Abstract
Water pollution can cause severe health hazards in living organisms since most of the contaminants are toxic, mutagenic, and carcinogenic. There is a critical need to decontaminate the water from industrial effluents preceding their discharge into water bodies. The current chapter explores the potential of various nanoparticle-based adsorbents with special reference to nano zero-valent iron (NZVI), iron oxide, titanium, alumina, and silica in the field of adsorptive hosting of inorganic and organic pollutants from aqueous solutions. The nano adsorbents exhibit greater adsorption capacity, rapid adsorption rate, and competence to host various pollutants, recyclability, and reusability when compared to conventional adsorbents. These properties emphasize the relevance of nano adsorbents for the remediation of water contaminated with heavy metal ions, dyes, and chlorinated organic compounds. This chapter gives an overview of the progress and application of bare and functionalized metal and metal oxide nanoparticles for this purpose. Moreover, the mechanism of heavy metal ions, dyes, and organic chlorinated compounds removal by nanoparticles has also been discussed. The present chapter offers advanced information about the imperative characteristics of some metal and metal oxide-based nanoparticles and demonstrates their advantages as adsorbents in water remediation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Extensive growth in the population of the world, along with urbanization, has lead to the rising demand for fresh water [1]. About 1.2 billion people in the world do not have access to hygienic and safe drinking water, and this issue is anticipated to augment in the years to come [2]. In addition, contamination of water with a variety of pollutants such as heavy metal ions, dyes, chlorinated organic compounds, pharmaceuticals, sediments, and radioactive contaminants has intensified the problem [3]. Among these contaminants, heavy metal ions, even in relatively small quantities, cause severe health vulnerability to human beings since they are poisonous, persistent, and non-biodegradable in nature. The effluence of heavy metal ions into water bodies caused by widespread industrialization and unsystematic removal results in contamination of aquatic ecosystems [4]. For instance, Pb2+ is employed in various industrial products, i.e., battery, pigments, printing, explosive, and fuel manufacturing. The Environmental Protection Agency (EPA) has recommended that the maximum Pb2+ contamination level in drinking water is 15 l µg L−1. The toxic effects of Pb2+ ions in human beings include inhibition of hemopoiesis, germinal cell dysfunction, hypertension, cognitive deformities, miscarriages, renal dysfunctions, and nervous disorders. Therefore, the elimination of Pb2+ from water is necessary to save living organisms [5]. Another heavy metal ion, Hg2+ is the common inorganic form of Hg, which can be transformed into more poisonous organic forms through biological methylation [6]. Undesirable effects of Cu2+ in human beings encompass an accumulation of this ion in the liver leading to Wilson’s disease, which further causes psycho-neurotic defects [7, 8]. The intake of Cu for humans should be below 5.0 mg d−1, as recommended by the European Commission [9] and made mandatory in the WHO guidelines. As a result, the concentration of Cu in drinking water has been stipulated to below 2.0 mg L−1 [10]. Similar to Cu2+, the devastating effects of numerous other heavy metal ions on the human body have been discussed by different scientists from time to time. In this regard, Cd leads to multiple chronic organ damage [11, 12], and Mn affects the CNS and respiratory system. Some of the ions like Ag+ and Zn2+ are important trace elements but turn toxic when taken in higher amounts. The removal of Ag could protect the resources and benefit human beings’ sustainable development [13]. Zn2+ in high doses can be toxic and cause detrimental health hazards to many biological and biochemical processes [14, 15].
Organic dyes constitute another category of pollutants that are released from several industries such as paint, cosmetics, textile, leather, pigment, paper, etc. [16]. Dyeing runoff has a dangerous impact on the environment since its presence in water causes carcinogenic effects. Moreover, colored effluents can decrease the level of O2 in water by affecting the photosynthesis of aquatic plants, and in extreme cases, leading to the suffocation of aquatic plants and animals [17]. Amongst the diverse kinds of dyes, Methylene blue (MB) and Rhodamine B (RhB) are mainly used in textile manufacture. These dyes may lead to eye burns and, in extreme cases, result in permanent damage to the vision of humans and animals [18]. Literature studies reveal that inhalation or ingestion of RhB may cause harm to the liver and thyroid [19]. The stability and persistence of the dyes in the water increase if not treated properly. Consequently, it is essential to eliminate the perilous dyes from wastewater before disposing of these into the aquatic ecosystem with an appropriate treatment process [20]. Malachite green (MG), a triphenylmethane cationic water-soluble dye [21], has carcinogenic, mutagenic, and teratogenic effects on humans. Noxious substances are produced on MG degradation that causes damage to the liver, lungs, and bones [22,23,24]. For that reason, it is obligatory to eliminate these contaminants from effluents prior to their discharge into water bodies.
With the increasing water pollution resulting from various kinds of pollutants, it becomes of utmost importance to develop and apply water remediation techniques to diminish the effects of contaminants. Significant efforts have been dedicated to developing effective physical and chemical treatment processes for removing inorganic and organic pollutants from contaminated water [25]. The methods such as adsorption, ion exchange, ozonation, precipitation, membrane separation, etc., are being employed (Fig. 9.1) to eliminate pollutants from aqueous runoffs [26]. However, some of these techniques have limitations, such as high processing cost, sludge formation, and inappropriateness at a large scale (Fig. 9.1) [27].
Amongst different methods, huge consideration has been gained by the adsorption phenomenon because of its benefits such as simplicity, easy operation, recyclability, uncomplicated removal, and reusability [28]. The characteristics of a particular adsorbent depend on its specific surface area, composition, and accessibility of various functional groups; therefore, the effectiveness of an adsorption process is controlled by these factors. Numerous adsorbents like ion-exchange resins, activated carbons [29,30,31,32], natural zeolites [33], chitosan [34], bio sorbents [35,36,37], and chelating materials [38, 39] have been investigated by the researchers for the elimination of pollutants from aqueous solutions. The ability of adsorbents depends on the presence of functional groups on their surfaces. For instance, an adsorbent with nitrogen-containing ligands (amino, amidoxime, hydrazine, and imidazole) are efficient in complexing metal ions [40,41,42,43,44,45,46].
In recent times, the production of nanomaterials with greater surface area, improved absorption capacity, and superior regeneration performance has exhibited immense potential in eliminating a wide range of pollutants [47]. For example, manganese oxides have been found to be excellent nano adsorbents for the uptake of heavy metal ions due to their greater adsorption ability, enhanced stability, and low price [48,49,50]. Also, the rational design and adsorbents possessing outstanding adsorption ability and easy separation are of paramount importance [51]. Compared with traditional adsorbents, magnetic nanoparticles have attracted intensive attention of many researchers and been widely used for the removal of heavy metals in wastewater treatment due to their excellent physical and chemical properties, such as super paramagnetism, high surface area, easy separation under external magnetic field and strong adsorption [52,53,54,55]. Based on these advantages, Fe3O4 nanoparticles were exploited to remove heavy metals from water by strong adsorption [56,57,58,59,60]. Not only heavy metal ions, metal oxide nano adsorbents also were extensively used for the hosting of dye pollutants owing to their enhanced surface area, improved photocatalytic properties, and reusability. The nanoparticle-based adsorbent can be recycled, thus leading to the production of very small volumes of sludge. A variety of metal and metal oxide nanoparticles like Fe/Ni [61], Ni [62], TiO2 [63,64,65,66], ZnO [67,68,69,70], Fe3O4, Fe2O3 [71,72,73,74], SnO2 [75, 76] ZnS [77], CdS [78], WO3 [79, 80] etc. have been utilized for hosting of both cationic and anionic dyes.
In addition to heavy metal ions and dyes, chlorinated organic compounds have also been effectively hosted mainly by metallic and bimetallic nanoparticles. Yet, there are fewer reports available on the use of metal oxide nanoparticles for the hosting of organo chlorines. The successful use of NZVI and Fe-based bimetallic in the hosting of organo chlorines has been demonstrated both in situ and ex situ, attributing to their high active surface area and higher number of adsorption sites. NZVI has been used for the effective hosting of heptachlor, lindane, pentachlorobenzene, and hexachlorobenzene [81].
The aim of this chapter is to explore the use of various types of metal and metal oxide-based nanomaterials for the adsorptive hosting of different water pollutants, particularly organo chlorinated compounds, dyes, and heavy metal ions, along with their mechanism of hosting. Further, the role and need for functionalization of these nanomaterials for the improved pollutant hosting performance has also been discussed in detail. The last section depicts the future perspective and research gaps regarding the use of these nanomaterials in the hosting of water pollutants.
9.2 Metal and Metal Oxide Nanoparticles
The hosting of water pollutants using metal and metal oxide-based nano adsorbents has emerged as the most promising approach for researchers. Many metal and metal oxide nanoparticles have been synthesized and employed for the removal of both organic (dyes and pesticides) and inorganic (heavy metal ions) pollutants present in the waste water. Among the metal nanoparticles, nanoscale zero-valent iron (NZVI) has emerged as the most successful nano adsorbent for hosting water pollutants, whereas a variety of metal oxide nanoparticles viz TiO2, iron oxides (Fe2O3 and Fe3O4), silicon dioxide (SiO2), aluminium oxide (Al2O3), etc. have also been fabricated for adsorptive removal of water pollutants (Fig. 9.2). The role of these nano adsorbents in the removal of pollutants from water has been proved very significant.
9.2.1 Nanoscale Zero-Valent Iron (NZVI)
Zero valent iron has been widely used for the breakdown of chlorinated organic compounds and inorganic pollutants for water remediation. Iron in zero oxidation state (Fe0) is known as zero-valent iron. Bulk zero-valent iron is a strong reducing agent that gets oxidized to Fe2+ upon reduction of pollutant molecules. Further, the properties of ZVI are improved when it is converted into nano-sized zero-valent iron. NZVI is of utmost importance owing to its improved properties like high surface area, greater thermal stability, less toxicity, better adsorption efficiency, and reducing properties as compared to bulk zero-valent iron. NZVI is obtained by both top-down and bottom-up approaches. Various studies describe the successful utilization of NZVI in the treatment of water containing pollutants viz heavy metals, dyes, and pesticides. An investigation [82] illustrated the use of NZVI for the complete removal of multiple heavy metal ions like Ni2+, Hg2+, and As5+ from water. In another study, the complete removal of As5+ from ground water was demonstrated [83] using NZVI, where adsorption of As5+ was followed by precipitation. NZVI was found to be more effective as compared to ZVI in terms of efficiency and time duration.
