Abstract
Millions of tons of iron precipitation residues, predominantly jarosite, are accumulating in the primary zinc or primary precious metals industry every year. Regardless of environmental concerns the material is land filled in almost any case, although valuables such as indium, silver, gold, nickel, or zinc are present in considerable amounts. Within the presented research, CO2-optimized multi-metal recovery from the residue jarosite by means of a selective chlorination extraction has been evaluated not only by executed experiments but also by a multidimensional simulation of possible process parameters, utilizing a Python algorithm in combination with automated FactSage process step simulation. This allows a simultaneous iteration of relevant reaction parameters such as temperature, pressure, stoichiometry, or variation of additives and with this offers a high degree of freedom in the choice of evaluated reactants. The paper will outline a selection of possibly recovered special metals and the best choice of additives and process parameters.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Selective chlorination
- Jarosite
- Iron precipitate
- Nickel industry
- Zinc industry
- Silver
- Zinc
- Lead
- Indium
- Nickel
Introduction
Throughout the nonferrous industry several examples are known where the element iron must be removed in course of the production of the main metal. In most cases, it forms or is a component of a generated residue, such as red mud from aluminium industry, fayalitic slag from copper industry, or precipitation residues from zinc or also nickel industry. While slags can find their utilization in various fields, such as building material, as sand blasting agent, or substitutes in cement clinker, the sludges obtained during hydrometallurgical precipitation processes are subject to be are often sent to landfill.
A common method to precipitate iron from sulphatic solution is the jarosite process, named after its formed mineral during the removal step [11]. This method is applied, next to other possible forms of precipitated mineral structure such as goethite or hematite, in the hydrometallurgical zinc production or also after the leaching of the nickel matte in course of the nickel and precious metals production [1]. Aside of the separation of iron as a basic iron sulphate also other present metals can be co-precipitated or adsorbed on the iron product and consequently removed from the remaining sulphatic solution. It is documented that an incorporation of valuable elements, such as indium, silver, nickel, or also arsenic and cobalt into the jarosite structure, can occur in the precipitation product [3, 4, 6, 8] in the same way as in naturally occurring jarosite minerals [2]. As a consequence, valuable and technologically important metals are lost, due to the dumping of such process residues.
Several strategies were investigated in the past to face increasing needs regarding ecological and economical improvements of the current situation, including concepts for immobilizing hazardous elements, recovering valuable elements, and minimizing the residual amount by utilizing the precipitate, for instance, as a substitute material in the building industry [7]. Till today only the jarofix process gained industrial scale but represents only an immobilizing strategy without any recovery of valuable elements.
Based on a previous pyrometallurgical investigated concept ([13], Wegscheider und Steinlechner) for the recovery of valuable metals from iron precipitation residues by mixing with electric arc furnace dust as a chlorine source and carbon as reducing agent, the present paper describes a concept, which waived the use of carbon as a reducing agent and therefore represents a CO2-optimized recovery strategy for the recovery of selected valuable elements. The approach followed is a selective chlorination of specific metals, while leaving behind the main constituent of jarosite, the iron compounds. Therefore, the thermochemical behavior that iron chlorides are only moderately stable compared to silver or indium chloride is fundamental.
Materials and Methods
The investigated materials from both zinc and nickel industries are iron precipitates in the form of a jarosite mineral, generated in course of an iron extraction from sulphatic process solution. While the adherent or co-precipitated elements are differing, the general mineral formular for both is the same, which is XFe3(SO4)2(OH)6•mH2O.
The basic iron sulphate has a monovalent cation on the X-position, which can be represented by K+, Na+, NH4+, Ag+, ½ Pb2+, and H3O+, for instance. Similar than in nature also the precipitate forms different kinds of jarosite depending on what cation is added or what impurities are present in the process solution.
The investigated jarosite from zinc industry represents mainly a sodium and potassium jarosite with a small rest amount of plumbo- and hydronium-jarosite. The one from nickel industry was precipitated as ammonium jarosite indicated by the absence of sodium and potassium, which can be seen in Table 1 summarizing the main elements from elemental analysis.
The related XRD plots summarizing the phases in the material are shown in Fig. 1, where (a) is the jarosite from zinc industry and (b) the one from nickel industry.
It is known from literature [6], Wegscheider und Steinlechner [5], that a thermal treatment leads to a stepwise decomposition of the jarosite structure, which was proved by own investigations carried out at a Netzsch STA 409 Pc under inert as well as oxidizing atmosphere for both materials. Figure 2 shows the result for the jarosite from nickel industry under inert (Argon) atmosphere, which was heated up to 1000 °C. Typically, three main steps can be observed, which partly vary in their temperature range because of different occurring jarosite types [7].
