Abstract
Healing of bony defects is a unique challenge due to the complex mechanical properties, irregular shapes, and critical size of missing bone. Bone defects that require surgery are typically caused by significant missing portions of bone and the methods of repair involve surgical implantation of a biomaterial. The gold standard for this repair is the use of autografts, but these can lead to bone morbidity. Alternatives include allografts and xenografts; however, multiple challenges associated with their use motivate tissue engineering efforts. To date, a wide spectrum of efforts have explored the design of osteogenic or osseoinductive biomaterials such as polymers, metals, and ceramics. Increasingly, efforts have also explored alternative fabrication approaches to create patient-specific implants. These efforts include adaptation of generative design principles and 3D printing to create unique geometries, methods to incorporate exogenous cell sources, and the use of conventional and exotic growth factor patterning and release approaches to further improve biological response. Here, we discuss the mechanisms of bone repair, the current available technologies, biomaterial design criteria, 3D printing as a fabrication method, and the influence of cells and growth factors on bone repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Bone Injury and Repair
1.1 Types of Injuries
In this chapter we will focus on the methods available to repair bone defects, focusing specifically on those that require surgical intervention to repair. These types of injuries include craniomaxillofacial defects, long bone segmental defects, and spinal fusion. Craniomaxillofacial injuries are classified as defects to the skull or jaw. These can arise from high energy impact trauma, cleft palate birth defects, and oral cancer [1,2,3,4]. Similar to craniofacial defects, long bone defects can arise from trauma, tumor resection, and nonunion [5]. Spinal fusions involve surgery to place an implant within the space of vertebrae to eliminate motion. Spinal fusion is used to treat spinal fractures, deformities, and instability [6]. Craniomaxillofacial and other segmental bone defects are particularly challenging due to their irregular size and shape and the amount of missing bone tissue. These types of defects are usually critical in size, in which the section of bone missing is too large for the body to regenerate. Biomaterial implants need to be optimized to repair these defects in order to promote new bone formation as well as avoid implant inflammation and infection, which is common in large missing portions of bone [7].
1.2 The Healing Cascade
In normal homeostasis, uninjured bone is constantly being remodeled. Bone is resorbed by a resident population of osteoclasts and new bone synthesized by resident osteoblasts in a precise balance [8]. This process is facilitated by mechanosensitive processes that respond to bone deformation and provide the stimuli to alternately produce or resorb more bone and maintain the mechanical support of soft tissues. In order to design materials for bone regeneration, the coupling of osteoclasts and osteoblasts needs to be recognized and kept in balance in order to avoid complete resorption of implants or unnecessary and often painful excess bone formation.
Bones of the body heal via either endochondral ossification or intramembranous ossification. The two methods have similar healing endpoints; however, endochondral ossification involves a cartilage intermediate and is typically the process involved in long bone healing, while intramembranous ossification does not involve cartilage formation and is the process by which the flat bones of the skull and jaw heal [9,10,11]. Bone healing occurs in stages; for segmental defects this can take several months to complete. Firstly, a hematoma is formed and inflammation occurs, bringing in various immune cells and bone progenitor cells. During a typical immune response, undifferentiated macrophages would migrate to the wound site and polarize to the M1 phenotype in the early stages (1–3 days) [12, 13]. This phenotype is considered “pro-inflammatory” and is responsible for the initial removal of any cellular debris and host defense mechanisms. After 3 days and continuing for weeks, M1 macrophages should shift in phenotype to the “anti-inflammatory” M2 macrophages, which remodel the tissue and deposit matrix [12, 13]. In the case of a biomaterial implant, M1 macrophages are responsible for graft resorption and rejection, while M2 macrophages are accountable for graft acceptance by the body. The M1 to M2 transition over the course of a week is important in avoiding persistent or chronic inflammation, which can lead to a foreign body reaction and ultimate need for a secondary surgery [14, 15]. The way in which mesenchymal stem cells and immune cells differentiate can be partly attributed to the pore size of implant materials. Pore size can determine how vessels form, how cells infiltrate and differentiate, whether inflammation or infection will occur, and how macrophages polarize [16], and suggest exciting opportunities to engineer biomaterial design to not only promote osteogenic activity but also modulate the immune and inflammatory cascade after injury. Ultimately, these macrophages and the topography of an implant can determine the success early-on in the wound healing process. After inflammation, cartilage formation occurs in long bones and vascular growth occurs within the cartilage [17]. Next, chondrocytes die off and cartilage is resorbed in order for mesenchymal stem cells to differentiate into osteoblasts. In intramembranous ossification this cartilage step is skipped and mesenchymal stem cells differentiate and mature, while blood vessels are formed during the primary bone formation step [17]. Finally, secondary bone formation occurs and bone is remodeled by osteoclasts in order to create the anisotropic nature of bone [17].
2 Current State of the Art in Repair: Bone Grafting
The gold standard to repair many bone defects is through the use of bone grafting. Autografts, allografts, and xenografts fall under this category. Grafting typically uses human or mammalian bone in order to repair a patient’s defect. Here, we will discuss the various types of bone grafting used to repair critical-sized bone defects.
2.1 Autografts
Autografts involve using bone from a secondary site in the patient’s own body in order to regenerate bone missing in the wound site. Multiple types of bone can be used, such as cancellous, cortical, vascularized bone, and bone marrow [18]. One of the most commonly used grafting sites is the iliac crest, a part of the pelvis. From this, one can take segments of cortical or cancellous bone for a variety of sized defects [18]. For craniofacial and long bone defects, bone can be repaired using iliac crest autografts with 70–95% success rates [19]. For repairing small bone defects, a chin graft or a retromolar graft from the area behind the third molar can be used [18, 20]. Other less commonly used grafts include tibial, rib, scapula, fascia, sternum, pedicled clavicle, and pedicled temporal bone [18, 20]. Unfortunately, defects longer than 6 cm have much lower success rates, and 50% failure rates have been reported for long bone defects [5, 19]. Drawbacks to removing the iliac crest include iliac fractures, pain, vascular and nerve injury, and persistent hematomas [18]. A popular cortical bone graft in craniofacial reconstruction is the calvarial graft, due to its slow resorption rate [18]. However, the thickness of this graft is highly variable and important vessels exist near this area of bone which should avoid being damaged. Removing this bone from a patient can cause deformity at the removal site and fracture of the bone. Although drawbacks limit the use of this graft, typically success rates are high. A study on 211 patients with calvarial grafts found that after 10–11 months there was a 95% chance of implant integration which matched with other findings of high success rates [21]. However, there was a high number of secondary procedures due to bone resorption, which was attributed to the need for a large amount of bone to be used as an autograft, and patient health differences [21].
General advantages of autografts include retention of some osteogenic cells and an immune response that does not persist [18]. Drawbacks to these methods include limited availability of bone and high chance of morbidity of bone at the site where the graft was taken from [18].
2.2 Allografts
Allografts use bone typically from a deceased donor, with cellular materials removed before implantation [18]. Repair using allografts involves demineralized bone matrix as particles, blocks, or sheets. This removal involves thorough treatment to eliminate any pathogenic agents and genetic material in order to minimize disease transmission. Removal of these pathogenic agents is necessary; however, in order to promote bone repair the extracellular matrix and collagen should not be removed [22]. A main drawback to using allografts is that the osteogenic properties of these vary from one commercial supplier to another due to the treatment and cleaning process [22, 23]. In general there can be high infection rates even after sterilization due to foreign substances remaining in the graft, but more vigorous removal of graft material ultimately leads to the bone being less likely to promote regeneration [20]. A study investigated four different allogenic bone matrices found that in all of the samples there were cells and cell residues before implantation, which in canine studies has shown to illicit an immune response [24]. Although cleaning of the bone matrix can be difficult, the implant survival rate is more than 95%, and new bone formation at 30% after 6 months [24].
2.3 Xenografts
Xenografts use bone from a mammalian source, typically bovine or porcine derived. Similar to allografts, infectious materials and cells must be removed from the bone prior to implantation. One study examined the structure of five different suppliers’ allograft and xenograft materials and discovered that three of the five bone substitutes failed to meet criteria the manufacturers had promised [22]. This was due to the grafts either containing cellular content, loss of lamellar bone structure, or no collagen present [22]. Xenografts do not repair as well as autografts, they have a slower integration with host bone than autografts, and disease transmission such as bovine spongiform encephalopathy is a concern [25, 26].
Given some of the disadvantages associated with existing autograft, allograft, or xenograft procedures, biomaterials for regenerative repair of bone have become increasingly popular conceptually. One advantage of biomaterial approaches is the ability to potentially generate shelf-stable implants in order to remove considerations regarding time between graft harvest and use.
3 Implant Design to Optimize Bone Regeneration
In the next sections we will discuss design strategies for biomaterial implants as alternatives to graft materials. We will discuss the properties of an such an implant, specifically what criteria need to be met in order to successfully regenerate bone. These criteria include selecting biocompatible materials that can promote bone and vessel formation, creating designs that can mimic the mechanical properties of bone and provide mechanical stability, altering the pore size and orientation through fabrication methods, and controlling degradation of the material. We will also discuss the variety of material classes available for implantation and the ways in which these can be modified to fit bone repair applications. These include polymers, both synthetic and natural, metals, and ceramics, focusing on their outcomes in vitro and in vivo and their specific advantages and disadvantages. We also highlight the method of 3D printing, which can be used to add functionality in shape, porosity, and release of biomolecules and cells. Finally, we will discuss cellular and growth factor additions to scaffold materials in order to improve bone formation.
4 Biomaterial Implants for Bone Regeneration
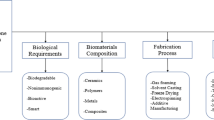
Biomaterial design criteria have to meet a wide range of benchmarks along with considerations of ease of surgical use and economic feasibility [20]. These criteria include biocompatibility, mechanical properties, pore size and orientation, and degradation and bioresorption. Presently, no biomaterial exists that meets all the following criteria. However, in Fig. 1 we outline a series of design criteria for biomaterial implants to address challenges in bone repair.
4.1 Biocompatibility
A biomaterial used for bone regeneration must be able to recruit cells from the surrounding tissues and provide nutrition and signals to support the vitality of these cells. There are many facets of biocompatibility related to bone repair; an implant should promote osteoconduction, osteoinduction, and osteogenesis. Cells should adhere to the biomaterial implant, enhance mineral formation and deposition of new bone, also known as osteoconduction. A variety of signals need to be provided to cells; ones that include osteoinduction, the promotion of differentiation of stem cells to mature bone cells. In addition to this, avoiding signals that may cause persistent inflammation, macrophage fusion, and foreign body response will lead to a more successful outcome. An implant also needs to promote osteogenesis, such that it attracts new cells from the surrounding tissue to the implant site to remodel and form bone [18]. A final important aspect of biocompatibility is the need for the implant to promote the formation of blood vessels. This should occur within a few weeks of biomaterial implantation to support nutrient transport and cell viability and induce osteoconduction, osteoinduction, and osteogenesis [27].
