Abstract
There is still controversy about the role of visual evoked potential (VEP) as an intraoperative neurophysiological technique. The development of new stimulus devices and improved anesthetic protocols led VEP to be more reproducible and reliable. Direct cortical and subcortical electrical stimulation is showing promising results in identifying visual pathways (VP) and their interconnections with other eloquent areas. In this chapter, we address the functional anatomy of VP, the different technical aspects of VEP and VP brain mapping during awake craniotomies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The light stimulus upon reaching the retina is converted into an electrical input that travels through the VP to the visual processing cortex in the occipital lobes. Optic nerves (ON), optic chiasm, optic tracts, and lateral geniculate bodies make up the anterior part of this pathway, while optic radiation and visual cortex make up its posterior part. The responses evoked by these stimuli are called VEP. Its landmark is a three-phase wave, whose main component is P100.
Tumor resection along the VP can cause lesions, resulting in partial or complete visual deficit. Hence, VEP is indicated in any procedure with risk to the visual structures, like orbital, transsphenoidal, parasellar, temporal, occipital, and parieto-occipital transition surgeries. Additionally, VP monitoring is useful in functional surgeries, including microelectrode placement in the globus pallidus for deep brain stimulation (DBS) and in stereotactic pallidotomies.
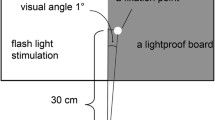
Classically, during intraoperative neurophysiology monitoring (IOM), visual function is studied through VEP with flash-light stimulation. First described in 1973 by Wright et al. [16], the technique was not promising, since under general anesthesia, the resulting potential was unstable and with low reproducibility. The spread of total intravenous anesthesia and the development of devices with higher luminescence improved the technique. Consequently, better correlation between VEP potential and postoperative visual function became more reliable.
Yet, intraoperative usage of VEP is still very challenging. VEP reflects the activation of fibers from the macular region of the retina. Peripheral retina is not fully activated with the technique. Changes in amplitude and latency have limited value in focal lesions in the optic radiation and associative cortex. As dependent on a polysynaptic pathway, VEP is susceptible to anesthetic agents and systemic factors. Hypotension, hypothermia, hypoxemia, hypovolemia, and hematocrit below 15% should be discharged when interpreting the amplitude drop of the N75-P100 complex [16, 20].
Mapping the VP in awake craniotomies aims to highlight the topography of the anatomical pathways and their functional connectivity with other eloquent areas, such as language [5,6,7, 11, 26, 44, 45, 48].
This chapter deals with the anatomy of the VP, intraoperative mapping, and monitoring techniques under general anesthesia and in the awake craniotomies. Technical limitations are also discussed.
Anatomy of the Visual Pathways
The light stimulus penetrating the pupil is captured by specialized cells in the retina (photoreceptors – cones and rods), converted into an electrical input, and transmitted via bipolar cells to the retinal ganglion cells, whose axons form the ON. After leaving the orbit through the optic canal in the lesser sphenoid wing the ON proceeds through the middle fossa. In the optic chiasm, the nasal fibers of each nerve cross the midline and join the temporal fibers of the other eye, constituting the optic tract. Each optic tract proceeds posteriorly to the lateral geniculate nucleus of the thalamus, where it synapses and initiates optic radiation, the final pathway to the primary cortical areas of visual processing (Fig. 7.1). Anatomy and physiology of the circadian reflex and the pupillary reflex are beyond the scope of this chapter.
The optical radiation is divided: one loop courses above and laterally to the inferior horn of the lateral ventricle. The other is Meyer’s loop, which courses anteriorly toward the pole of the temporal lobe before heading posteriorly to the cortex. Hence, surgeries in the temporo-occipital transition and in areas immediately anterior to the inferior horn of the lateral ventricle may damage the optic radiation. There is an incidence of an upper quadrantanopia ranging from 68 to 100% in anterior temporal lobectomies [52].
From the retina to the visual cortex there is a wide convergence of signals. In the peripheral retina, the signal from thousands of photoreceptors converges to one ganglion cell, while in the fovea this ratio is almost one to one [51]. This explains the high resolution of central vision and why the cortical representation of the fovea around the calcarine fissure is larger. Another important particularity is how information from the visual fields reaches the visual cortex. The light stimuli are refracted in the lens and when they reach the retina, the image is inverted both vertically and horizontally. Thus, the upper visual field is mapped below the calcarine fissure and the lower visual field above the calcarine fissure, besides the right-left inversion.
Due to the crossing of fibers in the optic chiasm and its topographic organization along the VP, different clinical presentations result from an injury to the VP, including amaurosis, hemianopia, and quadrantanopia. Their anatomical-functional correlation is illustrated in Figs. 7.2 and 7.3.
Correlation between injuries at different levels of the VP and visual deficits. (a) Injury to the ON causes disruption of nasal and temporal fibers of the same eye. This results in amaurosis ipsilateral to the injury. (b) Lesion of the optic chiasma interrupts the crossing of the nasal fibers in each eye, resulting in the loss of peripheral vision, called bi-temporal hemianopia. (c) Injury to the optic tract interrupts the ipsilateral and nasal temporal fibers contralateral to the injury. This results in homonymous hemianopia. (Adapted from Wilson-Pauwels, et al. [51] (with permission))
From the primary visual cortex (area 17), the signals are sent to the association visual cortex for interpretation (areas 18 and 19) and to the frontal ocular cortex, an area related to visual fixation. There may be differences in terms of cortical generator between reverse-pattern and flash-activated VEP. The former seems to be generated mainly in the primary visual cortex (area 17) while the latter is mediated by both the primary visual cortex and the association visual cortex. Flash-activated VEP seems not to be influenced by the point on the retina at which the stimulus occurs [30, 33].
Brain mapping during awake craniotomies has highlighted important anatomical functional correlations of the VP with other subcortical tracts, such as the inferior longitudinal fascicle (ILF), the inferior occipito-frontal fascicle (IFOF), and the U-fiber projection system, interconnecting different eloquent cortex. This issue will be detailed later in section “Awake Craniotomy: Visual Mapping and Monitoring”.
