Abstract
Taurine is an amino sulfonic acid that is implicated in numerous physiological functions, including the regulation of oxidative stress, which plays an important role in coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), together with other pathophysiological processes. The recent finding of decreased serum taurine levels in SARS-CoV-2-infected patients, in tandem with its potential modulatory role in COVID-19 due to its antiviral, antioxidant, anti-inflammatory, and vascular-related effects, provides a rationale for considering taurine as a beneficial supplement in patients suffering from COVID-19. Here, we reviewed the potential disease-modifying effects of taurine and combined these with the current knowledge on COVID-19 to clarify the potential role of taurine in this respiratory disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Taurine
- Coronavirus disease 2019 (COVID-19)
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
- Oxidative stress
- Inflammation
- Treatment
1 Introduction
Taurine , or 2-aminoethanesulfonic acid, is a semi-essential compound that is produced endogenously in the human body but is also taken up by diet since it is abundantly present in seafood and meat. Although not incorporated into proteins, taurine is classified as an amino acid, where it can be distinguished from “true” amino acids by the presence of a sulfonic acid moiety, instead of a carboxyl one. Taurine is widely distributed in human tissues, residing both intracellularly (especially in leukocytes, platelets, heart, retina, and brain) and extracellularly (Wójcik et al. 2010). In human plasma, concentrations normally range between 65 and 179 mmol/L (8–22 μg/mL) (Ghandforoush-Sattari et al. 2009). Taurine has many physiological functions, including involvement in the conjugation of bile acids, regulation of oxidative stress, mitochondrial membrane stabilization, and osmoregulation, as well as modulation of cardiovascular and neurological functions (Qaradakhi et al. 2020; Wójcik et al. 2010). Considering its versatile biological roles, taurine has been shown to exert promising effects on improving overall health and for treatment purposes in several disease states, such as hypertension and diabetes (Maleki et al. 2020a, b; Schaffer and Kim 2018; Sun et al. 2016). The therapeutic potential of taurine has also recently been implicated for coronavirus disease 2019 (COVID-19), the infectious respiratory disease induced by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that emerged in December 2019 and rapidly spread worldwide causing millions of deaths (Iwegbulem et al. 2021). Decreased serum taurine levels have been reported in SARS-CoV-2-infected subjects, where taurine levels were half the level compared to that of healthy controls, most notably in patients with moderate-to-high pro-inflammatory interleukin (IL)-6, with higher levels of IL-6 predictive of a severe disease course (Sabaka et al. 2021; Thomas et al. 2020). Furthermore, lower hypotaurine levels, the precursor of taurine, have been associated with an unfavorable prognosis of COVID-19 in an interventional metabolomics study (Danlos et al. 2021). These results have attracted our attention to the potential use of taurine as a beneficial supplement in taurine-depleted SARS-CoV-2-infected patients.
COVID-19 symptomatology ranges from asymptomatic or mild, self-limiting respiratory disease to severe disease, including (typical and atypical) acute respiratory distress syndrome (ARDS) and multi-organ failure (MOF) (Wang et al. 2021a). Some individuals are particularly at risk of developing severe disease, including the elderly (with associated “inflammaging” and “immunosenescence”), males, and those with comorbidities (e.g., diabetes, cardiovascular disease, obesity, chronic respiratory disease, immunodeficiency disorders) (van Eijk et al. 2021; Wang et al. 2021a). Progression of COVID-19 is assumed to result from multiple intertwined pathophysiological mechanisms, including (1) direct cytopathic effects of SARS-CoV-2 invasion, (2) a hyperactive immune response with hypercytokinemia, (3) an inflammation-driven “oxidative storm,” and (4) vascular-related effects, such as thrombotic microangiopathy and endothelial dysfunction (Cumpstey et al. 2021; van Eijk et al. 2021). We propose that the immune-modulating effects of taurine may be beneficial to each of these mechanisms and limit progression to severe disease considering its previously reported antiviral, (indirect) antioxidant, anti-inflammatory, and vascular-related effects.
Although COVID-19 was originally being considered a purely respiratory disease, we currently know that the virus also induces inflammation in and damage to other organ systems (Bourgonje et al. 2020a; van Eijk et al. 2021). Thus, the versatile effects of taurine may be widespread throughout the body. In addition, considering that a substantial subset of patients develop long-term symptoms after SARS-CoV-2 infection – so-called “chronic COVID syndrome,” “long-COVID” or “long-haulers” – taurine may also be beneficial in the postinfectious phase (Baig 2021). Herein we aim to clarify the potential role of taurine in COVID-19 by dissecting the various pathophysiological mechanisms of this disease. Finally, we explore the possibilities of taurine as a putative supplementary therapy for COVID-19.
2 SARS-COV-2 Life Cycle and the Antiviral Actions of Taurine
2.1 Animals
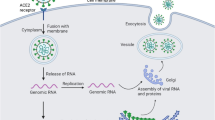
SARS-CoV-2 is thought to be primarily transmitted between people through respiratory droplets and aerosols (van Eijk et al. 2021). As shown in Fig. 1, the binding of SARS-CoV-2 to angiotensin-converting enzyme 2 (ACE2) at the cell membrane was found to be essential for viral cell entry (Hoffmann et al. 2020). In addition, transmembrane serine protease type 2 (TMPRSS2) was found to facilitate viral entry via endocytosis and be essential for direct fusion of the viral envelope to the cell membrane (Heurich et al. 2014; Hoffmann et al. 2020; Mahmoud et al. 2020). This route of viral entry matches that of SARS-CoV, the virus that caused an outbreak of severe acute respiratory syndrome in 2002−2004 (Heurich et al. 2014).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry and the involvement of angiotensin II (AngII) signaling in oxidative stress and inflammation in coronavirus disease 2019 (COVID-19). SARS-CoV-2 binding to ACE2 leads to viral entry into the host cell, which may be facilitated by transmembrane serine protease type 2 (TMPRSS2) and furin, among others. ADAM17 is a major sheddase of ACE2, resulting in soluble ACE2 that may bind to viral particles and potentially facilitate viral cell entry. Taurine stimulates ACE2, which functions as converter of Ang II into angiotensin 1-7 (Ang(1-7)) that in turn activates the Mas receptor (MasR). Ang(1-7)/MasR signaling stimulates endothelial nitric oxide synthase (eNOS) – in conjunction with its essential cofactor tetrahydrobiopterin (BH4) – to produce anti-inflammatory nitric oxide (NO). However, angiotensin II – converted from angiotensin I by angiotensin-converting enzyme (ACE) – activates the angiotensin II type 1 receptor (AT1R), which stimulates NADPH oxidase to oxidize BH4, thereby inducing eNOS to produce superoxide instead of NO. The production of reactive oxygen species (ROS) may cause endothelial dysfunction that contributes to COVID-19-related vascular pathology in various ways, including vasoconstriction-induced ischemia, dysregulated fibrinolysis, and the formation of NETs via complement activation
ACE2 is expressed in many tissues and cells throughout the human body, including a small population of lung cells – mainly type II alveolar epithelial cells (Wang et al. 2020b; Zou et al. 2020). Comparative studies investigating mRNA levels and protein expression of ACE2 demonstrated that other tissues besides the lung express high levels of ACE2, including the nasopharyngeal mucosa, suggesting that the main entry route of viral invasion is the nasal epithelium (Soni et al. 2021; Wang et al. 2020b). ACE2 was also found to be expressed on the enterocytes of the small intestine, as well as the vascular endothelium and arterial smooth muscle cells in various organs, supporting potential oral and blood-borne infectious routes, respectively (Hamming et al. 2004). In line with this wide tissue distribution of ACE2, the presence of SARS-CoV-2 has been demonstrated in various organs other than the lungs (Bourgonje et al. 2021b; Schurink et al. 2020; Song et al. 2021). For example, a cohort autopsy study examined the systemic SARS-CoV-2 distribution in postmortem organs from 26 COVID-19 patients and found SARS-CoV-2 in, among others, the lungs (92%), hilar lymph nodes (76%), small intestine (31%), colon (23%), heart (19%), and kidneys (15%) (Yao et al. 2021). Moreover, co-localization analysis demonstrated the co-expression of ACE2 and SARS-CoV-2 antigen in multiple organs (including the lungs, trachea, small intestine, heart, kidney, and pancreas), suggesting a potential correlation between membrane-bound ACE2 (mACE2) expression and SARS-CoV-2 tissue tropism (Liu et al. 2021). Alternative viral entry mechanisms include the presence of other viral entry receptors, such as furin, neuropilin-1, and cluster of differentiation 147 (CD147) – also known as extracellular matrix metalloproteinase inducer (EMMPRIN) or basigin – as well as the shedding of ACE2 into a soluble form, which provides a possible mechanism of dissemination of infection from the primary site of infection to peripheral locations (Kyrou et al. 2021; Yeung et al. 2021). Although CD147’s definite involvement in SARS-CoV-2 cell entry has not been established (Shilts et al. 2021), it was found to play a potential role in SARS-CoV-2 infection, either as a viral entry receptor (specifically in immune cells that do not express ACE2) or by reducing the expression of ACE2, which underscores a potential two-way contradictory effect of CD147 (Fenizia et al. 2021; Wang et al. 2020a).
