Abstract
Acid-base renal regulation is based on bicarbonate tubular reabsorption and H+ excretion. Molecular transmembrane mechanisms coupled to Na+Cl− and K+ reabsorption and excretion are involved.
Most sodium bicarbonate molecules filtered are reabsorbed by the proximal tubules, in exchange for H+ by an N+/H+ exchanger, and then reabsorbed at the basolateral membrane (Na+K+ATPase). Luminal H+ is sent downstream to the distal nephron. Na+Cl− and K+ become reabsorbed at the apical membrane, ascending limb Henle’s loop (Na+K+2Cl−). Sodium is reabsorbed in exchange for potassium at the next segment: aldosterone-sensitive distal nephron· (ASDN). The finest regulation of acid-base metabolism is by hydrogen excretion (connecting, collector tubules). “New bicarbonate” is formed and reabsorbed. Apical transporters: H+ATPase, H+K+ATPase. Basolateral membrane: kAE1 (anion exchanger).
Ammonium chloride (NH4+) may occupy the sites of H+ ions at the transporters; thus, ammonia excretion and reabsorption follow similar patterns as hydrogen excretion throughout the nephron. Rh glycoproteins (Rhgc, Rhbg) are also involved in this process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
As mentioned in the previous chapter, the lungs perform the task of removing organic volatile acids in the form of pCO2, in exchange for oxygen. The kidneys eliminate non-volatile organic acids that contain hydrogen (protons), which includes H+ from the intermediate metabolism, by which the dietary proteins participating in the formation of amino acids, such as leucine, isoleucine, valine, arginine, and methionine, are metabolized. Most of the H+ is excreted as buffer systems to be excreted into the urine. One such system, “titratable acid” (TA), is composed of the buffer pairs phosphoric acid/phosphates and sulfuric acid/sulfates. The other system is composed of ammonium/ammonia [1].
The excretion of hydrogen ions or protons and, in consequence, the formation of bicarbonate, depends on several factors, such as the glomerular filtration rate; the systemic pH (influencing H+ excretion along the nephron); the apical (luminal) pH; intracellular pH; concentration of HCO3− and the peritubular pCO2, as well as the action exerted by angiotensin II (Ag II) upon proximal reabsorption of Na+.
The renal tubular excretion of hydrogen ions is coupled to the excretion and reabsorption of sodium. Therefore, some physiological mechanisms that affect the renal handling of sodium will also affect the renal regulation of acid-base metabolism, being mainly the electrolyte composition and volume of the extracellular fluid (ECF); enzymatic and hormonal substances; transmembrane transport proteins; concentration gradient difference between the apical membranes of the renal tubular cell cytoplasm and luminal fluid, as well as between the cytoplasm and the peritubular fluid across the basolateral membrane [2]. Some of these mechanisms will be described below.
Hydrogen Excretion and Bicarbonate Reabsorption in the Proximal Tubule
The main function of the proximal tubule is the reabsorption of sodium chloride and other electrolytes, water, bicarbonate, glucose, calcium, magnesium, phosphates, sulfates, and small amounts of some amino acids and other chemicals filtered by the glomeruli. Most of these substances are recovered by the proximal tubules, through reabsorption in the three segments, S1, S2, and S3.
The proximal tubule’s main function regarding acid-base metabolism is the excretion of hydrogen ions and reabsorption of bicarbonate. The amount of H+ excreted by the kidneys is about 4200 mmol daily, in an adult subject with normal renal function. Most of the filtered bicarbonate is reabsorbed in the proximal tubules. Hydrogen ion excretion is accomplished by the production and excretion of TA and ammonia. The buffer pair ammonium/ammonia is produced mainly, but not exclusively, in the proximal tubule. The main area of excretion of ammonia is the final portion of the nephron, as will be described in the next section.
Some buffer substances within the ECF also undergo glomerular filtration. Bicarbonate, which is the main ECF buffer, is filtered as sodium bicarbonate. The apical membranes of the proximal tubular cells cannot reabsorb NaHCO3 molecules as such. Therefore, this molecule dissociates as Na+ and HCO3− molecules in the proximal luminal fluid. The Na+ molecule gets reabsorbed in the apical (luminal) membrane of the brush border cells by the action of several mechanisms, mainly a transmembrane transporter protein sodium/hydrogen exchanger (Na+/H+), named NHE3 , in an electroneutral exchange. Several NHE3 isoforms function on both the apical and basolateral membranes, depending on the physiological need of each site [3,4,5,6] (Fig. 2.1). Furthermore, the Na+ molecules are reabsorbed through Na+ channels, or by coupling with other molecules, such as glucose, phosphates, sulfates, amino acids, citrates, and other organic acids [7]. The proximal sodium reabsorption process ends up in the basolateral membrane by the action of the sodium/potassium transporter protein adenosine triphosphatase, also called sodium/potassium pump or, Na+K+ATPase, which exchanges 3 molecules of Na+ by 2 K+ molecules. Thus, this exchange favors the formation of a reduced Na+ concentration gradient and an intracellular electronegative gradient , a physiological scenario that facilitates the continuous entry of sodium into the cell and its reabsorption into the vasa recta, to the systemic circulation and the ECF [8].
Proximal tubule : Excretion of H+ and reabsorption of Na+HCO3- initiates in the brush border cells of the proximal tubule. The Na+HCO3 molecule splits in the tubular lumen, allowing the Na+ molecule to be exchanged for H+ through the apical membrane, by the exchanger protein NHE3. Acetazolamide inhibits whereas angiotensin II (AG II) accelerates the expression of NHE3. The H+ excreted in the tubular lumen binds to the HCO3- molecule which remained from the sodium bicarbonate filtered previously in the glomerulus. The equilibrium reaction takes place in the tubular lumen, rendering carbonic acid (H2CO3) and CO2 + H2O in the presence of the enzyme carbonic anhydrase IV (AC IV). CO2 and H2O are transported to the cytoplasm in the presence of aquaporins, mainly AQP 1. In the cytosol, the equilibrium reaction gets reversed, leading to reassemble H++ HCO3−. H+ is again excreted to the tubular lumen, while HCO3− is reabsorbed by the basolateral membrane, after binding Na +, favored by the NBCe1 cotransporter. Na+ molecules are also reabsorbed by the Na+K+ATPase protein, exchanging 3 Na+ molecules for 2 K+, facilitating intracellular electronegativity and Na+ reabsorption from the lumen into the peritubular space. Chromosomal alterations of the NHE3 counter transporter, AC, and the NBCe1 cotransporter lead to the development of Type II primary proximal renal tubular acidosis
Hydrogen ions go in the opposite direction, being extracted from the cytosol of the proximal cells toward the proximal tubular lumen, in exchange for Na+ molecules, by the NHE3 exchanger (Na+/H+ exchanger), as already mentioned. There, the hydrogen ions bind to HCO3− molecules that remained free in the tubular lumen to form carbonic acid (H2CO3). The equilibrium reaction starts this way within the tubular luminal fluid , a chemical reaction that is catalyzed in the presence of the enzyme carbonic anhydrase IV (AC IV), in the apical membrane of the brush border cells.
