Abstract
The objective of this study is to characterize physical and chemical properties and antioxidant activity of milk peptide fractions, resulting from partial hydrolysis with serine endopeptidases (alcalase and trypsin). Protein and peptide profile of the cleaved milk proteins was determined according to electrophoretis analysis in polyacrylamide gel and chromato-mass-spectrometry. Fluorometric method was used to determine antioxidant activity of hydrolysates and native dairy substrates. Comparative analysis of whey, milk and colostrum hydrolysates obtained with alcalase and trypsin was carried out. Based on experimental study, alcalase was chosen to produce colostrum hydrolysates with average and extensive degree of hydrolysis, and high antioxidant effect. New data on the features of enzymatic cleavage of whey and casein protein fractions from whey, milk and colostrum, and the level of their antioxidant activity were obtained. Milk protein partial hydrolysates with low antigenicity and high antioxidant potential are prospective components of specialized foodstuffs (infant, dietary and sport formulas).
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Proteolysis, or the cleavage of peptide bonds with specific enzymes (proteases), permits the development of protein hydrolysates with improved nutritional and bioactive characteristics [13, 27, 32]. Serine proteases, characterized by the presence of a serine residue in the catalytic center, belong to the most common class of peptidases with industrial importance [1, 25, 32]. Alcalase and trypsin, which are serine proteases of bacterial and animal origin, respectively, possess activity under neutral and alkaline conditions and exhibit wide substrate specificity. Trypsin predominantly cleaves peptide bonds in the carboxyl site of amino acids Arg and Lys, while alcalase has broad site-specificity (preferably a large uncharged residues) [4, 27, 28].
Milk proteins are potential source of bioactive peptides possessing antihypertensive, antioxidant, antithrombotic, opioid, and immunomodulating action. However, whey and casein fractions possess high allergenic potential, which is important in the development of safe milk products for infant food [2, 6, 31]. Biologically active properties of cleaved protein are determined by hydrolysis degree and enzyme used [3, 5]. It should be noted that careful choice of protease and filtration techniques can significantly reduce protein allergenicity. The molecular mass cut-off value of the filters must be in range 3–5 kDa to remove allergenicity of whey protein hydrolysates [30]. The previous study showed an increase in the antioxidant activity and the reduced antigenicity of whey and colostrum proteins with an enhancement in the degree of their proteolysis. The colostrum hydrolysates obtained with alcalase had a greater antioxidant potential than neutrase [7, 8]. In accordance with previous experimental [8] and literature data [16], temperature 50–60 ℃ and pH 8 are optimal for obtaining tryptic and alcalase hydrolysates of whey proteins with active acidity preferred for food components (pH 6.5–7.0 at the end of enzymatic process). The optimal technological process usually include the minimum number of stage, however ultrafiltration is obviously a necessary step to preparing safe hypoallergenic dairy hydrolysates [5, 6].
The aim of this work is a comparative study of partial hydrolysates of milk proteins obtained using serine proteases (alcalase and trypsin), characterization of their protein and peptide composition and antioxidant activity.
2 Materials and Methods
Concentrate of whey proteins (specifications BY 100377914.550–2008), dry defatted milk (STB 1858–2009), dry defatted colostrum (VNIMI, Moscow, Russia), alcalase (EC 3.4.21.62, Alcalase® 2.4L, activity 2.64 U/g; Sigma, USA), and trypsin (EC 3.4.21.4, ≥6.0 BAEE U/g; Sigma, USA) were applied in proteolysis reaction. Hydrolysis of milk proteins was carried out at enzyme/substrate ratio 0.25–8.0%, pH 7.4/8.0, temperature 50 ºC during 2–3 h. Filters Spin X–UF Concentrator 20 (cut-off value 5/10 kDa; Corning, UK) were used in samples fractionation.
