Abstract
Intraventricular tumors account for less than 2% of all intracranial tumors but are more common among children and adolescents. Colloid cysts are the most prevalent tumor of the third ventricle causing obstruction of the foramen of Monro, despite accounting for less than 2% of primary brain tumors overall. Resection of symptomatic and sometimes even incidental lesions is recommended due to potential for serious complications including sudden death. In current practice, the transcortical and transcallosal approaches are the two most heavily favored procedures for debulking the cyst and removing all cyst wall attachments, but preferences vary by the institution and individual surgeon. For tumors located in the lateral ventricles and fourth ventricles, several approaches may be utilized with the ultimate consideration being the preservation of eloquent brain regions. Techniques for removal of these lesions are reviewed with attention paid to complication profiles of different approaches in relation to ventricular anatomy. The relative advantages and limitations of endoscopic versus microsurgical approaches are discussed in the context of specific clinical scenarios. Open approaches to the subcortical spaces are briefly discussed as well, with attention to the benefits and risks of transgyral and transsulcal approaches.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Intraventricular tumor
- Colloid cyst
- Transcortical
- Transventricular
- Transforaminal
- Transcallosal
- Interhemispheric
- Parafascicular
- Third ventricle
- Fourth ventricle
- Lateral ventricles
- Surgical approaches
- Complications
Introduction to Intraventricular Tumors
Intraventricular tumors pose a unique surgical challenge due to their deep location and close association with perforating arteries and complex white matter tracts. One of the earliest descriptions of the treatment of intraventricular tumors comes from Walter E. Dandy’s monograph, Benign Tumors in the Third Ventricle of the Brain: Diagnosis and Treatment [19]. In this study, Dandy details a series of 21 cases with a mortality of 33%. Since then, there has been substantial research into the intricate neuroanatomy of the ventricles and their surrounding structures. In particular, tractography through diffusion tensor imaging has provided invaluable insight into the multidirectional architecture of white matter tracts [7].
Intraventricular tumors account for only 0.8–1.6% of all intracranial tumors but make up 16% of childhood and adolescent intracranial tumors [1]. These tumors can be broadly classified into primary intraventricular tumors or parenchymal tumors with exophytic growth. Primary intraventricular tumors originate from the lining of the ventricles [11]. This includes the ependymal or subependymal lining, septum pellucidum, choroid plexus, and arachnoid tissue. Parenchymal tumors with exophytic growth arise from regions surrounding the ventricles and grow more than two-thirds into a ventricle [1]. The following eight types of tumors account for more than 90% of intraventricular neoplasms: choroid plexus papillomas, chordoid plexus carcinomas, meningiomas, ependymomas, subependymomas, subependymal giant cell astrocytomas (SEGA), central neurocytomas, and metastases [1]. As summarized by Agarwal and Kanekar, each of these types of tumors are more likely to appear in certain ventricles [1]. Specifically, 50% of choroid plexus tumors occur in the lateral ventricles, and 40% occur in the fourth ventricle. Meningiomas commonly occur in the atria of the lateral ventricles. Ependymomas occur in the fourth ventricle in >60% of cases, while subependymomas occur in the fourth ventricle in 50–60% of cases and the lateral ventricular margins or septum in 30–40% of cases. SEGAs nearly always occur at the foramen of Monro, and central neurocytomas typically are found at the inferior septum pellucidum and anterior lateral ventricle. Intraventricular metastases account for only 0.9–4.6% of all cerebral metastases and are typically seen in the lateral ventricles [40]. Clinical signs and symptoms are usually secondary to cerebrospinal fluid (CSF) obstruction and elevated intracranial pressure. Infants may present with hydrocephalus, loss of appetite, and irritability [80]. Children and adults present with headache and vomiting with papilledema [22]. The specific location of the tumor may result in additional findings. For example, tumors of the posterior fossa may produce cerebellar dysfunction [1]. It should be noted that seizures and visual changes are not typically associated with intraventricular tumors [1].
Introduction to Colloid Cysts
In 1858, Heinrich Wallmann identified the first colloid cyst on autopsy in his manuscript titled Eine colloid cysts im dritten Hirnnventrikl und ein lipom im plexus chorioides [76]. The first successful operative removal would not occur until 1921 on a young female patient confirmed to have a third ventricular tumor on cerebral pneumography at the Johns Hopkins Hospital by Dandy [18]. Despite accounting for less than 2% of primary brain tumors overall, colloid cysts are now commonly recognized as the most prevalent tumor of the third ventricle causing CSF obstruction of the foramen of Monro [30, 72]. While the majority of colloid cysts are stable and found incidentally on imaging, patients can present with symptoms of obstructive hydrocephalus or even with dementia, and gait disturbances without elevated pressure, similar to normal pressure hydrocephalus [49]. Drop attacks and paradoxical headaches caused by changes in head position have also been described in the literature although this finding has not been proven to be pathognomonic [83]. Of all the possible outcomes associated with colloid cysts, the most feared is sudden death without signs of herniation or hydrocephalus speculated to arise from acute cardiac arrest associated with compression of hypothalamic cardiovascular regulatory centers [45, 75]. For this reason, neurosurgeons favor surgical treatment over conservative modalities such as observation for even asymptomatic colloid cysts when signs of cyst or ventricle enlargement are present [36].
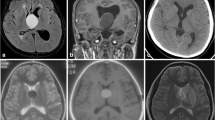
While these benign and congenital tumors have no illustrated genetic loci, familial inheritance has been posited through twin studies and generational reports in the literature [2, 9, 58, 62]. Embryologically, colloid cysts are believed to arise from either endodermal respiratory epithelium or components of the primitive neuroectoderm that give rise to the tela choroidea [41]. These theories are supported by the finding that they are immunohistochemically distinct in profile from choroid plexus epithelium and ependyma [43]. Colloid cysts are composed of an inner mucin-producing epithelial layer that stains PAS positive surrounded by an outer fibrinous layer that forms adhesions to surrounding ventricular structures anchored by a pedicle or broad sessile base [41, 44, 53]. In the overwhelming majority of cases, colloid cysts develop in the anterior portion of the third ventricle although rare cases in the septum pellucidum, retroforniceal region, and velum interpositum have rarely been reported [6]. The classic rostral presentation in the roof of the third ventricle occluding or even protruding through the foramen of Monro to occupy space in the lateral ventricles can easily be identified on CT imaging, in part due to the cyst’s proteinaceous contents composed of radiodense calcium ([50]; Fig. 6.1). MR imaging of colloid cysts may provide additional prognostic value as cyst T2 hyperintensity has been reported to correlate positively with risk of future growth and mass effect symptoms [8, 61]. However, CT scans are preferred as colloid cysts can variably appear isointense on MR imaging [51]. Colloid cysts range from 3 to 40 mm in diameter, with even larger cases reported in the literature [3, 5].