Carbonic acid dissociates in carbon dioxide (CO2) and H2O; both molecules penetrate the cell through the apical membrane favored by the action of H2O transport proteins, which in the proximal tubule is mainly aquaporin 1 (AQP1), which also functions as transmembrane gas transporter. In the present situation, AQP1 transports CO2 through the apical membrane, as well as water molecules, into the cytoplasm [9].
The reabsorption of H2O molecules is catalyzed by the presence of various aquaporins, throughout the nephron. Once the H2O and CO2 molecules enter the cytoplasm, the equilibrium reaction is reversed, leading to the intracellular production of H+ + HCO3−. Isoforms of carbonic anhydrase II and IV (AC II, AC IV) are important metalloenzymes in the regulation of acid-base metabolism, since they associate the respiratory component with the metabolic component, working simultaneously in both directions through the equilibrium reaction [10], as described below:
This way, CO2 is produced during the cellular metabolism , to be transported by the bloodstream and excreted by the lungs in the form of pCO2. On the other hand, in the equilibrium reaction H+ is eliminated by the kidneys, a process that leads to the production and reabsorption of bicarbonate. As this happens, carbonic anhydrase accelerates the equilibrium reaction hundreds of times per second, becoming an important mechanism of the buffer system in the EEC, as well as the renal regulation of the acid-base metabolism.
Although the CO2 is not an acid, it behaves as such due to its participation in the equilibrium reaction in a clockwise direction, mainly in clinical situations of overproduction and accumulation of pCO2 (respiratory acidosis). The increased production of CO2 makes the equilibrium reaction run to the right, leading to the formation of HCO3− and H+ ions. Bicarbonate molecules produced this way are used as a buffer, whereas the protons are excreted by the kidneys. The AC is essential in the reabsorption of bicarbonate and the excretion of hydrogen ions.
Once CO2 and H2O molecules enter the cytoplasm of the renal tubular cell through the apical membrane, the equilibrium reaction is reversed; the intracellular H2O molecule dissociates into a hydrogen ion (H+) and a hydroxyl group (OH−), facilitated by the intracellular carbonic anhydrase II (AC II). The OH− group binds to CO2 to form bicarbonate. Once the Na+HCO3− molecules are reassembled in the cytoplasm, they are reabsorbed as such through the basolateral membrane, facilitated by the presence of the sodium/bicarbonate exchanger, Na+ 3HCO3− (NBCe1), which transports 3 bicarbonate molecules, coupled by 1 sodium molecule [11] (Fig. 2.1). Besides, the excretion of H+ is further facilitated by the presence of a vacuolar proton transporter protein in the apical membrane, the H+ATPase or V-H+ATPase (vacuolar), although to a lesser extent in the proximal tubule than in the collecting tubule, where this transporter exercises its priority action on the α-intercalated cells [12]. Alterations or mutations of the NHE3, NBCe1, and AC II transporter proteins give rise to the development of hereditary proximal RTA, as described in the corresponding chapter of this text.
Hydrogen ion excretion and the consequent bicarbonate reabsorption are closely linked to sodium reabsorption in the proximal tubule. Under physiological conditions, the amount of Na+ reabsorbed, mainly as Na+Cl− and Na+ HCO3− at this site of the nephron, is about 70% of the glomerular filtration rate. Therefore, the glomerular filtration rate is a determining factor in the reabsorption of bicarbonate, sodium, chloride, and other electrolytes. Under physiological conditions, 50% to 75% of the filtered Na+ molecules are reabsorbed via the transcellular route; the remaining sodium by the paracellular route. Sodium reabsorption increases significantly during dehydration, hypovolemia, or shock [13].
In contrast, 80% to 90% of the NaHCO3− filtered by the glomeruli is reabsorbed in the proximal tubule. Of this amount, 80% is reabsorbed by the transcellular route and the remaining 20% by the paracellular route, by passive diffusion. Although urinary acidification begins in the proximal tubule, it represents only a minimal quantity, since the urinary pH at the end of the proximal segment 3 (S3) is reduced barely to 6.8–6.7, from the filtered fluid at pH 7.40, at the time of leaving the glomerulus. In contrast, maximum urinary acidification is achieved in the distal medullary collecting tubules, with a maximal reduction of the pH to 4.5–4.0 [14].
The main objective of the physicochemical functions described in the proximal tubule is the recovery of the filtered bicarbonate by the glomeruli, which otherwise would be lost in the final urine. In the event, this occurs the pathologic entity is named proximal RTA (type II), which is due to a reduction of the proximal tubule bicarbonate reabsorption threshold ; which is described at length in the corresponding chapter.
Ammonia Synthesis and Excretion in the Proximal Tubule
In the present section, the term ammonia is described as the buffer pair composed of ammonia (NH3) and ammonium (NH4+) molecules (pK ≅ 9.15). Since most of the body fluids are stable with a pH of 7.40 (±0.05), most of the compound is in the form of ammonium.
The purpose of the buffer systems is to prevent or minimize sudden pH changes in the ECF. Thus, the buffer function NH3 + H+ → NH4+ avoids the accumulation of H+, which will be later excreted by the kidneys. Most of the substances that participate in some way in the physiology of the kidneys are provided by the systemic circulation, whereas ammonia is produced (ammonia-genesis) inside the kidneys, throughout the epithelial cells of the nephron, including some of the glomerular epithelial cells (Fig. 2.2). Nevertheless, most of the production is in the mitochondria of the brush border cells of the S1, S2, and S3 segments of the proximal tubule , mainly as NH3. Ammonia formation originates from the amino acid glutamine metabolism. After several biochemical modifications, ammonia becomes transformed into α-ketoglutarate, rendering 3 bicarbonate and 2 ammonia molecules at the end of the process.
Proximal tubule : Synthesis and secretion of ammonia. NH3 molecules are exchanged for H+ in the cells of the proximal tubule by NHE3 in the apical membrane, as well as for K+ at the sites of various K+ channels, such as KCNA10, TWIK 1, KCNQ1, and KCNE1, going from the cytosol into the tubular fluid, where it binds to H+ to form NH4+. The bicarbonate formed in the cytoplasm during the equilibrium reaction is reabsorbed coupled to a Na+ molecule, by the sodium/bicarbonate co-transporter, NBCe1 located in the basolateral membrane. This is the same site of exchange of K+ for NH4+, which enters the cytosol from the peritubular fluid
Systemic acidosis is a powerful stimulus to increase ammonia production in the proximal tubules [15]. NH3 molecules are extracted from the cell at the apical membrane, mainly by the sodium/hydrogen exchanger (Na+/H+) (NHE3). The diuretic acetazolamide inhibits the action of carbonic anhydrase in the proximal tubule, favoring the development of metabolic acidosis. Besides, acetazolamide inhibits the NHE3 transporter protein; therefore, NH4+ excretion. The increased NHE3 expression and proximal tubular ammonium excretion seem to need the activation of the angiotensin II receptor (R-Ag II) [16].