Most of the studies demonstrate that when bare NZVI is exposed to an aquatic system containing dissolved oxygen, an oxide layer is formed on its surface, which provides core–shell nature to NZVI, where the shell forms the oxidized layer that protects the Fe0 state in the core. Consequently, the direct implication of using bare NZVI for hosting the pollutants leads to iron pollution as well as deactivation of NZVI. This issue can be resolved by functionalizing and entrapping the NZVI with some stabilizing agents, which not only prevents the quick oxidation of NZVI to magnetite but also provides functional groups for better hosting of contaminants. For example, NZVI can be entrapped into some non-toxic biomolecules like chitosan, cyclodextrins, cellulose, etc. One of the research studies demonstrated the use of functionalized NZVI, where it was entrapped into a complex of chitosan and cyclodextrin for the complete adsorptive hosting of Cr6+ and Cu2+ ions. The mechanism of metal ions hosting was supposed to be physisorption for both metal ions followed by their reduction i.e., Cr6+ and Cu2+ to Cr3+ and Cu0, respectively, by NZVI. In this case, NZVI itself gets oxidized from Fe0 to Fe3+ [84].
NZVI has also found a promising use in the hosting of organic pollutants, i.e., organochlorinated pesticides and dyes, followed by their degradation in the waste water. NZVI has been employed for more than 97% removal of many pesticides, including lindane, hexachlorobenzene, pentachlorobenzene, and hexachlorobutadiene [81]. Similarly, some experimental studies describe that bare and functionalized NZVI can act as effective adsorbents for remediation of water contaminated by colored pollutants, i.e., dyes. The studies for the removal of various dyes viz. Basic Blue-3 [85], MB [86], AB24 [87], Reactive Red [88] etc. have been reported from time to time.
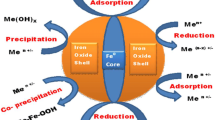
The possible mechanism of removal of heavy metal ions is based upon adsorption followed by reduction, precipitation, and co-precipitation depending upon the standard electrode potential of heavy metal ions (Fig. 9.3). The heavy metal ions like Pb2+, Ni2+ and Cd2+ which possess reduction potential slightly higher than Fe2+ are reduced by Fe0 core which is followed by their sorption on ferric hydroxide (FeOOH) shell (Eqs. 9.1 and 9.2)
On the other hand, for the heavy metal ions such as Cr6+, Se2+, Cu2+ and Hg2+ whose electrode potential is significantly higher than Fe2+ ions, the mechanism involves reduction by Fe0 core followed by precipitation or co-precipitation by ferric hydroxide (FeOOH) shell (Eqs. 9.3–9.5).
Further, the metal ions such as Ba2+ and Zn2+, whose standard electrode potential is lower than iron, get oxidized on the iron hydroxide shell (Eq. 9.6).
The mechanism of hosting of chlorinated pesticides and dyes generally comprises physisorption followed by reduction (Eq. 9.7)
The literature illustrates a variety of studies describing the use of bare as well as functionalized NZVI for the hosting of organic and inorganic water pollutants. In one of the reports [90], NZVI obtained from a plant extract of Syzygium jambos (Malabar plum) was employed for the effective hosting of Cr6+ with an adsorption capacity of 983.2 mg g−1. In another study [91], 99% of As5+ was also removed using NZVI. Even though the use of NZVI for waste water treatment has many advantages still there are certain shortcomings with their use. NZVI gets easily agglomerated, resulting in reduced surface area for adsorption. Also, their oxidation in water hinders the reduction process leading to their deactivation. In order to overcome these limitations, various functionalization strategies like a surface modification or doping (discussed in Sect. 9.2.5) have been developed. In a study, NZVI was surface functionalized by chitosan and employed for the removal of Cr6+ ions [92]. Also, the chitosan carboxymethyl β-cyclodextrin NZVI complex was fabricated for the effective removal of Cr6+ and Cu2+ ions [93]. The chitosan functionalized NZVI nanostructure possessed higher removal efficiency towards Cr6+ ions as compared to bare NZVI.
In addition to this, the use of NZVI for the decontamination of dyes and chlorinated organic compounds (R–Cl) from water has also been investigated. The hosting of these organic pollutants is based upon adsorption of pollutants on NZVI surface followed by their reductive degradation (Fig. 9.4). For example, an azo dye, i.e., Reactive Red A, was effectively removed using NZVI in the presence of hydrogen peroxide within 40 min [88]. The mechanism was based on the Fenton process where –OH free radicals are formed in two steps which lead to enhanced efficiency.
Mechanisms of hosting of different water pollutants by NZVI (adapted with permission from [3])
Adsorptive removal of a cationic dye, i.e., methylene blue with 100% efficiency using NZVI with adsorption capacity of 208.33 mg g−1 was reported [94], where the mechanism of removal was physisorption of dye onto the surface of NZVI via electrostatic interactions. Also, the NZVI supported on the surface of pillared clay for the effective hosting of Acid Red 315 dye where the removal efficiency of clay supported NZVI was found to be higher (100%) as compared to alone NZVI (80%), attributing to the enhanced surface area and higher number of functional groups [95].
NZVI alone or in combination with hydrogen peroxide has also been found effective in the hosting of chlorinated organic compounds from waste water in a Fenton-like process. The Fenton process using NZVI showed higher efficiency for hosting of pollutants attributing to the double formation of –OH radicals in contrast to conventional process which involves the use of bulk iron (Eqs. 9.8–9.10). This is the most important process for the removal of organic pollutants like dyes and organo chlorines.
9.2.2 Nano Titanium Dioxide (TiO2)
Nano TiO2 generally exists in three forms; two of them are active crystalline phases, i.e., anatase and rutile, and the third is an amorphous phase, i.e., brookite. The anatase phase is distinguished for its photo catalytic behavior and used in sunscreen for protection to the skin against UV rays. In contrast, the rutile phase of TiO2 is a stable phase. Photocatalytic behavior of nano TiO2 (anatase) depends upon the band gap of the material, which in turn is dependent upon the size and mode of synthesis. Nano titanium oxide has been comprehensively used for hosting various organic and inorganic contaminants via both adsorption and photocatalytic degradation. This can be attributed to its excellent stability, less toxicity, biocompatibility, and strong oxidizing nature. Bare nano-TiO2 has been employed for more than 95% removal of various organic dyes like Eriochrome Black T [96], Indigo Carmine [97], Methyl Orange [98], and Malachite Green [78]. The commercial use of bare TiO2 nanoparticles is limited because these can be activated only through UV radiation due to the high energy band gap of 3.2 eV. Moreover, their separation from the dispersion solution is very complicated. These limitations can be overcome by the functionalization of nano TiO2. For this, TiO2 NPs can be entrapped into a polymer matrix which not only increases the volume to surface area ratio of the polymer but also enhances the number of adsorption sites. A study illustrates the complete adsorptive removal of MB dye by employing a nanocomposite where TiO2 nanoparticles were filled into polyacrylamide-based hydrogel [99]. Here, the mechanism of adsorption was mainly based upon the ion exchange process accompanying the adsorption of dye molecules on the surface of the adsorbent.
In addition to the fact that nano TiO2 has been extensively employed for hosting of organic pollutants, it also found its use in the adsorptive removal of heavy metal ions. Many reports have described the use of mesoporous nano titania for the effective removal of Cr6+ [100], Pb2+, Cu2+, Fe3+, Cd2+, Zn2+ [101], Fe3+ and As5+ [102]. The functionalized nano titania has proved to be a superior adsorbent as compared to bare TiO2 NPs due to their greater surface area and easy separation. In addition, TiO2 embedded in the polymer matrix has also been employed for the hosting of heavy metal ions via adsorption. For example, in a study, polyvinyl alcohol (PVA) coated TiO2 was employed to host Cd2+, Ni2+, U6+, and Th4+ ions [103,104,105] from an aqueous solution.
The mechanism of removal of heavy metal ions by TiO2 usually consists of physical or chemical adsorption, whereas for organic dye pollutants, the adsorption process is followed by photocatalytic degradation (Eqs. 9.11–9.19).
Irradiation with UV light leads to the generation of electron–hole (hVB+–eCB−) pair in TiO2. The positively charged hole interacts with water to produce ·OH and H+ ions, whereas electrons interact with dissolved O2 to give superoxide ions (O2−·) which further reacts with water to give rise to hydroxide (OH−) ions and (·OOH) peroxide radicals. Further, OH is formed by the combination of H+ with ·OOH and hole with OH−. These ·OH free radicals, holes, and electrons are responsible for the degradation of organic pollutants.
Researchers have illustrated the enormous use of bare and modified TiO2 NPs and nanocomposites for the efficient hosting of heavy metal ions and organic dye pollutants. In this context, the mesoporous TiO2 nanoparticles were fabricated with the highest Cr6+ ion uptake capacity of 26.1 mg g−1 than ever reported [100]. Not only the nanoparticles, but nanowires of TiO2 having a diameter of 30–50 nm were also established for the adsorptive hosting of different heavy metal ions viz Pb2+, Fe3+, Cu2+, Cd2+, and Zn2+ ions. The TiO2 nanowires were found to be selectively effective for the maximum uptake, i.e., 97 and 80% towards Pb2+ and Fe3+ ions, respectively [101].
In addition to bare TiO2, functionalized and doped TiO2 nanocomposites have also been investigated for the adsorptive uptake of metal ion pollutants. The addition of metal and non-metal dopant or different supporting materials results in enhanced removal efficiency of TiO2 by reducing the band gap (Fig. 9.5). Razzaz et al. developed a nano fibrous nanocomposite of TiO2 with chitosan for the adsorptive hosting of Pb2+ and Cu2+ ions [107]. Another nanocomposite, i.e., TiO2− chitosan fabricated using microwave synthesis technique were used for the 88, and 72.56% adsorption of Cu2+ and Cd2+ ions, respectively, and maximum adsorption capacity was obtained to be 1,800 mol g−1 [92]. Also, the nano TiO2 coated with a biomolecule, i.e., starch, was also investigated for the hosting of Cd2+, Cu2+, Co2+, Ni2+ and Pb2+ ions with 90% removal efficiency [108]. Besides this, doped nano TiO2 has also been employed in heavy metal ions remediation, e.g., Fe-doped nano titania was used for the higher uptake of As5+ ions where the adsorption efficiency of doped nano titania was found to be higher than that of pure TiO2 attributing to the enhanced surface area by grain growth termination and photo catalytic response [102]. Further, pure and modified nano TiO2 can also be employed for the removal of organic dyes. The mechanism of removal of dyes is based on adsorption followed by photo catalytic degradation. In a batch adsorption experiment, Malachite Green dye was hosted by nano TiO2 with 85% removal efficiency [77]. Sood et al. and Yang et al. investigated the use of nano TiO2 for the 95% photo catalytic removal of Indigo Carmine dye [97] and 98.6% removal of Methyl Orange dyes [98]. Not only bare nano titania, functionalized or doped nano TiO2 have also been utilized for the removal of dyes. In a report, nano TiO2 doped with Niobium (Nb), i.e., TiO2: (Nb, N-x), were fabricated for the photocatalytic hosting of Methylene Blue dye (MB). The effect of doping was investigated on the removal efficiency, indicating the sixfold increased adsorption performance in doped NPs compared to bare ones [109], attributing to decreased band gap from 2.7 to 2.3 eV. In a batch experiment, TiO2 nanoparticles doped with 0.5–2.5% Bi3+ were employed for the removal of Alizarin Red S dye, where removal efficiency was found to be as high as 80% [110]. Similarly, Pd and N co-doped nano TiO2 were tested for the effective removal of Eosin Yellow dye [111]. Also, Ag+ doped TiO2was reported for 99% degradation of MB dye [112].
