Abstract
Upconversion nanoparticles (UCNPs) are excited in the near infrared (NIR) biological transparency window, but emits anti-Stokes shifted photons in the visible that are extremely photostable and ideally suited for biomedical imaging. First, we synthesize a UCNP-mOrange fluorescent protein conjugate that forms a Forster resonance energy transfer (FRET) pair with UCNP as the donor and mOrange as the acceptor to report intracellular pH by fluorescence imaging. The inactivity of the mOrange protein in acidic pH will decrease FRET efficiency making the emission green dominated by the UCNPs. In basic pH, the emission will be relatively orange. This is the first demonstration of ratiometric and self-ratiometric demonstration of intracellular pH imaging by the UCNPs. Next, we show a smart multifunctional nanoformulation of the UCNPs (NaGdF4: Yb, Er- 99mTc) using radioisotope labelling that shows potential for multimodal imaging using fluorescence, CT, and SPECT modalities. Intricate chemistry to engineer the UCNPs resulted in stabilizing the nanoformulation in physiological and aqueous environments with reproducible signal acquisition. This work gathers the expertise of physicists, chemists, and biologists in an interdisciplinary platform.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Biomolecular imaging techniques is a powerful tool for diagnosis of cancers, and may have high specificity and sensitivity [1]. Among different varieties of metallic/metaloxide nanoparticles, upconversion nanoparticles (UCNPs) are superior for fluorescence imaging as they shows anti-Stokes shifted emission upon low energy NIR excitation, and are extremely stable even in physiological conditions [2]. The visible fluorescence that forms the basis of bioimaging with the UCNPs can be tuned from blue to red by changing the dopant type and concentration to suit multiplexing and has been reported before. The NIR excitation of these UCNPs result is negligible autofluorescence that increases the imaging contrast. The fluorescence in the UCNPs are also accompanied by non-radiative decay that results in heat in the UCNPs. This heat can also be imaged using a simple infrared thermal camera. On the other hand, the size and composition of the UCNPs promote easy cellular uptake, and offers minimum cytotoxicity. The easy incorporation of rare-earth ions such as Gd3+ and Yb3+ into UCNPs makes them active under magnetic resonance imaging (MRI) [3] and computed tomography (CT) [2]. Besides, they also provide an excellent platform for further functionalization [2] to combine with radio isotopes for single-photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging, in order to improve the sensitivity of diagnosis. Such unique properties of these UCNPs makes them ideal candidate for the multimodal imaging which is essentially required to acquire biological information for identifying cancer at an early stage. UCNPs also found applications in photoenabled therapies. Overall, a control over the chemistry, surface functionalization, and knowledge of the photophysics in these UCNPs opens up a wide range of possibilities for biomedical applications.
In this work, we have done functional modification of the UCNPs with select proteins to achieve FRET which can be used for intracellular pH imaging. Next, we have modified the UCNP with another chelator (chl) protein and loaded radioisotopes therein to make the nanoformulation work as multimodal imaging agents.
2 Experimental Part
-
A.
Synthesis of UCNPs
First, oleic acid (OA) capped UCNPs (dispersible in cyclohexane) were synthesized following a published report [4]. Typically, GdCl3 (80%), YbCl3 (18%), ErCl3 (2%) were mixed with 14 mL of OA and 16 mL of octadecene in a three-necked flask, and heated to 150 ̊C under nitrogen atmosphere. Subsequently, 10 mL of methanol solution of NaOH (2.5 mmol) and NH4F (4 mmol) is added dropwise while stirring for 30 min maintaining reaction temperature at 50 ̊C. Finally, the mixture was heated to 300 ̊C for 1 h, and washed with ethanol, and the precipitate redispersed in cyclohexane.
Next, the OA-capping was replaced with citrate (Cit-UCNPs) to make the UCNPs hydrophilic by ligand exchange reaction [5].
-
B.
