Abstract
There is good evidence that some pharmacological treatments can effectively reduce symptoms of some trauma-related disorders. There is more evidence for posttraumatic stress disorder (PTSD) than for other specific trauma-related disorders. It is important that practitioners are aware of this and that appropriate prescribing of drugs occurs, when indicated. This chapter provides an overview of the evidence for the pharmacological treatment of PTSD and introduces the Cardiff PTSD prescribing algorithm, a tool designed to facilitate evidence-based prescribing.
The results of systematic reviews and meta-analyses support fluoxetine, paroxetine, sertraline and venlafaxine as the drugs with the best evidence for the treatment of PTSD when prescribed on their own. Prazosin and risperidone are the best evidenced drugs for augmentation. Based on these findings, the prescribing algorithm provides clear guidance with respect to dose, dose escalation, augmentation, common adverse effects, drug interactions and monitoring requirements. The final step in the algorithm involves considering changing to an alternative medication (amitriptyline, mirtazapine or phenelzine) that has a weaker evidence base but works in a different way to the other medications suggested.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Pharmacological treatment
- Fluoxetine
- Paroxetine
- Sertraline
- Venlafaxine
- Quetiapine
- Prazosin
- Amitriptyline
- Mirtazapine
- Phenelzine
1 Introduction
Pharmacological treatments have long been used for trauma-related psychological disorders, such as PTSD. Despite none of the main PTSD management guidelines recommending pharmacological treatments as strongly as trauma-focused psychological treatments (APA 2017; ISTSS 2018; NICE 2018; Phoenix Australia 2020a; VA/DoD 2017), medication is widely prescribed and widely taken for symptoms of PTSD.
The neurobiology of PTSD suggests that pharmacological approaches could be helpful. An enduring neurobiological hypothesis of PTSD concerns a dysfunctional hypothalamic-pituitary-adrenal axis resulting in adrenergic overactivity (Yehuda et al. 1991). Logically, addressing this by reducing adrenaline/nor-adrenaline levels, or by increasing cortisol levels, should represent an effective approach to both preventing and treating PTSD. Unfortunately, despite some signals of potential, the results of trials designed to demonstrate this with drugs such as propranolol and hydrocortisone have been disappointing (Astill Wright et al. 2019). However, as demonstrated in Chap. 4, the neurobiology of PTSD is complicated and it seems unlikely that medications currently available have precise enough actions to successfully treat all the symptoms and manifestations of PTSD.
Medication will continue to be used to treat PTSD, and justifiably so for some medications as there is strong evidence that they can reduce symptoms (e.g., Hoskins et al. 2021). Whilst we wait for further research to expand pharmacological treatment options, it is now critical that we take stock of the current evidence available and ensure it is appropriately used to inform prescribing practice. In common with psychological treatments, the evidence base with respect to trauma-related disorders is strongest for PTSD and much more limited or absent for other trauma-related disorders, not least for complex PTSD (Coventry et al. 2020).
2 The Evidence
There have been a number of robustly conducted systematic reviews of randomised controlled trials (RCTs) of pharmacological treatments for PTSD in recent years, not least those conducted to provide information to develop treatment guidelines (e.g., Hoskins et al. 2021). The results of these have much in common but, unsurprisingly, are not identical, given that the evidence is rapidly evolving. This led Australia (Phoenix Australia 2020a) to create its latest guidelines as “living guidelines” that are revised when relevant new research becomes available, rather than once every 5–10 years. For the first time, one of the major international treatment guidelines for PTSD will be updated within 2 years of its publication. This heralds a new era, and one to be welcomed as it should reduce the impact of understandable concerns about the reliability of ageing guidelines.
2.1 Results of Meta-Analyses
Hoskins et al. (2021) undertook a systematic review and meta-analyses of pharmacological treatments for PTSD that were used to inform the development of the International Society for Traumatic Stress Studies (ISTSS 2018) and Australian (Phoenix Australia 2020a) Guidelines for PTSD. Forty-nine monotherapy studies were included and data from 39 of these (4951 participants) were used in the meta-analyses undertaken. Participants were, on average, in their early forties and had been exposed to a wide variety of DSM A criterion fulfilling events with combat trauma being the commonest reported trauma type. Sexual and physical violence were the next most common traumatic experiences reported.
