Abstract
In Alphaproteobacteria, the initiation of chromosome replication is closely followed by chromosome segregation. In the early phases of chromosome segregation, the two newly replicated copies of the chromosomal centromere are separated and then directed toward opposite cell poles. Centromere translocation is an energy-dependent process that is carried out by the parABS system, the components of which are widely conserved through bacteriophage and bacterial kingdom, and are adapted for pole-directed chromosome segregation in this clade. The centromeres are lead elements in the process of chromosome segregation, and after they are tethered to the cell poles via a polar scaffolding protein called PopZ, the remaining parts of the chromosome fill in behind, in an ordered procession that is contemporaneous with ongoing DNA replication. The latter phases of segregation are mediated by nucleoid condensation proteins that are structurally and functionally analogous to chromosome organization factors that operate in all kingdoms of life.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Overview of Chromosome Segregation
A cell that replicates its genome is also faced with the challenge of segregating the DNA as two equal halves and ensuring that each daughter cell receives a complete copy. In a broad sense, all cells have similar means to accomplish this task. That is, they possess mechanisms for recognizing a defined location within each chromosome, called a centromere, and for directing force on those centromeres such that sister chromosomes are split apart and driven into separate daughter cells. Unlike eukaryotic cells, which separate DNA replication and chromosome segregation into discrete phases, bacteria begin to segregate chromosomal DNA soon after the initiation of DNA replication. This is possible because of the relative proximity of the origin of replication to the chromosome centromere, which is usually separated by just a few thousand bases, or 3% of the total size of the replicon (Livny et al. 2007). Thus, the centromere is present in two copies shortly after replication initiation, and the process of segregating these loci can begin. Subsequent to centromere segregation, the remaining sections of the chromosome follow in temporal and spatial order as the progress of the replication fork continues (Hong and McAdams 2011). The mechanisms for maintaining order during this bulk segregation phase are distinct from those involved in centromere segregation.
This chapter will focus on our knowledge of the molecular mechanisms of chromosome segregation in Alphaproteobacteria. An important factor that limits the breadth of this review is that experimental investigations on this subject have thus far been limited to a small number of species in this clade. Caulobacter crescentus, which is best known as a model organism for investigations in cell cycle regulation and bacterial cell biology, has been studied intensively with respect to chromosome segregation (Bowman et al. 2010; Easter and Gober 2002; Ebersbach et al. 2006; Figge et al. 2003; Laloux and Jacobs-Wagner 2013; Lim et al. 2014; Ptacin et al. 2010; Schwartz and Shapiro 2011; Shebelut et al. 2010; Surovtsev et al. 2016a; Taylor et al. 2017; Toro et al. 2008; Tran et al. 2017a; Viollier et al. 2004), and Agrobacterium tumefaciens (Ehrle et al. 2017; Howell et al. 2017; Kahng and Shapiro 2003), Sinorhizobium meliloti (Kahng and Shapiro 2003; Frage et al. 2016), and Brucella abortus (De Bolle et al. 2015; Deghelt et al. 2014) have been the subject of additional studies. In all of these species, centromeres that are not undergoing segregation are localized to a cell pole (Figge et al. 2003; Kahng and Shapiro 2003), and bacteria that place their centromeres in this manner are known to package the rest of the chromosome in an organized fashion, with the chromosome arms extending outward toward mid-cell (Viollier et al. 2004; Umbarger et al. 2011). In considering mechanisms for chromosome segregation, it is important to note that the Caulobacter crescentus genome consists of only one large circular chromosome, whereas S. meliloti and A. tumefaciens have two and four separate replicons, respectively. While the genomic sequences of these species suggest that each independent replicon has its own distinct set of chromosome segregation proteins, the extent to which their mechanisms are functionally and/or temporally interrelated is unknown.
Chromosome segregation in Caulobacter crescentus. (a) In new-born flagellated cells (sketch #1), the circular chromosome is arranged such that the origin of replication and nearby parS region are located at the flagellar pole, and the two chromosome arms extend across the cell to the opposite pole, where the replication terminus is located. Prior to the initiation of DNA replication, the cell establishes a gradient of DNA-bound ParA-ATP that emanates from the pole opposite the flagellum, which will become the destination for one of the newly replicated centromeres. Replication occurs during the “initiation” phase (sketch #1–#2), which is associated with a developmental transition that replaces the polar flagellum with a stalk and a different set of cell cycle regulatory proteins, and releases the parS region from the cell pole. After the parS region is duplicated, one of the two centromeres is chosen during the “distinction phase” (sketch #2–#3) as the substrate for rapid translocation to the opposite cell pole. During the subsequent “commitment phase” (sketch #3–#4), the translocating centromere is directed by the concentration gradient of ParA-ATP. The contributions of the polar proteins TipN and PopZ in setting up and maintaining the ParA gradient are discussed in the main text. When the centromere reaches the opposite pole (sketch #4–#5), it is anchored in place through interactions with PopZ. The initiation, distinction, and commitment phases finish long before the completion of S-phase, and the anchoring of the centromere to the pole is a geometric cue that establishes the global arrangement chromosomal DNA as replication continues (sketch #5–#6). (B) Fluorescence images of live Caulobacter crescentus cells, which express CFP-tagged ParB (in cyan) as a way of showing the location of the chromosome centromeres. The image panels show centromere localization through the initiation, distinction, and commitment phases. The whole cell cycle is around 90–120 min under these experimental conditions
Close observation and quantitative assessment of time-lapse movies of chromosome segregation in Caulobacter suggest that the process occurs in three distinct phases (Shebelut et al. 2010) (Fig. 1): (1) During “initiation,” the centromere is detached from the “old” cell pole shortly before it is duplicated by passage of the replication fork. (2) In the subsequent “distinction” phase, one of the two centromeres is chosen as the substrate for the chromosome segregation machinery, and the two centromeric foci are separated in physical space. (3) In the “commitment” phase, one of the centromeres is rapidly transported to the opposite pole (henceforth called the “destination” pole), while the other remains in the vicinity of the old pole. Notably, the rate of centromere travel is approximately 50% faster in the “commitment” phase than it is during the “distinction” phase, suggesting that the phases are mediated by distinct mechanisms.
2 Broad Conservation of the ParABS Centromere Translocation System
In all of the Alphaproteobacteria studied thus far, the centromere is observed to travel across the cell and become anchored to the destination pole during the commitment phase of chromosome segregation. Generally, the travel time is on the order of 10–60 min (Ehrle et al. 2017; De Bolle et al. 2015; Thanbichler and Shapiro 2006) and represents a fraction of the total cell cycle. The rapid, directional translocation of the centromere is accomplished by a highly conserved co-functioning set of genetic elements collectively known as the ParABS system, which will be covered in detail in this chapter.