Protein and peptide profile of obtained hydrolysates was defined by native and denaturing (with sodium dodecyl sulfate, SDS) electrophoresis in polyacrylamide gel [18]. Processing of gels was conducted using gel-documentation system Image Master VDS-SL and ImageMaster ID Software 4.20 (Amersham Bioscience, USA). Protein amount in experimental samples was determined according to calibration β-lactoglobulin (β-lg), α-lactalbumin (α-la), and bovine serum albumin (BSA) plots. The depth of proteolysis (%) was determined as the ratio of hydrolysed protein content in test sample to total protein amount in control sample. Total (TN) and α-amino nitrogen (AN) content in experimental samples were defined according to ISO 8968–1: 2014 [14] and ISO 11402: 2004 [20], respectively, while solids ratio – in accordance with ISO 6731: 2010 [15]. Chromato–MS system Agilent 1290 (Agilent, USA) with mass spectrometric detector Q–TOF 6550 (ESI+ mode) was used to obtain mass spectra, spectral recording range was 100–3200 m/z. Antioxidant activity (AOA) was evaluated with fluorimetric method according to the technique developed by E.I. Tarun (2014) [26], and ABTS•+ method as described by B. Hernández-Ledesma et al. (2007) [10]. The results of independent experiments were presented as the mean value ± confidence interval (p ≤ 0.05, n = 3).
3 Results
This work presents variants for obtaining hydrolysates of whey proteins with average degree of hydrolysis using serine proteases. Enzymatic cleavage of 2% whey solution with trypsin/alcalase was performed at protease amount 0.02%, pH 8, and temperature 50 C for 2 h. Obtained samples were analyzed by electrophoresis.
The protein and peptide profile of tryptic hydrolysates was studied depending on the reaction duration (15–120 min). According to native electrophoresis after 60 min of proteolysis, β-lg and α-la were not detected in the samples, whereas bovine serum albumin (BSA) was retained throughout the enzymatic process (Table 1). The SDS-electrophoregrams of whey samples hydrolyzed for 120 min reveal trace amounts of native whey proteins, except for BSA, whose content corresponds to that of the original level (Fig. 1A, lane 6).
Moreover, under these conditions trypsin most effectively cleaves β-lg, than α-la. Analysis of the peptide fraction of whey hydrolysate with trypsin showed the formation of intermediate peptides with 6.5 ≤ molecular range (mr) < 14.2 kDa, whose quantity decreased throughout the enzymatic process (Fig. 1A, lanes 2–6).
In the case of alcalase, proteolysis was performed under similar conditions. It was shown that within 90 min almost all β-lg and α-la were hydrolyzed (Table 1), and after 120 min of the enzymatic reaction only trace amounts of the predominant whey proteins and native BSA were detected (Fig. 1B, lane 6). Analysis of the peptide composition of whey hydrolysate with alcalase showed the formation of a discrete peptide fraction with mr < 6.5 kDa, the amount of which decreases significantly from 90 to 120 min of proteolysis (Fig. 1, lanes 2–6). Under the experimental terms, alcalase quite effectively cleaves β-lg and α-la, except for BSA.
According to electrophoretic analysis, the serine endopeptidases studied efficiently hydrolyze β-lg, which is the main milk allergen, and α-la. Different hydrolysis intermediates were detected in the whey samples obtained using alcalase and trypsin (Fig. 1A, lane 6; Fig. 1B, lane 6). However, the hydrolysis degree (AN/TN) with trypsin (18.2 ± 0.7) and alcalase (17.8 ± 1.1)% was comparable.
In the case of BSA hydrolysis, the use of these serine proteases is inefficient. Consequently, there was a necessity for an additional stage of BSA removal from hydrolysates using filters with a permeability ≤10 kDa. Ultrafiltrates of whey hydrolysates with trypsin and alcalase were obtained and analyzed. According to competitive ELISA, a decrease in residual antigenicity of hydrolysate samples as well as an increase in their antioxidant potential in the ABTS•+–Trolox test system were found (Table 1). In general, ultrafiltrates of whey hydrolysates (mr ≤ 10 kDa) represent a protein component with low antigenic potential and high AOA.
The features of milk proteins (casein and whey fractions) hydrolysis with alcalase and trypsin at enzyme/substrate ratio of 0.25–8.0%, pH 8, temperature 50 ℃ for 2 h were determined at the next stage. It was found that alcalase and trypsin effectively cleaved the casein fraction. Thus, native casein was not detected at an enzyme/substrate ratio of 0.25% in trypsin hydrolysate and 1% in alcalase sample (Fig. 2). The whey fraction was actively treated with alcalase and to a lesser extent with trypsin. Partial proteolysis products were detected in all trypsin hydrolysate samples (Fig. 2A), whereas protein compounds with mr < 6.5 kDa were not detected on the electrophoregram at alcalase concentration ≥4% (Fig. 2B).
