Abstract
Twin-screw granulation (TSG) is a pharmaceutical process that has gained increased interest for the development of oral dosage forms. The technology has evolved rapidly due to the flexibility of the equipment design, the selection of the process variables and the wide range of processed materials. Most importantly, TSG offers the benefits of both batch and continuous manufacturing for pharmaceutical products accompanied with excellent process control, high product quality which can be achieved through the implementation of quality by design (QbD) approaches and the integration of process analytical technologies (PAT). Here we present the basic concepts of the various twin-screw granulation techniques and present in detail their advantages and disadvantages. In addition, we discuss the detail of the instrumentation used for TSG and how the critical processing parameters (CPPs) affect the critical quality attributes (CQAs) of the produced granules. Finally, we present recent advances in TSG continuous manufacturing including paradigms of QbD approaches coupled with PAT monitoring for granule optimisation and process understanding.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Granulation
Granulation is a well-known powder processing technique, used in the pharmaceutical industry for the manufacturing of solid dosage forms [1]. This term is used to describe the processing of powders for particle enhancement with the aim to improve a range of properties such as flowability, compressibility, tabletability and homogeneous distribution of active ingredients, etc. [2, 3]. A deep understanding of the technology may aid in the prediction and preparation of good-quality granules along with tablet manufacturing. The transformation of powders to agglomerated particles is usually performed prior to tableting to ensure minimal aggregation and a uniform flow of the powder through the hopper to the die [2]. Typically, granulation is a particle size enlargement process where particles are processed to form larger multi-unit entities with a distribution in between 0.2 and 0.4 mm [4]. Even larger size granules (around 1–4 mm) are also prepared for the capsule manufacturing [5]. Depending on the final particle size, granules are further processed with other excipients and used as an intermediate before compression to form tablets, or for filling of hard gelatin capsules. Furthermore, granulation technology is used to reduce the generation of toxic dust during powder handling [6]. A good-quality granule exhibits a less non-friable behaviour and also produces less amount of fines during processing. Pharmaceutical powders have often uneven particle size distribution leading to segregation during storage which can be minimised and improve content uniformity via granulation. The bulk powders present several practical implications such as higher packaging, storage and transportation which can be tackled through the manufacturing of granules with higher densities. Traditionally, granulation of powders can be achieved by two different methods, classified as (a) dry granulation and (b) wet granulation.
In general, pharmaceutical manufacturing can be divided into two distinct stages: primary manufacturing (i.e. upstream operations) which involves the production of the active ingredient from the starting reagents and secondary manufacturing (i.e. downstream operations) which involves the conversion of the active drugs into products suitable for administration such as granules, capsules or tablets [7]. The secondary manufacturing involves integration of multiple processing units; thus, this stage requires a fundamental understanding of processing parameters to optimise a formulation [8,9,10].
The manufacturing of granules can be conducted via three different ways: dry, wet and melt granulation. The dry granulation involves aggregation of primary powders in the absence of any liquids under high pressure to facilitate bonding of the particles by direct contact and subsequent milling to attain the desired size [1]. This method is suitable for processing moisture-sensitive active pharmaceutical ingredients (APIs), while it is cost-effective due to the less processing steps, little or no material waste and low dust exposure. A common approach for dry granulation is roller compaction where powder is fed into two counterrotating rolls producing flat-shaped ribbons of compacted material. The use of a dry binder, such as cellulose, starch or povidone, in the powder blend is essential to achieve a stronger ribbon [11]. Subsequently, the ribbons can be milled to obtain the desired granule size. In many cases the use of roller compaction/dry granulation has been described as a ‘continuous production line’ [12]. The technique has been frequently used over the past two decades as it improves drug dosage weight control, content uniformity and powder flow while it is easy to scale up and facilitates continuous manufacturing [13]. In some cases the generation of fines makes the technology unfavourable or even unwanted for further processing and might disqualify or complicate the process for highly potent drugs [14].
A wide range of powder processing methods are employed in the pharmaceutical industry to produce granules with particular characteristics in terms of their physical and pharmaceutical properties (e.g. bioavailability) [15]. According to several studies, wet granulation provides better control of drug uniformity, improves flowability and increases bulk density and porosity [16,17,18]. Wet granulation requires volatile and non-toxic solvent (e.g. ethanol, isopropanol) for the processing of powder blends which are an alternative for moisture-sensitive APIs, and they tend to dry quickly. The use of aqueous solution is a common approach to create strong bonds between the particles that lock them together. However, when the water dries, the powders might break, and thus it is a prerequisite to form a dense mass. Following the production of granules, further processing is carried out by sieving, milling or mixing with additional components to manufacture a finished dosage form. Wet granulation technologies usually involve the use of high shear mixers and fluidised bed that produce dense and uniform granules.
Theoretically, granule mechanism requires the adhesion of particles for the formation of agglomerates and comprises of three key steps. The particle adhesion takes in various steps; as shown in Fig. 1, this involves wetting and nucleation, transition and ball growth [6]. Initially adhesion or cohesion forces take place through the formation of an immobile thin layer which results in the decrease of the interparticulate distance while increasing the contact area through van der Waals forces. This is the pendular state where water is distributed as mobile thin layer around the particles holding them together through lens-shaped rings. The transition state is entered by further addition of granulating liquid leading to the formation of stronger liquid bridges (funicular state) by filling up the interparticulate void space. This is usually the end of the wet granulation process, but further liquid saturation (80%) and air displacement lead to the capillary state where large snowballs are built up which are not suitable for pharmaceutical purposes.
Diagram of granule formation [19]
Melt granulation is similar to dry granulation process where binder solution is replaced by a meltable binder. Fluidised bed melt and melt extrusion or even high shear granulations are processing technologies that can be effectively used where the binder is melted/soften near or above its melting point or glass transition temperature in order to obtain agglomerated granules [20,21,22,23]. Usually the melting point of binders varies between 30 and 100 °C, and they either melt or become tacky followed by solidification upon cooling.
2 Instrumentation of a Twin-Screw Granulation (TSG) (Extruder)
Extrusion granulation equipment is almost identical to that used for typical hot melt extrusion (HME) and consists of the extruder, auxiliary equipment, downstream processing equipment and some other monitoring tools for performance and evaluation of product [24]. The extruder typically composed of hopper, single or twin screw, barrels, die and driving unit for screw. The auxiliary equipment equipped with a cooling and heating device is connected to the barrels, the conveyer belt to cool down the extrudates and the solvent delivery pump. The monitoring screen displays screw speed, temperature scales, torque monitor and pressure scales.
Overall extrusion process is divided into four sections [25]:
-
Feeding of the materials
-
Conveying of materials (mixing and particle size reduction)
-
Out flow from the extruder (no die)
-
Downstream processing
During processing, temperature is controlled by electrical heating bands and monitored by thermocouples displayed on monitoring screen. Usually, the extruder comprises of single or twin rotating screws placed inside the barrel. The barrel is composed of sections which is bolted and clamped together and described as zones in the extruder. A die plate is attached to the end of barrel, which determines the shape of the final extruded product (Fig. 2).
Schematic diagram of an extruder [26]
Currently the most common extruder types are (a) the single-screw and (b) the twin-screw extruders. The single-screw extruder consists of only single screw placed in the extruder barrel, while more advanced twin-screw extrusion system consists of either in a same direction (co-rotating) or opposite direction (counterrotating). Usually, the extruder screw is characterised by length/diameter (L/D) ratio of screw which typically ranges from 20 to 40:1.
The heat required (melt granulation) for the melting or fusing of the material is provided by the combination of electric or liquid clams on the barrels and friction of the materials produced by shear force between rotating screw and barrel wall [27].
The extrusion granulation process is fully controlled by applying the optimal temperature, screw speed and feed rate. The technique has a potential to control and process material at a specific requirement such as high shear extrusion or addition of solvent at solvent evaporation stage during processing. Screw configuration allows extruder to perform mixing and particle size rearrangement which performs a vital role for the dispersion of API into carrier matrices.
The material is fed from the hopper directly into the deeper flight or greater flight pitch. The geometry of flights (shown in Fig. 3) allows feeding materials to fall easily into rotating screw for conveying alongside the barrel. The helix angle and pitch of the screw control the constant rotation speed of screws. The materials fed as a solid into the transition zone where it is mixed, compressed, melted and plasticised. The compression process of materials into the barrel is regulated either by decreasing thread pitch but maintaining constant flight depth or maintaining constant thread pitch with decreasing flight depth [29]. This process creates pressure as the material moves into the barrel to the exit. The material moves in helical path as a result of some mechanisms such as transverse flow, pressure flow, drag flow and leakage, while movement of material in barrel is affected by two reverse mechanisms (a) screw diameter space and (b) width of the barrel. The material reaches the metering zones with uniform thickness and flowability which drag out the granulated material as uniform delivery of granules. The downstream processing is equipped with conveyer which permits extrudates to cool down at room temperature by applied air pressure.
Geometry of extruder screw [28]
In twin-screw extruders, screws can be rotated either in the same (co-rotating) or opposite (counterrotating) direction as shown in Fig. 4. Two screws are placed into the barrel side by side, and it is designed to control operating parameters such as the filling level of material, the screw speed, the feed time and the residence time [30]. In counterrotating extruder, twin screw rotates in opposite direction in barrel. This type of extruder is usually applied for high shear materials as material squeezed through the gaps between screws. Counterrotating screw provides an excellent dispersion of blended particles, while it also exhibits some disadvantages of air entrapment, high pressure and low output as a result of low maximum screw speed. This type of extruder does not produce pushing effect of materials into the barrel due to opposite direction of screw rotation.