Intraoperative Techniques
VEP – Asleep Techniques
The flash visual stimulus is the most commonly used mechanism for retinal activation during VP monitoring. Flash-VEP indicates VP integrity when present, rather than giving details of the visual system. Flash stimulation does not depend on patient compliance and activates luminescence-sensitive channels (cones and rods). Outpatient reverse-pattern stimulus activates more contrast-sensitive channels (rods) [30, 51]. In people with normal vision, the VEP is recorded eminently as a three-phase potential with a first negative component at 75 ms (N75), a positive component at 100 ms (P100), and a third negative component at 145 ms (N145).
After induction of anesthesia, eyes are taped with a transparent film dressing to avoid exposure and cornea abrasion. Subdermal needle electrodes are inserted in the lower and lateral or medial orbit for electroretinogram (ERG) recordings. ERG continuously evaluates the efficacy of the light stimulation of the retina. Needling of III, IV, and VI is done if indicated in IOM protocol (more details in Chap. 20). Afterward, a goggle-shaped stimulator (Fig. 7.4a) is placed over the closed eyes. Even further care must be taken not to displace the inserted needles. We kindly cover the region with gauze and use large adhesive tapes. There is a risk of goggle displacement by frontal skin flap during frontal and frontotemporal craniotomies or during patient’s positioning. Disk-type stimulators (Fig. 7.4b) are lighter, more flexible, and easily positioned, and tend to shift less [16, 19]. Yet, they are not available for all IOM machines. Newly released, adhesive low weight VEP electrodes are suitable for all IOM machines (Fig. 7.4c). They connect to a touch-proof electrical output and their luminescence relates to stimulus intensity. These VEP electrodes are available for white or red color flash.
Devices used for monitoring VEP: (a) Goggle-shaped stimulator. (b) Disk-type stimulator from Inomed GmBH, Germany. (c) Adhesive VEP electrodes for single-use and flash stimulation. Compatible with all IOM machines since they have touch-proof connections. Each eye stimulator should be connected to an electrical output for alternating stimulation. Manufactured by Spes Medica, Italy
Regardless of the device used, it is important that it is well fitted to the face not to press the eyeballs and to block the entrance of any light beam (Fig. 7.5). Total darkness is essential for the retina to be adequately stimulated only by the deflated flash, and in the operating room several light sources can disrupt the stimulation [14]. Displacement of stimulators is diagnosed by ERG loss.
Positioning of goggles: They are placed gently in order not to displace III, IV, and VI muscle needles. The needles should remain straight, leaning against the goggles. By doing so, needles do not damage the eyeballs. Needle cables bend on themselves and are covered by tape so they can’t be accidentally pulled off. Special care is taken to avoid goggle displacement with gauze and large adhesive tapes
LEDs with white light are preferable to LEDs with red light for the following reasons: (1) white contains all color spectra, being able to activate even cone-photoreceptors, whose maximum spectral sensitivity is for the colors red, green, and blue [51]; (2), all types of rods, consequently a larger area of the retina [14]; (3) white color keeps the retina adapted to light during long-lasting surgeries [34].
Paradoxically, although flash-VEP activates a wide retina area [14], the activation seems to predominate in fovea. The fovea is richer in cones and saturates less when exposed to bright light. This phenomenon might explain why quadrantanopia is poorly predicted by flash-VEP [20].
Stimulus and recording parameters vary widely among normative studies [53]. Most frequencies used to flash deflagration are inferior to 4 Hz. Most commonly, being around 1.1 Hz. Higher frequencies imply a reduction in the amplitude of the responses and, if done continuously, a greater risk of heat injury to the eyelids [16, 19]. Wiedemayer et al. [50] reported stable VEPs at a frequency of 8.5 Hz using goggles with red light-emitting diodes, but the signal-to-noise ratio was higher than that often obtained in skull surgery. There is an alternation of the stimulus between eyes.
The brightness of the flash is measured in illuminance, ranging between 10,000 and 20,000 lux (Lx). The intensity of the stimulus is measured in Candela (Cd) and ranges between 0 and 175 mCd, depending on the other parameter sets and differences between IOM machines. Since the retina has the ability to adapt to light, there can be a variation in the amplitude of the responses obtained after the initial stimuli.
For a stable activation of the retina, 1 minute of flash stimulus is recommended before VEP recording [34]. Stable and reproducible VEPs are obtained with stimuli of 10 to 20 ms duration. Kodama and Goto [19] have successfully described the use of stimuli with 40 ms duration. They were able to successfully identify P100 after 40 to 100 sweeps. Moreover, they could perform continuous stimulation for over 30 minutes, without skin burn events. Despite their good results, it is important to keep in mind that longer durations generate more heat, especially if done continuously for a long period of time. The balance between stimulus intensity, duration, frequency, and time is essential to ensure the safety of the technique.
Registration
ERG ensures that the retina is being adequately stimulated. A subdermal needle electrode is placed in the lower corner of the orbit of each eye and referenced to Fz or to ipsilateral medial/lateral orbit corner. Two main components can be identified in the ERG waveform, a negative component (N30 or A) related to photoreceptor activation and a positive component (P50 or B) related to retinal and ON activation [24].
Corkscrew electrodes are positioned at points Oz, O1, and O2 and referenced to Cz and Fz for registration of VEP on the scalp. Some authors suggest the placement of more recording electrodes, such as L10 and R10 (10 cm lateral to Oz), as a safety measure in case of displacement of any of the other electrodes during the approach [19, 34]. Usually, the highest amplitude responses are obtained in Oz-Fz or Oz-Cz, with inter-individual variability of the highest electronegativity.