ParABS genes are by no means unique to Alphaproteobacterial chromosomes. Close homologs are present in the chromosomes of nearly all bacterial genera (Livny et al. 2007), and a highly homologous set of genetic elements (often called RepABC) is responsible for the partitioning of a broad range of single-copy megaplasmids and related replicons across the phylogenetic spectrum (Austin and Abeles 1983; Castillo-Ramírez et al. 2009; Cevallos et al. 2008; Chai and Winans 2005; Koper et al. 2016; MacLellan et al. 2006; Petersen et al. 2009; Pinto et al. 2012). Interestingly, some or all of the chromosomally encoded ParABS components are lost in some bacterial lineages, including members of the Alphaproteobacteria (Livny et al. 2007). This scattered pattern of loss suggests that the ParABS system originated in a common ancestor in ancient evolutionary time, and that alternative mechanisms for chromosome segregation have arisen in multiple lineages at later times in evolution. One of the best characterized examples of chromosome segregation without ParABS occurs in Escherichia coli, a member of the Gammaproteobacteria clade. The mechanisms associated with this form of chromosome partitioning appear to be more closely related to the segregation of bulk DNA that follows ParABS-dependent segregation in Caulobacter crescentus, and are discussed elsewhere (Reyes-Lamothe et al. 2012; Woldringh et al. 2015).
Typical chromosomally encoded ParABS systems include three components. parA and parB are protein-encoding genes that work together to drive centromere segregation, and are nearly always present as adjoining loci on the chromosome. parS refers to the cis-acting DNA component of the ParABS chromosome translocation system.
3 Centromeres Are Defined by parS Nucleotide Sequences
In bacteria, the term “centromere” refers to the region of DNA that is acted upon directly by the ParABS chromosome partitioning mechanism, and it is the first part of the chromosome to move toward the destination pole. Centromeres are defined by the presence and distribution of one or more discrete nucleotide sequences called parS sites, which can be referred to collectively as a parS region. Each individual parS site is a 16 nucleotide inverted repeat consensus sequence, which is recognized and bound by a ParB dimer (Livny et al. 2007; Lin and Grossman 1998; Sanchez et al. 2015). Most parS regions include multiple parS sites (usually less than 10) that are arranged in a loosely defined cluster with spacing that ranges from tens of base pairs to several kb (Livny et al. 2007; Tran et al. 2017a; Jecz et al. 2015). Because DNA replication initiation is often in close temporal association with centromere duplication, parS regions are most often found within a few kb of the origin of replication, or 3% of the total size of the replicon (Livny et al. 2007). In Caulobacter crescentus, experimental evidence indicates that there are seven parS sites in a 10 kb cluster that is centered 8 kb from the origin of replication. parS sites with the highest affinity for ParB are situated 5 kb apart in the middle of the cluster, and lower affinity sites that are divergent in sequence by 6–8 base pairs lie at greater distances (Tran et al. 2017b).
Experimental evidence in Caulobacter crescentus indicates that the insertion of an additional parS site within the parS region is well tolerated, but that chromosome segregation defects occur when a parS site is inserted outside of this region (Tran et al. 2017b). parS insertion sites that are more than 500 kb from the natural parS region are not viable. This lethal phenotype has been used as the basis of a screen to identify parS sites in Caulobacter and other species. In these experiments, suspected sequences of chromosomal DNA are cloned into multicopy plasmids, and those that include the parS region are not stably maintained (Toro et al. 2008). Together, these studies suggest that the ParABS mechanism cannot properly segregate DNA if it is confused by the presence of an additional parS region at an ectopic locus.
By inserting recombination sites at specific locations in the chromosome, it is possible to invert sections DNA such that the origin of replication and the parS region are separated by a longer distance. Under laboratory conditions, Caulobacter crescentus maintains viability when this distance is increased from the normal 8 kb to 400 kb (Toro et al. 2008). This recombination has the expected effect of increasing the amount of time between replication initiation and chromosome segregation and of re-orienting the relative locations of chromosomal loci such that the centromeres remain at the poles whereas the origin is located farther toward the mid-cell region. Despite the viability of these recombinant strains, the information obtained from genomic sequencing of wildtype Alphaproteobacterial species makes it clear that large distances between replication origin and parS region are not favored in evolution.
The benefits that come from holding parS regions and origins of replication in proximity are unclear, but may be related to the ability to temporally coordinate replication initiation and chromosome segregation. Separating the chromosomes may be simpler when there is less DNA to separate, and an early start on chromosome segregation might allow more rapid cell division. Additionally, there is evidence that tethering of the centromere to the cell pole brings the replication origin close to polar regulatory proteins that control the timing of replication initiation (Chen et al. 2011; Lasker et al. 2016). Thus, the proximity of these two elements may be a device that physically connects mechanisms for cytoplasmic organization (i.e., polar tethering of centromeres and localization of polar signaling proteins) to those that regulate the timing of replication initiation.
4 ParB Assembles into a Complex Superstructure at the parS Region
In all ParABS systems, parS sequences are palindromes or near-palindromes that are recognized and bound directly by the protein ParB in its dimeric state. In cytoplasm, nearly all of the ParB is thought to be in dimeric form, and it binds to parS sites at relatively high affinity and also to non-specific DNA sequences at somewhat lower affinity (Sanchez et al. 2015; Song et al. 2017; Taylor et al. 2015). The assembly of ParB on bacterial DNA is analogous to the formation of kinetochores on eukaryotic chromosomes, in that these structures define the centromere and act as an interface between DNA and the segregation machinery that moves it. Chromosomally encoded ParB proteins in Alphaproteobacteria are essential for cell viability, and are members of a broad ParB sub-type called Type 1A, which is common in several other bacterial clades as well as phage and plasmids (Oliva 2016). This variant of ParB has three domains: a C-terminal dimerization domain, a central helix–turn–helix domain that binds to DNA, and an N-terminal domain that allows association between ParB dimers (Chen et al. 2015) and interaction with ParA (Scholefield et al. 2011).
Visualization of ParB in cells using fluorescence microscopy reveals one, two, or more tightly localized puncta, each corresponding to a chromosomal centromere (Ehrle et al. 2017; Bowman et al. 2008; Gruber and Errington 2009; Iniesta 2014). These observations must somehow be reconciled with the fact that there are far more ParB proteins in the cell [experimentally measured to be about 360 dimers/cell in Caulobacter (Lim et al. 2014)] than parS binding sites (7 sites, Tran et al. 2017b), and suggests that the centromeres are comprised of higher-order associations among many ParB proteins. A quantitative assessment of this question in a single-copy plasmid-based ParABS system indicates that more than 90% of ParB, or several hundreds of molecules, are localized in partitioning complexes (Sanchez et al. 2015).
