Abstract
Artificial oxygen ca obtain a stable mixture. Perfluorocarbons rriers are classified into two broad categories: hemoglobin-based and perfluorocarbon-based. Both provide oxygen transport to tissues. For several decades, perfluorocarbons have been explored as oxygen carriers in a variety of biological applications. Perfluorocarbons are chemically and physiologically inert, have excellent temperature and storage stability, represent little to no infectious danger, are commercially available, and have well-established gas transport qualities. Perfluorocarbons are compounds that have high solubility for many gases, but they are not suitable for direct injection into the vascular system and require emulsification to obtain a stable mixture. Perfluorocarbons may be classified into five categories based on the primary perfluorocarbon backbone utilized in the product: (1) perfluorodecalin, (2) perfluorooctyll bromide, (3) tertbutylperfluorocyclohexane, (4) dodecafluoropentane, and (5) perftoran. When combined with other blood-saving strategies, the use of perfluorocarbon-based oxygen carriers enables the performance of surgical procedures with increased blood loss while eliminating or lowering the need for allogeneic transfusion. Other applications have been described and are under investigation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Transfusion of red blood cells currently remains the most clinically viable means of increasing the oxygen-carrying capacity of blood in the setting of acute or chronic blood loss. Blood transfusion is a common procedure, with over 4.5 million in the US receiving blood transfusions each year, and the number of people in need of blood has surpassed the available donor pool. Not only is there a growing shortage of blood, but blood products are associated with substantial limitations and a low but not insignificant risk [1, 2]. Donated blood is processed to separate the red blood cell component from plasma and platelets. Blood must be stored at a low temperature, has a shelf life of only 42 days, and in most cases must undergo time-consuming compatibility testing to reduce the risk of transfusion reaction, a potentially fatal complication. In cases of acute anemia, an oxygen carrying substitute that can be administered quickly with no risk of incompatibility would have substantial impact in acute and critical care medicine. In chronic anemia the availability of artificial oxygen carriers could reduce the need for perioperative transfusion. Although artificial oxygen carriers would not replace the need for blood, they would help decrease the amount of blood required in many situations that traditionally necessitated blood transfusion.
There are two main categories of artificial oxygen carriers , hemoglobin-based or perfluorocarbon based [3]. These compounds do not provide all the functions of blood such as immune function, coagulation, and acid-base buffering, and therefore are not true blood substitutes. Instead, they replicate only the oxygen-carrying function of erythrocytes, and the term artificial oxygen carriers is preferred. This chapter will focus on the use of perfluorocarbons as oxygen carriers for support of oxygen transport to tissue and related applications.
Chemical and Physical Properties
Perfluorocarbons refer to a family of compounds that are hydrocarbons with fluorine atoms substituted for all hydrogen atoms, with a chemical structure abbreviated CxFy. Fluorine is the most electronegative element with one of the highest electron affinities and low polarizability. The chemical properties of fluorine contribute to the multiple physiological attributes that make perfluorocarbon-based oxygen carriers appealing to use as artificial oxygen carriers.
The low polarizability of fluorine and its slightly larger size compared to hydrogen makes perfluorocarbons conformationally different from their hydrocarbon counterparts. In contrast to hydrocarbons that have linear, zig-zag shapes and demonstrate conformational flexibility, perfluorocarbons based on this backbone take on a helical shape related to the steric repulsion of fluorine and become rigid, rod-like structures [4, 5]. The electronegativity of fluorine results in a strong C—F bond that is enhanced by the presence of adjacent bonds, yielding chemical and thermal stability, further strengthened by a dense electron sheath. The C—F bond is extremely polar; however, this does not result in polarization of the overall molecule as one would expect. In fact, perfluorocarbons are among the most nonpolar solvents that exist because all the dipole moments within the same molecule cancel each other out, which makes the overall compound nonpolar [4, 5].
