Abstract
Experiments were carried out in NaOH-H2O (50–50 wt%) electrolyte with a suspension of Fe2O3 particles at ~100 ℃. A rotating disk electrode of graphite or silver was used as the cathode. The current efficiency for iron deposition was consistently higher than 90%. Recent experiments were carried out by using iron-containing residue from industrial electrolysis processes for producing nickel and zinc. The challenge is to purify the residue before electrolysis.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Iron production and steel production are responsible for a large amount, about 9%, of the world's anthropogenic CO2 emissions [1]. Different scenarios and low emission pathways have been researched by steelmaking industries together with universities and research institutes to tackle this problem [2]. One of the most promising novel processes to replace the current steelmaking process is the low temperature electrodecomposition of iron oxide. This process is currently being developed under H2020-SIDERWIN project in the SPIRE European program. In this process, a suspension of iron oxide particles (20–50 wt%) in a highly alkaline electrolyte of 50 wt% NaOH in water is electrolysed. Hence, the iron oxide is electrochemically reduced to metallic iron at the cathode, while oxygen evolution occurs at the anode. Therefore, this process is capable of producing pure iron with a very low carbon footprint [3,4,5,6,7,8].
One of the advantages of this process is the fact that in addition to iron oxide (hematite), it is possible to feed this process with other iron-containing raw materials. One alternative raw material which is being studied to be used in this process is bauxite residue (also known as red mud); the waste material from the Bayer process for alumina production. Bauxite residue usually contains 30–45 wt% iron in the form of oxides and hydroxides. Besides, on average, 1–1.5 tons of bauxite residue is produced per ton of alumina [5]. Therefore, it is a considerable resource of iron. Also, during the purification processes for primary production of nickel and zinc prior to electrolysis in aqueous electrolytes, large amounts of containing waste materials rich in iron are removed during the treatment of the raw materials.
Experimental
Pure iron oxide, Fe2O3, and iron-rich waste materials from nickel and zinc purification processes were used as raw materials for electrodeoxidation of Fe2O3. The waste materials were treated by washing and calcination before electrolysis.
The electrolysis experiments were carried out in a polytetrafluoroethylene (PTFE) container (Ø 100 mm, 160 mm height). Different types of working electrodes were used in this study. A rotating disc electrode (RDE) was used for galvanostatic electrolysis experiments in the electrolyte of a suspension of particles of Fe2O3 or waste material in NaOH 50 wt% aqueous solution. Two grades of graphite G347 (grade 1) and G348 (grade 2) supplied by Tokai Carbon and a pure silver rod, 99.9% Alfa Aesar, were used as cathode materials. For cyclic voltammetry studies, a cavity electrode was used. This electrode was fabricated similar to the electrodes known as “cavity microelectrodes” in electrochemistry literature. Such electrodes are used for electrochemical studies of solid materials insoluble in the electrolyte [6]. The cavity electrode was made of a PTFE rod, Ø 10 and 40 mm long, where a hole of Ø 0.7 mm was drilled into it. A steel rod Ø 4 mm was inserted from the top into the PTFE rod. The powder was crushed in a mortar with a pestle and the cavity electrode was pushed on the powder and the cavity was filled with powder. The powder might be consumed after each scan. Between each sweep, the cavity electrode was removed from the electrochemical cell, cleaned, and reloaded with new powder. The experimental cell and details of the cavity electrode are shown in Fig. 1. The anode was of pure nickel in the form of a mesh surrounding the cathode.
Results and Discussion
Treatment of Iron Residue from Nickel Production
The waste product from Ni production was provided by Glencore Nikkelverk of Norway. Chlorine leaching is used to purify the nickel sulfate concentrate made by smelting. The composition of the main metallic element was analyzed by XRF and presented in Table 1. The material is fine with D50 < 10 µm. In the waste product, the species include iron (III) hydroxide, nickel and cobalt chlorides, and arsenic compound. Among them, the weight percentage of iron hydroxide is 60–100%.
Treatment of Iron Residue from Zn Production
The waste material from purifying the raw material for zinc electrowinning mostly contains the iron-rich compound of jarosite. The material was obtained from the Boliden Zinc plant at Odda, Norway. The results of the analysis of the iron residue by XRF are given in Table 2.
Thermal analysis is beneficial for the study of jarosite. Firstly, thermal decomposition of the jarosite waste was carried out using TG-DSC under nitrogen atmosphere (10 ml min−1). 11.774 mg of the sample was heated to 1000 °C with a heating rate of 10 °C min−1. The decomposition products depend on the treatment temperature of the jarosite. After thermal decomposition, the specific temperature was chosen to treat the jarosite waste, and then the product was washed by two-step processing to enrich hematite.