The proteolysis depth of milk proteins depending on the enzymatic process duration with alcalase was studied. Hydrolysis was carried out at defatted milk concentration 5%, enzyme/substrate ratio 1%, pH 8, and temperature 50 ℃ for 2 h. During the first 60 min of the enzymatic reaction, the casein fraction cleaved into intermediate peptides, whereas the whey fraction was almost completely hydrolyzed after 2 h (Fig. 2C). In addition, cleavage of the peptide fraction with increasing proteolysis duration up to 120 min, as well as an increase in the amount of α-amino nitrogen from 31.5 to 56 mg% were showed for alcalase hydrolysates.
The high site-specificity of trypsin (Arg and Lys) which accounts for the formation of intermediate high-molecular-mass hydrolysis products with mr > 6.5 kDa (Table 1, Fig. 1A) should be noted. At the same time, the broad site-specificity of alcalase determines the cleavage of substrates into multiple peptides with the formation of discrete peptide fractions, which undergo further cleavage with increasing duration of hydrolysis (Table 1; Fig. 1B, 2B) and endopeptidase concentration (Fig. 2C). Due to the high proteolytic activity of the commercial enzyme preparation alcalase, its broad substrate and site-specificity, stability during storage, and affordability, this endopeptidase was chosen to obtain partial hydrolysates of whey proteins with average as well as extensive hydrolysis degree.
A way of producing hydrolysate of defatted colostrum with alcalase was proposed. Enzymatic protein cleavage was carried out at protein substrate concentration 3% and enzyme/substrate ratio 1%, temperature 50 ℃, pH 8 for 3 h. Filters with separation capacity 10 kDa were used for fractionation of hydrolysates.
The electrophoregram shown in Fig. 2D reflects the typical composition of first milk. For samples of native colostrum, a relatively high content of immunoglobulins (Igs), the amount of casein comparable with milk, the presence of α-la, β-lg and other proteins of the whey fraction (BSA and lactoferrin) were shown.
According to SDS-electrophoregram (Fig. 1A), almost complete proteolysis of β-lg, α-la, and minor proteins into intermediate peptides was established in the whey hydrolysate. Numerous products of partial immunoglobulin proteolysis were detected in colostrum hydrolysate, and cleavage of casein fraction, β-lg, α-la, and minor whey proteins was established (Fig. 2D). According to total protein determination, unfiltered colostrum hydrolysate contains (29.1 ± 1.2)% of the fraction with mr ≤ 10 kDa. Thus, due to the content of partial proteolysis products of the Igs fraction with mr > 10 kDa, cleaved colostrum contains a smaller amount of low-molecular-mass component than whey hydrolysate.
As part of the optimization of obtaining enzymatic whey and colostrum hydrolysates with extensive cleavage degree, a higher enzyme/substrate ratio 5% as well as filters with a separating capacity 5 kDa were used to increase the content of the low-molecular-mass peptide fraction. Thus, according to an earlier experiment related to the defatted milk hydrolysate preparation, intermediate proteolysis products were not detected at enzyme/substrate ratio ≥4% (Fig. 2C).
During the process of preparing extensive colostrum hydrolysates enzymatic cleavage was carried out at enzyme/substrate ratio 5%, temperature 50 ℃, pH 7.4 for 3 h, the obtained samples were subjected to filtration with permeability 5 kDa. The content of low-molecular-mass fraction (mr ≤ 5 kDa) in unfiltered extensive hydrolysates of whey and colostrum was found to reach (39.6 ± 0.5) and (30.3 ± 0.2)%, respectively. The obtained data indicate an increase in the cleaved component proportion in whey hydrolysate by 1.3 times in compare to hydrolysed colostrum.
According to chromato-mass spectrometry, differences in the peptide fraction were found in the range 100–1500 Da. High signal level was established for single-charged ions with m/z values 680–900 Da, which is proportional to whey peptides of 6–8 amino acid residues length. The maximum signal per the mass spectra was established at m/z values 560–650 (5–6 amino acid residues). Thus, peptides with a lower molecular mass are characteristic of the colostrum sample. The obtained data are due to differences in the composition of the protein component of whey and colostrum, as well as to the peculiarities of substrate and site-specific of alcalase.