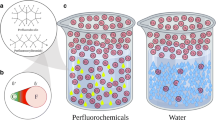
The low polarizability of fluorine accounts for the weak intermolecular forces witnessed in perfluorocarbons. Because perfluorocarbons are not as densely packed as their hydrocarbon counterparts, they have a greater ratio of surface area to volume occupied in water; this results in weak van der Waals interactions with water and thus highly hydrophobic nature of perfluorocarbons [4, 6]. Furthermore, the high polarity of C—F bonds prevents the formation of the induced dipoles and van der Waals forces necessary for lipid solubility, meaning perfluorocarbons are highly lipophobic as well [4, 7]. As a result of their simultaneous hydrophobic and lipophobic qualities, perfluorocarbons are biologically inert. They are not metabolized by the body and are removed by the reticuloendothelial system and via the lungs during exhalation [4, 8]. An example of their low water solubility and biological inertness in practice is how perfluorocarbons are used in commercially available contrast agents. These traits allow for the stabilization of injectable microparticles and prolong the existence of these contrast agents in vivo for ultrasonography [4, 9].
As noted, intermolecular forces between perfluorocarbon molecules are weak, yielding viscosities lower than water but higher densities, as much as twice that of water, and low surface tension. Due to their hydrophobic properties they are not miscible with water or blood [10]. In general perfluorocarbons are biologically and chemically inert but certain compounds can exhibit toxicity in vivo. Some perfluorocarbons have other halogens such as bromine partially substituted that alters some of the properties such as radiolucency.
Perfluorocarbons have high solubility for gases, including oxygen and carbon dioxide due to their weak intermolecular interactions, thereby their interest for use as artificial oxygen carriers [11]. In contrast to hemoglobin, perfluorocarbons are chemically inert and do not chemically bind to respiratory gases. The strong C—F bond results in stabilization of the perfluorocarbon molecule, and the steric shielding of the central carbon atom by fluorine atoms adds additional kinetic stability and inhibit reactivity [4, 12]. Because they are inert to oxidation, perfluorocarbons remain functional in the presence of carbon monoxide (CO) and even accelerate the recovery from CO poisoning by transporting excess CO [7, 13, 14]. Being chemically inert to respiratory gases also makes perfluorocarbons useful in the treatment of decompression sickness, which is caused by the supersaturation of respiratory gases, primarily nitrogen in blood and tissues followed by rapid decompression. Nitrogen comes out of solution as bubbles that obstruct small vessels. Animal studies have shown that perfluorocarbons have been effective for washing out nitrogen and preventing embolism [7, 15].
Perfluorocarbon Compounds
Perfluorocarbons may be classified into five categories based on the primary perfluorocarbon backbone utilized in the product: (1) perfluorodecalin, (2) perfluorooctyll bromide, (3) tertbutylperfluorocyclohexane, (4) dodecafluoropentane and (5) perftoran [7]. A summary of characteristics of these perfluorocarbons is provided in Table 16.1. These perfluorocarbons have been manufactured in several compounds for clinical development.
Fluosol-DA was granted FDA approval for coronary angioplasty in 1989, but the approval was later withdrawn in 1994 due to a limited shelf life and side effects related to complement activation [7]. Perftoran, a 7:3 mixture of perfluorodecalin and perfluoro-N-(4-methylcyclohexyl)-piperidine, was approved in several countries from 2005 to 2010 to treat blood loss, but isn’t currently approved for use [7]. Oxygent and Oxycyte reached human trials in the USA but, as of now, haven’t been approved by the FDA for use [7]. Oxycyte successfully completed phase II trials but was abandoned by the sponsor in 2014 due to lack of patient enrollment and financial reasons. Oxygent reached phase III trials, was transiently abandoned because of safety issues that later were disproved to be product-related [7]. During the initial phase III trial, it was thought that Oxygent caused excess bleeding and neurological events such as stroke-causing Alliance to suspend the trial [11]. Subsequent analysis of the clinical study data concluded that the conduct of the study and not the emulsion was responsible for the observed adverse events. It was demonstrated that the neurological and bleeding events were caused by excessive hemodilution in the patients receiving Oxygent instead of by Oxygent itself [11]. Furthermore, it was shown that inadequate management of blood pressure during the rapid autologous blood harvesting procedure resulted in decreased perfusion to the brain in some of these patients as well [11]. Related to these findings, new trials are being initiated in China and parts of Europe [7].