Mechanism of hosting of organic pollutant by pure and modified nano titania (adapted with permission from [106])
9.2.3 Nano Iron Oxide
Iron is the fourth most abundant element found in Earth’s crust. It exists in divalent and trivalent oxidation states. There are three types of iron oxides, i.e., hematite (α-Fe2O3), maghemite (y-Fe2O3), and magnetite (Fe3O4) whereas Fe exists in trivalent oxidation state in α-Fe2O3 and y-Fe2O3, while in Fe3O4, both divalent and trivalent states exist. Nano iron oxide has received the great attention of researchers for hosting both organic and inorganic water pollutants through adsorption due to its abundance and simplicity of fabrication.
Both hematite (α-Fe2O3) and maghemite (y-Fe2O3) nanoparticles possess great potential for the adsorptive removal of heavy metal ions from waste water. For this application, both have their own advantages. α-Fe2O3 is one of the most stable forms of nano iron oxide and exhibits corrosion resistant nature, whereas maghemite possesses a large surface area and is magnetically separable. Bare nano iron oxide (both α-Fe2O3 and y-Fe2O3) have been employed for the removal of heavy metal ions like Cr6+, Cd2+, Cu2+, Pb2+, Zn2+, Al3+, Ni2+ and Mn2+ions [113, 114] from the waste water. The mechanism of removal of heavy metal ions was based on electrostatic interactions, which were responsible for the adsorption of these metal ions. The surface of nano iron oxide is covered with FeOH due to water which forms either Fe−+ OH2 or FeO− depending upon the pH of the solution. The increased number of FeO− sites leads to increased adsorption of positively charged heavy metal ions and vice versa. Another simple, low cost and eco-friendly iron oxide, i.e., magnetite (Fe3O4), is also widely used as a nano adsorbent for hosting heavy metal ions. Magnetite also exhibits the property of magnetism which enables its easy separation from the solution after adsorption. Magnetite has also been used for the removal of Cr6+, Pb2+, Cu2+, Zn2+, Mn2+ [115,116,117] etc. Different values of adsorption capacities were obtained for diverse heavy metal ions using different iron oxide-based nano adsorbents. This can be accredited to the fact that various metal ions have different hydrated ionic radii in the aqueous solutions, which interact differently with dissimilar negatively charged adsorption sites. Metal ions with higher hydrated ionic radii adsorb weakly as compared to metal ions with lower hydrated ionic radii [118]. Scientific reports illustrate the extensive use of magnetic and non-magnetic nano iron oxide for the hosting of organic and inorganic pollutants. In a study carried out by Iconaru et al., magnetite, i.e., nano Fe3O4, was utilized for the removal of As5+ and Cu2+ ions where better efficiency of nano-magnetite was observed as compared to commercial magnetite [119]. In a batch adsorption experiment, nano-magnetite were demonstrated for the removal of Cu2+, Pb2+, Mn2+ and Zn2+ ions where highest and lowest adsorption capacities were observed towards Pb2+ and Mn2+, respectively [120]. This is attributed to the difference in hydrated ionic radii of metal ions resulting in different electrostatic interactions of metal ions on the surface of the nanoparticle. Similarly, non-magnetic haematite, i.e., nano α-Fe2O3, was investigated for the effective adsorption of Cd2+ and Cr6+ with the adsorption capacities of 146.41 and 16.17 mg g−1, respectively [121, 122].
Nevertheless, the use of bare iron oxide nanoparticles suffers from the limitation of their easy oxidation in water due to the presence of Fe2+ ions, the tendency to agglomerate, and corrosion by acids and bases as well. These limitations can be overcome by the functionalization of nanoparticles with organic and inorganic shell materials, which not only prevent their oxidation but also provide sites for enhanced metal ion adsorption. Functionalization not merely improves the adsorption efficiency of the adsorbent but also provides chemical binding to the pollutant via electrostatic and Van der Waals interactions for the adsorptive hosting of pollutants on the surface of the adsorbent [123, 124]. Enhanced adsorption capacities have been reported with polymer functionalized iron oxide nanoparticles. The surface modification of these nanoparticles combines the advantage of a magnetic core with organic and inorganic shell. Many materials like polypyrrole, polyaniline, polyvinyl imidazole, silica, polyethylene glycol, chitosan, tannin and surfactants have been used for the functionalization which improved the adsorption capacity and stability of nano iron oxide [125,126,127,128,129,130,131,132,133]. A variety of studies demonstrated the use of functionalized nano iron oxide for the improved adsorption efficiency towards heavy metal ions. In a scientific investigation, improvement in stability, mechanical strength, and removal performance was observed where nano Fe3O4 were functionalized with a polymer, i.e., polymeric mercaptoethylamino [134] for the removal of Ag+, Hg2+, Pb2+, and Cd2+ ions and with polyacrylic acid and diethylenetriamine for the uptake of Cu2+ and Cr6+ with higher removal efficiency towards Cu2+ as compared to Cr6+ [135]. The higher adsorption capacity was calculated for Cu2+ ions as compared to Cr6+ ions. Furthermore, the toxicity of nano iron oxide was minimized along with improved metal ion uptake capacity by functionalization with some biomolecules like glycine [136] and carboxy methyl cellulose [137] for improved hosting of Pb2+ ions. The incorporation of amino functional groups in the former and chelation in the latter was responsible for the enhanced removal performance. Also, some organic molecules have been used as chelating ligands to increase the adsorption capacity and selectivity of nano iron oxide. In a scientific investigation, nano Fe3O4 were amino-functionalized with a chelating ligand, i.e., triethylenetetramine [138] for the 85% removal of Cu2+ ions and with humic acid [139] for the removal of Hg2+, Pb2+, Cd2+, and Cu2+ ions where the adsorption capacity was found higher than bare nano-Fe3O4.
In addition, nano iron oxides have also found their application in the removal of dyes. Both bare and coated iron oxide nanoparticles have been investigated for dye adsorption. In a report, Reactive Red 2/A [140] was 95% removed adsorptively using nano-magnetite within 10 min. Another magnetite adsorbent functionalized by 3-aminopropyltriethoxysilane (APTES) was illustrated for the 96% adsorptive hosting of Sunset Yellow [141]. Similarly, surfactant i.e. sodium dodecyl sulphate (SDS) and hyperbranched polyglycerol modified Fe3O4 based nano adsorbent, were successfully demonstrated for the efficient removal of Crystal Violet [72] and MB [142] dyes, respectively. Scientists have also employed amino acids, i.e., L-arginine coated magnetite nano adsorbent for the elimination of Reactive Blue 19 dye [143] through adsorption. Not only bare and modified, but nano-magnetite supported on activated carbon surface have also proved as an efficient candidate for the dye pollutant hosting, attributing to the highly enhanced surface area of nano adsorbent. Magnetite mounted on activated carbon and Mn-doped magnetite doped on activated carbon have shown their implication in the elimination of MB [144] and MG [145] dyes with adsorption capacities of 117 and 87.5 mg g−1, respectively.
9.2.4 Nano Silicon Dioxide
Silicon dioxide i.e., silica is a known inorganic material due to its applications in chromatographic columns. It is a porous material with high specific surface area of 700 m2 g−1 which makes it suitable for the adsorptive removal of water pollutants. Nano silica exhibits great potential in adsorptive removal of organic and inorganic pollutants owing to its unique properties i.e. increased surface area, tailorable surface properties, and well-defined pore size. Further, the adsorption capacity and selectivity can be enhanced by modification of nano silica with different organic functional groups like –NH2, –SH, –OH, etc., or by using as support for other NPs and nanocomposites. Biofunctionalized silica nanospheres were fabricated and were modified with 3-aminopropyl and phenyl groups for the hosting of Cu2+ ions and a cationic dye, i.e., MB [146]. The adsorption capacity was increased towards Cu2+ ions and MB upon increasing the number of amino groups, which may be due to the rise in the number of binding sites on the surface of silica nanospheres. To enhance the adsorption capacity of nano silica, a nanocomposite of nano polyaniline and nano silica, as well as a crosslinked nano polyaniline immobilized on the surface of nano silica, was developed for the removal of different ions like Cu2+, Hg2+, Cd2+, and Pb2+. Higher adsorption capacities were noticed towards each metal ion in the case of crosslinked nanocomposite [147]. Not only the surface-modified but magnetic nano silica also gained considerable interest for hosting different pollutants from waste water. In this perspective, nano magnetite coated with silica showed their ability in this application at an industrial scale. In addition, core–shell nanoparticles of Ni@SiO2 were fabricated where magnetic properties of Ni were combined with porous silica for the enhanced and cost-effective removal of different dyes containing –OH group, i.e., Rhodamine B and Orange II and dye without –OH groups, i.e., Methylene Blue and Methyl Orange. Here, the effect of functional groups and charge on dye structures on the removal performance of nano adsorbent was examined, indicating higher adsorption capacity towards negatively charged dyes with –OH group with respect to the positively charged without –OH groups [148].