A major sheddase of ACE2 is a disintegrin and metalloproteinase 17 (ADAM17) , previously found to be activated when TMPRSS2 was not (Heurich et al. 2014). Notably, soluble ACE2 (sACE2) retains an intact interaction site for binding to SARS-CoV-2, which has recently been shown to form complexes with the SARS-CoV-2 spike protein (S) in a human kidney cell line (Yeung et al. 2021). In this study, the sACE2-S complex (either with or without interaction with vasopressin) facilitated cell entry via binding to angiotensin II type 1 receptor (AT1R) (or AVPR1B in case of vasopressin interaction), resulting in receptor-mediated endocytosis. These findings indicate a potential role for sACE2 as a co-receptor required for viral entry in cells lacking mACE2 and additionally highlight the involvement of the renin-angiotensin-aldosterone system (RAAS) in this cell entry mechanism.
SARS-CoV-2 infection is assumed to downregulate mACE2 either via shedding or endocytosis of the virus/ACE2 complex (Vieira et al. 2021). Normally, at the cell membrane, ACE2 converts angiotensin II (Ang II) – the main effector of RAAS causing blood pressure elevation – into angiotensin 1-7 (Ang(1-7)), which has opposing effects through binding to the Mas receptor (MasR), inducing the production of nitric oxide (NO) which lowers blood pressure. SARS-CoV-2-induced downregulation of ACE2 shifts this balance toward Ang II, which exerts its effects via AT1R. Apart from its main function as a regulator of blood pressure, Ang II also exerts pro-oxidative, pro-fibrotic, and pro-inflammatory effects (Benicky et al. 2009; Bourgonje et al. 2020a; Ruiz-Ortega et al. 2002). Interestingly, a study on neurogenic hypertension showed that Ang II-AT1R signaling promotes ACE2 internalization into the cell and subsequent lysosomal degradation (Deshotels et al. 2014). This finding supports the hypothesis that the endocytosis of the SARS-CoV-2/mACE2 complex is AT1R-mediated too, also considering the common occurrence of systemic hyperinflammation and the potential positive effects of AT1R blockers (ARBs) on the prognosis of COVID-19 – although still under debate (Baral et al. 2021; Duarte et al. 2021; Jarcho et al. 2020; Singh et al. 2021a). In this case, viral cell entry would not solely depend on surface ACE2 expression, but also on its endocytosis induced by Ang II-AT1R signaling. Taurine has previously been shown to upregulate mACE2 – albeit its magnitude still unknown – and attenuate the actions of Ang II (Lv et al. 2017; Schaffer et al. 2000). Taurine may therefore, in theory, impede viral cell entry by attenuating AT1R-mediated endocytosis of the SARS-CoV-2/ACE2 complex, thereby preserving ACE2 protective functions, primarily by converting Ang II to Ang1-7. High-risk groups for severe COVID-19 (e.g., the elderly, cardiovascular disease, diabetes, etc.) could especially benefit from these protective effects of taurine, as they tend to show decreased ACE2 expression and activity (Bourgonje et al. 2020a; van Eijk et al. 2021). However, the question remains whether taurine may also affect mACE2-independent viral cell entry. Albeit an inhibitory effect of taurine on CD147 has been reported, and its effect on other potential viral entry receptors or sACE2 requires further examination (Jin et al. 2018).
2.2 Viral Replication
Next to potentially limiting host cell entry of SARS-CoV-2, taurine may also exert antiviral actions during the process of viral replication. Upon entering the cell, viral uncoating follows, in which SARS-CoV-2 is disassembled to release its genomic RNA. After primary translation, the process of transcription and replication is initiated, mediated by the RNA-dependent RNA polymerase (RdRp, or nonstructural protein [nsp] 12). This is followed by translation of structural proteins, which assemble and encapsulate the newly formed genomic RNA, thereby generating new viral particles that are released from the host cell by exocytosis (V’Kovski et al. 2021). RdRp forms a complex with two nonstructural proteins, nsp7 and nsp8, which catalyze RNA synthesis, together forming a potential therapeutic target to inhibit SARS-CoV-2 replication. For instance, the antiviral actions of RdRp inhibitors (e.g., remdesivir) and hydrogen sulfide (H2S) donors (e.g., N-acetylcysteine) are thought to be mediated by acting on the RdRp/nsp7/nsp8 complex (Bourgonje et al. 2021a). Taurine has previously been shown to increase endogenous H2S levels and may therefore indirectly inhibit viral replication by inhibiting RdRp (Sun et al. 2016; Zhao et al. 2018).
When viruses exploit the hosts’ cellular machinery for their own replication, stress in cellular organelles such as the endoplasmic reticulum (ER) and mitochondria is induced, while host response mechanisms are inhibited (Ajaz et al. 2021; Li et al. 2015). Viral replication also requires large quantities of ER-produced proteins and lipids, which accumulate and induce ER stress whenever the amount exceeds the posttranslational process of protein folding (Li et al. 2015). ER stress was found to promote viral replication as was previously demonstrated for reovirus and hepatitis B virus, which potentially also relates to SARS-CoV-2 (Choi and Song 2019; Li et al. 2015). In fact, research on coronavirus-induced ER stress has been suggested to provide new targets for treating COVID-19 (Sureda et al. 2020). Previous studies have demonstrated that taurine inhibits ER-stress and its downstream effects, including apoptosis and activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome (Bian et al. 2018; Liu et al. 2019; Men et al. 2010).
Furthermore, taurine enhances mitochondrial function that may result in decreased viral replication due to its stimulating effect on interferon (IFN) production – which is initially compromised in COVID-19 (vide infra) (Bender et al. 2015; van Eijk et al. 2021). Mitochondrial antiviral-signaling proteins (MAVS), located on the outer mitochondrial membrane, peroxisomes, and the mitochondria-associated membrane of the ER, lead to the production of IFNs (Bender et al. 2015). A recent study on COVID-19 demonstrated that SARS-CoV-2 evades the antiviral type I IFN response by targeting MAVS (Wang et al. 2021b). These literature-based hypotheses on the antiviral effects of taurine provide a rationale for conducting future research (e.g., in SARS-CoV-2-infected cell lines) to test the actual effects of taurine on SARS-CoV-2 uptake and replication.
Finally, modulation of lipid metabolism by taurine could potentially interfere with the viral life cycle, considering lipids are the essential components of the SARS-CoV-2 envelope and double-membrane vesicles in the host cell involved in viral cell entry, viral replication, and viral propagation (Abu-Farha et al. 2020; Caterino et al. 2021). Taurine may modify the structure of the phospholipid bilayer through inhibition of phospholipid N-methyltransferase – an enzyme that regulates the ratio of phosphatidylethanolamine (PE) and phosphatidylcholine (PC) (Schaffer et al. 2010). As PE is normally situated on the outer side and PC on the inner side of the membrane, one can imagine that alterations in their ratio would influence the membrane’s structure and function (Schaffer et al. 2010). Other potential taurine-mediated effects on lipid-dependent viral replication include the modulation of molecules that impact lipids, such as cholesterol. Indeed, taurine modulates cholesterol metabolism with cholesterol-lowering effects, which may be explained through various mechanisms, including improved LDLR-binding capacity and increased formation of fecal bile acid via upregulating CYP7A1, among others (Chen et al. 2012).
3 Age-Related Mitochondrial Dysfunction in COVID-19 and the Indirect Antioxidant Actions of Taurine
There are multiple age-related contributors to the increased risk of severe disease in COVID-19, including (among others) an increased incidence of comorbidities in older patients, a decline in immune function affecting innate and adaptive immune responses (“immunosenescence”), and a chronic pro-inflammatory profile (“inflammaging”) (van Eijk et al. 2021). As seen in Fig. 2, inflammaging is associated with the deterioration of mitochondrial function, which entails mitochondria that are incompetent to produce sufficient amounts of adenosine triphosphate (ATP) to meet metabolic demands, while producing an excessive amount of reactive oxygen species (ROS), causing oxidative stress to the cell, resulting in chronic inflammation in the elderly. Mitochondrial dysfunction affecting immune cells is associated with immunosenescence, thereby contributing to excessive inflammation (with increased activation of NLRP3, NF-κB, and increased levels of IL-6) and impaired adaptive immunity as seen in COVID-19. Inflammation itself goes along with immune cell infiltration that generates and releases ROS, which normally function in cell signaling pathways important to their immunological functions (Bourgonje et al. 2020b). However, COVID-19 promotes the overproduction of ROS with antioxidant systems being overrun, causing harmful oxidative stress with resulting tissue damage and ongoing inflammation (Ganji and Reddy 2020). Ang II promotes ROS through the activation of NADPH oxidase, which is counteracted by conversion of Ang II into Ang(1-7) by ACE2 (Gwathmey et al. 2010; Rabelo et al. 2011). Notably, taurine was found to protect against oxidant-induced lung injury and pulmonary fibrosis (Gurujeyalakshmi et al. 2000; Schuller-Levis et al. 1994, 2003). Conversely, taurine concentrations were repeatedly observed to be decreased in oxidative stress-mediated disorders and during aging (Ito et al. 2012; Yoshimura et al. 2021). Although taurine does not appear to have inherent antioxidative capacity, it indirectly increases antioxidation by improving mitochondrial functions (by enhancing electron transport chain activity), thereby attenuating mitochondrial ROS production (Barbiera et al. 2020). Furthermore, taurine displays indirect antioxidant activity through the enhancement of endogenous H2S and glutathione (GSH) – a tripeptide synthesized from cysteine – availability, both of which are major antioxidants (Bourgonje et al. 2021a). H2S exerts its antioxidant effects through multiple mechanisms, including the direct scavenging of ROS, increasing levels of other antioxidant defense molecules (such as GSH), and by desulfuration generating sulfane sulfur species (Bourgonje et al. 2021a; Corsello et al. 2018). Finally, taurine is known to neutralize hypochlorous acid (HOCl), which is a major toxic oxidant generated by the myeloperoxidase-halide system within activated leukocytes and is involved in multiple age-related diseases (Casciaro et al. 2017; Chorazy et al. 2002; Goud et al. 2021).
