Abstract
Central sleep apnea is a manifestation of breathing instability in a variety of clinical conditions and is often bundled under the rubric of obstructive sleep apnea (OSA). Central sleep apnea occurs because of a transient cessation of ventilatory motor output, under several physiologic or pathologic conditions. This chapter will address the pathogenesis, clinical features, and management of central sleep apnea.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Central apnea
- Hypoventilation
- Hyperventilation
- Hypocapnia
- Cheyne–Stokes respiration
- CPAP
- Adaptive servo-ventilation
Central sleep apnea is a manifestation of breathing instability in a variety of clinical conditions and is often bundled under the rubric of obstructive sleep apnea. Central sleep apnea occurs because of a transient cessation of ventilatory motor output, under several physiologic or pathologic conditions. This chapter will address the pathogenesis, clinical features, and management of central sleep apnea.
Determinants of Central Apnea During NREM Sleep
Hypocapnia
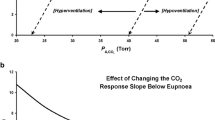
The sleep state (specifically non-rapid eye movement or NREM sleep) removes the wakefulness “drive to breathe” and renders respiration critically dependent on chemical influences, especially partial pressure of carbon dioxide (PCO2). Central apnea results if arterial PCO2 is lowered below a highly sensitive “apneic threshold.” [1, 2] Hypocapnia is a potent but not an omnipotent mechanism of reduced ventilatory motor output during NREM sleep. Several factors modulate and mitigate the effects of hypocapnia on ventilatory motor output and promote stability of respiration.
Short-Term Potentiation
Actively induced hyperventilation (such as hypoxic hyperventilation) is associated with activation of an excitatory neural mechanism referred to as short-term potentiation (STP) [ 3,4,5], which results in a gradual return of ventilation toward the baseline upon cessation of the stimulus to breathe. STP has been demonstrated in humans as well as in animals, and is unaffected by the state of consciousness. STP may play a significant role in preserving rhythmic respiration by preventing abrupt drop in ventilation during transient hypocapnia such as following brief hypoxia or transient arousal. In fact, central apnea rarely occurs following termination of brief hypoxia, despite hypocapnia at or below the apneic threshold [3,4,5]. Similarly, although hypocapnia occurs during transient arousals from sleep, the activation of STP may mitigate the occurrence of central apnea under these conditions [6]. However, prolonged hypoxia may abolish STP, which may explain the development of periodic breathing after 20–25 min of hypoxia and the occurrence of central apnea upon termination of prolonged hypoxic exposure [5, 7].
Duration of Hyperpnea
The duration of hyperpnea is another important determinant of reduced ventilatory motor output following hyperventilation. Central apnea does not usually occur following brief arousal in sleeping humans [8] or dogs [9] possibly due to insufficient reduction in PCO2 at the level of the central chemoreceptors.
In summary, the balance between hypocapnia and short-term potentiation determines the occurrence of post-hyperventilation apnea during stable sleep, while the duration of hyperventilation may determine whether the reduction in medullary PCO2 is enough for the development of central apnea.
Role of Upper Airway Reflexes
While hypocapnia is the most common influence leading to central apnea, other mechanisms may also induce central apnea. For example, negative pressure–induced deformation of the isolated upper airway causes central apnea in dogs during both wakefulness and sleep [10]. Whether such reflexes contribute to the developments of central apnea in sleeping humans remains speculative. Conversely, central apnea occurs more frequently in the supine position [11,12,13] and may be reversed with nasal continuous positive airway pressure (CPAP) [14]. Likewise, there is evidence of supine dependency including that the lateral position amelioration of severity of central apnea and Cheyne–Stokes respiration [11–13].
Mechanisms Perpetuating Breathing Instability
Central apnea does not occur as a single event, but as cycles of apnea/hypopnea alternating with hyperpnea. Ventilatory control during sleep operates as a negative-feedback closed-loop cycle to maintain homeostasis of blood gas tensions within a physiologic range. Many authors have adopted the engineering concept of “loop gain ” as a measure of ventilatory stability or susceptibility to central apnea and recurrent periodic breathing [15]. Loop gain represents the overall response of the plant (representing the lung and respiratory muscles); the controller (representing the ventilatory control centers and the chemoreceptors); and the delay, dilution, and diffusion inherent in transferring the signal between the plant and the controller. The formula for loop gain is as follows:
The formula can be expanded to account for pulmonary blood flow (Q, equivalent to cardiac output) and carbon dioxide–carrying capacity of the blood (β); the derivation for this expanded equation can be found in the study by Ghazanshahi and Khoo [16]. These two factors comprise the rate of carbon dioxide delivery to the chemoreceptors and the lungs, which, when delayed, can increase loop gain by producing lag between the disturbance (initial change in ventilation or carbon dioxide) and the response. A greater loop gain represents increased reactivity of the ventilatory circuit to disturbances and, consequently, ventilatory instability [17]. Central sleep apnea is associated with increased loop gain, which can be observed in conditions such as congestive heart failure (CHF – increased controller gain and prolonged circulation time) or obesity and tetraplegia (increased plant gain resulting from decreased lung volumes) [17,18,19]. Conversely, a lower loop gain corresponds to greater ventilatory stability, as is observed during REM sleep [20]. A detailed discussion of the dynamics of ventilatory control is beyond the scope of this chapter; however, there are several excellent reviews that have discussed this aspect in detail [21,22,23].
The occurrence of central apnea is associated with several consequences that conspire to promote further breathing instability:
-
Once ventilatory motor output ceases, rhythmic breathing does not resume at eupneic arterial PCO2 (PaCO2) due to inertia of the ventilatory control system; an increase in PaCO2 by 4–6 mmHg above eupnea is required for resumption of respiratory effort [24].
-
Central apnea is associated with narrowing or occlusion of the pharyngeal airway [25]. Thus, resumption of ventilation requires opening of a narrowed or occluded airway and overcoming tissue adhesion forces [26] and craniofacial gravitational forces.
