Abstract
Polymeric hydrogels are soft and hydrophilic materials of great interest for the engineering of soft tissues, as mimicking structures of the extracellular matrix; because of their 3D architecture and biological properties constitute an adequate environment for cells function. Biopolymers like collagen and hyaluronic acid have a good biological response but the mechanical characteristics make necessary the crosslinking of the networks in order to build resistant and easily workable structures. The paper presents preparation of new hydrogels based on methacrylated collagen and methacrylated hyaluronic acid/hyaluronic acid as natural-based networks with controlled morphology and biointeractivity. FTIR spectra confirmed the proposed structure and the scanning electron microscopy data indicated a porous morphology dependent of the ratio between polymers. The prepared hydrogels are highly swellable, bioadhesive and degradable by enzymes. In vitro cytotoxicity tests revealed a very good material response in contact with cells (fibroblasts).
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Collagen (Col) is the major component of the extracellular matrix that performs a number of functions in tissues, such as: maintaining the biologic and structural integrity; binding, storage and release of growth factors and cytokines, having an important role in tissue development and regeneration. Based on its excellent biocompatibility collagen is one of the most used biomaterials in soft tissue applications, as matrices for tissue regeneration, supports for the controlled release of active principles, implants etc. [1,2,3].
Along with collagen, hyaluronic acid (HA) is the basic substance of connective tissue, the extracellular matrix and the cytoskeleton. The chemical and stereometric structure of the molecule, high molecular weight, high viscosity of the solution, elasticity, hydrophilicity, the presence on the cell membrane of specific receptors determine the biological qualities of hyaluronic acid. HA has various functions, participating in the embryogenesis and morphogenesis, intercellular relationships and communication, mechanical resistance of tissues, water retention, transport and metabolism of substances, permeability of biological membranes [4, 5]. HA is used for skin protection, the formation of semipermeable barriers in the treatment of eczema and trophic ulcers. HA is widely used in wound healing, cosmetology - as an emollient in the composition of creams, lotions, gels. Various 3D networking scaffolds based on collagen and hyaluronic acids have been developed and the characteristics of the both biopolymers were combined in order to form adequate artificial ECM. The lower mechanical properties and fast degradability of the obtained structures required methods for crosslinking and physical or chemical processes were involved for preparing three-dimensional matrices with both physical-chemical and biological performances (support for cells infiltration into scaffold, their attachment, proliferation, and differentiation) [6, 7]. The product resulting from these interactions has a better ability to adhere to mucosal surfaces, an ideal feature for the treatment of an affected area.
The paper presents the obtaining and characterization of 3D scaffolds based on methacrylated collagen (ColMa) and methacrylate hyaluronic acid (HAMa)/hyaluronic acid and the materials architecture and properties have been compared in order to form EMC mimicking architectures.
2 Materials and Methods
2.1 Materials
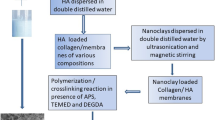
The hydrogels were obtained by using solution 0.5% (wt/vol, double distilled water) of methacrylate collagen (Cellink, Switzerland) and methacrylated hyaluronic acid/hyaluronic acid solution (0.5%, wt/vol, double distilled water). Hyaluronic acid was modified with methacrylic anhydride using the protocol described in [8]. Methacrylic anhydride (MA), initiating system (ammonium persulfate-APS, N, N, N’, N’-tetramethylethylene diamine - TEMED) were purchased from Sigma Aldrich. APS was purified by recrystallization from a mixture methanol/water (70/30, vol/vol).
For the citocompatibility tests, the following materials were used (Sigma Aldrich): MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide); Hank’s Balanced Salt Solution (HBSS); Dulbecco’s Modified Eagle Medium (DMEM) as culture medium; Penicillin-Streptomycin-Neomycin (PSN) and fetal bovine serum (FBS), sterile, suitable for cell culture, May-Grünwald and Giemsa staining solutions. All other chemical have been used as received.
2.2 Preparation of Hydrogels
Two types of hydrogels based on collagen modified with methacrylic anhydride (MA) have been prepared; in the first one modified hyaluronic acid (HA) with methacrylic anhydride was added and, respectively, in the second one the pure hyaluronic acid was used for scaffolds preparation. Briefly, a homogeneous mixture of methacrylated collagen and methacrylated hyaluronic acid/hyaluronic acid was adjusted to pH = 8, by adding 1N NaOH, and then an initiating system was added (ammonium persulphate (APS) and tetramethylethylenediamine (TEMED)), in the appropriate proportions, (1% mol/mol, reported to the methacrylate group) and stirred until a homogenous system was obtained. The mixtures were places in culture plates and exposed to ultraviolet radiation (UV-365 nm) for 10 min, washed with double distilled water and finally freeze-dried (−51 °C, 0.08 mBa). The hydrogels composition is presented in the Table 1.