Older models suggested that clusters are formed by “spreading,” where an initiating ParB dimer binds to a parS site, and subsequent binding of other ParB molecules is stabilized through lateral interactions (Murray et al. 2006). Based on newer evidence, current models propose that clusters are held together by ParB’s N-terminal domain, which facilitates interactions between ParB dimers bound stably at parS sites and other dimers bound non-specifically to random sites on the chromosome, which are often nearby in linear sequence but could be several kb distant (Sanchez et al. 2015; Broedersz et al. 2014). The models are supported by a crystal structure of the ParB–DNA complex, which shows that each N-terminal domain in a ParB dimer can separately form tetrameric complexes with three other ParB dimers (Chen et al. 2015). Further, ParB is able to compact parS-containing DNA sequences in vitro, but this activity is blocked by mutations in ParB’s N-terminal dimer interaction domain (Song et al. 2017).
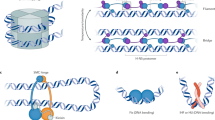
Because ParB-DNA networks are formed from a multivalent network of interactions, large centromeric assemblies can form even when the affinities between individual components (whether it is ParB binding non-specifically to DNA or an interaction between ParB dimers) are relatively weak (Fig. 2a). The result, which is known as the “nucleation and caging model,” is a dynamic cluster of ParB–DNA complexes (the cage) that centers on the parS region (the nucleation site), because the parS–ParB interaction is stable relative to other connections within the network (Sanchez et al. 2015). Although single parS sites are sufficient for the formation of a ParB cluster and centromere segregation in vivo (Jecz et al. 2015), genomic sequences of large replicons suggest that there are advantages in retaining several parS sites, perhaps as a mechanism for strengthening the ParB cluster. As discussed earlier in this chapter, placing an additional parS site outside of the cluster causes lethal defects (Toro et al. 2008; Tran et al. 2017b), probably because this creates a multicentric replicon.
Molecular mechanisms associated with chromosome segregation. (a) ParB-DNA structures define the centromere. ParB dimers bind DNA non-specifically, but bind to centromere-defining parS sites at significantly higher affinity. The N-terminal domain of ParB facilitates interactions between ParB dimers, both in cis- and trans-orientations. The combined effects of high affinity parS binding, multimerization, and lower affinity binding to non-specific DNA sequences enable ParB to form a dynamic cluster of molecules that cage parS and nearby DNA sequences. (b) ParABs-dependent centromere translocation. Contact between ParB and DNA-bound ParA-ATP stimulates ATP hydrolysis (step 1), which releases ParA from DNA and breaks the interaction. Upon release (step 2), the ParB cluster will continue to diffuse, though subsequent interactions with other ParA-ATP molecules (step 3) act like a ratchet in biasing net movement up the ParA-ATP concentration gradient. Continued directional translocation of the centromere requires a mechanism for controlling the distribution of ParA-ATP such that it is always highest in the direction of the destination pole. (c) ParA-ATP regeneration at destination pole. Monomeric ParA molecules do not bind DNA and diffuse rapidly within the cytoplasm. A current model holds that when ParA molecules encounter proteins that are specifically localized to the destination pole, such as the matrix-forming scaffold protein PopZ or the polar transmembrane protein TipN, they are induced to dimerize and bind ATP. As ParA-ATP molecules diffuse away from the PopZ matrix, they encounter DNA, to which they bind non-specifically. A gradient is formed because the most ParA-ATP molecules encounter and bind DNA soon after exiting the recycling point at the destination pole. (d) Smc and chromosome topology. Smc-ATP dimerizes and forms a ring that encloses two strands of DNA. In Caulobacter and other Alphaproteobacteria, this process is thought to facilitate the early stages of centromere partitioning. Shortly after the initiation of DNA replication (step 1), the replisomes (colored purple) duplicate the parS site. The parS sites on the newly replicated DNA are loading sites for new Smc loops (step 2). As replication continues, more Smc loops are added at each parS site (step 3), and the complexes of Smc loops hold the growing chromosome arms in topologically separated structures
5 The ParA ATPase Is Essential for Chromosome Segregation
The ParA component of the ParABS chromosome segregation system is a Walker A-type ATPase protein. In Alphaproteobacteria, the parA gene is essential for viability (Christen et al. 2011; Curtis and Brun 2014), and expressing ATPase deficient mutants has a dominant negative effect on the translocation of centromeres during chromosome segregation (Toro et al. 2008; Schofield et al. 2010). Chromosomally encoded ParA proteins are highly conserved throughout the bacterial kingdom (even more so than ParB), thus it is likely that all homologs share the same basic set of biochemical activities, including ATPase activity and ParB interaction. Our knowledge of the general mechanism of ParA-ATPase cycling is primarily derived from research in Bacillus (Scholefield et al. 2011; Hester and Lutkenhaus 2007; Leonard et al. 2005). ParA dimerizes after binding ATP, and in this state it binds to DNA non-specifically. When ParB interacts with DNA-bound ParA-ATP, the ATP is hydrolyzed, and ParA becomes monomeric and loses its affinity for DNA (Lim et al. 2014; Scholefield et al. 2011).
6 ParA Dynamics and ATP-Cycling Drive Centromere Translocation
A major question in the field is how ParB-mediated stimulation of ParA-ATP relates to the mechanical forces that drive chromosome segregation. In recent years, studies on ParABS systems that function on low-copy number plasmids (Hu et al. 2017) and on chromosome centromeres in Caulobacter (Lim et al. 2014; Surovtsev et al. 2016a) have converged on the same basic mechanism. Interestingly, the energy from ATP hydrolysis does not produce a mechanical force as it does in myosin motors and the like, but is instead used as a symmetry-breaking switch that provides directional guidance to what would otherwise be random motion. This phenomenon is generally described as a “burnt-bridge Brownian ratchet,” and is most clearly demonstrated in a reconstituted in vitro system that uses a minimal set of components: parS, ParB, and ParA from a plasmid system (Hwang et al. 2013; Vecchiarelli et al. 2013). In these systems, centromeres are created by coating a polystyrene bead with parS DNA and ParB. ParA-ATP is then laid across a carpet of non-specific DNA sequence. When the beads are placed on the carpet, they move across the carpet of ParA-ATP. Because contact with ParB stimulates ParA-ATPase activity, the areas where the bead has passed are devoid of ParA (the burnt-bridge component of the model). The consequence of this is that the polystyrene bead, which explores movement in all directions through Brownian motion, fails to get trapped by new ParA–ATP interactions when it diffuses in the retrograde direction, but is briefly held in place when it interacts with a part of the carpet that has ParA-ATP (the ratchet component of the model).