The follow-up periods for studies were relatively short with an average of just over 3 months. Although the studies followed standard, placebo controlled RCT methodology, some risk of bias was identified for most studies, although the degree was comparable to that found for RCTs of psychological treatments for PTSD. Of those compounds included where there were at least two RCTs available, fluoxetine, paroxetine, sertraline and venlafaxine were the only drugs found to be superior to placebo. Five other drugs were found to be superior to placebo in a single study (amitriptyline, mirtazapine, a neurokinin-1 antagonist, phenelzine and quetiapine). Of these, the quetiapine study was the only one in which more than 20 people were randomised to each arm; consequently, quetiapine was the only one of these drugs that received any form of positive recommendation in the ISTSS Guidelines (ISTSS 2018).
Hoskins et al. (2021) also considered pharmacological augmentation (i.e., adding another drug when a first drug had not worked). Thirty-four augmentation studies were identified and data from 30 of these (1566 participants) could be included in meta-analyses. Prazosin was the most commonly evaluated drug (10 studies) and, along with risperidone, was found to have a small positive effect when compared to augmentation with placebo. The only other drug evaluated for augmentation in two or more RCTs was topiramate, which was not found to be superior to placebo.
Similar meta-analyses to those undertaken by Hoskins et al. (2021) were undertaken for the other major guidelines produced similar results, but with a few key differences, for example VA/DoD (2017) did not find a convincing effect for prazosin. (Similarities and differences will be further considered in the section on guideline recommendations below.)
A relatively new advance in the statistical synthesis of RCTs is network meta-analysis. Network meta-analysis allows the comparison of both direct and indirect evidence from RCTs and, therefore, allows closer scrutiny of the likely effectiveness of some drugs with a lower number of RCTs (Rouse et al. 2017). Most meta-analyses of pharmacological treatments of PTSD, including Hoskins et al. (2021), did not use network meta-analysis but Cipriani et al. (2018) did. Although network meta-analysis involves a greater number of assumptions and, therefore, arguably a greater risk of erroneous results, than ordinary meta-analysis, the Cipriani et al. (2018) is worthy of further consideration.
Cipriani et al. (2018) used data from monotherapy and augmentation RCTs of pharmacological treatment for PTSD to develop a network including 37 different drugs, with data for eight-week follow-up from 51 RCTs. Statistically significant effects were found for (largest effect size first) phenelzine, desipramine, paroxetine, venlafaxine, fluoxetine, risperidone and sertraline. Phenelzine was found to have an effect size of 0.97 (95% CI = 1.68 to 0.27) over placebo but this was based on only one RCT that directly considered phenelzine.
Cipriani et al. (2018) correctly argued (and this is supported by the results of the Hoskins et al. (2021) and other reviews) that their work provides evidence that drugs from the same class do not have the same efficacy. This is important as it suggests that recommendations for use should be made at an individual drug level rather than according to drug class (e.g., selective serotonin reuptake inhibitors). There are interesting parallels here with cognitive behavioural treatments with a trauma focus; recent guidelines have recommended specific forms of these more than others (e.g., APA 2017; ISTSS 2018).
The RCT evidence for drugs suggests that those considered have been well tolerated overall by participants as judged by an absence of reported major adverse effects or greater dropout from studies of individuals allocated to an active drug rather than a placebo (Cipriani et al. 2018).
2.2 Key Points for Interpretation
The effect sizes found for the effective drugs in the meta-analyses considered were low; all below 0.5 (except for phenelzine and desipramine but these were from a network meta-analysis, with wide 95% confidence intervals and limited direct evidence, and should be treated more cautiously than the results from standard meta-analyses). It is important to remember that the effect sizes quoted for drugs are relative to placebo as opposed to wait list or treatment as usual, the standard comparators for psychological treatment RCTs. This is particularly important given the consistent very strong placebo effect achieved by placebo in RCTs of drugs for PTSD, which often approaches 50% itself (Davidson et al. 2006a, b). Consequently, the low effect sizes found in RCTs of drugs for PTSD are also likely associated with considerable benefit for individual participants. Many participants in RCTs of pharmacological treatments for PTSD will have experienced a greater than 50% reduction in their PTSD symptoms over the course of the study.