Termination of central apnea is associated with variable changes in arterial blood gases (hypoxia and hypercapnia) and transient EEG arousal, resulting in ventilatory overshoot, subsequent hypocapnia, and a recurrence of apnea/hypopnea. This sequence explains why apnea rarely occurs as a single event (i.e., “apnea begets apnea”) and why there is an overlap between central and obstructive apnea (upper airway obstruction often follows central apneas upon resumption of respiratory effort, i.e., mixed apnea).
Pathophysiologic Classification of Central Sleep Apnea
Central apnea syndrome may be present in a diverse group of conditions including heart failure and obstructive sleep apnea. The ICSD-3 lists several categories of central apnea: (1) Primary Central Sleep Apnea, (2) Central Sleep Apnea Due to Cheyne–Stokes Breathing Pattern, (3) Central Sleep Apnea Due to Medical Condition Not Cheyne–Stokes, (4) Central Sleep Apnea due to High Altitude Periodic Breathing, (5) Central Sleep Apnea Due to Drug or Substance Use, (6) Central Sleep Apnea of Infancy, (7) Central Sleep Apnea of Prematurity, and (8) Treatment-Emergent Central Sleep Apnea [27]. Central apneas are caused either by hyperventilation or hypoventilation. Primary central sleep apnea (CSA), Cheyne–Stokes respiration with central sleep apnea (CSA-CSR), and CSA at high altitude are examples of CSA-related to hyperventilation. Central sleep apnea due to drug or substance use is due to hypoventilation, whereas central apnea associated with other medical conditions may be due to either hyperventilation or hypoventilation. The underlying mechanisms influence the choice of therapy including optimization of medical therapy in central apnea associated with other conditions such as heart failure, hypothyroidism, or acromegaly.
The level of arterial PCO2 during wakefulness is often used to classify central apnea as hypercapnic or non-hypercapnic. However, such classification does not capture the underlying pathogenesis as apnea represents hypoventilation or a consequence of hyperventilation.
Central Sleep Apnea Secondary to Hypoventilation
The sleep state is associated with reduced ventilatory motor output, increased upper airway resistance, and hypoventilation. This physiologic constellation carries pathologic consequences in patients with an underlying abnormality in ventilatory control or impaired pulmonary mechanics. Most afflicted patients suffer from a central nervous system disease (e.g., encephalitis), neuromuscular disease (e.g., post-polio syndrome), or severe abnormalities in pulmonary mechanics (e.g., kyphoscoliosis [28]). Thus, the hallmark of this disease is alveolar hypoventilation representing nocturnal ventilatory failure or worsening of the underlying chronic disease. Arousal from sleep restores alveolar ventilation to a variable degree; resumption of sleep reduces ventilation in a cyclical fashion.
Central apnea secondary to hypoventilation does not necessarily meet the strict definition of “apnea,” since feeble ventilatory motor output may persist albeit below the thresholds required to preserve alveolar ventilation. Likewise, it may not meet the definition of “central” in patients with respiratory muscle disease or skeletal deformities. Consequently, the presenting clinical picture includes both features of the underlying ventilatory insufficiency (e.g., morning headache, cor pulmonale, peripheral edema, polycythemia, and abnormal pulmonary function tests) and features of the sleep apnea/hypopnea syndrome (e.g., poor nocturnal sleep, snoring, and daytime sleepiness).
A rare but interesting group of patients present with primary alveolar hypoventilation manifesting by daytime hypoventilation without an apparent identifiable cause and blunted chemo responsiveness [29, 30]. Congenital central hypoventilation syndrome (CCHS) results from a mutation in the gene that encodes the homeobox (PHOX) 2B gene.
The mechanism(s) responsible for hypercapnic central sleep apnea in a given patient influence(s) the management strategy, which aims to restore effective alveolar ventilation during sleep. Treatment of choice is assisted ventilation; nasal CPAP and supplemental oxygen are unlikely to alleviate the condition.
Central Apnea Secondary to Hyperventilation
Hypocapnia secondary to hyperventilation is the most common underlying mechanism of central apnea. A typical patient with non-hypercapnic central apnea has no evidence of a neuromuscular disorder, abnormal lung mechanics, or impaired responses to chemical stimuli. Accordingly, apnea is a result of a transient instability rather than a ventilatory control defect.
How does the first apnea begin? Several transient perturbations may trigger the initial event, including oscillation in sleep state [31], or transient hypoxia possibly due to retention of secretions or reduced lung volumes at sleep onset. Thus, hypoxia stimulates ventilation, subsequently leading to hypocapnia and apnea. The occurrence of apnea initiates the repetitive process of apnea–hyperpnea and leads to sustained breathing instability, manifested as periodic breathing (see above). In summary, non-hypercapnic central apnea is a heterogeneous entity that may be an idiopathic or a secondary condition. The pathogenesis may vary depending upon the clinical condition. However, hypocapnia secondary to hyperventilation is the common denominator in this group of disorders .
Central Apnea Risk Factors
Sleep State
Transient breathing instability and central apnea may occur during the transition from wakefulness to NREM sleep. As sleep state oscillates between wakefulness and light sleep [32,33,34], the level of PaCO2 is at or below the hypocapnic level required to maintain rhythmic breathing during sleep (i.e., the “apneic threshold”), resulting in central apnea. Recovery from apnea is associated with transient wakefulness and hyperventilation. The subsequent hypocapnia elicits apnea upon resumption of sleep. Consolidation of sleep alleviates the oscillation in sleep and respiration and stabilizes PaCO2 at a higher set point above the apneic threshold. Sleep onset is also associated with another type of central apnea, not preceded by hyperventilation. The transition from alpha to theta in normal subjects is associated with prolongation of breath duration [35].
