Abstract
Prostatic utricle (PU) is an enlarged diverticulum localized in the posterior urethra and derived by the persistence of Müllerian structures or decreased androgenic stimulation of the urogenital sinus.
It can be associated to other anomalies such as hypospadias, cryptorchidism, renal agenesis, and intersex disorders. Diagnosis can be provided by ultrasounds, voiding cysto-urethrogram (VCUG), and magnetic resonance imaging (MRI).
Treatment is a challenge due to the anatomic site of the prostatic utricle (PU) and the risk of injury to the surrounding structures and nerves. Many approaches have been described: conservative, endoscopic, open, and minimally invasive.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Prostatic utricle (PU) is an enlarged diverticulum localized in the posterior urethra and derived by the persistence of Müllerian structures or decreased androgenic stimulation of the urogenital sinus. It is a rare condition with an estimated prevalence of 5% in urologic patients and an incidence of 1% in autopsy [1, 2].
In males, secretion of anti-Müllerian hormone (AMH) by fetal testes causes regression of Müllerian structures. The only remnants of this system are the appendix testis and the prostatic utricle.
PU has a dual histologic origin that consists of an admixture of urogenital sinus and Wolffian cells caudally and Müllerian cells cranially [3, 4]. Utricular anomalies may result from incomplete regression of the Müllerian system or incomplete androgen-mediated regression and closure of urogenital sinus due to altered production or sensitivity to testosterone or AMH [5,6,7].
This explains why prostatic utricle can be associated to other anomalies such as hypospadias, cryptorchidism, renal agenesis, and intersex disorders [1, 2, 8], while only rarely is an isolated condition.
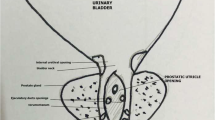
This anomaly can be classified as grade 0–III [9]. Grade 0 is limited to verumontanum, grade I is below the bladder neck, grade II is extended over the bladder neck, and grade III is the opening distal to the external sphincter.
Most of PUs are asymptomatic but they can also be manifested by urinary infections, urinary retention, epididymitis, stone formation, and postvoid dribbling. These symptoms are usually related to the compressive effect of the PU on surrounding structures [10].
2 Diagnosis
Ultrasounds are the first-line imaging method for PU providing diagnosis in most cases (Fig. 48.1a). VCUG also allows to see the utricular chamber filled with contrast medium and can be useful to detect the confluence between urethra and PU and its extension (Fig. 48.1b). Small utricles can be missed at ultrasounds and also at VCUG but are usually low-grade asymptomatic PU not requiring surgical treatment. After first-line diagnosis or in case of strong suspicion of PU, we recommend MRI which provides a better anatomic definition of the PU and the surrounding pelvic structures (Fig. 48.1c). Some authors mentioned the use of retrograde urethrogram, computed tomography, and also intravenous pyelogram as diagnostic tools [1, 10, 11], but radiation exposure should be considered and avoided when possible or limited in pediatric patients. Since imaging techniques are sensitive enough in diagnosing a PU, urethroscopy is not recommended as a routine investigation; however in selected cases, it is indicated to detect the coexistence of a uterine cervix [9].
(a–c) (a) US is the first-line imaging technique to diagnose a PU. PU appears as a fluid-filled cavity behind the bladder (B Bladder, U Utricle). (b) During voiding phase of a VCUG, the PU is filled with contrast medium and it is possible to identify the communication with the urethra. (c) MRI is a third-line imaging technique that allows a better anatomic definition of the malformation
3 Treatment
Nonsurgical approach can be attempted in case of UTI associated to PU using antimicrobial treatments but definitive treatment requires an endoscopic or surgical approach. Usually, symptomatic cases, grade III or grade II prostatic utricles but of conspicuous size, are considered an indication for surgery. Some authors report transrectal ultrasound (TRUS)-guided aspiration with or without injection of sclerosant therapy as an alternative [1, 12].