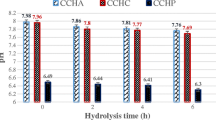
Comparative analysis of the antioxidant activity of whey and colostrum hydrolysates (ultrafiltrates 5 and 10 kDa) by ORAC and ABTS•+ methods is shown in Fig. 3. A decrease in IC50 (sample concentration corresponding to 50% inhibition of reactive oxygen species) indicates improving AOA of the tested compound. Calculations were made according to protein and solids content in the samples.
The differences in AOA level of whey and colostrum are caused by the peculiarities of protein-peptide composition (the ratio of casein and whey proteins) and the content of non-protein component. Thus, the amount of protein in whey reaches 80%, while defatted colostrum – 70%. In addition, colostrum is enriched with non-protein component (vitamins, minerals) with antioxidant potential.
Significant AOA increase in comparison with native substrates was found for samples of hydrolyzed whey and colostrum. The maximum antiradical effect (per protein content) was shown in experiments with filtrates of cleaved colostrum. In general, samples of hydrolysed whey and colostrum with different degree of hydrolysis were obtained, possessing a confirmed antioxidant potential.
4 Discussion
According to the literature [4, 16, 19] and own research [7,8,9], the choice of the suitable enzyme provides a specific peptide profile and the depth of hydrolysis of protein substrates. An increase in hydrolysis degree of milk proteins leads to the raise in their antioxidant activity and a decrease in residual antigenicity [17, 22, 29, 33, 34].
Extensive hydrolysate of whey proteins was characterized by Doucet et al. (2003) [4]. The 20% whey solution (pH 8.0) was hydrolyzed with alcalase at temperature 45 ℃ and enzyme/protein ratio 1:10 for 5 h. High proteolysis of whey isolate with alcalase caused gelation mainly through hydrophobic interactions. Aggregates were formed by low-molecular-mass peptides (<2 kDa) the share of which reached 80%. 130 peptides were identified in accordance with reversed-phase high-performance liquid chromatography (HPLC) and mass spectrometry [4].
M.V.T. Mota et al. (2004) [16] analyzed the hydrolysis products of whey proteins with alcalase, trypsin and pepsin obtained at different pH and temperature values (37 ℃ and pH 7–8–9, pH 8 and 30–37–50 ℃). Proteolysis of β-lg and α-la to intermediate peptides was retrieved in all experimental samples cleaved using alcalase. 12 major peptide fractions were revealed on HPLC profiles of alcalase hydrolysate, while 3 and 9 fractions for pepsin and trypsin, respectively [16].
According to studies [19, 34], the 5% whey solution was cleaved at 50 ℃, pH 5.5/7.0/8.0 for papain/neutrase/alcalase during 4–5 h, the enzyme ratio was 3% (relative to protein amount). The amino acid content of papain-treated hydrolysates was higher than that of alcalase, whereas free cysteine and proline were not detected after alcalase hydrolysis [19]. It was shown that cleavage with alcalase could reduce the antigenicity of β-lg and α-la effectively. Temperature and pH had the greatest effect on anti-α-la and anti-β-lg IgG binding inhibition, respectively [34].
Antioxidant activity of whey hydrolysates obtained with different proteases was evaluated [33]. Whey protein solution (3%) was preheated at 90 ℃ for 5 min, and then hydrolysis was performed using various proteases at enzyme/substrate ratio 2%, pH 8.0/50 ℃ (trypsin), pH 7.0/55 ℃ (papain), pH 8.5/65 ℃ (alcalase), pH 7.0/50 ℃ (protamex), pH 7.0/50 ℃ (flavourzyme), and pH 7.0/55 ℃ (protease N) during 4 h. The pretreatment increased the hydrolysis degree of whey protein with all enzymes, and alcalase-treated samples possessed the highest AOA [33]. In other study, peptides from whey proteins were liberated using neutrase, corolase PP, alcalase or flavourzyme. The obtained hydrolysates showed a high antiradical properties and a potential positive action on cultured human endothelial cells [17]. The maximum hydrolysis degree (63%) and the highest AOA were obtained for alcalase-treated whey as compared to flavourzyme and enzyme combination [22].
In the present study, greater antioxidant action was shown for the whey proteins cleaved with alcalase than for the tryptic hydrolysate (Table 1). The data on the high antiradical potential of the alcalase-treated samples are consistent with the results of several literature sources [17, 22, 29, 33]. The intensive hydrolysis of the casein and whey fractions using alcalase with the formation of numerous low-molecular-mass peptides presented in electrophoregrams (Fig. 1 and 2) should be noted, which explains the low antigenic potential of the proteolysis products (Table 1). The broad substrate and site-specificity of alcalase in the cleavage of milk proteins were uncovered in a number of scientific studies [4, 16, 19, 34].