Anatomy of the Lateral Ventricles
It is necessary to understand the anatomy of the ventricles in order to anticipate the risks and challenges of resecting intraventricular tumors. The lateral ventricles are a pair of curved structures composed of a body, atria, and three horns. These three horns are known as the anterior horn, posterior horn, and inferior horn. Both of the lateral ventricles communicate with the third ventricle through the foramen of Monro. The two lateral ventricles are separated by the thin septum pellucidum. The body of the lateral ventricles lies within the parietal lobe and extends anteriorly from the foramen of Monro to the splenium of the corpus callosum posteriorly [66]. Thus, the roof of the body is formed by the inferior portion of the body of the corpus callosum. The floor of the body is composed, from lateral to medial, of the body of the caudate nucleus, the stria terminalis and thalamostriate vein, the lateral part of the superior surface of the thalamus, and the choroid fissure [68]. The atria have a triangular shape and connect to the body, inferior horn, and posterior horn. The tapetum of the splenium of the corpus callosum forms the roof of the atrium, the floor is formed by the collateral trigone, and the medial wall is formed by the calcar avis of the calcarine fissure [66]. Similarly, the medial wall of the posterior horn is formed by the bulb of the corpus callosum and calcar avis. The floor of the posterior horn is formed by the collateral trigone. The roof and lateral walls are formed by the tapetum and separate the horn from the optic radiations [68]. The inferior horn extends from the atrium and terminates at the amygdala. Its floor is formed by the collateral eminence and hippocampus, with the hippocampus covered by the alveus. The roof of the inferior is formed laterally by the tapetum and medially by the tail of the caudate nucleus and stria terminalis [68]. The anterior horn’s anterior wall and roof are bounded by the genu of the corpus callosum, the floor of the horn is formed by the rostrum, and the lateral walls are formed by the head of the caudate nucleus. The medial wall of the frontal horn is formed by the columns of the fornix inferiorly [66].
Third Ventricular Anatomy
The approach for resection of third ventricular tumors, including colloid cysts, depends on the location, lesion size, and the clinical status of the patient. For instance, the transcortical approach favors tumors that require subchoroidal exposure and provides optimal access to tumors off the anterior third ventricle but would be inappropriate for those in the posterior compartment [27]. This approach is also favored in patients with larger ventricles or preexisting ventricular dilation and is more effective for voluminous tumors [55, 60]. Thorough knowledge of third ventricular anatomy is critical to appreciate the operative risks and tenets that must be at the fore of any open procedures targeting the colloid cysts. The third ventricle is a unilocular, narrow, midline cavity bearing semblance to a funnel in functionality and appearance [77]. The floor of the third ventricle is composed of the mamillary bodies, the infundibulum of the hypothalamus, the posterior perforated substance, and the midbrain tegmentum. The lateral walls are bound by the hypothalamus inferiorly and the thalamus superiorly. The choroid fissure is located between the thalamus and the body of the fornix and is the site of origin for the choroid plexus. The posterior wall is made of the pineal body, habenular commissure, pulvinar, posterior commissure, the splenium of the corpus callosum, and the aqueduct of Sylvius at its lowest point. Anteriorly, the third ventricular space is bound by the optic chiasm, lamina terminalis, and the rostrum of the corpus callosum [23, 78].
The roof of the third ventricle, which houses essential vessels, is especially important to spatially envision as colloid cysts frequently form fibrinous adhesions to this region. The roof consists of four layers (Fig. 6.2). The most superior layer is made of the body of the fornix anteriorly, with the posterior aspect encompassing the crura and hippocampal commissure. The next three layers include two thin layers of connective tissue derived from pia mater named tela choroidea, with a layer between them housing blood vessels. The choroid plexus in the roof of the third ventricle is attached to the deep layer of the tela. The velum interpositum, located between the two layers of the tela, contains the medial posterior choroidal arteries and the internal cerebral veins more anteriorly. The medial posterior and superior posterior choroidal arteries supply the tela choroidea [64, 82]. The anterior section of the roof of the third ventricle is cannulated by the foramen of Monro. Here, structures like the choroid plexus, medial posterior choroidal artery, internal cerebral vein, anterior septal vein, and thalamostriate vein all converge [26]. The foramen of Monro is accessed in order to debulk and remove colloid cysts in both the transcortical and transcallosal approaches.
Fourth Ventricle Anatomy
The fourth ventricle is a single, midline structure located anteriorly from the cerebellum and posteriorly from the medulla and pons. The ventricle is pyramid shaped, with the base of the pyramid composed of the dorsal side of the brainstem and the apex of the cone located in the anterior part of the cerebellar vermis [54]. The floor of the fourth ventricle is covered by a single-cell layer of ependyma, and the impressions of cranial nerve nuclei can be appreciated. The longitudinal median sulcus forms the vertical midline of the ventricle. The hypoglossal trigone can be observed at the medullary level, while the colliculus of the facial nerve can be seen at the pontic level [54]. The base of the pyramid is also characterized by four corners, or angles. The two lateral angles are the foramina of Luschka, the upper angle communicates with the aqueduct of Sylvius, and the lower angle continues as the central canal of the medulla and spinal cord. Notably, the obex forms the posterior wall of the lower angle. The apex of the fourth ventricle, the fastigium, is situated in the ventral surface of the cerebellar vermis and is bounded laterally by the cerebellar peduncles, superiorly by the lingula, and inferiorly by the nodule [54]. The superior walls of the fourth ventricle are formed by the superior cerebellar peduncle, while the lower walls are formed by the inferior cerebellar peduncles. Importantly, the lower portion of the roof supports the choroid plexuses of the fourth ventricle which are a set of paired structures that run from the obex to the lateral angles. The foramen of Magendie, a medial opening of the fourth ventricle that communicates with the subarachnoid space through the cisterna magna, is located at the bottom of the inferior medullary velum [54]. Generally speaking, the fourth ventricle is supplied by the basilar artery and cerebellar arteries. Specifically, the superior portion of the fastigium is vascularized by the superior cerebellar artery, which runs through the cerebellar-mesencephalic fissure [63]. The lower portion of the roof and choroid plexus is supplied by the posterior inferior cerebellar artery. The anterior inferior cerebellar artery, located along the cerebellar-pontine fissure, supplies the lateral angles of the base of the ventricle. The base is also supplied by perforating branches of the anterior spinal artery, basilar artery, and P1 of the posterior cerebral artery [63]. Venous drainage of the fourth ventricle is carried through the veins running with their corresponding arteries [54].