9.2.5 Nano Aluminum Oxide
Nano aluminum oxide is another low-cost and effective adsorbent for hosting waste water contaminants with efficient decontamination ability [149, 150]. Aluminium oxide possesses different crystalline structures like α, y, ƞ and θ where y-alumina (y-Al2O3) is most widely used. The bulk y-Al2O3 has been utilized as a conventional natural adsorbent for the elimination of water pollutants due to its interesting properties viz high compressive strength, resistance to corrosion, good thermal conductivity, and high electrical insulation [151]. The use of Al2O3 in the form of nano significantly enhances the capability as compared to bulk alumina. This can be accredited to the increased specific surface area, which leads to outstanding adsorption capacity and mechanical strength, low-temperature modification process [152]. The nano alumina has been used for hosting a number of organic and inorganic water pollutants. Tabesh et al. fabricated nano Al2O3 for the 97 and 87% adsorptive removal [153] of Pb2+ and Cd2+ ions, respectively. The maximum adsorption capacities of 47.08 and 17.22 mg g−1 were obtained for Pb2+ and Cd2+, respectively. The adsorption potential of nano alumina was also investigated for the removal of Zn2+, Cr6+, Ni2+, Cu2+, As2+, and Hg2+ [154,155,156,157,158] ions. Excellent adsorption capacities were observed for these metal ions. Further, the removal capacity of nano alumina can be enhanced by their surface modification with surfactants. The modification process not only improves the adsorption efficiency but also enhances their stability by preventing them from agglomeration. The surface of nano Al2O3 was modified with two different surfactants, i.e., SDS and Sodium tetra decyl sulphate (STS), for the enhanced removal of NH4+ and Cd2+ ions, respectively [159,160,161]. It was proposed that surface coating of anionic surfactant leads to alteration in surface charge which was responsible for the improvement in adsorption capacity. Nano alumina has also been found effective in boosting the adsorption performance of polyethersulfone (PES) membrane matrix towards Cu2+ ions [162].
Along with the hosting of heavy metal ions, nano-alumina has also found applications in the removal of organic pollutant dyes from the waste water. The high specific surface area and charged surface enable them to be effective in the adsorptive elimination of dyes. A number of scientific studies report the exclusion of different cationic and anionic dyes from the waste water. Scientists employed bare nano y-Al2O3, and SDS modified nano y-Al2O3 for the removal of a cationic dye i.e., Rhodamine B. The adsorption performance of SDS modified nano y-Al2O3 (97.7%) was found to be better as compared to bare y-Al2O3 (40%) [163]. This was due to the higher negative charge on the surface of SDS modified nano y-Al2O3 providing enhanced interaction towards cationic dye. Another cationic dye i.e., MB was also removed using nano y-Al2O3 with adsorption capacity of 1000 mg g−1 [164]. Electrostatic interactions between y-Al2O3 and MB were responsible for high adsorption capacity. In addition to cationic dyes, nano y-Al2O3 have also been found effective in the hosting of anionic dye i.e. Orange G where adsorption occurred via physisorption with 100% removal efficiency [165].
9.2.6 Bimetallic Nanoparticles
The name bimetallic nanoparticles indicate the blend of two different metals providing an assortment of new, different, and improved properties. Bimetallic nanoparticles are obtained in different forms like alloys, core–shell, and contact aggregate. In alloyed structure, two different metals are present homogeneously, whereas in core–shell type arrangement, one of the metals usually the inexpensive one is made core and the other acts as a shell. Bimetallic nanoparticles are fabricated by co-reduction or successive reduction of two metals. The properties of bimetallic nanoparticles depend upon the metals combined, mode of combination, size, and difference in reduction rates of two different metals. The process of bi-metallization results into improved and flexible electronic, structural, catalytic and surface properties. These bimetallic nanoparticles have received great attention among industrial and scientific areas owing to their novel properties. In the field of catalysis, bimetallic nanoparticles have demonstrated improved performance as compared to their monometallic counterparts. The mono metal and metal oxide nanoparticles exhibit the limitation of easy deactivation [166,167,168,169,170] and are easily affected by pH [171, 172] of the solution, which can be conquered by coating a small amount of noble metals on the surface of other active metals. Till date, a variety of bimetallic nanoparticles have been explored for the hosting of various organic compounds like chlorinated organic compounds, dyes, and inorganic metal ions from waste water. Fe-based bimetallic nanoparticles containing Ni, Cu, Al, and Pd as second metals have been investigated for the hydro dechlorination of polychlorine organic pollutants (Table 9.1).
The use of Fe-based bimetallic nanoparticles suffers from the certain limitations of spontaneous corrosion of Fe surface, which causes easy deactivation of nanoparticles system. This issue can be addressed by loading of bimetallic nanoparticles on carbon microspheres, or by adsorption of surfactants or polymers on the nanoparticles surface for prevention of agglomeration and corrosion [179] which can lead to increased cost-effectiveness of the process. Therefore, substantial attempts are being made to replace Fe with other active metals Al, Pd, Zn, Mg, etc. [180, 181]. Scientists have also synthesized Pd–Mg [182] and Pd–Al [183] bimetallic nanoparticles for the dechlorination of 2-chlorobiphenyl.
In addition to the hosting of chlorinated organic compounds, bimetallic nanoparticles have also emerged as a promising candidate for heavy metal removal. Bimetallic nanoparticles pose higher redox activity and adsorption capacity as compared to their mono metallic counterparts. Again, the Fe-based bimetallic nanoparticles containing other metals have achieved great success for the reductive hosting of Cu2+, Cd2+ and Cr6+ ions [184,185,186]. Further, the removal performance of bimetallic nanoparticles can be ameliorated by supporting them on polymeric surface. Enhanced removal capacity of Fe-nanoparticles supported on montmorillonite clay towards Cr6+ was observed because of the combined adsorption tendency of montmorillonite clay and reduction capacity of nanoparticles [187]. Other toxic metals like As5+ and Se4+ have also been successfully removed using bimetallic nanoparticles. Fe–Mn nanoparticles were synthesized [188] for the oxidation of As3+ to As5+ followed by complete removal of both ions owing to the oxidation capacity of Mn and adsorption efficiency of iron oxide. Similarly, Fe–Al nanoparticles (Fig. 9.6) were also employed for the complete elimination of As3+ to As5+ ions via simultaneous oxidation and reduction followed by adsorptive hosting [188]. The As5+ ions adsorbed on the Fe oxide layer get diffused to Al part where these get reduced to As3+ which further reduced to As0 via oxidation of Al and Fe into Al3+ and Fe2+ ions.
Possible mechanism of As ions hosting by Fe–Al bimetallic nanoparticles (adapted with permission from [188])
Bimetallic NPs have also gained a lot of attention from researchers for the removal of other organic and colored pollutants, i.e., dyes from the waste water. The scientific literature describes the outstanding behavior of bimetallic nanoparticles for the effective removal of dyes containing azo (–N = N–) functional groups, i.e., azo dyes. Scientists utilized Fe–Ni NPs for the effective hosting of Orange G in the waste water. The complete reductive degradation of dyes into by-products like aniline and naphthol was observed, which was followed by adsorption of byproducts [189]. Similarly, Fe–Cu [190] nanoparticles were developed for the complete decolorization of MB dye. Fe–Zn nanoparticles [191] were also exploited for the effective removal of Congo Red and MG dyes.
Further, to reduce the toxicity and threats caused by the leaching of toxic metal from bimetallic metals, these nanoparticles can be immobilized on many versatile catalytic supports. These nanoparticles supported on catalytic surfaces considerably enhance the removal performance and eco-friendliness of the system. Fe–Cu and Fe–Pd supported on agar gel were employed for the removal of Methylene Blue and Rhodamine B dyes with 90 and 80% efficiency, respectively [20].
9.3 Research Gaps
Although these nano adsorbents have emerged as the most promising candidates for effective hosting of contaminants from waste water, however, there are some issues in the bottleneck which are required to overcome for making them a superior host of water pollutants. Most metal oxide nanoparticles exhibit the limitation of instability, dispensability, and agglomeration, which lessens their removal performance. Also, their nano scale size makes separation from the waste water difficult. The use of modified and doped nano composites can provide the solution to these problems as it not only enhances the removal capacity but also provides stability and selectivity to the nano adsorbents. These metal oxide nanomaterials have been modified with a variety of polymeric, organic, and inorganic compounds to increase their removal capacity and selectivity towards the target contaminant. However, their synthesis and long-term performance is an issue. Similarly, bare NZVI and bimetallic nanoparticles containing iron suffer from the disadvantage of loss of activity due to corrosion and agglomeration. This problem has been solved by supporting nanomaterials on the surface of polymeric or surfactant support which combines the advantage of the high surface area of support with the removal activity of bimetallic nanoparticles by preventing them from agglomeration and corrosion. Although functionalized, doped, and supported metal-based nanoadsorbents have emerged as a promising solution. However, their synthesis and long-term performance are still an issue.
The commercial availability and use of nanoparticle-based adsorbents used for the hosting of water pollutants are rare; therefore, it is required to fabricate these nano adsorbents at an industrial scale. Also, from the economic point of view, the development and production cost of nano adsorbents should be optimized. Further, the application of nano adsorbents for onsite treatment is simple, more effective, and also reduces the operational cost of waste water treatment. Therefore, there is a strong need to develop the nano adsorbents which can be utilized and recycled for the onsite treatment of waste water.
Last but one, the escalated use of nano adsorbents at an industrial scale could impart toxicity to fauna and flora persistent in the environment. Therefore, the toxicity evaluation and biocompatibility of developed nano adsorbents towards the environment and human beings should be investigated prior to their implication in the field of hosting of water pollutants. The synthetic procedure for the development of nano adsorbents should meet the requirements of green chemistry. However, the reports on the toxicity evaluation of synthesized nano adsorbents are fewer.
9.4 Conclusions
The metal oxide-based nanostructures have been extensively utilized for the complete removal of inorganic and organic water pollutants owing to their excellent properties. The current chapter describes the extensive applicability of different metal, metal oxide-based nanomaterials, viz. NZVI, nano iron oxide nano alumina, nanosilica, nanotitania, and bimetallic nanomaterials for the removal of heavy metal ions, dyes, and chlorinated organic compounds from waste water. The mechanism of hosting of water pollutants using NZVI and bimetallic nanoparticles is usually based upon the adsorption followed by oxidative or reductive degradation of contaminants. Therefore, these have found great success in the complete removal of organic compounds, i.e., dyes and chlorinated organic compounds. The hosting of heavy metal ions using NZVI depends upon the standard reduction potential of heavy metal ions being hosted. Fewer reports on the reductive removal of metal ions, specially As5+, Cu2+, Cd2+, and Cr6+ involving the use of bimetallic nanoparticles are available. Among all the metal oxides, oxides of iron, i.e., Fe2O3 and Fe3O4 have been exploited the most for the adsorptive hosting of heavy metal ions owing to their magnetic properties. Further, ceramic-based nano adsorbents like nano silica and nano alumina have also established their higher applicability on the hosting of heavy metal ions as compared to organic contaminants. Nevertheless, the commercial availability of such nanomaterials and their toxicity imparted on fauna and flora are also the issue of concern. To minimize the harmful effects of nano adsorbents, regulatory measures on their use are also recommended. So, nano adsorbents have emerged as an excellent alternative to conventional adsorbents due to their unique and remarkable properties. Still, there is a long way to go to use nano adsorbents in practical applications of the hosting of waste water pollutants.