Central apnea at sleep onset if often considered “physiologic,” albeit not universal. Furthermore, events that occur during epochs scored as “wakefulness” are not captured. Whether sleep-onset central apnea is truly physiologic, or a reflection of increased loop gain is yet to be determined. The clinical implications and natural history of this “phenomenon” is unknown.
Central sleep apnea is uncommon during REM sleep as many studies suggest that breathing during REM sleep is impervious to chemical influences (REF), possibly due to increased ventilatory motor output during REM sleep [36, 37] relative to NREM sleep. In addition, there is evidence in animal studies that hypocapnia, per se, may decrease the amount of REM sleep [38]. The major barrier to answering this question in humans is the difficulty in conducting such experiments without disrupting REM sleep.
The loss of intercostal and accessory muscle activity during REM sleep leads to a reduction of alveolar ventilation. This may manifest as apparent central apnea or hypopnea in patients with compromised lung mechanics or neuromuscular disease. If severe diaphragm dysfunction is present, nadir tidal volume may be negligible and the event may appear as central apnea. Thus, central apnea during REM sleep represents transient hypoventilation rather than post-hyperventilation hypocapnia.
Age and Gender
Central sleep apnea is more prevalent in older adults relative to middle-aged individuals [39,40,41]. Physiologically, sleep state oscillations may precipitate central apnea in older adults [42]. Increased prevalence of comorbid conditions such as thyroid disease [43], congestive heart failure [44], atrial fibrillation [45], and cerebrovascular disease [46] may also contribute to increased susceptibility to develop central apnea in older adults.
Central sleep apnea is uncommon in premenopausal women [47]. There is evidence that women are less susceptible to the development of hypocapnic central apnea relative to men following mechanical ventilation. Physiologically, the hypocapnic apneic threshold is higher in men relative to women. Using nasal mechanical ventilation during stable NREM sleep, Zhou et al. [2] have shown that the apneic threshold was −3.5 versus −4.7 mmHg below room air level in men and women respectively. This difference was not due to progesterone. In fact, administration of testosterone to healthy premenopausal women for 12 days resulted in an elevation of the apneic threshold and a diminution in the magnitude of hypocapnic required for induction of central apnea during NREM sleep [48]. Conversely, suppression of testosterone with leuprolide acetate in healthy males decreases the hypocapnic apneic threshold and potentially stabilizing respiration [49]. Thus, male sex hormones are the most likely factor elevating the apneic threshold in men.
Medical Conditions
Sleep apnea is highly prevalent in patients with CHF [44, 50,51,52]. Javaheri et al. [51] demonstrated that 51% of male patients with CHF had sleep-disordered breathing, 40% had central sleep apnea, and 11% obstructive apnea. Risk factors for CSA in this group of patients include male gender, atrial fibrillation, age >60 years, and daytime hypocapnia (PCO2 < 38 mmHg during wakefulness) [53]. Risk factors for OSA differed by gender; the only independent determinant in men was body mass index (BMI), whereas age over 60 was the only independent determinant in women.
Hyperventilation is a common breathing pattern in patients with CHF, who demonstrate daytime hypocapnia and minimal or no rise in PET CO2 from wakefulness to sleep [54]. Chronic hyperventilation results in decreased plant gain [55, 56], which mitigates the magnitude of hypocapnia for a given increase in alveolar ventilation. In other words, steady-state hyperventilation and hypocapnia are potentially stabilizing rather than destabilizing as is commonly thought. Increased propensity to central apnea in patients with CHF is due to increased hypocapnic chemosensitivity (increased controller gain) and prolonged circulatory delay.
Sleep apnea is also common after a cerebrovascular accident (CVA) [46]; with central apnea being the predominant type in 40% of patients with sleep apnea after a CVA [57, 58]. Likewise, central apnea occurs in 30% of patients who are on stable methadone maintenance treatment [59]. Finally, several medical conditions predispose to the development of central apnea including hypothyroidism, acromegaly, and renal failure have an unexpectedly high prevalence of sleep apnea [60,61,62]. Nocturnal hemodialysis is associated with improvement in sleep apnea indices in patients with renal failure [62].
Cervical spinal cord injury (C-SCI) has also recently been identified as a risk factor for the development of central sleep apnea [63]. The mechanism underlying CSA in C-SCI is uncertain. Potential mechanisms include loss of intercostal muscle activity or decreased lung volume [64]. A reduction in lung volume results in increased plant gain, which causes an exaggerated change in PCO2 in response to changes in ventilation [65]. SCI has also been shown to increase peripheral chemosensitivity, possibly due to potentiation of the neurocircuitry regulating the production of serotonin, which is implicated in plasticity of the respiratory circuit [66]. The combination of increased plant gain and increased peripheral chemosensitivity may promote instability via ventilatory overshoot in response to minor derangements in ventilation or PCO2, leading to central apnea.
Some patients with central apnea have no apparent risk factor and are deemed to have “idiopathic central apnea.” Typically, these patients demonstrate increased chemo-responsiveness and sleep state instability [67]. It is plausible that these patients will have occult cardiac or metabolic disease. For example, idiopathic central sleep apnea is more prevalent in patients with atrial fibrillation [45].
Central sleep apnea can also develop during treatment of obstructive sleep apnea. Treatment-emergent central sleep apnea (TECSA) is primarily associated with the initiation of CPAP therapy, but has been observed in other OSA therapies, including mandibular advancement devices and surgical intervention [68, 69]. TECSA may be either transient or persistent, often resolving spontaneously with persistent positive airway pressure (PAP) therapy [69]. Possible mechanisms underlying TECSA include increased elimination of CO2 following relief of airway obstruction, hyperventilation due to PAP-related arousals, and over-titration causing activation of lung stretch receptors and subsequent inhibition of respiratory drive [68].