Open Transcortical, Transventricular, and Intraventricular Approach
Open surgical approaches for colloid cysts have not changed drastically since 1921, with Dandy favoring a posterior transcallosal approach followed by the development of a frontal transcortical approach by Greenwood in 1949 [19, 31]. Mckissock further refined this technique in 1951, paving a path for neurosurgeons to reach deeply located tumors in coordinates surrounded by critical neurovasculature [15]. With the advent of endoscopic technologies, enhanced microsurgical techniques, improved imaging, and reimagined navigation through frameless stereotaxy, the relatively high morbidity associated with early surgeries for these benign lesions has been greatly reduced [69]. Open surgery is now considered the gold standard for the categorical treatment of colloid cysts [56]. Stereotactically guided aspiration has been performed as an alternative to open surgery, but these procedures often fail to completely eradicate the cyst and are noted to have a high rate of recurrence [52]. For tumors of the third ventricle, the transcallosal and transcortical approaches are most often employed. The optimal procedure for removal of colloid cysts should minimize insults to brain parenchyma associated with prolonged retraction. In addition, patient-specific clinical status and ventricular anatomy should be taken into account. For instance, acute management of obtunded patients experiencing obstruction at the foramen of Monro routinely involves bilateral drainage of the lateral ventricles to avoid subfalcine herniation. However, in patients with GCS scores of 14 or 15, ventricular drainage should be deferred to allow for dilation and ease of operation [46]. Permissive dilation is particularly useful in the transcortical approach to the third ventricle.
The initiation of the transcortical approach for removal of colloid cysts begins with neuronavigation, ensuring that the entry pathway directly points toward the foramen of Monro. Once registration is completed, a small frontal burr hole craniotomy is performed with a cruciate dural incision made to reveal the middle frontal gyrus of the nondominant hemisphere, usually on the right side. The left side may be favored if the same-sided ventricle is dilated to a greater extent. Kocher’s point, 2.5 cm from the midline and 1 cm anterior to the coronal suture, is then identified [4]. A small resection is made 0.5–1.5 cm lateral to this landmark to allow for passage of a guiding catheter into the frontal horn of the lateral ventricle. Successful insertion of the catheter should bypass the motor strip and nearby eloquent structures including Broca’s area and subsequently guide dissection in later steps. In its initial stages, the transcortical approach should feel commonplace as it is a continuation of basic neurosurgical skills. Next, microdissection is performed through the white matter surrounding the catheter to create a 1–2 cm linear cerebrotomy. Minimal cortical opening is performed in order to decrease the likelihood of postoperative epilepsy [41]. Frameless stereotaxy is used throughout this process to ensure that the planned trajectory is maintained (Fig. 6.3).
It is the author’s preference to use tubular retractor systems that navigate critical subcortical tracts parafascicularly. Smaller variants of these tools are preferred in order to eliminate unnecessary disruption of adjacent structures. Before setting the retractors, the foramen of Monro must be visualized. A helpful method of triangulating this aperture is to search for the more obvious placement of the choroid plexus and to follow it anteriorly until the point of convergence with the septal and thalamostriate veins. The foramen of Monro lies just anterior to this point. Once identified, the retractors are set [46].
Prior to removing the cyst, which often causes bilateral ventricular dilation due to its position in the anterior third ventricle, a pellucidotomy is performed so that only unilateral shunting is needed postsurgically should the patient develop hydrocephalus [81]. Once completed, the colloid cyst is then targeted. If there is difficulty visualizing the wall of the cyst, the surgeon may opt for a more posterolateral angle to directly view the foramen of Monro. With this line of sight afforded from the lateral ventricle, larger colloid cysts can be immediately identified. Smaller cysts may be obstructed by the choroid plexus which requires readjusting in order to reveal the lesion; however, forniceal integrity must be preserved in order to safeguard the patient from suffering impairments in memory [42]. Any vessels originating from the choroid plexus that feed the colloid cysts are coagulated and then cut. The cyst is then punctured using a ringed curette with its contents carefully aspirated. Any attachments to the roof of the third ventricle or surrounding structures are also coagulated and cut with the cyst and then removed through the foramen of Monro as a whole instead of piecemeal. Using this approach, there is a surgical blind spot as the posterior compartment of the third ventricle may have a small piece of cyst capsule that remains attached. For this reason, an endoscope may be useful for observing the posterior aspect of the third ventricle and the contralateral foramen of Monro [10].