Thermal analysis of jarosite was carried out by thermogravimetry and differential scanning calorimetry. Hematite can be obtained by thermal decomposition of jarosite at 500–600 ℃. It may be assumed that the following reaction takes place during heat treatment of jarosite:
The product after thermal decomposition was washed by two-step processing. Firstly washing in water to remove solid matter such as zinc sulfate, followed by washing in aqueous chloride solution (more than 4 mol/l) to remove lead sulfate. It was found that the iron content increased to ~50% after this treatment. Some Si, Zn, and Al were still present but the contents of other elements were very low.
Electrochemical Studies
Pure Fe2O3
Experimental results have been obtained previously and presented in publications [3,4,5,6,7,8]. High purity iron was deposited on rotating disk electrodes of graphite and silver at current densities from 0.1–0.6 A/cm2 in aqueous NaOH (50 wt%) at 110 °C. The current efficiency for iron deposition was consistently higher than 90% and independent of rotation rate above 100 rpm. It can be estimated that such a process for producing iron will have an energy consumption of ~3 kWh/kg Fe for the electrolysis process.
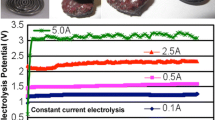
Figures 2 and 3 show typical results from an electrolysis experiment using a silver rotating cathode. The current efficiency determined from the weight increase of the cathode was found to be 95.0%. Smooth iron deposits were obtained on silver. Nickel was found to be an excellent anode material for oxygen evolution.
Iron Residue from Ni Production
Results from cyclic voltammetry at 10 mV/s are given in Fig. 4. A graphite rotating disc electrode (RDE) was used as the working electrode, and its rotating speed was 500 rpm. A mechanical stirrer at 100 rpm was placed in the electrolyte. Using a suspension of particles, the content of iron residue was defined by the ratio of solid (iron residue) to liquid (NaOH aqueous solution at 110 °C) [8], the value being equal to 1:90 g. The anodic current peak near −0.9 V (versus the reference electrode) is due to the oxidation of iron.
At higher proportions of solid particles, it was found that some nickel co-deposited with iron. Constant current electrolysis was carried out at 0.6 A/cm2. The cathode potential was found to be less negative when the content of solid particles increased. The deposit contained a small amount of nickel and no oxide. However, the current efficiency for iron deposition was only 7%. Further treatment to reduce the content of Ni and Co may be beneficial for improving the current efficiency.
Iron Residue from Zn Production
Figure 5 shows voltammetry obtained with a cavity electrode containing jarosite waste. Cathodic peaks are mainly due to iron deposition, but also oxygen reduction may contribute. In the anodic direction, oxidation of deposited iron may take place in two steps by the formation of Fe(II) as an intermediate. Oxidation of produced hydrogen may also take place.
Electrolysis was carried out by applying a constant cell voltage of 1.6 V for 4 h. Some metallic iron was detected in the deposit, but also iron oxide and lead were present. It shows that the efficiency of the iron deposition process depends very much on the purity of the iron oxide raw material.
Conclusions
Iron can be deposited by electrodecomposition of solid Fe2O3 particles suspended in a strong alkaline aqueous electrolyte of 50 wt% NaOH at ~100 °C. Pure oxygen is evolved on the anode. The current efficiency is normally above 90%, and the corresponding energy consumption is estimated to be ~3 kWh/kg Fe for the electrolysis process. Iron-rich waste materials from the electrowinning process for nickel and zinc can be purified and used as raw materials for electrodeposition of iron in a similar way. So far the efficiency of these processes is much lower due to the content of impurity elements.
References
Abdul Quader M, Ahmed S, Dawal SZ, Nukman Y (2016) Renew Sust Energ Rev 55
Yuan B, Kongstein OE, Haarberg GM (2009) Electrowinning of iron in aqueous alkaline solution using a rotating cathode. J Electrochem Soc 156:D64–D69
Yuan B, Haarberg GM (2009) Electrodeposition of iron in aqueous alkaline solution: an alternative to carbothermic reduction. ECS Trans 16:31–37
Allanore A, Lavelaine H, Valentin G, Birat JP, Lapicque F (2008) Iron metal production by bulk electrolysis of iron ore particles in aqueous media. J Electrochem Soc 155:E125–E129
Allanore A, Lavelaine H, Valentin G, Birat JP, Delcroix P (2010) Observation of the reduction of iron oxide particles to metal in alkaline solution by electrolysis. Electrochim Acta 55:4007–4013
Tang SH, Haarberg GM (2010) Electrowinning of iron from alkaline solution. ECS Trans 28(6):309–315
Haarberg GM, Khalaghi B (2020) Electrodeoxidation of iron oxide in aqueous NaOH electrolyte. ECS Trans 97(7):493–503
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Haarberg, G.M., Qin, B., Khalaghi, B. (2022). Electrochemical Reduction of Iron Oxides in Aqueous NaOH Electrolyte Including Iron Residue from Nickel and Zinc Electrowinning Processes. In: Ouchi, T., et al. Rare Metal Technology 2022. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92662-5_33
Download citation
DOI: https://doi.org/10.1007/978-3-030-92662-5_33
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92661-8
Online ISBN: 978-3-030-92662-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)