Clinical Features and Diagnosis
The clinical presentation includes features of the underlying disease and features of sleep apnea syndrome. Patients with central apnea secondary to hyperventilation may present with the usual symptoms of sleep apnea syndrome. Alternatively, they may present with insomnia and poor nocturnal addition. Frequent oscillation between wakefulness and stage 1 NREM sleep may promote sleep fragmentation and poor nocturnal sleep as the presenting symptoms.
Central sleep apnea may also be a found as an incidental polysomnographic finding in a patient with obstructive sleep apnea, either on the initial diagnostic study or after restoring upper airway patency with nasal CPAP. The latter is referred to as “complex sleep apnea,” implying a distinct clinical entity. However, it is likely that this phenomenon represents unmasking of the underlying breathing instability in patients with obstructive sleep apnea and may resolve spontaneously [70, 71].
Nocturnal polysomnography is the standard diagnostic method including measurement of sleep and respiration, and also including detection of flow, measurement of oxyhemoglobin saturation, and detection of respiratory effort. Detection of respiratory effort is important to distinguish central from obstructive apnea. Most clinical sleep laboratories utilize surface recording of effort to detect displacement of the abdominal and thoracic compartments instead of esophageal pressure recording.
The presence of cardiogenic oscillations (pulse artifacts) on the flow signal has been used as an indirect index of central etiology. The underlying rationale is the pulse artifacts represent transmission of a pulse waveform from the thorax, and hence indicates a patent upper airway that allows the transmission of cardiogenic oscillation. Morrell et al. [72] used fiber optic nasopharyngoscopy to evaluate upper airway patency during central apnea; cardiogenic oscillations were present even when the airway is completely occluded. Thus, the presence of cardiogenic oscillations does not prove upper airway patency or central etiology.
Management
Central sleep apnea is a disorder with protean manifestations and underlying conditions. The presence of comorbid conditions and concomitant obstructive sleep apnea influence therapeutic approach significantly. Specific therapeutic options include positive pressure therapy, pharmacologic therapy, and supplemental oxygen.
Positive Pressure Therapy
CPAP therapy is the initial treatment of choice for central sleep apnea. Published practice parameters by the American Academy of Sleep Medicine recommends CPAP as a standard therapy, based on the preponderance of evidence supporting its use [73]. Most of this evidence comes from investigations on central apnea related to congestive heart failure (CHF), although other subtypes of central sleep apnea appear to respond to CPAP as well, especially if it occurs in combination with episodes of obstructive or mixed apnea. In fact, “pure” central apnea with no concomitant obstructive events is uncommon. If a comorbid clinical condition is present, such as heart failure, hypothyroidism, or acromegaly, optimization of medical therapy is also required and may ameliorate the severity of central apnea. Likewise, central sleep apnea in patients with obstructive sleep apnea may resolve with alleviation of upper airway obstruction with positive pressure therapy. Many patients with idiopathic central sleep apnea receive a trial of nasal CPAP, which has been shown to reverse central sleep apnea, even in the absence of obstructive respiratory events [14], especially supine-dependent central sleep apnea. The response may be due to preventing upper airway occlusion during central apnea and subsequent ventilatory overshoot [25]. Prevention of ventilatory overshoot may explain the reported combination of reduced apnea frequency and increased PCO2 after CPAP [74]. Nasal CPAP is the initial option during a therapeutic titration study, despite the lack of systematic studies on nasal CPAP therapy in patients with idiopathic central apnea.
The exuberance regarding nasal CPAP therapy in patients with central apnea and CHF did not withstand the rigors of controlled clinical trials. The Canadian Continuous Positive Airway Pressure trial, or Can PAP [75] tested the hypothesis that CPAP would improve the survival rate without heart transplantation in patients with heart failure and central sleep apnea. This type of central apnea corresponds to Central Sleep Apnea Due to Cheyne–Stokes Breathing Pattern, in the International Classification of Sleep Disorders – Third Edition (ICSD-3). Participants were randomly assigned to nasal CPAP or no CPAP. There was no difference in the overall event rates (death and heart transplantation) between the two groups after a 2-year follow-up, despite greater improvement in the CPAP group at 3 months in several intermediate outcomes including apnea–hypopnea index, ejection fraction, mean nocturnal oxyhemoglobin saturation, plasma norepinephrine levels, and the distance walked in 6 min at 3 months. Thus, nasal CPAP had no measured effect on survival, despite the effect on the “severity” of central apnea and several intermediate outcome variables. Therefore, current evidence supports the use of CPAP to alleviate the severity of central sleep apnea and improve daytime function and quality of life.
Noninvasive positive pressure ventilation (NIPPV) using pressure support mode (bi-level nasal positive pressure) is effective in restoring alveolar ventilation during sleep. Clinical indications include nocturnal ventilatory failure and central apnea secondary to hypoventilation. There is evidence that NIPPV exerts a salutary effect on survival in patients with ventilatory failure secondary to amyotrophic lateral sclerosis [76]. It is unclear whether NIPPV exerts a similar effect in other neuromuscular conditions associated with nocturnal ventilatory failure. However, the overall evidence supports the use of NIPPV in a pressure support mode to treat central sleep apnea secondary to hypoventilation, such as neuromuscular or chest wall–related nocturnal hypoventilation. If the ventilatory motor output is insufficient to “trigger” the mechanical inspiration, adding a backup rate ensure adequate ventilation.
Treatment of central apnea secondary to hyperventilation using nasal pressure support ventilation in the bi-level mode may result in worsening of central apnea and breathing instability owing to augmented ventilatory overshoot and hypocapnia [77]. The work of Meza et al. [78] provides empiric evidence that pressure-support ventilation results in periodic breathing and recurrent central apnea when the pressure gradient is above 7 cm H2O. The addition of a backup rate would be required to maintain stable respiration, which would convert ventilatory support to controlled mechanical ventilation. In general, bi-level positive pressure therapy is unlikely to alleviate central apnea, without a backup rate. Nevertheless, bi-level PAP may ameliorate central apnea that accompanies severe obstructive apnea by preventing upper airway obstruction and ventilatory overshoot.
