Abstract
Selective bladder preservation (SBP) has emerged as an excellent treatment option for patients with muscle-invasive bladder cancer (MIBC) deemed eligible for consideration based on certain selection criteria. This chapter describes the clinical entity of MIBC, discusses the selection criteria utilized for evaluating candidacy for SBP, and addresses a multitude of bladder-sparing approaches that have been utilized both historically and in current clinical practice. An emphasis is placed on discussion of trimodality therapy (TMT) incorporating maximal transurethral resection of bladder tumor (TURBT) and concurrent chemoradiotherapy +/− immunotherapy. Discussion of SBP includes presentation of general treatment paradigms, a review of existing literature describing oncologic outcomes, discussion of quality of life considerations, and description of treatment planning techniques for high-quality radiotherapy (RT) delivery. Ongoing randomized clinical trials (RCTs) of interest will be underscored throughout as they pertain to each aspect of treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Background
Bladder cancer is the tenth most common cancer worldwide, with an incidence steadily increasing over time, especially in developed nations [1]. Muscle-invasive bladder cancer (MIBC) is defined as bladder cancer that has invaded at least to the depth of the muscularis propria of the bladder wall, characterized as pathologic T2 by the most recent edition of American Joint Commission on Cancer (AJCC) TNM staging [2]. MIBC comprises about 30% of bladder malignancies and encompasses histologies including urothelial (formerly known as transitional cell) carcinoma – which is the most common histology in the United States, accounting for 90% of diagnoses – as well as squamous cell carcinoma (accounting for most of the remaining 10%), adenocarcinoma, and neuroendocrine carcinoma [1]. Development of urothelial carcinoma is strongly linked to tobacco usage and environmental exposure in the developed world [3], whereas squamous cell carcinoma is frequently diagnosed in regions of Africa and the Middle East and manifests in the setting of chronic irritation, such as that secondary to the protozoan infection schistosomiasis [4].
The most common presentation of MIBC is painless gross hematuria, which is often assessed by urine cytology, computed tomography (CT), or magnetic resonance (MR) urography for complete imaging of the genitourinary tract and, ultimately, cystoscopy [5]. At the time of cystoscopy, which allows for direct visualization of the bladder lumen, transurethral resection of bladder tumor (TURBT) is commonly performed for both pathologic confirmation and tumor debulking. If muscular invasion is noted in the pathologic specimen (i.e., confirming pT2 disease), patients are additionally recommended to receive imaging to assess for metastases [6]. Table 18.1 describes AJCC 8th edition TNM classification staging for bladder cancer. For patients with muscle-invasive disease, definitive management is oft considered a subject of controversy; while surgical resection has been historically regarded as the most standard approach, bladder-sparing treatments are being increasingly utilized as an alternative, effective approach for select patients. Each of these treatment options will be addressed, with emphasis on and comparisons with bladder preservation.
Historical Approaches to MIBC
Radical Cystectomy
MIBC has historically been treated with radical cystectomy (RC), a surgery involving resection of the bladder, adjacent fat, distal ureters, and peritoneum with a pelvic lymph node dissection [7]. For men, the prostate and seminal vesicles are additionally removed; for women, the anterior vaginal wall, uterus, fallopian tubes, and ovaries are additionally removed. The standard pelvic lymph node dissection involves removal of the obturator nodes, external and internal iliac nodes, and the most inferiorly situated common iliac nodes [7]. An ongoing phase III randomized clinical trial (RCT) comparing standard pelvic lymphadenectomy to an extended lymphadenectomy for patients with pT2-T4a disease (SWOG S1011) is aiming to compare the disease-free survival (DFS) rates between these two surgical approaches [8], although results from the recently published LEA AUO AB 25/02 trial out of Europe suggest no reduction in the rate of locoregional recurrence (LRR) with extended pelvic lymph node dissection [9].
Following resection of the bladder, a urinary diversion is performed; both non-continent and continent options for urinary diversion are available. An ileal conduit is a non-continent diversion comprised of small bowel: a channel is created with the ureters attached to it, and it exits through the skin overlying the abdomen by a stoma emptying into a receptacle for urine collection [10]. An ileal conduit is the most commonly utilized type of urinary diversion following RC [10]. Two types of continent diversions include an Indiana pouch, which is a portion of ileum that is constructed to act as a urinary reservoir that allows for the patient to intermittently self-catheterize, and an orthotopic “neobladder,” which is a urinary pouch created from small or large bowel (ileum, ileo colon, or sigmoid colon) and then anastomosed to the distal urethra [11]. The benefits/drawbacks of one diversion versus another and/or picking the optimal approach for a patient are beyond the scope of this chapter.
