation of the default mode network (involved in self-referential processing) (Koch et al. 2016). This shift could reflect the neuronal correlate of increased assignment of salience to external events and impaired internal thoughts and memories.
3.2 Causes of PTSD-Related Cognitive and Brain Alterations
PTSD-related alterations in brain and cognition (1) may be a consequence of the disorder itself, (2) may be risk factors that facilitate PTSD symptoms after traumatic experiences, (3) may result from a multitude of other factors that lead to both PTSD and neurocognitive changes (e.g. childhood maltreatment, genetic and lifestyle factors), or (4) may be a combination of all these cause–effect relationships. There is evidence from animal and human studies that traumatic stress and PTSD itself affect brain and cognition (Fenster et al. 2018). These studies indicate an adverse—but to some degree reversible—effect of chronic and traumatic stress on brain and cognition (Lupien et al. 2009).
However, increasing evidence suggests that in addition to an effect of trauma on brain and cognition, brain and cognitive abnormalities may be risk factors for PTSD after trauma exposure. For example, longitudinal studies suggest that a major part of cognitive abnormalities were present before the onset of PTSD (Parslow and Jorm 2007). In this line, a twin study compared hippocampal volume in monozygotic twin pairs, in which one member was exposed to trauma (Vietnam combat exposure) and the other member, his brother, was not trauma-exposed (Gilbertson et al. 2002). In combat-exposed twin members with PTSD and, notably, in the non-combat-exposed co-twin members without PTSD smaller hippocampi were observed than in a control group. As genetically identical twin brothers with and without PTSD showed hippocampal abnormalities, this marker seems to be a risk factor rather than a consequence of PTSD. In addition, patients with PTSD displayed reduced intracranial volume (Bromis et al. 2018) but this feature usually manifests in childhood long before trauma exposure. As intracranial volume is an indicator of premorbid brain volume, reduced brain volume seems to be a risk factor for PTSD.
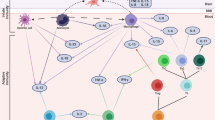
Taken together, PTSD may affect brain and cognition, and conversely, premorbid brain and cognitive alterations may increase the risk to develop PTSD. In the following, we discuss how additional factors such as childhood maltreatment, lifestyle, and genetic factors could affect brain, cognition, and psychopathology, which in turn could increase PTSD risk (see Fig. 4.2). In line with this notion, studies investigating the effect of early-life stress on cognition, psychopathology and the brain yielded highly similar findings as in patients with PTSD (Nakayama et al. 2020; Teicher et al. 2016). Studies on childhood maltreatment indicate a link with (1) general cognitive impairment, (2) increased general psychopathology, (3) volume loss in hippocampus and the medial prefrontal cortex, as well as (4) altered brain function such as increased amygdala response to threat and a less responsive reward system (see Teicher et al. 2016 for a review). Similarly, lifestyle factors such as physical exercise increase hippocampal volume (Opel et al. 2020), reduce measures of psychopathology (Caspi et al. 2014) and improve cognition (Karabatsiakis et al. 2014). Finally, genetic polymorphisms such as the apolipoprotein E—known to reduce hippocampal size and neurocognition—are linked with measures of psychopathology (Picard et al. 2018). That means, childhood maltreatment, lifestyle, and genetic factors may affect brain, cognition, and psychological health, thus increasing the risk for PTSD.
P and g factor model and its relevance for treatment. The p factor reflects general psychopathology and explains high correlations between different psychological symptoms, while the g factor represents a dimension of general intelligence that is thought to underlie high correlations between different cognitive functions. Thus, these two general factors of psychological and cognitive health describe general characteristics that underlie and contribute to symptoms of specific psychological and cognitive disorder categories such as PTSD (e.g. intrusions, altered arousal and reactivity, fragmented memories, and poor concentration) or dementia (e.g. depression, impaired memory). After accounting for the general factors of psychological and cognitive health, a part of symptoms that are specific for disorder categories are likely to remain unexplained (light blue). The general factors of p and g need to be accounted, to extract symptom-specific risk factors and brain correlates that go beyond and are independent from the general factors. This model has implications for PTSD treatment as interventions can be delineated in approaches that are transdiagnostic and target general factors of pathology (p and g factor) and treatments that target specific symptoms that are not explained by these two general factors
In line with the notion that brain, cognitive and general psychopathological alterations are a risk factor for PTSD, a meta-analysis suggests that brain region-specific alterations are only found when individuals with PTSD are compared with healthy controls but not when compared with depression (Bromis et al. 2018). Similarly, a large-scale study showed that a single cross-disorder factor score of brain structure explained 42–89% of the observed variance of four major psychological disorders, including major depressive disorder (MDD), bipolar disorder, schizophrenia, and obsessive-compulsive disorder (Opel et al. 2020). Abnormalities in brain structure highly correlated between all four disorder categories (r = 0.4–0.8). Regions that are altered in PTSD like the hippocampus, the amygdala, and anterior cingulum mainly drove the two most important factor scores of brain structure abnormalities in these four psychological disorders (Opel et al. 2020). This finding indicates that a large proportion of brain alterations between different psychological disorder categories are overlapping, and this may reflect transdiagnostic or cross-disorder brain alterations that may even be linked with cognitive impairment. Correspondingly, cognitive impairment is not only present in PTSD but also could be revealed in many psychological disorders and was linked with general psychopathology (Caspi et al. 2014). Taken together, findings suggest that brain and cognitive differences between PTSD and healthy controls may not be specific to PTSD but might be linked to general psychopathology that increase the risk to develop PTSD after trauma exposure.
Caspi et al. (2014) validated the concept of “general psychopathology” and called it the p factor—a rather stable dimension. The idea is that a general underlying factor explains the observation that individuals who score high in one psychological symptom (e.g. anxiety) usually display more symptoms of another disorder (e.g. depression). The p factor can be viewed in parallel with the g factor which is the well-known concept of a general intelligence factor.
3.3 The p and g Factor Model and Its Relevance for PTSD Treatment
We propose a novel p and g factor model of PTSD and depict its implication for treatment (see Fig. 4.2). In this model, we assume that genetic and environmental risk factors (e.g. poor diet, physical and social inactivity, childhood maltreatment) lead to a higher p factor (general psychopathology) and a lower g factor (intelligence). This symptomatology is reflected in brain correlates such as lower global brain volume as well as region-specific alterations (e.g. hippocampus, medial prefrontal cortex, insula, and amygdala). People with a high p and low g factor (which are reflected in a high amount of brain alterations) are believed to be more prone to develop PTSD after trauma exposure. This model partly explains why PTSD has a high comorbidity with other psychological disorders and is associated with brain and cognitive abnormalities.
In addition, this model yields important clinical implications, as it proposes two different kinds of therapeutic approaches (see bottom grey boxes in Fig. 4.2). On the one hand, so-called general factor, cross-disorder or transdiagnostic treatments should aim to improve the p and g factor and their underlying biological mechanisms. Macroscopic mechanisms such as global brain volume, hippocampal and medial prefrontal volume but also microscopic mechanisms may be the target. Examples for microscopic mechanisms include a dysregulated energy metabolism, altered mitochondrial respiration, oxidative stress, and inflammation (for details, see the following sections of this chapter). On the other hand, symptoms that go beyond the degree that is expected due to the p and g factor should be targeted with symptom-specific treatments (see light blue boxes in Fig. 4.2). This means, PTSD in a context of high general pathology of p and g might have a different biological underpinning than PTSD with low general pathology (e.g. larger hippocampal volume reduction in high vs. low general pathology). Therefore, it should be differently treated [e.g. with additional physical exercise as a potential general factor treatment that affects hippocampal volume and measures of p and g (Opel et al. 2020; Caspi et al. 2014; Karabatsiakis et al. 2014)]. Importantly, such general factor treatments have the potential for generalised, transdiagnostic effects on a wide range of symptoms. These general factor treatments should be accompanied by symptom-specific treatments if symptoms go beyond what is expected by general pathology (e.g. exposure therapy in phobias in a context of normal p and g factors). A novel perspective that integrates general factor approaches with symptom-specific treatments has not only the potential to reduce the burden of PTSD but also to reduce incidence of other psychopathologies, cognitive decline, and dementia in old age.