Recent technological advances allowed for variations in the mode of delivering positive pressure ventilation. One example is Adaptive Servo-Ventilation (ASV), which provides a small but varying amount of ventilatory support and a back-up rate, against a background of low level of CPAP. The device maintains ventilation at 90% of a running 3-min reference period; thus, changes in respiratory effort results in reciprocal changes in the magnitude of ventilatory support. There is evidence that ASV is more effective than CPAP, bi-level pressure support ventilation, or increased dead space in alleviating central sleep apnea [79, 80]. However, the Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure (SERVE-HF) trial demonstrated a significant increase in both all-cause and cardiac mortality in individuals with CHF with a left ventricular ejection fraction (LVEF) <45%, leading the American Academy of Sleep Medicine to recommend against the use of ASV in this population [81, 82]. ASV is still permissible for patients with CSA with CHF with LVEF >45% [82]. In patients for whom there are no absolute contraindications to ASV, the decision to initiate ASV hinges on the efficacy of the treatment in normalizing AHI, patient preference, payers’ preference, and the availability of the requisite support for adherence or troubleshooting.
Pharmacological Therapy
Pharmacologic therapy for central apnea remains elusive, and there are no controlled clinical trials demarcating the boundaries of effectiveness [83]. Several small clinical trials indicate that acetazolamide, theophylline, or zolpidem may be beneficial in the treatment of central apnea [84, 85]. Acetazolamide is a weak diuretic and a carbonic anhydrase inhibitor that causes mild metabolic acidosis. Acetazolamide ameliorates central sleep apnea when administered as a single dose of 250 mg before bedtime [18, 84]. Likewise, theophylline ameliorates the severity of Cheyne–Stokes respiration in patients with CHF [85], without adverse effect on sleep architecture. Zolpidem – a non-benzodiazepine sedative hypnotic – has been shown in one study to reduce the severity of central sleep apnea and improve sleep continuity [86]. However, there are no controlled studies demonstrating safety and efficacy; therefore, zolpidem cannot be recommended for the treatment of central apnea. Recently, serotonergic drugs have been investigated as possible therapies for central sleep apnea due to the modulatory role serotonin plays in the respiratory circuit. Buspirone, an anxiolytic and direct serotonin receptor agonist, has demonstrated some efficacy in treating central sleep apnea [87, 88]. Nevertheless, safety and efficacy of the pharmacologic agents await empiric proof. Pharmacologic therapy represents a major opportunity for future investigation.
Supplemental O2 and CO2
Several studies have demonstrated a salutary effect of supplemental O2 in patients with idiopathic central sleep apnea and patients with Central Sleep Apnea Due to Cheyne–Stokes Breathing Pattern [89]. Several potential mechanisms may explain the stabilizing effect of supplemental oxygen on respiration. Oxygen dampens peripheral chemoreceptor responsiveness and minimizes the subsequent ventilatory overshoot. In addition, prolonged hyperoxia stimulates respiration, perhaps by elevating cerebral PCO2 by the Haldane effect. Acute administration of oxygen is associated with diminished propensity to develop central apnea in normal subjects during sleep [90]. While long-term clinical trials are lacking, supplemental oxygen therapy is a promising adjunct for central apnea, especially in patients with CHF. Likewise, supplemental CO2 abolishes central apnea in patients with pure central sleep apnea, by raising PCO2 above the apneic threshold [91, 92]. However, this therapy is not practical given the need for a closed circuit to deliver supplemental CO2.
Transvenous Phrenic Nerve Stimulation
A recent development in the treatment of CSA is the use of implantable device-based therapy to pace the diaphragm in response to cessation of respiratory drive. Transvenous phrenic nerve stimulation (TPNS) involves an implantable 2-lead system, including a sensory lead which detects pauses in respiration and a stimulatory lead affixed to the phrenic nerve, which initiates contraction of the corresponding hemidiaphragm via a pulse generator implanted in the pectoral region [93]. TPNS significantly reduces the frequency of central apnea, nocturnal oxyhemoglobin desaturations, and arousals while improving sleep architecture and subjective sleep quality [94]. Benefits of TPNS include portability, effective control of central sleep apnea symptoms, and avoidance of nonadherence. The most common adverse effect of TPNS is discomfort, occurring in up to one-third of patients, but less than 5% of patients receiving TPNS elect to discontinue therapy [95]. As a recent innovation, there are no long-term clinical trials following the safety and efficacy of TPNS; however, the implementation of implantable devices represents an area of significant potential utility in the treatment of central sleep apnea.
A Suggested Approach
The heterogeneity of central sleep apnea dictates individualized treatment approach, including optimal treatment of underlying medical conditions and attention to potential medication effects. A trial of nasal CPAP in the sleep laboratory is warranted to ascertain the magnitude of improvement with CPAP alone. The use of BPAP in a pressure support mode is likely to aggravate the severity of central apnea, unless accompanied by a backup rate. While contraindicated in patients with CHF and LVEF <45%, ASV may be beneficial in patients with CSR secondary to CHF with LVEF >45% who do not respond to nasal CPAP alone. Supplemental O2 may be beneficial in patients with central apnea that persists on nasal CPAP, especially in patients with CHF-CSR.
Summary of Key Points
-
Sleep-related withdrawal of the ventilatory drive to breathe is the common denominator among all cases of central apnea, whereas hypocapnia is the final common pathway leading to apnea in non-hypercapnic central apnea.
-
The pathophysiologic heterogeneity may explain the protean clinical manifestations and the lack of a single effective therapy for all patients.
-
Central sleep apnea is not a single clinical entity; instead, it is a manifestation of breathing instability in a variety of clinical conditions. Central apnea syndrome may be present in a diverse group of conditions including heart failure and obstructive sleep apnea.
-
Central sleep apnea is caused either by hyperventilation or hypoventilation. Hypocapnia is the most potent and ubiquitous trigger of central sleep apnea. Central apnea rarely occurs as a single event; instead, it manifests by cycles of apnea/hypopnea alternating with hyperpnea.