There is a known method of whey protein hydrolysate production with average degree of hydrolysis [11], which includes preparation of 5% solution of whey protein (pH 7.9–8.3), hydrolysis with pancreatine at enzyme/substrate ratio 1.5–2.5%, temperature 48–54 ℃ for 3.0–3.5 h, and ultrafiltration with membrane permeability 20 kDa. The method is aimed at obtaining whey protein hydrolysate with high biological value, improved organoleptic properties and reduced allergenic potential.
A new method of obtaining partial hydrolysate of whey and colostrum proteins which includes using alcalase at enzyme/substrate 1%, hydrolysis at pH 7.4/8.0, temperature 50 ℃ for 2–3 h, and subsequent ultrafiltration with separation of the non-hydrolysed fraction with mr > 10 kDa was proposed. The resulting hydrolysate has reduced antigenicity and high antioxidant potential.
The method [23] also involves the use of alcalase, but in a larger amount (10% of protein mass). However, the application of 20 kDa filters [11, 23] does not allow the separation of residual non-cleaved whey proteins (β-lg and α-la with mr 18.4 and 14.2 kDa, respectively). It should be note the method [21], which includes hydrolysis with protamex or alcalase (4% by whey solids) at pH 7.5–8.0, temperature 40–55 ℃ for 4 h, and subsequent use of the membranes with discharge capacity 10 kDa.
Methods for obtaining hydrolysates of whey proteins with a high degree of hydrolysis were proposed [12, 24]. According to the method [12], proteolysis is carried out using the enzyme preparation flavourzyme at enzyme/substrate ratio 5% for 20 h. Then the resulting crude hydrolysate is ultrafiltrated on membranes with molecular masses of 5 kDa and 2 kDa. The final hydrolysate is lactose-free with more than 75% protein concentrated on low-molecular-mass fraction (0.5–2.0 kDa).
Other method of extensive whey hydrolysate production with low residual antigenicity [24] includes introduction of enzyme composition of pancreatin 1.5–2.0%, protamex 0.5–1.0% and flavourzyme 2.0–2.5%, performing hydrolysis at 45–48 ℃ during 18–24 h till amine nitrogen content 500–600 mg%. The resulting hydrolysate is low-lactose, and more than 80% of the protein fraction is presented by low-molecular-mass peptides and free amino acids (<2.0 kDa).
Preparation of extensive whey and colostrum hydrolysates proposed in present paper provides the use of highly active protease alcalase at enzyme/substrate ratio 5%, hydrolysis at pH 7.4/8.0, temperature 50 ℃ for 2–3 h, and subsequent ultrafiltration for separation of the fraction with mr > 5 kDa. The method makes it possible to obtain a hypoallergenic protein component with high antioxidant activity.
5 Conclusion
In this work, the use of serine proteases (alcalase and trypsin) for obtaining partial hydrolysates of whey and milk with average degree of hydrolysis was proposed, as well as the necessity of ultrafiltration with separation of fractions over 10 kDa was proved. At the same time, hydrolysis of colostrum is advisable to be performed with alcalase possessing broad site-specificity, which is directed to increase the amount of the cleaved whey fraction. Obtaining extensive hydrolysates of whey and colostrum involves the use of alcalase under optimized conditions, followed by ultrafiltration with separation of the fraction with a molecular mass over 5 kDa. Alcalase hydrolysis produces a wide range of low-molecular-mass peptides, while ultrafiltration removes the non-cleaved protein fraction. Experimental samples of whey and colostrum hydrolysates have reduced allergenic potential and confirmed antioxidant activity. In general, the work presents a comparative study of partial hydrolysates of dairy proteins (whey, milk and colostrum) obtained using alcalase and trypsin, characterization of their protein and peptide profile and antiradical properties.
The practical significance of the work consists in obtaining partial hydrolysates of milk proteins with different degree of hydrolysis, which can be used as a component with high nutritional and biological value to create new specialized products. The prospect of further research is connected with the characterization of bioactivities spectrum of partial whey and colostrum hydrolysates.