4.2 Mechanical Properties
Ideally, the mechanical properties of an implant would match the properties of the host bone at implant. However, this is extraordinarily difficult to meet, for a number of reasons. Firstly, bone is a multi-scale composite, with cortical and cancellous bone having vastly different mechanical properties. The Young’s modulus and compressive strength of cortical and cancellous bone can vary from a Young’s Modulus of 0.1–20 GPa (Table 1) [27]. Further, these properties reflect the mechanical properties of fully mature bone tissue with integrated vasculature; conceptually, biomaterials for bone repair may be much better suited having an environment designed for diffusive transport of nutrients and oxygen to facilitate cell penetration, proliferation, and extensive remodeling required to form new bone. An additional challenge with designing modulus-matched biomaterials is that many bone defects, notably craniomaxillofacial defects, are typically irregular in size and shape. This makes for difficulties shaping the implant to fit the defect site, which can affect mechanical stability. In general, the mechanical stability of the implant can also affect the healing outcome, as micromotion can directly inhibit osseointegration, so a mechanically stable implant is desired [28, 29].
4.3 Pore Size and Orientation
Typically, porous implants are used for bone regeneration as they provide a template for rapid cell infiltration and metabolic support via diffusion. The pore size of an implant greatly influences the cell behavior and ultimate success or failure of the surgery. There exists debate about optimal pore size to promote bone regeneration, as multiple cell types are involved in the healing process. Bose et al. suggest that pore sizes should be at least 100 μm in diameter for diffusion of nutrients and oxygen, and pore sizes ranging from 200 to 350 μm are optimal for the in-growth of bone tissue [27, 30]. As for macrophages, a pore size of 34 μm promotes a more pro-inflammatory phenotype [31], yet other sources have suggested that 30–40 μm pores promote a pro-healing phenotype and avoid a foreign body response [32, 33]. Bone is an anisotropic tissue, and thus the pore orientation is increasingly considered as an important design parameter to consider in implant design. Recent work have begun to describe the use of aligned pores to promote bone formation by structural guidance cues to increase blood vessel ingrowth, accelerate cellular migration, and guide osteogenic cell differentiation [34,35,36].
4.4 Degradation and Bioresorption
In order to fully repair bone, the implant must be able to degrade while still providing signals for the patient’s own cells to form new bone. This degradation time should match the time it takes for new bone to be formed in order to replace the implant. Different bones regenerate over different times, which are summarized in Table 1 [27, 37]. If a material degrades too quickly, then there will not be enough material to continue to promote host bone regeneration and mechanically support the implant site [38]. Conversely, if a material degrades too slowly, remaining material will block new bone formation, as seen in Fig. 2. Any degradation to a material leads to a loss of mechanical properties, and if this is controlled correctly, then load transfer from the implant to the host bone will occur [40,41,42]. Therefore, to create a biomaterial that can successfully regenerate bone, the design must have a controlled material degradation rate.
PCL and mineralized collagen implant in porcine ramus defect model. (a) Schematic of subcritical ramus defect locations along with 10 mm diameter, 10 mm thick mineralized collagen (CGCaP) scaffold, PCL support, and mineralized collagen-PCL composite implants. (b) Specimen locations were randomized on each side of the mandible and within each porcine animal model. Representative images of the subcritical ramus defect preimplant and postimplant. CGCaP collagen-glycosaminoglycan calcium phosphate, PCL polycaprolactone. (c) Representative μCT data showing partial penetration of the implant into the medullary cavity. Light regions represent bone mineral and dark regions represent no mineral or PCL still present within the implant. (Image adapted from [39])
5 Scaffolds: Mechanical, Chemical, and Biological Properties
Scaffolds are commonly thought of as an initial template that provides a constellation of structural, compositional, and mechanical signals to potentially accelerate the process of bone regeneration. Common scaffold materials include polymers, ceramics and hydroxyapatite materials, metals, and collagen-based implants. Some advantages of scaffolds are their ability to be tailored to specific patients and avoid the cellular material cleaning process that bone-derived graft materials require. When deciding between allograft or xenograft materials versus synthetic or other scaffold materials, sources have found a variety of results, ranging from better to worse healing outcomes [43, 44]. Alternatively, autograft materials have shown favorable healing and mechanics over scaffold materials, but autografts drawbacks outweigh their benefits [45,46,47]. Scaffolds do not require a secondary surgery as autografts do and do not suffer from a limited supply of material. If materials have the same or very similar healing outcomes, it is then favorable to use scaffolds over grafts due to their advantages over bone-derived materials. Scaffolds can also be patient-tailored, such as 3D printed or cast in the particular size and shape of the defect. In addition to this, patient-derived cells can be added to affect the outcome, and growth factors can be added to target specific cell functions to improve osteogenesis and angiogenesis [48]. A summary of clinically available implant materials and their outcomes in vitro and in vivo can be found in Table 2.
5.1 Synthetic Polymeric Scaffolds
Synthetic polymers are man-made polymers, commonly seen in household items such as plastics, rubbers, and glue. Synthetic polymers for tissue engineering must be biodegradable and biocompatible while avoiding a negative immune reaction and matching biomaterial properties as closely as possible. Much of this can be accomplished through modifying the polymer itself, and careful consideration must be made when examining the degradation byproducts. An advantage to using synthetic polymers are their large scale reproducibility with controlled mechanical properties, degradation, and structure [63].
A wide variety of techniques can be used to create porous scaffold architectures. These include casting and forming based methods such as solvent-casting, particulate leaching, and gas-foaming. Solvent-casting and particulate leaching techniques are simple, involving a water-soluble salt homogenously distributed through the polymer solution. The polymer is cast into shape and the solvent is removed by evaporation or lyophilization, while the salt is leached out by soaking in water to create an open-porous polymer [63]. Gas-foaming removes the need for organic solvents and instead carbon dioxide is used to create a polymer foam. In brief, the solid polymer is exposed to high pressure carbon dioxide, which is then saturated into the polymer, and then gas bubbles expand to create a closed-pore structure [63].
Increasingly, more exotic methods are also being used such as electrospinning, 3D printing, and thermally induced phase separation. Electrospinning can create polymeric fibers on the nanoscale by applying a high voltage and an electric field to a polymer solution on a collector which can be rotated in order to create various alignments of fibers. This method can easily create fine fibers; however, it can be difficult to create small diameter fibers with biocompatible materials, and creating 3D scaffolds and complex pore geometry still remain a challenge [64]. Scaffolds with nanofibers have shown to improve stem cell differentiation toward the osteogenic lineage and can be beneficial to bone repair due to their ability to mimic the type I collagen alignment in bone [64]. In addition, sacrificial nanofibers can be added to poly(caprolactone) fibers in order to align cells, direct the formation of extracellular matrix, increase tensile properties, and control the release of collagenase and growth factors to increase cellularity [65, 66]. 3D printing can be used to fabricate scaffolds with complex architectures; however, small pore sizes are difficult to achieve. Various methods of 3D printing exist, such as laser sintering, photopolymerization printing, and extrusion printing, which will be expanded on in Sect. 6. Thermally induced phase separation can be used to fabricate biodegradable 3D polymers by first dissolving the polymer in a solvent at high temperature and then phase separation occurs by lowering the temperature and final sublimation to create a porous polymer [63]. Ultimately, another advantage to this is the ability to modify the surface of polymers in order to alter cell interactions with the polymer surface.
The most extensively used polymeric material in cranioplasty is poly(methyl-methacrylate) (PMMA) . This is an easy to shape and lightweight material and does not radiate heat [20]. Polyethylene has also been used due to its porous nature, and if infections occur antibiotics can be used instead of complete removal of the implant [67,68,69]. Polyethylene glycol (PEG) hydrogels have been investigated for new bone formation due to their ability to slowly release growth factors. However, unlike other polymers, PEG hydrogels added to a mandibular defect saw no difference in new bone formation and did not have an osteogenic effect [70].
Other commonly used polymers in bone tissue engineering are poly(lactic acid) (PLA), poly(lactide-co-glycolide) (PLGA), 3-hydroxybutyric acid (PHB), and poly(caprolactone) (PCL) [39, 71,72,73,74]. PLA degradation byproducts are expected to be nontoxic; however, degradation by hydrolysis releases lactic acid and in a zygomatic fracture fixation, PLA caused swelling at the implant site in 60% of patients [75,76,77]. Some polymers used for bone regeneration, such as lactic acid based polymers, have caused fibrous tissue formation and foreign body responses [78]. Alternatively, PHB scaffolds have been shown to be highly compatible with osteoblasts and can induce ectopic, or abnormal, bone formation [79]. Benefits to using PLA, PCL, and PLGA are their FDA approval for certain use in humans and degradation rates can be tailored by altering the molecular weight and composition. However, drawbacks include poor mechanical properties compared to bone and the possibility of rejection by the body and foreign body responses. Mechanical properties can be tailored based on polymer crystallinity, and growth factor release can be added to these polymer systems, in the future these two factors can possibly eliminate the drawbacks of polymer systems.
5.2 Natural Polymeric Scaffolds
Natural polymers , such as collagen scaffolds, have been used extensively as an alternative material to heal bone defects. A common variant of the collagen scaffolds contains type I collagen, glycosaminoglycans such as chondroitin-6-sulfate, and acid [80,81,82,83,84]. These materials are homogenized together to create a liquid suspension and then freeze dried in order to create an open-porous structure that enables cell migration and penetration through the material. Other natural polymers, such as chitosan have also been investigated [51]. Chitosan is also highly biodegradable and biocompatible and can differentiate osteoblasts in vitro. However, this material is not osteoconductive and has caused allergic reactions [51]. Typically, collagen scaffolds without any mineral supplements are used to regenerate tendon or skin due to their poor ability to heal bone [51, 85,86,87,88]. A benefit to using collagen scaffolds is their tunable pore size and orientation, which can be achieved by using molds with different thermal properties in which scaffolds are lyophilized and altering the freezing rate and temperature [30, 81, 86, 89, 90]. Issues with using naturally derived polymers are that they may contain pathogenic impurities and produce a negative immune response, and it is harder to control the mechanical properties, however, they typically support cell adhesion and proliferation [63].