It is worth mentioning that ammonia molecules may be exchanged for Na+ or H+ molecules, at the transport site channels where these cations are transported through the cell membrane of the renal tubular cells. This phenomenon occurs since the hydrodynamic radius of these cations is similar to those of ammonia. Also, there are cellular transmembrane transporter proteins that are specific for ammonia, primarily for NH3, as will be described later. The largest amount of ammonia is extracted from the cell into the tubular lumen, where it binds to an H+ molecule to form NH4+, although a small amount gets reabsorbed through the cell’s basolateral membrane [17]. As mentioned before, the two ammonia molecules produced in the proximal tubule are excreted in the final urine, whereas the 3 bicarbonate molecules are restored to the ECF by the (NBCe1) cotransporter, contributing further to the regulation of the acid-base metabolic process. NBCe1 mutations give rise to proximal renal tubular acidosis, type II [18].
New K+ channels have recently been described in the brush border cells of the proximal tubules. They share potassium channel transport sites with other cations, mainly ammonia and, with less specificity, with sodium. They are the so-called “hyperpolarization-activated cation channels and cyclic nucleotides”, also known as HCN or pacemaker channels, which comprise 4 homologous subunits, HCN1 to HCN4. The HCN1 and HCN3 channels are located in the apical membrane of the proximal tubular cells and transport NH3 from the cytosol to the tubular lumen. There, they bind free H+ ions to form ammonia, which in turn, are transported in the luminal fluid to the collecting tubules. In addition to proximal hydrogen excretion, HCNs favor the ammonia genesis process.
HCN2 is expressed in the basolateral membrane of the α-intercalated cells of the collecting tubules, captures NH4+ which is transported to the cytosol; from there on to the tubular lumen for its final excretion. Furthermore, pacemaker channels are present in the α-intercalated cells of the collecting tubule, as well as in the thick ascending limb of the loop of Henle, as will be described later [19] (Fig. 2.3).
Proximal tubules : Ammonia excretion. Cationic channels activated by hyperpolarization and cyclic nucleotides (HCN). HCNs (pacemaker channels) comprise 4 homologous subunits, HCN1 to HCN4. HCN1 and HCN3 are expressed in the apical membrane of the proximal tubule brush border cells, being their main function is the excretion of K+ ions, which can be exchanged for other cations, such as NH3, from the cytoplasm to the tubular lumen. At this site, it binds an H+ ion to form NH4+, which descends via the proximal convoluted tubule into Henle’s descending loop. In the basolateral membrane, NH4+ occupies the K+ site of the Na+K+ATPase transporter protein
The mechanisms of hydrogen ion reabsorption and ammonia excretion in the proximal tubule allow the recovery of bicarbonate from the glomerular filtrate and its reabsorption into the extracellular space. Furthermore, urinary acidification begins in the proximal tubule, albeit moderately, reducing the initial filtrate pH from 7.40 to ±6.80.
Hydrogen Excretion and Bicarbonate Reabsorption in the Loop of Henle
The descending limb of Henle’s loop directs the glomerular filtrate from the proximal convoluted tubule to the renal medullary portion. While the fluid progresses downstream, NaCl is reabsorbed into the medullary interstitium, decreasing progressively its concentration inside the lumen. As the electrolyte concentration increases, NaCl has an important role in the countercurrent mechanism, a determining physiological function that participates in the maximum urinary concentration and dilution capacity. This mechanism also facilitates the concentration and recycling of ammonia in the medullary interstitium and, its final excretion in the urine, with an important effect on the systemic and renal regulation of acid-base metabolism [20].
The ascending limb of the loop of Henle reabsorbs approximately 15% of the bicarbonate filtered in the glomeruli and has unique physiological characteristics concerning the reabsorption of Na+ and other electrolytes, as well as in the regulation of acid-base metabolism. This area of the nephron is impermeable to water.
Sodium reabsorption is dependent on the cotransporter Na+ K+ 2Cl- (NKCC2), which transports sodium and potassium, 1 molecule each, plus 2 molecules of chloride, through the apical membrane of the tubular lumen [21, 22] (Fig. 2.4). Cl− molecules which enter the cytoplasm, leave the cell through the basolateral membrane by the K+/Cl− exchanger, whereas Na+ is extracted from the cytosol towards the interstitium by the action of the basolateral exchanger Na+K+ATP’ase (sodium/potassium pump), which, as in the proximal tubule, exchanges 3 Na+ molecules extracted from the cytosol, for 2 K+ molecules from the peritubular fluid. Both the entrance of potassium to the cell through the apical route (Na+K+2Cl−) and the exit of chloride through the basolateral membrane (K+Cl−) generate a positive electric charge in the tubular lumen, facilitating the transcellular and paracellular reabsorption of Na+. The concentration of cytoplasmic K+ increases due to entry into the cell by the basolateral membrane (Na+K+ATP’ase), plus the K+ entrance via the apical route (Na+K+2Cl−). These molecules return to the tubular lumen through the K+ channels localized in the apical membrane, called ROMK (Rat-Outer-Medullary K+ Channel). Hence, the intracellular and luminal fluid recirculation of K+ maintains the reabsorption cycle of Na+, K+, and Cl− operating continuously in the ascending limb of Henle’s loop [15, 22].
Thick ascending limb of the loop of Henle (TALH): Hydrogen excretion and bicarbonate reabsorption. This important part of the nephron, characterized by being impermeable to water, is the dilution site of the urine in the tubular lumen; it’s a relevant piece for the generation of a hypertonic interstitium and the development of the countercurrent mechanism. There is no aquaporins function in the apical membrane. Na+, K+, and Cl− are reabsorbed from the tubular lumen, by the Na+K+ 2Cl− cotransporter, which is inhibited by the use of loop diuretics. The K+ accumulating in the cytosol is excreted to the apical membrane through the K+ channels (ROMK). As in the proximal tubule, the NHE3 cotransporter also exchanges Na+ for H+ in the apical membrane. The Na+ entering the cell through the NKCC2 cotransporter gets reabsorbed in the basolateral membrane in exchange for 2 K+ molecules by the Na+K+ATP’ase anti-transporter. Sodium bicarbonate is reabsorbed in the basolateral membrane by the Na+ HCO3− cotransporters, NBCe1 and NBCn1, as well as by exchange with Cl- by the anionic counter transporter AKE1. Furthermore, in the basolateral membrane, the Na+ molecules are reabsorbed in exchange for H+ by the NHE4 anti-transporter, which function is similar to its counterpart NHE3 in the apical membrane
Another mechanism of Na+ reabsorption in exchange for H+ ions (NHE3) in the apical membrane, generating HCO3− molecules, which in turn become reabsorbed into the systemic circulation by the action of the Na+/HCO3− cotransporter or exchanger (NBC1), located at the basolateral membrane [23].