A similar set of mechanochemical interactions are thought to be at play during the segregation of chromosomal centromeres in Caulobacter cells, though there are some interesting differences in the proposed mechanisms compared to the in vitro reconstituted systems. ParA-ATP is distributed in a concentration gradient that increases in the direction of the destination pole (Schofield et al. 2010). This may facilitate directional guidance simply because the Brownian ratchet is even more strongly biased toward trapping the centromere when it happens to move toward an area of relatively high ParA-ATP concentration. It has also been proposed that the non-uniform distribution of ParA-ATP is a mechanism for providing a mechanical force that draws the centromere up the concentration gradient, as described by the “DNA-relay” model (Lim et al. 2014; Surovtsev et al. 2016a). According to this model, the elastic nature of DNA implies that some of the contacts between ParB and ParA-ATP are stretched out of equilibrium. As the tension is relaxed (and before ParA-ATP hydrolysis), the centromere is pulled in the direction of the most ParA-ATP contacts, which is up the concentration gradient. Because tethers can be formed in any direction, the Brownian nature of the system remains, and it is the energy put into the creation of the ParA-ATP concentration gradient that imparts directionality to the movement of the chromosome centromere. The energy boost that comes from DNA-relay type forces may be important for achieving a sufficient rate of travel as the centromere moves through the densely crowded environment (Le Gall et al. 2016) of the bacterial nucleoid.
7 Chromosome Segregation Is Under Control of Polar Guidance Cues
The models that consider the segregation of low-copy plasmids suggest that the nucleoid and ParABS components of the DNA-relay model are sufficient to explain all aspects of their movement within cells, including the oscillatory movement of single ParB foci between poles and the separation of newly replicated centromeres (Hu et al. 2017; Surovtsev et al. 2016b; Ebersbach and Gerdes 2004; Ringgaard et al. 2009). However, it is important to note that the chromosomal centromeres in Alphaproteobacteria (and also in other clades) display a significantly different pattern of movement. In Caulobacter, Brucella, and Agrobacterium, the centromere is located at the old cell when present as a single copy, and after chromosome replication, one of the centromeres remains in place while the other travels across the cell and becomes stably localized to the opposite pole (Fig. 1) (Ehrle et al. 2017; Deghelt et al. 2014; Thanbichler and Shapiro 2006). By contrast, the parS regions of low-copy plasmids are rarely found at the cell poles, even when present in multiple copies (Ebersbach and Gerdes 2004; Derman et al. 2008). This discrepancy suggests that the localization and movement of chromosomal centromeres are being subject to additional guidance cues outside of ParABS.
In Caulobacter, chromosome segregation is affected by two polar guidance cues, named PopZ and TipN. PopZ is localized to the new pole and the old pole. In vitro, it is capable of interacting with both ParA and ParB, and in popZ knockout cells the chromosomal centromeres drift in the cytoplasm instead of becoming anchored to cell poles (Bowman et al. 2008; Ebersbach et al. 2008; Ptacin et al. 2014). The centromere localization defect has also been observed in Agrobacterium (Ehrle et al. 2017). Together, these observations suggest that PopZ’s role is to tether the centromeres to the cell poles. Direct interaction between centromeric ParB and polar PopZ may account for a part of this mechanism. The significance of PopZ’s interaction with ParA is less clear, even though experiments in which wildtype popZ was replaced with protein-specific binding mutants suggest that the ParA–PopZ interaction is more important for chromosome segregation and polar localization than the ParB–PopZ interaction in vivo (Ptacin et al. 2014).
One compelling model for PopZ as a polar guidance factor is that it provides a specific location for regenerating ParA-ATP (Fig. 2d). In this model, ParA molecules that have fallen off of DNA in the wake of the moving centromere diffuse as monomers in cytoplasm until coming into contact with PopZ at the cell pole. The interaction between ParA monomers and polar PopZ may stimulate ATP binding and dimerization of ParA, or simply provide a place for ParA accumulation as ParA-ATP is regenerated at an intrinsic rate. As the regenerated population of ParA-ATP molecules diffuse out of the polar zone they will bind to DNA, thereby creating a gradient of DNA-bound ParA-ATP molecules that emanates from the pole.
This model is supported by time-lapse fluorescence microscope experiments, wherein the localizations of ParB/centromeres and ParA have been directly observed in live cells by expressing genetically encoded fluorescent proteins in fusion with ParA and ParB (as summarized in Fig. 1 and 2b). Similar observations have been made in Caulobacter crescentus (Schofield et al. 2010) and the Gammaproteobacteria Vibrio cholerae (Fogel and Waldor 2006). In each study, the centromeres are observed to move up a visible concentration gradient of nucleoid-bound ParA that emanates from the destination pole. As the centromeres move across the nucleoid, they are observed to leave an area of no ParA in their wake, while the concentration of ParA emanating from the destination pole becomes progressively higher.
The parS centromeres on low-copy number plasmids have also been observed to follow ParA concentration gradients across the nucleoid, with the notable difference that the centromeres do not move all of the way out to the cell poles, and the concentration of ParA re-forms in the area between the segregated centromeres (Ringgaard et al. 2009). Thus, it would appear that the chromosomal ParABS systems use the cell poles as locations for regenerating ParA-ATP, whereas this occurs spontaneously in the case of plasmid-based systems, and this distinction explains why these different systems have different patterns of centromere movement.
The other polar guidance cue that has been identified in Caulobacter is TipN, which is localized exclusively at the destination pole (Huitema et al. 2006; Lam et al. 2006; Yeh et al. 2010). TipN is a large transmembrane protein that interacts directly with ParA (Ptacin et al. 2010; Lam et al. 2006). In the absence of TipN, time-lapse fluorescence microscopy experiments show that the concentration of ParA at the destination pole is far less robust than in wildtype cells. Instead, ParA often accumulates behind the traveling ParB focus. This has the effect of increasing the frequency at which the traveling centromere reverses direction, thereby slowing the overall rate of chromosome segregation and frequently resulting in incomplete segregation (Schofield et al. 2010).
Based on these observations, it appears that TipN and PopZ play overlapping roles in directing chromosome segregation, and this view is further supported by evidence of genetic interactions between tipN and popZ in Caulobacter (Schofield et al. 2010). First, the centromere localization defect observed in a popZ knockout strain can be rescued by overproducing TipN, suggesting the overabundance of one polar cue can compensate for the loss of the other. Second, the combination of defects in tipN and popZ single mutants becomes inviable when they are combined as a tipN popZ double mutant, suggesting a lethal defect in chromosome segregation when both polar cues are absent. The lethal phenotype is consistent with the fact that parA and parB are both essential genes in Caulobacter (Christen et al. 2011; Mohl et al. 2001). Surprisingly, TipN is not as well conserved through Alphaproteobacteria as PopZ, suggesting that these two proteins are not co-evolving, and that TipN may only be important in special contexts. In Caulobacter, PopZ is localized to both cell poles, and the focus of TipN at the destination pole may help to provide an unambiguous directional cue. In contrast, PopZ is found only at the destination pole during centromere translocation in Agrobacterium and Brucella (Ehrle et al. 2017; Deghelt et al. 2014), and these species do not carry a tipN homolog, perhaps because an additional directional cue is not necessary.