Variants of collagen materials can be made in order to heal different tissues in the body, such as scaffolds containing calcium phosphate mineral in order to repair bone defects [83, 84, 91,92,93,94,95]. These scaffolds have been shown to be more appropriate for bone repair, due to their biocompatible, biodegradable, and bone formation-inducing behaviors. This has been demonstrated by mineral formation in vitro and bone formation in vivo without additional osteogenic supplements and inhibiting bone resorption [96,97,98]. Disadvantages to these scaffolds are their weak mechanical properties, due to their extremely porous nature. However, mechanical properties can be altered by adding additional materials during freeze drying, such as polymer reinforcements like PLA and PCL [99, 100]. These reinforcements can be 3D printed in various architectures, and one design in particular has been used to achieve shape-fitting in order to avoid micromotion upon implantation [99]. Mineralized collagen scaffolds combined with laser-sintered PCL have demonstrated a 6000-fold increase in Young’s Modulus compared to scaffolds alone [100], and in a porcine ramus defect model this composite material had greater bone repair than the scaffold or PCL construct alone [74]. Other elements such as allogenic tissues, growth factors, and other minerals can easily be added to these scaffolds by mixing into the suspension step before lyophilization [101,102,103]. Specifically, the amniotic membrane derived from placentas has been added to collagen and mineralized collagen scaffolds in order to control the wound healing process and avoid inflammation while increasing bone formation [101, 102, 104].
Increasingly, the delivery or endogenous production of growth factors has been investigated in collagen scaffolds. For example, PDGF-BB and IGF-I delivery was shown to influence migration into these scaffolds [87]. Additionally, current research is focused on sequestering and tethering these growth factors to collagen scaffolds or using micelles as controlled release mechanisms. Other minerals, most notably zinc, have been investigated to improve osteogenesis, and various glycosaminoglycans can be used in order to alter the mineral formation [103, 105]. Additionally, pore sizes and orientations have been investigated in collagen and mineralized collagen scaffolds in order to drive a response to increase viability of tenocytes or increase bone mineral formation [30, 34, 80, 81, 89, 105,106,107]. Finally, the important interaction of mesenchymal stem cells and osteoclasts has been investigated in these scaffolds, and research has shown that mineralized collagen scaffolds inhibit osteoclastogenesis by releasing osteoprotegerin [97, 98]. These scaffolds have the vast potential to be expanded on in order to achieve the criteria for bone regeneration. Whether it be altering the pore size and orientation, adding other minerals, glycoproteins, or tissue matrices, or adding growth factors and specific cell types, there is much work to be done to advance these mineralized scaffolds.
Commercially available natural polymers, such as the mineralized collagen material Healos®, have found comparable results in some cases to autografts. Healos® soaked in bone marrow aspirate without any exogenous factors demonstrated similar healing to autografts in posterolateral fusions. However, this same material performed poorly for interbody cages in spinal surgeries, due to volume of material and mechanical properties [62]. Thus, improvements still need to be made in order to increase mechanical strength and stability to repair other bone defects.
5.3 Metallic Scaffolds
Metal scaffold use is limited due to their ability to conduct heat, difficulty to shape during implantation, and radio-opacity [20]. Metal screws or plates can interfere with imaging of the defect site and monitoring the patient’s health. In addition to this, metals risk corrosion and fatigue over time, the stress shielding effect can cause bone atrophy, and it is difficult to have a metal implant fit well to the implant site without micromotion [75, 108, 109].
Titanium has been the metal of choice for use in large bone defects and like most metals is hard to shape, but resists infection and will be accepted by the body [20]. In order for titanium and its alloys to be successful in bone repair, typically surface modifications are necessary to promote cell attachment and integration. Various methods to do this include mechanical grinding or polishing the surface, physical vapor deposition, acid etching, or chemical vapor deposition [110].
Other metallic materials used include stainless steel 316 L, cobalt based alloys, porous tantalum, and magnesium. Disadvantages include their lack of biocompatibility, wear, and corrosion can release ions and particles that can lead to inflammation. Stainless steel specifically has a very high stiffness, so high in fact that it can lead to bone resorption due to the mismatch in mechanical properties of bone and the implant [111]. Unfortunately, in order to make porous metallic materials to mimic the natural structure of bone, these usually end up too weak to be a viable option [110]. Porous tantalum, however, has a high porosity, a Young’s modulus comparable to bone, and has been shown to be biocompatible in animal models [110]. Magnesium and its alloys are fully bioresorbable, have mechanical properties similar to native bone, do not induce a negative immune response, and promote bone growth [110]. Concerns of using magnesium are the hazards associated with rapid dissolution of the magnesium in the body. An alloy of titanium, nickel-titanium (Nitinol) can be used as a shape-memory material and has demonstrated biocompatibility and mechanical properties similar to bone. Studies have shown that nitinol is more biocompatible than stainless steel [110]; however, release of nickel ions poses a toxicity and allergy concern.
In vivo studies comparing metal implants have shown that porous nitinol had increased osseointegration compared to titanium alloys [112]. Of the metals available, nitinol and resorbable magnesium are the most promising due to low stiffness [111]. In general metals suffer from stress shielding, corrosion, and biofilm formation, all of which contribute to their concerns with clinical use. Overall, the use of metals is mostly desired for permanent implants at sites that need high mechanical loading or as fixation devices.
5.4 Ceramic and Hydroxyapatite Scaffolds
Hydroxyapatite and bioactive ceramics are the most widely used alternative to autografts and allografts in the preclinical and clinical settings [113]. One very common ceramic used in healing of bone defects are bioactive glasses. Bioglass is comprised of sodium, silicone, magnesium, potassium, oxygen, phosphorous, and calcium [114]. As far as healing results, a study examined two different versions of compressed hydroxyapatite scaffolds versus a xenogenic graft in mandibular defects and found no healing differences between the groups at the end of the study [43]. Another study used bioreactors to create bone over time in an autograft and a commercially available bioceramic and found that both were able to create mineral tissue, but autograft materials had more mature bone and mechanical properties more similar to bone [45]. In general, calcium phosphate or hydroxyapatite bone substitutes have less osteogenic potential than autografts [25, 45, 46]. However, hydroxyapatite coatings have different effects than used as a bulk material, and coatings promote cellular contact of osteoblasts [115]. Bioceramics can have various degradation times in the body, an example being hydroxyapatite and tricalcium phosphate (TCP), with hydroxyapatite scaffolds degrading after 2–5 years and TCP degrading within 1 year [113]. This degradation time impacts healing outcomes, as a clinical trial involving hydroxyapatite scaffolds demonstrated that after 15 months the scaffold was still present, and another study claimed the scaffolds were still present even after 7 years [37, 116]. In contrast to this, a β-TCP scaffold deposited new bone after 9 months but complete regeneration of the fibula was only found in 1 out of 14 patients [117].
An alternative to bioactive ceramics is bioinert nanoceramics. These include implants made of titanium, alumina, and zirconia [118]. These ceramics are not designed to regenerate the host bone due to their inert nature; however, they have high fracture toughness and mechanical strength at the implant site [118]. Titanium implants can be modified with Ca2+ ions in order to create titanium oxide, which helps prevent corrosion and absorb proteins to the surface of the material [118]. In addition, other treatments to titanium can be made to modify the surface to promote integration with the host bone, such as etching or sand blasting [118]. Similar to other bioinert ceramics, alumina does not promote osseointegration due to its inert nature, and thus coatings must be added, or the surface topography must be altered to enhance protein adhesion. Zirconia-yttria ceramics are often used as bone fillers due to the ability to prevent biofilms [119]. However, the drawback to these is their inert nature, and these ceramics will still remain in the body instead of host bone.
Bioceramics are thought of to be one of the preferred scaffolds for bone repair due to biocompatibility and high mechanical properties. However, due to the nature of ceramics, these materials can be brittle and only so much of the material can be resorbed by the body [115]. In order to achieve a biomaterial implant it is likely that a composite material will be needed that balances mechanical, chemical, and biological properties. Composite materials have already been discussed as better choices for tissue engineering applications, as no implant material exists today that includes all of the implant criteria [63].
6 3D Printing as a Tool to Improve Bone Formation
3D printing, also known as additive manufacturing, has been used to create materials in our daily lives as well as materials for the medical field. Various methods for creating designs and architectures that would be difficult or impossible using other methods can be accomplished by 3D printing. 3D printing involves a user-created design, which the printer then creates layer-by-layer. This approach overcomes the issue of irregular size and shape defects for bone repair, as the design can be tailored to fit a patient-specific shape. A patient’s defect can be scanned using MRI or CT technology to map the defect space, and subsequently this scan can be converted and used on a 3D printer to fill the defect space [120,121,122]. 3D printing methods can fall into four categories: extrusion, polymerization, laser sintering, and direct writing [123]. The extrusion method takes a solid polymer, extrudes the material through a nozzle by the application of heat and pressure, and allows the print to cool to room temperature to solidify. Fused deposition printing is an example of extrusion-based printing. Polymerization printing uses a bed of resin that is polymerized by lasers, for example, stereolithography [124]. Selective laser sintering involves a bed of polymer powder in which lasers are used to fuse the powder together to create a 3D print. Finally, direct writing uses powder and a regular inkjet printing head with binder, in which the binder is printed onto the loose powder. This method can be used to create interconnected pores; however, intensive optimization of the printing process for a new material is required [122, 123].
An expanding range of materials can be 3D printed, such as the polymers polyethylene, polylactic acid, and polycaprolactone, as well as ceramic materials such as TCP and HA. In addition to these, metals can also be 3D printed; however, this is less common, with an example being bioactive titanium scaffolds fabricated by inkjet 3D printing [125]. In this case, titanium was printed and then fired in order to strengthen the material, and the bioactivity was modified by the deposition of hydroxyapatite on the surface [125]. 3D printing can be used to make these materials very porous; however, a drawback to this is that the mechanical strength is lowered, which limits their use in load-bearing applications. Not only can 3D printing offer a better implant fit, it also can be modified with growth factors and cells. Growth factors and cells for use in 3D printing, also known as bioprinting, will be elaborated on in the following sections, but can be incorporated into polymers such as hydrogels for encapsulating cells and the slow release of biomolecules.