Synthesis of mOrange fluorescent proteins and construction of UCNPs-mOrange FRET nanoprobes
The His6-tagged mOrange fluorescent proteins were derived from pRSET-b mOrange plasmid. mOrange proteins were expressed in E. coli (BL21) competent cells. Later, we lysed the bacterial cell membrane, by performing mechanical lysis, using mechanical probe sonicator. Finally, we collected the proteins after centrifugation of the sample and purifird under known protocols. The Cit-UCNP-mOrange FRET nanoprobes were constructed via EDC (1-ethyl-3-(3-dimethylaminopropyl) carbadimide) (Sigma Aldrich, USA) chemistry [6]. For the FRET nanoprobe construction, we have used 2 mL of Cit-UCNPs (1.5 mg/mL) with 2 mL of mOrange FPs (0.35 mg/mL), and 3 mL of EDC (6 mg/mL) were incubated overnight at 4 ̊C with gentle shaking (200 rpm).
-
III.
Conjugation of radioisotope specific chelator (chl)
1 mg of EDC was added into 5 mL aqueous solution of cit-UCNPs (0.2 mg/ml), and the solution was stirred at 340 rpm for 1 h at room temperature. 2 mg of as-purchased Tc specific chelator was then added into the solution, and the solution was stirred under 200 rpm for 8 h at room temperature. The obtained UCNP@chl, formulation was collected by centrifuging the resultant mixture at 15,000 rpm for 30 min with the precipitation re-dispersed in 5 mL deionized water. SnCl2 (1 mg/ml) and Tc-pertechnetate in DI water were sequentially added to the UCNP@chl solution and allowed to react for 30 min with shaking at room temperature. The precipitate of 99mTc labeled UCNP@chl (UCNP@chl-99mTc) was redispersed in 0.5 mL DI water for final use.
-
IV.
Characterization of UCNPs
The morphology and crystal structures were imaged by High Resolution Transmission Electron Microscope (HR-TEM, JEM-2010F, JEOL, Japan). Fluorescence spectra were recorded by a Fluorolog spectrophotometer (JY Fluorolog 3 with iHR 320, Horiba, Japan), equipped with a 980 nm cw laser excitation (SDL, China). For the FRET imaging, we have used an inverted fluorescence microscope (Zeiss Axiovert 200) equipped with a 980 nm cw laser (CNI, China), and compatible optics and a wide-view dual-mode CMOS camera (Hamamatsu, Japan) to record high resolution images. 2 ml of as prepared UCNP@chl-99mTc (1 mg/mL) was taken in a quartz cuvette and 980 nm laser (SDL-5000 T, Shanghai, China) was used to observe the UCL. For the UCL imaging, the external 980 nm laser (SDL-5000 T, Shanghai China) along with optical fiber and a 10 mm laser collimator was installed in the IVIS (Xenogen IVIS 50, PerkinElmer, Inc. Massachusetts, U.S.A.).
-
E.
Cellular uptake and Intracellular pH imaging.
For in situ calibrations of the pH nanoprobes, 100 μL of nanoprobe solution were incubated with HeLa cells (104 cells/well) for 24 h at 37 ̊C, and 5% CO2. Later, intracellular pH was adjusted to that of the extracellular pH environment controlled by an ionophore ‘Nigericin’ (481,990, Calbiochem, Merck, USA). For this purpose, HeLa cells (incubated with UCNP-mOrange nanoprobes) were washed (by 1 × PBS) and incubated in pH (3.0, 5.0, and 7.0) controlled citrate/phosphate buffer medium. Afterwards, each well was treated with 20 μM of Nigericin and incubated for another 30 min at 37 ̊C, and 5% CO2 before heading for the microscopic imaging.
3 Results and Discussion
-
A.
Materials characterisations of UCNP, mOrange, and UCNP-mOrange FRET nanoprobes.
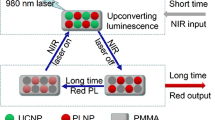
The UCNP formulation is schematically shown in Fig. 1. Figure 2 represents the morphological and FRET characteristics of UCNP-mOrange nanoprobes. Figure 2a shows the TEM image of Cit-UCNPs. The average size of this nanoparticles is 15 nm. Inset in Fig. 2a shows a colloidal solution of Cit-UCNPs (dispersed in DI water) emitting green upconversion luminescence (UCL) under 980 nm laser excitation. Figure 2b, shows the HR-TEM image of a single UCNP-mOrange nanoprobe. Under TEM, the UCNPs (yellow circle) appears darker than a layer of mOrange protein coated outside (red circle). The overall size of the nanoprobe has increased to 25 nm because of the mOrange protein shell of 4.8 nm thickness around Cit-UCNPs.