Endoscopic techniques include catheterization, aspiration, and utricle orifice incision [1, 13] or, as described by Husmann and Allen [14], electrofulguration of the mucosal layer, allowing coalescence of the deeper raw tissues to obliterate the cavity.
Several open techniques have been described, but the anatomic site of PU remains challenging because of the high risk of injury to the surrounding structures such as ejaculatory ducts, pelvic nerves, rectum, vas deferent, and ureters.
The open approaches reported are extraperitoneal or transperitoneal, suprapubical extravesical, transvesical transtrigonal, perineal, posterior trans-sacral, parasacral, posterior sagittal transrectal or pararectal, and anterior sagittal with or without rectal splitting [1, 3, 5, 15,16,17,18,19,20].
The most frequently used approaches will be described below.
4 Open Techniques
Transvesical transtrigonal approach : the anterior bladder wall is opened longitudinally exposing the trigone. A midline incision is done in the trigone revealing the PU behind the bladder. The PU is isolated till its confluence with the urethra and its neck is ligated at insertion point. A suprapubic tube is left for urinary drainage [3, 20].
Perineal approach : the patient lays in lithotomy position with flexed hips. A midline incision is made from the caudal end of the scrotum to the anterior margin of the anus with needle tip cautery and with the help of nerve and muscle stimulator. The incision is deepened through the perineal plane till the anterior wall of the rectum that is retracted posteriorly. Levator muscles are mobilized to expose the utricle that is isolated. Low utricles are easy to identify and to encircle the insertion point. In high and small ones, it can be safer to open the utricle in the midline to see and close the communication with urethra and remove the PU or decide to extirpate only the mucosal layer to avoid injuries [5].
Posterior sagittal trans-anorectal [17, 21, 22]: this approach has been widely described by Peña and is characterized by posterior incision with rectal splitting. This approach provides an excellent exposure but can require protective colostomy in case of insufficient bowel preparation and harvesting for 7–10 days postoperatively.
Alternatively, a posterior sagittal rectum retracting approach has been described [16]: the incision is made in the midline from the third sacral segment to about 1.3 cm posterior to the anus and deepened dividing the parasagittal muscles and elevator ani without cutting the external sphincter with the use of a muscle stimulator until the rectum. The investing fascia is divided in the midline and the rectum is retracted on the right. The PU is identified thanks to an internal catheter placed cystoscopically before the procedure. It is picked up and isolated carefully starting from the dome to its junction with the urethra.
Anterior sagittal : in the transanorectal approach [18, 19], the patient is placed in the prone knee-chest position and a ribbon impregnated with disinfectant is inserted in the rectum to reduce contamination. A midline sagittal incision is made from the anterior anal margin onto the perineum with needle tip cautery. Only the anterior rectal wall is opened and the incision is deepened in the perineal body remaining precisely in the midline until the retro-urethral space, where the PU lays, is exposed. Two self-retaining retractors are inserted to provide better exposure. The PU is dissected under direct vision till it enters the urethra and excised. The urethral defect is closed in layers. At the end of the PU removal, the rectal wall is closed in layers, the patient is turned in a lithotomy position, and a protective colostomy is created.
To avoid protective colostomy, Leite et al. introduced the possibility to perform a midline incision extended from the anterior margin of the anus to the scrotum without rectal splitting [15]. The anorectal sphincter can be sectioned if necessary and reconstructed at the end of the procedure.
5 Minimally Invasive Techniques
Laparoscopic approach [1, 23, 24]: the patient lays in supine position. At first, umbilical trocar 10 or 5 mm (according to the age of the patient) is placed for the optic. Two further trocars (5 mm or 3 according to the age) are placed at right and left flank, respectively. Once entered the abdominal cavity, the bladder is suspended to the abdominal wall to provide a better exposure of the retrovesical space. The peritoneum is opened and the PU is isolated until its communication with urethra. A ureteric catheter inserted in the utricle before the procedure helps the localization of the malformation. Alternatively, a cystoscope is inserted in the PU, so the light can guide the identification of the enlarged utricle. The communication with urethra can be closed with two endoloops or, in case of wide communication, with an endostapler (Fig. 48.2).