A further opportunity for 3D printing is the addition of these 3D prints to existing materials for bone regeneration. As it can be difficult to create load-bearing 3D prints with very porous structures, an alternative is to use 3D prints as mechanical supports and other biomaterials as the bioactive matrix. This has been demonstrated with mineralized collagen scaffolds and 3D printed polymers. The mineralized collagen acts as the bioactive and osteogenic matrix, and the polymer 3D print acts to give mechanical strength to the whole material in order to better match the mechanical properties of bone [29, 39, 74, 100]. This method provides another way to consider 3D printing; besides using the method to create a scaffold, 3D printing can be used to fabricate pieces of the overall structure. Overall, 3D printing is an extremely useful tool for creating patient-specific implants as it can create complex and porous shapes using a wide variety of materials and methods while also including the option of printing cells and growth factors. More research needs to be performed on optimizing 3D printed materials, as well as investigating combinations of 3D printing with other factors to create composites which can leverage multiple benefits.
7 Stem Cells: Biology and the Application for Tissue Regeneration
7.1 Stem Cells for Bone Repair
Multiple cell types are involved in the bone formation and remodeling process, such as osteocytes, osteoblasts, osteoclasts, and immune cells. Osteocytes maintain the existing bone and are considered mature bone cells. Osteoblasts are responsible for bone growth and can differentiate into osteocytes, while osteoclasts are responsible for bone resorption. Finally, immune cells are important for the healing outcome of the wound, as they clean the area and can lead to fibrous tissue formation or a foreign body reaction if a negative immune response persists [14, 15].
Based on literature, the addition of stem cells to implant materials before implantation has shown more success than implants without stem cells [126, 127]. Overall, the use of autologous or allogenic cells in combination with scaffolds for long bone repair has resulted in positive healing outcomes [5]. The most commonly used stem cells used are embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult mesenchymal stem cells (MSCs). ESCs are derived from embryos through in vitro fertilization, can proliferate infinitely, and can differentiate into any cell type. iPSCs are somatic cells that have been genetically reprogrammed to express the pluripotent properties similar to ESCs. Finally, MSCs, which are the most commonly used cell type for bone repair, are isolated from the liver, fetal blood, bone marrow, and umbilical cord. All of these cell types are able to differentiate into various bone cells, making them important in the bone repair process.
Typically, cells are cultured to a pre-confluent state and then added to graft materials and cultured for a short period of time before implantation into the defect space. Alternatively, cells can be injected directly into defects, which has shown some promise in vivo [128]. Cell death upon transplantation is a drawback; however, MSCs can be contained in spheroids to improve survival, and these have been injected into damaged tissues to promote repair [129, 130]. Interestingly, these have also shown that restricting MSC migration out of these spheroids can enhance the osteogenic potential of these spheroids [130]. In general, adult stem cells have a wide variety of results which can be due to the differences in donors, such as where the cells were sampled, the age of the donor, and life habits [115].
Of the mesenchymal stem cells used, bone marrow stromal cells are favored and can differentiate into almost all mesoderm-derived cell types, including cartilage, bone, hematopoietic stroma, tenocytes, and skeletal muscle cells [115]. However, loss of differentiation properties toward the adipocyte or chondrocyte lineage has been observed after multiple cell passages [131]. Pericytes have also been investigated and are derived from the peripheral blood. These cells are positive for some osteogenic markers and can differentiate along the osteogenic, chondrogenic, and adipogenic lineage [115]. Another commonly used cell line are adipose-derived stem cells, due to their being easy to acquire, abundant, and can differentiate into adipocytes, chondrocytes, osteoblasts, and myocytes. However, this cell line is more biased toward the osteogenic lineage, which can make for biased in vitro studies and have demonstrated less favorable outcomes compared to bone marrow stromal cells [132, 133]. In a study by Follmar et al., combining adipose-derived stem cells with allografts in a rabbit model demonstrated a foreign body response; however, these same cells in a porcine model accelerated bone healing [128, 134]. Another alternative is to use cells derived from pregnancies, such as umbilical cord and placental stem cells. Umbilical cord blood multilineage cells take longer to culture and express lower bone antigens, but exposure to osteoblast-conditioned media enhanced their rate of osteogenic differentiation [135, 136]. Placental stem cells have also been shown to have a bone marrow stromal cell-like behavior and possess multilineage differentiation potentials [115].
3D printing offers the unique opportunity to encapsulate cells into printed constructs and even encapsulate various cell types into the same print. These cells can be cultured and encapsulated into hydrogels, which can then be used in syringe pumps in bioprinters to print layer-by-layer. Mesenchymal stem cells and chondrocytes have been embedded into alginate hydrogels, and this hydrogel exhibited extracellular matrix formation both in vitro and in vivo [123]. Organ bioprinting, an approach to print fully capable organs, can be accomplished through printing a variety of cells and culturing the resultant scaffold post-printing. Firstly, the organ blueprint must be designed, next, stem cells required for the organ are isolated and differentiated, and then these are encapsulated into hydrogels or other medium to support the life of the cells, and finally, these are printed and placed into a bioreactor or incubator to continue cell growth [137]. Bioprinting enables cells to be printed in distinct areas using various nozzles containing hydrogels with different encapsulated cells. This can make for interesting studies comparing co-cultures in different compartments. Bioprinting with cells offers new complex architectures with a wide variety of cells; however, the material in which the cells are encapsulated within still needs to meet bioactivity requirements while being able to be printed. Cells must remain viable within these materials and further research needs to investigate improving these printers and materials to sustain cell viability.
7.2 Cells Involved in the Wound Healing Cascade
There are a wide variety of cells to consider using in biomaterial implants, and more research needs to be performed on using patient-derived cells in order to accelerate healing as well as the interactions of each cell type on the biomaterial implant. Maintaining the balance between osteoclasts and osteoblasts, creating a controlled environment for M1 to M2 macrophage phenotype transition, and allowing blood vessels to grow and deliver nutrients are all factors that need consideration in biomaterial implant design. The promise of better healing using biomaterial scaffold implants lies in the ability for these to be tailored to meet these requirements. In order to balance osteoclasts and osteoblasts, these cell types could be examined in a co-culture on the implant in order to determine the possible mechanisms and healing that may proceed in vivo. This has been performed on collagen-based scaffolds in order to determine that these scaffolds inhibit osteoclastogenesis [97, 98]. Similar studies should be carried out investigating this balance in other biomaterial implants as well. Uncovering the type of M1 to M2 macrophage transition in implants can be investigated by seeding M0 macrophages or monocytes on scaffolds in vitro. These transitions have been investigated by Spiller et al. [13, 138,139,140,141], and this can provide useful information over time about how these cells polarize in response to implant released factors and implant topography and composition. This phenotype transition could be helpful to elucidate whether inflammation may persist or if a foreign body response may occur before an in vivo experiment is undertaken. In addition to investigating these specific cells, placental-derived tissues have shown promise in modulating this transition and ultimately the immune response. The amnion and chorion membrane of the placenta have been investigated as an addition to scaffolds and have shown to dampen the pro-inflammatory immune response while promoting osteogenesis [101, 102, 104, 139, 142, 143]. Finally, angiogenesis is important for delivery of nutrients to the growing bone and inadequate vascularization of bone has been associated with a decrease in bone mass [144]. An interesting opportunity exists to test vessel formation in biomaterial implants for bone regeneration, an example being endothelial vessel formation created in hydrogels by co-culture of umbilical vein endothelial cells and normal lung fibroblasts [145]. This type of study could be expanded using released factors from implant or solely focusing on blood vessel formation in implants for bone regeneration. This may give a better understanding of how blood vessel formation would occur in vivo.
Overall there are many variables to consider when using stem cells and more research needs to be examined on the effect of adding these to implants. There exists potential for these cells to accelerate healing, and in combination with 3D printing even greater potential exists to improve bone repair with complex tissue architectures.
8 Growth Factors, Chemical Cues, Differentiating Agents for Bone
8.1 Growth Factors to Enhance Bone Repair
Growth factors are polypeptides and are used in bone regeneration to differentiate bone cells, promote angiogenesis, or promote migration and retention of cells to the implant site. These can act on the autocrine (influences the cell of origin), paracrine (influences nearby cells), or endocrine (influences the nearby microenvironment) systems. Growth factors bind to cell receptors and induce intracellular signal transduction which determines the biological response upon reaching the cell nucleus [146]. Additionally, a single growth factor may bind to different receptors. Growth factors are typically introduced to the body in one of the two methods, as a protein therapy or gene therapy. Protein therapy involves direct recombinant growth factor delivery to the site of interest, whereas gene therapy delivers growth factors to cells by gene encoding [146].
Most common growth factors interacting with the skeletal system are bone morphogenic proteins (BMPs), fibroblast growth factors (FGF), platelet-derived growth factor (PDGF), insulin-like growth factors (IGFs), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VGEF) to name a few. A summary of growth factors and their impact on bone and cartilage formation can be found in Table 3. Using growth factors to heal critically sized defects has shown to mostly improve the healing process; however, there have been reports that BMPs and TGFβ-3 did not improve healing [5].
Bone morphogenic proteins are typically considered the most promising approach to repair bone due to their osteoinductive nature. BMP-2, -4, -6, -7, and -9 have shown to have the greatest osteogenic success in vitro [157]. However, there have been mixed results with using BMPs. A review found that 11 results supported the use of BMPs, three results found no effect on bone repair, and two demonstrated negative outcomes [158]. This variability can be attributed to the variety of BMPs used and the treatment conditions. To repair fractures, recombinant human BMP-2 (rhBMP-2) is used most frequently, and rhBMP-7 is most commonly used for nonunion repairs [158]. Overall, rhBMPs have been shown to accelerate healing of tibial fractures and reduce infection rates [158]. There exist drawbacks to using BMPs, especially rhBMP-2 which has resulted in surgery complications, especially spinal surgeries. The majority of these complications stem from heterotopic ossification, or bone growth in areas of other tissues. Literature finds it difficult to compare the two BMPs, BMP-2 and -7, as most studies lack comparisons between the two which can elucidate differences. Another issue with BMPs and many growth factors is the delivery method. Due to their soluble nature, if these growth factors are not appropriately carried to the site of interest they can diffuse into nearby tissues and form bone in undesirable locations. In general, large doses of BMPs are required to achieve osteogenic effects, which can be both expensive and increase the risk of heterotopic ossification [158]. Thus, further research into BMP delivery needs to be performed in order to control the release of these factors better.
Fibroblast growth factors have been found to be suitable for regeneration of a wide range of tissues and are key regulators of bone development [148]. In particular, FGF-2, -9, and -18 are involved in bone development and FGF signaling can stimulate proliferation of osteogenic cells and angiogenesis [148]. Recombinant FGF-2 has shown to accelerate bone repair in rabbits, but its anabolic effect is limited to the first 24 h after fracture occurs [159]. In a rabbit model, FGF-2-coated hydroxyapatite scaffolds were shown to greatly enhance the osteoinductive effect compared to uncoated implants [160].