Twin-screw co-rotating and counterrotating screw [31]
On the other hand, a co-rotating screw rotates at the same directions, and it states the advantage of self-wiping and intermeshing design [25]. This type of extruder is mainly used in the industry which has competency to operate at high screw speed and provide excellent high output with intensive mixing and conveying of materials during the process. Co-rotating is subclassified as non-intermeshing and intermeshing.
Intermeshing twin-screw extruders are very popular as they provide self-wiping to minimise the non-motion of materials and prevent overheating of materials and superior mixing in respect over single-screw extruder. Intermeshing extruders operate on the principle of first in, first out where the material does not rotate along with screw to barrel [32]. Non-intermeshing extruders are used to extrude a highly viscous material which is not liable to produce high torque during processing [33].
3 TSG Processing Parameters
3.1 Temperature
Each of the barrels can have a unique set point; therefore, the process can be run using a single temperature value or a temperature profile, depending on specific requirements of the process. Temperature has an effect on processability of the formulation as viscosity is temperature dependent, as well as on the quality of the final product. The bed temperature for lubricated powders varies from 5 to 15 °C and depends on the flow rate, screw speed, L/S ratio and the amount of polymer in the binding solution [34, 35]. The temperature variations within the barrel can easily reach 70 °C, while the presence of long mixing zones increases the temperature of the formed granules. The temperature can be also influenced by the different wetting behaviour of the processed powders [36].
3.2 Screw Design
Screw configuration is designed based on the type, number and sequence of phases of which the extrusion comprises. At the entrance of the extruder, conveying screw elements of high-pitch sizes are placed to ensure proper powder feeding since bulk density of the inlet materials is much smaller than extrudate density. A standard configuration presents conveying elements up to two thirds of the screw length, followed by high shear mixing zone. Venting during extrusion process is sometimes needed to remove entrapped air or residue moisture from the final product. Vent requires a drop in pressure in that part of the extruder to prevent the exiting of the material through the opening. This is achieved by positioning of high-pitch conveying elements are positioned. For extrusion through the die and shaping of the product, the pressure should be increased so the pitch is reduced.
The elements on the screw shaft are interchangeable so a customised screw configuration can be assembled to match the specific requirements of each process. Elements differ in design to suit the various steps of processing, such as transporting, mixing, melting and shaping. Conveying elements have self-wiping geometry needed for transportation of the material along the screw, whereas the free volume of the screw and speed is modulated by pitch size (Fig. 5). Mixing (kneading) elements are used for mixing, melting and homogenisation, while they comprised of disc, which are staggered under certain angle. The angle determines the conveying and mixing properties; conveying properties decrease, whereas mixing properties increase with increasing offset angle. Additionally, longer discs impart more shear, while shorter improve dispersive mixing. Therefore, kneading elements can be classified as:
• Forwarding
• Neutral
• Reversing
Screw configuration has a critical impact on the product properties where insufficient mixing results in non-homogenous blends or leads to incomplete product conversion or reaction. On the contrary, if the mixing zone is too long and imparting too much shear, residence time can increase as well as product temperature both resulting in API degradation.
The use of conveying elements with different flight pitches can have a significant effect on the extruded granules. By increasing the flight pitch of the screws, the granule output is increased, while the fines are reduced due to the larger volume which is available for the wetted mass [37, 38]. The use of higher flight pitch facilitates the formation of porous granules, while the increased number of conveying elements results in increase of the granule strength. The presence of conveying elements right after a kneading zone plays a key role as it minimises the formation of large clumps and results in their breakage.
Furthermore, the kneading elements play a key role due to their capability to produce stronger compressive granules. Figure 6 shows a TSG process illustrating the granule development and compaction. When using high offset angles of kneading elements, granules appear larger and denser, while longer mixing zones produced less friable and breakable particles with narrow and controllable size.
On the contrary the usage of multiple kneading zones has a negligible effect on the granule particle size and distribution. Kneading elements control the morphology of extruded granules and usually produce elongated shapes compared to spherical-shaped granules obtained when only conveying screws are implemented [39, 40].
3.3 Feeding Rate and Screw Speed
The feeding rate is one of the most important process variables, and it has been found to influence the particle size and granule density/strength. High feeding rates are related to increased compressive forces which correspond to larger and dense granules. In the absence of kneading zones, high feed rates increase the formation of fines which are friable and easily break while the liquid distribution in the powder blend is non-uniform. Screw speed is related to the shear rate in a TSG, but it has low impact on the particle size of the granules and the residence time. By increasing the screw speed, only a minor size reduction has been observed only when high adhesive polymers are used in the granulation blend [41].
3.4 Mixing in a TSG
The powder mixing in a TSG is directly related to the screw configuration and the resulted shear rate as a function of the process settings and is classified as radial or axial based on the direction of spread. During the TSG process, radial mixing is a prerequisite for a homogeneous granule distribution at constant powder feeding, while axial mixing can also help to avoid inhomogeneities. The selection of feeding rate, screw speed and screw configuration determine the degree of mixing. The axial mixing is directly affected by the residence time distribution (RTD), screw speed and geometry. When suing high screw speeds, the axial mixing increases as estimated by the rise of the normalised variance (σ2θ) and the lowering of the Peclet number (Pe). However, there are contradictory studies suggesting that axial mixing is not affected by the processing parameters [42].
The most favourable feature of a TSG process is the capacity to processed high powder throughput in a short residence time (0–20s). Despite the possible gains of TSG over batch manufacturing, this is not always straightforward because there is a limited time frame to achieve homogeneous granule distribution, granule formation and breakage to obtain the final product. Hence, in order to achieve high TSG yield, the process should be designed carefully with longer residence time than the mixing time across the extruder barrel. The process is more complicated when mixing powders with the granulating liquid which requires homogeneous liquid distribution within the powder. Sayin et al. observed that the site of liquid addition and the periodicity of the peristaltic pump have a strong impact on the moisture content and distribution [43]. The use of more kneading zones can help to improve the distribution of granulation liquid, but further improvements required the addition of screw elements with modified geometries that can induce distributive mixing.
The addition of granulating liquid results in the formation of liquid bridges between the powder particles, and subsequently aggregation takes place to produce larger granules. The evolution of particle size and the primary shaping mechanism in the TSG is limited due to the capacity of the extruder. Extensive studies have shown that granule size increases not only after the kneading zones but also upstream (before) suggesting there are not only two granule formation mechanisms such as the dispersive and distributive mixing. In the third mechanism, the built-up material before the kneading zone (flow restriction) is forced-mixed with the inbound powder due to the throughput force. As a result, the increase in the throughput (feeding) leads to the increase of granule size assuming there is enough granulating liquid to form large agglomerates.
Kumar et al. investigated the interrelations of the residence time and mixing settings over the quality of extruded granules [44]. The study demonstrated that the increase of kneading elements had no influence on the yield fraction despite the improvement of powder mixing and residence time. The same was observed by increasing the L/S ratio which resulted only in the formation of larger granules.
4 Twin-Screw Granulation
TSG is an advanced processing technology that has been extensively used for granule production over the last couple of decades [4]. Several studies have been reported related to TSG optimisation by investigating the effect of the formulation composition and operational variables such as screw configuration, pitch and length of conveying element, thickness and angle of kneading element, and influence of kneading blocks. A schematic diagram of a TSG line is illustrated in Fig. 7.
Schematic representation of a continuous twin-screw granulation [45]
The first study for the development of pharmaceutical granules at a laboratory scale using a single-screw extruder was reported by Goodhart et al. [46]. The investigation was undertaken to evaluate various factors related to wet granulation such as the effect of granulating fluid, type of end plate, number of mixing anvils and screw speed. The studies were conducted to also understand the level of content uniformity during granulation. The use of water isopropanol as granulating fluid reduced the sugar solubility in the granulating fluid, creating increased torque during high-speed processing. The granules prepared by using this granulating fluid resulted in the compression of less gritty and smoother tablets compared to water. It was concluded that the water isopropanol granulating fluid created processing interruption but produced granules with low bulk densities.
Later on, in 1986 Gamlen and Eardley introduced the twin-screw extruder in pharmaceutical research, to produce paracetamol extrudates with a combination of excipients, i.e. Avicel, lactose and/or hydroxypropyl methylcellulose (HPMC), and using water as a granulating fluid [47]. The results revealed that addition of HPMC in the formulation influenced significantly on the extrusion properties. HPMC helped to retain water in its interstitial spaces, reducing frictional forces between extruder and the plate. Micrographs of both extruded formulations with or without HPMC showed similar appearances. Hence, the addition of HPMC improved the extrudability without affecting the extrudate quality. A year later, Lundeberg and later Kleinebudde (1988) also employed twin-screw extruders for the formation of effervescent granules [48,49,50,51,52]. Furthermore, TSG has been implemented to perform dry, wet and melt granulation process [53] including scaling up of continuous granulation coupled with process analytical technologies (PAT) by applying quality by design (QbD) approaches [54].
Dhenge et al. conducted an empirical study on a TSE with model pharmaceutical formulations focused on the physical properties of the final granules [42]. The authors investigated the effect of screw speed, powder feed rate and liquid-to-solid (L/S) ratio on the residence time and torque which was found to affect the particle size, strength, shape and structure of the granules. The most pronounced effect on granule properties was observed with a liquid-to-solid (L/S) ratio of 0.4, showing a monomodal distribution of granule sizes with a peak around the 1000 μm mark. At higher L/S, the extra liquid caused an increase in the residence time of material in the granulator, resulting in a reduction of undersized and oversized granules. The granule’s shape and hence the flow were improved due to the increased amount of granulating fluid which helped to achieve stronger liquid bridges between the particles. The powder feed rate influenced the transition and final state of granule properties such as size, shape, structure, porosity, strength and dissolution time. At low feed rate, the residence time became long, resulted in strong granules with an increased average granule size, whereas higher feed rate reduced the granule size. At a powder feed rate of 3.5 and 5 kg/h, the sphericity of the granules was found to be increased. The improved sphericity of granule was related to the increased filling at high powder feed rate which led to increase in shearing forces within the barrel and turned the processed powder to spherical agglomerates. The surface morphology of the granules became smooth by increasing the length of the screw while porosity was decreased. A high feed rate not only increased the granules’ morphological strength and stability but also affected the dissolution rates. It was concluded that TSG optimisation is a complex process and is affected significantly by the critical processing parameters.