Metabolism of Ammonia in the Loop of Henle
The ammonium from the proximal tubule is reabsorbed mainly in the form of ammonia (NH3) progressively as the filtrate advances to the renal medullary portion in the path of the descending loop of Henle, a process that is facilitated by the countercurrent mechanism. NH3 accumulates and is recycled in the medullary interstitial fluid, to finally be excreted in the collecting tubule. The purpose of increasing the concentration of NH4+ and NH3 in the tubular lumen and the peritubular fluid is to facilitate the excretion of NH4+ in the final urine through the collecting tubule.
Ammonia (NH3/NH4+) does not cross the cell membrane since it is not fat-soluble; therefore, it requires transporter proteins to carry out the reabsorption and tubular excretion processes (Fig. 2.5). The main mechanism of apical transmembrane transport in the ascending limb of the loop of Henle is the Na + K+ 2Cl− exchanger (NKCC2), which can transport ammonia as a substitute for K+ in the apical membrane. Therefore, it also participates in the regulation of acid-base metabolism [23, 24]. Luminal NH4+ competes with K+ for transport sites of the NKCC2 transporter protein. In the presence of hypokalemia or hyperkalemia, the concentration of K+ in the tubular lumen is altered and, thus, altering the transport of NH4+ at the same time [25, 26].
Thick ascending limb of the loop of Henle (TALH): Reabsorption and excretion of ammonia. Renal metabolism in the loop of Henle shows particular characteristics since the reabsorption of NH3 at the apical membrane predominates. NH3 accumulates and recycles in the medullary peritubular space, to its subsequent absorption and excretion in the collecting tubule. Only a minor amount of NH4 + (± 30%), remains in the tubular lumen, to continue its way to the distal tubule. In the apical membrane, NH4+ molecules may compete for the K+ sites at the Na+K+2Cl− cotransporter (NKCC2). In the ROMK channels, NH4+ is absorbed and K+ ions are removed from the cell into the lumen. Ammonia is also exchanged for H+ ions in the anti-transporter NHE3 of the apical membrane. In the basolateral membrane, NH3 molecules are exchanged at the K+ sites of the Na+K+ATPase transporter protein. Besides, the anti-transporter NH4 is expressed in the basolateral membrane, which exchanges Na+ ions (which enter the cytosol), for H+ ions, which are extracted into the peritubular fluid. NH4+ molecules are exchanged for H+ ions, thereby increasing the reabsorption of ammonia in the TALH. HCN3 pacemaker channel facilitates the reabsorption of NH4+ and stimulates the NBCn1 cotransporter, as well as the Na+K+ATPase transporter in the basolateral membrane. At the moment, chromosomal alterations in the TALH that may lead to the development of ATR are unknown
The thick ascending limb of Henle’s loop is characterized by increasing ammonium reabsorption and the recycling of ammonia in the renal medulla (Fig. 2.5). The high concentration of ammonia in the medullary interstitium is due to the reabsorption of ammonia in the descending limb and, the parallel reabsorption in the thick ascending limb of Henle, acting simultaneously in the form of a short circuit. The high concentration of ammonia that is reached in this area facilitates excretion into the collecting tubule and excretion of NH4+ in the final urine. The anti-transporter Na+/H+ or Na+/NH4+ (NHE4) is expressed in the basolateral membrane, the main action of which is to extract NH4+ from the cell to replace H+. This mechanism facilitates increasing the concentration of NH4+ in the medullary peritubular fluid; the result of which involves the reabsorption of a significant amount of ammonia in Henle’s thick ascending limb, thus, avoiding ammonia to proceed towards the distal tubule. Only 20 to 40% is transported to the distal tubule. Just a small amount of ammonia is reabsorbed in the distal tubule and about 10% to 15% goes downstream to the collecting tubule for its final excretion in the urine [27, 28].
Also, in the basolateral membrane are expressed the “cation channels activated by hyperpolarization and cyclic nucleotides” (HCN3), which participate in the reabsorption of NH4+ and the extraction of NH3 from the cytosol to the interstitium where it accumulates during the countercurrent mechanism. These channels stimulate the sodium/bicarbonate exchanger (NBCn1, NBCe1) and the Na+K+ATPase protein, so, the presence of HCN3 is important in the regulation of acid-base metabolism [29].
Hydrogen and Ammonium Excretion in the Distal and Connector Tubules
Tubular sodium reabsorption continues in the distal convoluted tubule, as well as in the principal cells of the connecting tubule and cortical collecting duct. This area of the nephron is known as the “aldosterone-sensitive distal nephron” (ASDN), where sodium reabsorption is through the epithelial sodium channels (ENaC). The apical reabsorption of sodium molecules in the distal tubule is mainly in exchange for K+ and to a lesser extent for hydrogen ions. This function is dependent on aldosterone, a hormone produced in the “zona glomerulosa” of the adrenal glands. Aldosterone stimulates the opening of the ENaC [30].
The scientific information available regarding the metabolism of ammonia in the distal tubule is scarce due to the difficulties in accessing this region of the kidney by micro-puncture studies. However, ammonia excretion in this tubular segment complies with some 10% to 15% of the total excretion of this buffer, under physiological conditions.
Hydrogen Excretion in the Collecting Tubule
Most hydrogen ions excretion and the consequent formation of “new bicarbonate” take place in the apical (luminal) membrane of the α-intercalated cells of the collecting tubule. In this area of the nephron, hydrogen ion excretion is independent of the reabsorption of sodium. The main excretory mechanism for H+ ions depends on the hydrogen pump or adenosine tri-phosphatase (H+ ATPase), which is also called vacuolar H+ ATPase (V-H+ ATPase) [30]. The H ATPase is made up of several subunits and it is energy-dependent. The B1 subunit is similar to the basolateral H+ ATPase of the proximal tubule, while the B2 subunit performs its function mainly in the collecting duct of the kidney. α-intercalated cells increase the production of V-H+ ATPase in response to several factors, such as the reduction of intracellular pH; the increase in the organic production of hydrogen ions and the presence of metabolic acidosis. This situation also occurs as a renal tubular metabolic compensation mechanism in the presence of secondary respiratory acidosis, with pCO2 retention [31,32,33,34] (Fig. 2.6).
