Abstract
In the present study, carbon-bonded alumina filters, normally used for the filtration of steel melts, have been investigated as a potential filter material for the filtration of aluminum. Short- and long-term pilot-scale filtration trials were conducted, and the filter behavior during filtration of aluminum alloy was determined by the use of Porous Disk Filtration Apparatus (PoDFA) for the short-term trials (with the casting alloy AlSi7Mg) and Liquid Metal Cleanliness Analyzer (LiMCA) for the long-term trials with wrought alloy 6xxx aluminum. All applied filters were also investigated after the filtration trial by Scanning Electron Microscopy (SEM) analysis. Furthermore, sessile drop experiments with capillary purification were performed to evaluate the wetting behavior as well as any reactions occurring between the filter material and the aluminum alloy being filtered.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
The application of ceramic foam filters is one of the state-of-the-art procedures for the removal of non-metallic inclusions (particles and films) from aluminum melt, which is important as the inclusions cause a reduction of the tensile strength and a sudden failure [1, 2]. Typically, silicon carbide (SiC) and alumina (Al2O3) filters are used. Recently, Voigt et al. [3] considered carbon-bonded alumina filter (Al2O3–C) as a new filter material for aluminum melts and obtained promising results regarding the filtration behavior.
Sessile drop measurements at 730 °C with capillary purification procedure showed a strong non-wetting behavior between aluminum and the Al2O3–C substrate and resulted in the roll-off of the aluminum droplet from the Al2O3–C substrate so that an interaction between the aluminum and Al2O3–C could not take place. Sessile drop measurements without capillary purification procedure, in contrast, require temperatures >950 °C for the removal of the oxide skin necessary for a reasonable measurement. However, the temperature of 950 °C (220 K higher than the temperature used for the filtration trials) entails further chemical reactions which cannot occur at lower temperatures during filtration [3].
Results from short-term filtration trials [3] show lower PoDFA values for Al2O3–C filters in comparison with Al2O3 reference filters standing for a better filtration behavior of the Al2O3–C filter material.
In this study, a modified sessile drop test setup with a capillary purification was used to achieve a lasting interface between aluminum and Al2O3–C for the microstructural investigations by means of scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDX). The aluminum filtration behavior of Al2O3–C filters was investigated with long-term filtration trials at a pilot filtration line whereby the filtration efficiency was measured by a liquid metal cleanliness analyzer (LiMCA).
Materials and Methods
Preparation of Ceramic Foam Filters and Substrates
For the preparation of alumina skeleton filters, the replica process using polyurethane foams was applied [4]. For the short-term filtration trials, skeleton filters in the form of square cuboids with dimensions of 50 × 50 × 20 mm3 and 20 ppi (pores per inch) were utilized. In the long-term filtration trials, skeleton filters in the form of a square frustum with a top base of 178 × 178 mm2 (aluminum melt inlet), a bottom base of 150 × 150 mm2 (aluminum melt outlet), a height of 48 mm, and 30 ppi were used.
The sintered alumina skeletons were coated with Al2O3–C slurry (see Table 1) using a combined dipping and centrifugation procedure (removal of excess slurry). After drying, the filters were coked at 800 °C in a steel retort filled with pet coke to provide a reducing atmosphere for 20 h. For the preparation of reference filters, the alumina skeleton foams were coated with an Al2O3 slurry (see Table 2) in order to obtain similar macro pore sizes as for the Al2O3–C filters. The Al2O3 reference filter was sintered at 1600 °C in air atmosphere.
The substrates for the sessile drop test were slip cast to cylindrical samples with a diameter of 55 mm and a height of 5 mm with the help of a mold consisting of a plastic ring and a plaster bottom. The composition of the substrate slurries as well as their thermal treatment followed the same pattern as for the coated filters; see Tables 1 and 2 for more details.