Platelet-derived growth factor is involved in the development of embryos but also plays important roles in bone repair in adults. Systemic application of PDGF has shown to result in increased bone mineral density and compressive properties in rat vertebrae [161], conversely, PDGF inhibited bone regeneration in rat calvarial defects [162]. However, with the appropriate carrier, the opposite was true and bone formation was increased in rat calvarial defects [163].
Insulin-like growth factors can influence both metabolic and growth activity in many cell and tissue types, and of the isoforms IGF-I and IGF-II, IGF-I has been typically only used in skeletal reconstruction [164]. IGF-I is the most abundant growth factor found in the skeletal system and regulates bone development and osteoblasts [165]. IGF-I has also been used to increase bone formation, but this did not have the desired effect in young animals [166]. IGF-I delivered via PLGA microparticles was shown to enhance new bone formation, but there was little therapeutic effect of using IGF-I alone for cartilage and bone repair in osteoarthritic joints [167, 168]. IGF-II is the most abundant growth factor in bone and both IGFs play important roles in stimulating osteoblast differentiation, deposition of bone, and collagen protein expression [169]. Insulin-like growth factors can be differentiated from one other by their functions, as IGF-II can induce proliferation and differentiation of MSCs to osteoblasts, while IGF-I cannot, and functions to maintain and grow bone [169].
Transforming growth factor beta is one of the most common cytokines and influences the development of various tissues [164]. The carrier of TGF-β plays an important role in its activity, as single doses of TGF-β1 had no effect in rabbit calvarial defects but gelatin capsules enhanced bone formation [170]. Similar to this, TGF-β hydrogels with very rapid or very slow degradation times had no effect on bone formation [171].
Finally, vascular endothelial growth factor not only controls vasculogenesis and angiogenesis, but is involved in recruitment and activity of bone forming cells [148]. VEGF had been shown to enhance blood vessel formation and ossification in murine femur fractures [172]. In addition, VEGFs have been shown to enhance bone formation when combined with other growth factors [148].
An alternative to growth factors is platelet rich plasma (PRP) , which is centrifuged autogenous blood that contains high concentrations of cells containing various growth factors such as PDGF, TGF-β, IGF, and VEGF [164]. PRP has been considered a better alternative to the single use of growth factors due to its composition of many growth factors and its cost-effective sourcing [173]. However, there are variabilities in success due to the preparation methods, concentration, and methods of application of PRP. In vitro, PRP has shown to induce proliferation of bone marrow stem cells and promote osteogenic differentiation [174]. In vivo studies have demonstrated various outcomes, with most improving the histological appearance of bone but some reporting harmful or non-significant effects [173].
Drawbacks to using recombinant growth factors in general are their roles in tumor formation or negative immune reactions, which has been demonstrated for BMP2 and VEGF [146, 175]. As with all growth factors, the design of the delivery system can greatly affect the outcome of the surgery. This adds another element to designing a biomaterial implant. If the biomaterial includes the release of a growth factor, then further consideration on the kinetics of release needs to be tailored to the wound of interest, whether it be a short or sustained release. Interestingly, combinations of scaffolds, cells, and growth factors have been shown both positive and negative results when compared to combinations of scaffolds and cells or growth factors [5].
8.2 Application of Growth Factors to Tissue Engineering
In addition to printing unique structures and multiple cell types, growth factors can be combined with bioprinting. Growth factors, like cells, can be added to the printing medium in order to drive cellular responses. Hydrogels have been effectively loaded with BMP-2 and VEGF in order to induce bone regeneration. In one study, BMP-2 was loaded into collagen hydrogels for a sustained release and VEGF was loaded into alginate and gelatin hydrogels for a burst release [176]. In this example, multiple print heads were used to create a scaffold with two different growth factors located in different regions of the scaffold that released at different rates based on material properties [176]. Bioprinting offers a simple way to incorporate various growth factors in order to study their interactions with cells; however, the material that these growth factors are encapsulated in determines their release. Further research needs to be performed in order to optimize these materials, especially materials other than hydrogels, as bioprinting is an incredibly useful tool if optimized.
There exist a wide variety of growth factors available to promote bone regeneration, and more research needs to be investigated on how to adequately deliver these and control cell fate. Again, factors that can control the balance of osteoclasts and osteoblasts, the M1 to M2 macrophage transition, and angiogenesis need to be examined. Research has demonstrated that osteoprotegerin plays a critical role in inhibiting osteoclastogenesis, which could be potentially used as a growth factor in order to maintain this balance between osteoclasts and osteoblasts [97, 98]. In addition to this, macrophage phenotype impacts the wound outcome and growth factors could be delivered in order to promote a more M1 or M2-like phenotype. Cytokines that can induce an M1 response include LPS and IFN-γ, while cytokines that can induce an M2 response include IL-4, IL-13, and IL-10 [13, 141, 177]. An interesting opportunity exists to combine these cytokines in 3D-printed scaffolds in order to drive a particular immune response depended on release rates and specific cytokines released. Finally, angiogenesis can be accomplished by introducing VEGF to scaffolds, and more research should involve examining blood vessel formation with and without this growth factor and its potential to induce vessel formation quicker in scaffolds. Overall, growth factor addition to implant materials holds promise, but more investigation must be performed on the negative outcomes of these factors, controlling delivery, and leveraging multiple growth factors in order to drive osteogenesis, wound healing, and angiogenesis.
9 Conclusions
There are many strategies to repair bones; however, no such strategy exists without its drawbacks. Autografts have the greatest potential to heal but require another surgery within the patient’s body. Allografts and xenografts have shown promising results, but processing methods can destroy important components in these materials. Other scaffold types are easy to manipulate and can be patient-specific; however, their results cannot yet compare to autografts. The future of bone regeneration involves combining these various methods to heal bone in order to achieve the properties of a biomaterial implant (Fig. 3): biocompatible materials, mechanics that match the properties of bone and prevent micromotion, a pore size and orientation that guides vessel formation and cell migration, and a material that degrades and allows new bone formation to occur. 3D printing can be used to print multiple material types, unique and challenging structures, and patient-specific implants. This can be useful in combination with the various materials, cells, and growth factors discussed here, to one day create a biomaterial implant that addresses all necessary criteria. Research efforts should also focus on targeting the balance between osteoclasts and osteoblasts, macrophage phenotype transition, and angiogenesis. These can be addressed by material design, studies investigating multiple cell-type interactions, and growth factor addition. Overall, there exists a vast amount of research and development left in the area of bone repair, and many factors need to be addressed. Optimizing materials, fabrication, cell types, and growth factors included in biomaterial implants must be accomplished in order to create the optimal biomaterial for bone repair.
References
Lew T, Walker J, Wenke J, Blackbourne L, Hale R. Characterization of craniomaxillofacial battle injuries sustained by United States service members in the current conflicts of Iraq and Afghanistan. J Oral Maxillofac Surg. 2010;68(1):3–7.
Fong A, Lemelman B, Lam S, Kleiber G, Reid R, Gottlieb L. Reconstructive approach to hostile cranioplasty: a review of the University of Chicago experience. J Plast Reconstr Aesthet Surg. 2015;68(8):1036–43.
Lee J, Kleiber G, Pelletier A, Reid R, Gottlieb L. Autologous immediate cranioplasty with vascularized bone in high-risk composite cranial defects. Plast Reconstr Surg. 2013;132(4):967–75.
Lee E, Chao A, Skoracki R, Yu P, DeMonte F, Hanasono M. Outcomes of calvarial reconstruction in cancer patients. Plast Reconstr Surg. 2014;133(3):675–82.
Roffi A, Krishnakumar GS, Gostynska N, Kon E, Candrian C, Filardo G. The role of three-dimensional scaffolds in treating long bone defects: evidence from preclinical and clinical literature — a systematic review. Biomed Res Int. 2017;2017:13.
Staff MC. Spinal fusion. Mayo Clinic. https://www.mayoclinic.org/tests-procedures/spinal-fusion/about/pac-20384523. Accessed on 23 Jan 2020
Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis. Curr Infect Dis Rep. 2008;10(5):394–403.
Rodan GA. Bone homeostasis. PNAS. 1998;95(23):13361–2.
Thompson E, Matsiko A, Farrell E, Daniel JK, O’Brien F. Recapitulating endochondral ossification: a promising route to in vivo bone regeneration. J Tissue Eng Regen Med. 2015;9:889–902.
Kruijt Spanjer EC, Bittermann GKP, van Hooijdonk IEM, Rosenberg AJWP, Gawlitta D. Taking the endochondral route to craniomaxillofacial bone regeneration: a logical approach? J Craniomaxillofac Surg. 2017;45(7):1099–106. https://doi.org/10.1016/j.jcms.2017.03.025.
Scott CK, Hightower JA. The matrix of endochondral bone differs from the matrix of intramembranous bone. Calcif Tissue Int. 1991;49(5):349–54.
Ribeiro M, Monteiro FJ, Ferraz MP. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter. 2012;2(4):176–94.
Spiller KL, Freytes DO, Vunjak-Novakovic G. Macrophages modulate engineered human tissues for enhanced vascularization and healing. Ann Biomed Eng. 2015;43(3):616–27.
Kim YK, Chen EY, Liu WF. Biomolecular strategies to modulate the macrophage response to implanted materials. J Mater Chem B. 2016;4(9):1600–9.
Gibon E, Lu LY, Nathan K, Goodman SB. Inflammation, ageing, and bone regeneration. J Orthop Transl. 2017;10:28–35. https://doi.org/10.1016/j.jot.2017.04.002.
Lee J, Byun H, Madhurakkat Perikamana SK, Lee S, Shin H. Current advances in immunomodulatory biomaterials for bone regeneration. Adv Healthc Mater. 2019;8:1–20.
Runyan CM, Gabrick KS. Biology of bone formation, fracture healing, and distraction osteogenesis. J Craniofac Surg. 2017;28(5):1380–9.
Elsalanty M, Genecov D. Bone grafts in craniofacial surgery. Craniomaxillofac Trauma Reconstr. 2009;2(03):125–34.
Pogrel M, Podlesh S, Anthony J, Alexander J. A comparison of vascularized and nonvascularized bone grafts for reconstruction of mandibular continuity defects. J Oral Maxillofac Surg. 1997;55(11):1200–6.