Another key issue with pharmacological research for PTSD is that much of it has been funded by drug companies that hold a patent for the drug being tested and generation of positive evidence should lead to monetization. Many of the drugs with limited evidence of effect have long since gone off-patent resulting in little, if any, incentivisation for drug companies to put large-scale funding into their further development. As with all interventions with limited research to explore their effectiveness, the adage that absence of evidence is not evidence of absence of effect is important to remember. For example, the superiority of amitriptyline, mirtazapine, a neurokinin-1 antagonist and phenelzine in the single RCTs undertaken for each of these agents suggests a requirement for further evaluation, not least for phenelzine given the network meta-analysis results. The same can be said for a number of other drugs where the only evidence against them being effective is from single, small, neutral trials.
3 Treatment Guidelines for PTSD
Despite different timings and different development committees, there is almost absolute consistency between current well-known and respected PTSD treatment guidelines in terms of their main pharmacological treatment recommendations (see Table 27.1). The consistency is far greater than for their main psychological treatment recommendations. All of the guidelines recommend pharmacological treatments but none of them at their highest level, which is reserved for some, but not all, trauma-focused psychological treatments.
Fluoxetine, paroxetine, sertraline and venlafaxine are in the highest rated group of medications by all guidelines although NICE recommends “venlafaxine or a selective serotonin reuptake inhibitor, such as sertraline”. Fluoxetine and paroxetine are both selective serotonin reuptake inhibitors (SSRIs) but NICE’s recommendation is somewhat concerning as there is currently insufficient evidence to recommend other commonly prescribed SSRIs such as citalopram and escitalopram.
There is considerable variation between the guidelines in terms of their second level pharmacological recommendations. This is likely to reflect the very limited evidence available, differences in the scopes of different guidelines. For example, pharmacological augmentation was outside the scope of the ISTSS guidelines (Bisson et al. 2019). NICE recommends the use of “antipsychotics such as risperidone” in some circumstances and ISTSS identifies quetiapine as a drug with emerging evidence of effect. None of the other guidelines recommend any antipsychotic; indeed, VA/DoD recommends against some antipsychotics as a monotherapy. Despite the results of the Cipriani et al. (2018) network meta-analysis, VA/DoD is the only guideline considered to recommend the use of phenelzine, albeit at a lower level than for other recommended drugs. Despite the results of the Hoskins et al. (2021) meta-analyses with respect to prazosin and risperidone, no drug is recommended for augmentation except, implicitly, for “antipsychotics such as risperidone” by NICE. VA/DoD found insufficient evidence to recommend any drug for augmentation and APA found insufficient evidence to recommend risperidone.
The PTSD guidelines considered are very helpful in as much as they give some clear indications as to which medications are most likely to help people with PTSD. They are less helpful in terms of helping clinicians know what next to prescribe and do not provide detailed descriptions of exactly how to prescribe and what dosages of medications are likely to be most helpful.
4 Pharmacological Prescribing for PTSD in Clinical Practice
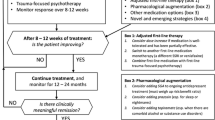
Most recommended psychological treatments for PTSD follow a relatively structured manual in terms of their delivery. The same cannot be said for pharmacological treatment beyond standard prescribing information such as dose ranges. This is likely to result in unhelpful variation that could be addressed by prescribing in a logical, standardised manner, informed by the evidence and the clinical presentation of the individual. A first step to achieving this was the development of the Cardiff PTSD prescribing algorithm (Bisson et al. 2020a). This is primarily based on the recommendations of the ISTSS guidelines and takes into account other evidence. The algorithm recognises that, faced with an absence of relevant evidence, clinicians are quickly faced with having to use evidence and clinical experience to inform practice rather than being able to be truly evidence-based.
Figure 27.1 provides a summarised version of the algorithm which can be downloaded in full from the British Journal of Psychiatry website as a supplementary document to the Bisson et al. (2020a) paper and as an appendix to the Australian guidelines in which it is included as “an example of an evidence-informed clinical tool for use in prescribing medications for PTSD” (Phoenix Australia 2020b).