Robot-assisted approach [25,26,27,28,29,30,31,32]: the child lays supine position with legs stretched out and abducted in order to allow for concomitant urethrocystoscopy which is performed to place a urethral catheter inside the utricle and a Foley catheter into the bladder to aid in identification. The optical port at the umbilicus is advanced into the peritoneum and two 8 mm working ports are placed on the para-rectal lines under direct vision (distance between each port and umbilicus 7 cm). An additional 5 mm assistant port is used. The table is put in Trendelenburg position so that the bowel glides out of the pelvis by gravity; finally the ports are secured to the robotic system. After pneumoperitoneum is achieved and PU dome identified, also through the help of the assistant providing movement of the catheter inside the utricle, the peritoneal reflection covering the dome is incised to free the dome. The PU is grasped with forceps and carefully dissected free of the surrounding tissues of the retrovesical space with a monopolar hook. A complete bloodless dissection is carried out for all the length of the diverticulum. Once completely dissected, the PU neck is secured with two preformed endoscopic loops and resected just a few millimeters above its junction with the urethra. By moving the bladder catheter forward and backward, it is possible to be sure not to have caused a stricture in the urethra (Fig. 48.3).
(a–f) Robot-assisted removal of PU. (a) Before the procedure, a ureteric catheter is placed in the PU. (b) We used one umbilical trocar for the optic, two operative trocars at the flanks, and one accessory trocar. (c) The peritoneum is opened and the PU is identified with the help of the catheter in the PU. (d) An endoloop is placed in the neck. (e, f) The PU is cut and exteriorized
6 Outcomes
6.1 Endoscopic Techniques
Schuhrke and Kaplan reported morbidity and high recurrence rate after endoscopic transurethral catheterization and aspiration, orifice dilatation, incision, or unroofing [13]. Coppens et al. reported 82% of success rate with transperineal or transrectal puncture, simple endoscopic section of the utricle meatus, or large marsupialization [33]. Ahmed and Palmer reported successful transperineal cyst aspiration and sclerotherapy by tetracycline under transrectal ultrasound guidance [12]. Husmann and Allen had 83% success rate with endoscopic electrofulguration of utricular mucosal layer [14].
6.2 Open Techniques
Schuhrke and Kaplan reported 58% of incomplete excision of PU by the suprapubic, retrovesical, or transvesical approach [13]. Monfort had no sequelae after transvesical approach [20], and Desautiel et al. had good results with transvesical transtrigonal approach in their series [3]. Good results with no recurrence have been reported with posterior sagittal approaches both transanorectal and rectum retracting [5, 16, 17]. Also the anterior sagittal approaches have proved effective as reported by Leite et al. and Rossi et al. [15, 19].
However, it must be considered that whatever the approach, the reported case series are quite small.
6.3 Minimally Invasive Techniques
Minimally invasive technique seems to have less associated morbidity when compared to open approaches. Laparoscopic and robotic approaches give excellent visualization and magnification which allow meticulous dissection of the PU and minimal manipulation of surrounding structures. In a recent review regarding mainly the laparoscopic approach, no intraoperative complications are reported nor recurrences, voiding dysfunctions, and malignant degeneration with a mean follow-up of 20.5 months [23].
Robotic experience is still limited to case reports; nevertheless, all authors report excellent visualization without intra- or postoperative complications [25,26,27,28,29,30,31,32].
7 Complications of Undiagnosed Prostatic Utricle
Asymptomatic, small PUs can remain undiagnosed. In literature, there are several cases of stone retention in these unknown PU [2, 34,35,36,37]. Stones are usually diagnosed in adulthood, but there are also cases reported in childhood [34]. In these cases, management may vary according to the dimension of the stone. Both open and endoscopic [37] approaches have been described.