Abuzayed B, Aydin S, Aydin S, Kucukyuruk B, Sanus G. Cranioplasty: review of materials and techniques. J Neurosci Rural Pract. 2011;2(2):162.
Depeyre A, Touzet-Roumazeille S, Lauwers L, Raoul G, Ferri J. Retrospective evaluation of 211 patients with maxillofacial reconstruction using parietal bone graft for implants insertion. J Craniomaxillofac Surg. 2016;44(9):1162–9. https://doi.org/10.1016/j.jcms.2016.06.034.
Ghanaati S, Barbeck M, Booms P, Lorenz J, Kirkpatrick CJ, Sader RA. Potential lack of “standardized” processing techniques for production of allogeneic and xenogeneic bone blocks for application in humans. Acta Biomater. 2014;10(8):3557–62. https://doi.org/10.1016/j.actbio.2014.04.017.
Bae H, Zhao L, Kanim L, Wong P, Delamarter R, Dawson E. Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976). 2006;31(12):1299–306.
Nelson K, Fretwurst T, Stricker A, Steinberg T, Wein M, Spanou A. Comparison of four different allogeneic bone grafts for alveolar ridge reconstruction: a preliminary histologic and biochemical analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(4):424–31. https://doi.org/10.1016/j.oooo.2014.05.020.
Athanasiou V, Papachristou D, Panagopoulos A, Saridis A, Scopa C, Megas P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: an experimental study in rabbits. Med Sci Monit. 2010;16(1):BR24–31.
Hartl A, Bitzan P, Wanivenhaus A, Kotz R. Faster integration of human allograft bone than of the bovine substitute Lubboc: non-randomized evaluation of 20 cases with benign tumors or tumor-like conditions. Acta Orthop Scand. 2004;75(2):217–20.
Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–54.
Dimitriou R, Babis GC. Biomaterial osseointegration enhancement with biophysical stimulation. J Musculoskelet Neuronal Interact. 2007;7(3):253–65.
Dewey MJ, Johnson EM, Weisgerber DW, Wheeler MB, Harley BAC. Shape-fitting collagen-PLA composite promotes osteogenic differentiation of porcine adipose stem cells. J Mech Behav Biomed Mater. 2019;95:21.
Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31(3):461–6. https://doi.org/10.1016/j.biomaterials.2009.09.063.
Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann Biomed Eng. 2014;42(7):1508–16.
Ratner BD. A pore way to heal and regenerate : 21st century thinking on biocompatibility. Regen Biomater. 2016;3:107–10.
Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. PNAS. 2010;107(34):15211–6.
Seong Y, Kang I, Song E, Kim H, Jeong S. Calcium phosphate – collagen scaffold with aligned pore channels for enhanced osteochondral regeneration. Adv Healthc Mater. 2017;6:1–11.
Bobbert FSL, Zadpoor AA. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J Mater Chem B. 2017;5(31):6175–92.
Lin W, Lan W, Wu Y, Zhao D, Wang Y, He X, et al. Aligned 3D porous polyurethane scaffolds for biological anisotropic tissue regeneration. Regen Biomater. 2019;7:19–27.
Marcacci M, Kon E, Moukhachev V, Lavroukov A, Kutepov S, Quarto R, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13(5):947.
Sheikh Z, Sima C, Glogauer M. Bone replacement materials and techniques used for achieving vertical alveolar bone augmentation. Materials (Basel). 2015;8:2953–93.
Weisgerber DW, Milner DJ, Lopez-Lake H, Rubessa M, Lotti S, Polkoff K, et al. A mineralized collagen-polycaprolactone composite promotes healing of a porcine mandibular defect. Tissue Eng Part A. 2017;24(11–12):943–54.
Sheikh Z, Najeeb S, Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials (Basel). 2015;8(9):5744–94.
Hutmacher D, Hurzeler MB, Schliephake H. A review of material properties of biodegradable and bioresorbable polymers and devices for GTR and GBR applications. Int J Oral Maxillofac Implants. 1996;11:667–78.
Chanlalit C, Shukla DR, Fitzsimmons JS, An KN, O’Driscoll SW. Stress shielding around radial head prostheses. J Hand Surg [Am]. 2012;37:2118–25.
Dau M, Kämmerer PW, Henkel KO, Gerber T, Frerich B, Gundlach KKH. Bone formation in mono cortical mandibular critical size defects after augmentation with two synthetic nanostructured and one xenogenous hydroxyapatite bone substitute - in vivo animal study. Clin Oral Implants Res. 2016;27(5):597–603.
Buser Z, Brodke D, Youssef J, Meisel H, Myhre S, Hashimoto R, et al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neuorosurg Spine. 2016;25(4):509–16.
Tatara AM, Koons GL, Watson E, Piepergerdes TC, Shah SR. Biomaterials-aided mandibular reconstruction using in vivo bioreactors. PNAS. 2019;116(14):6954–63.
Broggini N, Bosshardt DD, Jensen SS, Bornstein MM, Wang C, Buser D. Bone healing around nanocrystalline hydroxyapatite, deproteinized bovine bone mineral, biphasic calcium phosphate, and autogenous bone in mandibular bone defects. J Biomed Mater Res B Appl Biomater. 2015;103:1478–87.
Wang W, Yeung KWK. Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater. 2017;2(4):224–47.
Ghassemi T, Shahroodi A, Moradi A. Current concepts in scaffolding for bone tissue engineering. Arch Bone Jt Surg. 2018;6(2):90–9.
Peterson B, Whang P, Iglesias R, Wang J, Lieberman J. Osteoinductivity of commercially available demineralized bone matrix. J Bone Jt Surg. 2004;86(10):2243–50.
Acarturk T, Hollinger J. Commercially available demineralized bone matrix compositions to regenerate calvarial critical-sized bone defects. Plast Reconstr Surg. 2006;118(4):862–73.
Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res. 2014;9:18.
Goa T-J, Tuominen TK, Lindholm TS, Kommonen B, Lindholm TC. Morphological and biomechanical difference in healing in segmental tibia1 defects implanted with Biocoral(R) or tricalcium phbsphate cylinders. Biomaterials. 1997;18:219–23.
Galli C, Guizzardi S, Passeri G, Martini D, Tinti A, Mauro G, et al. Comparison of human mandibular osteoblasts grown on two commercially available titanium implant surfaces. J Periodontol. 2005;76(3):364–72.
Gredes T, Kubasiewicz-Ross P, Gedrange T, Dominiak M, Kunert-Keil C. Comparison of surface modified zirconia implants with commercially available zirconium and titanium implants: a histological study in pigs. Implant Dent. 2014;23(4):502–7.
Bansiddhi A, Sargeant T, Stupp S, Dunand D. Porous NiTi for bone implants: a review. Acta Biomater. 2008;4(4):773–82.
Bai X, Gao M, Syed S, Zhuang J, Xu X, Zhang X-Q. Bioactive hydrogels for bone regeneration. Bioact Mater. 2018;3(4):401–17.
Sanchez-Sotelo J, Munuera L, Madero R. Treatment of fractures of the distal radius with a remodellable bone cement. J Bone Jt Surg. 2000;82:856–63.
Lidfors N, Heikkila J, Koski I, Mattila K, Aho A. Bioactive glass and autogenous bone as bone graft substitutes in benign bone tumors. J Biomed Mater Res B. 2008;90B(1):131–6.
Crovace M, Souza M, Chinaglia C, Peitl O, Zanotto E. Biosilicate® — a multipurpose, highly bioactive glass-ceramic. In vitro, in vivo and clinical trials. J Non-Cryst Solids. 2016;432:90–110.
Leupold J, Barfield W, Han Y, Hartsock L. A comparison of ProOsteon, DBX, and collagraft in a rabbit model. J Biomed Mater Res B. 2006;79B(2):292–7.
Boyd D, Towler M, Wren A, Clarkin O. Comparison of an experimental bone cement with surgical Simplex® P, Spineplex® and Cortoss®. J Mater Sci Mater Med. 2008;19:1745–52.
Nee D, Noyes D, Shaw M, Gwilym S, Fairlie N, Birch N. Healos and bone marrow aspirate used for lumbar spine fusion: a case controlled study comparing healos with autograft. Spine (Phila Pa 1976). 2006;31(18):E636–40.
Liu X, Ma P. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32(3):477–86.
Gupte MJ, Ma PX. Nanofibrous scaffolds for dental and craniofacial applications. J Dent Res. 2012;91(3):227–34.
Qu F, Holloway J, Esterhai J, Burdick J, Mauck R. Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair. Nat Commun. 2017;8:1780.
Baker B, Shah R, Silverstein A, Esterhai J, Burdick J, Mauck R. Sacrificial nanofibrous composites provide instruction without impediment and enable functional tissue formation. PNAS. 2012;109(35):14176–81.
Abuzayed B, Tuzgen S, Canbaz B, Yuksel O, Tutunculer B, Sanus G. Reconstruction of growing skull fracture with in situ galeal graft duraplasty and porous polyethylene sheet. J Craniofac Surg. 2009;20(4):1245–9.
Abuzayed B, Dashti R, Turk O, Kaynar M. Aneurysmal frontal bone cyst in a child with history of acute lymphoblastic leukemia: a case of rare location and history. J Pediatr Hematol Oncol. 2010;32(1):e1–3.
Kucukyuruk B, Biceroglu H, Abuzayed B, Ulu M, Sanus G. Intraosseous meningioma: a rare tumor reconstructed with porous polyethylene. J Craniofac Surg. 2010;21(3):936–9.
Brockmeyer P, Kramer K, Krohn S, Kauffmann P, Mauth C, Dard M, et al. Influence of synthetic polyethylene glycol hydrogels on new bone formation during mandibular augmentation procedures in Goettingen minipigs. J Mater Sci Mater Med. 2015;26(6):1–7.
Gregor A, Filová E, Novák M, Kronek J, Chlup H, Buzgo M, et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng. 2017;11(1):1–21.
Zhang H, Mao X, Zhao D, Jiang W, Du Z, Li Q, et al. Three dimensional printed polylactic acid-hydroxyapatite composite scaffolds for prefabricating vascularized tissue engineered bone: an in vivo bioreactor model. Sci Rep. 2017;7:15255.
Park SA, Lee H-J, Park S-Y. In vivo evaluation of 3D-printed polycaprolactone scaffold implantation combined with β-TCP powder for alveolar bone augmentation in a beagle defect model. Materials (Basel). 2018;11(2):238.