References
Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM (2008) Science and technology for water purification in the coming decades. Nature 452:301–310
Manawi Y, Kochkodan V, Hussein MA, Khaleel MA, Khraisheh M, Hilal N (2016) Can carbon-based nanomaterials revolutionize membrane fabrication for water treatment and desalination? Desalination 391:69–88
Carroll DO, Sleep B, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resources 51:104–122
Kumar V, Jain A, Wadhawan S, Mehta SK (2018) Synthesis of biosurfactant-coated magnesium oxide nanoparticles for methylene blue removal and selective Pb2+ sensing. IET Nanobiotechnol 12(3):241–253
Dil EA, Ghaedi M, Asfaram A, Hajati S, Mehrabi F, Goudarzi A (2017) Preparation of nanomaterials for the ultrasound-enhanced removal of Pb2+ ions and malachite green dye: Chemometric optimization and modeling. Ultrason Sonochem 34:677–691
Mautner A, Kwaw Y, Wieland K, Mvubuc M, Bothac A, Jacob M, John MA, Siqueira G, Bismarck A (2019) Natural fibrenanocellulose composite filters for the removal of heavy metal ions from water. Ind Crops Prod 133:325–332
Araya M, Mc Goldrick MC, Klevay LM, Strain JJ, Robson P, Nielsen F, Olivares M, Pizarro F, Johnson L, Poirier KA (2001) Determination of an acute no-observed adverse effect level (NOAEL) for copper in water. Regul Toxicol Pharmacol 34:137–145
Zamani HA, Rajabzadeh G, Firouz A, Ganjali MR (2007) Determination of copper (II) in wastewater by electroplating samples using a PVC membrane copper (II) selective electrode. J Anal Chem 62:1080–1087
European-Comission (2003) Opinion of the scientific committee on food on the tolerable upper intake level of copper (SCF/CS/NUT/UPPLEV/57 Final)
WHO (2004) Copper in Drinking-Water (WHO/SDE/WSH/03.04/88).
Zhao GX, Li JX, Ren XM, Chen CL, Wang XK (2011) Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ Sci Technol 45:10454–10462
Jarup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208
Pourreza N, Rastegarzadeh S, Larki A (2014) Nano TiO2 modified with 2- mercaptobenzimidazole as an efficient adsorbent for removal of Ag (I) from aqueous solutions. J Ind Eng Chem 20:127–132
ATSDR (Agency for Toxic Substances and Disease Registry) (2005) Toxicological profile for zinc. (Available online: http://www.atsdr.cdc.gov/toxprofiles/tp60.pdf. Accessed on 20/03/2015)
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 929(3):407–418
Ghasemi E, Heydari A, Sillanp M (2017) Superparamagnetic Fe3O4@EDTA nanoparticles as an efficient adsorbent for simultaneous removal of Ag(I), Hg(II), Mn(II), Zn(II), Pb(II) and Cd (II) from water and soil environmental samples. Microchem J 131:51–56
Minh Chu TP, Nguyen NT, Vu TL, Dao TH, Dinh LC, Nguyen HL, Hoang TH, Le TS, Pham TD (2019) Synthesis, characterization, and modification of alumina nanoparticles for cationic dye removal. Materials 12:450
Mahmoud HR, Ibrahim SM, El-Molla SA (2016) Textile dye removal from aqueous solutions using cheap MgO nanomaterials: adsorption kinetics, isotherm studies and thermodynamics. Adv Powder Technol 27:223–231
Jain R, Sharma N, Bhargava M (2003) Electrochemical degradation of rhodamine dye in textile and paper industries effluent. J Sci Ind Res 62:1138–1144
Patra S, Roy E, Madhuri R, Sharma PK (2016) Agar based bimetallic nanoparticles as high-performance renewable adsorbent for removal and degradation of cationic organic dyes. J Ind Eng Chem 33:226–238
Bhagya NP, Prashantha PA, Raveendra RS, Sathyanarayani S, Ananda S, Nagabhushanad BM, Nagabhushanae RH (2016) Adsorption of hazardous cationic dye onto the combustion derived SrTiO3 nanoparticles: kinetic and isotherm studies. J Asian Ceramic Soc 4(1):68–74
Syed P, Shabudeen S (2011) Study of the removal of malachite green from aqueous solution by using solid agricultural waste research journal of chemical sciences res. J Chem Sci 1:88–104
Srivastava S, Sinha R, Roy D (2004) Toxicological effects of malachite green. Aquat Toxicol 66:319–329
Kushwaha AK, Gupta N, Chattopadhyaya MC (2010) Removal of cationic methylene blue and malachite green dyes from aqueous solution by waste materials of Daucuscarota. J Chem Pharm Res 2:34–45
Efome JE, Rana D, Matsuura T, Lan CQ (2019) Effects of operating parameters and coexisting ions on the efficiency of heavy metal ions removal by nano fibrous metal-organic framework membrane filtration process. Sci Total Environ 674:355–362
Martína DM, Faccinia M, García MA, Amantia D (2018) Highly efficient removal of heavy metal ions from polluted water using ion selective polyacrylonitrile nanofibers. J Environ Chem Eng 6:236–245
Naseem K, Begum R, Wu W, Usman M, Irfan A, Al-Sehemi AG, Farooqi ZH (2019) Adsorptive removal of heavy metal ions using polystyrene-poly isopropylmethacrylamide-acrylic acid core/shell gel particles: adsorption isotherms and kinetic study. J Mol Liq 277:522–531
Yuan X, Anc N, Zhu Z, Sun H, Zhengb J, Jia M, Lud C, Zhang W, Liu N (2018) Hierarchically porous nitrogen-doped carbon materials as efficient adsorbents for removal of heavy metal ions. Proc Saf Environ Prot 119:320–329
Cegłowski M, Gierczyk B, Frankowski M, Popend Ł (2018) A new low-cost polymeric adsorbents with polyamine chelating groups for efficient removal of heavy metal ions from water solutions. React Funct Polym 131:64–74
Kwiatkowski M, Broniek E (2017) An analysis of the porous structure of activated carbons obtained from hazelnut shells by various physical and chemical methods of activation, Colloids Surf A 529:443–453
Valderrama C, Barios JI, Caetano M, Farran A, Cortina JL (2010) Kinetic evaluation of phenol/aniline mixtures adsorption from aqueous solutions onto activated carbon and hyper crosslinked polymeric resin (MN200). React Funct Polym 70:142–150
Otero M, Grande CA, Rodrigues AE (2004) Adsorption of salicylic acid onto polymeric adsorbents and activated charcoal. React Funct Polym 60:203–213
Narkiewicz U, Pełech I, Podsiadły M, Cegłowski M, Schroeder G, Kurczewska J (2009) Preparation and characterization of magnetic carbon nanomaterials bearing APTS silica on their surface. J Mater Sci 45:1100–1106
Jian M, Liu B, Zhang G, Liu R, Zhang X (2015) Adsorptive removal of arsenic from aqueous solution by zeoliticimidazolate framework-8 (ZIF-8) nanoparticles. Colloid Surf A 465:67–76
Wadhawan S, Jain A, Nayyar J, Mehta S K (2020) Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: a review. J Water Process Eng 33:101038
Figueroa RA, MacKay AA (2005) Sorption of oxytetracycline to iron oxides and iron oxide-rich soils. Environ Sci Technol 39:6664–6671
Mon J, Flury M, Harsh JB (2006) Sorption of four triarylmethane dyes in a sandy soil determined by batch and column experiments. Geoderma 133:217–224
Wang S, Wang H (2015) Adsorption behavior of antibiotic in soil environment: a critical review. Front Environ Sci Eng 9:565–574
Maleki A, Hayati B, Najafi F, Gharibi F, Joo SW (2016) Heavy metal adsorption from industrial wastewater by PAMAM/TiO2nanohybrid: preparation, characterization and adsorption studies. J Mol Liq 224:95–104
Demirbilek C, Dinç CO (2012) Synthesis of diethylaminoethyl dextran hydrogel and its heavy metal ion adsorption characteristics. Carbohydr Polym 90:1159–1167
Bilba N, Bilba D, Moroi G (2004) Synthesis of a polyacrylamidoxime chelating fiber and its efficiency in the retention of palladium ions. J Appl Polym Sci 92:3730–3735
Gong B (2002) Synthesis of polyacrylaminoimidazole chelating fiber and properties of concentration and separation of trace Au Hg and Pd from samples. Talanta 57:89–95
Chang X, Su Q, Liang D, Wei X, Wang B (2002) Efficiency and application of poly (acryl dinitro phenyl amidrazone -dinitroacrylphenylhydrazine) chelating fiber for pre-concentrating and separating trace Au(III), Ru(III), In(III), Bi(III), Zr(IV), V(V), Ga(III) and Ti(IV) from solution samples. Talanta 57:253–261
Deng S, Bai R (2004) Removal of trivalent and hexavalent chromium with aminatedpolyacrylonitrile fibers: performance and mechanisms. Water Res 38:2424–2432
Deng S, Bai R, Chen JP (2003) Aminatedpolyacrylonitrile fibers for lead and copper removal. Langmuir 19:5058–5064
Ma N, Yang Y, Chen S, Zhang Q (2009) Preparation of amine group containing chelating fiber for thorough removal of mercury ions. J Hazard Mater 171:288–293
Martína DM, FacciniaM GMA, Amantiaa D (2018) Highly efficient removal of heavy metal ions from polluted water using ion selective polyacrylonitrile nanofibers. J Environ Chem Eng 6:236–245
Kim EJ, Lee CS, Chang YY (2013) Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl Mater Interfaces 5:9628–9634
Wang X, Ding X, Yao S, Vu X, Feng Q, Wang Z (2014) High super capacitor and adsorption behaviors of flower-like MoS2 nanostructures. J Mater Chem A 2:15958–15963
Guo YY, Guo H, Wang YP (2014) Designed hierarchical MnO2 microspheres assembled from nanofilms for removal of heavy metal ions. RSC Adv 4:14048–14054
Kim EJ, Lee CS, Chang YY, Chang YS (2013) Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl Mater Interfaces 5(19):9628–9634