However, the evaporation of moisture can be observed below 200 °C, the following split and removal of OH-group follows up to 450 °C and between 600 and 850 °C the SO3 is removed [10], illustrated by Eqs. 1, 2 and 3 exemplarily for a sodium jarosite. This in parallel leads to a release of incorporated elements in the jarosite structure, such as indium sitting on the iron position or silver as well as lead incorporated at the cation position. Also, other reactions of decomposition can occur in parallel, which then overlay the jarosite decomposition.
The decomposition of the jarosite structure forms the base for a number of accessible valuable side elements for the investigated selective chlorination process. The chlorination reactions take place without any addition of carbon as reducing agent which entails a significant reduction of the process greenhouse gas emissions compared to other research concepts.
The target of the present investigation is the evaporation of valuable elements from a jarosite material as a result of the formation of a volatile chloride compound. Furthermore, the iron should be left behind to form a high Fe-containing side product next to the collected valuable metal chloride dust.
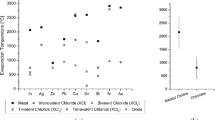
Due to the number of possible process parameter combinations and the aim to determine the optimum reaction mixtures, parameters, and influencing factors, the thermochemical software package FactSage with its integrated Equilibrium module was used together with a Python algorithm for data evaluation [9]. Non-chloride-containing additives, atmospheres, chlorine carriers, treatment temperatures, and stoichiometry factors were evaluated. The calculation assumes a thermal decomposed jarosite as starting condition. For the easier evaluation of the best conditions for a high extraction of valuables, a so-called Valex-factor was introduced, representing a monetarily weighted extraction rate of all available valuable metals, illustrated in Fig. 3 by the solid green circles. Additionally, the loss of iron to the gas phase was calculated, represented by the solid red circle. The black line represents a theoretical extraction of 100%.
Influence of temperature and chlorine stoichiometry on valex (green) and iron extraction (red) for a jarosite from zinc industry, the black circle represents a value of 1.00 [9] (Color figure online)
The parameter study in FactSage [9] showed that a temperature above 800 °C is required to obtain a satisfying valuable metal extraction yield by chlorination. It further was shown that trivalent chlorides have a better performance than monovalent or bivalent chlorides.
To verify the theoretical results, lab-scale experiments were performed. AlCl3 and MgCl2 were selected for further investigations. The design of experiment is shown in Table 2.
Summarizing the experimental procedure twofolds, fourfolds, or sixfolds, the calculated stoichiometric amount of the required chlorine compound, either AlCl3 or MgCl2, were added to the dry jarosite material. In the case of jarosite from zinc industry, a treatment temperature of 900 and 1100 °C was investigated, while in the case of jarosite from nickel industry only 1100 °C was chosen. All used chlorides were technical laboratory quality and due to their stability in hexahydrate form.
For the jarosite from zinc industry, the required amount is based on a total chlorination of Zn, Pb, Cu, Ag, and In and additionally assumed 5% of the iron amount, as this chlorination reaction cannot be avoided completely although it is the least stable chloride. In the case of precipitate from nickel industry Ni and 5% of the iron content was used as a base for calculation.
The jarosite and chlorination agent were intensively mixed in a mortar and 80 g of mixture per experiment filled in a MgO refractory crucible. The crucible was then charged into a resistance heated furnace operated under oxidizing atmosphere (air), which was already pre-heated to the treatment temperature of 900 or 1100 °C. After a treatment time of 30 min, the hot crucible was removed from the furnace and cooled down. The mass loss was measured during the experiment with a scale linked to the bottom of the furnace. The final step was the calculation of extraction rates based on the chemical analysis of the original and treated material, considering the decreased mass of material after the experiment.
Results and Conclusion
Figure 4a–c shows the obtained extraction rates of the lab-scale experiments of Pb and Zn for the treated jarosite from zinc industry, for the addition of magnesium chloride and aluminium chloride, at 900 as well as 1100 °C. Deviating from the FactSage calculation, a temperature of 900 °C does not lead to a complete extraction of valuables, which might be a subject of not perfectly mixed reaction material or also due to imperfect conditions leading to kinetic effects. As can be seen in the left segments in Fig. 4a, b, the extraction of iron in trials with AlCl3*6H2O is not given as it could not be qualitatively confirmed by analytical fluctuations. The values fluctuate around the value 0, it can therefore be stated that the extraction of iron was very low, compared to the experiments with MgCl2*6H2O. The increase of temperature in both cases of additives lead to a significant increase in the extraction of Zn and Pb. The iron loss increases with higher temperature and tendentially is higher in case of MgCl2*6H2O, at the same time the extraction rates of Zn and Pb in the case of magnesium chloride addition are significantly higher.