Vercruysse et al. investigated the operational parameters related to TSG and their effect on the manufacturing process using theophylline as model API [34]. There was no significant relationship of screw configuration and screw speed to the granules’ morphology, but a high number of kneading elements and increased throughput resulted in higher torque during granulation. The high torque resulted in temperature rise in the barrel which led to reduced amount of fines and less friable granules. The binder was found to be more effective when it was dissolved in the granulating fluid. Increasing the number of kneading elements yielded denser granules with a longer disintegration time and dissolution rate. The findings of this work suggested that the granule and tablet quality can be optimised by adjusting specific process variables. Khorsheed et al. also investigated the effect of TSG processing parameters on the powder and granule and tablet properties [55] using microcrystalline cellulose (MCC) or mannitol C160. Their study showed increasing MCC granule size and strength can reduce tabletability and vice versa. Although mannitol C160 granules did not affect tabletability, particle size reduction has shown significant compactibility improvement. They found a correlation between the yield pressure, plastic and elastic work of the initial powders and changes in tabletability performance as a result of the granulation process. Authors mentioned that increasing the strength and size of granules may cause their reduction of the tabletability.
The granule structure is closely related to tablet’s quality attributes, and it can be controlled by the selection of appropriate excipients as it was recently reported by Megarry et al. [56]. Allopurinol-granulated formulations were prepared by wet granulation using twin-screw and high shear processing. By using different mannitol grades (200SD and C160), it was found to present a polymorphic transition during processing, containing mostly β-mannitol. The 200SD granules presented needle-shaped morphology with high porosity and specific surface area, which led to poorer flow properties but higher tablet tensile strength. The study suggested that the understanding of specific excipient grade and their effect during processing is crucial to optimise tablet manufacturability.
Lute et al. investigated the influence of varying barrel fill levels on the mean residence time, granule properties and tensile strength of tables using MCC and lactose [57]. They reported that specific feed load or powder feed volume directly affects the granule size and shape. Increasing fill levels of MCC inside the extruder barrel caused shorter residence time along with decreased granule size. On the other hand, lactose maintained its granule size at all fill levels. Again, usage of specific pharma excipients and their processing parameters are crucial parts of the granulation optimisation process.
Twin-screw dry granulation is considered more effective for granule manufacturing as it limits heat exposure to only one-barrel zone, much shorter than melt granulation. Lui Y. et al. (2017) studied formulations containing different polymeric binders (AF15, Kollidon VA64, Soluplus, Kollidon SR), with glass transition temperature less than 130 °C [58]. Granulation of the primary powders with some degree of moisture was found to be beneficial for processed polymers with high glass transition temperatures. Selected polymer particles are more prone to soften and flow under frictional forces if their T g was closer to the barrel zone temperature in the kneading section. A higher zone temperature highly increases the opportunities for successful granulations. Screw speed was a major cause for friction heating, while the kneading block offset was only minor in its influence on the granulation process. According to their results, higher screw speed tended to increase the particles size, producing bigger chunks (> 3350 μm). Conversely, an increase of moisture content in the excipient resulted in smaller particle size distribution. So, successful granulation can be achieved by varying the processing parameters, and preformulation studies will aid to quickly optimise the process.
Ye et al. (2019) used twin-screw dry granulation to improve the flow property of moisture-sensitive materials [59]. They produced dry granule formulations using four different APIs processed with Klucel, Ethocel and magnesium stearate for sustained release. A DoE was employed to determine the effect of different processing parameters, i.e. screw speed, feeding rate, barrel temperature and screw configuration on the product properties (flow properties, particle size distribution and dissolution time). It was revealed that an increased screw speed is related to a higher percentage of medium size granules while a negative correlation was found between the amount of large size granules and screw speed. This was attributed to the decrease of the mean residence time due to the high screw speeds and the subsequent reduction of the kneading effect on primary powders and the formation of smaller granules. Higher feeding rates improve the flow properties of the powders, and decreased angles of repose were obtained. The morphology of granules was affected by the powder flow where long stripe shape exhibited poor flowability compared to round-shaped particles. Finally, drug release was affected by the binder content in the granule and presented significant variations, i.e. large stronger granules with more binder showed relatively slow release. On the other hand, small amount soft binder provided less particle adhesion which resulted in faster disintegration and hence faster dissolution rates. The continuous processing and simplicity of operation, in the absence of milling, suggest that TSDG is more effective compared to other conventional dry granulation approaches.
Kallakunta et al. applied heat-assisted dry granulation using a twin-screw extruder to formulate sustained-release granules [60]. Granulation feasibility was studied with different binders (e.g. Klucel EF, Kollidon VA64), sustained-release agents (e.g. Klucel MF, Eudragit RSPO) and diluents at various drug loads. The processing conditions were below the melting point or glass transition temperature of the formulation ingredients. They have found a size correlation to the binding capacity of the excipient. Formulations with Klucel produced granules with a size of around 250 μm, whereas Kollidon VA64F promoted finer granules related to its own particle size (20 μm). Hence, the good binding properties of the ingredients in the formulation facilitated easier granule formation and larger granule size. On the other hand, formulations consisting of only Klucel MF showed very minimal erosion (approximately 5%) over 24 h. The drug release was found to be incomplete in these formulations, as the high viscosity of Klucel MF might be the reason for the low drug release profiles. The excellent binding properties and viscosity of Klucel MF resulted in dosage forms with good matrix integrity, which then became one of the controlling factors for drug release. Conversely, formulations with Kollidon are reported to undergo greater erosion which destabilised the tablet matrix and led to fast drug release. In summary, heat-assisted dry granulation could be applied for continuous twin-screw granulation, which may ameliorate the process constraints and stability problems in conventional granulation techniques.
Unlike wet and dry granulation, melt granulation offers several advantages for processing pharmaceutical actives. Twin-screw melt granulation is carried out at higher temperatures than traditional batch melt granulation, and thermoplastic polymers can be used as binders. This is a clear benefit, considering the limited number of traditional binders that are suitable for use in conventional granulation processes. The process of twin-screw melt granulation is also advantageous as it eliminates the need for organic solvents and water, while it is cost-effective and environmentally friendly. As a totally water-free process, twin-screw melt granulation is suitable for drugs that undergo hydrolysis or degradation in the presence of water.
Van Melkebeke et al. studied twin-screw melt granulation for the manufacturing of immediate release formulation using two grades of polyethylene glycol (PEG 400 and 4000) as a binder [20]. Authors described the importance of granulation temperature on its dissolution properties. A high yield and fast dissolution rate were obtained only at a processing temperature near the melting point of PEG. Post-granulation characterisations showed a homogeneous dispersion of the BCS class II drug within the polymeric matrix created a micro-environment around the drug particles enhancing the dissolution rate. Addition of small surfactant amounts (polysorbate 80 or Cremophor) helped to achieve a complete drug release within 10 min. A high drug content required more PEG content and surfactant to obtain 100% drug release.
Batra A. et al. investigated polymeric binders with high melting points (180 °C) for improving tabletability of pharmaceutical APIs using twin-screw melt granulation [61]. For the purposes of the study, metformin hydrochloride and acetaminophen were used as active ingredients and several polymers, i.e. hydroxypropyl cellulose, hydroxypropyl methylcellulose, polyvinylpyrrolidone and methacrylate-based polymers, including Klucel EXF, Eudragit EPO and Soluplus, as binders. The prepared granules demonstrated good tablet tensile strength with even as low as 10% w/w polymer concentrations. As the melting temperature of acetaminophen is below 180 °C, its TSG temperature was achieved at 130 °C, and even at that temperature, extruded granules provided acceptable compatibility of >2 MPa, suggesting that compressed tablets could withstand manufacturing and end-use stresses during coating, packaging, transportation and handling. The work demonstrated that a pool of polymeric binders can be successfully used for twin-screw melt granulation and further exploited in the future.
Unlike wet or dry granulation, in melt granulation the growth mechanism involves an additional nucleation mechanism known as immersion or distribution as shown in Fig. 8.
Nucleation mechanism of melt granulation [62]
The mechanism was studied by Monteyne et al. for the development of immiscible drug-binder formulations [63]. They implemented thermal analysis, rheological characterisation and microscopic images to reach an in-depth understanding of material behaviour during agglomeration. They reported that the distribution of the binder in the immiscible blends caused a double T g and a clear loss peak in the thermogram. The binder was found to act as a separate phase favouring efficient binder distribution where a thin binder layer with restricted mobility is formed on the surface of the primary drug particles during granulation. Then it is covered by a second layer with improved mobility when the binder concentration is sufficiently high. The granules manufactured with 20% (w/w) Soluplus or higher became smaller and more spherical as a function of temperature, whereas a lower binder concentration resulted in larger and more needle-shaped granules. The study showed strong evidence of the binder distribution during TSG strongly affected the granule characteristics.
In another study, the same group carried out TSG studies with Soluplus and metoprolol or caffeine [64]. In this case, thermal analysis showed only one T g, indicating a highly miscible system. A high barrel temperature was used to process granules larger than 500 μm which also showed minimum torque fluctuation during granulation. Surprisingly, granules prepared with 20% or more binder concentration resulted in large aggregates at processing temperatures <90 °C. Prepared granules with caffeine-Soluplus blends remained broad size distribution over the different binder concentrations and were not affected by the process temperatures, whereas the granule size distribution of the MPT/SLP blends appeared narrower and shifted towards lower particle sizes with an elevated granulation temperature.