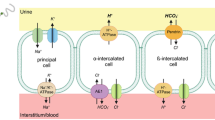
Connecting and collecting tubules, α-intercalated cell: Hydrogen excretion and bicarbonate reabsorption. This is an important part of the nephron in hydrogen excretion, which in turn is accompanied by bicarbonate molecule formation. This “new bicarbonate” is reabsorbed into the peritubular fluid and the extracellular fluid. The main function of “new bicarbonate” is to correct systemic acidosis. In this area, the excretion of H+ ions is independent of the reabsorption of Na+. In the apical membrane, the excretion of H+ depends mainly on the transporter proteins H+ ATPase (V H+ ATPase) or hydrogen bomb and H+K+ATPase, or potassium/hydrogen pump. Both transporter proteins excrete H+ into the tubular lumen; H+K+ATPase in exchange for K+ ions. H+ ions excreted in the tubular lumen bind phosphates to form phosphoric acid (HPO42 + H+ → H2PO4−) and sulfates to form sulfuric acid (HSO42 + H+ → H2SO4−), or titratable acid, to form ammonium (NH3 + H+ → NH4+). K+ entering the cell is extracted through the K+ channels (ROMK, Maxi-K, Kv1.3) into the tubular lumen. In the apical membrane, there is almost no AC IV; thus, CO2 and H2O molecules form slowly in the tubular space. However, intracellular AC II is abundant and sodium bicarbonate is produced and reabsorbed through the basolateral membrane by kAE1, which transports Cl− to the cytosol and HCO3− to the peritubular medullary fluid. Cl− ions are reabsorbed by Cl− channels, together with Na+ and K+ by the Na+K+2Cl− cotransporter, or NKCC1, in the basolateral membrane. NKCC1 is the counterpart of NKCC2, the latter expressed in the apical membrane of TALH. Chromosomal mutations of the H+ATPase, AC II, and the anti-transporter kAE1 are the etiology of primary distal ATR, type I
There is another mechanism of excretion of hydrogen through the apical membrane in the collecting tubules, which is the H+ K+ ATPase transporter protein, which exchanges an H+ molecule extracted from the cytoplasm, in exchange for a K+ molecule from the tubular lumen. This exchange is electroneutral, so it does not affect its function in the presence of transmembrane voltage changes. Two isoforms are expressed in the digestive system, gastric H+K+ ATPase, which is involved in the production of H+Cl− in the gastric mucosa and, colonic H+K+ ATPase. These isoforms are also expressed in the kidney. The presence of metabolic acidosis increases the production of H+K+ATPase. It is also stimulated in the presence of hypokalemia. H+K+ATPase exchanges K+ for H+ and can exchange Na+ molecules for H+; acting this way as a Na+K+ ATPase. It can also exchange NH4+ for K+ to excrete it. The reduction in blood K+ concentration as well as the presence of metabolic acidosis stimulate the secretion of H+K+ ATPase in the collecting tubule [35, 36].
The reabsorption of Na+ through the basolateral membrane is facilitated by the presence of the Na+K+ ATPase protein, as well as by the NKCC1 exchanger, which is the counterpart to the NKCC2, whose function is exerted in the apical membrane of the thick ascending limb of Henle. Likewise, its counterpart NKCC1 carries one Na+, one K+, and two Cl− molecules; but the molecular transportation takes place in the opposite direction, that is, from the α-intercalated cell cytoplasm to the fluid of the peritubular space.
Intracellular carbonic anhydrase (AC II) plays an important role as a source of intracellular H+ production (through the equilibrium reaction) which, in turn, is excreted by the transporter proteins V-H+ ATPase and H+K+ATPase. In contrast, extracellular AC IV is almost non-existent in the collecting tubule [37]. The new HCO3- produced in the α-intercalated cell is reabsorbed at the basolateral membrane by the presence of the anionic anti-transporter kAE1, which regulates the tight junction through the Claudin-4 protein [38].
Contrary to what happens in the presence of acidosis, when the intracellular or systemic pH increases, or in the presence of a fully developed clinical metabolic alkalosis, H+K+ ATP’ase translates the site of action toward the basolateral membrane at the β-intercalated cells, increasing the amount of bicarbonate excretion and conserving hydrogen ions that return to ECF, in the process of correcting or compensating the alkalosis. The H+ ATP’ase (V-H+ ATP’ase) transporter protein, which generally functions in the luminal membrane of the α-intercalated cells to excrete H+ ions, can also transfer its function to β-intercalated cells to excrete bicarbonate during an episode of systemic alkalosis, thus demonstrating the functionality of these transporter proteins [39, 40].
Mutations of AC II, V-H+ ATPase and anion exchanger kAE1 give rise to the development of distal tubular acidosis (Type I). The chromosomal mutations leading to the development of renal tubular acidosis are described in detail in the corresponding chapter.
H+ transporter molecules, mostly V-H+ATP’ase, excrete intracellular H+ into the tubular lumen, where hydrogen ions bind to phosphate to form phosphoric acid (HPO4−2/H2PO4−) and sulfates to form sulfuric acid (HSO4−2/H2SO4−), which are excreted as urinary buffers in the form of titratable acid [19]. Also, hydrogen ions bind ammonia (NH3) to form ammonium (NH4+). Under physiological conditions, approximately half the buffering is as titratable acid and the rest as ammonia/ammonium. However, when systemic acidosis develops, most of the buffering relies on ammonia/ammonium [41].
Excretion of Ammonia in the Collecting Tubule
About 80% ammonia excretion is in the α-intercalated cells of the collecting tubule, making this region of the nephron the most important site of regulation of the metabolic component of systemic acid-base balance [42].
Various transporter proteins participate in the collecting tubule to carry out the process of urinary acidification, consisting mainly of capturing NH3 from the medullary interstitium, introducing it into the cytosol of the α-intercalated cells, to its final urinary excretion as NH4+. Therefore, the process of reabsorption of ammonia is, as already mentioned, in the ascending limb of the loop of Henle, whereas the excretion is carried out in the opposite direction toward the tubular lumen in the α-intercalated cell of the collecting tubule, completing the recycling process of NH3/NH4+, as part of the countercurrent mechanism [43, 44].
Transmembrane transport proteins, such as V-H+ ATPase and H+K+ATPase, are expressed in the apical membrane of the α-intercalated cells of the collecting tubule. At this site, NH4+ may compete for the binding sites of the membrane for H+ and K+, to be excreted as a buffer in the final urine [45, 46] (Fig. 2.7).