-
Central sleep apnea is classified into the following specific categories according to the ICSD-3: (1) Primary Central Sleep Apnea, (2) Central Sleep Apnea Due to Cheyne–Stokes Breathing Pattern, (3) Central Sleep Apnea Due to Medical Condition Not Cheyne–Stokes, (4) Central Sleep Apnea due to High Altitude Periodic Breathing, (5) Central Sleep Apnea Due to Drug or Substance Use, (6) Central Sleep Apnea of Infancy, (7) Central Sleep Apnea of Prematurity, and (8) Treatment-Emergent Central Sleep Apnea. The underlying mechanisms influence the choice of therapy including optimization of medical therapy in central apnea associated with other conditions such as heart failure, hypothyroidism, or acromegaly.
-
Advanced age, male gender, and postmenopausal state in women are known determinants of central apnea. In contrast, central apnea is less common in REM sleep. Medical conditions which are associated with higher risk of central sleep apnea include CHF, CVA, chronic narcotics users, acromegaly, chronic renal failure, hypothyroidism, and spinal cord injury. Treatment-emergent central sleep apnea may also arise during treatment of obstructive sleep apnea.
-
Clinical features are a combination of sleep apnea features and comorbid conditions. The diagnosis requires nocturnal polysomnography. Specific therapeutic options include positive pressure therapy, pharmacologic therapy, supplemental oxygen, and transvenous phrenic nerve stimulation. Nasal CPAP is the recommended initial treatment of choice.
References
Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(3):813–22.
Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol (1985). 2000;89(1):192–9.
Tawadrous FD, Eldridge FL. Posthyperventilation breathing patterns after active hyperventilation in man. J Appl Physiol. 1974;37(3):353–6.
Eldridge FL, Gill-Kumar P. Central neural respiratory drive and after discharge. Respir Physiol. 1980;40(1):49–63.
Badr MS, Skatrud JB, Dempsey JA. Determinants of poststimulus potentiation in humans during NREM sleep. J Appl Physiol (1985). 1992;73(5):1958–71.
Badr MS, Morgan BJ, Finn L, et al. Ventilatory response to induced auditory arousals during NREM sleep. Sleep. 1997;20(9):707–14.
Berssenbrugge A, Dempsey J, Iber C, Skatrud J, Wilson P. Mechanisms of hypoxia-induced periodic breathing during sleep in humans. J Physiol. 1983;343:507–24.
Badr MS, Kawak A. Post-hyperventilation hypopnea in humans during NREM sleep. Respir Physiol. 1996;103(2):137–45.
Chow CM, Xi L, Smith CA, Saupe KW, Dempsey JA. A volume-dependent apneic threshold during NREM sleep in the dog. J Appl Physiol (1985). 1994;76(6):2315–25.
Harms CA, Zeng YJ, Smith CA, Vidruk EH, Dempsey JA. Negative pressure-induced deformation of the upper airway causes central apnea in awake and sleeping dogs. J Appl Physiol (1985). 1996;80(5):1528–39.
Sahlin C, Svanborg E, Stenlund H, Franklin KA. Cheyne-Stokes respiration and supine dependency. Eur Respir J. 2005;25(5):829–33.
Oksenberg A, Arons E, Snir D, Radwan H, Soroker N. Cheyne-Stokes respiration during sleep: a possible effect of body position. Med Sci Monit. 2002;8(7):Cs61–5.
Szollosi I, Roebuck T, Thompson B, Naughton MT. Lateral sleeping position reduces severity of central sleep apnea / Cheyne-Stokes respiration. Sleep. 2006;29(8):1045–51.
Issa FG, Sullivan CE. Reversal of central sleep apnea using nasal CPAP. Chest. 1986;90(2):165–71.
Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol Respir Environ Exerc Physiol. 1982;53(3):644–59.
Ghazanshahi SD, Khoo MC. Estimation of chemoreflex loop gain using pseudorandom binary CO2 stimulation. IEEE Trans Biomed Eng. 1997;44(5):357–66.
Sands SA, Mebrate Y, Edwards BA, et al. Resonance as the mechanism of daytime periodic breathing in patients with heart failure. Am J Respir Crit Care Med. 2017;195(2):237–46.
Ginter G, Sankari A, Eshraghi M, et al. Effect of acetazolamide on susceptibility to central sleep apnea in chronic spinal cord injury. J Appl Physiol (1985). 2020;128(4):960–6.
Bokov P, Essalhi M, Delclaux C. Loop gain in severely obese women with obstructive sleep apnoea. Respir Physiol Neurobiol. 2016;221:49–53.
Badr MS, Dingell JD, Javaheri S. Central sleep apnea: a brief review. Curr Pulmonol Rep. 2019;8(1):14–21.
Khoo MC. Determinants of ventilatory instability and variability. Respir Physiol. 2000;122(2–3):167–82.
Chapman KR, Bruce EN, Gothe B, Cherniack NS. Possible mechanisms of periodic breathing during sleep. J Appl Physiol (1985). 1988;64(3):1000–8.
Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91(2):295–305.
Leevers AM, Simon PM, Dempsey JA. Apnea after normocapnic mechanical ventilation during NREM sleep. J Appl Physiol (1985). 1994;77(5):2079–85.
Badr MS, Toiber F, Skatrud JB, Dempsey J. Pharyngeal narrowing/occlusion during central sleep apnea. J Appl Physiol (1985). 1995;78(5):1806–15.
Olson LG, Strohl KP. Airway secretions influence upper airway patency in the rabbit. Am Rev Respir Dis. 1988;137(6):1379–81.
Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–94.
Mezon BL, West P, Israels J, Kryger M. Sleep breathing abnormalities in kyphoscoliosis. Am Rev Respir Dis. 1980;122(4):617–21.