5 Twin-Screw Granulation and Continuous Manufacturing
Traditional manufacturing of medicinal products in the pharmaceutical industry uses batch processing, where every single unit operation is conducted separately. The adoption of continuous manufacturing (CM) from pharmaceutical industry is relatively new, and it takes place in a stepwise manner. On the other hand, food, petrochemical, polymer and oil refining industries have been undertaking continuous manufacturing operations for decades [13, 14, 65, 66] and produce large volume production at a cost-effective manner. The term ‘continuous’ means a required process may run for an extended period, with raw material constantly fed into the process. The pharmaceutical industry has faced many obstacles in attempting such a continuous production method on a day-to-day basis. The main perceived issue is the industry’s rigid structure, because of the strict supervision of regulatory agencies such as the FDA in the USA or the European Medicines Agency (EMA) in Europe [67, 68]. Despite the fact that there are no regulatory hurdles for implementing continuous processes, pharmaceutical industry needs to reform regulatory framework, pharmaceutical equipment design and operation.
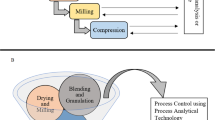
Some pharmaceutical companies plan to convert 70% of their production lines to continuous manufacturing to allow a more efficient product and process development as part of the product operation. Nevertheless, twin-screw granulation is an ideal example of such a process that has successfully been introduced for CM production. Recently, GEA introduced a continuous platform for oral solid dosage forms which integrates multiple operation units comprising of granulation, drying and tablet compression. As shown in Fig. 9, the process involves the dosing and mixing of raw materials using multiple feeders, followed by the high shear granulator for mixing, wetting and granulation through the coupling with a highly accurate dosing system (e.g. water, solvents, binder solutions). Subsequently the granules are continuously transferred (e.g. vacuum or gravity) to a fluidised bed dryer for drying and milled to achieve the desired particle size and content uniformity. The drying process is monitored with an on-line moisture LightHouse probe. Prior to tablet compression in a rotary tablet press, the dried powders are blended with the external phase (e.g. lubricants, disintegrants, fillers). The tablet press comprises of six compression modes such as compression to equal porosity, exchangeable compression module with a wash-off-line capability which are coupled with advanced in-line PAT sensors and a control system. The technology is versatile, and processed amounts vary from 500 g for R&D purposes up to 100 kg/h.
A similar processing technology known as MODCOS has been developed by Glatt for the conversion of the batch mode to a continuous fluid bed system. As shown in Fig. 10, loss in weight feeders is used for the dosing of the active drug substance and excipients through vacuum conveyor in a twin-screw extruder to produce medium- to high-density granules. The wet granules are pneumatically conveyed into the process insert of the fluid bed via a specially designed transfer line, where they are continuously dried to the required moisture level. Before the granules are compressed in tablets with other ingredients, a dry mixer is used to achieve homogeneous mixing. A key advantage of the MODCOS line is the narrow retention time distribution during drying, while a sophisticated discharge system facilitates complete emptying of the process chambers and thus prevents any cross-contamination with consistent product quality. The continuous line is coupled with a range of PAT tools including two NIR probes to determine content uniformity at the end of the extruder or the discharge system. A moisture probe is used at the fluid bed dryer discharge point and particle size probe at the powder discharge (feeders).
A unique feature of the continuous line is the intelligent control system where all the process parameters are controlled together and continuously monitored to provide the basis for automatic process control. The manufacturing process is controlled by recipes which include the distribution of residence times, while the data for all process units can be displayed as tables of graphs.
In batch manufacturing of solid dosage form, there is a limited process understanding and control which requires the application of more intensive and advanced manufacturing processes. Continuous granulation processing is fully automated, and thus scale-up issues related to batch manufacturing are no longer encountered. Often in batch granulation the active substances tend to agglomerate especially when they present highly cohesive properties. This problem can be easily mitigated in continuous granulation by combining high shear co-milling of the drug substances and excipients followed by low-shear blending. The powder blends are immediately compressed to produce tablets and unlike batch processing do not allow time for particles to re-agglomerate. CM lines can be built in a flexible way for the processing of multiple formulations (products) through careful consideration and optimal design. Continuous granulation lines can be easily adopted for both dry and wet processing which eventually results in a good return on the initial investment. Other advantages of continuous granulation processing include the following:
-
Enhanced development approach by implementing QbD approaches and incorporating PAT tools.
-
Reduce risk of manufacturing failure and prevent drug shortages.
-
Decrease the risk of out-of-specification failures both for intermediates and finished products.
-
Flexibility by using the same system to develop a manufacturing process.
-
Effect on supply chain by increasing supply speed and reacting to market demands.
-
Agility and reduced scale-up efforts.
-
Real-time quality assurance-secure quality attributes and measure critical quality parameters.
-
Reduction of capital and operational costs and environmentally friendly.
-
Cost reductions in R&D, product transfer and productivity.
-
Reduced system’s footprint.
5.1 Regulatory Aspects
The business of pharmaceutical industry and its regulatory environments are constantly evolving. Regulatory authorities are continuously reviewing their guidelines for the pharmaceuticals to achieve a continuous manufacturing process to understand the processing parameters and better monitoring of the production line. All these guidelines suggest continuous processing will be a key manufacturing approach for pharmaceuticals in the near future. This should allow a greater degree of control over the processing parameters during production.
Advances are also accompanied by worldwide regulatory initiatives, such as the FDA (Food and Drug Administration) and the ICH (International Conference on Harmonization). According to ICH Q8, quality is defined as ‘The suitability of either a drug substance or drug product for its intended use and this term includes such attributes as the identity, strength and purity’ [69]. The ICH Q8 provides an idea of the documentation process of the shear knowledge acquired during product and process development which can be used to analyse the product quality. It suggests that quality cannot be tested into a product; instead quality should be present due to the design of the product and process and testing is merely a method to confirm this.
The pharmaceutical industry relies on innovation and manufacturing development, where a quality by test (QbT) system is applied to maintain product quality using the following steps: raw material tests, fixed drug manufacturing processes and finished product tests. However, the development of the quality by design (QbD) concept, implemented by regulatory agencies, introduces higher level of product and process understanding but also potential regulatory flexibility. Hence, it can lead to increased successes rate in the development of finished products, process robustness, less manufacturing deviations and failed/reworked batches. In addition, it promotes easy of post-approval changes and real-time release testing with lower costs and cycle times. The QbD concept was first introduced by the quality pioneer Joseph M. Juran who introduced asset of universal processing steps to establish quality goals in order to avoid the loss of market share, failure of finished products and waste as a result of poor product quality planning [70]. Over the years, pharmaceutical QbD has been developed with the issuance of a series of guidelines such as ICH Q8 (pharmaceutical development), ICH Q9 (quality risk management), ICH Q10 (pharmaceutical quality system) and ICH Q11 (development and manufacture of drug substances) [69, 71,72,73]. Also, the FDA took notice of developments in other industries that are using continuous manufacturing to increase processing efficiency. Application of QbD approach into the pharmaceutical industry will certainly ensure improved pharmaceutical drug quality and safety and to achieve a desired pharmaceutical manufacturing process on the basis of scientific and engineering knowledge [74].
5.2 Quality by Design (QbD)
Quality by design (QbD) is defined in ICH Q8 guidelines as ‘A systemic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management’ [69]. Widely accepted elements of the foregoing QbD are as follows: quality target product profile (QTPP), critical quality attributes (CQAs), critical material attributes (CMAs) and critical process parameters (CPPs). The definitions of theses parameters are described below [69, 75].
Quality target product profile (QTPP). This is a prospective summary of the quality characteristics of a drug product such as dosage form, delivery systems, dosage strength, etc. The dosage strength and container closure system of the drug product have to be taken into account because these directly involve pharmacokinetics properties (e.g. dissolution) of the drug and drug product quality criteria (e.g. sterility, purity, stability).
Critical quality attributes (CQAs). Critical quality attributes are included with physical, chemical, biological or microbiological properties or characteristics of an output material including finished product. A potential CQA stipulation for a drug product derived from QTPP involves guidance relating to product and process development within an appropriate limit, range or distribution to ensure the desired product quality.
Critical material attributes (CMAs). This is included with the physical, chemical, biological or microbiological properties or characteristics of an input material. To ensure the desired quality of the drug substances, excipients or in-process materials, CMAs should be within an appropriate limit, range or distribution.
Critical process parameters (CPPs). This parameter helps to monitor before or in-process quality that influences the appearance, impurity and yield of the final product.
Steps for QbD implementation:
-
Define the desired performances of the product and identity the QTPPS.
-
Identify the CQAs.
-
Identify possible CMAs and CPPs.
-
Set up and execute the design of space, which is linked to CMAs, CPPs and also CQAs and to obtain enough information of how these parameters impact on QTPP. Hence, a process design space should be defined and lead to an end product with the desired QTPP.
-
Identify and control the sources of variability from the raw materials and the manufacturing process.
-
Continually monitor and improve the manufacturing process to assure consistent product quality.
Upon understanding the elements and the steps for QbD implementation, it is important to be familiar with the commonly used tools in QbD which is based on the science underlying the design and the science of manufacturing. This includes design of experiment (DoE), process analytical technology (PAT) and risk assessment [69].