4 Genetics of PTSD
Worldwide, 70.4% of individuals face a traumatic event at least once in their lifetime, whereas the lifetime prevalence of PTSD is around 4% (Kessler et al. 2017). This implies, some individuals might carry certain factors which make them vulnerable or resilient to develop the disorder. Individual differences such as family environment, personality, and biological risk factors can contribute to the vulnerability and resilience. Genetic factors can explain some of these individual differences. This makes PTSD genetically a complex trait, i.e. its variability must be explained by both genetic and environmental factors, and its aetiology is always a gene × environment interaction (G × E).
4.1 Heritability of PTSD Risk
Heritability estimates of PTSD risk following trauma range from 23.5% (True 1993) to 71% (Sartor et al. 2011). The large range of the heritability estimates can be explained by characteristics of the study population, namely ethnicity, sex distribution, age, and trauma type (Duncan et al. 2018a). Heritability estimates for PTSD risk might also comprise the susceptibility to be exposed to certain traumatic events such as childhood abuse (Dalvie et al. 2020; Pezzoli et al. 2019), assaults, or war traumas, but not non-assaultive traumas such as motor vehicle accidents or natural disasters (Ryan et al. 2016; Stein et al. 2002). Personality traits and certain behaviours such as risk taking might also be influenced by genetic factors (Ryan et al. 2016). Therefore, heritability estimates of PTSD risk should be interpreted with caution as susceptibility to trauma exposure should not be overlooked.
Association studies that aim to discover the genes that might contribute to a genetic trait either test hypothesis-driven candidate genes or assess hypothesis-free genetic variations in terms of single nucleotide polymorphisms (SNPs) in genome-wide association studies (GWAS) throughout the genomes of cases versus controls. Since PTSD can only be triggered by traumatic event exposure, it is reasonable to evaluate the effect of different genes while considering the type and frequency of experienced traumatic events (Conrad et al. 2017). Due to the difficulty in quantifying the environmental factor “traumatic load”, only few studies model this variable as a quantitative covariate in their statistical models.
4.2 Candidate Gene Studies
Hypothesis-driven association studies in PTSD have mainly targeted the genes of serotonergic and dopaminergic systems, stress response systems, and inflammatory responses. Most genes were selected due to their roles in fear response and memory modulation, namely for their involvement in the activation of the amygdala (Smoller 2016). Here, we will focus on some of the most influential ones.
A polymorphism (5-HTTLPR) in the regulatory promoter region of the serotonin transporter gene was one of the first genetic factors to be associated with anxiety (Lesch et al. 1996), and it is also of interest for PTSD research. Compared to the long allele (L) of 5-HTTLPR, its short allele (S) is associated with less expression of the serotonin transporter gene (SLC6A4), and less production of the serotonin transporter protein that leads to a decreased serotonin reuptake from the synaptic cleft. 5-HTTLPR S-allele homozygotes (SS genotype) were reported to be at increased risk to develop PTSD (Kolassa et al. 2010b). Other studies report the S-allele as a risk factor predicting PTSD symptoms, interacting with traumatic events such as blast exposure (Taylor et al. 2019). However, it has been shown that upon extremely high trauma exposure, genetic factors lost their importance as all genotype groups approached 100% likelihood to develop PTSD. Thus, it is highly important to model the covariate trauma load in the statistical models investigating the genetics of PTSD in terms of a G × E (Kolassa et al. 2010b; Wilker et al. 2018). Research also found L-allele to be a risk factor, as the number of L-alleles increases, prevalence of PTSD also increases in individuals with higher traumatic load (Grabe et al. 2009). In par with this, a study found that SS homozygosity in the 5-HTTLPR was a buffer against acquisition of certain PTSD symptoms among individuals who were victims of emotional abuse during childhood (Walsh et al. 2014), whereas other studies found no such effects (Kovacic Petrovic et al. 2016). Moreover, a meta-analysis provided inconclusive evidence on the role of 5-HTTLPR in PTSD (Gressier et al. 2013; Navarro-Mateu et al. 2013). A recent meta-analysis investigating the interaction between 5-HTTLPR and stress in predicting PTSD reported that 5-HTTLPR is a significant moderator, concluding that the presence of at least one S-allele is a risk factor in PTSD aetiology in combination with stress (Zhao et al. 2017). Notably, none of these meta-analyses considered traumatic load in their models. Based on these findings, the actual role of 5-HTTLPR in PTSD aetiology is not yet fully understood, although it seems to be a predictor of PTSD in G × E context.
As PTSD has been associated with alterations in the regulation of the endocrine stress-response system, specifically the hypothalamic-pituitary-adrenal (HPA) axis, research has aimed to investigate the relevance of genes coding for proteins involved in the HPA axis physiology. Among them, the FKBP5 protein modulates the sensitivity of the glucocorticoid receptor (GR), and more presence of the protein is associated with decreased GR sensitivity to cortisol. Thereby, the negative feedback loop of the HPA axis is less efficient and, thus, individuals might return to their normal endocrine stress response less effectively (Mehta and Binder 2012). There are certain polymorphisms that were associated with changing expression of the FKBP5 gene. These polymorphisms do not show main effects in predicting PTSD symptomatology (but see Watkins et al. 2016), and they are rather studied in G × E context. One of the initial G × E studies reported four different FKBP5 SNPs to interact with exposure to traumatic events during childhood but not in adulthood in regard to predicting PTSD symptomatology (Binder 2008). Carrying at least one risk allele (T) of one of these SNPs (rs1360780) was reported to moderate the influence of childhood trauma on PTSD risk in such a way that the T-allele carriers had increased PTSD risk, if they had a childhood trauma history compared to the ones who did not have. Conversely, in individuals not carrying the T-allele, childhood trauma history did not predict their PTSD risk (Klengel et al. 2013).
Two meta-analyses compiled a decade of research on the interplay of FKBP5 × traumatic life events and confirmed significant interactions between FKBP5 SNPs and presence of early-life stress (Wang et al. 2018) as well as presence of lifetime exposure to traumatic events (Hawn et al. 2019) in predicting PTSD risk. The meta-analytic studies concluded that certain combinations of genotype and being exposed to adverse environments constitute a particular risk for PTSD. Moreover, rs1360780 was also reported to condition the long-term effectiveness of exposure-based psychotherapy in PTSD (Wilker et al. 2014b). To conclude, a combination of FKBP5 and early-life adversity is among the relatively consistent genetic factors disposing for PTSD.
Immune system alterations have been associated with PTSD and certain immunomodulatory genes have been studied in PTSD. As for the genetic role of immune response elements, Michopoulos et al. (2015) identified a SNP within the CRP gene, which was further associated with increased C-reactive protein (CRP) levels, to be directly associated with the PTSD diagnosis as well as with the severity of PTSD symptoms. Another study found evidence for correlations of tumour necrosis factor alpha (TNF-α) serum levels and the TNF-α polymorphism rs1800629 with PTSD severity (Bruenig et al. 2017). Stress response elements are important in modulating immune system activity (Chrousos 1995), and therefore, stress response-related genetic factors (e.g. FKBP5) might contribute to immune alterations in PTSD and development of PTSD symptoms (i.e. memory formation) following immune system alterations (Wilker et al. 2014b; Zannas et al. 2016).
4.3 Genome-Wide Association Studies
Candidate genes associated with mental disorders explain small proportions of the variability in PTSD. Therefore, to find other genetic factors related to the disease, the Psychiatric Genetics Consortium-PTSD Group has conducted various hypothesis-free GWAS with large samples. Their first big attempt with 20,070 participants could neither identify SNPs relevant for PTSD nor replicate their previous results (Duncan et al. 2018b). In a GWAS meta-analysis (Nievergelt et al. 2019), they provided additional analyses for a diverse cohort of African and European ancestries, as well as for men and women. With a sample of almost 200,000 participants, they detected some SNPs to be linked to PTSD along with one in a Parkinson’s gene (PARK2) that has a role in the dopaminergic system. Moreover, the polygenic risk score that was computed to assemble the effects of all SNPs that the GWAS detected was shown to be significantly linked to PTSD. It was found to significantly predict PTSD symptomatology in yet another sample; however, the size of the observed association was small (~1%).