Large, retrospective, single-institution series out of the University of Southern California (USC) and Memorial Sloan Kettering Cancer Center (MSKCC) published in the early 2000s highlight outcomes after RC [12]. The USC experience reported on 633 patients with pT2-T4a disease managed with RC with a 5-year actuarial overall survival (OS) rate of 48% at a 5-year median follow-up and 32% at a 10-year median follow-up [7]. The MSKCC group studied 184 patients with pT2-T4 disease and found a 5-year OS rate of 36% and 27% at 10 years [13]. Later, the Southwest Oncology Group (SWOG), Eastern Cooperative Oncology Group (ECOG), and Cancer and Leukemia Group B (CALGB) published results of a trial investigating the implementation of neoadjuvant chemotherapy (NAC) with three cycles of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) in patients with cT2-T4a disease receiving RC (SWOG 8710) and found improved OS with addition of NAC [14], with a 5-year OS of 50% and a 10-year OS of 34% [12]. Data from the Advanced Bladder Cancer (ABC) meta-analysis collaboration analyzed over 3000 patients from 11 trials and reported a 5% absolute improvement in OS at 5 years with the addition of platinum-based combination chemotherapy [15]. Accompanying data additionally suggests that up to 30% of patients are unable to complete planned adjuvant systemic therapy due to perioperative morbidity [16], thereby solidifying the role for NAC as standard of care.
Postoperative Radiotherapy
For patients felt to be at high risk of LRR following RC, postoperative radiotherapy (PORT) following RC may additionally be considered [17, 18]. Patients receiving PORT may include patients with higher T stage (pT3-T4), positive surgical margins, and involved lymph nodes identified during surgical dissection [19]. To date, only a single study by the National Cancer Institute of Egypt has assessed administration of PORT following RC. Patients enrolled included 236 patients with pT3-T4 disease, and radiotherapy (RT) was administered either using a conventional fractionation scheme (daily RT to a total dose of 50 Gy over 5 weeks) or in a thrice daily fashion (at 1.25 Gy per fraction with 3 hours between fractions, to a total dose of 37.5 Gy given over the course of 12 days) [17]. While the study found improved local control (87–93% vs. 50%) and DFS (44–49% vs. 25%) for patients receiving PORT compared to RC only, 68% of the study population had squamous cell carcinoma secondary to bilharzia (schistosomiasis), and it remained unclear if findings of this study apply to non-squamous cell histologies as well.
As patients with extensive disease identified on pathology were often receiving adjuvant chemotherapy as well, a subsequent study was undertaken to assess PORT with chemotherapy. A phase II trial compared adjuvant chemotherapy to adjuvant chemotherapy with sandwiched RT in patients age 70 or younger with ≥pT3b disease, grade 3 disease, or positive nodes following RC with negative margins [18]. Patients received either four cycles of adjuvant gemcitabine and cisplatin chemotherapy (n = 45) or two cycles of gemcitabine and cisplatin chemotherapy before and after RT (n = 75), with RT consisting of 45 Gy in 1.5 Gy twice daily (BID) fractions using three-dimensional conformal radiotherapy (3DCRT) [18]. The investigators found a significant improvement in LRR-free survival with the addition of PORT at 2-year follow-up (96% vs. 69%, p < 0.01) with trends toward improvement in DFS (68% vs. 56%, p = 0.07) and OS (71% vs. 60%, p = 0.11) [18]. While the majority of patients enrolled on the trial had unfavorable disease characteristics, again, only 53% of patients had urothelial carcinoma, with a significant number of patients having squamous cell carcinoma due to the relatively higher incidence of schistosomiasis in Egypt.
Given the excellent outcomes demonstrated with the addition of PORT by Egyptian trials with uncertainty as to whether these results would be the same for patients with urothelial carcinoma, a randomized phase II trial was developed to assess pelvic recurrence-free survival with the addition of postoperative adjuvant intensity-modulated radiotherapy (IMRT) following RC for patients with pT3-T4 urothelial carcinoma (NRG GU-001) [20]. Unfortunately, the trial was closed 2 years after opening due to poor accrual. Consequently, there remains no existing prospective data regarding outcomes with PORT in the era of more novel treatment approaches with IMRT and image-guided radiotherapy (IGRT), both of which would improve efforts to minimize dose to pelvic organs at risk (OARs) such as the bowel and rectum. In a survey of 277 radiation oncologists in the United States of America regarding management of patients with node-negative MIBC, nearly half of surveyed radiation oncologists had used PORT for indications including gross residual disease, positive margins, pathological node involvement, pT3-T4 disease, lymphovascular invasion, and high-grade disease, with use of PORT significantly associated with using IMRT on multivariable regression [21]. Data from population-based analyses has suggested improvement in OS with PORT for patients with pT4, pathological node positivity, and positive surgical margins [22].
Surgical Morbidity
As with all oncologic treatment, the survival outcomes from RC must be viewed within the greater context of treatment-related morbidity and mortality, among other factors impacting operative candidates. While the mortality rate associated with RC is estimated to be 1–3% [23,24,25], the postoperative complication rate may be as high as 60% or greater [26, 27]. A series of over a thousand patients from a prospective complications database analyzed at a large, tertiary academic center found that 64% of patients experienced ≥1 complication, and of those, 83% of complications were graded 2–5, with 26% of patients requiring re-admission [26]. Even with efforts to decrease surgical morbidity by transitioning to laparoscopic and robot-assisted techniques (rather than an open cystectomy approach), data from a large systematic review demonstrated a 59% 90-day complication rate with 15% of complications being classified as high grade [27]. In addition to expected operative complications such as a urinary tract infection or wound infection leading to urosepsis, wound dehiscence, hemorrhage, rectal injury, and postoperative ileus leading to small bowel obstruction, the mean in-hospital stay of 9 days for all diversion types [27] carries the additional risk of venous thromboembolism – with potential to extend to pulmonary embolism – as well as hospital-acquired infection. For patients subsequently receiving PORT, treatment-related morbidity is even greater; a single-institution experience of 78 patients treated with a single dose of pre-operative RT and PORT reported a 37% bowel obstruction rate for patients receiving PORT as compared to 8% of patients who did not receive PORT [28].