Interrelationships between age-related mitochondrial dysfunction, oxidative stress, and taurine. Age-related inflammaging and immunosenescence are associated with mitochondrial dysfunction, leading to excessive amounts of reactive oxygen species (ROS). In addition, ROS is promoted by angiotensin II (Ang II) through the activation of NADPH oxidase. ROS in turn cause oxidative stress to the cell, resulting in tissue damage and ongoing inflammation. These effects may be alleviated by taurine because of its indirect antioxidant effects, including its stimulating effect on mitochrondrial functions and by enhancing endogenous hydrogen sulfide (H2S) and glutathione (GSH) availability
4 COVID-19 Immune Response and the Immune-Related Actions of Taurine
A maladaptive immune response is a hallmark of COVID-19, consisting of a hyperinflammatory innate immune response along with an inadequate adaptive response, eliciting both local and systemic tissue damage (van Eijk et al. 2021). Autopsy data show evidence of an extensive inflammatory response in various organs, including the lungs, kidneys, heart, brain, and liver (Schurink et al. 2020). The type and magnitude of the immune response highly depend on the phase of the disease course. The interstitial infiltrate in the lungs of patients with exudative diffuse alveolar damage (DAD), for example, was found to be CD4+-T-lymphocyte-mediated, whereas in patients with proliferative DAD, this response was primarily CD8+-T-lymphocyte-mediated (Schurink et al. 2020). Furthermore, early COVID-19 is characterized by a more pronounced viral presence and increased neutrophil infiltration, whereas increased macrophage and lymphoplasmacytic infiltration are more often observed when the disease progresses (Nienhold et al. 2020; Rendeiro et al. 2021). The disconnection between viral presence in early disease and inflammatory pathology in late COVID-19 underscores the role of self-perpetuating immunopathology in causing severe disease when the virus usually can no longer be detected.
Similar to other viral infections, the immune response against SARS-CoV-2 starts with the activation of pattern recognition receptors, such as Toll-like receptors (TLRs) that lead to the activation of transcription factors, ultimately resulting in the production of IFN-I, which exerts antiviral actions (Kumar et al., 2021). COVID-19 is characterized by an initial delay in IFN-I response, allowing the virus to replicate and disseminate uncontrollably within the infected host, thereby stimulating a strong activation of the immune response, which promotes hyperinflammatory injury during later stages of COVID-19 (van Eijk et al. 2021). SARS-CoV-2-induced activation of NLRP3 inflammasome was also shown to contribute to this hyperinflammation (Pan et al. 2021). The overactivation of the immune response is characterized by extensive hypercytokinemia – the so-called cytokine storm (Henderson et al. 2020). High serum levels of IL-6, IL-8, and tumor necrosis factor (TNF) at the time of hospitalization were found to be strongly and independently predictive of mortality (Del Valle et al. 2020). Furthermore, the change in ratio of pro-inflammatory IL-6 to anti-inflammatory IL-10 taken 4 days apart, called the Dublin-Boston score, was found to be of prognostic value in COVID-19 (McElvaney et al. 2020). Administration of taurine may be effective in reducing IL-6 and increasing IL-10, either directly or indirectly by affecting other inflammatory modulators, such as NLRP3 (Lak et al. 2015; Liu et al. 2019). Conversely, the SARS-CoV-2 infection especially in patients with moderate to high pro-inflammatory IL-6 levels was associated with decreased taurine levels (Thomas et al. 2020). This decrement could be potentially related to reduced taurine biosynthesis in COVID-19. For example, oxidative stress oxidizes the active form of vitamin B6, pyridoxal-5’-phosphate (PLP), which is involved as cofactor in endogenous taurine synthesis (Mahootchi et al. 2021). Another potential explanation to decreased serum taurine levels includes a shift in taurine distribution with increased body requirements due to its function as conjugator. This hypothesis is supported by a study in which increased levels of taurine were measured in peripheral blood mononuclear cells (PBMCs), where it can neutralize HOCl, thereby favoring anti-oxidation and anti-inflammation (Chorazy et al. 2002; Singh et al. 2021b). Other pro-inflammatory mediators have been shown to be inhibited by taurine, including Ang II, AT1R, TLR4, IL-1β, NADPH oxidase, and the NLRP3-inflammasome (Han et al. 2016; Liu et al. 2019; Schaffer et al. 2000; Younis et al. 2021). At the same time, taurine can increase anti-inflammatory ACE2 and Ang(1-7), in addition to IL-10 (Lv et al. 2017; Schaffer et al. 2000). Supplementation dosages vary greatly across human studies reporting on the effect of taurine supplementation on inflammatory markers. For instance, studies on obesity (3 gram/day for 8 weeks), type 2 diabetes (1000 mg 3 times/day for 8 weeks), and traumatic brain injury (30 mg/kg/day for 2 weeks) resulted in decreased levels of C-reactive protein (CRP), both TNF-α and CRP, and IL-6 respectively (Maleki et al. 2020b; Rosa et al. 2014; Vahdat et al. 2021).
The aforementioned downregulation of ACE2 with subsequent disrupted orchestration of the Ang II:Ang(1-7) ratio may contribute to the excessive inflammatory response in COVID-19 patients. Ang II binding to AT1R for instance induces the activation of pro-inflammatory mediators, including TLR4, IL-6, Il-1β, NF-κB, NADPH oxidase, and NLRP3-inflammasome (Fazeli et al. 2012; Wen et al. 2016). Together, these mediators induce the production of superoxide (O2˙-), increase vascular permeability, and facilitate leukocyte and thrombocyte adhesion as an excessive response to the infection (Jin et al. 2020; Mittal et al. 2014). The inhibition of Ang II/AT1R-signaling by taurine may prevent the ACE2-bound virus from entering the cell, limit the pro-inflammatory response, and facilitate anti-inflammation. During a moderate innate immune response, Ang(1-7) activates the MasR, which induces endothelial nitric oxide synthase (eNOS ) to produce anti-inflammatory NO (Sampaio et al. 2007). Interestingly, taurine has been found to increase eNOS expression and phosphorylation (Guizoni et al. 2020). Ang II/AT1R-signaling , however, activates superoxide-producing NADPH oxidase, which causes oxidation of the essential eNOS cofactor tetrahydrobiopterin (BH4), inducing eNOS to produce O2˙-rather than NO – a process called eNOS uncoupling (Bowers et al. 2011; Fazeli et al. 2012). Inhibition of NADPH oxidase by taurine may therefore limit O2˙- production, thereby resulting in an increased availability of BH4 for the activation of eNOS to produce anti-inflammatory NO (Myojo et al. 2014).
Taurine also inhibits TLR4, which has the dual function of initially activating a pro-inflammatory response by way of pro-inflammatory cytokines and an anti-inflammatory response after it is endocytosed into the cell (Kim et al. 2013). Inside the cell, TLR4 activates anti-inflammatory cytokines, such as IL-10, after which TLR4-signaling is ended (Chang et al. 2009; Guven-Maiorov et al. 2015; Kim et al. 2013). In leukocytes, taurine traps and reacts with HOCl to produce taurine chloramine, which inhibits the generation of inflammatory mediators, such as IL-6 and TNF-α (Chorazy et al. 2002). Finally, alteration of the gut microbiota (with its immunomodulatory potential) in response to SARS-CoV-2 infection has been linked to increased levels of inflammatory markers and more severe COVID-19 (Yeoh et al. 2021). Taurine has a modulatory role on the gut microbiota by potentiating the production of sulfide, an inhibitor of pathogen respiration key to viral invasion into the host, thereby enhancing the resistance against viral infection (Stacy et al. 2021).
5 COVID-19 Vascular Pathology and the Vascular-Related Actions of Taurine
5.1 Prothrombotic State
COVID-19 predisposes individuals to a prothrombotic state, characterized by elevations in D-dimer and fibrinogen levels, both correlates of a poor outcome (Di Micco et al. 2020; He et al. 2021). Consistent with this prothrombotic state, both pulmonary and extrapulmonary microthrombotic and thromboembolic complications are common among severely affected COVID-19 patients, findings which are thought to contribute to disease symptomatology and MOF (Fahmy et al. 2021). For example, microthrombi in the lungs could lead to pulmonary vascular redistribution, thereby contributing to the gas exchange abnormalities observed in COVID-19 (Thillai et al. 2021). The microvascular thrombi have been shown to contain neutrophil extracellular traps (NETs) associated with platelets and fibrin, indicating inflammation as an underlying mechanism for the observed thrombotic complications in COVID-19 – a process also known as “dysregulated immunothrombosis” (Ackermann et al. 2021; Nicolai et al. 2020). Although intravascular microthrombosis is not specific to COVID-19 as it may also occur in sepsis-induced disease states, it was previously shown to occur more frequently in COVID-19-induced respiratory failure when compared to patients with influenza by a ninefold increase (Ackermann et al. 2020). The pathophysiology of the observed thrombosis is complex and assumed to result from an interplay between various underlying mechanisms, including endothelial damage, platelet dysfunction, complement activation associated with the formation of thrombogenic NETs, hypercytokinemia (including IL-6-mediated platelet abnormalities and thrombogenesis), and abnormal blood flow (e.g., due to hyperviscosity or impaired microcirculation in response to hypoxia) (Ackermann et al. 2021; Ahmed et al. 2020; van Eijk et al. 2021).