5.3 Design of Experiment (DoE)
A design of experiment is an excellent tool, which allows pharmaceutical scientists to determine the relationship among factors that influence the output of a process. This methodology was first reported by Fisher in his book The Design of Experiment [76]. The application of DoE in QbD helps to gain the maximum information about a pharmaceutical process, factors affecting the raw material attributes (e.g. particle size) and process parameters (e.g. screw speed, time) which helps to reduce the number of experiments required involved in quality attributes such as blend uniformity, tablet hardness, thickness and friability. It is almost impossible to assess each operation individually because each unit operation has many different input and output variables as well as process parameters. Thus, the results are investigated by DoE and help to identify optimal conditions, the critical factors that influence most CQAs and those which do not as well as the existence of interactions and synergies between factors [77].
5.4 QbD Approaches in Twin-Screw Granulation
The benefits of using QbD approaches in pharmaceutical manufacturing have been recognised and promoted by regulatory bodies such as the Food and Drug Administration (FDA) combined with PAT principles and closed loop quality assurance. Continuous manufacturing lines have been implemented in pharmaceutical industry such as the drug product Orkambi by Vertex for the treatment of cystic fibrosis in 2015. There are only a few studies in literature related to QbD approaches coupled with PAT tools related to twin-screw granulation.
The first report was conducted by Fonteyene et al. (2014) who investigated the effects of variation in raw material properties on the CQAs of granules produced by wet granulation followed by tableting [78]. By using a powder-to-tablet wet granulation line, a model formulation of theophylline–lactose–PVP (30%–67.5%–2.5%) was investigated while the process parameters are kept steady. For the purposes of the study, seven grades of theophylline were processed, and the features of granules/tablets were evaluated. The granule particle size for all experiments showed a bimodal distribution with granules being in-spec containing either large amounts of fines (>150 μm) or large amounts of oversized particles (>1400 μm). The differences were directly correlated to the initial particle size of the theophylline grade. The granules obtained with fine powders presented higher bulk density and lower tapped density compared to the initial powder blend suggesting that no tapped volume reduction can be produced when granule powders have high tapped densities.
As shown in Fig. 11, the granule morphology showed needle-shaped, while for the small size fractions, more spherical particles were observed without however having been able to identify any differences for the granules of the various theophylline grades.
PCA applied for the content uniformity showed that smaller granules present more lactose monohydrate while larger granules more theophylline. The investigations on the processability showed that theophylline powders play a key role in feeding with large powders pushing the injectors out of the granulator barrel. Regarding the tableting process, a direct relation between the granule size and compression forces was observed with small size fractions (<150 μm) require higher compaction forces.
Maniruzzaman et al. applied a QbD approach by introducing for the first time a design of experiment to investigate the effect of formulation composition in a wet extrusion granulation process [79]. Twin-screw granulation was conducted by using blends of polymer/inorganic excipients (hydroxypropyl methylcellulose and magnesium aluminometasilicate–MAS) as carriers and PEG as the binder to produce ibuprofen granules. The MAS/polymer ratio, PEG amount (binder) and liquid-to-solid (L/S) ratios were set as the independent variables and the dissolution rates, mean particle size, the dissolution rates, mean particle size and the loss on drying (LoD) of the extruded granules (D50) and the loss on drying (LoD) of the extruded granules as the dependent variables. The morphology of the obtained granules appeared spherical for all processed batches compared to the needle-shaped IBU with the D50 particle size varying from 100 to 300 μm. Dynamic vapour sorption analysis showed a reversible water uptake of all batches and provided evidence that the inorganic excipient prevents significant water uptake. The content uniformity was assessed using confocal Raman mapping and demonstrated excellent ibuprofen distribution within the granules which was partially amorphous as a result of the processing during twin-screw granulation. The DoE analysis revealed that the PEG amounts and the L/S ratio had a significant effect on both the ibuprofen release and the LOD. The granule particle size distribution was found to be affected significantly by the MAS/polymer ratios but also the binder amounts.
The same group conducted an identical study by using a DoE to investigate the effect of the excipient composition, binder amount and liquid-to-solid (L/S) ratio (independent variables) on drug dissolution rates, median particle size diameter and specific surface area (dependent variables), shown in Fig. 12 [80]. This time ethanol was used as granulating liquid instead of water.
The use of a different liquid resulted in the formation of larger granules with D50 varying from 200 to 583 μm. For most of the batches, a monomodal particle size distribution was obtained, while the granule morphology appeared spherical as before. This time all independent variables were found to have a significant effect on the drug dissolution rates and particle size distribution, while the two-way interactions identified after the data integration suggested a complex granulation process.
The granule’s specific surface area was only affected by the MAS/polymer ratios and the PEG amounts at a significant level. Interestingly the water-insoluble ibuprofen demonstrated relatively fast release rates with 2 h (pH 1.2) due to the increased amorphous content which resulted due to the solubilisation effect of ethanol and the drug absorption within the porous network of the inorganic magnesium aluminometasilicate excipient.
Another comprehensive study was conducted by Grymonpre et al., who investigated the impact of critical process parameters (CPPs) and critical material parameters (CMPs) on the CQAs in twin-screw melt granulation process followed by milling and tableting of the formed granules [81]. Two active substances, acetaminophen (APAP) and hydrochlorothiazide (HCT), were co-processed with a range of hydrophilic polymers such as Kollidon VA64, Soluplus (SOL), Eudragit EPO and Affinisol grades (15 LV, 4 M). The processing temperatures were affected by the glass transition of the binders, and for the HPMC grades, higher temperatures were used due to their complex viscosities.
The milled granules presented lower moisture content (<1%) with regular shape and good flowability. More fines were received when EPO and Affinisol were used as binders, but overall, all polymers were suitable for melt granulation processing. SOL and VA64 outperform the rest of the binders due to their high milling efficiency and resistance to form fines. Compatibility studies showed identical performance for both drugs irrespective of the polymeric binder. The tabletability studies (Fig. 13) showed significant improvement of melt granulated products compared to physical mixtures. The T g of the polymer was found to affect the extrusion processing especially for VA64 which resulted in increased torque, while binders with low T g (e.g. SOL, EPO) facilitated a smoother granulation process. Similarly, the tableting process was also affected by the binder grade where EPO showed less fragmentation and higher elastic deformation during tablet compaction in comparison with SOL and VA64. Overall, the study demonstrated that twin-screw melt granulation was robust for both low and high drug loading processes and CQAs were well identified.
PC1 vs. PC2 bi-plot of the determined compaction properties (loadings) for the experimental runs of the DoE (scores). The numbers represent corresponding experimental run of HCT-EPO formulations (blue triangles), HCTSOL formulations (dark red circles) and HCT-VA64 formulations (orange boxes), plotted against loadings (star shaped) for which PF represents the plasticity factor, IER the anti-correlated in-die elastic recovery, PF slope the slope of the plasticity factor over four compaction pressures, Py the Heckel value and Db the fragmentation factor
In another study the same group applied a QbD methodology by using the continuous ConsiGma extrusion line to investigate the formulation optimisation of twin-screw granulated composition of various binary filler/binder grades and ratios. By using multiple linear regression models, the authors were able to understand the impact of the filler/binder properties on the granule and tablet CQAs. A DoE with 27 batches was combined with PCA plot to reduced large data sets and used to identify similarities or differences of materials with different chemical characteristics. The overall scope of the study was to identify the impact of materials on CQAs and consequently develop predicting formulation models with suitable characteristics for the processing of APIs with unfavourable properties. For example, the study demonstrated that the granule particle size was not affected by the original particle size or the water uptake of the filler. The granule flowability was found to be affected by the binder higher concentration amounts but not of the filler properties. The granule friability was decreased when PVP was used at higher concentrations. Finally, the tabletability was not affected by the grade of the filler which showed no impact at all. On the contrary the binder grade and the use of higher PVP concentrations demonstrated a significant effect on the improved tabletability. When compared to other binder grades, it was identified that the bulk density and specific surface play a role on the deformation mechanisms and hence tabletability.
5.5 Process Analytical Technology (PAT)
PAT is defined as ‘Tools and systems that utilize, analyze and control real-time measurements of raw and processed materials during manufacturing, to ensure optimal processing condition are used to produce final product that consistently conforms to established quality and performance standards’ [71]. The goal of PAT is to enhance understanding of and control the manufacturing process, which is consistent with our current drug quality system. So, quality cannot be tested into products; it should either be built-in or by design.
Design space is defined by the key critical process parameters identified from process characterisation studies and their acceptable ranges. These parameters are the primary focus of on-, in- or at-line PAT applications [74]. In most cases, spectroscopic techniques, such as Raman spectroscopy, UV-Vis spectroscopy and NMR (nuclear magnetic resonance), are commonly used. Besides the foregoing, near-infrared spectroscopy (NIR) [82], nano metric temperature measurement (MTM) [83] and tunable diode laser absorption spectroscopy (TDLAS) are widely applied tools in the pharmaceutical manufacturing field and play important roles in real-time monitoring of the processes used. Among these, NIR has drawn great attention in industry because it is a rapid, non-invasive analytical technique, and there is no need for extensive sample preparation [84,85,86,87]. In most research, it is used for the identification and characterisation of raw materials and intermediates, analysis of dosage forms, manufacturing and prediction of one or more variables in the process line or the final product stream(s) on the basis of on-line, in-line or at-line spectroscopic measurement [88]. The fast growth of interest in QbD and its tools DoE, PAT and risk assessment approaches makes the QbD available and feasible to the pharmaceutical field to achieve a better understanding of the material and process parameters used.
5.6 Process Analytical Technology (PAT) in Extrusion Granulation
The framework of PAT is intended to support innovation and efficiency in pharmaceutical processes, manufacturing and quality assurance [89]. This includes systems that can be employed to design, analyse and control manufacturing by measuring critical quality and performance attributes in a timely manner. This involves measurements of raw and in-process materials aiming to ensure the quality of the finished products. PAT is not only away to implement real-time release testing but also to effectively detect failures and help understand the manufacturing processes. Due to the versatility of PAT tools, relevant information can be obtained by monitoring a range of physical, chemical and biological attributes. This can be achieved mainly by four key components which include:
-
Multivariate tools for design, data acquisition and analysis which helps to build scientific understanding and identify CPPs and CMAs which eventually leads to process understanding while the generated information is integrated into the process control.