Connecting and collecting tubules, α.intercalated cell: Ammonium excretion. In the presence of systemic acidosis, the highest excretion of H+, up to 80%, occurs as ammonia, in the α-intercalated cell of the collecting tubules. In the apical membrane, the ammonia (NH3) molecules compete for the H+ sites at the H+ATPase transporter, and mainly for the K+ sites at the H+K+ ATPase transporter. NH3 is excreted in the tubular lumen, where it binds to H+ to form NH4+. The Rhesus glycoproteins (Rh) are expressed in the α-intercalated cell as the subunits Rhbg and Rhcg, which are gas transport proteins, including carbon dioxide, ammonia, and nitric oxide in various organs, including the kidney. Rhcg glycoprotein, which participates in NH3 excretion, is expressed in the apical membrane. In the basolateral membrane, both Rh glycoproteins facilitate the capture of NH4+ molecules from the peritubular fluid, into the cytosol of the α-intercalated cell. Furthermore, NH4+ competes for the K+ sites in the NKCC1 transporter (Na+K+2Cl−) in the basolateral membrane. Hyperpolarization-activated cation channels and cyclic nucleotides (pacemaker channels) are expressed in the α-intercalated cell and, in the principal cells of the collecting tubules. The HCN2 isoform captures NH4+ from the peritubular fluid and releases it into the cytosol, to be excreted through the apical membrane into the final urine
Within the most inner area of the renal medulla, the apical membranes of the α-intercalated cells are devoided of extracellular carbonic anhydrase IV (AC IV) [47]. Nonetheless, intracellular α-intercalated cells are very abundant in anhydrase II carbonic acid enzyme, which participates in the equilibrium reaction in the cytosol, resulting in the formation of bicarbonate and hydrogen ions. These protons allow the urinary acidification process to keep going, by H+ leaving the α-intercalated cell cytosol towards the tubular lumen through the action of V-H+ATPase and the H+K+ ATP’ase in the apical membrane [48]. On the other hand, HCO3− gets reabsorbed from the cytosol into the peritubular fluid through the basolateral membrane, in the presence of the kAE1 anion exchanger, which exchanges HCO3− for Cl− [49].
NH4+ is exchanged for Na+ or K+ molecules at the K+ sites of the Na+K+ATPase and NKCC1 transporters, to be transported from the peritubular fluid to the cytosol and then to the tubular lumen. Therefore, it participates in hydrogen ion excretion, although to a lesser extent than the other mechanisms already mentioned [50].
Hyperpolarization-activated cation channels and cyclic nucleotides (HCN) are also expressed in the α-intercalated cells in different tissues in rats and other mammals, where pacemaker HCN activity is regulated. In studies conducted in rats, it is suggested that HCN participates in the activation of the Na+K+ATPase protein and the Na+/HCO3− (NBCn1) cotransporter, thus, participating in the regulation of acid-base metabolism. HCN2 is expressed in the α-intercalated cells of the collecting tubule, whose function is to capture NH4+ from the peritubular fluid to its transportation and release in the cytosol. Further excretion to the luminal fluid is carried out by Na+K+ATPase and V-H+ATPase [51].
Contrary to the previously held concept that water and gases, like CO2, cross cell membranes by passive diffusion through the bilipid membranes. However, water and gas channels have been detected in some cell membranes. Two families of H2O and CO2 transmembrane transporter proteins are known to date, aquaporins (AQPs) and Rhesus (Rh) glycoproteins. The first known function of AQPs was the transmembrane transport of H2O, and subsequently, transport of CO2 was known. This information led to an important change in the understanding of acid-base metabolism, since cellular transport of CO2 and H2O requires the presence of AQPs, both systemically and in the epithelial cells of the renal tubules [52, 53]. Other investigations reported that AQP 1 possesses the ability to transport nitric oxide (NO) through certain cell membranes, mainly in vascular endothelial cells, demonstrating the importance of this protein on the regulation of blood pressure [54].
Together with other ammonium transporter proteins, such as H+ATPase and H+K+ATPase, Rhesus glycoproteins represent the main site of excretion of ammonia. The ammonia/ammonium buffer system is the main site for proton binding and excretion; its contribution is of the greatest importance in the regulation of acid-base metabolism [55]. Rhesus glycoproteins are expressed in several tissues of plants, animals and numerous microorganisms. These glycoproteins are gas transporters, mainly CO2 and NH3 [56,57,58].
Rhesus glycoproteins (Rhgc, Rhbg) capture and transport CO2 and NH4 from the peritubular fluid (hypertonic, with a high concentration of ammonia) to the cytosol. This mechanism ensues at the basolateral membrane of the α-intercalated cells of the collecting tubule. On the other hand, Rhgc, but not Rhbg, is expressed at the apical membrane, and participates in the α-intercalated cells of the collecting tubule excreting NH3 and CO2 [59] (Fig. 2.7).
The Physiological Impact of Urinary Buffers in the Excretion of Hydrogen
A buffering substance contains a weak acid and a weak base, with their respective salts; can minimize sudden changes in pH in a solution.
A normal subject, under physiologic normal status, needs to excrete 80 to 100 mEq of hydrogen ions, daily. It would be necessary to drop the urine pH to 1.5, to excrete such an amount of non-volatile acids. On the contrary, to excrete an alkaline charge, it is required to increase the urinary pH to 8.0. This situation is not feasible, since the renal capacity to acidify and alkalinize the urine, falls within a 4.0 to 8.0 pH range, which corresponds to an [H+] of 1/0.0001 (10-4) and 1/0.00000001 (10-8).
Therefore, the kidneys utilize buffers to excrete the non-volatile H+ ions. The renal excretion of excess hydrogen (H+) is achieved by titratable acid (TA) and ammonia excretion. These buffers are of minimal physiological importance at the systemic level, but in the urine, their role is most important to excrete protons. The largest amount of systemic hydrogen ions are derived from the intermediate metabolism of amino acids. The concentration of free H+ [H+] in the ECF is 0.0000000398 (nm/l). The logarithmic expression of this value is 3.98 × 10−8, corresponding to a pH of 7.40 (±0.05).
Most of the ammonia is produced mainly in the proximal tubule and is excreted as ammonium molecules in the urine. The presence of buffer systems in the urine leads to a reduction of the amount of free H+, maintaining the urinary pH stable at 6.0–6.5, under physiological conditions. The production of urinary buffers, mostly ammonia, is increased in the presence of metabolic acidosis, which occurs in the α-intercalated cells of the connecting and collecting tubules. On the contrary, when bicarbonate accumulates in the ECF (metabolic alkalosis), the excess is excreted in the β-intercalated cells. From this, it can be deduced that the urinary pH is not an indicator of the quantity of excreted buffers, but rather of the urinary quantity of free hydrogens (H+), in the case of systemic acidosis, or of hydroxyls (OH_), which are transformed in HCO3_, in the case of systemic metabolic alkalosis.
Once the hydrogen ions that are extracted from the α-interspersed cells into the lumen of the connecting and collecting tubules, they undergo the buffering process in the form of titratable acid (phosphoric and sulfuric acids) and ammonium (NH4+), which are excreted by the urine. Only a small amount of free hydrogen ions remain in the urine, determining the urinary pH within the physiological range of 6.0–6.5 [60].
The ammonia/ammonium buffer pair, which is been produced in the brush border of the proximal tubular cells, is the main mechanism of regulation of the acid-base metabolism since it excretes from 50% to 70% of the hydrogen produced in the body. This amount increases to 80% or 90% when the systemic production of H+ rises, even slightly; when the renal intracellular pH is reduced or in the presence of a fully developed systemic acidosis, either of metabolic or respiratory origin (in this last instance as a compensatory mechanism). The renal response to increasing the urinary excretion of H+ is by augmenting the expression of the cotransporters NBCn1 and NBC3 [60].