2.3 Structural and Morphological Characterization
The Fourier transform infrared spectra (FT-IR) were registered on dried hydrogels using a spectrophotometer (Bio-Rad Win-IR instrument, USA) by scanning within the range of 400–4000 cm−1, 16 scans at a resolution of 4 cm−1. Gold coated cross-sections of hydrogels were examined using scanning electron microscopy – SEM (Tescan Vega SBH II, Czech Republic) at an accelerating voltage of 30 kV.
2.4 Swelling Properties, Bioadhesion and in Vitro Degradability
The swelling study was performed in phosphate buffer solution (PBS, pH = 7.4) and simulated wound exudate (SWE, pH = 7.4), at 37 °C, using the volumetric method; the swelling degree (SD, %) was calculated with the Eq. 1:
where Wo is the weight of the dry hydrogel, Wt is the weight of the swollen hydrogel and SDeq is the swelling degree at equilibrium. The in vitro degradation properties were evaluated by incubating the hydrogels with a collagenase solution (1 × 10–2%, in PBS solution, pH = 7.4, at 37 °C) and the spectrophotometrically monitoring (reaction with ninhydrin reagent - 420nm). The bioadhesion tests were performed on a TA.XT Plus® analyser (Stable Micro Systems - Godalming, UK) on a hydrated dialysis tubing membrane (cellulose, Visking DTV14000), in PBS (pH 7.4), at 37 °C.
2.5 In Vitro Cytotoxicity Tests
In vitro evaluation of citocompatibility (normal fibroblasts from albino rabbit) was also performed by the direct contact method, supported by morphological analysis of cell culture [9]. The cells were cultured in DMEM with 10% FBS and a mixture of penicillin, neomycin and streptomycin (1%), at 37 °C, in a humidified atmosphere of 5% CO2. A number of 1 × 104 cells per well in a 24-well culture plate was maintained in contact with each hydrogel (sterilized by exposure to UV light for 20 min and then incubated for 24 h in DMEM). Four parallel samples from each hydrogel were put in direct contact with the cell culture in the wells. For reference purpose, cells were seeded in the same condition. At 24, 48 and 72 h the MTT assay was performed. The formazan resulted was detected by a Tecan Sun-rise Plate Reader (570 nm). The cell viability was calculated by the following equation:
where Ah is absorbance of samples and Ac is control absorbance. For cells morphology analysis a double staining with May-Grünwald solution and Giemsa solution was performed.
3 Results and Discussions
3.1 Hydrogel Structure and Morphology
The main markers for collagen identification are amides A, I and II (Fig. 1). Amide A can be observed in the absorption zone with wavelengths between 2800 and 3500 cm−1, with the peak located at the value of 3437.3 cm−1 which indicates the presence of N-H type bonds. Another peak at a wavelength of 1647.0 cm−1 and 1643.9 cm−1, respectively, indicates the presence of the C = O double bonds representing the absorption zone of amide I. Amide II can also be found near this zone, having the peaks at 1558.7 cm−1 and 1560.1 cm−1.
Regarding the peaks 1030.2 cm−1 and 1042.4 cm−1, respectively, which are relatively isolated from the areas mentioned above, it can be noted that their wavelengths correspond to the simple C-O and C-O-C connections. The band at 1413 cm−1 is attributed to the stretching of COO −, which refers to the acid group of molecule HA [10].
Following the SEM analysis, it was possible to observe the structural differences of the prepared hydrogels: both hydrogels, with methacrylated HA (Fig. 2a) and pure HA (Fig. 2b) have a porous morphology and the predominant appearance was the nanofiber. The difference between the two is given by the accentuated uniform distribution of pores in the ColMa-HA material, while the ColMa-HAMa material did not show this homogeneity, being mostly fibrous.
3.2 Swelling Characteristics, Bioadhesion and in Vitro Degradability
The prepared hydrogels swell extensively in PBS and the highest values for the swelling degree at equilibrium were recorded for the material containing collagen-Ma and HA (modified or not with Ma) with an increased content of polysaccharide; as was expected the hydrophilic groups from HA improved water interactions with in the 3D network. In SWF, the highest value for SD was measure for the hydrogel ColMaHA2. The swelling behavior is an important characteristic for hydrogels and their capacity to absorb fluids is due to morphology, such as the network porosity, and due to chemical structure and the presence of hydrophilic groups such as carboxyl (-COOH), hydroxyl (-OH), amine(NH2) amides (-CO-NH-, -CO-NH2) from collagen and hyaluronic acid [10]. The bioadhesion tests revealed that all hydrogels are bioadhesive and hyaluronic acid improved this property.