An additional consideration of great importance for patients receiving surgical management – unrelated to patient characteristics such as age, performance status, and comorbidities – is the impact of treatment facility type and case volume on oncologic outcomes and morbidity. For patients receiving RC, high hospital volume and surgical expertise have been associated with improved overall survival, with the combined effect of both being shown to decrease the risk of long-term mortality by 20% [29]. By contrast, population-based analyses on patients with MIBC receiving bladder preservation have suggested no such benefit, indicating that all types of centers may more readily offer this approach [30]. While reasonable outcomes have been demonstrated with RC, with RT classically reserved as an option for only those refusing RC or deemed inoperable, a great interest has emerged in bladder preservation options that may allow for patients to maintain a functional bladder, thereby improving quality of life.
Introduction to Bladder Preservation
Selective bladder preservation (SBP) first emerged in the 1980s with the performance of single-institution retrospective cohort studies demonstrating reasonable oncologic outcomes for patients who had been administered neoadjuvant RT with or without chemotherapy followed by cystoscopic response assessment [31]. Patients who demonstrated a complete pathologic response to neoadjuvant treatment were then designated as eligible for a bladder preservation approach and received further/completion radiotherapy, whereas those with an incomplete response on interim evaluation proceeded to RC [31]. With the rise of this new treatment paradigm came the ultimate question for appropriate patient selection: Which patients would be the best candidates for consideration of this type of treatment, as opposed to proceeding with RC upfront?
Selection Criteria
Strict selection criteria have been proposed in determining which medically operable patients are best suited for bladder preservation and include the following [32]:
-
cT2-T3a disease
-
Patients with unifocal disease (no definitive cutoff for size, but often ≤5–6 cm)
-
Patients without extensive carcinoma in situ
-
Patients who have received maximal, visibly complete TURBT
-
Patients without tumor-associated hydronephrosis
Additional considerations when assessing candidacy for SBP include favorable baseline bladder function, with the idea that the bladder should only be preserved if pre-treatment capacity and voiding ability are intact, as well as adequate renal function to allow for administration of concurrent radiosensitizing platinum-based chemotherapy (cisplatin alone or as part of a combination). The rationale underlying these selection criteria will be addressed in detail in the discussion of trimodality therapy.
Selective Bladder Preservation vs. Radical Cystectomy
A number of challenges have arisen in efforts to establish SBP as an alternative treatment paradigm for patients deemed eligible for consideration based on the aforementioned selection criteria. In the absence of prospective, randomized data directly comparing SBP to RC in the management of MIBC, a multitude of factors come into play in making the final determination as to which treatment the patient will receive. Depending on the practice environment in which the patient is being evaluated, patients may be subjected to referral bias, as patients would require referral to a clinician familiar with the modality to have a discussion about SBP [33]. An additional determination that is often made at the time of surgical consultation is the patient’s operability, which carries inherent selection bias that confounds any comparisons between SBP and RC, as patients who are medically frail or less likely to perform well postoperatively are more likely to be offered SBP over RC than those with minimal comorbidity and excellent performance status [34].
Yet another factor that must be taken into consideration is the difference between clinical and pathologic staging; clinical staging does not necessarily possess high accuracy for the detection of advanced disease, as historical data suggest up to 76% of patients with MIBC may have a discrepancy between clinical T stage at TURBT and final pathologic T stage at RC [35, 36]. While these data do not reflect recent advances in modern imaging techniques, with a trend toward increasing utilization of multi-parametric MRI [37, 38], they highlight that pitfalls exist in staging information available for patients receiving SBP.
While there are currently no prospective, randomized data directly comparing outcomes from SBP to RC, data from high-quality retrospective series have suggested similar survival outcomes to RC for patients receiving bladder-sparing trimodality therapy (TMT). A study of 112 patients with MIBC evaluated at a multidisciplinary clinic (in which both RC and SBP were presented as treatment options) at Princess Margaret Cancer Center utilized propensity score matching for retrospective survival analyses and reported a 5-year disease-specific survival (DSS) rate of 73.2% for patients receiving RC vs. 76.6% for patients receiving TMT, with a salvage cystectomy rate of 10.7% for patients failing TMT [39]. Accompanying these data are those from population-based analyses; a National Cancer Database (NCDB) analysis using propensity score matching for patients with cT2-3N0M0 urothelial carcinoma treated definitively with either RC or TMT found no significant difference in OS (4 year OS of 42.6% for RC vs. 39.1% for TMT, p = 0.15) with report of a time-varying hazard ratio [40]. Meta-analysis data reviewing 19 studies on 12,380 patients has additionally found no significant difference in OS, DSS, or progression-free survival when comparing SBP to RC [41].