Perfluorocarbon-Based Oxygen Delivery
In 1966 Clark and Gollan were the first to demonstrate that perfluorocarbon liquids have a tremendous capacity for carrying and delivering oxygen [4, 7, 11]. Perfluorodecalin, one of the most studied perfluorocarbons, has 1000 times the molecular solubility for oxygen than that of water [7]. The combination of strong intramolecular forces with weak intermolecular forces allows perfluorocarbon liquids to behave almost like ideal fluids in that they’re easily able to dissolve gases with similar, low cohesivity properties such as O2, CO2, NO, etc. [4]. The mechanism of perfluorocarbons using loose van der Waals forces to dissolve oxygen for delivery contrasts with the way hemoglobin transports oxygen, using chemical bonding between the oxygen molecule and the iron atom in heme. Oxygen uptake by perfluorocarbon solutions is linear in contrast to the sigmoidal uptake by hemoglobin characteristic of the varying affinity for oxygen as oxygen is bound [11]. This linear relationship represents perfluorocarbon solubility following Henry’s law, which states that the amount of gas dissolved in a liquid is directly proportional to the partial pressure of the gas. One consequence of following Henry’s law is that having an elevated arterial oxygen partial pressure maximizes the benefits of the oxygen-carrying capacity of perfluorocarbon solutions [4]. Another consequence is the rapid oxygen uptake by perfluorocarbons and the rapid and extensive oxygen extraction when needed by tissues [4, 5, 7]. When compared to RBCs, oxygen loading and unloading by perfluorocarbons occurs at twice the rate, and the rate of oxygen extraction from perfluorocarbons is three times higher because perfluorocarbons can release over 90% of loaded oxygen [4, 16]. Furthermore, when perfluorocarbon emulsions are added to the plasma, the solubility of oxygen is increases by factors of 10–50 [17, 18].
Related to their hydrophobic and immiscible nature, perfluorocarbons cannot be directly administered intravenously but must be emulsified to generate thermodynamically metastable but kinetically stable mixtures [9]. Characteristics desirable for intravascular oxygen transport include emulsion stability and low vapor pressure with rapid excretion. These ideal properties are not common among perfluorocarbons, but those with slightly lipophilic properties such as F-octyl bromide (perflubron) are more suitable.
While perfluorocarbons have several attractive features for potential use as RBC substitutes in the setting of blood loss and surgery, the hydrophobic nature of perfluorocarbons that make them immiscible in aqueous media such as blood. To use perfluorocarbons in the setting of acute blood loss, perfluorocarbons must be manufactured into a stable, emulsified, or encapsulated form for intravascular use [4, 5, 16]. This has led to the development of multiple generations of perfluorocarbon formulations.
Clinical Applications
Research into RBC substitutes has become increasingly important given the limited availability of RBC concentrates and the growing demand to accommodate an aging population [19, 20]. Utilizing purely synthetic compounds as oxygen carriers has the goal to provide a substitute with an unlimited supply, universal compatibility, lack of disease transmittance, and avoidance of transfusion-related reactions and complications [7, 16].