Taurine may be protective in thrombotic complications of COVID-19 by acting on these different underlying mechanisms. For example, the vasorelaxant functions of taurine (vide infra) may restore hypoxia-induced impaired microcirculation, thereby attenuating thrombosis induced by abnormal blood flow (Ahmed et al. 2020; Nishida and Satoh 2009). Furthermore, the above-described anti-inflammatory effects of taurine will likely attenuate thrombosis associated with the cytokine storm. In COVID-19, cytokine-induced (and possibly virus-induced) endothelial damage, as well as increased Ang II/AT1R signaling, may stimulate the release of plasminogen activator inhibitor 1 (PAI-1), an inhibitor of fibrinolysis and a risk factor of thrombosis, from primarily endothelial cells lining the blood vessels (Ahmed et al. 2020). Elevated PAI-1 levels have previously been observed among intensive care unit (ICU)-admitted COVID-19 patients, and impaired fibrinolytic activity has further been demonstrated by prolonged clot lysis time in critically ill COVID-19 patients (Nougier et al. 2020; Wright et al. 2020). In ARDS, elevated PAI-1 levels have been shown to be an independent risk factor for poor outcomes and appear to play a role in fibrin deposition leading to fibrosis (Whyte et al. 2020). Taurine may hamper PAI-1 release (e.g., possibly through attenuating cytokine-induced endothelial damage or inhibiting AT1R), as was previously demonstrated in animal studies, and could therefore restore the fibrinolytic shutdown in severe COVID-19 (Lee et al. 2005; Ruan et al. 2016). Simultaneously, due to its stimulating effect on ACE2, taurine favors the Ang(1-7)/Mas pathway, activation of which leads to the inhibition of platelet adherence and aggregation via the release of NO (Ahmed et al. 2020). Furthermore, ACE2 itself has antithrombotic effects, partly through the activation of tissue plasminogen activator (tPA), a serine protease found on endothelial cells involved in fibrinolysis (Ahmed et al. 2020). Paradoxically, a recent study in 118 hospitalized COVID-19 patients found elevations in not only PAI-1 levels but also tPA levels, findings that are similar to sepsis-induced coagulopathy (Schmitt et al. 2019; Zuo et al. 2021). These results indicate that fibrinolytic homeostasis in COVID-19 is complicated and provide an explanation for the enhanced bleeding risk among critically ill patients, in addition to the well-known enhanced thrombotic risk (Al-Samkari et al. 2020). An animal study exploring the effect of taurine in combination with delayed tPA on embolic stroke found that this treatment prevented tPA-associated hemorrhage, as well as fibrin/fibrinogen and platelet deposition in (micro)vessels, which could be linked to the inhibition of CD147 by taurine (Jin et al. 2018). Thus, taurine may be protective by regulating fibrinolytic activity, preventing both hemorrhage and thrombosis, through its inhibitory effect on CD147. In addition to improving microvascular patency, the inhibitory effects of taurine on CD147 may potentially limit SARS-CoV-2 uptake and COVID-19-associated hyperinflammation, considering its role in increasing inflammation and fibrosis after a pro-inflammatory insult (Fenizia et al. 2021; Jin et al. 2019; Wang et al. 2020a; Zhu et al. 2014). Although most data come from animal models, the antithrombotic properties of taurine have also been demonstrated in human studies (Santhakumar et al. 2013; Franconi et al. 1995; Ijiri et al. 2013). Findings included a decrease platelet aggregation (Santhakumar et al. 2013; Franconi et al. 1995), a prolongation in prothrombin clotting time (Santhakumar et al. 2013), and an increase in endogenous thrombolytic activity (Ijiri et al. 2013).
5.2 Vasoconstriction
As the endothelial cell layer is a key regulator of vascular homeostasis through its production of vasodilators (e.g., NO, prostaglandins, and endothelium-derived hyperpolarizing factor) and vasoconstrictors, including endothelin-1 (ET-1), endothelial damage in COVID-19 could lead to a prothrombotic and pro-inflammatory state of vasoconstriction (Varga et al. 2020). Release of ET-1 and platelet-activating factor shift the vascular equilibrium toward more vasoconstriction, leading to a reduction in tissue perfusion with resultant ischemic-related tissue damage, thereby further activating inflammation and cytokine release (Eltzschig and Carmeliet 2011; Indranil Biswas 2019). Interestingly, concentrations of ET-1 and ET-1-receptor expression are increased by Ang II, resulting in an increased production of ROS by NADPH oxidase (Lin et al. 2014; Loomis et al. 2005; Moreau et al. 1997). Prevention of conversion of Ang II into Ang(1-7) due to ACE2 deficiency may limit the activation of eNOS via Ang(1-7)/MasR signaling, thereby inhibiting the production of vasodilatory NO. Furthermore, hypoxia is a common feature in severe COVID-19, which itself induces impaired microcirculation in affected organs. For example, hypoxic pulmonary vasoconstriction (i.e., contraction of the vascular smooth muscle of small intrapulmonary arteries in response to hypoxia) likely is a partial contributor to the impaired gas exchange in COVID-19 (Thillai et al. 2021).
Taurine may be beneficial in COVID-19-related vascular dysfunction due to its known modulatory role in homeostatic function of vascular smooth muscle. Through its attenuating effect on Ang II signaling, taurine is assumed to target the vasoconstrictive effects that Ang II exerts via the AT1R (Schaffer et al. 2000). Furthermore, the increased production of NO as a result of taurine not only exerts anti-inflammatory effects but is also assumed to cause vasorelaxation. NO may interact with H2S to generate inorganic hydrogen polysulfides (H2Sn) that activate protein kinase G (PKG)1α, which has vasorelaxant functions (Bourgonje et al. 2021a). NO could also induce relaxation of vascular smooth muscle cells via activation of guanylate cyclase, which is downregulated by increased levels of intracellular Ca2+ (Murad et al. 1987; Serfass et al. 2001). Depending on cellular Ca2+ concentrations, taurine either promotes vasoconstriction to maintain blood pressure or exerts vasodilatory actions during hypoxia , thereby increasing tissue perfusion (Nishida and Satoh 2009). In a study on individuals with prehypertension (i.e., an early stage in the development of hypertension), administration of taurine significantly improved endothelium-dependent and endothelium-independent vasodilation (Sun et al. 2016). Therein, experimental studies on hypertensive rats showed that administration of taurine inhibited transient receptor potential channel 3 (TRPC3) expression in the vasculature, whereas TRPC3 antagonist treatment enhanced H2S donor-induced vascular relaxation, indicating that taurine exerts vascular relaxation by targeting TRPC3-mediated calcium influx, which is regulated by AT1R (Sun et al. 2016; Yamaguchi et al. 2018). Next to smooth muscle in the vasculature, taurine has previously been shown to exert a vasorelaxant effect on pulmonary smooth muscle in rats (Ammer et al. 2013). This finding may be especially interesting for long-COVID-19 patients, in which bronchodilators have been suggested as a treatment option to facilitate breathing – even in patients without concomitant obstructive lung disease (Maniscalco et al. 2021).
6 Taurine as Putative Supplementary Therapy for COVID-19
The role of taurine as an immunomodulator has been well-described in the literature. Considering its antiviral, anti-inflammatory, and vascular modulatory effects, as well as its favorable safety profile, taurine could be regarded as a putative beneficial supplement in taurine-deficient patients with COVID-19. To date, no clinical trials of taurine as a treatment for COVID-19 have been conducted. However, other researchers have previously suggested the therapeutic potential of taurine and its derivatives in COVID-19 (Iwegbulem et al. 2021). Potentially suitable derivatives that were mentioned by the authors included taurolidine (TRD) and 1.4.5-oxathiazinane-4,4-dioxide (OTD), both of which contain various administration options (e.g., intravenous, oral, cutaneous) and are well-tolerated. Previously, taurine has been shown to be effective as treatment for diseases such as diabetes and hypertension (Ito et al. 2012; Maleki et al. 2020a; Sun et al. 2016). For example, a clinical trial on 120 prehypertensive individuals reported that taurine supplementation of 1.6 g/day resulted in significant reductions in systolic and diastolic blood pressures (Sun et al. 2016). Furthermore, in a double-blind placebo-controlled study on patients with type 2 diabetes mellitus, taurine supplementation of 3 g/day for 8 weeks improved glycemic control (by reducing fasting blood sugar and insulin levels) and lipid profiles (through decreased total cholesterol and low-density lipoprotein cholesterol levels) (Maleki et al. 2020a). High doses have also been studied, including in a clinical trial in patients with stroke-like episodes of MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes) in which doses of 9 g/day and 12 g/day were used (Ohsawa et al. 2019). The European Food Safety Agency (EFSA) considers up to 1 gram of taurine per kg of bodyweight per day to be safe (European Food Safety Authority (EFSA), 2009). Moreover, a study on the pharmacokinetics of taurine on healthy volunteers after oral administration of 4 g taurine demonstrated an absorption phase of 1−2.5 h (Ghandforoush-Sattari et al. 2010). In this study, the maximum plasma taurine concentration was 86.1 ± 19.0 mg/L (0.69 ± 0.15 mmol), which returned to normal 6−8 h following digestion. Taurine supplementation may be used in COVID-19 considering its low cost, wide availability, and high safety profile with minimal side effects, all of which contribute to the feasibility of conducting randomized controlled trials to test its clinical effects. Since taurine is hypothesized to reduce SARS-CoV-2 infection, taurine may be recommended in every subject with COVID-19, although the greatest benefit is expected in high-risk groups for severe COVID-19 considering their higher baseline levels of inflammation, oxidative stress, and vascular dysfunction. Based on studies in other disease states, beneficial effects may be expected to occur at dosages of 3 g/day for 8 weeks, although its high safety profile legitimizes its use throughout the entire COVID-19 disease course (Maleki et al. 2020a, b; Rosa et al. 2014). Future studies should be conducted to examine the involvement and applicability of taurine supplementation in COVID-19.