8 Segregation Activities Outside of ParABS
Despite the broad conservation of the ParABS mechanism across the prokaryotic kingdom (Livny et al. 2007) end even into archaea (Schumacher et al. 2015), these components are not essential for successful chromosome partitioning or survival in many bacterial species. In the Gammaproteobacteria Vibrio cholerae, for example, deletions in either parA or parB produce viable strains. In these mutants, the chromosomes are correctly partitioned between daughter cells even though the centromeres fail to complete the journey to their normal anchoring point at the destination pole (Fogel and Waldor 2006; Kadoya et al. 2011). In Gram positive Bacillus subtilis, deletion of the parA homolog (called soj) has no detectable effect on chromosome partitioning, whereas deletion of the parB homolog (spo0J) results in a 100-fold increase in the production of anucleate cells (Ireton et al. 1994). Notably, E. coli and related Enterobacteriales species manage chromosome segregation entirely without the ParABS system. Together, these and other examples suggest that the bacterial kingdom includes additional mechanisms for chromosome partitioning that operate along with or in place of ParABS.
An increasing body of literature is revealing ParABS-independent chromosome partitioning activities in Caulobacter. Part of the evidence for these mechanisms has come from quantitative assessment of chromosome segregation, which suggests that segregation occurs in multiple distinct phases (Shebelut et al. 2010) (Fig. 1). Notably, the rate of centromere travel is approximately 50% faster in the ParABS-mediated commitment phase than it is during the distinction phase, and neither centromere detachment from the old pole nor the separation of centromeres is blocked by expression of a dominant-negative ParA mutant protein that fully inhibits the commitment step (Shebelut et al. 2010). This suggests that the early phases of chromosome partitioning occur by ParABS-independent mechanisms, but these are not well understood. It is possible that the short-distance movements of the centromeres at early stages occur by the release of tension within the nucleoid, as may occur after breaking the PopZ-dependent polar anchor, and by the creation of new tension during the replication of DNA. In both cases, the source of the tension could be proteins that mediate the compaction of bulk DNA.
9 SMC/Condensin and Topologically Associated Domains
SMC is a DNA-associated protein that can mediate DNA compaction and may play a role in the early phases of chromosome partitioning. SMC proteins (also known as condensins) have been a subject of significant interest in bacterial cell biology because they are found in nearly all prokaryotes, and are strikingly homologous in structure and function to a broad class of eukaryotic proteins with the same name. Eukaryotic condensins organize chromatin by binding ATP and subsequently forming dimers that hold DNA strands together in a closed loop (Haering et al. 2002).
The strongest evidence that SMC is involved in bacterial chromosome segregation comes from smc mutant phenotypes in species outside of Alphaproteobacteria. In B. subtilis, for example, ∆smc strains produce a large fraction of anucleate cells, indicating failure in chromosome segregation (Gruber et al. 2014; Wang et al. 2014). B. subtilis SMC is loaded onto origin-proximal regions of the chromosome by a ParB-dependent process (Gruber and Errington 2009). Recent chromosome capture studies have compared chromosomal architecture in wild type and smc mutants, and found that the contacts between chromosome arms are fewer in number and more widely distributed in smc mutants, suggesting that the arms are misaligned when SMC is not loaded onto the chromosome (Wang et al. 2015). Restoring SMC loading restores the alignment of chromosome arms, initially at the parS site where SMC is loaded and later through the rest of the chromosome as the area of SMC coverage spreads from its loading site (Wang et al. 2017). A model that describes the role of SMC in chromosome partitioning, called “loop extrusion,” holds that SMC is loaded onto DNA at parS regions shortly after the passage of the replisome, and that the collective looping and DNA condensation activities of multiple SMC molecules on each of the newly replicated DNA segments result in the formation of two separate topologically associated domains (TADs). Progressive addition of SMC molecules on the two separating parS sites drives further extrusion of the TADs, thus driving the initial phase of chromosome partitioning (Wang et al. 2014).
E. coli also requires a nucleoid-associated SMC-like ATPase called MukB for chromosome partitioning. mukB deletion mutants are defective not only in chromosome segregation but also in DNA compaction (Danilova et al. 2007; Lioy et al. 2018), which is consistent with the idea that an SMC-like protein also establishes chromosomal TAD’s in this organism. Unlike SMC, MukB will load onto DNA non-specifically, and instead of being loaded onto DNA at parS sites, it is restricted to the area around the origin of replication(Danilova et al. 2007) by the exclusionary activity of a different TAD that forms at the terminal region (Nolivos et al. 2016). Instead of segregating newly replicated DNA by loop extrusion, the TAD’s established by MukB may drive partitioning through entropy-driven demixing (discussed below).
In Caulobacter, SMC is loaded onto the chromosome at parS sites through interaction with ParB, where it holds the chromosome arms in close proximity (Tran et al. 2017a) (Fig. 2c). Consistent with this, chromosome arms are misaligned in ∆smc mutants (Le et al. 2013), suggesting that Caulobacter SMC forms a TAD in the region of the chromosomal centromere, much as it does in B. subtilis. Currently, the most direct evidence that SMC affects chromosome partitioning in Caulobacter is that the expression of a dominant-negative ATPase deficient form of SMC causes replicated centromeres to be held in close proximity (Schwartz and Shapiro 2011). Presumably, either the distinction or the commitment step in centromere segregation is blocked because DNA strands are linked by SMC loops that are too numerous or too strong to allow partitioning to move forward. However, efforts to gain a deeper understanding of SMC’s role have been hampered by the lack of an obvious chromosome partitioning phenotype in ∆smc knockout strains, which suggests that the TAD formed by SMC is not necessary for segregation in Caulobacter. Instead, the parABS elements, which are essential in Caulobacter but not in B. subtilis, appear to be the dominant players in chromosome segregation in this species.