Sessile Drop Measurements
Sessile drop tests with aluminum are a challenging task due to the affinity of aluminum to oxygen and thus the formation of an oxide skin which alters the results of the contact angle measurements [6, 7]. Therefore, removal of the oxide skin is necessary, and can be achieved by a capillary purification method [7] or an improved sessile drop method [8]. In this study, a simple sessile drop setup with a dropping unit made of boron nitride (Henze Boron Nitride Products AG, Germany) was used [9]. The dropping unit consists of a hopper for the melting of aluminum and a steel plunger pulling the melted aluminum through a bottleneck to retain the oxide skin. The hopper filled with a cylindrical piece of Al99.7 (Rheinfelden, Germany) was placed on the substrate and positioned in the hot stage microscope. The kiln was heated with 10 K/min to a temperature of 730 °C with a holding time of 10 min. During thermal treatment, the kiln chamber was flushed with argon to minimize the oxygen level.
The substrate/Al couple was weighted after the cooling down and with the help of the mass of the ceramic substrate before the experiment, the droplet weight was calculated. The droplet weight was between 300 and 600 mg, which is significantly higher than typical droplet weights (<100 mg) for conventional sessile drop tests [3, 6]. Considering the measured contact angles, it has to be kept in mind that this setup produces droplets with a high weight which is necessary such that the dropping can take place. The higher droplet weight influences the contact angle due to the higher gravity of the droplet. Furthermore, the dropping temperature cannot be precisely adjusted due to the missing of a mechanical trigger leading to significant differences in the reached dropping temperatures and low temperatures (<700 °C). However, the primary goal for using this sessile drop setup was to enable the formation of the interface between Al2O3–C and aluminum, and not to measure the contact angle between Al2O3–C.
To reach an Al/Al2O3–C couple, a small deepening was introduced in the substrate by grinding to hinder the droplet to roll off.
After the sessile drop measurements, microsections of the substrate/Al couples were made to investigate the interface between the substrate material and solidified aluminum by means of the scanning electron microscope (SEM) Philips XL 30 (FEI, Eindhoven, Germany) equipped with an energy dispersive X-ray spectroscopy (EDX) device (Phoenix, USA).
Short-Term Aluminum Filtration Trials
The short-term filtration trials were conducted at the metal foundry Georg Herrmann Metallgiesserei (Muldenhütten, Germany) with the aluminum alloy AlSi7Mg (EN AC-42100) from Rheinfelden Alloys (Rheinfelden, Germany) whereby the melt comprised 50% ingots and 50% scrap (recycled aluminum consisting of solidified feeders and runners) for the introduction of non-metallic inclusions to the melt. AlSi7Mg alloy was melted, skimmed, and cast into a sand mold consisting of a joint sprue, four horizontal runners with filter chambers, and vertical steel molds [3]. The used filters were cut out and embedded with epoxy resin, ground, polished, and analyzed with SEM/EDX. The aluminum in the steel mold was separated from the feeder and then analyzed regarding non-metallic inclusions with the help of a cold Porous Disk Filtration Apparatus analysis (PoDFA) performed by HOESCH Metallurgical Service (Niederzier, Germany).
Long-Term Aluminum Filtration Trials
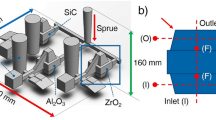
The long-term filtration trials were conducted in a pilot filtration line at Hydro Aluminium AS primary aluminum plant (Sunndal, Norway), which consists of a melting furnace, a launder system, a filter box, and a lifting pump. The line is equipped with two LiMCA II units (ABB Ltd., Canada), which allow the continuous monitoring of inclusion number and size in the front and behind the filter box. As the filter box is designed for 584 mm filters while only 178 mm Al2O3–C filters were producible for the filtration tests, a refractory adapter holding the four similar 178 mm filters was used. In order to avoid floating of the filters during the inverse priming procedure [10], all four filters were weighed down with a steel adapter. Before the inverse priming started, the filters were preheated using a hot air blower positioned below the filters.