7 Conclusion
The amino sulfonic acid taurine has extensive regulatory versatility and is hypothesized to play a modulatory role in COVID-19. Existing evidence supports antiviral, antioxidant, anti-inflammatory, and vascular modulatory effects of taurine, which could target the multifaceted nature of COVID-19 pathophysiology (Fig. 3). These possible effects are diverse and range from inhibiting viral invasion on account of involvement in (AT1R-mediated) SARS-CoV-2/ACE2 endocytosis to reducing immunopathology and vascular injury because of its complex involvement in both inflammatory and coagulation pathways. As with other infections, SARS-CoV-2 is associated with decreased levels of taurine, thereby limiting its protective properties normally occurring under physiologic circumstances. Next to its endogenous production, taurine can easily and safely be supplemented to improve body functions. Altogether, taurine should be regarded as a promising supplementary therapeutic option in COVID-19, although future clinical studies are warranted to explore its definite suitability in this disease.
Abbreviations
- ACE2:
-
angiotensin-converting enzyme 2
- ADAM17:
-
a disintegrin and metalloproteinase 17
- Ang(1-7):
-
angiotensin 1-7
- Ang II:
-
angiotensin II
- ARBs:
-
AT1R blockers
- ARDS:
-
acute respiratory distress syndrome
- ATP:
-
adenosine triphosphate
- AT1R:
-
angiotensin II type 1 receptor
- BH4:
-
tetrahydrobiopterin
- COVID-19:
-
coronavirus disease 2019
- CD147:
-
cluster of differentiation 147
- CRP:
-
c-reactive protein
- DAD:
-
diffuse alveolar damage
- EFSA:
-
The European Food Safety Agency
- EMMPRIN:
-
extracellular matrix metalloproteinase inducer
- eNOS:
-
endothelial nitric oxide synthase
- ER:
-
endoplasmic reticulum
- ET-1:
-
endothelin-1
- GSH:
-
glutathione
- HOCl:
-
hypochlorous acid
- H2S:
-
hydrogen sulfide
- H2Sn:
-
hydrogen polysulfides
- ICU:
-
intensive care unit
- IFN:
-
interferon
- IL:
-
interleukin
- mACE2:
-
membrane-bound ACE2
- MasR:
-
Mas receptor
- MAVS:
-
mitochondrial antiviral-signaling proteins
- MELAS:
-
mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes
- MOF:
-
multi-organ failure
- NLRP3:
-
NLR family pyrin domain containing 3
- NO:
-
nitric oxide
- nsp:
-
nonstructural protein
- NETs:
-
neutrophil extracellular traps
- O2˙-:
-
superoxide
- OTD:
-
1.4.5-oxathiazinane-4,4-dioxide
- PAI-1:
-
plasminogen activator inhibitor 1
- PDH:
-
pyruvate dehydrogenase
- PKG:
-
protein kinase G
- RAAS:
-
renin-angiotensin-aldosterone system
- RdRp:
-
RNA-dependent RNA polymerase
- ROS:
-
reactive oxygen species
- S:
-
spike
- sACE2:
-
soluble ACE2
- SARS-CoV-2:
-
severe acute respiratory syndrome coronavirus 2
- TLRs:
-
Toll-like receptors
- TMPRSS2:
-
transmembrane serine protease type 2
- TNF:
-
tumor necrosis factor
- tPA:
-
tissue plasminogen activator
- TRD:
-
taurolidine
- TRPC3:
-
transient receptor potential channel 3
References
Abu-Farha M, Thanaraj TA, Qaddoumi MG, Hashem A, Abubaker J, Al-Mulla F (2020) The role of lipid metabolism in COVID-19 virus infection and as a drug target. Int J Mol Sci 21(10):3544
Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 383(2):120–128
Ackermann M, Anders HJ, Bilyy R, Bowlin GL, Daniel C, De Lorenzo R, Egeblad M, Henneck T, Hidalgo A, Hoffmann M, Hohberger B, Kanthi Y, Kaplan MJ, Knight JS, Knopf J, Kolaczkowska E, Kubes P, Leppkes M, Mahajan A, Manfredi AA, Maueroder C, Maugeri N, Mitroulis I, Munoz LE, Narasaraju T, Naschberger E, Neeli I, Ng LG, Radic MZ, Ritis K, Rovere-Querini P, Schapher M, Schauer C, Simon HU, Singh J, Skendros P, Stark K, Sturzl M, van der Vlag J, Vandenabeele P, Vitkov L, von Kockritz-Blickwede M, Yanginlar C, Yousefi S, Zarbock A, Schett G, Herrmann M (2021) Patients with COVID-19: in the dark-NETs of neutrophils. Cell Death Differ May 24:1–15
Ahmed S, Zimba O, Gasparyan AY (2020) Thrombosis in coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin Rheumatol 39(9):2529–2543
Ajaz S, McPhail MJ, Singh KK, Mujib S, Trovato FM, Napoli S, Agarwal K (2021) Mitochondrial metabolic manipulation by SARS-CoV-2 in peripheral blood mononuclear cells of patients with COVID-19. Am J Phys Cell Physiol 320(1):C57–C65
Al-Samkari H, Leaf KRS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, Bendapudi PK, Bornikova L, Gupta S, Leaf DE, Kuter DJ, Rosovsky RP (2020) COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 136(4):489–500
Ammer EM, Shaaban AA, Ghonem HA, Elkashef HA (2013) Effect of taurine on the respiratory system of rats. J Food Pharm Sci 1(2):22–29
Baig AM (2021) Chronic COVID syndrome: Need for an appropriate medical terminology for long-COVID and COVID long-haulers. J Med Virol 93(5):2555–2556
Baral R, Tsampasian V, Debski M, Moran B, Garg P, Clark A, Vassiliou VS (2021) Association between renin-angiotensin-aldosterone system inhibitors and clinical outcomes in patients with COVID-19: a systematic review and meta-analysis. JAMA Netw Open 4(3):e213594
Barbiera A, Sorrentino S, Lepore E, Carfì A, Sica G, Dobrowolny G, Scicchitano BM (2020) Taurine attenuates catabolic processes related to the onset of sarcopenia. Int J Mol Sci 21(22):8865
Bender S, Reuter A, Eberle F, Einhorn E, Binder M, Bartenschlager R (2015) Activation of type I and III interferon response by mitochondrial and peroxisomal MAVS and inhibition by hepatitis C virus. PLoS Pathog 11(11):e1005264
Benicky J, Sánchez-Lemus E, Pavel J, Saavedra JM (2009) Anti-inflammatory effects of angiotensin receptor blockers in the brain and the periphery. Cell Mol Neurobiol 29(6-7):781–792
Bian Y, Wang H, Sun S (2018) Taurine alleviates endoplasmic reticulum stress in the chondrocytes from patients with osteoarthritis. Redox Rep 23(1):118–124
Bourgonje AR, Abdulle AE, Timens W, Hillebrands JL, Navis GJ, Gordijn SJ, Bolling MC, Dijkstra G, Voors AA, Osterhaus AD, van der Voort PHJ, Mulder DJ, van Goor H (2020a) Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J Pathol 251(3):228–248
Bourgonje AR, Feelisch M, Faber KN, Pasch A, Dijkstra G, van Goor H (2020b) Oxidative stress and redox-modulating therapeutics in inflammatory bowel disease. Trends Mol Med 26(11):1034–1046
Bourgonje AR, Offringa AK, van Eijk LE, Abdulle AE, Hillebrands JL, van der Voort PHJ, van Goor H, van Hezik EJ (2021a) N-Acetylcysteine and hydrogen sulfide in coronavirus disease 2019. Antioxid Redox Signal (ahead of print)
Bourgonje AR, van Linschoten RCA, West RL, van Dijk MA, van Leer-Buter CC, Kats-Ugurlu G, Pierik MJ, Festen EAM, Weersma RK, Dijkstra G (2021b) Treatment of severe acute ulcerative colitis in SARS-CoV-2 infected patients: report of three cases and discussion of treatment options. Ther Adv Gastroenterol 14:17562848211012595
Bowers MC, Hargrove LA, Kelly KA, Wu G, Meininger CJ (2011) Tetrahydrobiopterin attenuates superoxide-induced reduction in nitric oxide. Front Biosci (Schol Ed) 3:1263–1272
Casciaro M, Di Salvo E, Pace E, Ventura-Spagnolo E, Navarra M, Gangemi S (2017) Chlorinative stress in age-related diseases: a literature review. Immun Ageing 14:21
Caterino M, Gelzo M, Sol S, Fedele R, Annunziata A, Calabrese C, Fiorentino G, D’Abbraccio M, Dell’Isola C, Fusco FM, Parella R, Fabbrocini G, Gentile I, Andolfo I, Capasso M, Costanzo M, Daniele A, Marchese E, Polito R, Russo R, Missero C, Ruoppolo M, Castaldo G (2021) Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci Rep 11(1):2941
Chang J, Kunkel SL, Chang CH (2009) Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci USA 106(43):18327–18332
Chen W, Guo JX, Chang P (2012) The effect of taurine on cholesterol metabolism. Mol Nutr Food Res 56(5):681–690
Choi JA, Song CH (2019) Insights into the role of endoplasmic reticulum stress in infectious diseases. Front Immunol 10:3147
Chorazy M, Kontny E, Marcinkiewicz J, Maśliński W (2002) Taurine chloramine modulates cytokine production by human peripheral blood mononuclear cells. Amino Acids 23(4):407–413
Corsello T, Komaravelli N, Casola A (2018) Role of hydrogen sulfide in NRF2- and sirtuin-dependent maintenance of cellular redox balance. Antioxidants (Basel) 7(10):129
Cumpstey AF, Clark AD, Santolini J, Jackson AA, Feelisch M (2021) COVID-19: a redox disease-what a stress pandemic can teach us about resilience and what we may learn from the reactive species interactome about its treatment. Antioxid Redox Signal (ahead of print)