The number of measured responses varies between 40 and 200, depending on the signal-to-noise ratio. The most important is to obtain stable and reliable VEPs promptly. Recommended filtering ranges from 1-30 Hz (low-frequency filter) and 300–1000 Hz (high-frequency filter) [16, 19, 34, 53]. We generally use 1-100 Hz bandpass filter, sensitivity of 20 μV/division, and 200–300 ms analysis time for adults and 500 ms for infants.
During occipital craniotomies, VEP potentials might be recorded with a strip electrode positioned directly over the visual cortex (cortical VEP) (Fig. 7.6). Ota et al. [33] were able to register P100 with good detectability and stability using a strip. They demonstrated consistency between postoperative clinical changes and findings during surgery. Shahar et al. [39] recorded P100 with subdural recording during awake craniotomies. They stated that P100 presented greater amplitude and spatial resolution than scalp recording.
Female , 65 y.o., breast cancer metastasis in the left occipital region. No previous visual deficit. Flash-VEP recording electrodes at Oz-Fz’. Filters: 5-100 Hz Complete IOM protocol included: TES-MEP: C3-Cz and C4-Cz with recordings at APB and AH/SSEP for ulnar and tibial nerves bilaterally/subcortical motor mapping (monopolar & cathodal) and EEG. (a) Baselines (bsl) with normal P100 (110.9 ms). (b) After dura mater opening, a strip electrode was placed directly over the left occipital cortex: cortical P100 showed 10 ms shorter latency compared to bsl. During tumor resection: cortical P100 latency increased 10 ms. (c) After resection and back to corkscrew recordings: P100 latency was delayed (128.9 ms). Total delay = 14 ms in relation to previous corkscrew recordings. P100 amplitude variation <50%. Patient had no new deficit. (d) MRI at presentation
Kikuchi et al. [18] studied the correlation between P100 amplitude and the cross-sectional area of the ON. They stimulated ON in the vicinity of the orbit and registered a potential near the chiasma, about 8 mm distance. In this experimental study, the amplitude of the potential is related directly to the cross-sectional area of the nerve, i.e., a reduction of its amplitude with the gradual narrowing of the nerve. The study did not assess the correlation between visual function and changes in the ON potential. Møller et al. [28] recorded ON potential after flash stimulation. The response showed low amplitude and reproducibility, with intraoperative fluctuations. In both techniques, only the exposed ON was evaluated, restricting its use to very specific approaches.
Interpretation and Warning Signs
ERG translates that retina is properly being stimulated and serves as a control technique. A drop in amplitude of the VEP associated with the loss of ERG waves indicates a failure in the visual stimulus, either by inadequate deflagration of the flash, or by stimulator displacement (Fig. 7.7).
Male, 83 y.o., lung cancer metastasis in the right supraorbital region. Flash-VEP with recording electrodes at Oz-Fz and Oz-Cz’. ERG: inferior orbit-Fz. Filters: LFF - 1 Hz, HFF - 800 Hz. Sweeps: 100. Baselines showed replicable N30(A)/P50(B) and P100 with normal latencies. Right N30/P50 disappeared, as did P100 at the same eye. Due to the wide frontal craniotomy, skin flap displaced the goggles. The diagnose of the situation was fully dependent on ERG recordings. (a) Displacement of goggle after frontal skin repair with loss of ERG (black line). Baselines in green. (b) Loss of P100 on the right. (c) MRI – right supraorbital lesion
VEP is indicated in all surgery in which the VP is at risk, either due to proximity, direct invasion, or compression of its fibers (Fig. 7.8). In this context, the P100 is the most robust, stable, and reliable parameter for VEP analysis during IOM. Each VEP wave relates to a specific generator. Most probably, P40 reflects activation of the ON [28], and P50 and N80 are generated in central structures distally to the optic chiasm. The origin of N75 has not yet been completely clarified, but it is likely that N75 and P100 have their functional and anatomical origin in different neuronal structures [30, 42]. Although some indications suggest that P100 is generated in the primary visual cortex, studies with magnetoencephalography and depth electrodes have suggested an important contribution from the dorsal extrastriate cortex of the middle occipital gyrus, lingual gyrus, and ventral portion of the fusiform gyrus [33]. Hayashi et al. [16] showed N75-P100-N145 to have more reproducibility under anesthesia than other VEP components.
Male , 39 y.o, left ophthalmic artery aneurysm. Flash-VEP: recording electrodes at Oz-Fz and Oz-Cz’. ERG: inferior orbit-Fz. Filters: 1-100 Hz. Sweeps: 35. (a) Baseline: absent left P100 (7:27 a.m.). No change with temporary clip. (b) Left P100 showing up about 10 minutes after definitive clamping. (c) Final left P100. (d) Right VEP with normal P100 from baseline to final recordings
The use of VEP as a predictor of postoperative visual deficit is still controversial. Some studies have described specific findings to have positive relation to the postoperative outcome. Yet, others showed opposite results and technical difficulties interfering with its reliability and reproducibility. Cedzich et al. [3] reported inconsistent results in their report with great fluctuation of latencies, amplitudes, and even loss of potentials that did not correlate with a significant clinical change. The development of new anesthetic protocols and devices with higher luminescence led to a greater correlation between intraoperative findings and postoperative visual outcomes.
Yet, previous visual impairment or large visual field defects directly affect intraoperative VEP recording feasibility. If obtained, P100 shows low amplitude and reproducibility and is unstable [1, 25, 33]. Hence, a corrected visual acuity greater than 0.4 is an important factor for the acquisition of reliable potentials [16, 20].
To overcome interindividual variability, P100 is compared with patient’s intraoperative baseline. P100 amplitude deterioration >50% has been shown to be the most reliable parameter [38]. Transient P100 loss with recovery does not seem to indicate irreversible VP damage. Harding et al. [15] showed that P100 loss for more than 4 minutes related to an increased risk of postoperative deficit. This temporal analysis still has to be confirmed by other studies.
Pattern-reversal P100 latency shows great variability between healthy and affected eyes. Yet, flash-VEP P100 latency varies little, resulting in less importance as a diagnostic value [16]. Latency was shown to have an intraoperative spontaneous variation. Therefore, it is ineffective for this purpose (Harding et al., 2015).