Figure 4d shows the result of the experiment with nickel jarosite at 1100 °C. Higher extraction of both iron and nickel was achieved when using MgCl2*6H2O compared to the addition of AlCl3*6H2O, similar to the results with material from zinc industry. The highest nickel extraction (45%) was achieved using the sixfold of the stoichiometric ratio.
a-c Extraction rates for Zn, Pb, and Fe for the experiments with jarosite from zinc industry at 900 and 1100 °C for a two times, b four times, and c six times stoichiometric addition of chlorine carrier; d Extraction rates for Ni and Fe for the experiments with jarosite from nickel industry at 1100 °C for two to six times stoichiometric addition of chlorine carrier
Comparing the results of zinc and lead with the nickel extraction rate it is obvious that the extraction via selective chlorination is more efficient at given conditions for zinc and lead.
Considering the theoretical achievable iron content of the remaining iron compound (treated jarosite) also an iron chloride addition is meaningful from the point of view of no new introduced impurity, which limits a possible utilization. Also, the low stability of iron chloride compared to the other targeted formed valuable metal chlorides and its trivalent state promise good extraction yields but must be proofed by an additional campaign.
References
Davenport WG (2011) Extractive metallurgy of nickel, cobalt, and platinum group materials. Elsevier, Amsterdam
Desborough GA, Smith KS, Lowers HA, Swayze GA, Hammarstrom JM, Diehl SF et al (2010) Mineralogical and chemical characteristics of some natural jarosites. In: Geochimica et Cosmochimica Acta 74 (3):1041–1056. https://doi.org/10.1016/j.gca.2009.11.006
Dutrizac JE (1984) The behavior of impurities during Jarosite Precipitation. In: Renato G. Bautista (Hg.): Hydrometallurgical process fundamentals. Tre behavior of impurities during jarosite precipitation. Boston, MA: Springer US, S. 125–169
Dutrizac JE, Chen TT (2013) The behaviour of gallium during jarosite precipitation. Canadian Metallurgical Quarterly 39(1):S. 1–14. https://doi.org/10.1179/cmq.2000.39.1.1
Frost RL, Wills RA, Kloprogge JT, Martens WN (2006) Thermal decomposition of hydronium jarosite (H3O)Fe3(SO4)2(OH)6. J Therm Anal Calorim 83 (1):S. 213–218. https://doi.org/10.1007/s10973-005-6908-0
Frost RL, Wills RA, Weier ML, Musumeci AW, Martens W (2005) Thermal decomposition of natural and synthetic plumbojarosites. Importance in ‘archeochemistry’. Thermochimica Acta 432 (1):S. 30–35. https://doi.org/10.1016/j.tca.2005.04.001
Hoeber L, Steinlechner S (2021) A comprehensive review of processing strategies for iron precipitation residues from zinc hydrometallurgy. Cleaner Eng Technol 4:S. 100214. https://doi.org/10.1016/j.clet.2021.100214
Pappu A, Saxena M, Asolekar SR (2006) Jarosite characteristics and its utilisation potentials. Sci Total Environ 359(1–3):S. 232–243. https://doi.org/10.1016/j.scitotenv.2005.04.024
Lerche R (2021) Evaluation of the mechanisms of CO2-optimised chlorination reactions during the multi-metal recovery from precipitation residues of the zinc industry. Master’s thesis. Montanuniversität, Leoben. Chair of Nonferrous Metallurgy
Steinlechner S, Antrekowitsch J (2018) Thermodynamic considerations for a pyrometallurgical extraction of indium and silver from a jarosite residue. Metals 8. https://doi.org/10.3390/met8030xxx
Sinclair RJ (2005) The extractive metallurgy of zinc. 1. ed. Carlton, Vic.: AusIMM (Spectrum series/Australasian Institute of Mining and Metallurgy, 13)
Wegscheider S, Steinlechner S (2016) Residues from zinc industry-a potential future resource for silver and indium. In: Proceedings of COM 2016
Wegscheider S, Steinlechner S, Leuchtenmüller M (2017) Innovative concept for the recovery of silver and indium by a combined treatment of Jarosite and electric arc furnace dust. JOM 69(2):S. 388–394. https://doi.org/10.1007/s11837-016-2192-7
Funding
This work was funded by the Christian Doppler Research Association; the Austrian Federal Ministry for Digital and Economic Affairs; and the National Foundation for Research, Technology and Development.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Steinlechner, S., Höber, L. (2022). CO2-Optimized Recovery of Special Metals from Precipitation Residue by Selective Chlorination. In: Ouchi, T., et al. Rare Metal Technology 2022. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92662-5_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-92662-5_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92661-8
Online ISBN: 978-3-030-92662-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)