10 Potential Roles for Other Nucleoid-Associated Proteins
SMC is just one of several nucleoid-associated proteins that may be involved in establishing TADs or otherwise participating in chromosome segregation in Alphaproteobacteria, but at the current time, the roles of these other factors are even less clear. The Caulobacter genome includes two genes that encode the histone-like nucleoid-associated protein HU, but the double knockout strain exhibits only a minor degree of chromosome de-compaction (Le et al. 2013) and has not been reported to affect chromosome segregation. Three recent publications have described the function of another nucleoid-associated protein called GapR (Taylor et al. 2017; Arias-Cartin et al. 2017; Ricci et al. 2016). GapR is highly conserved among Alphaproteobacteria and is essential in Caulobacter under normal growth conditions. A significant fraction of cells in gapR mutant cultures are anucleate or otherwise lacking DNA in polar regions, suggesting defects in DNA compaction or chromosome partitioning (Arias-Cartin et al. 2017). However, the phenotype of gapR mutants is highly pleiotropic, and also includes delayed chromosome replication and differences in gene expression across the chromosome, which has made it difficult to assess the direct role of GapR in chromosome segregation. Quantitative fluorescence microscopy has shown that the passage of the replisome displaces GapR from DNA, leaving a broad section of GapA-free nucleoid in its wake (Arias-Cartin et al. 2017), in a manner that is analogous to the clearance of ParA after passage of the centromeres. Although it is tempting to speculate that GapA is associated with a replication-dependent DNA compaction activity that facilitates chromosome segregation, current evidence is not sufficient to support this notion.
11 Partitioning Origin-Distal DNA
In Caulobacter, as well as in other species, the origin of replication or the closely associated parS region is the first part of the chromosome to be partitioned during chromosome segregation. How is the rest of the chromosome partitioned after the ParABS-dependent movement has completed? Theoretical simulations predict that this aspect of chromosome partitioning is an entropy-driven process, in which the demixing of the intertwined DNA polymers is the lowest energy outcome (Jun and Mulder 2006). While complete partitioning is not spontaneous and probably requires energy input (Minina and Arnold 2014), there is disagreement over where energy is added to the system. Modeling studies that are based on the E. coli system suggest that differential compaction of TADs leads to a non-uniform distribution of forces within the nucleoid that drives partitioning (Junier et al. 2014). Alternatively, another model suggests that supercoiling imposes a certain plectonemic structure on chromosomal DNA, and that in vivo crowding prevents the lowest energy conformation. Here, initial centromere displacement by ParABS, in conjunction with formation of relatively open, nucleoid-free space due to cell elongation and local DNA compaction in the vicinity of the replisome, provides sufficient entropic force for the partitioning of the chromosome toward the destination pole (Hong et al. 2013). An interesting demonstration of partitioning forces in the origin-distal parts of the chromosome comes from the observation of double-strand break repair in Caulobacter cells (Badrinarayanan et al. 2015). Here, a single double-strand break causes the surrounding ~300 kb section of the chromosome to become highly mobile within the cytoplasm, and after it pairs with its homologous partner for recombination-repair, the traveling section of DNA rapidly returns to its normal position in the cell, via a ParA-independent mechanism that presumably involves the same entropic forces that are at play during chromosome segregation.
12 Concluding Remark
In this chapter, we discuss the ParABS system as the central mechanism for generating the force to drive chromosome segregation in Alphaproteobacteria. Although the vast majority of information on chromosome segregation in this clade has come from studies that focused exclusively on Caulobacter crescentus, the high degree of conservation among ParABS and the limited number of studies that have considered chromosome in other Alphaproteobacteria species suggest that, for the most part, the lessons from Caulobacter can be broadly applied. There are, however, some notable exceptions to this idea. Some Alphaproteobacteria lack one or more ParABS components, and the mechanisms that compensate for such deficiencies are not known. Similarly, the polar factors that facilitate ParABS function in Caulobacter are not as well conserved as ParABS itself, and the question of how chromosome segregation is supported in the absence of these factors has not been explored. Another important difference between Caulobacter and many other Alphaproteobacteria species is the number of chromosomes being segregated. For those that harbor two or more replicons, each usually carries its own complete ParABS mechanism. It will be interesting to learn how these species’ physiology has changed to accommodate these additions.
Owing to the fact that ParABS systems have been characterized in detail in a wide range of systems across the bacterial kingdom, much is known about this core mechanism. However, there are gaps in our understanding of the interfacing between core ParABS operations and additional factors, which seem to have evolved in a clade-specific manner. Consequently, current models are incomplete. For example, the establishment and maintenance of a cytoplasmic gradient of DNA-bound ParA would seem to require a pole-localized regenerator of ParA-ATP, but the molecular mechanism is not known. Additionally, if there is a pole-directed directional cue, the mechanism by which this machinery reliably chooses one, but not both centromeres has not been adequately described. In a broader sense, the fact that segregation is limited to one centromere and one time per cell cycle suggests that the system is also controlled by negative regulation. However, this and other intriguing possibilities await further investigation.
References
Arias-Cartin R et al (2017) Replication fork passage drives asymmetric dynamics of a critical nucleoid-associated protein in Caulobacter. EMBO J 36:301–318
Austin S, Abeles A (1983) Partition of unit-copy miniplasmids to daughter cells. I. P1 and F miniplasmids contain discrete, interchangeable sequences sufficient to promote equipartition. J Mol Biol 169:353–372
Badrinarayanan A, Le TBK, Laub MT (2015) Rapid pairing and resegregation of distant homologous loci enables double-strand break repair in bacteria. J Cell Biol 210:385–400
Bowman GR et al (2008) A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole. Cell 134:945–955
Bowman GR et al (2010) Caulobacter PopZ forms a polar subdomain dictating sequential changes in pole composition and function. Mol Microbiol 76:173–189