Barlow PB, Bartlett D Jr, Hauri P, et al. Idiopathic hypoventilation syndrome: importance of preventing nocturnal hypoxemia and hypercapnia. Am Rev Respir Dis. 1980;121(1):141–5.
Weese-Mayer DE, Rand CM, Berry-Kravis EM, et al. Congenital central hypoventilation syndrome from past to future: model for translational and transitional autonomic medicine. Pediatr Pulmonol. 2009;44(6):521–35.
Pack AI, Cola MF, Goldszmidt A, Ogilvie MD, Gottschalk A. Correlation between oscillations in ventilation and frequency content of the electroencephalogram. J Appl Physiol (1985). 1992;72(3):985–92.
Trinder J, Whitworth F, Kay A, Wilkin P. Respiratory instability during sleep onset. J Appl Physiol (1985). 1992;73(6):2462–9.
Dunai J, Wilkinson M, Trinder J. Interaction of chemical and state effects on ventilation during sleep onset. J Appl Physiol (1985). 1996;81(5):2235–43.
Dunai J, Kleiman J, Trinder J. Ventilatory instability during sleep onset in individuals with high peripheral chemosensitivity. J Appl Physiol (1985). 1999;87(2):661–72.
Thomson S, Morrell MJ, Cordingley JJ, Semple SJ. Ventilation is unstable during drowsiness before sleep onset. J Appl Physiol (1985). 2005;99(5):2036–44.
Orem J. Neuronal mechanisms of respiration in REM sleep. Sleep. 1980;3(3–4):251–67.
Orem J, Lovering AT, Dunin-Barkowski W, Vidruk EH. Tonic activity in the respiratory system in wakefulness, NREM and REM sleep. Sleep. 2002;25(5):488–96.
Lovering AT, Fraigne JJ, Dunin-Barkowski WL, Vidruk EH, Orem JM. Hypocapnia decreases the amount of rapid eye movement sleep in cats. Sleep. 2003;26(8):961–7.
Phillips B, Cook Y, Schmitt F, Berry D. Sleep apnea: prevalence of risk factors in a general population. South Med J. 1989;82(9):1090–2.
Phillips BA, Berry DT, Schmitt FA, Magan LK, Gerhardstein DC, Cook YR. Sleep-disordered breathing in the healthy elderly. Clinically significant? Chest. 1992;101(2):345–9.
Ancoli-Israel S, Kripke DF, Klauber MR, Mason WJ, Fell R, Kaplan O. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14(6):486–95.
Pack AI, Silage DA, Millman RP, Knight H, Shore ET, Chung DC. Spectral analysis of ventilation in elderly subjects awake and asleep. J Appl Physiol (1985). 1988;64(3):1257–67.
Kapur VK, Koepsell TD, deMaine J, Hert R, Sandblom RE, Psaty BM. Association of hypothyroidism and obstructive sleep apnea. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1379–83.
Bradley TD, Floras JS. Sleep apnea and heart failure: part II: central sleep apnea. Circulation. 2003;107(13):1822–6.
Leung RS, Huber MA, Rogge T, Maimon N, Chiu KL, Bradley TD. Association between atrial fibrillation and central sleep apnea. Sleep. 2005;28(12):1543–6.
Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22(2):217–23.
Bixler EO, Vgontzas AN, Lin HM, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–13.
Zhou XS, Rowley JA, Demirovic F, Diamond MP, Badr MS. Effect of testosterone on the apneic threshold in women during NREM sleep. J Appl Physiol (1985). 2003;94(1):101–7.
Mateika JH, Omran Q, Rowley JA, Zhou XS, Diamond MP, Badr MS. Treatment with leuprolide acetate decreases the threshold of the ventilatory response to carbon dioxide in healthy males. J Physiol. 2004;561(Pt 2):637–46.
Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–9.
Javaheri S, Parker TJ, Wexler L, et al. Occult sleep-disordered breathing in stable congestive heart failure. Ann Intern Med. 1995;122(7):487–92.
Javaheri S. Central sleep apnea-hypopnea syndrome in heart failure: prevalence, impact, and treatment. Sleep. 1996;19(10 Suppl):S229–31.
Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–6.
Xie A, Skatrud JB, Puleo DS, Rahko PS, Dempsey JA. Apnea-hypopnea threshold for CO2 in patients with congestive heart failure. Am J Respir Crit Care Med. 2002;165(9):1245–50.
Dempsey JA, Sheel AW, Haverkamp HC, Babcock MA, Harms CA. [The John Sutton Lecture: CSEP, 2002]. Pulmonary system limitations to exercise in health. Can J Appl Physiol. 2003;28 Suppl:S2–24.
Dempsey JA, Smith CA, Przybylowski T, et al. The ventilatory responsiveness to CO(2) below eupnoea as a determinant of ventilatory stability in sleep. J Physiol. 2004;560(Pt 1):1–11.
Bassetti C, Aldrich MS, Chervin RD, Quint D. Sleep apnea in patients with transient ischemic attack and stroke: a prospective study of 59 patients. Neurology. 1996;47(5):1167–73.
Parra O, Arboix A, Bechich S, et al. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161(2 Pt 1):375–80.
Grunstein RR, Ho KY, Berthon-Jones M, Stewart D, Sullivan CE. Central sleep apnea is associated with increased ventilatory response to carbon dioxide and hypersecretion of growth hormone in patients with acromegaly. Am J Respir Crit Care Med. 1994;150(2):496–502.
Grunstein RR, Ho KY, Sullivan CE. Sleep apnea in acromegaly. Ann Intern Med. 1991;115(7):527–32.
Grunstein RR, Sullivan CE. Sleep apnea and hypothyroidism: mechanisms and management. Am J Med. 1988;85(6):775–9.
Hanly PJ, Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102–7.
Sankari A, Bascom AT, Chowdhuri S, Badr MS. Tetraplegia is a risk factor for central sleep apnea. J Appl Physiol (1985). 2014;116(3):345–53.