Danlos FX, Grajeda-Iglesias C, Durand S, Sauvat A, Roumier M, Cantin D, Colomba E, Rohmer J, Pommeret F, Baciarello G, Willekens C, Vasse M, Griscelli F, Fahrner JE, Goubet AG, Dubuisson A, Derosa L, Nirmalathasan N, Bredel D, Mouraud S, Pradon C, Stoclin A, Rozenberg F, Duchemin J, Jourdi G, Ellouze S, Levavasseur F, Albiges L, Soria JC, Barlesi F, Solary E, Andre F, Pene F, Ackerman F, Mouthon L, Zitvogel L, Marabelle A, Michot JM, Fontenay M, Kroemer G (2021) Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis 12(3):258
Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, Lavin Y, Swartz TH, Madduri D, Stock A, Marron TU, Xie H, Patel M, Tuballes K, van Oekelen O, Rahman A, Kovatch P, Aberg JA, Schadt E, Jagannath S, Mazumdar M, Charney AW, Firpo-Betancourt A, Mendu DR, Jhang J, Reich D, Sigel K, Cordon-Cardo C, Feldmann M, Parekh S, Merad M, Gnjatic S (2020) An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 26(10):1636–1643
Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM (2014) Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension 64(6):1368–1375
Di Micco P, Russo V, Carannante N, Imparato M, Cardillo G, Lodigiani C (2020) Prognostic Value of Fibrinogen among COVID-19 Patients Admitted to an Emergency Department: An Italian Cohort Study. J Clin Med 9(12):4134
Duarte M, Pelorosso F, Nicolosi LN, Salgado MV, Vetulli H, Aquieri A, Azzato F, Castro M, Coyle J, Davolos I, Criado IF, Gregori R, Mastrodonato P, Rubio MC, Sarquis S, Wahlmann R, Rothlin RP (2021) Telmisartan for treatment of Covid-19 patients: An open multicenter randomized clinical trial. EClinicalMedicine 37:100962
Eltzschig HK, Carmeliet P (2011) Hypoxia and inflammation. N Engl J Med 364(7):656–665
European Food Safety Authority (EFSA) (2009) EFSA adopts opinion on two ingredients commonly used in some energy drinks. Retrieved July 14th, 2021 from https://www.efsa.europa.eu/en/news/efsa-adopts-opinion-two-ingredients-commonly-used-some-energy-drinks
Fahmy OH, Daas FM, Salunkhe V, Petrey JL, Cosar EF, Ramirez J, Akca O (2021) Is microthrombosis the main pathology in coronavirus disease 2019 severity?-A systematic review of the postmortem pathologic findings. Crit Care Explor 3(5):e0427
Fazeli G, Stopper H, Schinzel R, Ni CW, Jo H, Schupp N (2012) Angiotensin II induces DNA damage via AT1 receptor and NADPH oxidase isoform Nox4. Mutagenesis 27(6):673–681
Fenizia C, Galbiati S, Vanetti C, Vago R, Clerici M, Tacchetti C, Daniele T (2021) SARS-CoV-2 Entry: At the crossroads of CD147 and ACE2. Cell 10(6):1434
Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M, Gironi A, Anichini R, Seghieri G (1995) Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: effects of taurine supplementation. Am J Clin Nutr 61(5):1115–1119
Ganji R, Reddy PH (2020) Impact of COVID-19 on mitochondrial-based immunity in aging and age-related diseases. Front Aging Neurosci 12:614650
Ghandforoush-Sattari M, Mashayekhi S, Nemati M, Routledge PA (2009) A rapid determination of taurine in human plasma by LC. Chromatographia 69(11):1427–1430
Ghandforoush-Sattari M, Mashayekhi S, Krishna CV, Thompson JP, Routledge PA (2010) Pharmacokinetics of oral taurine in healthy volunteers. J Amino Acids 2010:346237
Goud PT, Bai D, Abu-Soud HM (2021) A multiple-hit hypothesis involving reactive oxygen species and myeloperoxidase explains clinical deterioration and fatality in COVID-19. Int J Biol Sci 17(1):62–72
Guizoni DM, Vettorazzi JF, Carneiro EM, Davel AP (2020) Modulation of endothelium-derived nitric oxide production and activity by taurine and taurine-conjugated bile acids. Nitric Oxide 94:48–53
Gurujeyalakshmi G, Wang Y, Giri SN (2000) Taurine and niacin block lung injury and fibrosis by down-regulating bleomycin-induced activation of transcription nuclear factor-kappaB in mice. J Pharmacol Exp Ther 293(1):82–90
Guven-Maiorov E, Keskin O, Gursoy A, Van Waes C, Chen Z, Tsai CJ, Nussinov R (2015) The architecture of the TIR domain signalosome in the Toll-like Receptor-4 signaling pathway. Sci Rep 5:13128
Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC (2010) Angiotensin-(1-7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension 55(1):166–171
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637
Han Z, Gao LY, Lin YH, Chang L, Wu HY, Luo CX, Zhu DY (2016) Neuroprotection of taurine against reactive oxygen species is associated with inhibiting NADPH oxidases. Eur J Pharmacol 777:129–135
He X, Yao F, Chen J, Wang Y, Fang X, Lin X, Long H, Wang Q, Wu Q (2021) The poor prognosis and influencing factors of high D-dimer levels for COVID-19 patients. Sci Rep 11(1):1830
Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, Caricchio R, Mahmud S, Hazen MM, Halyabar O, Hoyt KJ, Han J, Grom AA, Gattorno M, Ravelli A, De Benedetti F, Behrens EM, Cron RQ, Nigrovic PA (2020) On the alert for rytokine storm: immunopathology in COVID-19. Arthritis Rheum 72(7):1059–1063
Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S (2014) TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 88(2):1293–1307
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280
Ijiri Y, Ikarugi H, Tamura Y, Ura M, Morishita M, Hamada A, Mori M, Mori H, Yamori Y, Ishii H, Yamamoto J (2013) Antithrombotic effect of taurine in healthy Japanese people may be related to an increased endogenous thrombolytic activity. Thromb Res 131(2):158–161
Indranil Biswas GAK (2019) Endothelial dysfunction in cardiovascular diseases. In: Shad KF, Bilgrami NL, Saravi SSS (eds) Basic and clinical understanding of microcirculation. IntechOpen. https://doi.org/10.5772/intechopen.89365. Available from: https://www.intechopen.com/books/basic-and-clinical-understanding-of-microcirculation/endothelial-dysfunction-in-cardiovascular-diseases
Ito T, Schaffer SW, Azuma J (2012) The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids 42(5):1529–1539
Iwegbulem O, Wang J, Pfirrmann RW, Redmond HP (2021) The role of taurine derivatives in the putative therapy of COVID-19-induced inflammation. Ir J Med Sci Feb 18:1–2
Jarcho JA, Ingelfinger JR, Hamel MB, D’Agostino RB Sr, Harrington DP (2020) Inhibitors of the renin-angiotensin-aldosterone system and Covid-19. N Engl J Med 382(25):2462–2464
Jin R, Xiao AY, Liu S, Wang M, Li G (2018) Taurine reduces tPA (tissue-type plasminogen activator)-induced hemorrhage and microvascular thrombosis after embolic stroke in rat. Stroke 49(7):1708–1718
Jin R, Zhong W, Liu S, Li G (2019) CD147 as a key mediator of the spleen inflammatory response in mice after focal cerebral ischemia. J Neuroinflammation 16(1):198
Jin Y, Ji W, Yang H, Chen S, Zhang W, Duan G (2020) Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther 5(1):293
Kim KS, Kim JS, Park JY, Suh YH, Jou I, Joe EH, Park SM (2013) DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum Mol Genet 22(23):4805–4817
Kumar P, Sobhanan J, Takano Y, Biju V (2021) Molecular recognition in the infection, replication, and transmission of COVID-19-causing SARS-CoV-2: an emerging interface of infectious disease, biological chemistry, and nanoscience. NPG Asia Materials 13(1):14
Kyrou I, Randeva HS, Spandidos DA, Karteris E (2021) Not only ACE2-the quest for additional host cell mediators of SARS-CoV-2 infection: Neuropilin-1 (NRP1) as a novel SARS-CoV-2 host cell entry mediator implicated in COVID-19. Signal Transduct Target Ther 6(1):21
Lak S, Ostadrahimi A, Nagili B, Asghari-Jafarabadi M, Beigzali S, Salehi F, Djafarzadeh R (2015) Anti-inflammatory effect of taurine in burned patients. Adv Pharm Bull 5(4):531–536
Lee EA, Seo JY, Jiang Z, Yu MR, Kwon MK, Ha H, Lee HB (2005) Reactive oxygen species mediate high glucose-induced plasminogen activator inhibitor-1 up-regulation in mesangial cells and in diabetic kidney. Kidney Int 67(5):1762–1771
Li S, Kong L, Yu X (2015) The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit Rev Microbiol 41(2):150–164
Lin YJ, Kwok CF, Juan CC, Hsu YP, Shih KC, Chen CC, Ho LT (2014) Angiotensin II enhances endothelin-1-induced vasoconstriction through upregulating endothelin type A receptor. Biochem Biophys Res Commun 451(2):263–269