For every trial, about 8 mT wrought aluminum alloy 6082 (main alloying elements of Si ~ 0.95%, Fe ~ 0.2%, Mn ~ 0.6%, and Mg ~ 0.65%) was transferred to the melting furnace and circulated in the loop with the help of the lifting pump. For the introduction of inclusions every 10 min, 4 kg of chips (made of compacted aluminum sawdust) were added behind the lifting pump. Altogether, two long-term filtration trials were conducted: one trial with a reference filter (labeled Al2O3) and one trial with carbon-bonded alumina filters labeled as Al2O3–C. The aluminum melt temperature at the priming procedure was 740 °C for both trials.
The employed filters were cut out and embedded in epoxy resin, ground, polished, and analyzed with SEM/EDX.
Results
Analysis of Ceramic Foam Filters
The filters and the substrates were prepared and examined whereby the structure of the Al2O3–C coating was examined by SEM, and the chemical composition was measured by EDX (which will be presented in “Long-Term Aluminum Filtration Trials”). A SEM micrograph of a virgin filter for the long-term filtration shows a heterogeneous microstructure of the Al2O3–C coating evoked by the different grain size distributions of the carbon raw materials (Fig. 1).
Sessile Drop Measurements
The contact angles determined by means of the above-described setup possess discrepant results (Table 3) compared to the measurements at 730 and 950 °C performed by Voigt et al. [3]. The underlying causes for the differences are manifold: The intended flat deepening on the part of the substrate, a relative high mass of the aluminum droplet as well as a low dropping temperature of <700 °C. The contact angles measured using sessile drop measurement with capillary purification at 730 °C [3] are therefore more reliable.
After cooling to room temperature, preliminary tests revealed no adhesion between the aluminum droplet and the Al2O3–C substrate. To hinder a premature release of the solidified aluminum droplet from the substrate during handling and/or sample preparation, a small amount of superglue was used.
The SEM investigation of the interface showed no adhesion of the Al droplet to the substrate but a pronounced interface/reaction zone in the Al2O3–C substrate (dark area in the center left); see Fig. 2b, c. EDX analysis of two marked zones in Fig. 2b showed only slight differences in the chemical composition, particularly regarding silicon and carbon. Within the reaction zone (EDX 2), the silicon peak is higher than in the unreacted zone (EDX 1). Conversely, the carbon peak is lower in the reacted zone (EDX 2) in comparison with the unreacted zone (EDX 1). The origin of the detected silicon is unknown as pure aluminum (99.7%) was used for the sessile drop measurements. It should be mentioned that the carbon detection with EDX is afflicted with an error due to the low atomic number of carbon. But the clear contrast in the SEM image taken in a backscattered electron mode (Fig. 2c) shows that there is a difference in the chemical composition of EDX 1 and EDX 2, although the difference in the height of the carbon peak is very small. The microstructural investigations by SEM revealed a similar amount and size of large pores (>5 µm) (Fig. 3). Otherwise, a different quantity of small pores (<1 µm) and hence a different polishing behavior due to the surface roughness were observed. This is approved by clear differences in surface morphology between the affected and unaffected zones in Fig. 2b.
Short-Term Aluminum Filtration Trials
The short-term filtration trial took approximately 16 s and showed an equal filling of the aluminum [3]. The PoDFA index (given in the area of inclusions per kilogram analyzed aluminum) yields the sum of total detected inclusions. The lower PoDFA index indicates a smaller number of non-metallic inclusions in the aluminum sample and hence the better is the melt quality. The PoDFA analyses found Al2O3 films, carbides, magnesium oxide, spinel, refractory material, iron and manganese oxides as well as grain refiners in the castings. The PoDFA index of 0.106 mm2/kg of the Al2O3–C filter sample was half of the PoDFA index of the Al2O3 reference filter sample with 0.246 mm2/kg. This indicates an improved filtration when using the Al2O3–C filter.