Another issue concerns the possible onset of tumors in undiagnosed PU. Usually the diagnosis is done in adults, but also an adolescent case has been reported [38]. Schuhrke and Kaplan reported a 3% incidence of malignancy in a case series of 88 patients with PU [13]. Reported malignancies in the prostatic utricle include urothelial carcinoma, clear cell adenocarcinoma, prostatic duct carcinoma (called endometrial carcinoma in the past), and squamous cell carcinoma [38,39,40,41,42].
8 Infertility Issues
The presence of an enlarged PU is associated to subfertility in adult males. This happens probably because of compressive effect of the PU on ejaculatory duct [3]. If the PU remains undiagnosed till the adult age, infertility constitutes an indication for treatment.
Müllerian and Wolffian structures have separate embryologic origin, so there should not be communication between the two systems; nevertheless in some cases the vas deference has an ectopic insertion in the cavity. In these cases, complete resection of the PU requires vasectomy. The detached vas can be reimplanted in the posterior urethra or in the bladder [3, 5, 20], but fertility is compromised.
On the other hand, if there is no communication between the two systems, the vas deferens can be separated from the Müllerian derivatives. However, sometimes it is necessary to leave a small part of the PU wall in close proximity to the deferens to avoid the risk of damage.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- MRI:
-
Magnetic resonance imaging
- PU:
-
Prostatic utricle
- TRUS:
-
Transrectal ultrasound
- US:
-
Ultrasounds
- VCUG:
-
Voiding cysto-urethrogram
References
Liu B, He D, Zhang D, Liu X, Lin T, Wei G. Prostatic utricles without external genital anomalies in children: our experience, literature review, and pooling analysis. BMC Urol. 2019; https://doi.org/10.1186/s12894-019-0450-z.
Wang W, Wang Y, Zhu D, Yan P, Dong B, Zhou H. The prostatic utricle cyst with huge calculus and hypospadias: a case report and a review of the literature. Can Urol Assoc J. 2015; https://doi.org/10.5489/cuaj.2381.
Desautel MG, Stock J, Hanna MK. Mullerian duct remnants: surgical management and fertility issues. J Urol. 1999; https://doi.org/10.1016/S0022-5347(01)68050-9.
Glenister TW. The development of the utricle and of the so-called “middle” or “median” lobe of the human prostate. J Anat. 1962;96
Krstić ZD, Smoljanić Ž, Mićović Ž, Vukadinović V, Sretenović A, Varinac D. Surgical treatment of the Müllerian duct remnants. J Pediatr Surg. 2001; https://doi.org/10.1053/jpsu.2001.23958.
Devine CJ, Gonzalez-Serva L, Stecker JF, Horton CE. Utricular configuration in hypospadias and intersex. J Urol. 1980; https://doi.org/10.1016/S0022-5347(17)55959-5.
Verma SK, Shetty BS, Kanth L. A boy with acute urinary retention: a Mullerian duct remnant (2006, 3b). Eur Radiol. 2006; https://doi.org/10.1007/s00330-005-0116-y.
Shebel HM, Farg HM, Kolokythas O, El-Diasty T. Cysts of the lower male genitourinary tract: embryologic and anatomic considerations and differential diagnosis. Radiographics. 2013; https://doi.org/10.1148/rg.334125129.
Ikoma F, Shima H, Yabumoto H. Classification of enlarged prostatic utricle in patients with hypospadias. Br J Urol. 1985; https://doi.org/10.1111/j.1464-410X.1985.tb06356.x.
Hester AG, Kogan SJ. The prostatic utricle: an under-recognized condition resulting in significant morbidity in boys with both hypospadias and normal external genitalia. J Pediatr Urol. 2017; https://doi.org/10.1016/j.jpurol.2017.01.019.
Kojima Y, Hayashi Y, Maruyama T, Sasaki S, Kohri K. Comparison between ultrasonography and retrograde urethrography for detection of prostatic utricle associated with hypospadias. Urology. 2001; https://doi.org/10.1016/S0090-4295(01)00954-2.