-
Process analytical chemistry tools can effectively provide real-time and in situ data for the processed materials and process. The process measurements can also be near real time (e.g. on-, in- and at-line) in an invasive or non-invasive manner.
-
Process monitoring and control involves the design of process controls and development of mathematical relations that provide adjustments to achieve control of all critical attributes.
-
Continuous process optimisation and knowledge management relate to data collection and analysis from generated databases over the life cycle of the finished product.
PAT tools have been successfully implemented in continuous wet granulation production line, namely, ConsiGma developed by GAE Pharma [90]. The process consists of five locations where the critical quality attributes are measured. The first and the third measure the blend uniformity of the processed powders and the moisture content of the dried granules, while the second monitors the moisture distribution of the formed granules. A NIR sensor is coupled to the first location to allow accurate control of the powder feeding and blending and if possible to provide feedback for controlling blending operations [91,92,93,94]. Similarly, NIR probes were used to measure the moisture content and distribution.
Fonteyne et al. introduced a combination of PAT tools by using Raman, NIR and photometric imaging technique for the evaluation of the powder-to-tablet ConsiGma production line [95]. The aim of the study was to acquire solid-state information and granule size distribution data and in turn use them to predict a range of granule properties such as moisture content, bulk/tapped density and flowability. As shown in Fig. 14, the Raman and NIR spectra were collected to apply principal component analysis (PCA) and determine whether the formed granules contained theophylline monohydrate. Similarly, a PC plot was used to analyse all data collected through 11 DoE experiments when using the FlashSizer 3D process for the estimation of granule size and particle distribution.
(a) Raman spectra, PC 1 (47.12%) versus PC 2 (26.95%) scores plot; (b) Raman spectra, PC 2 (26.95%) versus PC 3 (16.24%) scores plot; (c) Raman spectroscopy, PC A loadings plots of PC 1, PC 2 and PC 3; (d) NIR spectroscopy, second derivative of NIR spectra. Coloured applied as above for Cluster A (dashed line) and Cluster B (full line)
The same group conducted a separate study in order to obtain an in-depth understanding of the twin-screw extrusion granulation and fluidised bed drying processes by using Batch Statistical Process Monitoring (BSPM) principles [96]. For the purposes of the study, the group implemented multivariate data analysis in terms of PCA, partial least squares regression and its various extensions such as multiblock PCA/PLS and orthogonal PLS. The work demonstrated how multivariate data analysis can be routinely used to generate data from the variable monitored by the univariate sensors for a continuous granulation process and also how the BSMP concepts are used to monitor variables in order to identify operational variations.
Madarász et al. studied real-time feedback control of twin-screw wet granulation by using dynamic image analysis [97]. In a typical granulation process of lactose and starch blends, a process camera was coupled with image analysis to monitor the particle size distribution of the obtained granules. The real-time feedback control was implemented by controlling the feeding rate of the granulating liquid (peristaltic pump) through a PC.
As shown in Fig. 15, the image analysis software consisted of three main stages:
-
(a)
Preprocessing: Greyscale filter and binarisation.
-
(b)
Post-processing: Excluding particles on the edges of the image, edge detection and removing noise.
-
(c)
Analysing and classification: Particle count, determining particle characteristics (minimum and maximum calliper diameter, aspect ratio), classification and summarisation.
The peristaltic pump is controlled through the image analysis software by using a manual RPM or an auto mode (Fig. 16) where the software controls the pump’s rotation speed via a P controller. By setting the desired granule particle size (e.g. D50: 1200 μm), the granulation process was tested by simulating different events including system startup and pump malfunction. Eventually the system could automatically adjust the granule particle size at the set value.
In-line monitoring via image analysis was carried out by Sayun et al., who used a twin-screw granulator with two different screw configurations and various liquid-to-solid (L/S) ratios [98]. The real-time high-speed imaging system features a red–green–blue light that targets the sample creating 3D images and can record particles with size distributions from 50 to 3000 μm. The work revealed that the fraction of fines increased with increasing L/S ratio suing both screw configurations.
It was also found that the screw configuration imparts a strong effect on the granule porosity while increases in L/S ratio result in decreasing porosity. The authors observed that the small window of imaging (Fig. 17 for capturing granule particles) resulted in measurement fluctuations originated from powder and liquid feeding methods. The recorded d10 values presented less variations compared to d50 and d90 but were prone to L/S variations.
Rehrl et al. introduced the concept of using soft PAT sensor in order to control the three different continuous processing lines such as hot melt extrusion, direct compression and wet granulation [99]. By measuring the concentration of the API at specific locations using NIR probes, for example, directly after granulation, it was able to predict the concentration of the drug in the feeder. The concentration prediction from on-line spectral measurements (at specific regions) can be done by constructing calibration curves at various w/w % and combined PLS regression models. The developed PLS model had a R2 of 98.3% and validation experiments carried out at flow rates of 20–20 kg/h. The experiments revealed the dependence of the wet granulation process on the feeder excitation.
6 Conclusions
Despite the fact that twin-screw granulation is a relatively new process in pharmaceutical industry, it represents an excellent paradigm of pharmaceutical processing that combines principles of QbD and PAT monitoring for process control and quality while translating the existing batch processing to continuous manufacturing. However, there is still a lack of adequate association between the experimental findings and theoretical prediction regarding material transport and kinetics in twin-screw granulation. Nevertheless, TSG is one of the few pharmaceutical processes that has proved its potential and applicability for the commercialisation of finished products through the implementation of continuous manufacturing.
References
D.M. Parikh, Handbook of pharmaceutical granulation technology, CRC Press, 2016.
G.M. Walker, Chapter 4 Drum Granulation Processes, in: A.D. Salman, M.J. Hounslow, J.P.K.B.T.-H. of P.T. Seville (Eds.), Granulation, Elsevier Science B.V., 2007: pp. 219–254.
G.K. Bolhuis, H. de Waard, Compaction properties of directly compressible materials, Pharm. Powder Compact. Technol. 2 (2011) 154.
T.C. Seem, N.A. Rowson, A. Ingram, Z. Huang, S. Yu, M. de Matas, I. Gabbott, G.K. Reynolds, Twin screw granulation—A literature review, Powder Technol. 276 (2015) 89–102.
M.E. Aulton, K. Taylor, Pharmaceutical preformulation, Aulton’s Pharm. Des. Manuf. Med. Elsevie r Heal. Sci. Edinburgh. (2013).
M.E. Aulton, Pharmaceutics. The Science of Dosage Form Design. 2nd edn. Churchill Livingstone, (2005).
J. Rantanen, J. Khinast, The future of pharmaceutical manufacturing sciences, J. Pharm. Sci. 104 (2015) 3612–3638.
S. Mascia, P.L. Heider, H. Zhang, R. Lakerveld, B. Benyahia, P.I. Barton, R.D. Braatz, C.L. Cooney, J.M.B. Evans, T.F. Jamison, End-to-end continuous manufacturing of pharmaceuticals: integrated synthesis, purification, and final dosage formation, Angew. Chemie Int. Ed. 52 (2013) 12359–12363.
R. Lakerveld, B. Benyahia, P.L. Heider, H. Zhang, A. Wolfe, C.J. Testa, S. Ogden, D.R. Hersey, S. Mascia, J.M.B. Evans, The application of an automated control strategy for an integrated continuous pharmaceutical pilot plant, Org. Process Res. Dev. 19 (2015) 1088–1100.
R. Lakerveld, B. Benyahia, R.D. Braatz, P.I. Barton, Model-based design of a plant-wide control strategy for a continuous pharmaceutical plant, AIChE J. 59 (2013) 3671–3685.
H. Mangal, M. Kirsolak, P. Kleinebudde, Roll compaction/dry granulation: Suitability of different binders, Int. J. Pharm. 503 (2016) 213–219.
R. Singh, M. Ierapetritou, R. Ramachandran, An engineering study on the enhanced control and operation of continuous manufacturing of pharmaceutical tablets via roller compaction, Int. J. Pharm. 438 (2012) 307–326.
P. Kleinebudde, Roll compaction/dry granulation: pharmaceutical applications, Eur. J. Pharm. Biopharm. 58 (2004) 317–326.
Y. Funakoshi, T. Asogawa, E. Satake, The use of a novel roller compactor with a concavo-convex roller pair to obtain uniform compacting pressure, Drug Dev. Ind. Pharm. 3 (1977) 555–573.
S.M. Iveson, J.D. Litster, K. Hapgood, B.J. Ennis, Nucleation, growth and breakage phenomena in agitated wet granulation processes: a review, Powder Technol. 117 (2001) 3–39.
C. Wang, S. Hu, C.C. Sun, Expedited development of a high dose orally disintegrating metformin tablet enabled by sweet salt formation with acesulfame, Int. J. Pharm. 532 (2017) 435–443.
L. Cai, L. Farber, D. Zhang, F. Li, J. Farabaugh, A new methodology for high drug loading wet granulation formulation development, Int. J. Pharm. 441 (2013) 790–800.
S.-H. Kim, K.-M. Hwang, C.-H. Cho, T.-T. Nguyen, S.H. Seok, K.-M. Hwang, J.-Y. Kim, C.-W. Park, Y.-S. Rhee, E.-S. Park, Application of continuous twin screw granulation for the metformin hydrochloride extended release formulation, Int. J. Pharm. 529 (2017) 410–422.