Ota et al. [33] stated that direct cortical recordings improve spatial resolution. They were able to register the best cortical P100 in the vicinity of the calcarine fissure. Noachtar et al. [30] showed a variability of lateral extent of area 17 ranging from 4 mm to 24 mm. Hence, it would result in considerable topographic variability of the primary visual cortex.
Type of stimulus, electrodes, and recording parameters directly affect the quality of intraoperative P100. The diversity of reported techniques hinders the direct comparison of efficacy. Hence, it is understandable that controversy about intraoperative VEP still exists.
Special Techniques
Stimulation of the optic tract might be performed in functional surgeries for Parkinson’s disease and primary dystonia refractory to drug treatment. The internal globus pallidus is the therapeutic target in some cases of pallidotomies and deep brain stimulation (DBS), and its posterior-inferior-medial part lies a few millimeters above the optic tract. Hence, stimulation of the VP at this point is important for recognition and prevention of injury of the anatomical therapeutic target previously identified by stereotaxis.
For this purpose, Landi et al. [21] described the use of a monopolar electrode for stimulation of the optic tract under general anesthesia. Stimulus parameters were single-phase square pulse with 50 μs duration, 3 Hz frequency, 1 to 4 mA intensity, and 60 sweeps. Recording electrodes were positioned at Oz, O1, and O2 with a non-cephalic reference. They obtained biphasic potentials N40-P70 in scalp. Manohar et al. [27] could successfully reproduce Landi’s protocol. In both studies, no patient had visual changes postoperatively.
Regarding parasellar tumor resection, Benedicic and Bosnjak [1] reported a cohort of 11 cases, in which they performed a dual stimulation technique. VEP was elicited by epidural stimulation of the optic nerves (using one needle electrode with a ball tip at each side of the optic nerve) and by flash with scalp recording (Oz-Fz as a control). Direct optic nerve stimulation used 0.2 to 5 mA, pulse duration of 0.1–0.3 ms at a rate of 2 Hz. The responses obtained with direct nerve stimulation consisted of three-phase potentials characterized by a first positive deflection at 20 ms (P20), a negative at 30 ms (N30), and a third small positive deflection at 40 ms (P40). They used as warning signal an amplitude deterioration greater than 30% for P20 and N30. Reversible reduction in responses during manipulation of the anterior VP had statistical significance in preventing postoperative visual deterioration. No deterioration of P100 was registered during the procedure.
We present a case of three-phase wave P20-N30-P40 complex recorded at scalp after subcortical stimulation using a DBS probe (Fig. 7.9). This case covers both methodologies described above and points to possible new frontiers of VP evaluation.
Male, 14 y.o., with dystonia due to pantothenate kinase-associated neurodegeneration (PKAN). DBS approach. IOM protocol: 1. Flash-VEP / 2. MEP (due to proximity to internal capsule: bilateral muscles recordings: Orbicularis oris, Deltoid, Extensor digitorum communis, Abductor pollicis brevis, Vastus medialis, Tibialis anterior and Abductor hallucis) / 3. Optic tract mapping using DBS electrode. (a) Googles and patient positioning. (b) Baseline. Flash-VEP recording electrodes at Oz-Fz, O1-Fz and O2-Fz. Filters: 30-100 Hz. Sweeps: up to 1000. (c) After positioning the DBS electrode, electrical stimulation was performed through the most distal contact to assess the proximity of the optic tract. Parameters: intensity 0.2 to 4 mA, frequency 2 Hz, duration 0.2 ms, sweeps: 50, recordings at Oz-Fz / O1-Fz / O2-Fz. A three-phase wave P20-N30-P40 complex showed up with 3 mA and is greater with 4 mA (last line). (Source: Denise Spinola Pinheiro, MD, PhD and Murilo Martinez Marinho, MD. Unpublished personal data (with permission))
Interestingly, Böex et al. [2] used subcortical-cortical evoked potentials in twelve patients under general anesthesia. They recorded bilateral cortical responses at central, parietal, parieto-occipital, occipital regions. Bipolar stimulation parameters were biphasic pulse of 0.2 ms/phase, 1.3 Hz, 30 sweeps, 5-4000 Hz, and up to 10 mA for optic radiation and Meyer’s loop and 5 mA for ON. They aimed to set a correlation between stimulation point and distance to VP. Biphasic P1(2.4–5.4 ms)-P2(15–21.6 ms) complex was successfully evoked at a distance up to 4.5 mm. P1-P2 were registered at ipsilateral occipital cortex after temporal and parietal stimulations and bilateral occipital responses after ON stimulation.
Stimulation along the VP with scalp recordings seems to be a promising technique. The methodology needs to be correlated with outcome in larger cohorts to validate and standardize its application.
Awake Craniotomy: Visual Mapping and Monitoring
Real-time identification of functional networks during brain tumor resection has improved the functional and oncological outcomes. The role of awake craniotomy and eloquent-areas mapping is already well known [4, 9, 32, 47]. New frontiers in cortical and subcortical direct electrical stimulation (DES) of non-motor and non-verbal connections are being searched [6, 8, 11, 48]. VP and DES is the next big thing.
Of utmost interest for DES is optic radiation and visual cortex. The fibers of the optical radiation take a three-dimensional path involving the temporal and occipital horns of the lateral ventricle and terminate in a retinotopic organization in the visual cortex. The central beam of optic radiation contains the macular fibers (central vision) and terminates at the lips of the calcarine fissure, while the anterior (Meyer’s loop) and posterior beams represent the upper and lower quadrants of the retina [12, 35] (Figs. 7.2 and 7.3). Optic radiation mapping is important when operating the posterior half of temporal lobe, inferior parietal lobe, and occipital lobe. There is a somatotopic organization of the primary and associative visual cortex regarding shape, color, and motion.