Ahmed M, Palmer JW. Large symptomatic Mullerian duct cyst treated by tetracycline sclerotherapy. Int Urol Nephrol. 1995; https://doi.org/10.1007/BF02551319.
Schuhrke TD, Kaplan GW. Prostatic utricle cysts (Mullerian duct cysts). J Urol. 1978; https://doi.org/10.1016/S0022-5347(17)57627-2.
Husmann DA, Allen TD. Endoscopic management of infected enlarged prostatic utricles and remnants of rectourethral fistula tracts of high imperforate anus. J Urol. 1997; https://doi.org/10.1016/S0022-5347(01)64898-5.
Leite MTC, Fachin CG, De Albuquerque Maranhão RF, Shida MEF, Martins JL. Anterior sagittal approach without splitting the rectal wall. Int J Surg Case Rep. 2013; https://doi.org/10.1016/j.ijscr.2013.05.013.
Meisheri IV, Motiwale SS, Sawant VV. Surgical management of enlarged prostatic utricle. Pediatr Surg Int. 2000; https://doi.org/10.1007/s003830050722.
Alam S, Levitt MA, Sheldon CA, Peña A. The posterior sagittal approach for recurrent genitourinary pathology. J Urol. 2007; https://doi.org/10.1016/j.juro.2007.03.189.
Dòmini R, Rossi F, Ceccarelli PL, De Castro R. Anterior sagittal transanorectal approach to the urogenital sinus in adrenogenital syndrome: preliminary report. J Pediatr Surg. 1997; https://doi.org/10.1016/S0022-3468(97)90012-9.
Rossi F, de Castro R, Ceccarelli PL, Domini R. Anterior sagittal transanorectal approach to the posterior urethra in the pediatric age group. J Urol. 1998; https://doi.org/10.1097/00005392-199809020-00058.
Monfort G. Transvesical approach to utricular cysts. J Pediatr Surg. 1982; https://doi.org/10.1016/S0022-3468(82)80499-5.
Siegel JF, Brock WA, Pena A. Pediatric urology: transrectal posterior sagittal approach to prostatic utricle (Mullerian duct cyst). J Urol. 1995; https://doi.org/10.1016/S0022-5347(01)67722-X.
Keramidas DC, Kapouleas GP, Papandreou E. The posterior sagittal approach for the excision of a prostatic utricle cyst. Br J Urol. 1995; https://doi.org/10.1111/j.1464-410X.1995.tb07723.x.
Raicevic M, Saxena AK. Laparoscopic management of Müllerian duct remnants in the paediatric age: evidence and outcome analysis. J Minim Access Surg. 2018; https://doi.org/10.4103/jmas.JMAS_213_16.
Aminsharifi A, Afsar F, Pakbaz S. Laparoscopic management of müllerian duct cysts in infants. J Pediatr Surg. 2011; https://doi.org/10.1016/j.jpedsurg.2011.04.008.
Lima M, Maffi M, Di Salvo N, Ruggeri G, Libri M, Gargano T, Lardy H. Robotic removal of Müllerian duct remnants in pediatric patients: our experience and a review of the literature. Pediatr Medica e Chir. 2018; https://doi.org/10.4081/pmc.2018.182.
Najmaldin A, Antao B. Early experience of tele-robotic sugery in children. Int J Med Robot Comput Assist Surg. 2007; https://doi.org/10.1002/rcs.150.
Hong YK, Onal B, Diamond DA, Retik AB, Cendron M, Nguyen HT. Robot-assisted laparoscopic excision of symptomatic retrovesical cysts in boys and young adults. J Urol. 2011; https://doi.org/10.1016/j.juro.2011.07.113.
Wu JA, Hsieh MH. Robot-assisted laparoscopic hysterectomy, gonadal biopsy, and orchiopexies in an infant with persistent mullerian duct syndrome. Urology. 2014; https://doi.org/10.1016/j.urology.2013.10.006.