Zeng T, Yu Y, Li Z, Zuo J, Kuai Z, Jin Y, Wang Y, Wu A, Peng C (2019) 3D MnO2 nanotubes reduced graphene oxide hydrogel as reusable adsorbent for the removal of heavy metal ions. Mater Chem Phys 231:105–108
Kharissova OV, Dias HVR, Kharisov BI (2015) Magnetic adsorbents based on micro and nano-structured materials. RSC Adv 5(9):6695–6719
Reddy DHK, Lee SM (2013) Application of magnetic chitosan composites for the removal of toxic metal and dyes from aqueous solutions. Adv Colloid Interface Sci 201–202:68–93
Xu PA, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Liu ZF (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10
Zhang XM, Liu JY, Kelly SJ, Huang XJ, Liu JH (2014) Biomimetic snowflake shaped magnetic micro-/nanostructures for highly efficient adsorption of heavy metal ions and organic pollutants from aqueous solution. J Mater Chem A 2(30):11759–11767
Fan H, Mab X, Zhou S, Huang J, Liu Y, Liu Y (2019) Highly efficient removal of heavy metal ions by carboxymethyl cellulose immobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohydr Polym 213:39–49
Huang XG, Zhan XZ, Wen CL, Xu F, Luo LJ (2018) Amino-functionalized bacterial cellulose activated carbon composite for Pb2+ and methyl orange sorption from aqueous solution. J Mater Sci Technol 34(5):855–863
Mahdavi S, Jalali M, Afkhami A (2012) Removal of heavy metals from aqueous solutions using Fe3O4, ZnO and CuO nanoparticles. J Nanopart Res 14:846–863
Pylypchuk IV, Kessler VG, Seisenbaeva GA (2018) Simultaneous removal of acetaminophen, diclofenac, and Cd(II) by trametes versicolor laccase immobilized on Fe3O4/SiO2-DTPA hybrid nanocomposites. ACS Sustain Chem Eng 6(8):9979–9989
Yu Y, Li Y, Wang YQ, Zou BF (2018) Self-template etching synthesis of urchin like Fe3O4 microspheres for enhanced heavy metal ions removal. Langmuir 34(32):9359–9365
Liu Y, Zeng G, Tang L, Cai Y, Pang Y, Zhang Y, Yang G, Zhou Y, He X, He Y (2015) Highly effective adsorption of cationic and anionic dyes on magnetic Fe/Ni nanoparticles doped bimodal mesoporous carbon. J Colloid Interface Sci 448:451–459
Pandian CJ, Palanivel R, Dhananasekaran S (2015) Green synthesis of nickel nanoparticles using Ocimum sanctum and their application in dye and pollutant adsorption. Chin J Chem Eng 23:1307–1315
Gamra MA, Ahmed MA (2015) TiO2 nanoparticles for removal of malachite green dye from waste water. Adv Chem Eng Sci 5:373–388
Kansal SK, Sood S, Umar A, Mehta SK (2013) Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J Alloys Comp 581(25):392–397
Sood S, Umar A, Mehta SK, Kansal SK (2015) Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J Colloid Interface Sci 450:213–222
Sood S, Umar A, Mehta SK, Sinha ASK, Kansal SK (2015) Efficient photocatalytic degradation of brilliant green using Sr-doped TiO2 nanoparticles. Ceramic Int 41(3):3533–3540. https://www.sciencedirect.com/science/article/pii/S027288421401743X
Kataria N, Garg VK, Jain M, Kadirvelu K (2016) Preparation, characterization and potential use of flower shaped Zinc oxide nanoparticles (ZON) for the adsorption of Victoria Blue B dye from aqueous solution. Adv Powder Technol 27(4):1180–1188
Saharan P, Chaudhary GR, Lata S, Mehta SK, Mor S (2015) Ultra fast and effective treatment of dyes from water with the synergistic effect of Ni doped ZnO nanoparticles and ultrasonication. Ultrason Sonochem 22:317–325
Chaudhary GR, Saharan P, Ahmad U, Mehta SK, Mor S (2013) Well-Crystalline ZnO nanostructures for the removal of acridine orange and C. brilliant blue R-250 hazardous dyes, Sci Adv Mater 5(12):1886–1894
Kansal SK, Lamba R, Mehta SK, Umar A (2013) Photocatalytic degradation of Alizarin Red S using simply synthesized ZnO nanoparticles Mater. Lett 106(1):385–389
Rajabi HR, Arjmand H, Hoseini SJ, Nasrabadi H (2015) Surface modified magnetic nanoparticles as efficient and green sorbents: synthesis, characterization, and application for the removal of anionic dye. J Magn Magn Mater 394:7–13
Aashima US, Arora A, Gautam S, Singh S, Choudhary RJ, Mehta SK (2019) Magnetically retrievable Ce-doped Fe3O4 nanoparticles as scaffolds for the removal of azo dyes. RSC Adv 9:23129
Saharan P, Chaudhary GR, Mehta SK, Umar A (2016) Efficient photocatalytic degradation of victoria blue R and fast green FCF dyes using γ-Fe2O3 and Fe3O4 nanoparticles. Nanosci Nanotechnol Lett 8(11):965–971
Chaudhary GR, Saharan P, Kumar A, Mehta SK, Mor S, Ahmad U (2013) Adsorption studies of cationic, anionic and azo-dyes via monodispersed Fe3O4 nanoparticles. J Nanosci Nanotechnol 13(5):3240–3245
Lamba R, Umar A, Mehta SK, Kansal SK (2015) Well-crystalline porous ZnO–SnO2 nanosheets: an effective visible-light driven photocatalyst and highly sensitive smart sensor material. Talanta 131:490–498
Lamba R, Umar A, Mehta SK, Kansal SK (2015) ZnO doped SnO2 nanoparticles hetero junction photo-catalyst for environmental remediation. J Alloys Comp 653(25):327–333
Kaur S, Sharma S, Umar A, Singh S, Mehta SK, Kansal SK (2017) Solar light driven enhanced photocatalytic degradation of brilliant green dye based on ZnS quantum dots. Superlattices Microstruct 103:365–375
Kaur M, Mehta SK, Kansal SK (2018) Visible light driven photocatalytic degradation of ofloxacin and malachite green dye using cadmium sulphide nanoparticles. J Environ Chem Eng 6(3):3631–3639
Shukla S, Chaudhary S, Umar A, Chaudhary GR, Kansal SK, Mehta SK (2016) Surfactant functionalized tungsten oxide nanoparticles with enhanced photocatalytic activity. Chem Eng J 288:423–443
Shukla S, Chaudhary S, Umar A, Chaudhary GR, Mehta SK (2015) Dodecyl ethyl dimethyl ammonium bromide capped WO3 nanoparticles: efficient scaffolds for chemical sensing and environmental remediation. Dalton Trans 44:17251–17260
Simkovic K, Derco J, Valicova M (2015) Removal of selected pesticides by nano zero-valent iron. Acta Chim Slov 8(2):152–155
Zhang WX (2003) Nano scale iron particles for environmental remediation: an overview. J Nanopart Res. 5:323–332
Kanet SR, Greneche JM, Choi H (2006) Arsenic (V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier. Mater Environ Sci Technol 40:2045–2050
Sikder MT, Mihara Y, Islam MS, Saito T, Tanaka S, Kurasaki M (2015) Preparation and characterization of chitosan-caboxymethyl-β-cyclodextrin entrapped nanozero-valent iron composite for Cu (II) and Cr (IV) removal from wastewater. Chem Eng J 236:378–387
Khan MS, Khan AA, Bangash FU, Shah SS, Khan P (2013) Removal of basic dye from aqueous solutions using nano scale zero valent iron (NZVI) as adsorbent, J Chem Soc Pak 35(3)
Arabi S, Sohrabi MR (2014) Removal of methylene blue, a basic dye, from aqueous solutions using nanozerovalent iron Water science and technology. Water Sci Technol 70(1):24–31
Lin TY, Weng HC, Chen YF (2008) Effective removal of AB24 dye by nano/micro-size zero-valent iron, separation and purification effective removal of AB24 dye by nano/micro-size zero-valent iron. Sep Purif Technol 64(1):26–30
Shojaei S, Khammarnia S, Shojaei S, Sasani M (2017) Removal of reactive red 198 by nanoparticle zero valent iron in the presence of hydrogen peroxide. J Water Environ Nanotechnol 2(2):129–135
Li S, Wang W, Yi Y, Zhnag LW (2014) Zero-valent iron nanoparticles (nZVI) for the treatment of smelting wastewater: a pilot-scale demonstration. Chem Eng J 254:115–123
Xiao ZH, Xu Y, Yuan M, Jing X, Huang J, Li Q, Sun D (2017) Ultra-efficient removal of chromium from aqueous medium by biogenic iron based nanoparticles. Sep Purif Technol 174:466–473
Kanet SR, Greneche JM, Choi H (2006) Arsenic (V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier. Environ Sci Technol 40:2045–2050
Zimmermann AC, Mecabo A, Fagundes T, Rodrigues CA (2010) Adsorption of Cr (VI) using Fe-crosslinked chitosan complex (Ch–Fe). J Hazard Mater 179:192–196
Sikder MT, Miharac Y, Islam MS, Saitod T, Tanaka S, Kurasaki M (2014) Preparation and characterization of chitosan-caboxymethyl-β-cyclodextrin entrapped nano zero-valent iron composite for Cu (II) and Cr (IV) removal from wastewater. Chem Eng J 236:378–387
Arabi S, Sohrabi MR (2014) Removal of methylene blue, a basic dye, from aqueous solutions using nano-zerovalent iron. Water Sci Technol 70(1):24–31
Hassan MMA, Hamad HA, Shther DE (2019) Treatment of contaminated water with industrial dyes by using nano zero valent iron (NZVI) and supported on pillared clay. Adv Anal Chem 9(1):1–7
Kansal SK, Sood S, Umar A, Mehta SK (2013) Photocatalytic degradation of Eriochrome Black T dye using well-crystalline anatase TiO2 nanoparticles. J Alloys Compd 581:392–397
Sood S, Kumar S, Umar A, Kaur A, Mehta SK, Kansal SK (2015) TiO2 quantum dots for the photocatalytic degradation of indigo carmine dye. J Alloys Compd 650:193–198
Yang Y, Flatebo C, Liang J, Dong P, Yuan J, Wang T, Zhang J, Chen W, Wu J, Ajayan PM, Ci L, Li Q, Lou J (2016) Towards methyl orange degradation by direct sunlight using coupled TiO2 nanoparticles and carbonized cotton T-shirt. Appl Mater Today 3:57–62