The United Kingdom (UK) Medical Research Council (MRC) developed a multi-center feasibility pilot study addressing Selective bladder Preservation Against Radical Excision (SPARE), which attempted to randomize patients with cT2-3N0M0 urothelial carcinoma status post three cycles of NAC to RC or SBP [42]. Patients were randomized to the study intervention prior to a cystoscopy following NAC with plan for a fourth cycle of NAC followed by radiotherapy or RC for patients with ≤T1 residual tumor (whereas all non-responders would immediately proceed with RC following the third cycle of NAC) [42]. Unfortunately, the trial was closed due to poor accrual, leaving the need for a phase III trial assessing for non-inferiority between the two approaches.
Bladder Preservation Treatment Paradigms
While conventional treatment for SBP generally involves a multimodality approach incorporating maximal surgical resection and RT administered concurrently with radiosensitizing systemic therapy, bladder-sparing unimodality treatment approaches may be employed for certain patients. These will be addressed briefly in turn prior to discussion regarding multimodality treatment options.
Surgical Monotherapy
Transurethral Resection of Bladder Tumor
Following maximal TURBT, the clinical complete response (CR) rate for patients with cT2-T3 disease (solitary lesions, no CIS) based on repeat cystoscopic assessment (performed 3 weeks following initial TURBT) has been found to range from 10% to 20% based on small series performed in the 1980s [43, 44]. A retrospective cohort study out of MSKCC comparing 99 patients receiving TURBT as definitive therapy (of which 57% of patients had a preserved bladder) to 52 patients receiving RC found a non-significant 10-year DSS (76% for TURBT vs. 71% for RC, p = 0.30) [45]. Of note, most patients were found to have cT0 disease on repeat cystoscopy, and these patients had significantly better survival than the patients with residual T1 disease on restaging TURBT (p = 0.003) [45]. Among patients with residual tumors, 69%demonstrated relapsed disease within the bladder, of which only 53% of patients were successfully salvaged with RC [45]. These results indicate that, while some patients with no residual disease on restaging TURBT demonstrate favorable outcomes with maximal TURBT alone as definitive treatment, many patients will have relapsed disease within the bladder (of which not all cases can be salvaged), thereby making TURBT alone a suboptimal choice for definitive treatment. Small series performed from 1950 to 1970 comparing TURBT alone to RC have demonstrated consistently inferior survival rates for TURBT as monotherapy, with an estimated 5-year OS of approximately 30% [46, 47].
Partial Cystectomy
For certain patients, partial cystectomy may be a viable treatment option for those pursuing surgical management while seeking bladder preservation. Patients under consideration for this approach must be very carefully selected: the ideal would have a solitary lesion of small size, without evidence of CIS, situated in a portion of the bladder amenable to complete excision with a widely negative margin (of at least 1 cm but preferably 2 cm) [48]. Prior to partial cystectomy, the bladder would need to be adequately sampled by random biopsy (including the prostatic urethra) with no evidence of tumor involvement elsewhere in the bladder [49]. Importantly, the remaining portion of the bladder following partial cystectomy would still need to have adequate capacity to allow maintenance of normal voiding [48, 49]. For this reason, patients would not be considered optimal candidates for partial cystectomy if they had tumors involving the bladder neck, ureteral orifices, or trigone (areas in which ureteral re-implantation would be required to achieve an adequate margin) [50]. Patients would therefore also be viewed as suboptimal candidates for this approach if they had history of a recurrent bladder tumor. A handful of single-institution retrospective series, each with a relatively small cohort, has suggested a 5-year OS rate of approximately 70% with a bladder preservation rate of 65% for well-selected patients receiving partial cystectomy [51,52,53]. Alas, given the relatively strict selection criteria, less than 10% of patients with MIBC receive partial cystectomy [54], and even for those patients, ~25% may still require salvage RC following recurrence [55].
Radiotherapy Monotherapy
External Beam Radiotherapy
External beam radiotherapy (EBRT) alone has been utilized for patients with cT2-T4 disease; while frequently reserved for patients with significant comorbidities precluding surgery or administration of systemic therapy in the United States, this treatment option was explored in the definitive setting in Europe from 1970 to 1990 with multiple published experiences. The earliest and largest of these was a study out of Edinburgh, Scotland, reporting on 963 patients with muscle-invasive urothelial carcinoma receiving RT alone, which found a 5-year OS across all T stages of 30.3%, with worse survival associated with age 80 or greater, cT4 disease, ulcerated lesions, grade 3 disease, and size ≥7 cm [56]. These patients were treated with 4, 6, or 9 MV photon irradiation using a small field measuring 10 × 10 cm including the whole bladder in the target volume to a dose of 55 Gy in 20 fractions, and severe RT-related complications were seen in about 15% of patients [56, 57]. Another large, retrospective study out of Glasgow, Scotland, reported on 709 patients receiving radical RT, including administered doses up to 60–64 Gy in 30 fractions; treatment was designed using a four-field technique for the first 4 weeks of treatment followed by a bladder boost for the last 2 weeks [58]. Patients in this study additionally received pelvic nodal irradiation, with doses of 40.42.5 Gy [58]. The crude 5-year OS rate was reported to be 24.7%, with 5-year OS of 86.9% for T1 tumors, 49.1% for T2 tumors, 27.7% for T3 tumors, and 1.8% for T4 tumors; of interest, patients with urothelial carcinoma demonstrated improved survival compared to those with squamous cell carcinoma, and pelvic nodal irradiation did not confer an OS benefit [58]. Similar studies were undertaken in the United States; a series by Pollack et al. analyzed 135 patients treated with an average dose of 6588 ± 475 cGy with an average fractional dose of 207 ± 18 cGy and found a 5-year OS rate of 26%, consistent with the survival outcomes from other studies [59]. Across studies, 5-year local control was estimated to be 30–50%, with prognostic factors including T stage, tumor size, tumor histology, extent of resection by TURBT, and presence of hydronephrosis/CIS [56,57,58,59].