In large samples compiled from different studies, it is difficult to control the effect of traumatic load or previous traumatic event history, which can partially explain the difficulties in finding a consistent genetic factor predicting the PTSD risk. To overcome this, Wilker and colleagues (Wilker et al. 2018) performed a cases-only PTSD GWAS with Northern Ugandan rebel-war survivors (N = 924) along with Rwandan genocide survivors (N = 370) as the replication sample, and included traumatic load in their analyses as a covariate. They reported five significant SNPs in their discovery sample and could replicate one of them (rs3852144, A > G) in their replication sample. This indicates that as the number of rs3852144 minor G-alleles increased, the PTSD risk after trauma decreased. They provided initial evidence that rs3852144 could be linked to differences in the therapy-related decrease of PTSD symptoms. The biological mechanism of this SNP from a non-coding region is yet to be discovered.
To conclude, the genetic factors which have been associated with PTSD contribute little to explain the variability of the disease and its severity among patients. Therefore, genetic research in PTSD has so far not provided substantial contribution to the understanding of the disorder’s aetiology or to develop novel treatment strategies. A reason for the limited success of genetic studies on PTSD might be the heterogeneity of the disease among individuals. Considering varying symptom profiles among individuals with PTSD, it is reasonable to assume that the individuals with diverse PTSD symptomatology might have different genetic risk factors, which leads to difficulties in finding a consistent genetic or epigenetic risk factor for all PTSD cases in a particular study. Other reasons include complex physiological mechanisms underlying PTSD (e.g. inflammation, oxidative stress) influencing memory formation and p factor which are associated with hundreds of different genetic factors, complex environmental predictors such as individual trauma history and traumatic load which are difficult to assess, as well as individual differences related to personality, ethnicity, and sociodemographic background. Studies should also analyse individuals with similar psychological symptoms, along with similar biological manifestations of the disease together.
4.4 Epigenetic Alterations in PTSD
Epigenetic alterations are changes in the chemical structure of DNA that do not affect the gene sequence. Epigenetic markers influence gene expression, i.e. whether a gene is activated or silenced, and how much they are expressed. Epigenetic mechanisms are evolution’s shut-down tools for the genetic material; e.g. they silence the second X chromosome in human females, switch off the unnecessary genes in specialised cells, and shut down “outdated” genes from our evolutionary history that are redundant for us. Epigenetic alterations are heritable but also prone to change based on variety of environmental conditions. Not all epigenetic markers from parents pass onto the later generations.
Epigenetic changes can be additions or deletions of a chemical group to/from the DNA strand or the histone proteins that help pack the DNA in the form of chromosomes. The most studied epigenetic marker in stress research is DNA methylation, i.e. the addition of a methyl (-CH3) group to (mostly) cytosine bases of DNA. If the cytosine is found next to a guanine, a so-called CpG site is formed. When CpG sites are common in one region of the gene, this region is called a CpG island. If DNA methylation occurs at a regulatory region of a gene (i.e. promoter), where transcription factors bind to control gene expression, it can prevent the transcription factor from binding and, thus, influence gene transcription and protein production.
Stress presents an important environmental factor that leads to dynamic changes in DNA methylation (Zhang and Meaney 2010). Therefore, DNA methylation has been a focus for PTSD epigenomics research. As for genetic studies, epigenetic studies may be hypothesis-driven and hypothesis-free. As for hypothesis-driven approaches, the same genes that were found to interact with adverse environment to predict PTSD symptomatology were studied in DNA methylation context. Researchers also conducted hypothesis-free epigenome-wide association studies (EWAS) to compare the methylation status of thousands of CpG sites in cases versus controls.
As for the hypothesis-driven studies, Koenen et al. (2011) found higher PTSD risk in individuals exposed to higher number of traumatic events, if they had lower serotonin transporter gene (SLC6A4) promoter methylation. However, a study assessing the impact of mindfulness intervention in PTSD reported no association between PTSD and SLC6A4 promoter methylation before or after the intervention (Bishop et al. 2018).
Allele-dependent methylation, i.e. the occurrence of methylation patterns according to particular alleles (Meaburn et al. 2010), was commonly observed in FKBP5. In childhood-trauma survivors who carry an FKBP5 rs1360780 risk allele (T), methylation of a CpG island on an important regulatory region was lower (which leads to increased FKBP5 gene expression) than in individuals who do not carry the risk allele or who do not have a history of childhood trauma (Klengel et al. 2013). In another study, recovery following psychotherapy predicted decreased methylation in veterans with PTSD when compared to methylation levels before therapy (Yehuda et al. 2013). Moreover, higher FKBP5 expression due to epigenetic changes related to stress and ageing has been associated with increased inflammatory responses that can also partially explain observed immune system alterations in PTSD (Zannas et al. 2019).
As for hypothesis-free studies, in different cohorts the PTSD EWAS results revealed many differentially methylated CpG sites but did not replicate each other. A recent meta-analysis which used data from civilian and veteran samples revealed less methylation in a CpG site of aryl-hydrocarbon receptor repressor gene (AHRR) in PTSD cases (Smith et al. 2020). The gene can contribute to immune system alterations in PTSD. Another recent EWAS replicated the finding in AHRR in US veterans, and reported other significant sites in genes that might be involved in pathogen response (Logue et al. 2020). Longitudinal EWAS concerning PTSD development and treatment were performed in military cohorts. Comparing epigenomic profiles before and after deployment, Rutten et al. (2018) reported PTSD-associated decreases in DNA methylation in three genes ZFP57, RNF39, and HIST1H2APS2. Interestingly, increase in ZFP57 methylation was later associated with successful treatment of PTSD in another sample (Vinkers et al. 2021). ZFP57 protein is associated with epigenetic regulation (Li et al. 2008) and susceptibility to stress (Jakobsson et al. 2008).
Altogether, epigenomics results on PTSD aetiology point towards the role of stress axes, inflammation, and neuromodulatory processes. However, most of the reported methylation changes have not yet been replicated and offer limited explanation of individual variability in PTSD severity and prevalence. This could be explained with the heterogeneity of the disease, individual differences in personality and physiology, and the varying degrees of exposure to traumatic events and lifetime trauma history (Morrison et al. 2019). Supporting the idea of the possible effect of heterogeneity of PTSD symptomatology and differences in physiological manifestation of the disease on PTSD (epi-)genetics, researchers recently identified two PTSD epigenetic biotypes with EWAS data from samples of veteran male cohorts, and their 3-year follow-up (Yang et al. 2021). The two epigenetic biotypes show different methylation patterns, oppositely dysregulated in certain signalling pathways, and have distinct PTSD symptom manifestations. Furthermore, most epigenetic analyses are performed on blood samples which contain many different types of immune cells, whose composition might be associated with the disease, and likely have different methylation profiles. Methylation profiles differ across tissues (e.g. blood vs. saliva), and possibly contribute to nonreplicable results.
Future research should attempt to adequately model traumatic load and reduce the symptom heterogeneity in participants through creating symptom clusters or recruiting individuals with similar psychological and physiological manifestations. These measures may help to identify clinical and biological subtypes of PTSD and might contribute to novel classifications or treatments of PTSD.
5 Trauma-Related Alteration in Mitochondrial Functioning Leads to Energy Deficiency and Inflammation
Persistent alterations in the regulation of the immune system are arising as a deciding factor of mental health. The immune system consists of two major branches: innate and adaptive immune response. The innate immune response presents the body’s fast-acting, pathogen-unspecific defence against infectious threats and injury. It is mainly mediated by leukocytes, including macrophages/glia cells, monocytes, neutrophils, basophils, and eosinophils. The adaptive immune response presents a delayed, but prolonged and threat-specific defence against pathogens. It mainly constitutes lymphocytes, including T cells which ought to destroy pathogen-infected cells. Besides, B cells produce and release antibodies to destroy recognised pathogens (Murphy and Weaver 2018).