Alternative routes to the intraforaminal approach to the third ventricle have been utilized to reach challenging colloid cysts. The transchoroidal approach was developed to gain access to the velum interpositum and then the bottom layers of the roof of the third ventricle by dissecting through the choroidal fissure, the area between the fornix and the thalamus [37]. Wen et al. have advocated for use of a transchoroidal approach as it takes advantage of microsurgical anatomy by travelling through a naturally occurring cleft. The top layer of the taenia choroidea is dissected through the choroidal fissure to reveal the two internal cerebral veins where further dissection between these vessels can be performed due to lack of bridging veins. This ultimately allows for exposure of the third ventricle after continuing through the bottom layer of taenia choroidea and the choroid plexus [77, 79]. However, this approach risks abruption of blood flow through retraction of the internal veins and the fornix [34]. Subchoroidal and suprachoroidal methods have also been recommended for the resection of colloid cysts. In the subchoroidal approach, the taenia choroidea is opened inferolaterally to the choroid plexus alongside the thalamus. The risk of damaging the thalamostriate vein is greatly increased in this approach as the route to the third ventricle passes within 5–10 mm of the vessel [56]. In the suprachoroidal approach, an incision in the tenia fornices is performed to reveal the internal cerebral veins and the remaining layers of the third ventricular roof. Both routes are performed nonroutinely due to their proximity to the thalamus, fornix, and aforementioned vasculature. However, in select cases, these approaches may be the preferred choice.
Open Transcallosal, Interhemispheric Approach
The transcallosal approach serves as the counterpart to the transcortical entry in modern neurosurgery. Guided by literature reporting less seizure activity, this interhemispheric craniotomy has grown in favor among colleagues as the preferred route for colloid cyst resection as well as for tumors located within the frontal horn, the body of the lateral ventricle, and the anterior third ventricle [80]. The transcallosal approach begins with adjusting the patient so that the superior sagittal sinus lines up parallel to the floor to leverage gravity retraction for when it becomes necessary to dissociate the sinus and falx away from the ipsilateral hemisphere [16]. A craniotomy anterior to the coronal suture is performed followed by a U-shaped dural incision to uncover the superior sagittal sinus [15, 73]. The sinus is then retracted to allow for access to the midline. Special care is taken to avoid parasagittal bridging veins; however, smaller veins may be sacrificed despite the best efforts. The corpus callosum is then targeted for microdissection after separation of the right superior frontal gyrus from the falx and circumnavigation around larger bridging cortical veins (Fig. 6.4). A 1–2 cm incision is made in the corpus callosum with microdissection performed painstakingly so as to spare the cingulate gyrus and any encountered pericallosal arteries [32]. After entry into the lateral ventricle is achieved, the same routes to the third ventricle used in the transcortical approach can be initiated for colloid cyst removal. For tumors located in the frontal horns and anterior portion of the body, the goal of resection is central enucleation and subsequent peripheral dissection. It can be beneficial to place cotton sponges into the trigone to prevent tumor cells from moving into the occipital and temporal horns [80]. The transcallosal approach is beneficial for patients without hydrocephalus, and recent advances in microsurgery have minimized the risk of vascular damage. Furthermore, the transcallosal approach avoids injury to white matter tracts located within the cortical and subcortical space.
Pterional Transsylvian Approach
The pterional transsylvian approach is commonly performed for tumors of the temporal horn of the lateral ventricle [80]. The patient is placed in dorsal decubitus position, and a pterional craniotomy is performed at the level of the Sylvian fissure. Likewise, the dura is opened with a central incision along the Sylvian fissure, and the fissure is then opened from the pars opercularis to the inferior frontal gyrus [59]. As the frontal lobe is separated from the temporal lobe, care is taken to mobilize the middle cerebral artery. The Sylvian artery must be displaced to better visualize the limiting sulcus of the insula [59]. A small corticotomy is then performed at the limiting sulcus of the insula behind the limen insulae, affording access to the temporal horn [13].
Parieto-Occipital Interhemispheric Approach
The parieto-occipital interhemispheric approach may be employed for tumors of the posterior third ventricle, atrium, and occipital horn [80]. The patient is placed in sitting position, and burr holes are made a few millimeters lateral of the midline on the contralateral side of the superior sagittal sinus. Incision of the dura should be performed to preserve the veins draining into the superior sagittal sinus. Dissection is then performed toward the splenium of the corpus callosum. An incision through the precuneal gyrus anterior to the parieto-occipital sulcus is then made, which provides access to the atria and occipital horns.
Telovelar Approach for the Fourth Ventricle
The median inferior suboccipital cerebellomedullary fissure approach, also known as the telovelar approach, allows access to the fourth ventricle for tumor removal [80]. With the patient in prone position, an incision is made from the C2–C3 spinous processes to the external occipital protuberance, and a burr hole is placed below the protuberance. This ensures that the craniotomy is performed below the transverse sinus. C1 laminectomy may be performed if this improves the working angle, especially when needing to reach the rostral aspects of the fourth ventricle [29]. Subsequently, an opening is made through the vallecula followed by separation of the cerebellar tonsils to access the tonsillouveal sulcus. At this point, the foramen of Magendie, tela choroidea, and inferior medullary velum may be observed. The telovelum is then opened to the level necessary to remove the tumor. This incision allows excellent access to the lateral angles, caudal half of the ventricle, and superolateral recess [29]. The most rostral aspects of the fourth ventricle may be more challenging to access, but as previously stated, C1 laminectomy allows for an improved angle of approach.
Complications and Controversies
The transcortical and transcallosal approaches to deep regions of the brain are complex and technically challenging procedures with their own inherent risk profiles. Complications shared between them include risk for damage to the thalamostriate vein, septal vein, fornices, and internal cerebral veins. Disruption of the thalamostriate vein can lead to hemorrhagic infarction of the thalamus and basal ganglia, which carries the risk of developing parkinsonism [47]. Damage to this vessel can also cause mutism, hemiplegia, and drowsiness [15]. While early reports advocated for sacrifice of this vein due to its strong collateral support in order to enlarge the foramen of Monro, the degree of collateral support has not been shown to prognosticate which patients would go on to develop postoperative complications [35, 74]. Therefore, active preservation of this structure is paramount. In cases where the colloid cyst projects superiorly to cause lateral separation of the fornices, the interforniceal route had been proposed as an alternative to the third ventricle. This too has fallen by the wayside in contemporary neurosurgical practice after poorer outcomes than initially anticipated result.