One of the first-generation perfluorocarbons emulsions developed was Fluosol-DA, which is a perfluorodecalin product incorporating Pluronic-68 and egg yolk phospholipids for emulsification. Fluosol-DA is the only perfluorocarbon to have achieved FDA approval, available from 1989 to 1994, with an indication for percutaneous transluminal coronary balloon angioplasty for reperfusion of ischemic tissue [21, 22]. First-generation perfluorocarbon emulsions had several drawbacks such as less effectiveness in oxygen delivery, short intravascular half-life, storage at freezing temperatures, and complement activation by emulsifying agents [22]. Second-generation perfluorocarbon emulsions like Oxygent, a perfluorooctyl bromide-based product, addressed some of these issues by having a higher perfluorocarbon content, using natural phospholipids for emulsification, and not requiring freezing for storage [22]. However, these products had similar side effects such as the cytokine-mediated processes and platelet sequestration [22].
One promising product to treat hemorrhagic shock that has milder adverse effects is Perftoran. Created in Russia and then approved for use in 1996 to treat hemorrhage and various ischemic conditions, Perftoran is a perfluorodecalin-based product that uses Proxanol 268 instead of egg yolk phospholipid as the emulsifier [23]. According to reports, Perftoran has been administered over 35,000 times and has demonstrated increased benefit with milder adverse events reported due to the product’s smaller size [23]. As of now, FluorO2 Therapeutics, Inc plans to manufacture Perftoran (rebranded as Vidaphor) in the United States for clinical trials [24]. Another promising perfluorocarbon solution being investigated is composed of a perfluorodecalin core surrounded by a biocompatible albumin capsule. These albumin-derived perfluorocarbon solutions demonstrated functionality as artificial oxygen carriers and biocompatibility by lacking severe side effects in animal models [11]. An additional preclinical study demonstrated that these perfluorocarbon solutions acted as life-saving RBC substitutes in the setting of massive hemodilution [25]. Although there are currently no perfluorocarbon solutions being regularly used for humans as the setting of blood substitution, clinical investigations are still underway. Further improvements in perfluorocarbon products may allow for synthetic blood substitutes in the future.
The use of perfluorocarbon-based oxygen carriers, used in the setting of other blood-saving techniques would allow for surgical procedures with risk of blood loss to be performed while simultaneously avoiding or reducing the amount of allogeneic transfusion [3]. A major field of application for perfluorocarbons is clearly the replacement of blood transfusions in trauma or surgical setting requiring large volume blood loss [5].
Future Directions
Liquid Ventilation
Humans, along with other air-breathing species, exchange oxygen and carbon dioxide through lungs, a respiratory organ with an air-blood interface. In fish and other vertebrates and invertebrates, gills serve as the respiratory organ able to extract oxygen from water. In human acute lung injury, the resulting inflammatory injury to the alveolar epithelium and loss of surfactant with elevation of alveolar surface tension leads to alveolar collapse and/or filling of alveoli with edema fluid. It was hypothesized that filling the lungs with fluid rather than air would eliminate the air-fluid interface (and surface tension along with it), reduce atelectasis, allow restoration of alveolar structure, and improve gas exchange. For this to be successful, the fluid used for installation into the lungs would have to be capable of carrying sufficient oxygen to meet oxygen transfer needs. Perfluorocarbons have the characteristics required to meet these needs. The use of perfluorocarbons in this manner is termed liquid ventilation, wherein the lungs are insufflated with an oxygenated perfluorochemical liquid rather than an oxygen-containing gas mixture. The use of perfluorocarbons as the biochemically inert carrier of oxygen and carbon dioxide, rather than nitrogen, has several theoretical benefits for treating acute lung damage.
There have been two major approaches to liquid ventilation: total liquid ventilation and partial liquid ventilation. Total liquid ventilation involves filling all potential air spaces including airway dead space volume with liquid. Perfluorocarbons have a kinematic viscosity close to that of water and are roughly twice as dense as water. Since perfluorocarbons are denser and more viscous than gas, with a slower spreading rate and higher diffusion coefficients, the work of liquid breathing exceeds the respiratory muscles to maintain ventilation for extended periods of time. A special mechanical ventilator to deliver liquid tidal volumes , replenish oxygen in the perfluorocarbon, and remove carbon dioxide are necessary to maintain pulmonary gas exchange [26]. Total liquid ventilation is a technologically challenging process that is still in pre-clinical experimentation and evaluation and has not entered clinical trials.