4.1 Using the Algorithm
Medication should only be prescribed by clinicians with the necessary knowledge and skills to do so. Before prescribing medication, it is important that a person with PTSD has been fully assessed, their goals and needs have been fully identified, and that they have been made aware of all possible treatment options to co-produce an appropriate management plan with their clinician. With specific respect to pharmacological treatment, it is vital to ensure that the person with PTSD understands what is being offered, including its potential benefits, possible adverse effects, monitoring requirements and likely duration of treatment, in order to make a fully informed decision about whether or not to proceed.
The algorithm provides clear notes about common adverse effects and drug interactions of the medications recommended along with more detailed guidance on initiating prazosin as a result of the risk of severe first-dose hypotension. Monitoring requirements are also included. For example, for fluoxetine, paroxetine, sertraline and venlafaxine, if suicidal ideation is present prior to starting treatment this should be monitored on a weekly basis initially. For the prescription of quetiapine or risperidone, blood tests should be undertaken to check urea and electrolytes, full blood count, lipids (fasting if possible), glucose (fasting if possible) and prolactin.
Acknowledging the frequency of agitation and insomnia and the unlikely immediate impact on these symptoms of the first-line recommended drugs, it is recommended that adjunctive quetiapine (if marked agitation is present) and trazadone or mirtazapine (if insomnia is present) be considered. As described in Fig. 27.1, a key feature of the algorithm is to increase doses on a monthly basis according to clinical response and tolerability and, if ongoing clinically significant symptoms are present at the maximum tolerated dose then the next step should be followed which involves changing medication or adding another medication as augmentation.
The rationale for dose escalation according to response and tolerability was based on the finding that the mean daily dose of medications used in RCTs of pharmacological treatments for PTSD is high (e.g., fluoxetine 41.4 mg, paroxetine 35.1 mg, sertraline 136.7 mg, venlafaxine 223.1 mg and quetiapine 258.0 mg) (Bisson et al. 2020b). This strongly suggests that starting doses alone are unlikely to provide the majority of people with PTSD an optimal treatment response. The rationale is also informed by the positive findings of using a measurement/outcomes-based approach to prescribing for depression (Guo et al. 2015).
The final step in the algorithm involves considering changing to an alternative medication with a weaker evidence base but that works in a different way to the other medications suggested. Amitriptyline, mirtazapine and phenelzine all have some, albeit limited, evidence that they may be effective for symptoms of PTSD. These drugs are now off-patent and have not been subjected to the further evaluation their signals of efficacy could have justified. Amitriptyline and phenelzine are older antidepressants; amitriptyline in the tricyclic family of antidepressants and phenelzine a monoamine oxidase inhibitor. Both were originally approved by the US Food and Drug Administration in 1961 and need to be prescribed very cautiously due to the risk of adverse effects and interactions.
4.1.1 Algorithm Notes
-
1.
If a person with PTSD is already on psychotropic medication, this should be reduced and stopped as per BNF guidance before starting an alternative.
-
2.
From the start of treatment consider adjunction of SSRI with:
-
Quetiapine—If marked agitation present.
-
Trazadone 50 mg–100 mg night/Mirtazapine 15 mg night—If insomnia present.
-
-
3.
Side effect profile is similar for all SSRIs, however notable considerations to make when choosing SSRI:
-
Sertraline: Generally fewer side effects.
-
Fluoxetine: More alerting—potentially less suited if person with PTSD is agitated at start.
-
Paroxetine: Greater risk of discontinuation symptoms.
-
-
4.
SSRIs/SNRIs have many drug interactions—even with common drugs used to manage rudimentary illnesses. Therefore, it is important to be fully aware of what concomitant medications the person with PTSD is on before initiating treatment.
Here is a brief outline of some common drug interactions with SSRIs/SNRIs and their potential consequences if co-prescribed:
-
Other serotonergic drugs = Increased risk of serotonin syndrome.
-
Drugs that affect haemostasis (e.g. Aspirin and NSAIDs) = Increased risk of bleeding (especially Upper GI).
-
Drugs inducing hyponatraemia (e.g. Diuretics) = Increased risk of developing hyponatraemia.
-
Other drugs metabolised by CYP2D6.
For a full and detailed outline of the drug interactions for SSRIs/SNRIs and for the other drugs named in the algorithm, please visit https://bnf.nice.org.uk.
-
-
5.
Initiating Prazosin:
As there is a risk of severe first-dose hypotension, the first and second doses should be taken whilst sitting on a bed just before lying down. It is important to keep well hydrated whilst taking prazosin and to get up slowly—initially sitting up on the bed and then slowly standing up. For the first two nights, it is important to sit on the toilet to pass water rather than stand up.