The transcallosal route carries unique sequelae as a result of its pathway to the lateral ventricle. Retraction of the cortex for extended periods can lead to postoperative bouts of contralateral leg weakness. However, improvements in microsurgery have minimized the need for prolonged insult. The greatest concern is that of venous infarction due to sacrifice of large cortical bridging veins or drawn-out retraction of the superior sagittal sinus, especially in patients with hypercoagulable states [28, 36, 48]. The likelihood that pericallosal arteries may be damaged is also heightened given that anatomical variants such as one or three pericallosal arteries may exist that are not apparent on preoperative MR imaging [24, 33, 39]. Disconnection syndrome has also been reported although limiting the incision to 2.5 cm or less within the corpus callosum has largely prevented this complication [70]. Furthermore, damage to the supplementary motor cortex or thalamus along the interhemispheric route may also contribute to mutism [57]. In terms of technical difficulty, the transcallosal approach can be disorienting for surgeons with respect to ventricular anatomy as different head placements may be set. Additionally, the working space available through the interhemispheric corridor may be narrower than in the transcortical approach.
In the transcortical approach, technical challenges include less space to maneuver when operating in patients with smaller or less dilated ventricles and the availability of only one foramen of Monro to access the third ventricle. Intraventricular complications related to this approach are the same as in the transcallosal approach except that there is less risk of damaging the fornix and thalamostriate vein (Laidlaw). Edema due to retraction of the cortex in this approach has been associated with contralateral, self-resolving hemiparesis [49]. Conflicting reports have been cited in the literature pertaining to cognitive ramifications arising from cerebrotomy. In 2002, Desai et al. discovered in their single institutional experience that of 30 patients who underwent a transcortical approach for removal of a colloid cyst, 26% had seizures [21]. Another group reported no seizures postoperatively after performing the same approach in 27 patients and corroborated these findings with a literature review revealing a seizure rate of 8.3% across 238 transcortical cases [25]. In direct contrast to commonly held sentiment, Milligan et al. found that out of the 127 patients who had removal of intraventricular lesions, postoperative seizures arose in 25% of those who underwent a transcallosal, interhemispheric approach versus 8% in the transcortical cohort (P = 0.1; [55]). These publications showcase the ongoing debate still surrounding the optimal approach to deep lesions of the third ventricle.
Between the transcortical and transcallosal approaches, the larger question looms as to whether endoscopic or microsurgical techniques offer better outcomes for resection of colloid cysts. Connolly et al. found that out of 483 patients, split almost evenly between endoscopic and microsurgical approaches, the seizure rate was 14.7% for the microsurgical group and 5.4% in the endoscopic group (P = 0.001). The 30-day readmission rate was also higher for the microsurgical group (P = 0.015) [17]. Contrastingly, Brostigen et al. found that there was no statistically significant difference between microsurgery and endoscopic surgery for colloid cysts related to the grade of resection or postoperative complications in 32 consecutive patients [12]. In corroboration with this finding, a more comprehensive meta-analysis of 1278 patients showed that a higher gross total resection rate and lower rate of occurrence was achieved using microsurgical techniques over the endoscopic method in transcranial operations. No significant differences existed in mortality rate between these two groups, but morbidity was higher in the microsurgical cohort [67]. As microsurgical and endoscopic techniques continue to evolve, the march of superiority alternates in stride. Endoscopic techniques that were once considered ineffective at completely removing the cyst wall have now emerged as a compelling option. Microsurgical approaches that previously produced higher seizure rates and mutism are now improved with neurosurgeons reducing retraction on the cortex and minimizing transgressions against eloquent fiber tracts aided by tools such as diffusion tensor imaging [71]. Both approaches can play a cooperative role in the removal of colloid cysts.
For instance, in cases where it is uncertain whether the entire cyst wall was removed en bloc, an endoscope can be used to illuminate the operative blind spot. Reciprocally, microsurgical techniques might be employed if the cyst wall is not completely removed by endoscopic procedures alone.
Approaches to the Subcortical Space
It is possible for intraaxial tumors to lie below the cortical surface but outside the ventricles, within the subcortical space. Broadly speaking, approaches to the subcortical space can be divided into transgyral or transsulcal approaches. Due to the deep-seated nature of these subcortical lesions, it is necessary to traverse through the cortex and surrounding white matter. The use of preoperative imaging, functional magnetic resonance imaging (fMRI), diffusion tensor imaging (DTI), and cortical mapping is therefore employed to minimize damage to eloquent regions [14].
A transgyral approach necessitates performing a corticectomy that will dissect through the overlying pia, gray matter, and white matter until the tumor is reached. It is therefore critical to choose a gyrus that will have minimal impact to the patient. Examples of such gyri include the anterior frontal lobe, superior parietal lobule, and inferior temporal gyrus [14]. This approach avoids damaging sulcal vessels and results in less damage to the subcortical “U” fibers [65].
For a transsulcal approach, the sulcus that is chosen is typically the one that reaches furthest to the lesion while avoiding critical regions. A craniotomy is performed with the dural opening centered over the sulcus. The arachnoid membrane overlying the sulcus is then carefully dissected [20]. Separation of the surround gyri is maintained with dynamic or fixed retraction as a corticectomy is performed to reach the lesion [14]. The transsulcal approach is considered a parafascicular approach since it is performed parallel to the major white matter tracts [38]. Furthermore, traversing the sulcus itself allows for minimal damage to the brain parenchyma although special care must be taken to avoid damaging sulcal vessels.