5.1 Immune Cell Composition and PTSD
Regarding the cellular immune response, PTSD has been linked to altered numbers of leukocytes and lymphocytes (Lindqvist et al. 2017a) although there is conflicting evidence of the precise nature of these alterations. To address this inconsistency, Sommershof et al. (2009) distinguished between functionally differing T cell subpopulations, and found lowered naïve cytotoxic CD3+ and CD8+ T lymphocyte counts, elevated memory CD3+ and CD8+ T lymphocyte counts, as well as lower counts of CD4+ regulatory T cells in PTSD patients as compared to trauma-exposed individuals and non-traumatised controls. A reduction in naïve T cells can imply a higher susceptibility to infectious diseases. A shortage of regulatory T cells is critical for immune-regulatory imbalances. Importantly, there is preliminary evidence that the altered proportion of CD4+ regulatory T cells could be partially reversible by trauma-focused psychotherapy (Morath et al. 2014a).
5.2 Low-Grade Inflammation and Cytokine Levels in PTSD
Immune cells communicate by releasing signalling proteins, e.g. cytokines, which also present major communicators between the immune and nervous systems. There are various types of cytokines which can exert pro-inflammatory effects (i.e. increasing immune activity) as well as anti-inflammatory effects (i.e. decreasing immune activity) (Murphy and Weaver 2018). Research frequently investigated pro-inflammatory cytokines like interleukin (IL-) 1β, IL-6, the tumour necrosis factors (TNF-) α and β, and interferon (IFN-) α, β, and γ, as well as anti-inflammatory cytokines like IL-4 and IL-10. Elevating levels of IL-6 and TNF-α trigger the liver to produce C-reactive protein (CRP) presenting a biomarker of acute-phase inflammation (Murphy and Weaver 2018).
Higher levels of pro-inflammatory cytokines have also been found in blood of individuals with stress-related mental health problems, indicating a chronic low-grade activity of their immune system. In PTSD, meta-analyses and systematic reviews provided evidence for elevated blood levels of IL-1β, IL-6, TNF-α, CRP, IL-4, and IL-10 as compared to healthy controls (Hori and Kim 2019; Speer et al. 2018; Yuan et al. 2019). However, there is inconclusive evidence on elevated IFN-γ levels in blood serum of PTSD patients (Lindqvist et al. 2017b).
To date, it is ambiguous whether inflammation is a (potential) vulnerability marker for PTSD onset after traumatic stress or whether it manifests because of trauma exposure and/or due to PTSD itself. Notably, elevated inflammatory markers are no specific biomarker of PTSD. Instead, various mental and physical conditions involve elevated cytokine levels. In fact, research found elevated inflammatory activity in a variety of mental health problems (e.g. depression, bipolar disorder) or after stress exposure (e.g. chronic caregiving stress, early-life stress, intimate partner violence) but could not identify disorder-specific markers of inflammation (Yuan et al. 2019; Tursich et al. 2014). At the same time, chronically elevated, unspecific inflammatory activity applies to almost all ageing-related non-communicable diseases such as cardiovascular diseases, diabetes, and even cancer (Duan et al. 2019; Franceschi and Campisi 2014; Grivennikov et al. 2010). Likewise, individuals with stress-related mental health problems such as PTSD exhibit an elevated vulnerability for the premature onset of such non-communicable physical health problems (Pacella et al. 2013). Investigating the shared immunological correlates of PTSD will enable advanced understanding of the multiple adverse consequences of PTSD and will open new perspectives on effective treatment approaches.
5.3 Cellular Energy Metabolism and Oxidative Stress in PTSD
Chronic inflammatory activity manifested as elevated levels of pro-inflammatory cytokines exerts wide-spread alterations in the metabolism of cells, disturbs their oxidative balance, and can impair the cellular energy production by mitochondria. These alterations emerge as key mechanisms underlying the development of a variety of chronic diseases and also apply to several mental health problems such as PTSD and depression (Hitzler et al. 2019; Karabatsiakis and Schönfeldt-Lecuona 2020). Mitochondria, the powerhouses of our cells, are intracellular organelles, which have their own mitochondrial DNA (mtDNA), and are the main producers of biochemical energy in humans. Moreover, immune cells release various molecules to regulate the inflammation reaction and fight pathogens, including reactive oxygen/nitrogen species (ROS/RNS), i.e. highly reactive oxygen and nitrogen molecules (Lugrin et al. 2014). Normally, ROS and RNS are rapidly neutralised by antioxidants or detoxification mechanisms of cells. Upon imbalance between levels of ROS and antioxidants—a state called oxidative stress—, ROS/RNS cause considerable damage to essential cell compartments, including mitochondria, DNA, cell membranes, and essential enzymes (Turrens 2003). ROS are physiological by-products of oxidative phosphorylation (OXPHOS), a process to produce biochemical energy in the form of adenosine triphosphate (ATP), which takes place in mitochondria.
Few studies have so far investigated markers of oxidative stress in PTSD. There is initial evidence of elevated levels of lipid peroxidation and lowered antioxidant enzymes in blood serum of earthquake-survivors with PTSD as compared to earthquake-exposed healthy controls (Atli et al. 2016). Using a combined metabolomics and lipidomics approach, Karabatsiakis et al. (2015) identified several metabolites in blood serum that allowed to discriminate between PTSD patients and healthy controls, including lowered levels of two metabolites with antioxidant properties, i.e. a bilirubin isomer and pantothenic acid (vitamin B5). Besides, studies also characterised possible consequences of oxidative stress in PTSD: Morath and colleagues (2014b) observed a higher level of DNA double-strand breaks in leukocytes of PTSD patients and traumatised adults as compared to non-traumatised healthy controls. Importantly, successful trauma-focused psychotherapy was able to normalise DNA strand breaks among PTSD patients. Further research is needed to draw firm conclusions regarding the associations of PTSD with oxidative stress.
Mitochondria themselves are of pivotal relevance in initiating, regulating, and resolving immune responses and inflammatory processes (Mills et al. 2017). As for their immunomodulatory role, altered (and possibly impaired) mitochondrial functioning is gaining attention in the explanation of various psychopathologies such as depression (Karabatsiakis and Schönfeldt-Lecuona 2020). Mitochondria are essentially involved in several physiological processes that are disrupted in PTSD; i.e. mitochondria were linked to abnormal fear learning, brain circuit activities, synaptic plasticity, the production of steroid hormones, as well as the regulation of central and peripheral inflammation (Mills et al. 2017; Miller 2013). Chronic and traumatic stress are not only important triggers of PTSD and related mental health problems, but were also linked to altered mitochondrial functioning (Boeck et al. 2016; Gumpp et al. 2020; Picard and McEwen 2018).
To date, direct studies of mitochondrial functioning in PTSD have not yet been conducted in humans. By now, metabolomics studies identified several metabolites involved in pathways related to mitochondrial activity which enabled to discriminate between PTSD patients and healthy controls: Karabatsiakis et al. (2015) identified 13 metabolites including glycerophospholipids, fatty acid metabolites, nucleosides, bile acids and derivates, monosaccharides, and antioxidants, which displayed significant changes in PTSD. In another metabolomics study, Mellon et al. (2019) found differences between PTSD subjects and controls in pathways related to glycolysis and fatty acid uptake and metabolism as well as in pathways related to urea cycle and amino acid metabolism. These data indicate changes in the metabolic profile of individuals with PTSD with an involvement of mitochondrial alterations.