Subsequently, RT monotherapy in the modern treatment setting has been compared to the RT plus concurrent chemotherapy in both retrospective cohort studies and prospective, randomized trials like BC2001 [60, 61]. While these data will be addressed in greater detail with discussion of TMT, given their results favoring concurrent chemotherapy administration for improved survival outcomes, RT alone has fallen out of favor for treatment in the definitive setting for patients able to receive systemic therapy. For patients who are not able to receive systemic therapy due to comorbidities, contraindications, or patient preference, reasonable outcomes may be achieved with radical RT with the understanding that (1) recurrent disease will require treatment with salvage cystectomy and (2) not all pelvic recurrences may necessarily be able to undergo successful salvage treatment.
Brachytherapy
Use of brachytherapy for radical RT was initially reported on in the 1940s and was largely utilized in Europe at its peak popularity [62,63,64,65]. The earliest reports of utilization of brachytherapy included use of permanent radon seeds, with later series reporting on use of interstitial iridum-192 [62, 63, 65]. This practice largely fell out of favor due to treatment-related toxicity, including urinary leakage in the acute/sub-acute setting and late side effects of stenosis, stricture, or fistula formation. Though EBRT was therefore often the preferred radiotherapeutic technique for patients receiving unimodality treatment with RT, brachytherapy was later studied in the 1990s as a boost treatment in combination with EBRT +/− TURBT or partial cystectomy [66]. Small retrospective series have indicated excellent outcomes for well-selected patients, with estimated 5-year local control of 70%, 5-year OS ranging from 60% to 70%, and a 5-year bladder preservation rate of 90–95% [67, 68]. When administered with low-dose preoperative EBRT of 10–11 Gy (administered in 2–3 fractions, such as 3 fractions of 3.5 Gy) for prevention of iatrogenic scar formation, brachytherapy doses range from 30 to 50 Gy [66,67,68].
Combined Modality Treatment
Partial Cystectomy/TURBT and Chemotherapy
To improve outcomes with TURBT and partial cystectomy, clinicians additionally have considered the use of adjuvant chemotherapy, as has been done for patients receiving RC. The addition of chemotherapy was studied in both the neoadjuvant and adjuvant settings. A series out of Italy of 104 patients with cT2-4N0M0 urothelial carcinoma who had received three cycles of neoadjuvant methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) found that 60% of patients who received TURBT following NAC were alive at median follow-up of 4.5 years, with 44% of patients maintaining a functional bladder [69]. A similarly sized sample population was reported on by MSKCC, with 111 patients receiving 4 cycles of neoadjuvant MVAC, of which 26 (23.4%) were selected for partial cystectomy based on favorable response on repeat cystoscopy; though 12 patients (46%) developed bladder recurrences, patients with no residual tumor (pT0) or non-invasive residual disease (pTis) were found to have a 5-year OS of 87% [70]. Patients included in these studies were those with unifocal, solitary tumors measuring ≤5 cm with a significant response noted following NAC [70, 71]. These results encourage the use of NAC when possible if pursuing TURBT or partial cystectomy for bladder preservation, although a significant number of patients will require salvage treatment for recurrences, which account for about half of the patient population receiving this treatment.
For patients receiving chemotherapy administered in the adjuvant setting, this paradigm has also demonstrated improvements in local control compared to TURBT alone. A small retrospective analysis of 50 patients with cT2-4 disease (of which 36 patients had T3 disease) treated with TURBT followed by 2–6 cycles of cisplatin/methotrexate found a 76% post-treatment CR rate with 5-year local control of 60% [72]. Further, a phase II nonrandomized trial comparing patients receiving RC (n = 71) to those receiving TURBT with three cycles of adjuvant platinum-based chemotherapy (n = 75) found no significant difference in 5-year and 10-year cancer-specific survival (p = 0.54), which was reported as 64.5% and 59.8%, respectively, for patients receiving bladder preservation [73]. For the patients receiving TURBT and adjuvant chemotherapy with clinical response, 40 patients (53%) achieved an initial CR, although 56% ultimately developed recurrence or progression and 45% received salvage RC [73].