Conclusion
Since the first successful removals of colloid cysts and intraventricular tumors in the early 1920s, burgeoning technologic advancements and increasingly innovative techniques have been developed to ensure favorable outcomes for patients. Today, most neurosurgeons opt to perform either the transcortical or transcallosal approach to reach the foramen of Monro and the third ventricle for debulking and removal of colloid cysts, with important advantages and drawbacks to either approach. Conflicting reports of postoperative seizure complication rates recently shifted the operative paradigm to favor the transcallosal approach; however, it is the authors’ opinion that the transcortical, transventricular approach is just as safe and effective as the transcallosal, interhemispheric approach for resection of benign colloid cysts. Once inside the lateral ventricle, the intraforaminal route provides the safest corridor for removal of the cyst as it utilizes a naturally occurring cannulation and spares injury to the thalamus, fornix, and thalamostriate vein. Transchoroidal, subchoroidal, and suprachoroidal approaches are not advocated except in experienced hands or cases where the intraforaminal approach would render the planned resection untenable. In addressing the controversy between microsurgical and endoscopic techniques, the effectiveness will ultimately depend on the comfort and skill level of the operator as both technologies have proven successful at achieving the same end result. As a result of these neurosurgical advances, the majority of patients with colloid cysts are completely cured after resection. For intraventricular tumors, the precise location within the ventricular system dictates which approach should be utilized. Generally speaking, the anterior transcallosal, interhemispheric approach is used for frontal horn tumors, the pterional transsylvian approach for temporal horn tumors, the telovar approach for fourth ventricle tumors, and the parieto-occipital interhemispheric approach for tumors of the posterior third ventricle, occipital horn, and atria. Finally, for tumors lying within the subcortical space, transgyral or transsulcal approaches may be used. The choice of approach is largely dependent on whether an eloquent region will be at risk and if the surgeon feels comfortable maneuvering sulcal vessels. Lesions of the subcortical space and ventricular system are deep seated and require a thorough understanding of neuroanatomy in order to appreciate the approach that must be utilized and the risks of such an approach.
References
Agarwal A, Kanekar S. Intraventricular tumors. Semin Ultrasound CT MR. 2016;37(2):150–8.
Aggarwal A, Corbett A, Graham J. Familial colloid cyst of the third ventricle. J Clin Neurosci. 1999;6(6):520–2.
Agrawal A, Santhi V, Umamaheswara RV. Giant colloid cyst of the third ventricle: challenges in management. Chin Neurosurg J. 2016;2(1):11.
Aref M, Martyniuk A, Nath S, Koziarz A, Badhiwala J, Algird A, et al. Endoscopic third ventriculostomy: outcome analysis of an anterior entry point. World Neurosurg. 2017;104:554–9.
Armao D, Castillo M, Chen H, Kwock L. Colloid cyst of the third ventricle: imaging-pathologic correlation. Am J Neuroradiol. 2000;21(8):1470–7.
Azab WA, Salaheddin W, Alsheikh TM, Nasim K, Nasr MM. Colloid cysts posterior and anterior to the foramen of Monro: anatomical features and implications for endoscopic excision. Surg Neurol Int. 2014;7:5.
Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44(4):625–32.
Beaumont TL, Limbrick DD, Rich KM, Wippold FJ, Dacey RG. Natural history of colloid cysts of the third ventricle. J Neurosurg. 2016;125(6):1420–30.
Benoiton LA, Correia J, Kamat AS, Wickremesekera A. Familial colloid cyst. J Clin Neurosci. 2014;21(3):533–5.
Birski M, Birska J, Paczkowski D, Furtak J, Rusinek M, Rudas M, et al. Combination of neuroendoscopic and stereotactic procedures for total resection of colloid cysts with favorable neurological and cognitive outcomes. World Neurosurg. 2016;85:205–14.
Boudreau RP. Primary intraventricular tumors. Radiology. 1960;75(6):867.
Brostigen CS, Meling TR, Marthinsen PB, Scheie D, Aarhus M, Helseth E. Surgical management of colloid cyst of the third ventricle. Acta Neurol Scand. 2017;135(4):484–7.
Campero A, Ajler P, Garategui L, Goldschmidt E, Martins C, Rhoton A. Pterional transsylvian-transinsular approach in three cavernomas of the left anterior mesiotemporal region. Clin Neurol Neurosurg. 2015;130:14–9. https://doi.org/10.1016/j.clineuro.2014.12.013. PMID: 25576880.
Chaichana L. Kaisorn, chapter 22 – transsulcal versus transgyral approaches for subcortical tumors. In: Chaichana K, Quiñones-Hinojosa A, editors. Comprehensive overview of modern surgical approaches to intrinsic brain tumors. Academic Press; 2019. p. 379–91.
Cikla U, Swanson KI, Tumturk A, Keser N, Uluc K, Cohen-Gadol A, et al. Microsurgical resection of tumors of the lateral and third ventricles: operative corridors for difficult-to-reach lesions. J Neuro-Oncol. 2016;130(2):331–40.
Cohen-Gadol A. Interhemispheric craniotomy. In: Neurosurgical atlas. Neurosurgical Atlas, Inc.; 2016. [cited 2019 Jan 12]. Available from: http://www.neurosurgicalatlas.com/volumes/cranial-approaches/interhemispheric-craniotomy.
Connolly ID, Johnson E, Lamsam L, Veeravagu A, Ratliff J, Li G. Microsurgical vs. endoscopic excision of colloid cysts: an analysis of complications and costs using a longitudinal administrative database. Front Neurol. 2017;8:259. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5465269/
Dandy W. The Johns Hopkins hospital. Bull Johns Hopkins Hosp. 1922;32:562.
Dandy W. Benign tumors in the third ventricle of the brain: diagnosis and treatment. Arch Neur Psychiatry. 1935;33(1):242.
Day JD. Transsulcal parafascicular surgery using brain path® for subcortical lesions. Neurosurgery. 2017;64(CN_suppl_1):151–6.
Desai KI, Nadkarni TD, Muzumdar DP, Goel AH. Surgical management of colloid cyst of the third ventricle—a study of 105 cases. Surg Neurol. 2002;57(5):295–302.
Duong H, Sarazin L, Bourgouin P, Vézina JL. Magnetic resonance imaging of lateral ventricular tumours. Can Assoc Radiol J. 1995;46(6):434–42.
Duvernoy HM. The human brain: surface, three-dimensional sectional anatomy with MRI, and blood supply. Springer Science & Business Media; 2012. p. 492.
Ehni G. Interhemispheric and percallosal (transcallosal) approach to the cingulate gyri, intraventricular shunt tubes, and certain deeply placed brain lesions. Neurosurgery. 1984;14(1):99–110.
Eichberg DG, Sedighim S, Buttrick S, Komotar RJ. Postoperative seizure rate after transcortical resection of subcortical brain tumors and colloid cysts: a single surgeon’s experience. Cureus. 2018;10(1):e2115. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5871436/
Fonseca RB, Black PM, Filho HA. Approaches to the third ventricle. Arq Bras Neurocir. 2012;31(1):3–9.