As previously mentioned, the main function of the proximal tubule concerning the regulation of acid-base metabolism is the recovery of bicarbonate filtered by the glomerulus, while the distal tubule has as a priority the formation of new bicarbonate, which happens as a consequence of the excretion of hydrogen ions bound to the buffer systems, mainly titratable acid and excretion of ammonia [61].
The ammonia/ammonium buffer system is formed by weak acid and its base, with a dissociation constant (pK) of ~9.15. The lower this value, the greater the amount of the buffer solution that is in the form of acid, NH4+, according to the following reaction:
Therefore, in urine with a physiological pH of 6.0 to 7.0, the ammonium (acid form) excreted from the cell to the tubular lumen would be trapped until its final expulsion in the urine. As formerly described, this NH4+ “entrapment theory” is correct, but incomplete, due to the existence of NH3/NH4+ transporter molecular proteins expressed throughout the nephron [61].
References
Chan JCM. Nutrition and acid-base metabolism. Fed Proc. 1981;40:2423–8.
Rodriguez-Soriano J. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol. 2002;13:2160–70.
Goyal S, Vanden Heuvel G, Aronson PS. Renal expression of novel Na+/H+ exchanger isoform NHE8. Am J Physiol Renal Physiol. 2003;284(3):F467–73. Epub 2002 Oct 29.
Biemesderfer D, Pizzonia J, Abu-Alfa A, Exner M, Reilly R, Igarashi P, Aronson PS. NHE3: a Na+/H+ exchanger isoform of renal brush border. Am J Phys. 1993;265(5 Pt 2):F736–42.
Halperin ML, Kamel KS, Goldstein MB. Polyuria. In: Halperin ML, Kamel KS, Goldstein MB, editors. Fluid, electrolyte, and acid-base physiology. 4th ed. Philadelphia: Saunders Elsevier; 2010. p. 403–22.
Burckhardt G, DiSole F, Helmle-Kolb C. The Na+/H+ exchanger gene family. J Nephrol. 2002;15(Suppl 5):S3–S21.
Wang T, Hropor M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Phys. 2001;281:F1120–8.
Jorgensen PL. Structure, function and, regulation of Na+, K+ATPase in the kidney. Kidney Int. 1986;29:10–20.
Nielsen S, Agre P. The aquaporin family of water channels. Kidney Int. 1995;48(4):1057–106.
Silverman DN, Lindskoo S. The catalytic mechanism of carbonic anhydrase: implications of a rate-limiting protolysis of water. A Chem Res. 1988;21(1):30–6.
Soleimani M. Na+HCO3 cotransporter (NBC): expression and regulation in the kidney. J Nephrol. 2002;15(Suppl 5):S32–40.
Nakhoul NL, Hamm LL. Vacuolar H+ATPase in the kidney. J Nephrol. 2002;12(Suppl 5):S22–31.
Trachtman H. Sodium metabolism. In: Avner ED, Harmon WE, Niaudet P, editors. Pediatric nephrology. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. p. 125–45.
Dubose TD Jr, Cogan MG, Rector FC. Acid-base disorder. In: Brenner BM, Rector FC, editors. Brenner and Rector’s the kidney. 5th ed. Philadelphia: WB Saunders; 1996. p. 929–98.
Hebert SC, Gamba G, Kaplan M. The electroneutral Na+ -(K+)-Cl cotransport family. Kidney Int. 1996;49:1638–41.
Nagami GT. Luminal secretion of ammonia in the mouse proximal tubule perfused in vitro. J Clin Invest. 1988;81:159–64.
Weiner ID, Verlander JW. Renal ammonia metabolism and transport. Compr Physiol. 2013;3(1):201–20. https://doi.org/10.1002/cphy.c120010.
Li HC, Szigligeti P, Worrell RT, Matthews JB, Conforti L, Soleimani M. Missense mutations in Na+:HCO3- cotransporter NBC1 show abnormal trafficking in polarized kidney cells: a basis of proximal renal tubular acidosis. Am J Physiol Renal Physiol. 2005;289(1):F61–71.
López-González Z, Cosete Ayala-Aguilera C, Martinez-Morales F, Galicia-Cruz O, Salvador-Hernández C, Pedraza-Chaverri J, Medeiros M, Hernández AM, Escobar L. Immunolocalization of hyperpolarization-activated cationic HCN1 and HCN3 channels in the rat nephron: regulation of HCN3 by potassium diets. Histochem Cell Biol. 2015;144(4) https://doi.org/10.1007/s00418-015-1375-6.
Trachtman H. Sodium and water. En: Avner ED, Harmon WE, Niaudet P. eds., Pediatric nephrology, 5th ed., Lippincott Williams & Wilkins, Philadelphia, 2004: 125–145.
Chan JCM, Mak RHK. Acid-base homeostasis. En: Avner ED, Harmon WE, Niaudet P. eds., Pediatric nephrology, 5th ed., Lippincott Williams & Wilkins, Philadelphia, 2004:189–208.
Weinstein AM, Krahn TA. A mathematical model of rat ascending Henle limb. II Epithelial function. Am J Physiol Renal Physiol. 2010;298:F525–42.
Amemiya M, Loffing J, Lotscher M, Kaissling B, Alpern RJ, Moe OW. Expression of NHE-3 in the apical membrane of rat renal proximal tubule and thick ascending limb. Kidney Int. 1996;48:1206–15.
Kikeri D, Sun A, Zeidel ML, Hebert SC. Cell membranes impermeable to NH3. Nature. 1989;339:478–80.
Attmane-Elakeb A, Amlal H, Bichara M. Ammonium carriers in medullary thick ascending limb. Am J Physiol Renal Physiol. 2001;280:F1–9.
DuBose TD, Good DW. Chronic hyperkalemia impairs ammonium transport and accumulation in the inner medulla of the rat. J Clin Invest. 1992;90:1443–9.
Good DW. Effects of potassium on ammonia transport by medullary thick ascending limb of the rat. J Clin Invest. 1987;80:1358–65.
Good DW. Active absorption of NH4_ by rat medullary thick ascending limb: inhibition by potassium. Am J Physiol Renal Fluid Electrolyte Physiol. 1988;255:F78–87.
Good DW, Knepper MA, Burg MA. Ammonia and bicarbonate transport by thick ascending limb of rat kidney. Am J Phys. 1984;247:F35–44.
Quinn S, Harvey BJ, Thomas W. Rapid aldosterone actions on epithelial sodium channel trafficking and cell proliferation. Steroids. 2014;81:43–8. https://doi.org/10.1016/j.steroids.2013.11.005. Epub 2013 Nov 20.