Time | Morning (mg) | On going to bed (mg) |
|---|---|---|
Days 1–2 | Nil | 1 |
Days 3–7 | Nil | 2 |
Week 2 | 1 | 4 |
Week 3 | 2 | 6 |
Week 4 | 2 | 10 |
-
6.
Risperidone also has evidence to be used instead of Prazosin or Quetiapine in adjunctive therapy.
-
7.
Quetiapine has been used at a maximum dosage of 800 mg/day in PTSD research studies. However, the mean dose of Quetiapine used in people with PTSD in the research studies was 258 mg/day, therefore a lower maximum dose has been recommended in this algorithm although some individuals may benefit from higher doses. It may therefore be appropriate to use higher doses in some instances; the decision should be made based on the clinician’s judgement.
4.1.2 Common Adverse Effects


Case Examples
The four case examples below provide brief summaries of four people with PTSD/complex PTSD who experienced symptom improvement associated with pharmacological treatment. Not everyone will benefit from pharmacological treatment, even though their adherence is excellent. In such instances, having exhausted all indicated and/or desired steps of the prescribing algorithm, it is important to help individuals to reduce and stop medication that is not working. No medication is risk-free and it is not in anyone’s best interests to be taking medication that is not beneficial. Indeed, sometimes people will be better off not taking medication that is helping to a degree, as the impact of adverse effects outweighs the impact of benefits. Undertaking a cost-risk benefits analysis of taking a drug can be a very helpful way to determine if continuation is appropriate.
Bronwyn
Bronwyn, a 40-year-old nurse, developed PTSD after witnessing the unexpected death of a patient she was looking after during the COVID-19 pandemic. She consulted her General Practitioner who diagnosed her PTSD and described the treatment options available, including trauma-focused psychological therapy. After considering the options, Bronwyn decided that, despite its stronger recommendation, she did not want to pursue psychological therapy at that juncture but would rather try medication in the first instance. She was not taking any other medication and it was agreed that she would commence sertraline 50 mg daily, to be taken in the morning after food. A month later she returned to her doctor. She had experienced some nausea in the first 2 weeks of taking sertraline, but this had settled, and she was pleased that her PTSD symptoms had reduced to a degree. She did, however, describe some ongoing symptoms of PTSD. She and her doctor agreed that she should increase the dose of sertraline to 100 mg, which she did. A month later she was continuing to tolerate sertraline and described feeling much better, with mild residual PTSD symptoms only. Subject to her continuing to tolerate sertraline, her doctor recommended that she continue it for a year and, if she remained well, to gradually reduce and try to stop it thereafter.
Jeff
Jeff, a 60-year-old army veteran, described multiple traumatic events during his military service during the 1980s that precipitated PTSD. He had been taking paroxetine 40 mg daily for many years and had recently experienced some benefit from a course of cognitive behaviour therapy with a trauma focus. He and his therapist had decided that he was unlikely to make further significant gains with further psychological treatment and he was keen to explore alternative pharmacological treatment approaches to further reduce his PTSD symptoms. He met with a psychiatrist and they discussed treatment options. He had tried a higher dose of paroxetine once before but did not tolerate this and, therefore, was keen to try an alternative medication. It was agreed that it would be appropriate to slowly reduce his paroxetine and cross-taper with venlafaxine after checking his blood pressure.
Two months later, Jeff had successfully stopped paroxetine and was now taking and tolerating venlafaxine 150 mg daily. Unfortunately, he reported no real change in his PTSD symptoms. Indeed, he had felt a bit worse whilst changing medications. It was agreed that he should increase venlafaxine to 225 mg; a month later he reported a slight improvement and it was agreed he should increase venlafaxine to 300 mg but this was associated with more nausea and sexual dysfunction and when seen a month later he had reduced back to 225 mg of his own accord. After further discussion, it was agreed that augmentation would be tried with prazosin. Following the algorithm, he was able to increase prazosin to 2 mg in the morning and 6 mg at night with some benefit but was not keen to further increase the dose. It was noted that he had achieved a further small reduction in his symptoms and was happy with the current level of control he had achieved. He was keen to continue on this combination of medication, without making further changes, and it was agreed that he would be referred back to his General Practitioner to provide ongoing monitoring and prescribing.