Fujii K, Lenkey C, Rhoton AL. Microsurgical anatomy of the choroidal arteries: lateral and third ventricles. J Neurosurg. 1980;52(2):165–88.
Garrido E, Fahs GR. Cerebral venous and sagittal sinus thrombosis after transcallosal removal of a colloid cyst of the third ventricle case report. Neurosurgery. 1990;26(3):540–2.
Ghali MGZ. Telovelar surgical approach. Neurosurg Rev. 2019;44(1):61–76.
Glastonbury CM, Osborn AG, Salzman KL. Masses and malformations of the third ventricle: normal anatomic relationships and differential diagnoses. Radiographics. 2011;31(7):1889–905.
Greenwood J. Paraphysial cysts of the third ventricle: with report of eight cases. J Neurosurg. 1949;6(2):153–9.
Hendricks B, Cohen-Gadol A. Colloid cyst (transcallosal approach). In: Neurosurgical atlas. Neurosurgical Atlas, Inc.; 2016. Available from: http://www.neurosurgicalatlas.com/volumes/brain-tumors/intraventricular-tumors/third-ventricular-tumors/colloid-cyst/colloid-cyst-transcallosal-approach.
Hernesniemi J, Romani R, Dashti R, Albayrak BS, Savolainen S, Ramsey C, et al. Microsurgical treatment of third ventricular colloid cysts by interhemispheric far lateral transcallosal approach—experience of 134 patients. Surg Neurol. 2008;69(5):447–53.
Herrmann H-D. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42(6):1217–8.
Hirsch JF, Zouaoui A, Renier D, Pierre-Kahn A. A new surgical approach to the third ventricle with interruption of the striothalamic vein. Acta Neurochir. 1979;47(3):135–47.
Horn EM, Feiz-Erfan I, Bristol RE, Lekovic GP, Goslar PW, Smith KA, et al. Treatment options for third ventricular colloid cystscomparison of open microsurgical versus endoscopic resection. Neurosurgery. 2007;60(4):613–20.
Ibáñez-Botella G, Domínguez M, Ros B, De Miguel L, Márquez B, Arráez MA. Endoscopic transchoroidal and transforaminal approaches for resection of third ventricular colloid cysts. Neurosurg Rev. 2014;37(2):227–34. discussion 234
Jackson C, Gallia GL, Chaichana KL. Minimally invasive biopsies of deep-seated brain lesions using tubular retractors under exoscopic visualization. J Neurol Surg A Cent Eur Neurosurg. 2017;78(6):588–94. https://doi.org/10.1055/s-0037-1602698. PMID: 28482372.
Kapu R, Symss NP, Pande A, Vasudevan MC, Ramamurthi R. Management of pediatric colloid cysts of anterior third ventricle: a review of five cases. J Pediatr Neurosci. 2012;7(2):90–5.
Koeller KK, Sandberg GD. Armed forces institute of pathology. From the archives of the AFIP. Cerebral intraventricular neoplasms: radiologic-pathologic correlation. Radiographics. 2002;22(6):1473–505.
Kondziolka D, Lunsford LD. Microsurgical resection of colloid cysts using a stereotactic transventricular approach. Surg Neurol. 1996;46(5):485–92.
Konovalov AN, Pitskhelauri DI, Shkarubo M, Buklina SB, Poddubskaya AA, Kolycheva M. Microsurgical treatment of colloid cysts of the third ventricle. World Neurosurg. 2017;1(105):678–88.
Kuchelmeister K, Bergmann M. Colloid cysts of the third ventricle: an immunohistochemical study. Histopathology. 1992;21(1):35–42.
Lach B, Scheithauer BW, Gregor A, Wick MR. Colloid cyst of the third ventricle. A comparative immunohistochemical study of neuraxis cysts and choroid plexus epithelium. J Neurosurg. 1993;78(1):101–11.
Lagman C, Rai K, Chung LK, Nagasawa DT, Beckett JS, Tucker AM, et al. Fatal colloid cysts: a systematic review. World Neurosurg. 2017;1(107):409–15.
Laidlaw J, Kaye AH. 44 – colloid cysts. In: Kaye AH, Laws ER, editors. Brain tumors. 3rd ed. Edinburgh: W.B. Saunders; 2012. p. 849–63. Available from: http://www.sciencedirect.com/science/article/pii/B9780443069673000442.
Lanciego JL, Luquin N, Obeso JA. Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med. 2012;2(12):a009621. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543080/
Lega BC, Yoshor D. Postoperative dural sinus thrombosis in a patient in a hypercoagulable state: case report. J Neurosurg. 2006;105(5):772–4.
Little JR, MacCarty CS. Colloid cysts of the third ventricle. J Neurosurg. 1974;40(2):230–5.
Maeder PP, Holtås SL, Basibüyük LN, Salford LG, Tapper UA, Brun A. Colloid cysts of the third ventricle: correlation of MR and CT findings with histology and chemical analysis. AJR Am J Roentgenol. 1990;155(1):135–41.
Mamourian AC, Cromwell LD, Harbaugh RE. Colloid cyst of the third ventricle: sometimes more conspicuous on CT than MR. Am J Neuroradiol. 1998;19(5):875–8.
Mathiesen T, Grane P, Lindquist C, von Holst H. High recurrence rate following aspiration of colloid cysts in the third ventricle. J Neurosurg. 1993;78(5):748–52.
Mckissock W. The surgical treatment of colloid cyst of the third ventricle; a report based upon twenty-one personal cases. Brain. 1951;74(1):1–9.
Mercier P, Bernard F, Delion M. Microsurgical anatomy of the fourth ventricle. Neurochirurgie. 2018;S0028-3770(18):30067–5.
Milligan BD, Meyer FB. Morbidity of transcallosal and transcortical approaches to lesions in and around the lateral and third ventricles: a single-institution experience. Neurosurgery. 2010;67(6):1483–96.
Nair S, Gopalakrishnan CV, Menon G, Easwer HV, Abraham M. Interhemispheric transcallosal transforaminal approach and its variants to colloid cyst of third ventricle: technical issues based on a single institutional experience of 297 cases. Asian J Neurosurg. 2016;11(3):292–7.