Morphology and FRET characteristics of UCNP-mOrange nanoprobes. a TEM image of Cit-UCNPs. Inset shows a colloidal solution of Cit-UCNP (DI water) emitting green emission when illuminated with 980 nm laser. b High-resolution TEM image of a single UCNP-mOrange nanoprobe. The yellow circle shows the single Cit-UNCP, and a layer or mOrange protein with red circle. c Spectral overlapping between the emission of FRET donor (Cit-UCNPs), excitation of FRET acceptor (mOrange), respectively. d Convoluted and deconvolution FRET emission spectrum of UCNP-mOrange, under the excitation of 980 nm laser, which shows a sensitized emission band at 566 nm, and is coming from mOrange FPs. Reproduced with permission from Ref [7]. Copyright 2020 Elsevier B.V.
Figure 2c shows the spectral overlap between UCNP (FRET donor) emission, and mOrange (FRET acceptor) excitation spectrum. We can observe that the broad excitation spectrum of mOrange fluorescent proteins is straddling the green emission band of Cit-UCNPs, satisfying the first condition of FRET. Figure 2d presents the spectroscopic FRET characteristics of UCNP-mOrange nanoprobe. Under the illumination of 980 nm laser, 5 distinct emission band has been observed. Out of these bands, peaks at 520, 521, 540, and 548 nm resembles to the characteristic peaks of UCNPs, and emission peak it 566 nm corresponds to the sensitized emission from mOrange fluorescent proteins. The confirmation of energy transfers between UCNPs and mOrange fluorescent protein is therefore established.
3.1 Intercellular pH Sensing Using UCNP-MOrange FRET Nanoprobe
Next, in addition to spectroscopic confirmation of FRET, we have investigated the in-vitro FRET imaging under different pH environment. To demonstrate the sensitivity of the nanoprobes to estimate the intracellular pH accurately, we have used Nigericin to modulate the intracellular pH with that of extracellular medium. Figure 3 presents the FRET images of HeLa cells in different pH (7.0, 5.0, and 3.0) buffers. The use of Nigericin ionophore would ensure an identical pH value inside and outside of the cells.
Nigericin mediated in situ pH imaging in HeLa cells. Fluorescence imaging of UCNP-mOrange nanoprobes internalized HeLa cell(s) with nigericin modulated pH of a 7.0, b 5.0, and c 3.0, respectively. From left to right (L to R): Bright field image, green (UCNP) channel, red (mOrange) channel, and dark field overlay of the red and green channel images. Common scale bar of 10 μm. Reproduced with permission from Ref [7]. Copyright 2020 Elsevier B.V.
At alkaline pH (7.0, Fig. 3a), we could observe the individual signals from both UCNPs (green channel), and mOrange (red channel) from the nanoprobes uptaken in the HeLa cells. The FRET ratio (red to green channel signal) obtained is ~ 0.71. For the case of mildly acidic pH (5.0, Fig. 3b), the FRET intensity ratio dropped to ~ 0.52 as mOrange loses their ability to absorb energy from UCNP. At the high acidic pH (~3.0, Fig. 3c), the FRET ratio is observed to as low as ~ 0.30. Other than the drop in the FRET ratio at low pH, the overlay emission color has also been affected. The inactivity of the mOrange protein in acidic pH (3.0) would decrease the energy transfer efficiency making the emission green (Fig. 3c) dominated by the UCNPs. In basic pH, the emission will be relatively orange (Fig. 3a). The above results indicate that the UCNP-mOrange FRET nanoprobes can be used for accurate estimation of intracellular pH.