Mittal H, Ray SS (2016) A study on the adsorption of methylene blue onto gum ghatti/TiO2 nanoparticles-based hydrogel nanocomposite. Int J Biol Macromol 88:66–80
Seisenbaeva GA, Daniel G, Nedelec JM, Gunko YK, Kessler VG (2012) High surface area ordered mesoporous nano-titania by a rapid surfactant-free approach. J Mater Chem 22:20374–20380
Malhat FM, Youssef A (2014) Selective removal of heavy metal from drinking water using titanium oxide nanowire. Macromol Symp 337:96–101
Deedar N, Irfan A, Qazi IA (2009) Evaluation of the adsorption potential of titanium dioxide nanoparticles for arsenic removal. J Environ Sci 21:402–408
Abbasizadeh S, Keshtkar AR, Mousavian MA (2013) Preparation of a novel electrospun polyvinyl alcohol/titanium oxide nanofiber adsorbent modified with mercapto groups for uranium (VI) and thorium (IV) removal from aqueous solution. Chem Eng J 220:161–171
Abbasizadeh S, Keshtkar A, R, Mousavian M A, (2014) Sorption of heavy metal ions from aqueous solution by a novel cast PVA/TiO2nanohybrid adsorbent functionalized with amine groups. J Ind Eng Chem 20:1656–1664
Him C, Tsang A, Zeng KY, Zhao W, Zhang T, Zhan Y, Ruijie X, Leung DYC, Huang H (2019) Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: overview and forecast. Environ Int 125:200–228
Tsang CHA, Zeng KY, Zhao W, Zhang T, Zhan Y, Xie R, Dennis YC, Huang LH (2019) Titanium oxide based photocatalytic materials development and their role of in the air pollutants degradation: overview and forecast. Environ Int 125:200–228
Razzaz A, Ghorban S, Hosayni L, Irani M, Aliabadi M (2016) Chitosan nanofibers functionalized by TiO2 nanoparticles for the removal of heavy metal ions. J Taiwan Inst Chem E 58:333–343
Baysal A, Kuznek C, Ozcan M (2018) Starch coated titanium dioxide nanoparticles as a challenging sorbent to separate and pre concentrate some heavy metals using graphite furnace atomic absorption spectrometry, Int J Environ Anal Chem 98(1):45–55
Breault TM, Bartlett BM (2013) Composition dependence of TiO2: (Nb, N)-x compounds on the rate of photocatalytic methylene blue dye degradation. J Phys Chem C 117:8611–8618
Sood S, Mehta SK, Umar A, Kansal SK (2014) The visible light-driven photocatalytic degradation of Alizarin red S using Bi-doped TiO2 nanoparticles, New. J Chem 38:3127–3136
Kuvarega AT, Krause RWM, Mamba BB (2011) Nitrogen/palladium Co doped TiO2 for efficient visible light photocatalytic dye degradation. J Phys Chem C 115:22110–22120
Sahoo C, Gupta AK, Pillai IMS (2012) Photocatalytic degradation of methylene blue dye from aqueous solution using silver ion-doped TiO2 and its application to the degradation of real textile waste water. J Environ Sci Health A Tox Hazard Subst Environ Eng 47(10):1428–1438
Akhbarizadeh R, Shayestefar DMRE (2014) Competitive removal of metals from wastewater by maghemite nanoparticles: a comparison between simulated wastewater and AMD. Mine Water Environ 33:89–96
Rajput S, Singh LP, Jr CUP, Mohan D (2017) Lead (Pb2+) and copper (Cu2+) remediation from water using Super paramagnetic maghemite (–Fe2O3) nanoparticles synthesized by Flame Spray Pyrolysis (FSP). J Colloid Interface Sci 492:176–190
Watts MP, Coker VS, Parry SA, Pattrick RAD, Thomas RAP, KalinR LJR (2015) Biogenic nano-magnetite and nano-zero valent iron treatment of alkaline Cr(VI) leachate and chromite ore processing residue. Appl Geochem 54:27–42
Shan C, Ma Z, Tong M, Ni J (2015) Removal of Hg(II) by poly(1-vinylimidazole)-grafted Fe3O4 @SiO2 magnetic nanoparticles. Water Res 69:252–260
Mahmoud ME, Abdelwahab MS, Abdou AEH (2016) Enhanced removal of lead and cadmium from water by Fe3O4 cross linked-O-phenylenediaminenano-composite. Sep Sci Technol 51:237–247
Lide DR (1992) Handbook of chemistry and physics. 73rd edn. CRC Press, Boca Raton, FL, pp 12–18
Iconaru SL, Guégan R, Popa CL, MotelicaHeino M, Ciobanu CS, Predoi D (2016) Magnetite (Fe3O4) nanoparticles as adsorbents for As and Cu removal. Appl Clay Sci 134:128–135
Giraldo L, Erto A, Carlos J, Piraján M (2013) Magnetite nanoparticles for removal of heavy metals from aqueous solutions: synthesis and characterization. Adsorption19(2):465–474
Zhu X, Song T, Lv Z, Ji G (2016) High-efficiency and low-cost α-Fe2O3 nanoparticles coated volcanic rock for Cd (II) removal from wastewater. Process Saf Environ 104:373–381
Ren T, He P, Niu W, Wu Y, Ai L, Gou X (2013) Synthesis of α-Fe2O3 nanofibers for applications in removal and recovery of Cr(VI) from wastewater. Environ Sci Pollut Res 20:155–162
Patwardhan SV (2012) Chemistry of aqueous silica nanoparticles surfaces and the mechanism of selective peptide adsorption. J Am Chem Soc 134:6244–6256
Wang X, Guo Y, Yang L, Han M, Zhao J, Cheng X (2012) Nanomaterials as sorbents to remove heavy metal ions in water treatment. J Environ Analyt Toxicol 2:2–7
Takafuji M, Ide S, Ihara H, Xu ZH (2004) Preparation of poly(1-vinylimidazole)-grafted magnetic nanoparticles and their application for removal of metal ions. Chem Mater 16:1977–1983
Song J, Kong H, Jang J (2011) Adsorption of heavy metal ions from aqueous solution by polyrhodanine encapsulated magnetic nanoparticles. J Colloid Interface Sci 359:505–511
Chávez-Guajardo AE, Medina-Llama JC, Maqueira L, Andrade CAS, Alves KGB, Melo CPD (2015) Efficient removal of Cr(VI) and Cu(II) ions from aqueous media by use of polypyrrole/maghemite and polyaniline/maghemite magnetic nanocomposites. Chem Eng J 281:826–836
Madrakian T, Afkhami A, Zolfigol MA, Ahmadi M, Koukabi N (2012) Application of modified silica coated magnetite nanoparticles for removal of iodine from water samples. Nano-Micro Lett 4:57–63
Adeli M, Yamini Y, Faraji M (2017) Removal of copper, nickel and zinc by sodium dodecyl sulphate coated magnetite nanoparticles from water and wastewater samples. Arab J Chem 10:S514–S521
Magnet C, Lomenech C, Hurel C, Reilhac P, Giulieri F, Chaze AM, Persello J, Kuzhir P (2017) Adsorption of nickel ions by oleate-modified magnetic iron oxide nanoparticles. Environ Sci Pollut R 24:7423–7435
Madrakian T, Afkhami A, Rezvani-jalal N, Ahmadi M (2014) Removal and preconcentration of lead(II), cadmium(II) and chromium(III) ions from wastewater samples using surface functionalized magnetite nanoparticles. J Iran Chem Soc 11:489–498
Behbahani NS, Rostamizadeh K, Yaftian MR, Zamani A, Ahmadi H (2014) Covalently modified magnetite nanoparticles with PEG: preparation and characterization as nano-adsorbent for removal of lead from wastewater. J Environ Health Sci 12:103
Rahbar N, Jahangiri A, Boumi S, Khodayar MJ (2014) Mercury removal from aqueous solutions with chitosan-coated magnetite nanoparticles optimized using the Box-Behnken design. Jundishapur J Nat Pharm Prod 9(2)
Madrakian T, Afkhami A, ZadpourB AM (2015) New synthetic mercaptoethylaminohomopolymer-modified maghemite nanoparticles for effective removal of some heavy metal ions from aqueous solution. J Ind Eng Chem 21:1160–1166
Huang SH, ChenD H (2009) Rapid removal of heavy metal cations and anions from aqueous solutions by anamino-functionalized magnetic nano-adsorbent. J Hazard Mater 16:174–179
Verma M, Tyagi I, Chandra R, Gupta VK (2017) Adsorptive removal of Pb (II) ions from aqueous solution using CuO nanoparticles synthesized by sputtering method. J Mol Liquids 225:936–944
Fan H, Ma X, Zhou S, Huang J, Liu Y, Liu Y (2019) Highly efficient removal of heavy metal ions by carboxymethylcelluloseimmobilized Fe3O4 nanoparticles prepared via high-gravity technology. Carbohydr Polym 213:39–49
Gao J, He Y, Zhao X, Ran X, Wuc Y, Su Y, Dai J (2016) Single step synthesis of amine-functionalized mesoporous magnetite nanoparticles and their application for copper ions removal from aqueous solution. J Colloid Interface Sci 481:220–228
Liu JF, Shanzhao Z, Binjiang G (2008) Coating of Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ Sci Technol 42:6949–6954
Lin C, Lin YS, Ho JM (2016) Adsorption of Reactive Red 2 from aqueous solutions using Fe3O4 nanoparticles prepared by co-precipitation in a rotating packed bed. J Alloy Comp 666:153–158
Muthukumaran C, Sivakumar VM, Thirumarimurugan M (2016) Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J Taiwan Inst Chem Eng 63:354–362
He Y, Cheng Z, Qin Y, Xu B, Ning L, Zhou L (2015) Facile synthesis and functionalization of hyperbranched polyglycerol capped magnetic Fe3O4 nanoparticles for efficient dye removal. Mater Lett 151:100–103
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi MS, Nazmara A, Mahvi H (2016) Modeling of reactive blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: Optimization, reusability, kinetic and equilibrium studies. J Mag Mag Mater 404:179–189
Wu R, Liu JH, Zhao L, Zhang X, Xie J, Yu B, Ma X, Yang ST, Wang H, Liu Y (2014) Hydrothermal preparation of magnetic Fe3O4@C nanoparticles for dye adsorption. J Environ Chem Eng 2:907–913
Asfaram A, Ghaedi M, Hajati S, Goudarzi A, Dil EA (2017) Screening and optimization of highly effective ultrasound-assisted simultaneous adsorption of cationic dyes onto Mn-doped Fe3O4 nanoparticle-loaded activated carbon. Ultrason Sonochem 34:1–12