Peter
Peter, a 45-year-old man who was subjected to repeated sexual abuse by a member of staff at the boys’ club he attended between the ages of 10 and 15, was diagnosed with complex PTSD by a psychiatrist who assessed him. He had received a lot of psychological and pharmacological treatments over the years, including high doses of selective serotonin reuptake inhibitors and venlafaxine, along with antipsychotic medication in the form of olanzapine. Some skills training work had helped him better regulate his emotions and feel more comfortable with interpersonal relationships, but he did not feel that any medication had helped him, and he did not like the side effects of the drugs he had taken. He had stopped all prescribed medication around 6 months previously and was not using any psychoactive substances. He was keen to try a different pharmacological treatment for his symptoms.
Treatment alternatives were discussed with Peter. After considerable discussion, he decided that he would like to try phenelzine. The psychiatrist provided Peter with detailed information about phenelzine, not least about its potential to interact with certain foodstuffs and alcoholic beverages resulting in potentially fatal raised blood pressure, and side effects such as postural hypotension. Peter remained keen to try phenelzine and was clear that he would adhere to the dietary restrictions whilst taking it. Phenelzine was commenced at 15 mg three times a day.
Peter experienced some reduction in his symptoms within 2 weeks of starting phenelzine and this improvement was maintained thereafter. Peter continued to experience ongoing symptoms of complex PTSD but described phenelzine as “taking the edge” off them and making them more manageable. He was better able to tolerate phenelzine than other medications he had taken and reported no problematic side effects. Peter agreed with his psychiatrist that he would continue on phenelzine for the foreseeable future, with regular monitoring of his mental and physical health.
Cerys
Cerys, a 30-year-old woman, was subjected to a horrific rape 2 years previously and developed PTSD as a result. She felt totally unable to discuss what had happened and could not engage in trauma-focused psychological therapy; she had tried to on one occasion but felt so overwhelmed she did not continue with this. Cerys had, however, developed a strong therapeutic relationship with her therapist and managed to engage in some non-trauma-focused work. She found controlled breathing techniques and deep muscular relaxation helpful. She and her therapist were very concerned by the impact her ongoing PTSD symptoms were having on her and, having not initially wanted to try pharmacological treatment, Cerys decided she would now consider it.
Cerys was referred to a psychiatrist by her therapist and, after discussion, agreed that she would try pharmacological treatment in line with the prescribing algorithm. She initially tried fluoxetine and then sertraline but stopped both of these within a few weeks of starting them, as they were associated with her experiencing some suicidal thoughts. After further discussion with the psychiatrist, it was agreed that it would not be appropriate to try paroxetine or venlafaxine as they have similar side-effect profiles. Cerys did not want to try quetiapine but was interested in trying mirtazapine instead. Mirtazapine was commenced at 15 mg at night and Cerys immediately reported some improvement in her sleep but described ongoing significant symptoms of posttraumatic stress disorder when reviewed by the psychiatrist at 1 month. They agreed that she would increase the dose to 30 mg at night and a month later Cerys noticed that her PTSD symptoms had reduced and, although still present, were more manageable.
Cerys remained in contact with her therapist and 3 months after starting mirtazapine was able to engage in trauma-focused psychological therapy. Having completed this psychological treatment 4 months later she was feeling much better with some mild residual symptoms of PTSD and was keen to continue to take mirtazapine 30 mg at night.
5 Summary
There is strong evidence to suggest that some pharmacological treatments are effective at reducing symptoms of trauma-related psychological disorders and have a role in their management. Despite the need for further research, there is ample knowledge to inform an evidence-based approach to prescribing and this is likely to harness the positive potential of pharmacological treatment for PTSD and other trauma-related psychological disorders.
Notes
- 1.
Taylor D, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. Newark: John Wiley & Sons, Incorporated; 2018.