P. Thapa, J. Tripathi, S.H. Jeong, Recent trends and future perspective of pharmaceutical wet granulation for better process understanding and product development, Powder Technol. 344 (2019) 864–882.
B. Van Melkebeke, B. Vermeulen, C. Vervaet, J.P. Remon, Melt granulation using a twin-screw extruder: a case study, Int. J. Pharm. 326 (2006) 89–93.
B. Mu, M.R. Thompson, Examining the mechanics of granulation with a hot melt binder in a twin-screw extruder, Chem. Eng. Sci. 81 (2012) 46–56.
J.M. Keen, C.J. Foley, J.R. Hughey, R.C. Bennett, V. Jannin, Y. Rosiaux, D. Marchaud, J.W. McGinity, Continuous twin screw melt granulation of glyceryl behenate: development of controlled release tramadol hydrochloride tablets for improved safety, Int. J. Pharm. 487 (2015) 72–80.
T. Monteyne, J. Vancoillie, J.-P. Remon, C. Vervaet, T. De Beer, Continuous melt granulation: Influence of process and formulation parameters upon granule and tablet properties, Eur. J. Pharm. Biopharm. 107 (2016) 249–262.
H.F. Mark, J.I. Kroschwitz, Encyclopedia of polymer science and engineering, 1985.
J. Breitenbach, Melt extrusion: from process to drug delivery technology, Eur. J. Pharm. Biopharm. 54 (2002) 107–117.
D. Ridhurkar, A. Vajdai, Z. Zsigmond, Hot-melt extrusion (HME) and its application for pharmacokinetic improvement of poorly water soluble drugs., Pharmacol. Toxicol. Biomed. Reports. 2 (2016).
C. Martin, Guidelines for Operation of Leistritz Twinscrew Extruder, Am. Leistritz Corp. Somerv. (2001) 21–25.
M. Maniruzzaman, D. Douroumis, S.J. Boateng, J.M. Snowden, Hot-melt extrusion (HME): from process to pharmaceutical applications, Recent Adv. Nov. Drug Carr. Syst. (2012).
P.S. Johnson, Developments in extrusion science and technology, Rubber Chem. Technol. 56 (1983) 575–593.
M. Mollan, Historical overview, DRUGS Pharm. Sci. 133 (2003) 1–18.
P. Pitayachaval, P. Watcharamaisakul, A review of a machine design of chocolate extrusion based co-rotating twin screw extruder, in: IOP Conf. Ser. Mater. Sci. Eng., IOP Publishing, 2019: p. 12012.
W. Thiele, Twin-screw extrusion and screw design, DRUGS Pharm. Sci. 133 (2003) 69–98.
I. Ghebre-Sellassie, I. Ghebre-Selassie, C.E. Martin, F. Zhang, J. DiNunzio, C. Martin, Melt-Extruded Molecular Dispersions, in: Pharm. Extrus. Technol., CRC Press, 2003: pp. 264–279.
J. Vercruysse, D.C. Díaz, E. Peeters, M. Fonteyne, U. Delaet, I. Van Assche, T. De Beer, J.P. Remon, C. Vervaet, Continuous twin screw granulation: influence of process variables on granule and tablet quality, Eur. J. Pharm. Biopharm. 82 (2012) 205–211.
M.R. Thompson, S. Weatherley, R.N. Pukadyil, P.J. Sheskey, Foam granulation: new developments in pharmaceutical solid oral dosage forms using twin screw extrusion machinery, Drug Dev. Ind. Pharm. 38 (2012) 771–784.
K.E. Rocca, S. Weatherley, P.J. Sheskey, M.R. Thompson, Influence of filler selection on twin screw foam granulation, Drug Dev. Ind. Pharm. 41 (2015) 35–42.
U. Shah, Use of a modified twin-screw extruder to develop a high-strength tablet dosage form, Pharm. Technol. 29 (2005) 52–66.
D. Djuric, P. Kleinebudde, Impact of screw elements on continuous granulation with a twin-screw extruder, J. Pharm. Sci. 97 (2008) 4934–4942.
R.M. Dhenge, J.J. Cartwright, M.J. Hounslow, A.D. Salman, Twin screw granulation: Steps in granule growth, Int. J. Pharm. 438 (2012) 20–32.
M.R. Thompson, J. Sun, Wet granulation in a twin-screw extruder: Implications of screw design, J. Pharm. Sci. 99 (2010) 2090–2103.
M.R. Thompson, K.P. O’Donnell, “Rolling” phenomenon in twin screw granulation with controlled-release excipients, Drug Dev. Ind. Pharm. 41 (2015) 482–492.
R.M. Dhenge, R.S. Fyles, J.J. Cartwright, D.G. Doughty, M.J. Hounslow, A.D. Salman, Twin screw wet granulation: Granule properties, Chem. Eng. J. 164 (2010) 322–329.
R. Sayin, A.S. El Hagrasy, J.D. Litster, Distributive mixing elements: towards improved granule attributes from a twin screw granulation process, Chem. Eng. Sci. 125 (2015) 165–175.
A. Kumar, M. Alakarjula, V. Vanhoorne, M. Toiviainen, F. De Leersnyder, J. Vercruysse, M. Juuti, J. Ketolainen, C. Vervaet, J.P. Remon, Linking granulation performance with residence time and granulation liquid distributions in twin-screw granulation: An experimental investigation, Eur. J. Pharm. Sci. 90 (2016) 25–37.
N. Kittikunakorn, T. Liu, F. Zhang, Twin-screw melt granulation: Current progress and challenges, Int. J. Pharm. 588 (2020) 119670.
F.W. Goodhart, J.R. Draper, F.C. Ninger, Design and use of a laboratory extruder for pharmaceutical granulations, J. Pharm. Sci. 62 (1973) 133–136.
M.J. Gamlen, C. Eardley, Continuous extrusion using a raker perkins MP50 (multipurpose) extruder, Drug Dev. Ind. Pharm. 12 (1986) 1701–1713.
P. Kleinebudde, H. Lindner, Experiments with an instrumented twin-screw extruder using a single-step granulation/extrusion process, Int. J. Pharm. 94 (1993) 49–58.
P. Kleinebudde, A.J. Sølvberg, H. Lindner, The power-consumption-controlled extruder: A tool for pellet production, J. Pharm. Pharmacol. 46 (1994) 542–546.
N.-O. Lindberg, C. Tufvesson, L. Olbjer, Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins MPF 50 D, Drug Dev. Ind. Pharm. 13 (1987) 1891–1913.
N.-O. Lindberg, M. Myrenas, C. Tufvesson, L. Olbjer, Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins MPF 50D. Determination of mean residence time, Drug Dev. Ind. Pharm. 14 (1988) 649–655.
N.-O. Lindberg, C. Tufvesson, P. Holm, L. Olbjer, Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins MPF 50 D. Influence on intragranular porosity and liquid saturation, Drug Dev. Ind. Pharm. 14 (1988) 1791–1798.
M.R. Thompson, Twin screw granulation-review of current progress, Drug Dev. Ind. Pharm. 41 (2015) 1223–1231.
F.R. Parker, Department of Health and Human Services, US Food and Drug Administration: Authority and Responsibility, in: FDA Adm. Enforc. Man., CRC Press, 2005: pp. 21–60.
B. Khorsheed, I. Gabbott, G.K. Reynolds, S.C. Taylor, R.J. Roberts, A.D. Salman, Twin-screw granulation: Understanding the mechanical properties from powder to tablets, Powder Technol. 341 (2018) 104–115.
A. Megarry, A. Taylor, A. Gholami, H. Wikström, P. Tajarobi, Twin-screw granulation and high-shear granulation: The influence of mannitol grade on granule and tablet properties, Int. J. Pharm. 590 (2020).
S. V. Lute, R.M. Dhenge, A.D. Salman, Twin screw granulation: An investigation of the effect of barrel fill level, Pharmaceutics. 10 (2018) 1–21.
A.S. Liu Y, Thompson MR, O’Donnell KP, Heat Assisted Twin Screw Dry Granulation, AIChE J. 63 (2017) 4748–4760.
X. Ye, V. Kallakunta, D.W. Kim, H. Patil, R. V. Tiwari, S.B. Upadhye, R.S. Vladyka, M.A. Repka, Effects of Processing on a Sustained Release Formulation Prepared by Twin-Screw Dry Granulation, J. Pharm. Sci. 108 (2019) 2895–2904.
V.R. Kallakunta, H. Patil, R. Tiwari, X. Ye, R.S. Vladyka, S. Sarabu, D.W. Kim, S. Bandari, M.A. Repka, Exploratory studies in heat-assisted continuous twin-screw dry granulation: A novel alternative technique to conventional dry granulation, HHS Public Access. 555 (2019) 380–393.
A. Batra, D. Desai, A.T.M. Serajuddin, Investigating the Use of Polymeric Binders in Twin Screw Melt Granulation Process for Improving Compactibility of Drugs, J. Pharm. Sci. 106 (2017) 140–150.
T. Schfer, C. Mathiesen, Melt pelletization in a high shear mixer, VIII Eff. Bind. Viscosity. Int. J. Pharm. 139 (1996) 125–138.
T. Monteyne, L. Heeze, S.T.F.C. Mortier, K. Oldörp, R. Cardinaels, I. Nopens, C. Vervaet, J.P. Remon, T. De Beer, The use of Rheology Combined with Differential Scanning Calorimetry to Elucidate the Granulation Mechanism of an Immiscible Formulation During Continuous Twin-Screw Melt Granulation, Pharm. Res. 33 (2016) 2481–2494.
T. Monteyne, L. Heeze, S.T.F.C. Mortier, K. Oldörp, I. Nopens, J.P. Remon, C. Vervaet, T. De Beer, The use of rheology to elucidate the granulation mechanisms of a miscible and immiscible system during continuous twin-screw melt granulation, Int. J. Pharm. 510 (2016) 271–284.