Nakasu Y, Isozumi T, Nioka H, Handa J. Mechanism of mutism following the transcallosal approach to the ventricles. Acta Neurochir. 1991;110(3):146–53.
Niknejad HR, Samii A, Shen S-H, Samii M. Huge familial colloid cyst of the third ventricle: an extraordinary presentation. Surg Neurol Int. 2015;6(Suppl 11):S349–53.
Park JH, Cho HR, Seung WB, Lee SH, Park YS. The pterional-transsylvian approach for tumor in the temporal horn: a case report. Brain Tumor Res Treat. 2015;3(2):118–21. https://doi.org/10.14791/btrt.2015.3.2.118.
Pendl G, Öztürk E, Haselsberger K. Surgery of tumours of the lateral ventricle. Acta Neurochir. 1992;116(2):128–36.
Pollock BE, Schreiner SA, Huston J. A theory on the natural history of colloid cysts of the third ventricle. Neurosurgery. 2000;46(5):1077–81. discussion 1081-1083
Ravnik J, Bunc G, Grcar A, Zunic M, Velnar T. Colloid cysts of the third ventricle exhibit various clinical presentation: a review of three cases. Bosn J Basic Med Sci. 2014;14(3):132–5.
Rhoton AL Jr. Cerebellum and fourth ventricle. Neurosurgery. 2000;47(3 Suppl):S7–27.
Rhoton AL. The lateral and third ventricles. Neurosurgery. 2002;51(suppl_4):S1-207–71.
Sampath R, Katira K, Vannemreddy P, Nanda A. Quantifying sulcal and gyral topography in relation to deep seated and ventricular lesions: cadaveric study for basing surgical approaches and review of literature. Br J Neurosurg. 2014;28(6):713–6. https://doi.org/10.3109/02688697.2014.913771. PMID: 24836819.
Scelsi CL, Rahim TA, Morris JA, Kramer GJ, Gilbert BC, Forseen SE. The lateral ventricles: a detailed review of anatomy, development, and anatomic variations. AJNR Am J Neuroradiol. 2020;41(4):566–72.
Sheikh AB, Mendelson ZS, Liu JK. Endoscopic versus microsurgical resection of colloid cysts: a systematic review and meta-analysis of 1,278 patients. World Neurosurg. 2014;82(6):1187–97.
Shenoy SS, Lui F. Neuroanatomy, ventricular system. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2020.
Stachura K, Grzywna E. Neuronavigation-guided endoscopy for intraventricular tumors in adult patients without hydrocephalus. Wideochir Inne Tech Maloinwazyjne. 2016;11(3):200–7.
Symss NP, Ramamurthi R, Kapu R, Rao SM, Vasudevan MC, Pande A, et al. Complication avoidance in transcallosal transforaminal approach to colloid cysts of the anterior third ventricle: an analysis of 80 cases. Asian J Neurosurg. 2014;9(2):51.
Szmuda T, Słoniewski P, Szmuda M, Waszak PM, Starzyńska A. Quantification of white matter fibre pathways disruption in frontal transcortical approach to the lateral ventricle or the interventricular foramen in diffusion tensor tractography. Folia Morphol (Warsz). 2014;73(2):129–38.
Tenny S, Thorell W. Cyst, brain, colloid. In: StatPearls [Internet]. Treasure Island: StatPearls Publishing; 2018. Available from: http://www.ncbi.nlm.nih.gov/books/NBK470314/.
Tomasello F, Cardali S, Angileri FF, Conti A. Transcallosal approach to third ventricle tumors: how I do it. Acta Neurochir. 2013;155(6):1031–4.
Türe U, Yaşargil MG, Al-Mefty O. The transcallosal—transforaminal approach to the third ventricle with regard to the venous variations in this region. J Neurosurg. 1997;87(5):706–15.
Turillazzi E, Bello S, Neri M, Riezzo I, Fineschi V. Colloid cyst of the third ventricle, hypothalamus, and heart: a dangerous link for sudden death. Diagn Pathol. 2012;18(7):144.
Wallmann H. Eine Colloidcyste im dritten Hirnventrikel und ein Lipom im Plexus chorioides. Archiv f pathol Anat. 1858;14(3):385–8.
Wen HT, Rhoton AL, de Oliveira E. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42(6):1205–17.
Yamamoto I, Rhoton AL, Peace DA. Microsurgical anatomy of the third ventricle: part 2. In: Brock M, editor. Modern neurosurgery 1. Berlin, Heidelberg: Springer Berlin Heidelberg; 1982. p. 205–14. https://doi.org/10.1007/978-3-662-08801-2_24.
Yasargil MG. Transchoroidal approach to the third ventricle: an anatomic study of the choroidal fissure and its clinical application. Neurosurgery. 1998;42(6):1218–9.
Yaşargil MG, Abdulrauf SI. Surgery of intraventricular tumors. Neurosurgery. 2008;62(6 Suppl 3):1029–40. https://doi.org/10.1227/01.neu.0000333768.12951.9a. discussion 1040-1. PMID: 18695523
Zhou JJ, Mooney MA, Farber SH, Bohl MA, Little AS, Nakaji P. Bedside iohexol ventriculography for patients with obstructive colloid cysts: a protocol to identify auto-fenestration of the septum pellucidum. World Neurosurg. 2018;122:e279–84.
Zohdi A, Elkheshin S. Endoscopic anatomy of the velum interpositum: a sequential descriptive anatomical study. Asian J Neurosurg. 2012;7(1):12–6.
Zohrevandi B, Monsef Kasmaie V, Asadi P, Tajik H. Third ventricle colloid cyst as a cause of sudden drop attacks of a 13-year-old boy. Emerg (Tehran). 2015;3(4):162–4.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kondajji, A., Romiyo, P., Duong, C., Kim, W., Yang, I. (2022). Open Approaches to Intraventricular Tumors, Colloid Cysts, and the Subcortical Space. In: Zada, G., Pradilla, G., Day, J.D. (eds) Subcortical Neurosurgery. Springer, Cham. https://doi.org/10.1007/978-3-030-95153-5_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-95153-5_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-95152-8
Online ISBN: 978-3-030-95153-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)