The SEM images of the used filters of the short-term filtration trials show large inclusions consisting of mainly Al, Mg, Si, and O captured by the filters. The structure of the Al2O3–C coating is comparable to Al2O3–C coating of the unused filter; see Figs. 1 and 4.
Long-Term Aluminum Filtration Trials
The N20 values of the LiMCA measurements of the Al2O3 and the Al2O3–C filter possessed a comparable behavior before and after the filter box. The N20 values before the filter box decreased at the beginning of the trial until they reached a relatively stable level. It should be noted that the stable area was shorter for the Al2O3–C trial due to an addition of 67 kg of extra chips for testing the filtration behavior with high inclusions load. The N20 level before the filter box increased with the addition of the chips, but an increase of the N20 level after the filter box is not observed.
The N20 values after the filter box showed a decrease at the beginning of the experiment which is a common behavior observed at other filtration experiments [11, 12]. It is obvious that the N20 values after the filter box possessed a higher standard deviation than the N20 values in the front of the filter. The N20 levels before and after the filter box are comparable for the Al2O3 and the Al2O3–C filtration trials. For the stable filtration areas, the filtration efficiency was calculated with 91.2 ± 6.7% (for the Al2O3 between 23.1 and 45 min) and 88.8 ± 12.3% (for the Al2O3–C between 15 and 25.9 min). So, the filtration behavior of the two trials is comparable (Fig. 5).
An intensive SEM investigation of the used filters revealed a few typical inclusions captured in the filter. Nearly, no Al2O3 films, carbides, magnesium oxide, spinel, and refractory material were found; see Fig. 6. Furthermore, a large number of iron-rich intermetallics were found due to the high iron content of the aluminum melt. The iron-rich intermetallic phases were often connected to the filter independent of the filter surface chemistry; see Fig. 6c. The affinity of iron-rich intermetallics to the filter surface would suggest that removal of iron with the help of filters could be effective.
Thus, results from the SEM investigations indicate that the addition of the compacted aluminum sawdust chips does not seem to be a successful way of introducing a comparable level of typical inclusion to the melt because no typical inclusions were detected in the filters. Upon closer examination, it becomes evident that the Al2O3–C coating possessed different structures (Fig. 6) which are not comparable to the unused filter in Fig. 1. Figure 6c shows a very homogenous, dense, and compact coating, and nearly no pores or larger carbon structures are visible. In contrast, Fig. 6d shows Al2O3–C coating with large pores. The large structures of the unused filter (Fig. 1) are not empty—they are filled with carbon raw materials, for example, graphite. No dependence on the location of the different structures in the filter was observed. Figure 6b shows both structures next to each other. It appears that only thinner areas of the coating have been affected by this phenomenon. EDX analysis showed that the denser the coating, the lower is the carbon peak (see Fig. 7). This observation implicates that in the denser structures, the carbon is partly disappeared and left behind pores (Fig. 6d) or a very dense structure made of mostly alumina particles (Fig. 6c). The latter is very surprising due to the high temperature needed to compact alumina grains (>1400 °C). The temperature during the filtration trials was not higher than 750 °C, i.e., significantly lower than the required compaction temperature. Further in-depth investigations towards the observations made in this study are necessary for more clarification (Fig. 7).
Conclusion
In the present study, carbon-bonded alumina filters, normally used for the filtration of steel melts, have been investigated as a potential filter material for the filtration of aluminum. Short- and long-term pilot-scale filtration trials were conducted.
The sessile drop trials with a simple dropping unit generated Al2O3–C/Al couples which were examined by SEM and EDX. Despite a missing adhesion between aluminum droplet and Al2O3–C substrate, a reaction zone with a thickness of around 500 µm next to the aluminum melt was observable.
The short-term trial showed a better filtration performance of the Al2O3–C filter compared to the Al2O3 reference filter. In contrast, the long-term filtration trials did not reveal the beneficial filtration effect of carbon-bonded alumina filters regarding the LiMCA results.