Nguyen A, Arora H, Reese J, Kaouk J, Rhee A. Robot-assisted laparoscopic excision of prostatic utricle in a 3-year old. J Pediatr Urol. 2018; https://doi.org/10.1016/j.jpurol.2018.07.015.
Macedo A, Del Debbio Di Migueli R, Ottoni SL, Leal da Cruz M, Manzano JP. Robotic-assisted excision of a prostatic utricle cyst in a 12-month boy with proximal hypospadia and 45X0/46XY karyotype. J Pediatr Urol. 2020; https://doi.org/10.1016/j.jpurol.2020.08.020.
Khoder WY, Kretschmer A, Gratzke C, Becker A, Stief C. Robotic-assisted excision of giant prostatic utricular cysts: technique, outcomes and follow-up. Surg Technol Int. 2019;35
Goruppi I, Avolio L, Romano P, Raffaele A, Pelizzo G. Robotic-assisted surgery for excision of an enlarged prostatic utricle. Int J Surg Case Rep. 2015; https://doi.org/10.1016/j.ijscr.2015.03.024.
Coppens L, Bonnet P, Andrianne R, De Leval J. Adult müllerian duct or utricle cyst: clinical significance and therapeutic management of 65 cases. J Urol. 2002; https://doi.org/10.1016/S0022-5347(05)65190-7.
Spence HM, Chenoweth VC. Cysts of prostatic utricle (müllerian duct cysts); report of two cases in children, each containing calculi, cured by retropubic operation. J Urol. 1958; https://doi.org/10.1016/S0022-5347(17)66274-8.
Valdevenito JP, Valdevenito R, Cuevas M, Espinoza A, Guerra J. Quiste del utrículo prostático: Reporte de un caso complicado de litiasis gigante. Arch Esp Urol. 2002;55
Şalvarcl A, Istanbulluoʇlu O. Monosymptomatic persistent hematospermia due to rarely encountered prostatic utricle stones. Urol Int. 2015; https://doi.org/10.1159/000354766.
Bagrodia A, Gerecci D, Ramirez D, Decker DB, Hudak SJ. Holmium laser endourethrotomy and litholapaxy of an occult prostatic utricle calculus. Can J Urol. 2012;19
Gualco G, Ortega V, Ardao G, Cravioto F. Clear cell adenocarcinoma of the prostatic utricle in an adolescent. Ann Diagn Pathol. 2005; https://doi.org/10.1016/j.anndiagpath.2005.02.006.
Pineault K, Alam R, Meyer A, Johnson MH. Urothelial carcinoma within the prostatic utricle of an adult with hypospadias and Fanconi anemia. Urol Case Rep. 2020; https://doi.org/10.1016/j.eucr.2019.101043.
Zhang C, Li X, He Z, Xiao Y, Li S, Zhou L. Squamous cell carcinoma of the enlarged prostatic utricle in an adult. Urology. 2012; https://doi.org/10.1016/j.urology.2011.06.017.
Melicow MM, Tannenbaum M. Endometrial carcinoma of uterus masculinus (prostatic utricle). Report of 6 cases. J Urol. 1971; https://doi.org/10.1016/S0022-5347(17)61430-7.
Vale JA, Patel A, Ball AJ, Hendry WF, Chappell ME, Fisher C. Endometrioid carcinoma of the prostate: a misnomer? J R Soc Med. 1992; https://doi.org/10.1177/014107689208500708.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Lima, M., Maffi, M. (2022). Enlarged Prostatic Utricle Associated to Hypospadias. In: Hadidi, A.T. (eds) Hypospadias Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-94248-9_48
Download citation
DOI: https://doi.org/10.1007/978-3-030-94248-9_48
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-94247-2
Online ISBN: 978-3-030-94248-9
eBook Packages: MedicineMedicine (R0)