References
American Psychological Association (2017) Clinical practice guideline for the treatment of PTSD. https://www.apa.org/ptsd-guideline/ptsd.pdf. Accessed 3 Feb 2021
Astill Wright L, Sijbrandij M, Sinnerton R, Lewis C, Roberts NP, Bisson JI (2019) Pharmacological prevention and early treatment of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Transl Psychiatry 9:334
Bisson JI, Berliner L, Cloitre M, Forbes D, Jensen TK, Lewis C et al (2019) The International Society for Traumatic Stress Studies new guidelines for the prevention and treatment of PTSD: methodology and development process. J Trauma Stress 32:475–483
Bisson JI, Baker A, Dekker W, Hoskins M (2020a) Evidence-based prescribing for post-traumatic stress disorder. Br J Psychiatry 216:125–126
Bisson JI, Hoskins MD, Stein DJ (2020b) Pharmacological and other biological treatments of PTSD in adults. In: Forbes D, Bisson JI, Monson CM, Berliner L (eds) Effective treatments for PTSD, 3rd edn. Guilford, New York
Cipriani A, Williams T, Nikolakopoulou A, Salanti G, Chaimani A, Ipser J et al (2018) Comparative efficacy and acceptability of pharmacological treatments for post-traumatic stress disorder in adults: a network meta-analysis. Psychol Med 48:1975–1984
Coventry PA, Meader N, Melton H, Temple M, Dale H, Wright K et al (2020) Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: Systematic review and component network meta-analysis. PLoS Med 17:e1003262
Davidson J, Rothbaum BO, Tucker P (2006a) Venlafaxine extended release in posttraumatic stress disorder: a sertraline and placebo controlled study. J Clin Psychopharmacol 26:259–267
Davidson J, Baldwin D, Stein DJ, Kuper E, Benattia I, Ahmed S (2006b) Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6 month randomized controlled trial. Arch Gen Psychiatry 63:1158–1165
Department of Veterans Affairs and Department of Defense (VA/DoD) (2017) VA/DoD clinical practice guideline for the management of posttraumatic stress disorder and acute stress disorder. https://www.healthquality.va.gov/guidelines/MH/ptsd/VADoDPTSDCPGFinal012418.pdf. Accessed 3 Feb 2021
Guo T, Xiang Y, Xiao L, Hu C, Chiu HFK, Ungvari GS et al (2015) Measurement-based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry 172:1004–1013
Hoskins MD, Bridges J, Sinnerton R, Nakamura A, Underwood JFG, Slater A et al (2021) Pharmacological therapy for post-traumatic stress disorder: a systematic review and meta-analysis of monotherapy, augmentation and head-to-head approaches. Eur J Psychotraumatol 12(1):1802920
International Society for Traumatic Stress Studies (ISTSS) (2018) ISTSS PTSD prevention and treatment guidelines: methodology and recommendations. https://istss.org/getattachment/Treating-Trauma/New-ISTSS-Prevention-and-Treatment-Guidelines/ISTSS_PreventionTreatmentGuidelines_FNL-March-19-2019.pdf.aspx. Accessed 3 Feb 2021
National Institute for Health and Care Excellence (2018) Post-traumatic stress disorder NICE guideline [NG116]. https://www.nice.org.uk/guidance/ng116. Accessed 3 Feb 2021
Phoenix Australia (2020a). Australian guidelines for the prevention and treatment of acute stress disorder, posttraumatic stress disorder and complex PTSD. https://www.phoenixaustralia.org/australian-guidelines-for-ptsd. Accessed 3 Feb 2021
Phoenix Australia (2020b) Medication prescribing algorithm for the treatment of PTSD. https://www.phoenixaustralia.org/wp-content/uploads/2020/07/Chapter-6.-Appendix-Medication-prescribing-algorithm.pdf. Accessed 3 Feb 2021
Rouse B, Chaimani A, Li T (2017) Network meta-analysis: an introduction for clinicians. Intern Emereg Med 12:103–111
Yehuda R, Giller EL, Southwick SM, Lowy MT, Mason JW (1991) Hypothalamic-pituitary-adrenal dysfunction in posttraumatic stress disorder. Biol Psychiatry 30:1031–1048
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bisson, J.I. (2022). Pharmacological Treatment for Trauma-Related Psychological Disorders. In: Schnyder, U., Cloitre, M. (eds) Evidence Based Treatments for Trauma-Related Psychological Disorders. Springer, Cham. https://doi.org/10.1007/978-3-030-97802-0_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-97802-0_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-97801-3
Online ISBN: 978-3-030-97802-0
eBook Packages: MedicineMedicine (R0)