Halperin ML, Kamel KS, Goldstein MB: Principles of acid-base physiology. En Halperin ML, Kamel KS, Goldstein MB (eds): Fluid, electrolyte, and acid-base physiology 4th ed. Saunders Elsevier, Philadelphia, 2010:3–28.
Doucet A, Horisberger J. Renal ion-translocating ATPases. The P-type family. In: Seldin D, Giebisch G, editors. The kidney physiology and pathophysiology. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 140–70.
Gluck S. Nelson r: the role of the V-ATPase in renal epithelial H+ transport. J Exp Biol. 1992;172:205–18.
Cavingston TL, Campbell WG, Wingo CS, Cainn BD. Molecular identification of the renal H+K+ -ATPases. Semin Nephrol. 1999;19:431–7.
Silver RB, Mennit PA, Satlin LM: Stimulation of H+, K+ATPase in intercalated cells of cortical collecting duct with chronic metabolic acidosis. Am J Physiol. 1996;270:F539–47. The kidney anion exchanger 1 affects tight junction properties via claudin-4.
Garg LC. Respective roles of H-ATPase and H-K-ATPase in ion transport in the kidney. J Am Soc Nephrol. 1991;2(5):949–60.
Wall SM, Fischer MP. Contribution of the Na_-K_-2Cl_ cotransporter (NKCC1) to transepithelial transport of H_, NH4_, K_, and Na_ in rat outer medullary collecting duct. J Am Soc Nephrol. 2002;13:827–35.
Lashhab R, Rumley AC, Arutyunov D, Rizvi M, You C, Dimke H, Touret N, Zimmermann R, Jung M, Chen XZ, Alexander T, Cordat E. The kidney anion exchanger 1 affects tight junction properties via claudin-4. Sci Rep. 2019;9:Article number: 3099.
Codina J, Dubose TD Jr. Molecular regulation and physiology of the HKATPases in the kidney. Semin Nephrol. 2006;26:345–51.
Gumz ML, Lynch IJ, Greenlee MM, Cain BD, Wingo CS. The renal-ATPases: physiology, regulation, structure. Am J Physiol Renal Physiol. 2010;298:F12–21.
McCance RA, von Finck MA. The titratable acidity, pH, ammonia and phosphates in the urines of very young infants. Arch Dis Child. 1947;22(112):200–9.
Koeppen BM, Steinmetz PR. Basic mechanisms of urinary acidification. Med Clin North Am. 1983;67(4):753–70.
Knepper MA. NH4 transport in the kidney. Kidney Int. 1991;40:S95–S102.
Knepper MA, Good DW, Burg MB. Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol Renal Fluid Electrolyte Physiol. 1984;247:F729–38.
Kurtz I, Balaban RS. Ammonium as a substrate for Na_-K_-ATPase in rabbit proximal tubules. Am J Physiol Renal Fluid Electrolyte Physiol. 1986;250:F497–502.
Attmane-Elakeb A, Sibella V, Vernimmen C, Belenfant X, Hebert SC, Bichara M. Regulation by glucocorticoids of expression and activity of rBSC1, the Na_-K_ (NH4_)-2Cl_ cotransporter of medullary thick ascending limb. J Biol Chem. 2000;275:33548–53.
Brown D, Zhu XL, Sly WS. Localization of membrane-associated carbonic anhydrase type IV in kidney epithelial cells. Proc Natl Acad Sci U S A. 1990;87(19):7457–61.
Dobyan DC, Bulger RE. Renal carbonic anhydrase. Am J Phys. 1982;243(4):F311–24.
Vichot AA, Zsengellér ZK, Shmukler BE, Adams ND, Dahl NK, Alper SL. Loss of kAE1 expression in collecting ducts of end-stage kidneys from a family with SLC4A1 G609R-associated distal renal tubular acidosis. Clin Kidney J. 2017;10(1):135–40. https://doi.org/10.1093/ckj/sfw074. Epub 2016 Aug 31.
Wall SM, Fischer MP. Contribution of the Na(+)-K(+)-2Cl(−) cotransporter (NKCC1) to transepithelial transport of H(+), NH(4)(+), K(+), and Na(+) in rat outer medullary collecting duct. Am Soc Nephrol. 2002;13(4):827–35.
Carrisoza-Gaytan R, Rangel C, Salvador C, Saldaña-Meyer R, Escalona C, Satlin LM, Liu W, Zavilowitz B, Joyce Trujillo J, Bobadilla NA, Escobar LI. The hyperpolarization-activated cyclic nucleotide-gated HCN2 channel transports ammonium in the distal nephron. Kidney Int. 2011;80:832–40. https://doi.org/10.1038/ki.2011.230.
Itel F, Al-Samir S, Öberg F, Chami M, Kumar M, Supuran CT, Deen PM, Meier W, Hedfalk K, Gros G, Endeward V. CO2 permeability of cell membranes is regulated by membrane cholesterol and protein gas channels. FASEB J. 2012;26(12):5182–91. https://doi.org/10.1096/fj.12-209916. Epub 2012 Sep 10.
Boron WF, Cooper GJ. Effect of DIDS on the CO2 permeability of the water channel AQP1. FASEB J. 1998;12:A374.
Herrera M, Hong NJ, Garvin JL. Aquaporin-1 transports NO across cell membranes. Hypertension. 2006;2006(48):157–64.
Silver RB, Mennit PA, Satlin LM. Stimulation of H+, K+ ATPase in intercalated cells of cortical collecting duct with chronic metabolic acidosis. Am J Physiol. 1996;270:F539–47.
Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 2008;22:64–73.
Weiner ID, Hamm LL. Molecular mechanisms of renal ammonia transport. Annu Rev Physiol. 2007;69:317–40.
Muñoz-Arizpe R, Escobar L, Medeiros M. Acidosis tubular renal en niños: conceptos actuales de diagnóstico y tratamiento. Bol Med Hosp Infant Mex. 2013;70(3):178–94.
Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Chérif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R. Expression of RhCG, a new putative NH3/NH+4 transporter, along the rat nephron. J Am Soc Nephrol. 2002;13:1999–2008.
Kwon TH, Fulton C, Wang W, Kurtz I, Frøkiær J, Aalkjaer C, Nielsen S. Chronic metabolic acidosis upregulates rat kidney Na-HCO cotransporters NBCn1 and NBC3 but not NBC1. Am J Physiol Renal Physiol. 2002;282:F341–51.
Alpern RJ, Rector FC. Renal acidification mechanisms. In: Brenner BM, editor. Brenner & Rector’s, the kidney. 5th ed. Philadelphia: WB Saunders Co.; 1996. p. 408–71.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Muñoz, R. (2022). Renal Regulation of Acid-Base Metabolism. In: Muñoz, R. (eds) Renal Tubular Acidosis in Children. Springer, Cham. https://doi.org/10.1007/978-3-030-91940-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-91940-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-91939-9
Online ISBN: 978-3-030-91940-5
eBook Packages: MedicineMedicine (R0)