Furthermore, there is initial evidence that mitochondrial alterations may contribute to PTSD symptomatology and increase susceptibility to PTSD (Preston et al. 2018). One preliminary study showed altered gene expression of mitochondria-related genes, including six genes of the OXPHOS pathway, in the prefrontal cortex of post-mortem brains from six PTSD patients and six controls (Su et al. 2008). Another study identified genes associated with mitochondrial function that were differentially methylated in PTSD compared to trauma-exposed control subjects (Hammamieh et al. 2017). Moreover, lower mtDNA copy number as a marker for the cellular mitochondrial density was found in male combat veterans with PTSD (Bersani et al. 2016). Another study analysed SNPs in the mtDNA and showed significant correlation between PTSD severity and the heteroplasmy levels of two mtDNA SNPs in genes coding for proteins in the respiratory chain (Flaquer et al. 2015).
These findings altogether suggest that mitochondrial alterations play a role in the aetiology of PTSD. Longitudinal studies are needed to determine whether mitochondrial dysregulation precedes or follows PTSD onset and if a causal relationship exists between PTSD and mitochondrial alterations (Bersani et al. 2020). Further research also needs to measure mitochondrial function and mitochondrial oxygen consumption related to ATP production in cells of individuals with and without PTSD.
6 Implication of Gut Microbiota in Stress and PTSD
Perturbations in the microbiota-gut-brain axis (MGBA) have been linked to illnesses both physical (e.g. gastrointestinal disorders) and psychological (e.g. depression, anxiety) (Smith et al. 2019). The MGBA is a complex, bidirectional, and interactive network that connects gut and brain. The underlying mechanism of MGBA involves gut microbiota, central nervous system (CNS), enteric nervous system (ENS), immune system, hypothalamic-pituitary axis (HPA), etc. (Dinan and Cryan 2012). Gut microbiota refers to the approximately 100 trillion diverse microorganisms inhabiting the human gastrointestinal (GI) tract such as archaea, fungi, eukaryotes, protozoa, bacteriophages, viruses, and predominantly bacteria (beneficial and pathogenic bacteria) (Thursby and Juge 2017). Gut microbiota along with their genes and metabolites are termed as the human gut microbiome (Berg et al. 2020). Indeed, vastly present microbial communities in the GI tract play a crucial role in modulating the immune system, the metabolomic responses, stress regulation, and our health homeostasis (Cryan et al. 2019; Danneskiold-Samsøe et al. 2019).
Alterations and imbalance in the composition as well as metabolic capacity of gut microbiota is known as gut dysbiosis (Zeng et al. 2017) and it is a major factor linked to MGBA perturbation. Exposure to stress as well as other factors such as antibiotics, dietary changes, changes in pH levels in gut, etc. are attributed to cause gut dysbiosis (Zeng et al. 2017; Fröhlich et al. 2016; Ilhan et al. 2017; Madison and Kiecolt-Glaser 2019). Animal model studies show alterations in the composition of gut microbiota due to exposure to different types of psychological stress like maternal separation, chronic social defeat, restraint conditions, etc. (Rea et al. 2020). On the one hand, increased inflammation associated with stress could trigger “blooms” of pathogenic bacteria which promotes dysbiosis (Madison and Kiecolt-Glaser 2019). On the other hand, gut dysbiosis could affect the regulation of the stress response by intensifying HPA activity, and causing variations in neurotransmitters and inflammation (Johnson 2020). Gut dysbiosis is especially linked to abnormal immune responses and resultant abnormal production of inflammatory cytokines (Lin et al. 2019). Both these are observed in PTSD (Toft et al. 2018).
A better health status is associated with a higher diversity of bacterial composition; however, gut dysbiosis is commonly associated with loss of microbiota diversity (LOMD) (Mosca et al. 2016), and congruently, an increased level of anxiety and depression is associated with LOMD (Johnson 2020). This is further reflected in an exploratory study which found no substantial difference in overall microbial community diversity between trauma-exposed and PTSD participants; but, in participants with PTSD, a decrease in the relative abundance of certain bacterial phyla (i.e. Actinobacteria, Lentisphaerae, and Verrucomicrobia) were found (Hemmings et al. 2017). PTSD is associated with liver cirrhosis in veterans, and a study observed, gut dysbiosis characterised with reduced microbial diversity in cirrhotic veterans with PTSD when compared with non-PTSD veterans (Bajaj et al. 2019). The intestinal mucosal barrier prevents microbes, toxins, food antigens to leave the gut lumen and enter other body systems (Ghosh et al. 2020). However, stress can impact intestinal mucosal barrier and may trigger a state of “leaky gut” characterised with severe dysfunctions of the intestinal mucosal barrier and increased intestinal permeability, this can potentially permit entry of pathogenic bacteria and bacterial toxins into systemic circulation (Kelly et al. 2015). For instance, systemic presence of lipopolysaccharides (LPS; which are a major constituent of the outer membrane of gram-negative bacteria) can elevate inflammation as well as oxidative stress, and a slight increase in systemic LPS itself could trigger depressive symptoms (Selhub et al. 2014).
Stress, alterations in the gut microbial composition (gut dysbiosis), a state of leaky gut (gut permeability), and associated inflammation may potentially contribute to the development and exacerbations of PTSD symptomatology and comorbidities. Therefore, future studies on PTSD should consider the pathophysiological role of MGBA in their respective research framework. Importantly, emerging knowledge on the role of gut microbiota in stress-related disorders can tremendously contribute to innovative treatment and disease prevention approaches (see: Cryan et al. 2019).
7 Conclusion and Future Perspectives
The most well-replicated finding is the dose-response effect of traumatic stress load on PTSD risk, which seems to be similarly present in both biological sexes. Traumatic stress load not only affects the aetiology of PTSD or other psychological disorders but also the risk for adverse physical health outcomes often associated with PTSD. Genetic studies indicate that the contribution of single SNPs to overall PTSD risk is small, and research is yet to yield conclusive evidence. G × E interaction studies clearly demonstrate the need to consider lifetime traumatic load in genetic studies on PTSD, because genetic risk factors might lose their importance with exposure to traumatic stress, and anyone could develop PTSD with sufficient trauma load.
PTSD is consistently associated with chronic low-grade inflammation and altered immune regulation, and this may further contribute to the overall health decline. Therefore, we consider the biomolecular process modulating inflammation and its systemic consequences as a promising research focus for PTSD. An important hinge factor related to the regulation of inflammation may be alterations in mitochondrial bioenergetics and related oxidative stress in cells. Indeed, inflammation, oxidative stress, and mitochondrial functioning might be a biological correlate of a general psychopathological dimension, the so-called p factor, or in other words, the common variance between a diverse set of psychological symptoms. Hence, mitochondrial and immune system functioning might represent a common underlying mechanism of the aetiology of a wide range of psychological disorders including PTSD. This potential cross-disorder mechanism might explain why brain and cognitive alterations (e.g. volume reduction in hippocampus and anterior cingulum as well as deficits in executive function and episodic memory) found in PTSD are also found in several other psychological disorders and in people who experienced risk factors of psychopathology such as childhood maltreatment (CM). New therapeutic interventions that target these common mechanisms between disorders have the beneficial potential not only for patients with PTSD but also for other psychological and neurocognitive disorders.
Stress-induced elevation in inflammation and resultant higher levels of oxidative stress could alter the gut microbiota (gut dysbiosis) and a subsequent state of “leaky gut”. This may induce perturbations in MGBA which is implicated in the development or exacerbation of several diseases such as anxiety, MDD, PTSD, gastrointestinal disorders, cancer, etc. Further, microbiota alterations may increase inflammation and can potentially contribute to a compromised regulation of the immune system and metabolic processes. Endeavours are required to develop an integrative knowledge on the interplay of brain activity, immune regulation, cellular energy homeostasis, mitochondrial metabolism, and gut microbiota as hinge factors to investigate the biological effects of chronic and traumatic stress exposure in diseases like PTSD and its comorbidities. In the long run, this integrative perspective may result in developing innovative evidence-based psychobiological interventions that will serve as effective add-ons to existing evidence-based trauma-focused psychotherapies with the aim to ensure sustainable health outcomes for individuals with a history of CM or severe stress and trauma exposure.