The extensive SEM investigations of the used Al2O3 and Al2O3–C filters did not detect a large amount of typical non-metallic inclusions in the solidified aluminum. Furthermore, the SEM analyses disclosed a partial transformation of the Al2O3–C coating layer from a heterogenous and porous layer into a dense and compact layer. EDX analyses revealed a correlation between the density and the carbon content of the Al2O3–C coating layer. The denser the layer, the lower was the carbon peak in the EDX analyses.
The missing improvement of the filtration behavior and the unstable structure of the Al2O3–C coating show that Al2O3–C (in its presented form) is not suitable for long-term filtration tasks. Further investigations towards the filtration behavior of Al2O3–C filters and the chemical and morphological alteration of Al2O3–C during filtration are necessary.
References
Campbell J (2003) Castings, Butterworth-Heinemann ISBN 0 7506 4790 6: 282–301
Samuel FH, Liu H, Samuel AM (1993) Effect of Melt Cleanliness on the Properties of an Al-10 Wt Pct Si-10 Vol Pct SiC(p) Composite. Metallurgical Transactions A 24A: 1631–1645
Voigt C, Hubálková J, Zienert T, Fankhänel B, Stelter M, Charitos A, Aneziris CG (2020) Aluminum Melt Filtration with Carbon Bonded Alumina Filters. Materials 13: 20203962
Voigt C, Zienert T, Schubert P, Aneziris CG, Hubálková J (2014) Reticulated porous foam ceramics with different surface chemistries. J. Am. Ceram. Soc. 97, 7: 2046–2053
Emmel M, Aneziris CG (2012) Development of novel carbon bonded filter compositions for steel melt filtration. Ceramics International 38: 5165–5173
Eustathopoulos, N; Nicholas MG; Drevet B (1999) Wettability at High Temperatures, Pergamon; ISBN: 9780080421469
Sobczak N, Singh M, Asthana R (2005) High-temperature Wettability in Metal/Ceramic Systems – Some Methological Issues. Current Opinion in Solid State and Materials Science 9, 241-253
Shen P, Fujii H, Matsumoto T, Nogi K (2004) Critical Factors Affecting the Wettability of α-Alumina by Molten Aluminum. Journal of the American Ceramic Society 87 7:1265-1273
Malczyk P, Zienert T, Kerber F, Weigelt C, Sauke SO, Semrau H, Aneziris CG (2020) Corrosion-Resistant Steel–MgO Composites as Refractory Materials for Molten Aluminum Alloys, Materials, 13 4737
Bao S, Syvertsen M, Syvertsen F, Gihleengen BE, Tundal U, Pettersen T (2019) Laboratory Scale Study of Reverse Priming in Aluminium Filtration, Light Metals 2019, 1105–1111
Voigt C, Jäckel E, Taina F, Zienert T, Salomon A, Wolf G, Aneziris CG, Brun P (2017) Filtration efficiency of functionalized ceramic foam filters for aluminum melt filtration. Metall Mater Trans B 48B: 497-505
Syvertsen M, Kvithld A, Bao S, Nordmark A, Johansson A (2014) Parallel Laboratory and Industrial Scale Aluminium Filtration Test with Al2O3 and SiC Based Filters, Light Metals 2014, 1041-1046
Acknowledgements
The authors thank the German Research Foundation (DFG) for supporting these investigations as part of the Collaborative Research Centre 920 “Multi-Functional Filters for Metal Melt Filtration—A Contribution towards Zero Defect Materials” (Project-ID 169148856) sub-projects A02 and S01. The authors also acknowledge the support of Pyrotek.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Voigt, C. et al. (2022). Short- and Long-Term Aluminum Filtration Trials with Carbon-Bonded Alumina Filters. In: Eskin, D. (eds) Light Metals 2022. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-030-92529-1_82
Download citation
DOI: https://doi.org/10.1007/978-3-030-92529-1_82
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92528-4
Online ISBN: 978-3-030-92529-1
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)