3.2 Multimodal Imaging Using UCNP-chl- 99 m Tc Nanoformulation
Multi-modal imaging property of the UCNP@chl-99mTc is demonstrated by observing the UCL from the nanoformulation dispersed in water under 980 nm laser irradiation (Fig. 4a). UCL property of this nanocomposite was further investigated under small animal in vivo imaging system (IVIS) where only water containing glass vial shows no signal (Fig. 4b) under GFP filter, however, the vial containing 1 mg/mL of UCNP@chl-99mTc nanoformulation shows strong luminescence (Fig. 4c).
The Eppendorf containing the UCNP formulation without the radiolabel 99mTc (Fig. 4d) only shows the CT contrast because of the Yb3+ content in the formulation. The final formulation with the radio label (UCNP@chl-99mTc) shows strong SPECT signals (Fig. 4e). These results show proof-of-concept that the formulation could be used for multimodal imaging.
4 Conclusions
In conclusion, we demonstrate two UCNP based nanoformulation having functional imaging capabilities under deep penetrating 980 nm illumination. First, we have constructed a highly sensitive FRET based nanoprobe using UCNP as the donor and mOrange fluorescent protein as the acceptor. The UCNPs are internalized in HeLa cells. Upon changing the cellular pH by using an ionophore the emission from the UCNP-mOrange conjugate change from green at acidic pH and orange at alkaline pH. This UCNP based FRET imaging provides accurate estimation of intracellular pH. The results are reproducible, stable, and reversible indicating usability in early detection of cancer and other pH malfunctions in cells.
The second nanoformulation showed strong green fluorescence image contrast under 980 nm excitation. The component Yb3+ in the host NaGdF4 of the UCNP makes the nanoformulation CT active. Radiolabelling of the UCNP obtained by chelating Tc in the protein network enabled SPECT/CT imaging. In short, the UCNP nanoformulation could demonstrate multimodal imaging.
References
Ashok AH, Mizuno Y, Howes OD (2019) Tobacco smoking and dopaminergic function in humans: a meta-analysis of molecular imaging studies. Psychopharmacology 236(4):1119–1129
Gupta A, Lam CW, Wu CT, Yang DM, Chattopadhyay S (2018) Photothermal disintegration of 3T3 derived fat droplets by irradiated silica coated upconversion nanoparticles. Part Part Syst Charact 35:1800294
Bi H, He F, Dai Y, Xu J, Dong Y et al (2018) Quad-model imaging- guided high-efficiency phototherapy based on upconversion nanoparticles and ZnFe2O4 integrated graphene oxide. Inorg Chem 57(16):9988–9998
Ghosh S, Chiang WC, Fakhri MY, Wu CT, Chen RS, Chattopadhyay S (2020) Ultrasensitive broadband photodetector using electrostatically conjugated MoS2-upconversion nanoparticle nanocomposite. Nano Energy 67:104258
Cao T, Yang T, Gao Y, Yang Y, Hu H, Li F (2010) Water-soluble NaYF4:Yb/Er upconversion nanophosphors: synthesis, characteristics and application in bioimaging. Inorg Chem Commun 13:392–394
Dennis AM, Rhee WJ, Sotto D, Dublin SN, Bao G (2012) Quantum dot-fluorescent protein FRET probes for sensing intracellular pH. ACS Nano 6:2917–2924
Ghosh S, Chang YF, Yang DM, Chattopadhyay S (2020) Upconversion nanoparticle-mOrange protein FRET nanoprobes for self-ratiometric/ratiometric determination of intracellular pH, and single cell pH imaging. Bios Bioelectron 155:112115
Acknowledgements
The authors would like to thank Ministry of Science and Technology, Taiwan (MOST-107-2112-M-010-003-MY3) for the research funding.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Chattopadhyay, S., Ghosh, S., Akhtar, N., Yang, DM., Chen, C.L., Liu, RS. (2022). Upconversion Nanoformulation for Functional Imaging. In: Lin, KP., Liu, RS., Yang, BH. (eds) Future Trends and Challenges of Molecular Imaging and AI Innovation. FASMI 2020. Springer Proceedings in Physics, vol 272. Springer, Cham. https://doi.org/10.1007/978-3-030-92786-8_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-92786-8_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92785-1
Online ISBN: 978-3-030-92786-8
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)