Broedersz CP et al (2014) Condensation and localization of the partitioning protein ParB on the bacterial chromosome. Proc Natl Acad Sci U S A 111:8809–8814
Castillo-Ramírez S, Vázquez-Castellanos JF, González V, Cevallos MA (2009) Horizontal gene transfer and diverse functional constrains within a common replication-partitioning system in Alphaproteobacteria: the repABC operon. BMC Genomics 10:536
Cevallos MA, Cervantes-Rivera R, Gutiérrez-Ríos RM (2008) The repABC plasmid family. Plasmid 60:19–37
Chai Y, Winans SC (2005) RepB protein of an agrobacterium tumefaciens Ti plasmid binds to two adjacent sites between repA and repB for plasmid partitioning and autorepression. Mol Microbiol 58:1114–1129
Chen YE et al (2011) Spatial gradient of protein phosphorylation underlies replicative asymmetry in a bacterium. Proc Natl Acad Sci U S A 108:1052–1057
Chen B-W, Lin M-H, Chu C-H, Hsu C-E, Sun Y-J (2015) Insights into ParB spreading from the complex structure of Spo0J and parS. Proc Natl Acad Sci U S A 112:6613–6618
Christen B et al (2011) The essential genome of a bacterium. Mol Syst Biol 7:528
Curtis PD, Brun YV (2014) Identification of essential alphaproteobacterial genes reveals operational variability in conserved developmental and cell cycle systems. Mol Microbiol 93:713–735
Danilova O, Reyes-Lamothe R, Pinskaya M, Sherratt D, Possoz C (2007) MukB colocalizes with the oriC region and is required for organization of the two Escherichia coli chromosome arms into separate cell halves. Mol Microbiol 65:1485–1492
De Bolle X, Crosson S, Matroule J-Y, Letesson J-J (2015) Brucella abortus cell cycle and infection are coordinated. Trends Microbiol 23:812–821
Deghelt M et al (2014) G1-arrested newborn cells are the predominant infectious form of the pathogen Brucella abortus. Nat Commun 5:4366
Derman AI, Lim-Fong G, Pogliano J (2008) Intracellular mobility of plasmid DNA is limited by the ParA family of partitioning systems. Mol Microbiol 67:935–946
Easter J, Gober JW (2002) ParB-stimulated nucleotide exchange regulates a switch in functionally distinct ParA activities. Mol Cell 10:427–434
Ebersbach G, Gerdes K (2004) Bacterial mitosis: partitioning protein ParA oscillates in spiral-shaped structures and positions plasmids at mid-cell. Mol Microbiol 52:385–398
Ebersbach G et al (2006) Regular cellular distribution of plasmids by oscillating and filament-forming ParA ATPase of plasmid pB171. Mol Microbiol 61:1428–1442
Ebersbach G, Briegel A, Jensen GJ, Jacobs-Wagner C (2008) A self-associating protein critical for chromosome attachment, division, and polar organization in caulobacter. Cell 134:956–968
Ehrle HM et al (2017) Polar organizing protein PopZ is required for chromosome segregation in agrobacterium tumefaciens. J Bacteriol 199
Figge RM, Easter J, Gober JW (2003) Productive interaction between the chromosome partitioning proteins, ParA and ParB, is required for the progression of the cell cycle in Caulobacter crescentus. Mol Microbiol 47:1225–1237
Fogel MA, Waldor MK (2006) A dynamic, mitotic-like mechanism for bacterial chromosome segregation. Genes Dev 20:3269–3282
Frage B et al (2016) Spatiotemporal choreography of chromosome and megaplasmids in the Sinorhizobium meliloti cell cycle. Mol Microbiol 100:808–823
Gruber S, Errington J (2009) Recruitment of condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell 137:685–696
Gruber S et al (2014) Interlinked sister chromosomes arise in the absence of condensin during fast replication in B. subtilis. Curr Biol 24:293–298
Haering CH, Löwe J, Hochwagen A, Nasmyth K (2002) Molecular architecture of SMC proteins and the yeast cohesin complex. Mol Cell 9:773–788
Hester CM, Lutkenhaus J (2007) Soj (ParA) DNA binding is mediated by conserved arginines and is essential for plasmid segregation. Proc Natl Acad Sci U S A 104:20326–20331
Hong S-H, McAdams HH (2011) Compaction and transport properties of newly replicated Caulobacter crescentus DNA. Mol Microbiol 82:1349–1358
Hong S-H et al (2013) Caulobacter chromosome in vivo configuration matches model predictions for a supercoiled polymer in a cell-like confinement. Proc Natl Acad Sci U S A 110:1674–1679
Howell M et al (2017) Absence of the polar organizing protein PopZ results in reduced and asymmetric cell division in agrobacterium tumefaciens. J Bacteriol 199
Hu L, Vecchiarelli AG, Mizuuchi K, Neuman KC, Liu J (2017) Brownian ratchet mechanism for faithful segregation of low-copy-number plasmids. Biophys J 112:1489–1502
Huitema E, Pritchard S, Matteson D, Radhakrishnan SK, Viollier PH (2006) Bacterial birth scar proteins mark future flagellum assembly site. Cell 124:1025–1037
Hwang LC et al (2013) ParA-mediated plasmid partition driven by protein pattern self-organization. EMBO J 32:1238–1249
Iniesta AA (2014) ParABS system in chromosome partitioning in the bacterium Myxococcus xanthus. PLoS One 9:e86897
Ireton K, Gunther NW, Grossman AD (1994) spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol 176:5320–5329
Jecz P, Bartosik AA, Glabski K, Jagura-Burdzy G (2015) A single parS sequence from the cluster of four sites closest to oriC is necessary and sufficient for proper chromosome segregation in Pseudomonas aeruginosa. PLoS One 10:e0120867
Jun S, Mulder B (2006) Entropy-driven spatial organization of highly confined polymers: lessons for the bacterial chromosome. Proc Natl Acad Sci U S A 103:12388–12393
Junier I, Boccard F, Espéli O (2014) Polymer modeling of the E coli genome reveals the involvement of locus positioning and macrodomain structuring for the control of chromosome conformation and segregation. Nucleic Acids Res 42:1461–1473
Kadoya R, Baek JH, Sarker A, Chattoraj DK (2011) Participation of chromosome segregation protein ParAI of Vibrio cholerae in chromosome replication. J Bacteriol 193:1504–1514
Kahng LS, Shapiro L (2003) Polar localization of replicon origins in the multipartite genomes of agrobacterium tumefaciens and Sinorhizobium meliloti. J Bacteriol 185:3384–3391
Koper P, Żebracki K, Marczak M, Skorupska A, Mazur A (2016) RepB proteins of the multipartite Rhizobium leguminosarum bv trifolii genome discriminate between centromere-like parS sequences for plasmid segregational stability. Mol Microbiol 102:446–466
Laloux G, Jacobs-Wagner C (2013) Spatiotemporal control of PopZ localization through cell cycle-coupled multimerization. J Cell Biol 201:827–841
Lam H, Schofield WB, Jacobs-Wagner C (2006) A landmark protein essential for establishing and perpetuating the polarity of a bacterial cell. Cell 124:1011–1023
Lasker K, Mann TH, Shapiro L (2016) An intracellular compass spatially coordinates cell cycle modules in Caulobacter crescentus. Curr Opin Microbiol 33:131–139
Le Gall A et al (2016) Bacterial partition complexes segregate within the volume of the nucleoid. Nat Commun 7:12107