Lee et al. [23] demonstrated that there are specific areas for one type of function, while others relate to more than one image feature. Certainly, there is an inter-individual anatomical and functional variability. They stimulated the lateral temporo-occipital region or temporo-parieto-occipital junction and elicited scotomas or large visual field defects on the contralateral side. The basal temporo-occipital cortical region produced micropsia (shrunken image), macropsia (enlarged image), and metamorphopsia (image distortion). Stimulation of the fusiform gyrus and lingual gyrus produced colorful shapes, and stimulation of areas such as the basal temporo-occipital junction, mesial parieto-occipital junction, or temporo-parieto-occipital junction and occipital pole produced shapes with motion. Complex shapes associated with auditory responses were produced upon stimulation of the superior temporal cortex.
The subcortical DES exposes the interneural connections that associate the captured images with memories, language, and other cognitive aspects. This network is fundamental for perceiving and understanding the surrounding environment.
The processing of the written word, as well as recognition of images and people, and the access to the meaning of this information depends on the connections of the visual system to the other eloquent areas.
The optic radiation is located medially and above the ILF, just below the IFOF. This region of high connectivity between the optic radiation, IFOF, and ILF is called the Sagittal Striatum (SS) and should be considered as a functional boundary in occipito-temporal tumor resections [5]. The posterior dorsal portion of ILF is part of the ventral indirect semantic language pathway and connects the occipital lobe with the basal posterior-temporal cortex. This is the area for visual word and visual object formation, visual recognition, and reading [10]. The IFOF is part of the direct ventral semantic language pathway, and relates to written word comprehension, color, and letter recognition [40]. In the right hemisphere, IFOF also seems to be involved in the recognition of facial emotions, especially for feelings of sadness, anger, and fear [48]. The function of the right hemisphere should not be overlooked, as referenced in Chap. 19.
The depth of the temporo-occipito-parietal junction also deserves special attention, since the optic radiation bundles are emerging from the lateral geniculate body at this point.
We use several tasks to evaluate visual network, such as the visual field, Verst-Maldaun-Language-Assessment (VMLA)-Semantic [44, 46], stroop test, line bi-section, drawing, description of acts, and reading (Table 7.1). Some of these tests are available for free use at vemotests.com. More details are in Chap. 18.
There is a large inter-individual variability in the shape of the optical radiation. Its surgical manipulation without causing any visual deficits can be challenging [12, 13, 35]. Usually, DES of optical radiation is referred to by the patient as visual blurring, phosphenes, shadows, and less frequently, visual hallucinations. We usually add visuospatial assessment for optic radiation. This task is compounded by objects placed in different quadrants on the screen. Thus, one image should be positioned in the quadrant under interest and another in the opposite quadrant [13]. It is important to ask the patient to fix his gaze on the center of the screen.
Reading and verbal and non-verbal semantic tasks associated with cadence of hand movement complement the evaluation of optical radiation interconnected to Geschwind area.
Despite advances in optical radiation mapping, intraoperative recognition of quadrantanopia is still a frequent limitation. Conti Nibali et al. [29] proposed a new approach for visual field assessment. The numbers 0, 3, or 7 are presented randomly, one at a time and at different points of the extreme peripheral portion of the screen (upper, middle, or lower) for 0.3–0.5 seconds during subcortical mapping. Like by Double Picture Naming Task (DPNT), the patient is encouraged to keep his/her gaze fixed to the center of the screen. Errors of detection and visual discrimination could be separated according to the stimulus of optic radiation and ILF respectively. Naming of a number is a strongly acquired automatism, which decreases the occurrence of errors due to language alteration. Hence, the technique showed great specificity for optic radiation mapping and was able to reduce visual deficits when compared to the control group, submitted to DPNT.
The visual cortex also has important connections with the thalamus, which acts as a gateway to the frontal eye field, supplementary ocular cortex, and lateral prefrontal cortex. Déjerine in 1901 has showed the relationships of pulvinar nucleus with posterior temporal and parietal adjacent regions (cited by [31]). And later on, Walker in 1938 reported pulvinar relation to temporo-parietal occipital association cortex [31]. The dorsomedial, ventrolateral, ventroanterior, and dorsolateral thalamus nuclei act in the control of self-generated eye movements, saccades, and visuospatial attention [22, 41]. Such functional aspects were explored by the author in a recent case (Fig. 7.10, Video 7.1). Awake craniotomy and DES might be useful tools in identifying these connections.
Male, 47 y.o. Left thalamic cavernoma. The patient underwent awake craniotomy for intraoperative visual field and semantic testing. [tests available at vemotests.com]. (a) Tractography demonstrating the corticospinal tract in blue. (b) Video: During cavernoma manipulation, the patient referred diplopia in the left quadrants. He was instructed to gaze to the center of the screen and name the objects in the quadrants. This case illustrates the role of the thalamus and its connections to eye movement and visuospatial attention
Both bipolar stimulation at 50-60 Hz and monopolar stimulation at 250–500 Hz might be used for VP mapping. The choice of mapping technique should be based on team experience (neurophysiologist and neurosurgeon). Additionally, the patient’s clinical presentation, such as presence or absence of seizures, degree of tumor infiltration, edema, and delineation of the tumor borders should also be taken into consideration. More details are in Chap. 18.
The patient should be comfortably positioned and with wide face exposure. If patient uses glasses, they should be prepared for intraoperative use by removing one or both straps as necessary. A previous clinical evaluation and tractography images analysis are important to set IOM protocol. Switching between appropriate tests directed to the functions of the anatomical resection area optimizes the results. Continuous communication between the tester and the surgeon is of paramount importance.
Mapping of VP during awake craniotomies is a good technique to recognize interindividual anatomical heterogeneity and anatomical-functional relationships [10, 12, 35, 52]. The functional connectivity cannot be assessed with flash-PEV. Tractography has limitations in identifying fiber crossing, underestimating the anterior extent of Meyer’s loop, for example. We reported a case, in which we performed awake craniotomy and flash-VEP during two different approaches for left and right occipital lesions [44, 45]. We had the unique opportunity of analyzing the outcome of the same patient after either technique. Awake craniotomy was performed for resection of the left lesion, without new deficits. Asleep approach using flash-VEP was used for the right lesion. The patient evolved with left inferior temporal quadrantanopia.