References
Atli A, Bulut M, Bez Y, Kaplan İ, Özdemir PG, Uysal C et al (2016) Altered lipid peroxidation markers are related to post-traumatic stress disorder (PTSD) and not trauma itself in earthquake survivors. Eur Arch Psychiatry Clin Neurosci 266:329–336
Bajaj JS, Sikaroodi M, Fagan A, Heuman D, Gilles H, Gavis EA et al (2019) Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am J Physiol Gastrointest Liver Physiol 317:G661–G669
Behnke A, Rojas R, Karabatsiakis A, Kolassa I-T (2020) Childhood maltreatment compromises resilience against occupational trauma exposure: a retrospective study among emergency medical service personnel. Child Abuse Negl 99:104248
Berg G, Rybakova D, Fischer D, Cernava T, Vergès M-CC, Charles T et al (2020) Microbiome definition re-visited: old concepts and new challenges. Microbiome 8:103
Bersani FS, Morley C, Lindqvist D, Epel ES, Picard M, Yehuda R et al (2016) Mitochondrial DNA copy number is reduced in male combat veterans with PTSD. Prog Neuro-Psychopharmacol Biol Psychiatry 64:10–17
Bersani FS, Mellon SH, Lindqvist D, Kang JI, Rampersaud R, Somvanshi PR et al (2020) Novel pharmacological targets for combat PTSD—metabolism, inflammation, the gut microbiome, and mitochondrial dysfunction. Mil Med 185:311–318
Binder EB (2008) Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 299:1291
Bishop JR, Lee AM, Mills LJ, Thuras PD, Eum S, Clancy D et al (2018) Methylation of FKBP5 and SLC6A4 in relation to treatment response to mindfulness based stress reduction for posttraumatic stress disorder. Front Psych 9:418
Boeck C, Koenig AM, Schury K, Geiger ML, Karabatsiakis A, Wilker S et al (2016) Inflammation in adult women with a history of child maltreatment: the involvement of mitochondrial alterations and oxidative stress. Mitochondrion 30:197–207
Bromis K, Calem M, Reinders AATS, Williams SCR, Kempton MJ (2018) Meta-analysis of 89 structural MRI studies in posttraumatic stress disorder and comparison with major depressive disorder. AJP 175:989–998
Bruenig D, Mehta D, Morris CP, Harvey W, Lawford B, Young RM et al (2017) Genetic and serum biomarker evidence for a relationship between TNFα and PTSD in Vietnam war combat veterans. Compr Psychiatry 74:125–133
Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S et al (2014) The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clin Psychol Sci 2:119–137
Chrousos GP (1995) The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. N Engl J Med 332:1351–1363
Conrad D, Wilker S, Pfeiffer A, Lingenfelder B, Ebalu T, Lanzinger H et al (2017) Does trauma event type matter in the assessment of traumatic load? Eur J Psychotraumatol 8:1344079
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M et al (2019) The microbiota-gut-brain axis. Physiol Rev 99:1877–2013
Dalvie S, Maihofer AX, Coleman JRI, Bradley B, Breen G, Brick LA et al (2020) Genomic influences on self-reported childhood maltreatment. Transl Psychiatry 10:38
Danneskiold-Samsøe NB, de Freitas D, Queiroz Barros H, Santos R, Bicas JL, Cazarin CBB, Madsen L et al (2019) Interplay between food and gut microbiota in health and disease. Food Res Int 115:23–31
Dinan TG, Cryan JF (2012) Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37:1369–1378
Duan L, Rao X, Sigdel KR (2019) Regulation of inflammation in autoimmune disease. J Immunol Res 2019:1–2
Duncan LE, Cooper BN, Shen H (2018a) Robust findings from 25 years of PTSD genetics research. Curr Psychiatry Rep 20:115
Duncan LE, Ratanatharathorn A, Aiello AE, Almli LM, Amstadter AB, Ashley-Koch AE et al (2018b) Largest GWAS of PTSD (N=20 070) yields genetic overlap with schizophrenia and sex differences in heritability. Mol Psychiatry 23:666–673
Elbert T, Schauer M, Neuner F (2015) Narrative exposure therapy (NET): reorganizing memories of traumatic stress, fear, and violence. In: Schnyder U, Cloitre M (eds) Evidence based treatments for trauma-related psychological disorders. Springer, Cham, pp 229–253
Fenster RJ, Lebois LAM, Ressler KJ, Suh J (2018) Brain circuit dysfunction in post-traumatic stress disorder: from mouse to man. Nat Rev Neurosci 19:535–551
Flaquer A, Baumbach C, Ladwig K-H, Kriebel J, Waldenberger M, Grallert H et al (2015) Mitochondrial genetic variants identified to be associated with posttraumatic stress disorder. Transl Psychiatry 5:e524
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69:S4–S9
Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B et al (2016) Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav Immun 56:140–155
Ghosh P, Swanson L, Sayed IM, Mittal Y, Lim BB, Ibeawuchi S-R et al (2020) The stress polarity signaling (SPS) pathway serves as a marker and a target in the leaky gut barrier: implications in aging and cancer. Life Sci Alliance 3:e201900481
Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP et al (2002) Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 5:1242–1247
Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S et al (2009) Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. AJP 166:926–933
Gressier F, Calati R, Balestri M, Marsano A, Alberti S, Antypa N et al (2013) The 5-HTTLPR polymorphism and posttraumatic stress disorder: a meta-analysis: 5-HTTLPR and PTSD: a meta-analysis. J Trauma Stress 26:645–653
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Gumpp AM, Boeck C, Behnke A, Bach AM, Ramo-Fernández L, Welz T et al (2020) Childhood maltreatment is associated with changes in mitochondrial bioenergetics in maternal, but not in neonatal immune cells. Proc Natl Acad Sci U S A 117:24778–24784
Günak MM, Billings J, Carratu E, Marchant NL, Favarato G, Orgeta V (2020) Post-traumatic stress disorder as a risk factor for dementia: systematic review and meta-analysis. Br J Psychiatry 217:600–608
Hammamieh R, Chakraborty N, Gautam A, Muhie S, Yang R, Donohue D et al (2017) Whole-genome DNA methylation status associated with clinical PTSD measures of OIF/OEF veterans. Transl Psychiatry 7:e1169
Hawn SE, Sheerin CM, Lind MJ, Hicks TA, Marraccini ME, Bountress K et al (2019) GxE effects of FKBP5 and traumatic life events on PTSD: a meta-analysis. J Affect Disord 243:455–462
Hayes JP, Hayes SM, Mikedis AM (2012) Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord 2:9
Hemmings SMJ, Malan-Müller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG et al (2017) The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med 79:936–946
Hitzler M, Karabatsiakis A, Kolassa I-T (2019) Biomolekulare Vulnerabilitätsfaktoren psychischer Erkrankungen: Einfluss von chronischem und traumatischem Stress auf Immunsystem, freie Radikale und Mitochondrien. Psychotherapeut 64:329–348
Hopper JW, Frewen PA, van der Kolk BA, Lanius RA (2007) Neural correlates of reexperiencing, avoidance, and dissociation in PTSD: symptom dimensions and emotion dysregulation in responses to script-driven trauma imagery. J Traum Stress 20:713–725
Hori H, Kim Y (2019) Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci 73:143–153
Ilhan ZE, Marcus AK, Kang D-W, Rittmann BE, Krajmalnik-Brown R (2017) pH-mediated microbial and metabolic interactions in fecal enrichment cultures. mSphere 2:12
Jakobsson J, Cordero MI, Bisaz R, Groner AC, Busskamp V, Bensadoun J-C et al (2008) KAP1-mediated epigenetic repression in the forebrain modulates behavioral vulnerability to stress. Neuron 60:818–831
Johnson KV-A (2020) Gut microbiome composition and diversity are related to human personality traits. Human Microb J 15:100069
Karabatsiakis A, Schönfeldt-Lecuona C (2020) Depression, mitochondrial bioenergetics, and electroconvulsive therapy: a new approach towards personalized medicine in psychiatric treatment—a short review and current perspective. Transl Psychiatry 10:226
Karabatsiakis A, Böck C, Salinas-Manrique J, Kolassa S, Calzia E, Dietrich DE et al (2014) Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Transl Psychiatry 4:e397