Liu X, Zhang YR, Cai C, Ni XQ, Zhu Q, Ren JL, Chen Y, Zhang LS, Xue CD, Zhao J, Qi YF, Yu YR (2019) Taurine alleviates schistosoma-induced liver injury by inhibiting the TXNIP/NLRP3 inflammasome signal pathway and yroptosis. Infect Immun 87(12):e00732–e00719
Liu J, Li Y, Liu Q, Yao Q, Wang X, Zhang H, Chen R, Ren L, Min J, Deng F, Yan B, Liu L, Hu Z, Wang M, Zhou Y (2021) SARS-CoV-2 cell tropism and multiorgan infection. Cell Discov 7(1):17
Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS (2005) Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther 315(3):1058–1064
Lv Q, Yang Q, Cui Y, Yang J, Wu G, Liu M, Ning Z, Cao S, Dong G, Hu J (2017) Effects of taurine on ACE, ACE2 and HSP70 expression of hypothalamic-pituitary-adrenal axis in stress-induced hypertensive rats. Adv Exp Med Biol 975(Pt 2):871–886
Mahmoud IS, Jarrar YB, Alshaer W, Ismail S (2020) SARS-CoV-2 entry in host cells-multiple targets for treatment and prevention. Biochimie 175:93–98
Mahootchi E, Raasakka A, Luan W, Muruganandam G, Loris R, Haavik J, Kursula P (2021) Structure and substrate specificity determinants of the taurine biosynthetic enzyme cysteine sulphinic acid decarboxylase. J Struct Biol 213(1):107674
Maleki V, Alizadeh M, Esmaeili F, Mahdavi R (2020a) The effects of taurine supplementation on glycemic control and serum lipid profile in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Amino Acids 52(6-7):905–914
Maleki V, Mahdavi R, Hajizadeh-Sharafabad F, Alizadeh M (2020b) The effects of taurine supplementation on oxidative stress indices and inflammation biomarkers in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Diabetol Metab Syndr 12:9
Maniscalco M, Ambrosino P, Fuschillo S, Stufano S, Sanduzzi A, Matera MG, Cazzola M (2021) Bronchodilator reversibility testing in post-COVID-19 patients undergoing pulmonary rehabilitation. Respir Med 182:106401
McElvaney OJ, Hobbs BD, Qiao D, McElvaney OF, Moll M, McEvoy NL, Clarke J, O’Connor E, Walsh S, Cho MH, Curley GF, McElvaney NG (2020) A linear prognostic score based on the ratio of interleukin-6 to interleukin-10 predicts outcomes in COVID-19. EBioMedicine 61:103026
Men X, Han S, Gao J, Cao G, Zhang L, Yu H, Lu H, Pu J (2010) Taurine protects against lung damage following limb ischemia reperfusion in the rat by attenuating endoplasmic reticulum stress-induced apoptosis. Acta Orthop 81(2):263–267
Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20(7):1126–1167
Moreau P, d’Uscio LV, Shaw S, Takase H, Barton M, Lüscher TF (1997) Angiotensin II increases tissue endothelin and induces vascular hypertrophy: reversal by ET(A)-receptor antagonist. Circulation 96(5):1593–1597
Murad F, Waldman S, Molina C, Bennett B, Leitman D (1987) Regulation and role of guanylate cyclase-cyclic GMP in vascular relaxation. Prog Clin Biol Res 249:65–76
Myojo M, Nagata D, Fujita D, Kiyosue A, Takahashi M, Satonaka H, Morishita Y, Akimoto T, Nagai R, Komuro I, Hirata Y (2014) Telmisartan activates endothelial nitric oxide synthase via Ser1177 phosphorylation in vascular endothelial cells. PLoS One 9(5):e96948
Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, Scherer C, Rudelius M, Zoller M, Hochter D, Keppler O, Teupser D, Zwibler B, von Bergwelt-Baildon M, Kaab S, Massberg S, Pekayvaz K, Stark K (2020) Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation 142(12):1176–1189
Nienhold R, Ciani Y, Koelzer VH, Tzankov A, Haslbauer JD, Menter T, Schwab N, Henkel M, Frank A, Zsikla V, Willi N, Kempf W, Hoyler T, Barbareschi M, Moch H, Tolnay M, Cathomas G, Demichelis F, Junt T, Mertz KD (2020) Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat Commun 11(1):5086
Nishida S, Satoh H (2009) Vascular modulation of rat aorta by taurine. Adv Exp Med Biol 643:37–46
Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, David JS, Bonnet A, Negrier C, Dargaud Y (2020) Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost 18(9):2215–2219
Ohsawa Y, Hagiwara H, Nishimatsu SI, Hirakawa A, Kamimura N, Ohtsubo H, Fukai Y, Murakami T, Koga Y, Goto YI, Ohta S, Sunada Y, KN01 Study Group (2019) Taurine supplementation for prevention of stroke-like episodes in MELAS: a multicentre, open-label, 52-week phase III trial. J Neurol Neurosurg Psychiatry 90(5):529–536
Pan P, Shen M, Yu Z, Ge W, Chen K, Tian M, Xiao F, Wang Z, Wang J, Jia Y, Wang W, Wan P, Zhang J, Chen W, Lei Z, Chen X, Luo Z, Zhang Q, Xu M, Li G, Li Y, Wu J (2021) SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat Commun 12(1):4664
Qaradakhi T, Gadanec LK, McSweeney KR, Abraham JR, Apostolopoulos V, Zulli A (2020) The anti-inflammatory effect of taurine on cardiovascular disease. Nutrients 12(9):2847
Rabelo LA, Alenina N, Bader M (2011) ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens Res 34(2):154–160
Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, Park J, Foox J, Hether T, Warren S, Kim Y, Reeves J, Salvatore S, Mason CE, Swanson EC, Borczuk AC, Elemento O, Schwartz RE (2021) The spatial landscape of lung pathology during COVID-19 progression. Nature 593(7860):564–569
Rosa FT, Freitas EC, Deminice R, Jordão AA, Marchini JS (2014) Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr 53(3):823–830
Ruan Y, Li M, Wang T, Yang J, Rao K, Wang S, Yang W, Liu J, Ye Z (2016) Taurine supplementation improves erectile function in rats with streptozotocin-induced type 1 diabetes via amelioration of penile fibrosis and endothelial dysfunction. J Sex Med 13(5):778–785
Ruiz-Ortega M, Ruperez M, Lorenzo O, Esteban V, Blanco J, Mezzano S, Egido J (2002) Angiotensin II regulates the synthesis of proinflammatory cytokines and chemokines in the kidney. Kidney Int Suppl 82:S12–S22
Sabaka P, Koščálová A, Straka I, Hodosy J, Lipták R, Kmotorková B, Kachlikova M, Kušnírová A (2021) Role of interleukin 6 as a predictive factor for a severe course of Covid-19: retrospective data analysis of patients from a long-term care facility during Covid-19 outbreak. BMC Infect Dis 21(1):308
Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM (2007) Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49(1):185–192
Santhakumar AB, Fozzard N, Perkins AV, Singh I (2013) The synergistic effect of taurine and caffeine on platelet activity and hemostatic function. Food Public Health 3(3):147–153
Schaffer S, Kim HW (2018) Effects and mechanisms of taurine as a therapeutic agent. Biomol Ther (Seoul) 26(3):225–241
Schaffer SW, Lombardini JB, Azuma J (2000) Interaction between the actions of taurine and angiotensin II. Amino Acids 18(4):305–318
Schaffer SW, Jong CJ, Ramila KC, Azuma J (2010) Physiological roles of taurine in heart and muscle. J Biomed Sci 17 Suppl 1(Suppl 1):S2
Schmitt FCF, Manolov V, Morgenstern J, Fleming T, Heitmeier S, Uhle F, Al-Saeedi M, Hackert T, Bruckner T, Schochl H, Weigand MA, Hofer S, Brenner T (2019) Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: results of an observational pilot study. Ann Intensive Care 9(1):19
Schuller-Levis G, Quinn MR, Wright C, Park E (1994) Taurine protects against oxidant-induced lung injury: possible mechanism(s) of action. Adv Exp Med Biol 359:31–39
Schuller-Levis GB, Gordon RE, Wang C, Park E (2003) Taurine reduces lung inflammation and fibrosis caused by bleomycin. Adv Exp Med Biol 526:395–402
Schurink B, Roos E, Radonic T, Barbe E, Bouman CSC, de Boer HH, de Bree GJ, Bulle EB, Aronica EM, Florquin S, Fronczek J, Heunks LMA, de Jong MD, Guo L, du Long R, Lutter R, Molenaar PCG, Neefjes-Borst EA, Niessen HWM, van Noesel CJM, Roelofs JJTH, Snijder EJ, Soer EC, Verheji J, Vlaar APJ, Vos W, van der Wel NN, van der Wal AC, van der Valk P, Bugiani M (2020) Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe 1(7):e290–e299
Serfass L, Carr HS, Aschenbrenner LM, Burstyn JN (2001) Calcium ion downregulates soluble guanylyl cyclase activity: evidence for a two-metal ion catalytic mechanism. Arch Biochem Biophys 387(1):47–56
Shilts J, Crozier TWM, Greenwood EJD, Lehner PJ, Wright GJ (2021) No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. Sci Rep 11(1):413