Trimodality Therapy
Treatment Overview
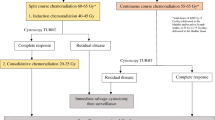
TMT for SBP consists of maximal safe TURBT with examination under anesthesia followed by concurrent chemoradiotherapy. TURBT allows for tumor debulking, which is especially useful for management of a relatively radioresistant tumor histology, allowing further local therapy to be delivered with adjuvant RT to ideally address residual macroscopic or microscopic disease. Chemotherapy is administered concurrently with RT to both (1) enhance radiosensitivity for the purpose of increasing fractional cell kill (local function) and (2) address any sites housing micrometastatic disease (systemic function). The most commonly utilized chemotherapy regimens for TMT include [61, 74, 75]:
-
Cisplatin 35 mg/m2, weekly up to 6 weeks
-
Cisplatin-based regimens with 5-FU (fluorouracil 400 mg/m2 on days 1–3 and 8–10 with cisplatin 15 mg/m2 on days 1,2, 8, and 9) or paclitaxel (paclitaxel 50 mg/m2 on days 1 and 8 and cisplatin 15 mg/m2 on days 1, 2, 8, and 9)
-
5-FU/mitomycin-C (500 mg/m2 5-FU days 1–5 and 16–20 with mitomycin-C 12 mg/m2 on day 1)
-
Gemcitabine 27 mg/m2, twice weekly up to 6 weeks
A considerable amount of variation exists in radiotherapeutic management with regard to both target volume and dose: some clinicians treat a partial bladder vs. the full bladder; some pursue nodal irradiation, while others target the bladder only; some clinicians attempt to plan a focal boost to the bladder tumor; and some clinicians administer treatment using a hypofractionated regimen (55 Gy in 20 fractions), while others treat using a conventional fractionation scheme up to 64–64.8 Gy in 1.8–2 Gy daily fractions. These treatment-related considerations will be addressed in detail with discussion of radiation techniques.
Visibly completed TURBT has been strongly advocated for in patient selection based on evidence suggesting that maximal TURBT is associated with higher rates of CR, lower rate of salvage RC, and improved OS [76, 77]. A retrospective analysis of 415 patients treated at the University of Erlangen found that early tumor stage and complete TURBT were the most important factors in predicting for CR and survival [76]. However, while maximal TURBT has historically been regarded as a strict selection criterion, there is a growing body of evidence to suggest that similar outcomes may still be achieved with incomplete TURBT [38, 78]. In a retrospective series out of MGH, while the salvage RC rate was higher for patients with incomplete TURBT vs. visibly complete TURBT (42% vs. 22%, p < 0.001), a CR was still achieved in 57% of patients with incomplete TURBT [78]. This idea is additionally represented by a portion of the patients enrolled in the BC2001 trial, of whom greater than a third received biopsy only or an incomplete TURBT [61]. More recently, research efforts have even been directed toward the possibility of forgoing TURBT due to concern for iatrogenic tumor spread, such as with initiation of the BladderPath study out of the United Kingdom: BladderPath is a phase II/III trial randomizing patients with possible MIBC to TURBT or multiparametric MRI for clinical staging [38].
Another essential factor for appropriate patient selection is the patient’s T stage, as higher T stage is associated with less likelihood of CR following TMT. Results from the MGH experience have demonstrated that the clinical CR rate for cT2 tumors was about 80% as compared to 64% for cT3-T4 disease, suggesting that patients with cT2-T3a disease have the greatest likelihood of optimal outcomes with SBP [78]. However, patients with cT4a disease were included in the vast majority of large, retrospective series exploring SBP as well as BC2001. With regard to in situ disease, early data published in the early 1990s found that extensive CIS was associated with much higher risk of LRR (40% vs. 6%, p = 0.075) and that absence of CIS was a significant predictor for clinical CR (p = 0.03); these data guided the general recommendation encouraging absence of CIS for TMT [79]. Similarly, data from the pioneering experiences at MGH have suggested that tumor-related hydronephrosis is associated with worse OS and DSS, and these results were corroborated by findings of a lesser likelihood to achieve CR for patients with tumor-related hydronephrosis in RTOG 8903 [78, 80].
Given the relatively higher incidence of urothelial carcinoma compared to other forms of MIBC, such as squamous cell carcinoma or adenocarcinoma, it is unclear if patients with non-urothelial carcinoma histologies have differences in outcomes following SBP. While prospective studies investigating patients treated with SBP such as BC2001 have limited inclusion criteria to those with urothelial carcinoma, retrospective data comparing outcomes between patients with non-urothelial carcinoma (22%, n = 66) and urothelial carcinoma (78%, n = 237) found no significant difference in CR rate (82–83%, p = 0.9), 10–year DSS (64–67%, p = 0.39), or 10-year OS (42%, p = 0.21) [81].
Outcomes and Literature Review
SBP was first developed following pioneering single-institution experiences by the University of Erlangen, University of Paris, and Massachusetts General Hospital (MGH) [82,83,84]. The University of Paris experience represents one of the earliest efforts at bladder preservation, as clinicians there initially studied concurrent chemoradiotherapy as a preoperative regimen prior to RC. After finding that 18 consecutive patients demonstrated 100% pathologic CR upon analysis of the final cystectomy specimen, it was determined that concurrent chemoradiotherapy may be utilized toward SBP [49]. The French experience reported on a cohort of 54 patients with operable cT2-T4 MIBC, all of whom were managed with concurrent chemoradiotherapy with cisplatin/5-FU and BID RT administered in a split course fashion as both induction (24 Gy) and consolidation (with an additional 20 Gy) following TURBT [82]. Re-staging cystoscopy and TURBT were performed 4–6 weeks following completion of induction chemoradiation, and consolidation treatment was administered only to those with CR; any patients with residual disease after induction received RC. At post-induction cystoscopy, 40 patients (74%) were found to have a CR; at mean follow-up of 27 +/− 12 months, three patients receiving SBP with initial CR developed recurrent disease in the pelvis, and the overall 3-year DFS rate was reported to be 62% with no significant survival difference noted between patients receiving SBP and those ultimately receiving RC [82].