L.D. Bruce, N.H. Shah, A.W. Malick, M.H. Infeld, J.W. McGinity, Properties of hot-melt extruded tablet formulations for the colonic delivery of 5-aminosalicylic acid, Eur. J. Pharm. Biopharm. 59 (2005) 85–97.
C. Martin, Twin screw extrusion for pharmaceutical processes, in: Melt Extrus., Springer, 2013: pp. 47–79.
S. Byrn, M. Futran, H. Thomas, E. Jayjock, N. Maron, R.F. Meyer, A.S. Myerson, M.P. Thien, B.L. Trout, Achieving continuous manufacturing for final dosage formation: challenges and how to meet them. May 20–21, 2014 continuous manufacturing symposium, J. Pharm. Sci. 104 (2015) 792–802.
S.L. Lee, T.F. O’Connor, X. Yang, C.N. Cruz, S. Chatterjee, R.D. Madurawe, C.M. V Moore, X.Y. Lawrence, J. Woodcock, Modernizing pharmaceutical manufacturing: from batch to continuous production, J. Pharm. Innov. 10 (2015) 191–199.
Q. ICH, Pharmaceutical development, Q8, Curr. Step. 4 (2009).
J.M. Juran, Juran on quality by design: the new steps for planning quality into goods and services, Simon and Schuster, 1992.
I.C.H. ICH, Q9 Quality Risk Management, in: Proc. Int. Conf. Harmon. Tech. Requir. Regist. Pharm. Hum. Use, 2005.
I.C.H.H.T. Guideline, Pharmaceutical quality system q10, Curr. Step. 4 (2008).
R. Ogilvie, ICH Q11: Development and manufacture of drug substance, ICH Qual. Guidel. An Implement. Guid. (2017) 639–665.
Food and Drug Administration, Guidance for industry, PAT-A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance, http://http//www.fda.gov/cder/guidance/published.html (2004).
X.Y. Lawrence, G. Amidon, M.A. Khan, S.W. Hoag, J. Polli, G.K. Raju, J. Woodcock, Understanding pharmaceutical quality by design, AAPS J. 16 (2014) 771–783.
T. Seidenfeld, RA Fisher on the design of experiments and statistical estimation, in: Founders Evol. Genet., Springer, 1992: pp. 23–36.
A. Gawade, S. Chemate, A. Kuchekar, Pharmaceutical Quality by Design: A new approach in product development, Res. Rev. J. Pharm. Pharm. Sci. 2 (2013) 5–12.
M. Fonteyne, H. Wickström, E. Peeters, J. Vercruysse, H. Ehlers, B.-H. Peters, J.P. Remon, C. Vervaet, J. Ketolainen, N. Sandler, J. Rantanen, K. Naelapää, T. De Beer, Influence of raw material properties upon critical quality attributes of continuously produced granules and tablets, Eur. J. Pharm. Biopharm. 87 (2014) 252–263.
M. Maniruzzaman, A. Nair, M. Renault, U. Nandi, N. Scoutaris, R. Farnish, M.S.A. Bradley, M.J. Snowden, D. Douroumis, Continuous twin-screw granulation for enhancing the dissolution of poorly water soluble drug, Int. J. Pharm. 496 (2015).
M. Maniruzzaman, S.A. Ross, T. Dey, A. Nair, M.J. Snowden, D. Douroumis, A quality by design (QbD) twin—Screw extrusion wet granulation approach for processing water insoluble drugs, Int. J. Pharm. 526 (2017) 496–505.
W. Grymonpré, G. Verstraete, V. Vanhoorne, J.P. Remon, T. De Beer, C. Vervaet, Downstream processing from melt granulation towards tablets: In-depth analysis of a continuous twin-screw melt granulation process using polymeric binders, Eur. J. Pharm. Biopharm. 124 (2018) 43–54.
M.T. Islam, M. Maniruzzaman, S.A. Halsey, B.Z. Chowdhry, D. Douroumis, Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach, Drug Deliv. Transl. Res. 4 (2014) 377–387.
V. Lourenço, D. Lochmann, G. Reich, J.C. Menezes, T. Herdling, J. Schewitz, A quality by design study applied to an industrial pharmaceutical fluid bed granulation, Eur. J. Pharm. Biopharm. 81 (2012) 438–447.
T.R.M. De Beer, P. Vercruysse, A. Burggraeve, T. Quinten, J. Ouyang, X. Zhang, C. Vervaet, J.P. Remon, W.R.G. Baeyens, In-line and real-time process monitoring of a freeze drying process using Raman and NIR spectroscopy as complementary process analytical technology (PAT) tools, J. Pharm. Sci. 98 (2009) 3430–3446.
L. Saerens, L. Dierickx, T. Quinten, P. Adriaensens, R. Carleer, C. Vervaet, J.P. Remon, T. De Beer, In-line NIR spectroscopy for the understanding of polymer–drug interaction during pharmaceutical hot-melt extrusion, Eur. J. Pharm. Biopharm. 81 (2012) 230–237.
F. De Leersnyder, E. Peeters, H. Djalabi, V. Vanhoorne, B. Van Snick, K. Hong, S. Hammond, A.Y. Liu, E. Ziemons, C. Vervaet, Development and validation of an in-line NIR spectroscopic method for continuous blend potency determination in the feed frame of a tablet press, J. Pharm. Biomed. Anal. 151 (2018) 274–283.
M.T. Islam, N. Scoutaris, M. Maniruzzaman, H.G. Moradiya, S.A. Halsey, M.S.A. Bradley, B.Z. Chowdhry, M.J. Snowden, D. Douroumis, Implementation of transmission NIR as a PAT tool for monitoring drug transformation during HME processing, Eur. J. Pharm. Biopharm. 96 (2015) 106–116.
R. Singh, A.D. Román-Ospino, R.J. Romañach, M. Ierapetritou, R. Ramachandran, Real time monitoring of powder blend bulk density for coupled feed-forward/feed-back control of a continuous direct compaction tablet manufacturing process, Int. J. Pharm. 495 (2015) 612–625.
P.A.T. FDA, A Framework for Innovative Pharmaceutical Development, Manufacturing and Quality Assurance. Guidance for Industry (2004), (n.d.).
S. Laske, A. Paudel, O. Scheibelhofer, S. Sacher, T. Hoermann, J. Khinast, A. Kelly, J. Rantannen, O. Korhonen, F. Stauffer, A review of PAT strategies in secondary solid oral dosage manufacturing of small molecules, J. Pharm. Sci. 106 (2017) 667–712.
A.U. Vanarase, M. Alcalà, J.I.J. Rozo, F.J. Muzzio, R.J. Romañach, Real-time monitoring of drug concentration in a continuous powder mixing process using NIR spectroscopy, Chem. Eng. Sci. 65 (2010) 5728–5733.
A.U. Vanarase, M. Järvinen, J. Paaso, F.J. Muzzio, Development of a methodology to estimate error in the on-line measurements of blend uniformity in a continuous powder mixing process, Powder Technol. 241 (2013) 263–271.
L. Martínez, A. Peinado, L. Liesum, G. Betz, Use of near-infrared spectroscopy to quantify drug content on a continuous blending process: influence of mass flow and rotation speed variations, Eur. J. Pharm. Biopharm. 84 (2013) 606–615.
V. Kehlenbeck, Use of near infrared spectroscopy for in-and off-line performance determination of continuous and batch powder mixers: opportunities & challenges, Procedia Food Sci. 1 (2011) 2015–2022.
M. Fonteyne, S. Soares, J. Vercruysse, E. Peeters, A. Burggraeve, C. Vervaet, J.P. Remon, N. Sandler, T. De Beer, Prediction of quality attributes of continuously produced granules using complementary pat tools, Eur. J. Pharm. Biopharm. 82 (2012) 429–436.
A.F. Silva, J. Vercruysse, C. Vervaet, J.P. Remon, J.A. Lopes, T. De Beer, M.C. Sarraguça, European Journal of Pharmaceutics and Biopharmaceutics Process monitoring and evaluation of a continuous pharmaceutical twin-screw granulation and drying process using multivariate data analysis, (n.d.).
L. Madarász, Z.K. Nagy, I. Hoffer, B. Szabó, I. Csontos, H. Pataki, B. Démuth, B. Szabó, K. Csorba, G. Marosi, Real-time feedback control of twin-screw wet granulation based on image analysis, Int. J. Pharm. 547 (2018) 360–367.
R. Sayin, L. Martinez-Marcos, J.G. Osorio, P. Cruise, I. Jones, G.W. Halbert, D.A. Lamprou, J.D. Litster, Investigation of an 11 mm diameter twin screw granulator: screw element performance and in-line monitoring via image analysis, Int. J. Pharm. 496 (2015) 24–32.
J. Rehrl, A.-P. Karttunen, N. Nicolaï, T. Hörmann, M. Horn, O. Korhonen, I. Nopens, T. De Beer, J.G. Khinast, Control of three different continuous pharmaceutical manufacturing processes: Use of soft sensors, Int. J. Pharm. 543 (2018) 60–72.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Nandi, U., Dey, T., Douroumis, D. (2022). Continuous Twin-Screw Granulation Processing. In: Fytopoulos, A., Ramachandran, R., Pardalos, P.M. (eds) Optimization of Pharmaceutical Processes. Springer Optimization and Its Applications, vol 189. Springer, Cham. https://doi.org/10.1007/978-3-030-90924-6_6
Download citation
DOI: https://doi.org/10.1007/978-3-030-90924-6_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90923-9
Online ISBN: 978-3-030-90924-6
eBook Packages: Mathematics and StatisticsMathematics and Statistics (R0)