References
Chandrasekaran, M. (ed.): Enzymes in Food and Beverage Processing (S. I, II). Taylor & Francis Group, London, UK (2016)
Chourasia, R., Phukon, L.C., Singh, S.P., Rai, A.K., Sahoo, D.: Role of enzymatic bioprocesses for the production of functional food and nutraceuticals. Biomass, Biofuels, Biochemicals, pp. 309–334. Elsevier B.V, Amsterdam, Netherlands (2020)
Dos Santos Aguilar, J.G., Sato, H.H.: Microbial proteases: production and application in obtaining protein hydrolysates. Food Res. Int. 103, 253–262 (2018)
Doucet, D., Otter, D.E., Gauthier, S.F., Foegeding, E.A.: Enzyme-induced gelation of extensively hydrolyzed whey proteins by alcalase: peptide identification and determination of enzyme specificity. J. Agric. Food Chem. 51(21), 6300–6308 (2003)
Du Toit, G., et al.: Identifying and managing cow’s milk protein allergy. Arch. Dis. Childhood – Educ. Pract. 95(5), 134–144 (2010)
El-Agamy, E.I.: The challenge of cow milk protein allergy. Small Rumin. Res. 68(1–2), 64–72 (2007)
Halavach, T.M., Dudchik, N.V., Tarun, E.I., Zhygankov, V.G., Kurchenko, V.P., Romanovich, R.V., et al.: Biologically active properties of hydrolysed and fermented milk proteins. J. Microbiol. Biotechnol. Food Sci. 9(4), 714–720 (2020)
Halavach, T.M., Kurchenko, V.P.: Milk protein hydrolysis with enzyme preparation and proteolytic systems of lactic acid bacteria. Proc. BSU 7(1–2), 106–126 (2012). (in Russian)
Halavach, T.N., Kurchenko, V.P., Zhygankov, V.G., Evdokimov, I.A.: Determination of physicochemical, immunochemical and antioxidant properties, toxicological and hygienic assessment of whey protein concentrate and its hydrolysate. Foods Raw Mater. 3(2), 105–114 (2015)
Hernández-Ledesma, B., Quirós, A., Amigo, L., Recio, I.: Identification of bioactive peptides after digestion of human milk and infant formula with pepsin and pancreatin. Int. Dairy J. 17(1), 42–49 (2007)
Kruglik, V.I., Zorin, S.N., Gmoshinskij, I.V., Ponomarev, D.V., Nikitina, N.E., Abramova, A.A., et al.: Patent of Russia 2375910 (2009)
Kruglik, V.I., Zorin, S.N., Gmoshinskiy, I.V., Nikitina, N.Ye., Volkova, I.N., Revyakina, N.V., et al.: Patent of Russia 2428047 (2011)
Lee, A., Pontin, M.C.F., Kosmerl, E., Jimenez-Flores, R., Moretti, D.B., Ziouzenkova, O.: Assessment of adipogenic, antioxidant, and anti-inflammatory properties of whole and whey bovine colostrum. J. Dairy Sci. 102, 8614–8621 (2019)
Milk and milk products: Determination of nitrogen content. Part 1: Kjeldahl principle and crude protein calculation. ISO 8968–1:2014. International Organization for Standardization, Geneva, Switzerland (2014)
Milk, cream and evaporated milk: ISO 6731:2010. Determination of total solids content (Reference method). International Organization for Standardization, Geneva, Switzerland (2010)
Mota, M.V.T., Ferreira, I.M.P.L.V.O., Oliveira, M.B.P., Rocha, C., Teixeira, J.A., et al.: Enzymatic hydrolysis of whey protein concentrates: peptide HPLC profiles. J. Liq. Chromatogr. Relat. Technol. 27(16), 2625–2639 (2004)
O’Keeffe, M.B., FitzGerald, R.J.: Antioxidant effects of enzymatic hydrolysates of whey protein concentrate on cultured human endothelial cells. Int. Dairy J. 36(2), 128–135 (2014)
Osterman, L.A.: Electrophoresis. Isoelectric focusing ultracentrifugation. Methods of Protein and Nucleic Acid Research, vol. 1. Springer, Heidelberg (1984). ISSN: 978-3-642-87487-1
Ou, K., Liu, Y., Zhang, L., Yang, X., Huang, Z., Nout, M.J.R., et al.: Effect of neutrase, alcalase, and papain hydrolysis of whey protein concentrates on iron uptake by Caco-2 cells. J. Agric. Food Chem. 58(8), 4894–4900 (2010)