The University of Erlangen initiated prospective study of SBP in the early 1980s, initially by assessing patients receiving TURBT followed by EBRT alone to 50–56 Gy in 2 Gy daily fractions; following treatment of over 100 consecutive patients in this manner, radiosensitizing platinum monotherapy (cisplatin or carboplatin) was added for treating patients thereafter [76]. A German group reported on outcomes for 415 patients, of which 79% had cT2-T4 disease, 30.3% were treated with RT alone following TURBT, and 69.6% received concurrent chemoradiotherapy following TURBT; they found a CR rate of 72% and reported that the local control following CR without muscle-invasive recurrence was 64% at 10 years [76]. The 10-year DSS was 42% for their cohort, and the bladder preservation rate was 80% [76]. Administration of radiosensitizing chemotherapy concurrently with RT was found to improve both CR rate and survival, and patients who required salvage RC due to disease persistence or recurrence still maintained a 10-year DSS of 45%, comparable to the 10-year DSS for the cohort at large [76]. Of note, patients in this German study received the entire course of concurrent chemoradiotherapy without mid-evaluation cystoscopy to evaluate response; re-staging was performed 6–8 weeks following completion of all definitive treatment. Further, the patients routed to salvage RC were only those with poorly differentiated, superficial tumors, or persistent/residual invasive disease, and patients with well-differentiated, superficial disease remaining (e.g., CIS) were allowed to continue with SBP while receiving endoscopic treatment with TURBT/intravesical therapy [76].
At a similar time in the mid-1990s, pioneers at MGH reported on an initial experience with 53 patients with cT2-4N0M0 MIBC treated consecutively with TURBT and adjuvant concurrent chemoradiotherapy to a dose of 40 Gy (using a daily fractionation scheme) with concurrent cisplatin followed by cystoscopic evaluation and further treatment to 64.8 Gy for patients with a CR or those deemed unsuitable for RC [85]. The study found an initial CR rate of 53% with 89% of patients having a functioning bladder, and the 4-year DFS was found to be 45% for the entire cohort [85]. MGH has subsequently reported on patients treated over 20 years with long-term follow-up; in their cohort of 348 patients with cT2-T4aN0M0 MIBC, all of whom were treated with maximal TURBT and concurrent chemoradiotherapy to 64–65 Gy with cisplatin (with patients receiving response assessment following 40 Gy and some patients receiving additional chemotherapy administered adjuvantly or neoadjuvantly), their findings were as follows at nearly 8-year median follow-up [78]:
-
Initial CR rate: 72%
-
Cystectomy rate: 29% (native bladder preservation: 71%)
-
12% – invasive tumor recurrence noted on post-treatment surveillance
-
17% – incomplete response noted following concurrent chemoradiotherapy
-
-
5-year OS, 52%; 10-year OS, 35%
-
5-year DSS, 64%; 10-year DSS, 59%
-
10-year rates of recurrence (for patients with initial CR):
-
Non-invasive: 29%
-
Invasive: 16%
-
Pelvic: 11%
-
Distant: 32%
-
The two most important factors predicting for OS and DSS were clinical T stage and initial CR following induction therapy [78]. NAC was not found to be associated with OS on multivariable regression analysis, and no patients required RC due to toxicity secondary to treatment from SBP [78].
Following promising results from the aforementioned single institution experiences, data obtained from cooperative group experiences undertaken by the Radiation Therapy Oncology Group (RTOG) confirmed such outcomes. These trials are briefly summarized in Table 18.2 [49]. The first published RTOG trial was RTOG 8512, which was a phase II study analyzing 42 patients with cT2-T4N0-2M0 disease receiving 40 Gy to the pelvis with 2 cycles of concurrent cisplatin followed by an additional 24 Gy with another cycle of cisplatin in the event of CR (whereas patients with residual tumor following 40 Gy and 2 cycles of cisplatin received RC) [86]. The study found an initial CR of 66%, and 42% of patients were alive with an intact bladder at 5 years [86]. This study was followed by RTOG 8802, which sought to investigate outcomes with the addition of MCV chemotherapy following TURBT but prior to concurrent chemoradiotherapy [87]. Of 91 patients studied, the 4-year risk of LRR was found to be 43%, which was similar to the reported 4-year rate of surviving with an intact bladder of 44% [87]. This was then followed by RTOG 8903, which was a phase III trial aiming to compare concurrent cisplatin with RT (standard arm) to the standard arm plus the addition of neoadjuvant MCV chemotherapy; however, this study was closed early due to high rate of severe leukopenia witnessed in patients receiving MCV [80]. Based on the 123 patients analyzed, neoadjuvant MCV was not found to be associated with CR rate, OS, or freedom from distant metastases; based on these findings, later RTOG did not incorporate NAC [80].