Karabatsiakis A, Hamuni G, Wilker S, Kolassa S, Renu D, Kadereit S et al (2015) Metabolite profiling in posttraumatic stress disorder. J Mol Psych 3:2
Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP (2015) Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci 9:392
Kessler RC, Aguilar-Gaxiola S, Alonso J, Benjet C, Bromet EJ, Cardoso G et al (2017) Trauma and PTSD in the WHO world mental health surveys. Eur J Psychotraumatol 8:1353383
Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM et al (2013) Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 16:33–41
Koch SBJ, van Zuiden M, Nawijn L, Frijling JL, Veltman DJ, Olff M (2016) Aberrant resting-state brain activity in posttraumatic stress disorder: a meta-analysis and systematic review. Depress Anxiety 33:592–605
Koenen KC, Uddin M, Chang S-C, Aiello AE, Wildman DE, Goldmann E et al (2011) SLC6A4 methylation modifies the effect of the number of traumatic events on risk for posttraumatic stress disorder. Depress Anxiety 28:639–647
Kolassa I-T, Ertl V, Eckart C, Kolassa S, Onyut LP, Elbert T (2010a) Spontaneous remission from PTSD depends on the number of traumatic event types experienced. Psychol Trauma 2:169–174
Kolassa I-T, Ertl V, Eckart C, Glöckner F, Kolassa S, Papassotiropoulos A et al (2010b) Association study of trauma load and SLC6A4 promoter polymorphism in posttraumatic stress disorder: evidence from survivors of the Rwandan genocide. J Clin Psychiatry 71:543–547
Kolassa I-T, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ-F (2010c) The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val158Met polymorphism. Biol Psychiatry 67:304–308
Kovacic Petrovic Z, Nedic Erjavec G, Nikolac Perkovic M, Peraica T, Pivac N (2016) No association between the serotonin transporter linked polymorphic region polymorphism and severity of posttraumatic stress disorder symptoms in combat veterans with or without comorbid depression. Psychiatry Res 244:376–381
Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S et al (1996) Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274:1527–1531
Li X, Ito M, Zhou F, Youngson N, Zuo X, Leder P et al (2008) A maternal-zygotic effect gene, Zfp57, maintains both maternal and paternal imprints. Dev Cell 15:547–557
Lin C-H, Chen C-C, Chiang H-L, Liou J-M, Chang C-M, Lu T-P et al (2019) Altered gut microbiota and inflammatory cytokine responses in patients with Parkinson’s disease. J Neuroinflammation 16:129
Lindqvist D, Mellon SH, Dhabhar FS, Yehuda R, Grenon SM, Flory JD et al (2017a) Increased circulating blood cell counts in combat-related PTSD: associations with inflammation and PTSD severity. Psychiatry Res 258:330–336
Lindqvist D, Dhabhar FS, Mellon SH, Yehuda R, Grenon SM, Flory JD et al (2017b) Increased pro-inflammatory milieu in combat related PTSD—a new cohort replication study. Brain Behav Immun 59:260–264
Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS et al (2018) Smaller hippocampal volume in posttraumatic stress disorder: a multisite enigma-pgc study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry 83:244–253
Logue MW, Miller MW, Wolf EJ, Huber BR, Morrison FG, Zhou Z et al (2020) An epigenome-wide association study of posttraumatic stress disorder in US veterans implicates several new DNA methylation loci. Clin Epigenetics 12:46
Lugrin J, Rosenblatt-Velin N, Parapanov R, Liaudet L (2014) The role of oxidative stress during inflammatory processes. Biol Chem 395:203–230
Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009) Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 10:434–445
Madison A, Kiecolt-Glaser JK (2019) Stress, depression, diet, and the gut microbiota: human–bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci 28:105–110
Meaburn EL, Schalkwyk LC, Mill J (2010) Allele-specific methylation in the human genome: implications for genetic studies of complex disease. Epigenetics 5:578–582
Mehta D, Binder EB (2012) Gene × environment vulnerability factors for PTSD: the HPA-axis. Neuropharmacology 62:654–662
Mellon SH, Bersani FS, Lindqvist D, Hammamieh R, Donohue D, Dean K et al (2019) Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS One 14:e0213839
Metcalfe J, Jacobs WJ (1996) A “Hot-system/cool-system” view of memory under stress. PTSD Res Quart 7:8
Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO et al (2015) Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatr 172:353–362
Miller WL (2013) Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol 379:62–73
Mills EL, Kelly B, O’Neill LAJ (2017) Mitochondria are the powerhouses of immunity. Nat Immunol 18:488–498
Moores KA, Clark CR, McFarlane AC, Brown GC, Puce A, Taylor DJ (2008) Abnormal recruitment of working memory updating networks during maintenance of trauma-neutral information in post-traumatic stress disorder. Psychiatry Res 163:156–170
Morath J, Gola H, Sommershof A, Hamuni G, Kolassa S, Catani C et al (2014a) The effect of trauma-focused therapy on the altered T cell distribution in individuals with PTSD: evidence from a randomized controlled trial. J Psychiatr Res 54:1–10
Morath J, Moreno-Villanueva M, Hamuni G, Kolassa S, Ruf-Leuschner M, Schauer M et al (2014b) Effects of psychotherapy on DNA strand break accumulation originating from traumatic stress. Psychother Psychosom 83:289–297
Morrison FG, Miller MW, Logue MW, Assef M, Wolf EJ (2019) DNA methylation correlates of PTSD: recent findings and technical challenges. Prog Neuro-Psychopharmacol Biol Psychiatry 90:223–234
Mosca A, Leclerc M, Hugot JP (2016) Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol 7:455. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4815357/
Murphy KM, Weaver C (2018) Janeway immunologie. Akad. Verlag, Spektrum
Nakayama M, Hori H, Itoh M, Lin M, Niwa M, Ino K et al (2020) Possible long-term effects of childhood maltreatment on cognitive function in adult women with posttraumatic stress disorder. Front Psych 11:344
Navarro-Mateu F, Escámez T, Koenen KC, Alonso J, Sánchez-Meca J (2013) Meta-analyses of the 5-HTTLPR polymorphisms and post-traumatic stress disorder. PLoS One 8:e66227
Nawijn L, van Zuiden M, Frijling JL, Koch SBJ, Veltman DJ, Olff M (2015) Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev 51:189–204
Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T (2004) Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry 4:34
Neuner F, Elbert T, Schauer M (2020) Narrative exposure therapy for PTSD. In: Bufka LF, Wright CV, Halfond RW (eds) Casebook to the APA clinical practice guideline for the treatment of PTSD. American Psychological Association, Washington pp 187–205
Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen C-Y, Choi KW et al (2019) International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun 10:4558
Opel N, Goltermann J, Hermesdorf M, Berger K, Baune BT, Dannlowski U (2020) Cross-disorder analysis of brain structural abnormalities in six major psychiatric disorders: a secondary analysis of mega- and meta-analytical findings from the enigma consortium. Biol Psychiatry 88:678–686
Pacella ML, Hruska B, Delahanty DL (2013) The physical health consequences of PTSD and PTSD symptoms: a meta-analytic review. J Anxiety Disord 27:33–46
Parslow RA, Jorm AF (2007) Pretrauma and posttrauma neurocognitive functioning and PTSD symptoms in a community sample of young adults. AJP 164:509–515
Pezzoli P, Antfolk J, Hatoum AS, Santtila P (2019) Genetic vulnerability to experiencing child maltreatment. Front Genet 10:852
Picard M, McEwen BS (2018) Psychological stress and mitochondria: a systematic review. Psychosom Med 80:141–153
Picard M, McEwen BS, Epel ES, Sandi C (2018) An energetic view of stress: focus on mitochondria. Front Neuroendocrinol 49:72–85
Preston G, Kirdar F, Kozicz T (2018) The role of suboptimal mitochondrial function in vulnerability to post-traumatic stress disorder. J Inherit Metab Dis 41:585–596