Phenolic, amino and condensation resins – Determination of free-formaldehyde content. ISO 11402:2004. International Organization for Standardization, Geneva, Switzerland (2004)
Prosekov, A.J., Ul'rikh, E.V., Poturaeva, N.L., Koroleva, O.V., Budrik, V.G., Botina S.G., et al.: Patent of Russia 2528068 (2014)
Souza, R.S.C. de, Tonon, R.V., Stephan, M.P., Silva, C.M., Penteado, A.L., Cabral, L.M.C., et al.: Evaluation of the antioxidant potential of whey protein concentrated by ultrafiltration and hydrolyzed by different commercial proteases. Braz. J. Food Technol. 22, e2018021 (2019)
Sviridenko, J.J., Abramov, D.V., Myagkonosov, D.S., Ovchinnikova, E.G., Tutelyan, V.A., Mazo, et al.: Patent of Russia 2663583 (2018)
Sviridenko, J.J., Abramov, D.V., Myagkonosov, D.S., Tutelyan, V.A., Mazo, V.K., Zorin, S.N.: Patent of Russia 2529707 (2014)
Tacias-Pascacio, V.G., Morellon-Sterling, R., Siar, E.-H., Tavano, O., Berenguer-Murcia, Á., Fernandez-Lafuente, R.: Use of Alcalase in the production of bioactive peptides: a review. Int. J. Biol. Macromol. 165, 2143–2196 (2020)
Tarun, E.I.: Comparison of antioxidant activities of gallic, coffee and chlorogenic acids. Proc. BSU 9, 186–191 (2014). (in Russian)
Tavano, O.L.: Protein hydrolysis using proteases: an important tool for food biotechnology. J. Mol. Catal. B Enzym. 90, 1–11 (2013)
Torkova, A., Ryazantzeva, K., Agarkova, E.Yu., Tsentalovich, M., Kruchinin, A., Fedorova, T.V.: Cheese whey catalytic conversion for obtaining a bioactive hydrolysate with reduced antigenicity. Curr. Res. Nutr. Food Sci. 4(SI.2), 182–196 (2016)
Torkova, A.A., Ryazantseva, K.A., Agarkova, E.Y., Kruchinin, A.G., Tsentalovich, M.Y., Fedorova, T.V.: Rational design of enzyme compositions for the production of functional hydrolysates of cow milk whey proteins. Appl. Biochem. Microbiol. 53(6), 669–679 (2017). https://doi.org/10.1134/S0003683817060138
Van Beresteijn, E.C.H., Peeters, R.A., Kaper, J., Meijer, R.J.G.M., Robben, A.J.P.M., Schmidt, D.G.: Molecular mass distribution immunological properties nutritive value of whey protein hydrolysates. J. Food Prot. 57(7), 619–625 (1994)
Vandenplas, Y., Munasir, Z., Hegar, B., Kumarawati, D., Suryawan, A., Kadim, M., et al.: A perspective on partially hydrolyzed protein infant formula in nonexclusively breastfed infants. Korean J. Pediatr. 62(5), 149–154 (2019)
Whitehurst, R.J., van Oort, M. (eds.): Enzymes Food Technology, vols. 1, 13, 2nd edn. Blackwell Publishing Ltd, Iowa, USA
Zhang, Q.-X., Wu, H., Ling, Y.-F., Lu, R.-R.: Isolation and identification of antioxidant peptides derived from whey protein enzymatic hydrolysate by consecutive chromatography and Q-TOF MS. J. Dairy Res. 80(3), 367–373 (2013)
Zheng, H., Shen, X., Bu, G., Luo, Y.: Effects of pH, temperature and enzyme-to-substrate ratio on the antigenicity of whey protein hydrolysates prepared by Alcalase. Int. Dairy J. 18(10–11), 1028–1033 (2008)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Halavach, T. (2022). Proteolysis of Bovine Whey, Milk and Colostrum with Serine Endopeptidases. In: Kurchenko, V., Lodygin, A., Machado da Costa, R.M., Samoylenko, I. (eds) Intelligent Biotechnologies of Natural and Synthetic Biologically Active Substances. ICAETT 2021. Lecture Notes in Networks and Systems, vol 408. Springer, Cham. https://doi.org/10.1007/978-3-030-96641-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-96641-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96640-9
Online ISBN: 978-3-030-96641-6
eBook Packages: EngineeringEngineering (R0)