Kotsyuda SS, Tomina VV, Zub YL, Furtat IM, Melnyk IV (2017) Bifunctional silica nanospheres with 3-aminopropyl and phenyl groups. Synthesis approach and prospects of their applications. Appl Surf Sci 420:782–791
Mahmoud ME, Fekry NA, El-Latif MMA (2016) Nanocomposites of nanosilica-immobilized-nanopolyaniline and cross linked nanopolyaniline for removal of heavy metals. Chem Eng J 304:679–691
Jiang Z, Xie J, Jiang D, Yan Z, Jing J, Liu D (2014) Enhanced adsorption of hydroxyl contained/anionic dyes on non functionalized Ni@SiO2core–shell nanoparticles: kinetic and thermodynamic profile. Appl Surface Sci 292:301–310
Giles D, Mohapatra M, Issa TB, Anand S, Singh P (2011) Iron and aluminium based adsorption strategies for removing arsenic from water. J Environ Manag 92:3011–3022
Prabhakar R, Samadder SR (2018) Low cost and easy synthesis of aluminium oxide nanoparticles for arsenite removal from groundwater: a complete batch study. J Mol Liquids 250:192–201
Hojamberdiev M, Daminova SS, Kadirova ZC, Sharipov KT, Mtalo F, Hasegawa M (2018) Ligand-immobilized spent alumina catalyst for effective removal of heavy metal ions from model contaminated water. J Environ Chem Eng 6:4623–4633
Saadi Z, Saadi R, Fazaeli R (2013) Fixed-bed adsorption dynamics of Pb (II) adsorption from aqueous solution using nanostructured alumina. J Nanostruct Chem 3:1–8
Tabesh S, Davar F, LoghmanEstarki RM (2018) Preparation of Al2O3 nanoparticles using modified sol-gel method and its use for the adsorption of lead and cadmium ions. J Alloys Compd 730:441–449
Stietiya MH, Wang JJ (2014) Zinc and cadmium adsorption to aluminum oxide nanoparticles affected by naturally occurring ligands. J Environ Qual 43:498
Poursani AS, Nilchi A, Hassani AH, Shariat M, Nouri J (2015) A novel method for synthesis of nano Al2O3: Study of adsorption behavior of chromium, nickel, cadmium and lead ions. Int J Environ Sci Technol 12:2003–2014
Mahdavi S, Jalali M, Afkhami A (2015) Heavy metals removal from aqueous solutions by Al2O3 nanoparticles modified with natural and chemical modifiers. Clean Technol Environ Policy 17:85–102
Patra AK, Dutta BAA (2012) Self-assembled mesoporous Al2O3 spherical nanoparticles and their efficiency for the removal of arsenic from water. J Hazard Mater 201:170–177
Wang X, Zhan C, Kong B, Zhu X, Liu J, Xu W, Cai W, Wang H (2015) Self-curled coral like Al2O3 nanoplates for use as an adsorbent. J Colloid Interface Sci 453:244–251
Nguyen TMT, Do TPT, Hoang TS, Nguyen NV, Pham HD, Nguyen TD, Pham TNM, Le TS, Pham TD (2018) Adsorption of anionic surfactants onto alumina: characteristics, mechanisms, and application for heavy metal removal. Int J Polym Sci 2830286(11)
Pham T D, Tran T T, Le V A, Pham T T, Dao T H, Le T S (2019)Adsorption characteristics of molecular oxytetracycline onto alumina particles: the role of surface modification with an anionic surfactant. J Mol Liquids 287:110900
Pham T, Do TU, Pham TT, Nguyen TAH, Nguyen TKT, Vu ND, Le TS, Vu CM, Kobayashi M (2019) Adsorption of poly (styrenesulfonate) onto different-sized alumina particles: characteristics and mechanisms. Colloid Polym Sci 297:13–22
Ghaemi N (2016) A new approach to copper ion removal from water by polymeric nanocomposite membrane embedded with γ-alumina nanoparticles. Appl Surface Sci 364:221–228
Chu TPM, Nguyen NT, Vu TL, Dao TH, Dinh LC, Nguyen HL, Hoang TH, Le TS, Pham TD (2019) Synthesis, characterization, and modification of alumina nanoparticles for cationic dye removal. Mater 12:450
Ali S, Abbas Y, Zuhra Z, Butlerc IS (2019) Synthesis of y-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv 1:2–13
Banerjee S, Dubey S, Gautam RK, Chattopadhyaya MC, Sharma YC (2019) Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye, Orange G from aqueous solutions. Arab J Chem 12(8):5339–5354
Huang CC, Lo SL, Lien HL (2015) Vitamin B12-mediated hydro dechlorination of dichloromethane by bimetallic Cu/Al particles. Chem Eng J 273:413–420
Wang HW, Peng P, Fennell DE (2015) Rapid dechlorination of 1,2,3,4-TCDD by Ag/Fe bimetallic particles. Chem Eng J 273:465–471
Hong HJ, Farooq W, Yang JS, Yang JW (2010) Separ Sci Technol 45(688):1975–1981
Chen LH, Huang CC, Lien HL (2008) Bimetallic iron–aluminum particles for dechlorination of carbon tetrachloride. Chemosphere 73:692–697
Lin CJ, Liou YH, Lo SL (2009) Supported Pd/Sn bimetallic nanoparticles for reductive dechlorination of aqueous trichloroethylene. Chemosphere 74(2):314–319
Ma L, Ding Z, Gao T, Zhou R, Xu W, Liu J (2004) Discoloration of methylene blue and wastewater from a plant by a Fe/Cu bimetallic system. Chemosphere 55(9):1207–1212
Fang W, Xu C, Zheng J, Chen G, Jiang K (2015) Fabrication of Cu–Ag bimetal nanotube-based copper silicates for enhancement of antibacterial activities. RSC Adv 5(694):39612–39619
Zhang Z, Shen Q, Cissoko N, Wo J, Xu X (2010) Catalytic dechlorination of 2,4-dichlorophenol by Pd/Fe bimetallic nanoparticles in the presence of humic acid. J Hazard Mater 182(867):252–258
Choi JH, Kim YH (2009) Reduction of 2, 4, 6-trichlorophenol with zero-valent zinc and catalyzed zinc. J Hazard Mater 166:984–991
Yang B, Deng S, Yu G, Lu Y, Zhang H, Xiao J, Chen G, Cheng X, Shi L (2013) Pd/Al bimetallic nanoparticles for complete hydro dechlorination of 3-chlorophenol in aqueous solution. Chem Eng J 219:492–498
Lin Y, Shih, Mac Farlane J (2015) Amphiphilic compounds enhance the dechlorination of pentachlorophenol with Ni/Fe bimetallic nanoparticles. Chem Eng J 262:59–67
Nagpal V, Bokare AD, Chikate RC, Rode CV, Paknikar KM (2010) Reductive dechlorination of γ-hexachlorocyclohexane using Fe–Pd bimetallic nanoparticles. J Hazard Mater 175:680–687
Bleyl S, Kopinke FD, Mackenzie K (2012) Carbo-Iron Synthesis and stabilization of Fe(0)-doped colloidal activated carbon for in situ groundwater treatment. Chem Eng J 191:588–595
DeVor R, Knighton KC, Aitken B, Maloney P, Holland E, Talalaj L, FidlerR ES, Clausen CA, Geiger CL (2008) Dechlorination comparison of mono-substituted PCBs with Mg/Pd in different solvent systems. Chemosphere 73:896–900
Yang B, Zhang Y, Deng S, Yu G, Lu Y, Wu J, Xiao J, Chen G, Cheng X, Shi L (2013) Reductive degradation of chlorinated organic pollutants-contaminated water by bimetallic Pd/Al nanoparticles: effect of acidic condition and surfactants. Chem Eng J 234:346–353
Agarwal S, Al-Abed SR, Dionysiou DD (2009) Impact of organic solvents and common anions on 2-chlorobiphenyl dechlorination kinetics with Pd/Mg. Appl Catal B: Environ 92:17–22
Yang B, Deng S, Yu G, Zhang H, Wu J, Zhuo Q (2011) Bimetallic Pd/Al particles for highly efficient hydrodechlorination of 2-chlorobiphenyl in acidic aqueous solution. J Hazard Mater 189:76–83
Kuang Y, Du J, Zhou R, Chen Z, Megharaj M, Naidu R (2015) Calcium alginate encapsulated Ni/Fe nanoparticles beads for simultaneous removal of Cu (II) and monochlorobenzene. J Colloid Interface Sci 447:85–91
WengX LS, Zhong Y, Chen Z (2013) Chitosan stabilized bimetallic Fe/Ni nanoparticles used to remove mixed contaminants-amoxicillin and Cd (II) from aqueous solutions. Chem Eng J 229:27–34
Huguet MR, Marshall WD (2009) Reduction of hexavalent chromium mediated by micro- and nano-sized mixed metallic particles. J Hazard Mater 169:1081–1087
Kadu BS, Sathe YD, Ingle AB, Chikate RC, Patil KR, Rode CV (2011) Efficiency and recycling capability of montmorillonite supported Fe–Ni bimetallic nanocomposites towards hexavalent chromium remediation. Appl Catal B Environ 104:407–414
Zhang G, Qu J, Liu H, Liu R, Wu R (2007) Preparation and evaluation of a novel Fe–Mn binary oxide adsorbent for effective arsenite removal. Water Res 41:1921–1928
Cheng Z, Fu F, Dionysiou DD, Tang B (2016) Adsorption, oxidation, and reduction behavior of arsenic in the removal of aqueous As (III) by mesoporous Fe–Al bimetallic particles Water Res 96:22–31
Bokare AD, Chikate RC, Rode CV, Paknikar KM (2008) Iron-nickel bimetallic nanoparticles for reductive degradation of azo dye Orange G in aqueous solution. Appl Catal B Environ 79:270–278
Ma LM, Ding ZG, Gao TY, Zhou RF, Xu WY, Liu J (2004) Discoloration of methylene blue and wastewater from a plant by a Fe/Cu bimetallic system. Chemosphere 55:1207–1212
GautamR K, Rawat V, Banerjee S, Sanroman MA, Soni S, Singh SK, Chattopadhyaya MC (2015) Synthesis of bimetallic Fe–Zn nanoparticles and its application towards adsorptive removal of carcinogenic dye malachite green and Congo red in water. J Mol Liq 212:227–236
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jain, A., Wadhawan, S., Mehta, S.K. (2022). Nanoparticles-Based Adsorbents for Water Pollutants Removal. In: Das, R., Saha, B.B. (eds) Rapid Refrigeration and Water Protection. Springer Water. Springer, Cham. https://doi.org/10.1007/978-3-030-93845-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-93845-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93844-4
Online ISBN: 978-3-030-93845-1
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)