Awake craniotomy plays an important role in VP mapping and monitoring.
Anesthesia and Systemic Factors
Like other polysynaptic evoked potentials, VEP suffers wide interference from anesthetic drugs. Under total intravenous anesthesia (TIVA), VEP shows less amplitude and latency variability, compared to other anesthetic regimens [16, 43, 49]. Kodama and Goto [19] reported a 97% successful rate for flash-VEP recordings under TIVA in twelve years’ experience.
Inhaled anesthetics such as nitric oxide, sevoflurane, and isoflurane markedly depress the amplitude of P100, even at low doses [3]. Conflictingly, Ota et al. [33] reported stable P100 in 14 of 17 patients using sevoflurane and propofol. Kamada et al. [17] used only sevoflurane, and P100 was successfully recorded over the cortex. However, in most surgeries where VEP monitoring is indicated, motor evoked potential is also required, and in this context, inhalational anesthesia should be avoided.
Other venous agents such as Thiopental increase the latency and decrease the amplitude of the VEP in a dose-dependent manner. Its suppressive effect is already considerable at low doses. Ketamine only slightly increases latency, but markedly attenuates amplitude [16].
The brain is an organ of high metabolism, with high and constant demand for oxygen and nutrients. Therefore, alterations in systemic homeostasis compromise the detection and stability of VEP, since they hinder synaptic transmission and/or axonal conduction. Hypoxemia, hypotension, and hemodilution, especially hematocrit below 15%, decrease oxygen transport to the central nervous system, with probable effect on VEP latency increase and amplitude deterioration. In somatosensory evoked potentials (SSEPs), hypocapnia generates changes in pH and ions’ balance, with impaired conduction velocity [16]. As a polysynaptic pathway, we could infer that the same changes occur in obtaining P100. Hypothermia, in turn, interferes in synaptic transmission. Russ et al. [37] evidenced that a drop in body temperature by 1° reduces the central conduction of SSEPs by 15%. The increase in the latency of VEPs with hypothermia has already been shown [36].
Conclusion
Different visual stimulus and recording parameters have been described for VEP IOM in the past decade. It led to improvement of the technique, more stable recordings, and improved correlation to postoperative visual outcome. However, differences in reported techniques and interpretation parameters hinder reliability and consensus about intraoperative VEP.
Regarding awake craniotomies for VP cortical and subcortical mapping, reports point to its usefulness for identifying functional interconnections beyond the limitations of flash-VEP technique.
Flash-VEP presents low sensibility for detecting small optical radiation lesions and resulting quadrant impairment.
As with other neurophysiological techniques, VEP is sensitive to the effects of inhaled anesthetic agents and changes in systemic homeostasis. Hence, TIVA is recommended.
Team communication is an essential factor in mitigating existing technical and environmental biases.
Abbreviations
- AH:
-
Abductor hallucis
- APB:
-
Abductor pollicis brevis
- Bsl:
-
Baseline
- BR:
-
Brachioradialis
- DELT:
-
Deltoid
- DES:
-
Direct electrical stimulation
- DBS:
-
Deep brain stimulation
- EDC:
-
Extensor digitorum communis
- ERG:
-
Electroretinogram
- IFOF:
-
Inferior occipito-frontal fascicle
- ILF:
-
Inferior longitudinal fascicle
- IOM:
-
Intraoperative neurophysiological monitoring
- MEP:
-
Motor evoked potential
- OO:
-
Orbicular oris
- OP:
-
Optic nerve(s)
- PKAN:
-
Pantothenate kinase-associated neurodegeneration
- SEF:
-
Spectral EEG frequency
- SSEP:
-
Somatosensory evoked potential
- SS:
-
Sagittal Striatum
- TA:
-
Tibialis anterior
- TES:
-
Transcranial electrical stimulation
- TIVA:
-
Total intravenous anesthesia
- VEP:
-
Visual evoked potential(s)
- VM:
-
Vastus medialis
- VP:
-
Visual pathways
References
Benedicic M, Bosnjak R. Intraoperative monitoring of the visual function using cortical potentials after electrical epidural stimulation of the optic nerve. Acta Neurochir. 2011;153:1919–27. https://doi.org/10.1007/s00701-011-1098-y.
Boëx C, Goga C, Bérard N, Al Awadhi A, Bartoli A, Meling T, Bijlenga P, Schaller K. Intraoperative subcortical-cortical evoked potentials of the visual pathway under general anesthesia. Clin Neurophysiol. 2021; https://doi.org/10.1016/j.clinph.2021.02.399.
Cedzich C, Schramm J, Mengedoht CF, Fahlbusch R. Factors that limit the use of flash visual evoked potentials for surgical monitoring. Electroencephalogr Clin Neurophysiol. 1988;71:142–5.
Chang EF, Raygor KP, Berger MS. Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg. 2015;122:250–61. https://doi.org/10.3171/2014.10.JNS132647. Epub 2014 Nov 28.
Chang-Seng E, Moritz-Gasser S, Duffau H. Awake mapping for low-grade gliomas involving the left saggital stratum: anatomofunctional and surgical considerations. J Neurosurg. 2014;120:1069–77.
Coello AF, Moritz-Gasser S, Martino J, Martinoni M, Matsuda R, Duffau H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J Neurosurg. 2013;119:1380–94.
Duffau H. New concepts in surgery of WHO grade gliomas: functional brain mapping, connectionism and plasticity – a review. J Neuro-Oncol. 2006;79:77–115. https://doi.org/10.1007/s11060-005-9109-6.
Duffau H. Awake Surgery for Nonlanguage Mapping Neurosurgery. 2010;66:523–9. https://doi.org/10.1227/01.NEU.0000364996.97762.73.