Biodegradation is an essential feature for hydrogels with applications in tissue engineering. In vitro degradation studies of the obtained supports demonstrated their tendency to lose structural integrity in the prepared degradation solution (0.01% collagenase, pH 7.2). In the graphical representation in Fig. 3 can be observed that the highest values were found for the material ColMa-HAMa with a ratio of 6:1. Although collagen provides strength and stability to the mixtures in which it is found, the values of these traits increasing in direct proportion to its concentration in the product, in this case, the main difference was the change with the presence or absence of MA in hyaluronic acid.
3.3 In vitro citocompatibility
Biocompatibility is an important feature for materials designed to be used in various engineering applications. In this sense, the evaluation of cell viability by MTT method was performed in order to determine the cytotoxicity of the obtained hydrogels. The study resulted in a value of cell viability that met current standards, both materials having viability of over 95%, both at 48 and 72 h of culture (Fig. 4). They were also close to the value of the control, at 48 h still showing a higher viability. These results pointed out that hydrogels are not cytotoxic, but they stimulate cell growth.
After staining the samples (Fig. 5), it was found that the cells had a normal morphology, specific to fibroblasts, some areas showing a tendency to populate the material.
It can be noticed that this tendency of the cells led to overpopulation, hence the pronounced shape and their high density, the materials representing an environment favorable to cell proliferation, comparatively better than control. Finally, the results of the microscopic analysis confirm those of the cytotoxicity tests by MTT method, both types of supports having a high citocompatibility with fibroblast cells.
4 Conclusions
In the present paper, methacrylated collagen and methacrylated hyaluronic acid were UV crosslinked and high swellable hydrogels in PBS and SWF fluids were obtained. The hydrogels are bioadhesive and degradable in presence of collagenase these properties being strongly dependent of the content of hyaluronic acid. The hydrogels are citocompatible for fibroblasts and stimulate their growth and proliferation.
References
Lai, V.K., Nedrelow, D.S., Lake, S.P., et al.: Swelling of collagen-hyaluronic acid co-gels: an in vitro residual stress model. Ann. Biomed. Eng. 44(10), 2984–2993 (2016)
Abdeen, A.A., Saha, K.: Manufacturing cell therapies using engineered biomaterials. Trends Biotechnol. 35, 971–982 (2017)
Dai, C., Shawn, S., Amor, K.: Skin substitutes for acute and chronic wound healing: an updated review. J. Dermatol. Treat. 31(6), 639–648 (2020)
Davidenko, N., Campbell, J.J., Thian, E.S., Watson, C.J., Cameron, R.E.: Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 6, 3957–3968 (2010)
Gonzalez, S.R., Wolter, K.G., Yuen, J.C.: Infectious complications associated with the use of integra: a systematic review of the literature. Plast Reconstr Surg Glob Open. 8(7), e2869 (2020)
Shahrokhi, S., Arno, A., Jeschke, M.G.: The use of dermal substitutes in burn surgery: acute phase. Wound Repair Regen. 22(1), 14–22 (2014)
Vig, K., Chaudhari, A., Tripathi, S., et al.: Advances in skin regeneration using tissue engineering. Int. J. Mol. Sci. 18(4), 789 (2017)
Spearman, B.S., Agrawal, N.K., Rubiano, A., Simmons, C.S., Mobini, S., Schmidt, C.E.: Tunable methacrylated hyaluronic acid-based hydrogels as scaffolds for soft tissue engineering applications. J. Biomed. Mater. Res. 108A, 279–291 (2020)
Stockert, J.C., Horobin, R.W., Colombo, L.L., Blázquez-Castro, A.: Tetrazolium salts and formazan products in cell biology: viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 120(3), 159–167 (2018)
Yousefi, F., Kandel, S., Pleshko, N.: Infrared spectroscopic quantification of methacrylation of hyaluronic acid: a scaffold for tissue engineering applications. Appl. Spectrosc. 72, 1455–1466 (2018)
Acknowledgment
This work was supported by a grant of the Ministry of Research, Innovation and Digitalization, CCCDI - UEFISCDI, Romania, “New hybrid polymer/peptide hydrogels as innovative platforms designed for applications in cell cultures (HYPCELGEL)”, project number PN-III-P2-2.1-PED-2019-2743, within PNCDI III.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Raicu, A., Cobzariu, I., Vasilache, A.L., Peptu, C.A., Butnaru, M., Verestiuc, L. (2022). New Hydrogels Based on Methacrylated Collagen and Hyaluronic Acid for Soft Tissue Engineering. In: Tiginyanu, I., Sontea, V., Railean, S. (eds) 5th International Conference on Nanotechnologies and Biomedical Engineering. ICNBME 2021. IFMBE Proceedings, vol 87. Springer, Cham. https://doi.org/10.1007/978-3-030-92328-0_48
Download citation
DOI: https://doi.org/10.1007/978-3-030-92328-0_48
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92327-3
Online ISBN: 978-3-030-92328-0
eBook Packages: EngineeringEngineering (R0)