Weisgerber DW, Milner DJ, Lopez-Lake H, Rubessa M, Lotti S, Polkoff K, et al. A mineralized collagen-polycaprolactone composite promotes healing of a porcine mandibular ramus defect. Tissue Eng Part A. 2017;24(11–12):943–54.
Gredes T, Kunath F, Gedrange T, Kunert-Keil C. Bone regeneration after treatment with covering materials composed of flax fibers and biodegradable plastics: a histological study in rats. Biomed Res Int. 2016;2016:1–8.
Athanasiou K, Niederauer G, Agrawal CM. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials. 1996;17(2):93–102.
Athanasiou K, Agrawal C, Barber F, Burkhart S. Orthopaedic applications for PLA-PGA biodegradable polymers. Anthrosc J Arthrosc Relat Surg. 1998;14(7):726–37.
Kroeze RJ, Helder MN, Smit TH. Biodegradable polymers in bone tissue engineering. Materials (Basel). 2009;2(3):833–56.
Mai R, Hagedorn MG, Gelinsky M, Werner C, Turhani D, Spath H, et al. Ectopic bone formation in nude rats using human osteoblasts seeded poly(3)hydroxybutyrate embroidery and hydroxyapatite-collagen tapes constructs. J Craniomaxillofac Surg. 2006;34(2):101–9.
Harley BA, Leung JH, Silva ECCM, Gibson LJ. Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater. 2007;3(4):463–74.
O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26(4):433–41.
Florent D, Levingstone T, Schneeweiss W, de Swartw M, Jahns H, Gleeson J, et al. Enhanced bone healing using collagen– hydroxyapatite scaffold implantation in the treatment of a large multiloculated mandibular aneurysmal bone cyst in a thoroughbred filly. J Tissue Eng Regen Med. 2015;9:1193–9.
Cunniffe G, Dickson G, Partap S, Stanton K, O’Brien FJ. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J Mater Sci Mater Med. 2010;21:2293–8.
Al-Munajjed A, Gleeson J, O’Brien F. Development of a collagen calcium-phosphate scaffold as a novel bone graft substitute. Stud Heal Technol Inf. 2008;133:11–20.
Ren X, Bischoff D, Weisgerber DW, Lewis MS, Tu V, Yamaguchi DT, et al. Osteogenesis on nanoparticulate mineralized collagen scaffolds via autogenous activation of the canonical BMP receptor signaling pathway. Biomaterials. 2015;50:107–14.
Caliari SR, Harley BAC. Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Adv Healthc Mater. 2014;3:1086–96.
Caliari SR, Harley BAC. Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen-GAG scaffolds. Tissue Eng Part A. 2012;19(9–10):1100–12.
Kanungo BP, Gibson LJ. Density-property relationships in collagen-glycosaminoglycan scaffolds. Acta Biomater. 2010;6(2):344–53. https://doi.org/10.1016/j.actbio.2009.09.012.
O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25(6):1077–86.
Murphy CM, O’Brien FJ. Understanding the effect of mean pore size on cell activity in collagen-glycosaminoglycan scaffolds. Cell Adhes Migr. 2010;4(3):377–81.
Weisgerber DW, Caliari SR, Harley BAC. Mineralized collagen scaffolds induce hMSC osteogenesis and matrix remodeling. Biomater Sci. 2015;3(3):533–42.
Yamaguchi DT, Lee JC, Tu V, Ren X, Harley BAC, Weisgerber DW, et al. Osteogenesis on nanoparticulate mineralized collagen scaffolds via autogenous activation of the canonical BMP receptor signaling pathway. Biomaterials. 2015;50:107–14. https://doi.org/10.1016/j.biomaterials.2015.01.059.
Kanungo BP, Silva E, Van Vliet K, Gibson LJ. Characterization of mineralized collagen-glycosaminoglycan scaffolds for bone regeneration. Acta Biomater. 2008;4(3):490–503.
Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J Biomed Mater Res A. 2010;92(3):1066–77.
Lyons FG, Gleeson JP, Partap S, Coghlan K, O’Brien FJ. Novel microhydroxyapatite particles in a collagen scaffold: a bioactive bone void filler? Clin Orthop Relat Res. 2014;472(4):1318–28.
Ren X, Tu V, Bischoff D, Weisgerber DW, Lewis MS, Yamaguchi DT, et al. Nanoparticulate mineralized collagen scaffolds induce in vivo bone regeneration independent of progenitor cell loading or exogenous growth factor stimulation. Biomaterials. 2016;89:67–78.
Ren X, Dewey MJ, Bischoff D, Miller TA, Yamaguchi DT, Harley BAC, et al. Nanoparticulate mineralized collagen glycosaminoglycan materials directly and indirectly inhibit osteoclastogenesis and osteoclast activation. J Tissue Eng Regen Med. 2019;13:823–34.
Ren X, Zhou Q, Foulad D, Tiffany AS, Dewey MJ, Bischoff D, et al. Osteoprotegerin reduces osteoclast resorption activity without affecting osteogenesis on nanoparticulate mineralized collagen scaffolds. Sci Adv. 2019;5:1–12.
Dewey MJ, Weisgerber DW, Johnson E, Wheeler MB, Harley BAC. Shape-fitting collagen-PLA composite promotes osteogenic differentiation of porcine adipose stem cells. J Mech Behav Biomed Mater. 2019;95:21.
Weisgerber DW, Erning K, Flanagan CL, Hollister SJ, Harley BAC. Evaluation of multi-scale mineralized collagen-polycaprolactone composites for bone tissue engineering. J Mech Behav Biomed Mater. 2016;61:318–27. https://doi.org/10.1016/j.jmbbm.2016.03.032.
Hortensius RA, Ebens JH, Dewey MJ, Harley BAC. Incorporation of the amniotic membrane as an immunomodulatory design element in collagen scaffolds for tendon repair. ACS Biomater Sci Eng. 2018;4(12):4367–77.
Hortensius RA, Ebens JH, Harley BAC. Immunomodulatory effects of amniotic membrane matrix incorporated into collagen scaffolds. J Biomed Mater Res A. 2016;104(6):1332–42.
Tiffany AS, Gray DL, Woods TJ, Subedi K, Harley BAC. The inclusion of zinc into mineralized collagen scaffolds for craniofacial bone repair applications. Acta Biomater. 2019;93:86–96. https://doi.org/10.1016/j.actbio.2019.05.031.
Dewey M, Johnson E, Slater S, Milner D, Wheeler M, Harley B. Mineralized collagen scaffolds fabricated with amniotic membrane matrix increase osteogenesis under inflammatory conditions. Regen Biomater. 2020;7:247.
Dewey M, Nosatov A, Subedi K, Harley B. Anisotropic mineralized collagen scaffolds accelerate osteogenic response in a glycosaminoglycan-dependent fashion. RSC Adv. 2020;10:15629.
Offeddu GS, Ashworth JC, Cameron RE, Oyen ML. Multi-scale mechanical response of freeze-dried collagen scaffolds for tissue engineering applications. J Mech Behav Biomed Mater. 2015;42:19–25.
Klaumünzer A, Leemhuis H, Schmidt-Bleek K, Schreivogel S, Woloszyk A, Korus G, et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat Commun. 2018;9(1):4430. https://doi.org/10.1038/s41467-018-06504-7.
Radzi S, Cowin G, Schmutz B. Metal artifacts from titanium and steel screws in CT, 1.5T and 3T MR images of the tibial Pilon: a quantitative assessment in 3D. Quant Imag Med Surg. 2014;4(3):163–72.
Terjesen T, Nordby A, Arnulf V. Bone atrophy after plate fixation: compute tomography of femoral shaft fractures. Acta Orthop Scand. 1985;56(5):416–8.
Alvarez K, Nakajima H. Metallic scaffolds for bone regeneration. Materials (Basel). 2009;2(3):790–832.
Moghaddam NS, Andani MT, Amerinatanzi A, Haberland C, Huff S, Miller M, et al. Metals for bone implants: safety, design, and efficacy. Biomanufact Rev. 2016;1:1.
Assad M, Jarzem P, Leroux M, Coillard C, Chernyshov AV, Charette S, et al. Porous titanium‐nickel for intervertebral fusion in a sheep model: Part 1. Histomorphometric and radiological analysis. J Biomed Mater Res Part B Appl Biomater. 2003;64B(2):107.
Bohner M. Physical and chemical aspects of calcium phosphates used in spinal surgery. Eur Spine J. 2001;10:S114–21.
Durgalakshmi D, Subhathirai S, Balakumar S. Nano-bioglass: a versatile antidote for bone tissue engineering problems. Proc Eng. 2014;92:2–8.
Scaglione S, Quarto R, Giannoni P. Stem cells and tissue scaffolds for bone repair. In: Cellular response to biomaterials. Cambridge: Woodhead Publishing Limited; 2008. p. 291–312. https://doi.org/10.1016/B978-1-84569-358-9.50012-0.
Werber K, Brauer R, Weiss W, Becker K. Osseous integration of bovine hydroxyapatite ceramic in metaphyseal bone defects of the distal radius. J Hand Surg [Am]. 2000;25(5):833–41.
Arai E, Nakashima H, Tsukushi S, Shido Y, Nishida Y, Yamada Y, et al. Regenerating the fibula with beta-tricalcium phosphate minimizes morbidity after fibula resection. Clin Orthop Relat Res. 2005;431:233–7.
Devendran S, Namashivayam S, Ambigapathi M, Nagarajan S, Tsai W-B, Sethu SN, et al. Nanoceramics on osteoblast proliferation and differentiation in bone tissue engineering. Int J Biol Macromol. 2017;98:67–74. https://doi.org/10.1016/j.ijbiomac.2017.01.089.
Roualdes O, Duclos M-E, Gutknecht D, Frappart L, Chevalier J, Hartmann D. In vitro and in vivo evaluation of an alumina–zirconia composite for arthroplasty applications. Biomaterials. 2010;31(8):2043–54.
Canzi P, Marconi S, Benazzo M. From CT scanning to 3D printing technology: a new method for the preoperative planning of a transcutaneous bone-conduction hearing device. Acta Otorhinolaryngol Ital. 2018;38(3):251–6.
Haleem A, Javaid M. Role of CT and MRI in the design and development of orthopaedic model using additive manufacturing. J Clin Orthop Trauma. 2018;9(3):213–7.
Brunello G, Sivolella S, Meneghello R, Ferroni L, Gardin C, Piattelli A, et al. Powder-based 3D printing for bone tissue engineering. Biotechnol Adv. 2016;34(5):740–53.
Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16(12):496–504.
Trombetta R, Inzana J, Schwarz E, Kates S, Awad H. 3D printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann Biomed Eng. 2017;45(1):23–44.