Duffau H, Moritz-Gasser S, Mandonnet E. A re-examination of neural basis of language processing: proposal of a dynamic hodotopical model from data provided by brain stimulation mapping during picture naming. Brain & Language. 2014;131:1–10.
Duffau H, Schotten MT, Mandonnet E. White matter functional connectivity as an additional landmark for dominant temporal lobectomy. J Neurol Neurosurg Psychiatry. 2008;79:492–5. https://doi.org/10.1136/jnnp.2007.121004.
Duffau H, Velut S, Mitchell MC, Gatignol P, Capelle L. Intra-operative mapping of the subcortical visual pathways using direct electrical stimulations. Acta Neurochir. 2004;000:1–6. https://doi.org/10.1007/s(0701-003-0199-7.
Ebeling U, Reulen HJ. Neurosurgical topography of the optic radiation in the temporal lobe. Acta Neurochir. 1988;92:29–36.
Gras-Combe G, Moritz-Gasser S, Herbet G, Duffau H. Intraoperative subcortical electrical mapping of optic radiations in awake surgery for glioma involving visual pathways. J Neurosurg. 2012;117:466–73.
Gutzwiller EM, Cabrilo I, Radovanovic I, Schaller K, Boëx C. Intraoperative monitoring with visual evoked potentials for brain surgeries J Neurosurg. 2018; https://doi.org/10.3171/2017.8.JNS171168. Epub 2018 March 30.
Harding GFA, Bland JDP, Smith VH. Visual evoked potential monitoring of optic nerve function during surgery. J Neurol Neurosurg Psychiatry. 1990;53:890–5.
Hayashi H, Kawaguchi M. Intraoperative monitoring of flash visual evoked potential under general anesthesia. Korean J Anesthesiol. 2017;70(2):127–35.
Kamada K, Todo T, Morita A, Masutani Y, Aoki S, Ino K, Kawai K, Kirino T. Functional monitoring for visual pathway using real-time visual evoked potentials and optic-radiation tractography. Neurosurgery. 2005;57(ONS Suppl 1):ONS-121–7.
Kikuchi Y, Sasaki T, Matsumoto M, Oikawa T, Itakura T, Kodama N. Optic nerve evoked potentials elicited by electrical stimulation – experimental and clinical studies. Neurol Med Chir. 2005;45:349–55.
Kodama K, Goto T. Neurophysiology of the visual system: basics and intraoperative neurophysiology techniques. Deletis V, Shils JL, Sala F, Seidel K, Neurophysiology in Neurosurgery – A modern approach. Elsevier; 2020.53–64.
Kodama K, Goto T, Sato A, Sakai K, Tanaka Y, Hongo K. Standard and limitation of intraoperative monitoring of the visual evoked potential. Acta Neurochir. 2010;152:643–8.
Landi A, Pirillo D, Cilia R, Antonini A, Sganzerla EP. Cortical visual evoked potentials recorded after optic tract near field stimulation during GPi-DBS in non-cooperative patients. Clin Neurol Neurosur. 2011;113:119–22.
Lappi O. Eye movements in the wild: oculomotor control, gaze behavior & frames of reference. Neurosci Biobehav Rev. 2016;69:49–68.
Lee HW, Hong SB, Seo DW, Tae WS, Hong SC. Mapping of functional organization in human visual cortex: electrical cortical stimulation. Neurology. 2000;54:849–54. https://doi.org/10.1212/WNL.54.4.849.
Lowitsch K, Hopf HC, Buchner H, Claus D, Jörg J, Rappelsberger P, Tackmann W. Visuell evozierte Potentiale (VEP). In: EP-Buch D, editor. . 1st ed. Stuttgart: Georg Thieme Verlag; 2000.
Luo Y, Regli L, Bozinov O, Sarnthein J. Clinical utility and limitations of intraoperative monitoring of visual evoked potentials. PLoS One. 2015;10(3):e0120525. https://doi.org/10.1371/journal.pone.0120525.
Mandonnet E, Gatignol P, Duffau H. Evidence for an occipito-temporal tract underlying visual recognition in picture naming. Clin Neurol Neurosur. 2009;111:601–5.
Manohar N, Thakkar K, Balasubramaniam A, Palan A. Globus pallidus internum targeted deep brain stimulation placement using optic tract stimulated visual evoked potentials and corticospinal tract stimulation in a case of severe dystonia. Neurol India. 2019;67:600–1.
Møller AR, Burgess JE, Sekhar LN. Recording compound action potentials from the optic nerve in man and monkeys. Electroencephalogr Clin Neurophysiol. 1987;67:549–55.
Conti Nibali M, Leonetti A, Puglisi G, Rossi M, Sciortino T, Gay LG, Arcidiacono UA, Howells H, Viganò L, Zito PC, Riva M, Bello L. Preserving visual functions during gliomas resection: feasibility and efficacy of a novel intraoperative task for awake brain surgery. Front Oncol. 2020;10:1485. https://doi.org/10.3389/fonc.2020.01485.
Noachtar S, Hashimoto T, Lüders H. Pattern visual evoked potentials recorded from human occipital cortex with chronic subdural electrodes. Electroencephalogr Clin Neurophysiol. 1993;88:435–46.
Ojemann GA, Fedio P, van Buren JM. Anomia from pulvinar and subcortical parietal stimulation. Brain. 1968;91:99–116. https://doi.org/10.1093/brain/91.1.99.
Ojemann GA. The neurobiology of language and verbal memory: observations from awake neurosurgery. Int J Psychophysiol. 2003;48:141–6. https://doi.org/10.1016/S0167-8760(03)00051-5.
Ota T, Kawai K, Kamada K, Kin T, Saito N. Intraoperative monitoring of cortically recorded visual response for posterior visual pathway. J Neurosurg. 2010;112:285–94. https://doi.org/10.3171/2009.6.JNS081272.