Partial liquid ventilation differs from total liquid ventilation by filling only the functional residual capacity with perfluorocarbon (approximately 30 mL/kg) and using a conventional mechanical ventilator to deliver gas tidal volumes. This approach entered early clinical trials using perflubron as the perfluorocarbon [27] but was associated with complications such as barotrauma related to the large tidal volumes that were common at the time.
Non-respiratory applications such as pulmonary medication administration and radiographic imaging may have potential clinical use [20].
Albumin-Derived Perfluorocarbon Solutions
Most hemoglobin-based artificial oxygen carriers were associated with severe side effects in clinical trials and therefore further development was suspended. While hemoglobin-based oxygen carriers must maintain a specific size to avoid local nitric oxide scavenging from the endothelium, perfluorocarbon-based oxygen carrier emulsified particles tend to grow because of Oswald ripening and flocculation. Wrobeln et al., successfully synthesized nanoscaled perfluorocarbon-based oxygen carriers with a perfluorodecalin core surrounded by a biocompatible albumin shell (capsules) [14]. In a rat model of large-scale hemodilution with exchange of approximately 95% of the blood volume with either capsules in a plasma-like solution (treatment) or the plasma-like solution without capsules (control), hypoxia sensitive organs such as the small intestine and kidney were protected. This study served as a “proof of concept” that capsules are a potentially life-saving erythrocyte substitute. With these capsules tissue hypoxia, even at critically low hematocrit, can be avoided [25].
Summary
Artificial oxygen carriers are divided into two categories: one based on hemoglobin and the second based on perfluorocarbons. Neither are a complete substitute for blood but do provide the ability to carry clinically useful amounts of oxygen to tissues. Perfluorocarbons have been investigated as oxygen carriers in a range of biological applications for several decades. These compounds are chemically and physiologically inert, have temperature and storage stability, provide little to no infectious risk, and have well-established gas transport properties. Perfluorocarbons are gas-dispersing chemicals that are not suited for direct injection into the vascular system but require preparation such as emulsification or other stable nanoparticle generation. When used in conjunction with other blood-saving measures, perfluorocarbon-based oxygen carriers have the potential to compensate for blood loss during surgical operations while avoiding or reducing the need for allogeneic transfusion.
References
Delaney M, Wendel S, Bercovitz RS, Cid J, Cohn C, Dunbar NM, et al. Transfusion reactions: prevention, diagnosis, and treatment. Lancet. 2016;388(10061):2825–36.
Goodnough LT. Risks of blood transfusion. Crit Care Med. 2003;31(12 Suppl):S678–86.
Henkel-Hanke T, Oleck M. Artificial oxygen carriers: a current review. AANA J. 2007;75(3):205–11.
Riess JG. Understanding the fundamentals of perfluorocarbons and perfluorocarbon emulsions relevant to in vivo oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2005;33(1):47–63.
Spahn DR. Blood substitutes. Artificial oxygen carriers: perfluorocarbon emulsions. Crit Care. 1999;3(5):R93–7.
Dalvi VH, Rossky PJ. Molecular origins of fluorocarbon hydrophobicity. Proc Natl Acad Sci U S A. 2010;107(31):13603–7.
Jagers J, Wrobeln A, Ferenz KB. Perfluorocarbon-based oxygen carriers: from physics to physiology. Pflugers Arch. 2021;473(2):139–50.
Haldar R, Gupta D, Chitranshi S, Singh MK, Sachan S. Artificial blood: a futuristic dimension of modern day transfusion sciences. Cardiovasc Hematol Agents Med Chem. 2019;17(1):11–6.
Schutt EG, Klein DH, Mattrey RM, Riess JG. Injectable microbubbles as contrast agents for diagnostic ultrasound imaging: the key role of perfluorochemicals. Angew Chem Int Ed Engl. 2003;42(28):3218–35.