Rauch SL, Shin LM, Phelps EA (2006) Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research—past, present, and future. Biol Psychiatry 60:376–382
Rea K, Dinan TG, Cryan JF (2020) Gut microbiota: a perspective for psychiatrists. Neuropsychobiology 79:50–62
Rutten BPF, Vermetten E, Vinkers CH, Ursini G, Daskalakis NP, Pishva E et al (2018) Longitudinal analyses of the DNA methylome in deployed military servicemen identify susceptibility loci for post-traumatic stress disorder. Mol Psychiatry 23:1145–1156
Ryan J, Chaudieu I, Ancelin M-L, Saffery R (2016) Biological underpinnings of trauma and post-traumatic stress disorder: focusing on genetics and epigenetics. Epigenomics 8:1553–1569
Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE et al (2011) Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol Med 41:1497–1505
Schauer M, Neuner F, Elbert T (2005) Narrative exposure therapy. A short-term intervention for traumatic stress disorders after war, terror, or torture. Crisis 26:194–195
Schneider A, Pfeiffer A, Conrad D, Elbert T, Kolassa I-T, Wilker S (2020) Does cumulative exposure to traumatic stressors predict treatment outcome of community-implemented exposure-based therapy for PTSD? Eur J Psychotraumatol 11:1789323
Scott JC, Matt GE, Wrocklage KM, Crnich C, Jordan J, Southwick SM et al (2015) A quantitative meta-analysis of neurocognitive functioning in posttraumatic stress disorder. Psychol Bull 141:105–140
Selhub EM, Logan AC, Bested AC (2014) Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. J Physiol Anthropol 33:2
Smith RP, Easson C, Lyle SM, Kapoor R, Donnelly CP, Davidson EJ et al (2019) Gut microbiome diversity is associated with sleep physiology in humans. PLoS One 14:e0222394
Smith AK, Ratanatharathorn A, Maihofer AX, Naviaux RK, Aiello AE, Amstadter AB et al (2020) Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies methylation changes in AHRR. Nat Commun 11:5965
Smoller JW (2016) The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology 41:297–319
Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg E-M et al (2009) Substantial reduction of naive and regulatory T cells following traumatic stress. Brain Behav Immun 23:1117–1124
Speer K, Upton D, Semple S, McKune A (2018) Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. J Inflamm Res 11:111–121
Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ (2002) Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am J Psychiatry 159:1675–1681
Su YA, Wu J, Zhang L, Zhang Q, Su DM, He P et al (2008) Dysregulated mitochondrial genes and networks with drug targets in postmortem brain of patients with posttraumatic stress disorder (PTSD) revealed by human mitochondria-focused cDNA microarrays. Int J Biol Sci 4:223–235
Taylor MK, Hernández LM, Stump J, Tschiffely AE, Goforth CW, Laver DC et al (2019) Blast exposure interacts with genetic variant 5HTTLPR to predict posttraumatic stress symptoms in military explosives personnel. Psychiatry Res 280:112519
Teicher MH, Samson JA, Anderson CM, Ohashi K (2016) The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci 17:652–666
Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474:1823–1836
Toft H, Bramness JG, Lien L, Abebe DS, Wampold BE, Tilden T et al (2018) PTSD patients show increasing cytokine levels during treatment despite reduced psychological distress. Neuropsychiatr Dis Treat 14:2367–2378
True WR (1993) A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry 50:257
Turrens JF (2003) Mitochondrial formation of reactive oxygen species. J Physiol 552:335–344
Tursich M, Neufeld RWJ, Frewen PA, Harricharan S, Kibler JL, Rhind SG et al (2014) Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry 4:e413
Vinkers CH, Geuze E, van Rooij SJH, Kennis M, Schür RR, Nispeling DM et al (2021) Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Mol Psychiatry 26(4):1264–1271
Walsh K, Uddin M, Soliven R, Wildman DE, Bradley B (2014) Associations between the SS variant of 5-HTTLPR and PTSD among adults with histories of childhood emotional abuse: results from two African American independent samples. J Affect Disord 161:91–96
Wang Q, Shelton RC, Dwivedi Y (2018) Interaction between early-life stress and FKBP5 gene variants in major depressive disorder and post-traumatic stress disorder: a systematic review and meta-analysis. J Affect Disord 225:422–428
Watkins LE, Han S, Harpaz-Rotem I, Mota NP, Southwick SM, Krystal JH et al (2016) FKBP5 polymorphisms, childhood abuse, and PTSD symptoms: results from the National Health and Resilience in Veterans Study. Psychoneuroendocrinology 69:98–105
Wilker S, Kolassa I-T (2013) The formation of a neural fear network in posttraumatic stress disorder: insights from molecular genetics. Clin Psychol Sci 1:452–469
Wilker S, Elbert T, Kolassa I-T (2014a) The downside of strong emotional memories: how human memory-related genes influence the risk for posttraumatic stress disorder—a selective review. Neurobiol Learn Mem 112:75–86
Wilker S, Pfeiffer A, Kolassa S, Elbert T, Lingenfelder B, Ovuga E et al (2014b) The role of FKBP5 genotype in moderating long-term effectiveness of exposure-based psychotherapy for posttraumatic stress disorder. Transl Psychiatry 4:e403
Wilker S, Schneider A, Conrad D, Pfeiffer A, Boeck C, Lingenfelder B et al (2018) Genetic variation is associated with PTSD risk and aversive memory: evidence from two trauma-exposed African samples and one healthy European sample. Transl Psychiatry 8:251
Wilker S, Catani C, Kolassa S, Ibrahim H, Rajan V, Pfeiffer A et al (n.d.) Sex differences in PTSD risk: evidence from post-conflict populations challenges the general assumption of increased vulnerability in females. Eur J Psychotraumatol. https://www.tandfonline.com/doi/epub/10.1080/20008198.2021.1930702
Yang R, Gautam A, Getnet D, Daigle BJ, Miller S, Misganaw B et al (2021) Epigenetic biotypes of post-traumatic stress disorder in war-zone exposed veteran and active duty males. Mol Psychiatry 26(8):4300–4314
Yehuda R, Daskalakis NP, Desarnaud F, Makotkine I, Lehrner AL, Koch E et al (2013) Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front Psych 4:118
Yuan N, Chen Y, Xia Y, Dai J, Liu C (2019) Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry 9:233
Zannas AS, Wiechmann T, Gassen NC, Binder EB (2016) Gene-stress-epigenetic regulation of FKBP5: clinical and translational implications. Neuropsychopharmacology 41:261–274
Zannas AS, Jia M, Hafner K, Baumert J, Wiechmann T, Pape JC et al (2019) Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB–driven inflammation and cardiovascular risk. Proc Natl Acad Sci U S A 116:11370–11379
Zeller AC, Conrad D, Schneider A, Behnke A, Pfeiffer A, Blum GF et al (2020) A combination of combat experience, early abduction, and severe traumatization fuels appetitive aggression and violence among abductees of rebel war in Northern Uganda. Aggress Behav 46:465–475
Zeng MY, Inohara N, Nuñez G (2017) Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol 10:18–26
Zhang T-Y, Meaney MJ (2010) Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol 61:439–466, C1-3
Zhao M, Yang J, Wang W, Ma J, Zhang J, Zhao X et al (2017) Meta-analysis of the interaction between serotonin transporter promoter variant, stress, and posttraumatic stress disorder. Sci Rep 7:16532
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Varadarajan, S., Behnke, A., Gumpp, A.M., Mavioglu, R.N., Fissler, P., Kolassa, IT. (2022). An Integrative View on the Biopsychology of Stress and Posttraumatic Stress Disorder. In: Schnyder, U., Cloitre, M. (eds) Evidence Based Treatments for Trauma-Related Psychological Disorders. Springer, Cham. https://doi.org/10.1007/978-3-030-97802-0_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-97802-0_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-97801-3
Online ISBN: 978-3-030-97802-0
eBook Packages: MedicineMedicine (R0)