Rajan S, Simon MV. Intraoperative neurophysiologic monitoring and mapping of the visual pathways. In: Simon MV, editor. Intraoperative Neurophysiology – a comprehensive guide to monitoring and mapping. Demos Medical Publishing; 2019. p. 303–11.
Rubino PA, Rhoton AL, Tong X, Oliveira E. Three-dimensional relationships of the optic radiation. Neurosurgery. 2005;57(ONS Suppl 3):ONS-219–27. https://doi.org/10.1227/01.NEU.0000176415.83417.16.
Russ W, Kling D, Loesevitz A, Hempelmann G. Effect of hypothermia on visual evoked potentials (VEP) in humans. Anesthesiol. 1984;61:207–10.
Russ W, Sticher J, Scheld H, Hempelmann G. Effects of hypothermia on somatosensory evoked responses in man. Br J Anaesth. 1987;59:1484–91.
Sasaki T, Itakura T, Susuki K, Kasuya H, Munakata R, Muramatsu H, Ichikawa T, Sato T, Endo Y, Sakuma J, Matsumoto M. Intraoperative monitoring of visual evoked potential: introduction of a clinically useful method. J Neurosurg. 2010;112:273–84.
Shahar T, Korn A, Barkay G, Biron T, Hadanny A, Gazit T, Nossek E, Ekstein M, Kesler A, Ram Z. Elaborate mapping of the posterior visual pathway in awake craniotomy. J Neurosurg. 2017; https://doi.org/10.3171/2017.2.JNS162757. Epub 2017 August 25.
Surbeck W, Hildebrandt G, Duffau H. The evolution of brain surgery on awake patients. Acta Neurochir. 2015;157:77–84. https://doi.org/10.1007/s00701-014-2249-8.
Tanaka M, Kunimatsu J. Thalamic roles in eye movements. In: The Oxford Handbook of Eye Movements; 2012. https://doi.org/10.1093/oxfordhb/9780199539789.013.0013.
Tobimatsu S, Shima F, Ishido K, Kato M. Visual evoked potentials in the vicinity of the optic tract during stereotactic pallidotomy. Electroencephalogr Clin Neurophysiol. 1997;104:274–9.
Uribe AA, Mendel E, Peters ZA, Shneker BF, Abdel-Rasoul M, Bergese SD. Comparison of visual evoked potential monitoring during spine surgeries under total intravenous anesthesia versus balanced general anesthesia. Clin Neurophysiol. 2017;128:2006–13.
Verst SM, Castro I, Junior WS, Melo MN, Oliveira JR, Almeida SS, Alvarez NRC, Sucena ABC, Barros MR, Marrone CD, Battistella LR, Vasconcellos MAK, Alves AS, Maldaun MVC. Verst-Maldaun language assessment (VMLA) validation process database. Mendeley Data. 2020;V2 https://doi.org/10.17632/zjhfk7mm7v.2.
Verst SM, Melo MN, Caivano AS, Fonseca US, Mathias LR Jr, Vilasboas T. Awake surgery versus VEP in tumors of visual pathway: case report. Interdiscip Neurosurg. 2020;20:100675.
Verst SM, Castro I, Scappini-Junior W, Melo MN, Oliveira JR, Almeida SS, Alvarez NRC, Sucena ACB, Barros MR, Marrone CD, Maldaun MVC. Methodology for creating and validating object naming and semantic tests used by Verst-Maldaun language assessment during awake craniotomies. Clin Neurol Neurosurg. 2021;202:106485.
Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, Mazoyer B, Tzourio-Mazoyer N. Meta-analyzing left hemisphere language areas: phonology, semantics and sentence processing. NeuroImage. 2006;30:1414–32.
Vilasboas T, Herbet G, Duffau H. Challenging the myth of right nondominant hemisphere: lessons from corticosubcortical stimulation mapping in awake surgery and surgical implications. World Neurosurg. 2017;103:449–56.
Watson KR, Shah MV. Clinical comparison of ‘single agent’ anaesthesia with sevoflurane versus target-controlled infusion of propofol. Br J Anaesth. 2000;85:541–6.
Wiedemayer H, Fauser B, Sandalcioglu IE, Armbruster W, Stolke D. Observations on intraoperative monitoring of visual pathways using steady-state visual evoked potentials. Eur J Anaesthesiol. 2004;21:429–33.
Wilson-Pauwels L, Akesson EJ, Stewart PA, Spacey SD. Optic Nerve. In: Decker BC, editor. Cranial Nerves in health and disease. 2nd ed; 2002.
Yogarajah M, Focke NK, Bonelli S, Cercignani M, Acheson J, Parker GJM, Alexander DC, McEvoy AW, Symms MR, Koepp MJ, Duncan JS. Defining Meyer’s loop-temporal lobe resections, visual field deficits and diffusion tensor tractography. Brain. 2009;132:1656–68.
York D. Visual evoked potentials during surgery. In: Nuwer MR, editor. Intraoperative monitoring of neural function. Handb Clin Neurophysiol. Elsevier; 2008. p. 172–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Electronic Supplementary Material
Male, 47 y.o. Left thalamic cavernoma. The patient underwent awake craniotomy for intraoperative visual field and semantic testing [tests available at vemotests.com]. (a) Tractrography demonstrating the corticospinal tract in blue. (b) Video: During cavernoma manipulation, the patient referred diplopia in the left quadrants. He was instructed to gaze to the center of the screen and name the objects in the quadrants. This case illustrates the role of the thalamus and its connections to eye movement and visuospatial attention (MOV 25573 kb)
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
de Melo, M.N., Verst, S.M. (2022). Evoked Potential – Visual Pathways Approach beyond Visual Evoked Potentials. In: Verst, S.M., Barros, M.R., Maldaun, M.V.C. (eds) Intraoperative Monitoring. Springer, Cham. https://doi.org/10.1007/978-3-030-95730-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-95730-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-95729-2
Online ISBN: 978-3-030-95730-8
eBook Packages: MedicineMedicine (R0)