Lemal DM. Perspective on fluorocarbon chemistry. J Org Chem. 2004;69(1):1–11.
Riess JG. Perfluorocarbon-based oxygen delivery. Artif Cells Blood Substit Immobil Biotechnol. 2006;34(6):567–80.
Kirsch P. Modern fluoroorganic chemistry: synthesis, reactivity, applications. 2nd ed. John Wiley & Sons, Inc; 2013.
Yokoyama K. Effect of perfluorochemical (PFC) emulsion on acute carbon monoxide poisoning in rats. Jpn J Surg. 1978;8(4):342–52.
Wrobeln A, Laudien J, Gross-Heitfeld C, Linders J, Mayer C, Wilde B, et al. Albumin-derived perfluorocarbon-based artificial oxygen carriers: a physico-chemical characterization and first in vivo evaluation of biocompatibility. Eur J Pharm Biopharm. 2017;115:52–64.
Mayer D, Ferenz KB. Perfluorocarbons for the treatment of decompression illness: how to bridge the gap between theory and practice. Eur J Appl Physiol. 2019;119(11–12):2421–33.
Khan F, Singh K, Friedman MT. Artificial blood: the history and current perspectives of blood substitutes. Discoveries (Craiova). 2020;8(1):e104.
Ferenz KB, Steinbicker AU. Artificial oxygen carriers-past, present, and future-a review of the most innovative and clinically relevant concepts. J Pharmacol Exp Ther. 2019;369(2):300–10.
Spiess BD. Perfluorocarbon gas transport: an overview of medical history with yet unrealized potentials. Shock. 2019;52(1S Suppl 1):7–12.
Eggleton CD, Roy TK, Popel AS. Predictions of capillary oxygen transport in the presence of fluorocarbon additives. Am J Physiol. 1998;275(6):H2250–7.
Wrobeln A, Schluter KD, Linders J, Zahres M, Mayer C, Kirsch M, et al. Functionality of albumin-derived perfluorocarbon-based artificial oxygen carriers in the Langendorff-heart (dagger). Artif Cells Nanomed Biotechnol. 2017;45(4):723–30.
Bachert SE, Dogra P, Boral LI. Alternatives to transfusion. Am J Clin Pathol. 2020;153(3):287–93.
Williamson LM, Devine DV. Challenges in the management of the blood supply. Lancet. 2013;381(9880):1866–75.
Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs. 2010;34(8):622–34.
Latson GW. Perftoran (Vidaphor)-Introduction to Western medicine. Shock. 2019;52(1S Suppl 1):65–9.
Jahr JS, Guinn NR, Lowery DR, Shore-Lesserson L, Shander A. Blood substitutes and oxygen therapeutics: a review. Anesth Analg. 2021;132(1):119–29.
Wrobeln A, Jagers J, Quinting T, Schreiber T, Kirsch M, Fandrey J, et al. Albumin-derived perfluorocarbon-based artificial oxygen carriers can avoid hypoxic tissue damage in massive hemodilution. Sci Rep. 2020;10(1):11950.
Hirschl RB, Conrad S, Kaiser R, Zwischenberger JB, Bartlett RH, Booth F, et al. Partial liquid ventilation in adult patients with ARDS: a multicenter phase I-II trial. Ann Surg. 1998;228(5):692–700.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kaye, A.D., Samaniego, K., Miriyala, S., Miller, B.C., Cornett, E.M., Conrad, S.A. (2022). Perfluorocarbon-Based Oxygen Carriers. In: Liu, H., Kaye, A.D., Jahr, J.S. (eds) Blood Substitutes and Oxygen Biotherapeutics. Springer, Cham. https://doi.org/10.1007/978-3-030-95975-3_16
Download citation
DOI: https://doi.org/10.1007/978-3-030-95975-3_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-95974-6
Online ISBN: 978-3-030-95975-3
eBook Packages: MedicineMedicine (R0)