Singh S, Offringa-Hup AK, Logtenberg SJJ, Van der Linden PD, Janssen WMT, Klein H, Waanders F, Simsek S, de Jager CPC, Smits P, van der Feltz M, Beumer GJ, Widrich C, Nap M, Pinto-Sietsma SJ (2021a) Discontinuation of antihypertensive medications on the outcome of hospitalized patients with severe acute respiratory syndrome-coronavirus 2. Hypertension 78(1):165–173
Singh Y, Trautwein C, Fendel R, Krickeberg N, Berezhnoy G, Bissinger R, Ossowski S, Salker MS, Casadei N, Riess O, Deutsche COVID-19 OMICS Initiative (DeCOI) (2021b) SARS-CoV-2 infection paralyzes cytotoxic and metabolic functions of the immune cells. Heliyon 7(6):e07147
Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, Lu P, Weizman OE, Liu F, Dai Y, Szigeti-Buck K, Yasumoto Y, Wang G, Castaldi C, Heltke J, Ng E, Wheeler J, Alfajaro MM, Levavasseur E, Fontes B, Ravindra NG, Van Dijk D, Mane S, Gunel M, Ring A, Kazmi SAJ, Zhang K, Wilen CB, Horvath TL, Plu I, Haik S, Thomas JL, Louvi A, Farhadian SF, Huttner A, Seilhean D, Renier N, Bilguvar K, Iwasaki A (2021) Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med 218(3):e20202135
Soni S, Jiang Y, Tesfaigzi Y, Hornick JL, Çataltepe S (2021) Comparative analysis of ACE2 protein expression in rodent, non-human primate, and human respiratory tract at baseline and after injury: a conundrum for COVID-19 pathogenesis. PLoS One 16(2):e0247510
Stacy A, Andrade-Oliveira V, McCulloch JA, Hild B, Oh JH, Perez-Chaparro PJ, Sim CK, Lim AI, Link VM, Enamorado M, Trinchieri G, Segre JA, Rehermann B, Belkaid Y (2021) Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 184(3):615–627
Sun Q, Wang B, Li Y, Sun F, Li P, Xia W, Zhou X, Li Q, Wang X, Chen J, Zeng X, Zhao Z, He H, Liu D, Zhu Z (2016) Taurine supplementation lowers blood rressure and improves vascular function in prehypertension: randomized, double-blind, placebo-controlled study. Hypertension 67(3):541–549
Sureda A, Alizadeh J, Nabavi SF, Berindan-Neagoe I, Cismaru CA, Jeandet P, Los MJ, Clementi E, Nabavi SM, Ghavami S (2020) Endoplasmic reticulum as a potential therapeutic target for covid-19 infection management? Eur J Pharmacol 882:173288
Thillai M, Patvardhan C, Swietlik EM, McLellan T, De Backer L, Lanclus M, De Backer W, Ruggiero A (2021) Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID-19. Thorax 76(2):182–184
Thomas T, Stefanoni D, Reisz JA, Nemkov T, Bertolone L, Francis RO, Hudson KE, Zimring JC, Hansen KC, Hod EA, Spitalnik SL, D’Alessandro A (2020) COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight 5(14):e140327
Vahdat M, Hosseini SA, Soltani F, Cheraghian B, Namjoonia M (2021) The effects of taurine supplementation on inflammatory markers and clinical outcomes in patients with traumatic brain injury: a double-blind randomized controlled trial. Nutr J 20(1):53
van Eijk LE, Binkhorst M, Bourgonje AR, Offringa AK, Mulder DJ, Bos EM, Kolundzic N, Abdulle AE, van der Voot P, Hj RMGO, van der Hoeven JG, den Dunnen WF, Hillebrands JL, van Goor H (2021) COVID-19: immunopathology, pathophysiological mechanisms, and treatment options. J Pathol 254(4):307–331
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395(10234):1417–1418
Vieira C, Nery L, Martins L, Jabour L, Dias R, Simões ESAC (2021) Downregulation of membrane-bound angiotensin converting enzyme 2 (ACE2) receptor has a pivotal role in COVID-19 immunopathology. Curr Drug Targets 22(3):254–281
V’Kovski P, Kratzel A, Steiner S, Stalder H, Thiel V (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol 19(3):155–170
Wang K, Chen W, Zhang Z, Deng Y, Lian JQ, Du P, Wei D, Zhang Y, Sun XX, Gong L, Yang X, He L, Zhang L, Yang Z, Geng JJ, Chen R, Zhang H, Wang B, Zhu YM, Nan G, Jiang JJ, Li L, Wu J, Lin P, Huang W, Zhang J, Fu L, Yang XM, Zhao Z, Sun S, Gu H, Wang Z, Wang CF, Lu Y, Liu YY, Wang QY, Bian H, Zhu P, Chen ZN (2020a) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct Target Ther 5(1):283
Wang Y, Wang Y, Luo W, Huang L, Xiao J, Li F, Qin S, Song X, Wu Y, Zheng Q, Jin F, Wang Y (2020b) A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci 17(11):1522–1531
Wang C, Wang Z, Wang G, Lau JY, Zhang K, Li W (2021a) COVID-19 in early 2021: current status and looking forward. Signal Transduct Target Ther 6(1):114
Wang S, Dai T, Qin Z, Pan T, Chu F, Lou L, Zhang L, Yang B, Huang H, Lu H, Zhou F (2021b) Targeting liquid-liquid phase separation of SARS-CoV-2 nucleocapsid protein promotes innate antiviral immunity by elevating MAVS activity. Nat Cell Biol 23(7):718–732
Wen Y, Liu Y, Tang T, Lv L, Liu H, Ma K, Liu B (2016) NLRP3 inflammasome activation is involved in Ang II-induced kidney damage via mitochondrial dysfunction. Oncotarget 7(34):54290–54302
Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ (2020) Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost 18(7):1548–1555
Wójcik OP, Koenig KL, Zeleniuch-Jacquotte A, Costa M, Chen Y (2010) The potential protective effects of taurine on coronary heart disease. Atherosclerosis 208(1):19–25
Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC Jr (2020) Fibrinolysis shutdown correlation with thromboembolic events in severe COVID-19 infection. J Am Coll Surg 231(2):193–203
Yamaguchi Y, Iribe G, Kaneko T, Takahashi K, Numaga-Tomita T, Nishida M, Birnbaumer L, Naruse K (2018) TRPC3 participates in angiotensin II type 1 receptor-dependent stress-induced slow increase in intracellular Ca(2+) concentration in mouse cardiomyocytes. J Physiol Sci 68(2):153–164
Yao XH, Luo T, Shi Y, He ZC, Tang R, Zhang PP, Cai J, Zhou XD, Jiang DP, Fei XC, Huang XQ, Zhao L, Zhang H, Wu HB, Ren Y, Liu ZH, Zhang HR, Chen C, Fu WJ, Li H, Xia XY, Chen R, Wang Y, Liu XD, Yin CL, Yan ZX, Wang J, Jing R, Li TS, Li WQ, Wang CF, Ding YQ, Mao Q, Zhang DY, Zhang SY, Ping YF, Bian XW (2021) A cohort autopsy study defines COVID-19 systemic pathogenesis. Cell Res 31(8):836–846
Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS, Chan V, Ling L, Joynt G, Hui DSC, Chow KM, Ng SSS, Li TCM, Ng RW, Yip TC, Wong GLH, Chan FK, Wong CK, Chan PK, Ng SC (2021) Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70(4):698–706
Yeung ML, Teng JLL, Jia L, Zhang C, Huang C, Cai JP, Zhou R, Chan KH, Zhao H, Zhu L, Siu KL, Fung SY, Yung S, Chan TM, To KKW, Chan JFW, Cai Z, Lau SKP, Chen Z, Jin DY, Woo PCY, Yuen KY (2021) Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 184(8):2212–2228
Yoshimura T, Inokuchi Y, Mutou C, Sakurai T, Nagahama T, Murakami S (2021) Age-related decline in the taurine content of the skin in rodents. Amino Acids 53(3):429–434
Younis NS, Ghanim AMH, Elmorsy MA, Metwaly HA (2021) Taurine ameliorates thioacetamide induced liver fibrosis in rats via modulation of toll like receptor 4/nuclear factor kappa B signaling pathway. Sci Rep 11(1):12296
Zhao H, Qu J, Li Q, Cui M, Wang J, Zhang K, Liu X, Feng H, Chen Y (2018) Taurine supplementation reduces neuroinflammation and protects against white matter injury after intracerebral hemorrhage in rats. Amino Acids 50(3-4):439–451
Zhu X, Song Z, Zhang S, Nanda A, Li G (2014) CD147: a novel modulator of inflammatory and immune disorders. Curr Med Chem 21(19):2138–2145
Zou X, Chen K, Zou J, Han P, Hao J, Han Z (2020) Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14(2):185–192
Zuo Y, Warnock M, Harbaugh A, Yalavarthi S, Gockman K, Zuo M, Madison JA, Knight JS, Kanthi Y, Lawrence DA (2021) Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep 11(1):1580
Acknowledgments
The authors would like to thank the scientific and medical illustrator Dr. Nikola Kolundzic (King’s College London, UK) for his valuable help in the graphical design of the figures.
Disclosure
The authors declare that they have no conflicts of interest. This review was realized without funding.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
van Eijk, L.E., Offringa, A.K., Bernal, ME., Bourgonje, A.R., van Goor, H., Hillebrands, JL. (2022). The Disease-Modifying Role of Taurine and Its Therapeutic Potential in Coronavirus Disease 2019 (COVID-19). In: Schaffer, S.W., El Idrissi, A., Murakami, S. (eds) Taurine 12. Advances in Experimental Medicine and Biology, vol 1370. Springer, Cham. https://doi.org/10.1007/978-3-030-93337-1_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-93337-1_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-93336-4
Online ISBN: 978-3-030-93337-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)