Le TBK, Imakaev MV, Mirny LA, Laub MT (2013) High-resolution mapping of the spatial organization of a bacterial chromosome. Science 342:731–734
Leonard TA, Butler PJ, Löwe J (2005) Bacterial chromosome segregation: structure and DNA binding of the Soj dimer--a conserved biological switch. EMBO J 24:270–282
Lim HC et al (2014) Evidence for a DNA-relay mechanism in ParABS-mediated chromosome segregation. elife 3:e02758
Lin DC, Grossman AD (1998) Identification and characterization of a bacterial chromosome partitioning site. Cell 92:675–685
Lioy VS et al (2018) Multiscale structuring of the E. coli chromosome by nucleoid-associated and condensin proteins. Cell 172:771–783.e18
Livny J, Yamaichi Y, Waldor MK (2007) Distribution of centromere-like parS sites in bacteria: insights from comparative genomics. J Bacteriol 189:8693–8703
MacLellan SR, Zaheer R, Sartor AL, MacLean AM, Finan TM (2006) Identification of a megaplasmid centromere reveals genetic structural diversity within the repABC family of basic replicons. Mol Microbiol 59:1559–1575
Minina E, Arnold A (2014) Induction of entropic segregation: the first step is the hardest. Soft Matter 10:5836–5841
Mohl DA, Easter J, Gober JW (2001) The chromosome partitioning protein, ParB, is required for cytokinesis in Caulobacter crescentus. Mol Microbiol 42:741–755
Murray H, Ferreira H, Errington J (2006) The bacterial chromosome segregation protein Spo0J spreads along DNA from parS nucleation sites. Mol Microbiol 61:1352–1361
Nolivos S et al (2016) MatP regulates the coordinated action of topoisomerase IV and MukBEF in chromosome segregation. Nat Commun 7:10466
Oliva MA (2016) Segrosome complex formation during DNA trafficking in bacterial cell division. Front Mol Biosci 3:51
Petersen J, Brinkmann H, Pradella S (2009) Diversity and evolution of repABC type plasmids in Rhodobacterales. Environ Microbiol 11:2627–2638
Pinto UM, Pappas KM, Winans SC (2012) The ABCs of plasmid replication and segregation. Nat Rev Microbiol 10:755–765
Ptacin JL et al (2010) A spindle-like apparatus guides bacterial chromosome segregation. Nat Cell Biol 12:791–798
Ptacin JL et al (2014) Bacterial scaffold directs pole-specific centromere segregation. Proc Natl Acad Sci U S A 111:E2046–E2055
Reyes-Lamothe R, Nicolas E, Sherratt DJ (2012) Chromosome replication and segregation in bacteria. Annu Rev Genet 46:121–143
Ricci DP et al (2016) Cell cycle progression in Caulobacter requires a nucleoid-associated protein with high AT sequence recognition. Proc Natl Acad Sci U S A 113:E5952–E5961
Ringgaard S, van Zon J, Howard M, Gerdes K (2009) Movement and equipositioning of plasmids by ParA filament disassembly. Proc Natl Acad Sci U S A 106:19369–19374
Sanchez A et al (2015) Stochastic self-assembly of ParB proteins builds the bacterial DNA segregation apparatus. Cell Syst 1:163–173
Schofield WB, Lim HC, Jacobs-Wagner C (2010) Cell cycle coordination and regulation of bacterial chromosome segregation dynamics by polarly localized proteins. EMBO J 29:3068–3081
Scholefield G, Whiting R, Errington J, Murray H (2011) Spo0J regulates the oligomeric state of Soj to trigger its switch from an activator to an inhibitor of DNA replication initiation. Mol Microbiol 79:1089–1100
Schumacher MA et al (2015) Structures of archaeal DNA segregation machinery reveal bacterial and eukaryotic linkages. Science 349:1120–1124
Schwartz MA, Shapiro L (2011) An SMC ATPase mutant disrupts chromosome segregation in Caulobacter. Mol Microbiol 82:1359–1374
Shebelut CW, Guberman JM, van Teeffelen S, Yakhnina AA, Gitai Z (2010) Caulobacter chromosome segregation is an ordered multistep process. Proc Natl Acad Sci U S A 107:14194–14198
Song D, Rodrigues K, Graham TGW, Loparo JJ (2017) A network of cis and trans interactions is required for ParB spreading. Nucleic Acids Res 45:7106–7117
Surovtsev IV, Lim HC, Jacobs-Wagner C (2016a) The slow mobility of the ParA partitioning protein underlies its steady-state patterning in Caulobacter. Biophys J 110:2790–2799
Surovtsev IV, Campos M, Jacobs-Wagner C (2016b) DNA-relay mechanism is sufficient to explain ParA-dependent intracellular transport and patterning of single and multiple cargos. Proc Natl Acad Sci U S A 113:E7268–E7276
Taylor JA et al (2015) Specific and non-specific interactions of ParB with DNA: implications for chromosome segregation. Nucleic Acids Res 43:719–731
Taylor JA, Panis G, Viollier PH, Marczynski GT (2017) A novel nucleoid-associated protein coordinates chromosome replication and chromosome partition. Nucleic Acids Res 45:8916–8929
Thanbichler M, Shapiro L (2006) MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell 126:147–162
Toro E, Hong S-H, McAdams HH, Shapiro L (2008) Caulobacter requires a dedicated mechanism to initiate chromosome segregation. Proc Natl Acad Sci U S A 105:15435–15440
Tran NT, Laub MT, Le TBK (2017a) SMC progressively aligns chromosomal arms in Caulobacter crescentus but is antagonized by convergent transcription. Cell Rep 20:2057–2071
Tran NT et al (2017b) Permissive zones for the centromere-binding protein ParB on the Caulobacter crescentus chromosome. Nucleic Acids Res. https://doi.org/10.1093/nar/gkx1192
Umbarger MA et al (2011) The three-dimensional architecture of a bacterial genome and its alteration by genetic perturbation. Mol Cell 44:252–264
Vecchiarelli AG, Hwang LC, Mizuuchi K (2013) Cell-free study of F plasmid partition provides evidence for cargo transport by a diffusion-ratchet mechanism. Proc Natl Acad Sci U S A 110:E1390–E1397
Viollier PH et al (2004) Rapid and sequential movement of individual chromosomal loci to specific subcellular locations during bacterial DNA replication. Proc Natl Acad Sci U S A 101:9257–9262
Wang X, Tang OW, Riley EP, Rudner DZ (2014) The SMC condensin complex is required for origin segregation in Bacillus subtilis. Curr Biol 24:287–292
Wang X et al (2015) Condensin promotes the juxtaposition of DNA flanking its loading site in Bacillus subtilis. Genes Dev 29:1661–1675
Wang X, Brandão HB, Le TBK, Laub MT, Rudner DZ (2017) Bacillus subtilis SMC complexes juxtapose chromosome arms as they travel from origin to terminus. Science 355:524–527
Woldringh CL, Hansen FG, Vischer NOE, Atlung T (2015) Segregation of chromosome arms in growing and non-growing Escherichia coli cells. Front Microbiol 6:448
Yeh Y-C, Comolli LR, Downing KH, Shapiro L, McAdams HH (2010) The caulobacter Tol-pal complex is essential for outer membrane integrity and the positioning of a polar localization factor. J Bacteriol 192:4847–4858
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wang, H., Bowman, A.I., Bowman, G.R. (2022). Chromosome Segregation in Alphaproteobacteria . In: Biondi, E. (eds) Cell Cycle Regulation and Development in Alphaproteobacteria. Springer, Cham. https://doi.org/10.1007/978-3-030-90621-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-90621-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-90620-7
Online ISBN: 978-3-030-90621-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)