Sankari A, Vaughan S, Bascom A, Martin JL, Badr MS. Sleep-disordered breathing and spinal cord injury: a state-of-the-art review. Chest. 2019;155(2):438–45.
Naughton MT. Loop gain in apnea. Am J Respir Crit Care Med. 2010;181(2):103–5.
Sankari A, Bascom AT, Riehani A, Badr MS. Tetraplegia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long-term facilitation. J Appl Physiol (1985). 2015;119(10):1183–93.
Xie A, Rutherford R, Rankin F, Wong B, Bradley TD. Hypocapnia and increased ventilatory responsiveness in patients with idiopathic central sleep apnea. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1950–5.
Zhang J, Wang L, Guo H-J, Wang Y, Cao J, Chen B-Y. Treatment-emergent central sleep apnea: a unique sleep-disordered breathing. Chin Med J. 2020;133(22)
Zeineddine S, Badr MS. Treatment-emergent central apnea: physiologic mechanisms informing clinical practice. Chest. 2021;159(6):2449–57.
Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest. 2007;132(1):81–7.
Wang D, Teichtahl H, Drummer O, et al. Central sleep apnea in stable methadone maintenance treatment patients. Chest. 2005;128(3):1348–56.
Morrell MJ, Badr MS, Harms CA, Dempsey JA. The assessment of upper airway patency during apnea using cardiogenic oscillations in the airflow signal. Sleep. 1995;18(8):651–8.
Aurora RN, Chowdhuri S, Ramar K, et al. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep. 2012;35(1):17–40.
Naughton MT, Benard DC, Rutherford R, Bradley TD. Effect of continuous positive airway pressure on central sleep apnea and nocturnal PCO2 in heart failure. Am J Respir Crit Care Med. 1994;150(6 Pt 1):1598–604.
Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med. 2005;353(19):2025–33.
Aboussouan LS, Khan SU, Banerjee M, Arroliga AC, Mitsumoto H. Objective measures of the efficacy of noninvasive positive-pressure ventilation in amyotrophic lateral sclerosis. Muscle Nerve. 2001;24(3):403–9.
Johnson KG, Johnson DC. Bilevel positive airway pressure worsens central apneas during sleep. Chest. 2005;128(4):2141–50.
Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol (1985). 1998;85(5):1929–40.
Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614–9.
Morgenthaler TI, Gay PC, Gordon N, Brown LK. Adaptive servoventilation versus noninvasive positive pressure ventilation for central, mixed, and complex sleep apnea syndromes. Sleep. 2007;30(4):468–75.
Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servo-ventilation for central sleep apnea in systolic heart failure. N Engl J Med. 2015;373(12):1095–105.
Aurora RN, Bista SR, Casey KR, et al. Updated adaptive servo-ventilation recommendations for the 2012 AASM guideline: “The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses”. J Clin Sleep Med. 2016;12(5):757–61.
Hudgel DW, Thanakitcharu S. Pharmacologic treatment of sleep-disordered breathing. Am J Respir Crit Care Med. 1998;158(3):691–9.
DeBacker WA, Verbraecken J, Willemen M, Wittesaele W, DeCock W, Van deHeyning P. Central apnea index decreases after prolonged treatment with acetazolamide. Am J Respir Crit Care Med. 1995;151(1):87–91.
Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep-disordered breathing in heart failure. N Engl J Med. 1996;335(8):562–7.
Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009;5(2):122–9.
Maresh S, Prowting J, Vaughan S, et al. Buspirone decreases susceptibility to hypocapnic central sleep apnea in chronic SCI patients. J Appl Physiol (1985). 2020;129(4):675–82.
Giannoni A, Borrelli C, Mirizzi G, Richerson GB, Emdin M, Passino C. Benefit of buspirone on chemoreflex and central apnoeas in heart failure: a randomized controlled crossover trial. Eur J Heart Fail. 2021;23(2):312–20.
Javaheri S, Ahmed M, Parker TJ, Brown CR. Effects of nasal O2 on sleep-related disordered breathing in ambulatory patients with stable heart failure. Sleep. 1999;22(8):1101–6.
Chowdhuri S, Sinha P, Pranathiageswaran S, Badr MS. Sustained hyperoxia stabilizes breathing in healthy individuals during NREM sleep. J Appl Physiol (1985). 2010;109(5):1378–83.
Xie A, Rankin F, Rutherford R, Bradley TD. Effects of inhaled CO2 and added dead space on idiopathic central sleep apnea. J Appl Physiol (1985). 1997;82(3):918–26.
Badr MS, Grossman JE, Weber SA. Treatment of refractory sleep apnea with supplemental carbon dioxide. Am J Respir Crit Care Med. 1994;150(2):561–4.
Ding N, Zhang X. Transvenous phrenic nerve stimulation, a novel therapeutic approach for central sleep apnea. J Thorac Dis. 2018;10(3):2005–10.
Costanzo MR, Javaheri S, Ponikowski P, et al. Transvenous phrenic nerve stimulation for treatment of central sleep apnea: five-year safety and efficacy outcomes. Nat Sci Sleep. 2021;13:515–26.
Schwartz AR, Goldberg LR, McKane S, Morgenthaler TI. Transvenous phrenic nerve stimulation improves central sleep apnea, sleep quality, and quality of life regardless of prior positive airway pressure treatment. Sleep Breath. 2021;25(4):2053–63.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Badr, M.S., Ginter, G. (2022). Central Sleep Apnea: Pathophysiology and Clinical Management. In: Badr, M.S., Martin, J.L. (eds) Essentials of Sleep Medicine. Respiratory Medicine. Humana, Cham. https://doi.org/10.1007/978-3-030-93739-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-93739-3_8
Published:
Publisher Name: Humana, Cham
Print ISBN: 978-3-030-93738-6
Online ISBN: 978-3-030-93739-3
eBook Packages: MedicineMedicine (R0)