Maleksaeedi S, Wang JK, El-Hajje A, Harb L, Guneta V, He Z, et al. Toward 3D printed bioactive titanium scaffolds with bimodal pore size distribution for bone ingrowth. Proc CIRP. 2013;5:1558–163.
Scarano A, Crincoli V, Di Benedetto A, Cozzolino V, Lorusso F, Podaliri Vulpiani M, et al. Bone regeneration induced by bone porcine block with bone marrow stromal stem cells in a minipig model of mandibular “critical size” defect. Stem Cells Int. 2017;2017:9082869.
Walmsley GG, Ransom RC, Wan DC. Stem cells in bone regeneration. Stem Cell Rev. 2016;12(5):524–9.
Wilson SM, Goldwasser MS, Clark SG, Monaco E, Bionaz M, Hurley WL, et al. Adipose-derived mesenchymal stem cells enhance healing of mandibular defects in the ramus of swine. J Oral Maxillofac Surg. 2012;70(3):e193–203. https://doi.org/10.1016/j.joms.2011.10.029.
Ho S, Murphy K, Leach K. Increased survival and function of mesenchymal stem cell spheroids entrapped in instructive alginate hydrogels. Stem Cells Transl Med. 2016;5(6):773–81.
Ho S, Keown A, Leach K. Cell migration and bone formation from mesenchymal stem cell spheroids in alginate hydrogels are regulated by adhesive ligand density. Biomacromolecules. 2017;18(12):4331–40.
DiGirolamo C, Stokes D, Colter D, Phinney D, Class R, Prockop D. Propagation and senescence of human marrow stromal cells in culture: a simple colony‐forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 2001;107(2):275–81.
Im GI, Shin YW, Lee KB. Do adipose tissue-derived mesenchymal stem cells have the same osteogenic and chondrogenic potential as bone marrow-derived cells? Osteoarthr Cartil. 2005;13:845–53.
Luby A, Ranganathan K, Lynn J, Nelson N, Donneys A, Buchman S. Stem cells for bone regeneration: current state and future directions. J Craniofac Surg. 2019;30(3):730–5.
Follmar K, Prichard H, DeCroos F, Wang H, Levin L, Klitzman B, et al. Combined bone allograft and adipose-derived stem cell autograft in a rabbit model. Ann Plast Surg. 2007;58(5):561–5.
Kubo M, Sonoda Y, Muramatsu R, Usui M. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539–46.
Hutson E, Boyer S, Genever P. Rapid isolation, expansion, and differentiation of osteoprogenitors from full-term umbilical cord blood. Tissue Eng. 2005;11(9–10):1407.
Ventola CL. Medical applications for 3D printing: current and projected uses. Pharm Ther. 2014;39(10):704–11.
Graney PL, Zreiqat H, Spiller KL, Spiller KL. In vitro response of macrophages to ceramic scaffolds used for bone regeneration. J R Soc Interface. 2016;13:20160346.
Witherel CE, Yu T, Concannon M, Dampier W, Spiller K. Immunomodulatory effects of human cryopreserved viable amniotic membrane in a pro-inflammatory environment in vitro. Cell Mol Bioeng. 2017;10:451.
Weingarten MS, Witherel CE, Spiller KL, Graney PL, Freytes DO. Response of human macrophages to wound matrices in vitro. Wound Repair Regen. 2016;24(3):514–24.
Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–88. https://doi.org/10.1016/j.biomaterials.2014.02.012.
Go YY, Kim SE, Cho GJ, Chae S, Song J. Differential effects of amnion and chorion membrane extracts on osteoblast-like cells due to the different growth factor composition of the extracts. PLoS One. 2017;12(8):1–20.
Go YY, Kim SE, Cho GJ, Chae S-W, Song J-J. Promotion of osteogenic differentiation by amnion/chorion membrane extracts. J Appl Biomater Funct Mater. 2016;14(2):e171–80.
Carano R, Filvaroff E. Angiogenesis and bone repair. Drug Discov Today. 2003;8(21):980–9.
Ngo M, Harley B. The influence of hyaluronic acid and glioblastoma cell coculture on the formation of endothelial cell networks in gelatin hydrogels. Adv Healthc Mater. 2017;6(22)
Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. Chir Organi Mov. 2008;92:161–8.
Laflamme C, Curt S, Rouabhia M. Epidermal growth factor and bone morphogenetic proteins upregulate osteoblast proliferation and osteoblastic markers and inhibit bone nodule formation. Arch Oral Biol. 2010;55(9):689–701.
Yun Y-R, Jang JH, Jeon E, Kang W, Lee S, Won J-E, et al. Administration of growth factors for bone regeneration. Regen Med. 2012;7(3):369.
Hossain M, Irwin R, Baumann M, McCabe L. Hepatocyte growth factor (HGF) adsorption kinetics and enhancement of osteoblast differentiation on hydroxyapatite surfaces. Biomaterials. 2005;26(15):2595–602.
Kawasaki T, Niki Y, Miyamoto T, Horiuchi K, Matsumoto M, Aizawa M, et al. The effect of timing in the administration of hepatocyte growth factor to modulate BMP-2-induced osteoblast differentiation. Biomaterials. 2010;31(6):1191–8.
Deng M, Zhang B, Wang K, Liu F, Xiao H, Zhao J, et al. Mechano growth factor E peptide promotes osteoblasts proliferation and bone-defect healing in rabbits. Int Orthop. 2011;35(7):1099–106.
Dai Z, Wu F, Yeung E, Li Y. IGF-IEc expression, regulation and biological function in different tissues. Growth Hormon IGF Res. 2010;20(4):275–81.
Young CS, Bradica G, Hollinger JO. Preclinical toxicology studies of recombinant human platelet-derived growth factor-bb either alone or in combination with beta-tricalcium phosphate and type I collagen. J Tissue Eng. 2011;2010:246215.
Chang P-C, Seol Y-J, Cirelli J, Pellegrini G, Jin Q, Franco L, et al. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene Ther. 2010;17:95–104.
Andrew J, Hoyland J, Andrew S, Freemont A, Marsh D. Demonstration of TGF-β1 mRNA by in situ hybridization in normal human fracture healing. Calcif Tissue Int. 1993;52(2):74–8.
Bourque W, Gross M, Hall B. Expression of four growth factors during fracture repair. Int J Dev Biol. 1993;37(4):573–9.
Cheng H, Jiang W, Phillips F, Haydon R, Peng Y, Zhou L, et al. Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Jt Surg Am. 2003;85(8):1544–52.
Krishnakumar GS, Roffi A, Reale D, Kon E, Filardo G. Clinical application of bone morphogenetic proteins for bone healing: a systematic review. Int Orthop. 2017;41(6):1073–83.
Chen W-J, Jingushi S, Aoyama I, Anzai J, Hirata G, Tamura M, et al. Effects of FGF-2 on metaphyseal fracture repair in rabbit tibiae. J Bone Miner Metab. 2004;22(4):303–9.
Draenert G, Draenert K, Tischer T. Dose-dependent osteoinductive effects of bFGF in rabbits. Growth Factors. 2009;27(6):419–24.
Mitlak B, Finkelman R, Hill E, Li J, Martin B, Smith T, et al. The effect of systemically administered PDGF‐BB on the rodent skeleton. J Bone Miner Res. 1996;11(2):238.
Marden L, Ran R, Pierce G, Reddi A, Hollinger J. Platelet-derived growth factor inhibits bone regeneration induced by osteogenin, a bone morphogenetic protein, in rat craniotomy defects. J Clin Inven. 1993;92(6):2897–905.
Chung C, Kim D, Park Y, Nam K, Lee S. Biological effects of drug-loaded biodegradable membranes for guided bone regeneration. J Peridontal Res. 1997;32:172–5.
Schliephake H. Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg. 2002;31:469–84.
Canalis E. Growth factor control of bone mass. J Cell Biochem. 2009;108:769–77.
Specer E, Liu C, Si E, Howard G. In vivo actions of insulin-like growth factor-I (IGF-I) on bone formation and resorption in rats. Bone. 1991;12:21–6.
Akeno N, Robins J, Zhang M, Czyzyk-Krzeska M, Clemens T. Induction of vascular endothelial growth factor by IGF-I in osteoblast-like cells is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2alpha. Endocrinology. 2002;143(2):420–5.
Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger M, Auer J, et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33(4):660–72.
Hayrapetyan A, Jansen JA, van den Beucken JJJP. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng Part B Rev. 2014;21(1):75–87.
Hong L, Tabata Y, Miyamoto S, Yamada K, Aoyama I, Tamura M, et al. Promoted bone healing at a rabbit skull gap between autologous bone fragment and the surrounding intact bone with biodegradable microspheres containing transforming growth factor-β1. Tissue Eng. 2004;6(4):331.
Yamamoto M, Tabata Y, Hong L, Miyamoto S, Hashimoto N, Ikada Y. Bone regeneration by transforming growth factor β1 released from a biodegradable hydrogel. J Control Release. 2000;64(1–3):133–42.
Street J, Bao M, DeGuzman L, Bunting S, Peale FJ, Ferrara N, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61.
Oryan A, Alidadi S, Moshiri A. Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther. 2016;16:213.
Zou J, Yuan C, Chunshen W, Cao C, Yang H. The effects of platelet-rich plasma on the osteogenic induction of bone marrow mesenchymal stem cells. Connect Tissue Res. 2014;55(4):304.
Raida M, Heymann A, Gunther C, Niederwieser D. Role of bone morphogenetic protein 2 in the crosstalk between endothelial progenitor cells and mesenchymal stem cells. Int J Mol Med. 2006;18:735–9.
Park JY, Shim JH, Choi S-A, Jang J, Kim M, Lee SH, et al. 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J Mater Chem B. 2015;2:5415–25.
Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials. 2015;37:194–207.
Acknowledgements
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs Broad Agency Announcement for Extramural Medical Research through the Award No. W81XWH-16-1-0566 (BACH). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. This work was also supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number R21 DE026582 (BACH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We are grateful for the funding for this study provided by the NSF Graduate Research Fellowship DGE-1144245 (MJD). The authors would like to acknowledge Vasiliki Kolliopoulos and Andrey Nosatov for assistance with manuscript editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dewey, M.J., Harley, B.A.C. (2022). Biomaterial Design Principles to Accelerate Bone Tissue Engineering. In: Guastaldi, F.P., Mahadik, B. (eds) Bone Tissue Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-92014-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-92014-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-92013-5
Online ISBN: 978-3-030-92014-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)