Subsequent RTOG trials explored utilization of BID RT, as was done by clinicians at the University of Paris (and, separately, by investigators in Egypt using PORT) with concurrent chemotherapy administration. RTOG 9506 reported on 34 patients with cT2-T4N0M0 MIBC without hydronephrosis receiving TURBT followed by induction chemoradiotherapy to 24 Gy administered BID at 3 Gy per fractions with concurrent cisplatin/5-FU; following cystoscopy and re-biopsy 4 weeks later, patients with CR received consolidation chemoradiotherapy with BID RT to the bladder to 20 Gy (for a cumulative dose of 44 Gy) [88]. While the study reported a 3-year OS of 83% with 66% of patients maintaining an intact bladder, the grades 3–4 hematologic toxicity rate of 21% declared this regimen as relatively toxic in spite of encouraging oncologic outcomes [88]. The RTOG turned to investigation of adjuvant chemotherapy with its next trial, RTOG 9706, which analyzed 52 patients with cT2-T4aN0M0 MIBC patients who received induction chemoradiotherapy (administered BID with 1.8 Gy to the pelvis in the morning followed by a 1.6 Gy boost to the tumor 4–6 hours later) with concurrent cisplatin, cystoscopic evaluation 3–4 weeks following induction, consolidation chemoradiation in the event of CR (given in 1.5 Gy BID fractions to a total dose of 45.6 Gy to the pelvis/bladder and 64.8 Gy to the tumor), and, finally, three cycles of adjuvant MCV [89]. The authors found that 74% of patients achieved CR, with only 11% of patients experiencing grades 3–4 hematologic toxicity (unlike the nearly double rate noted in RTOG 9506); however, only 45% of patients were able to receive the full three cycles of adjuvant MCV, and of the patients who received the full three cycles, 41% developed grades 3–4 hematologic toxicity [89]. Consequently, this treatment regimen was also felt to be very toxic, although the logic underlying condensing the induction phase into a shorter time frame with BID RT was sound. The subsequently performed RTOG 9906 trial also assessed BID RT but added paclitaxel to induction cisplatin and utilized an adjuvant chemotherapy regimen consisting of gemcitabine/cisplatin [90].
The RTOG trials performed most recently have continued to evaluate different variations of systemic therapy administration. RTOG 0233 was a phase II study reporting on a group of 93 patients randomized to receive cisplatin/paclitaxel or cisplatin/5-FU administered concurrently with induction RT to 40.3 Gy following TURBT, with patients then receiving consolidation chemoradiation to 64.3 Gy with the same chemotherapy given during induction in the event of downstaging to T0, Tcis, or Ta disease [74]. Patients then went on to receive adjuvant chemotherapy with gemcitabine (1000 mg/m2), paclitaxel (50 mg/m2), and cisplatin (35 mg/m2) all administered on days 1 and 8. Results showed comparable rates of 5-year OS between the two arms (paclitaxel, 71%; 5-FU, 75%) with 5-year bladder-intact survival rates of 67–71% [74]. However, the study reported marked rates of toxicity, with 16 patients (35%) treated with paclitaxel and 19 (44%) treated with 5-FU developing late grades 3–4 toxicity (of which 11% and 6%, respectively, were attributed to RT) [74].
Following completion of RTOG 0233, a pooled analysis of RTOG 8802, 9506, 9706, 9906, and 0233 was published in 2014 and reported on 468 patients across the 5 studies, with clinical T stage of T2 in 61%, T3 in 35%, and T4a in 4% of patients. With median follow-up of 4.3 years among all patients and 7.8 years among survivors, the study found [91]:
-
CR rate: 69%
-
5-year OS, 57%; 10-year OS, 36%
-
5-year DSS, 71%; 10-year DSS, 65%
-
10-year estimate of muscle-invasive LRR: 14%
-
10-year estimate of non-muscle invasive LRR: 36%
-
10-year estimate of distant metastasis: 35%
Most recently, RTOG 0712 reported on SBP using either cisplatin/5-FU with BID RT or gemcitabine with once daily RT following TURBT as part of induction to 40 Gy as well as consolidation to 64 Gy for those patients achieving CR on interim cystoscopic assessment; this was then followed by adjuvant cisplatin/gemcitabine [75]. Twice weekly gemcitabine emerged as an attractive systemic therapy option following completion of a phase I trial by the University of Michigan establishing good response, survival, and bladder preservation rates of this regimen, with a maximum tolerated dose of 27 mg/m2 [92]. While not statistically powered to make a comparison between cisplatin/5-FU with BID RT and gemcitabine with once daily RT, RTOG 0712 demonstrated rates of freedom from distant metastasis exceeding 75% in both arms (cisplatin/5-FU with BID RT, 78%; gemcitabine with daily RT, 84%) with post-induction CR rates of 88% for cisplatin/5-FU with BID RT and 78% for gemcitabine with daily RT. [75] These results have encouraged further utilization of gemcitabine with daily RT as an alternative to the prior platinum-based RTOG regimens with BID RT, especially for patients with renal function precluding use of agents like cisplatin.
The largest prospective, randomized study performed in patients with MIBC is BC2001; this trial was performed in the United Kingdom and reported on 360 patients with MIBC randomized to receive either RT alone (n = 178) or RT with concurrent 5-FU/mitomycin-C (n = 182) [61]. In addition, patients were randomized to receive whole bladder radiotherapy or treatment of a partial bladder volume using a partial 2-by-2 factorial design, with permission of 2 RT schedules: (1) a conventionally fractionated schedule to 64 Gy in 32 fractions over the course of 6.5 weeks or (2) a hypofractionated approach of 55 Gy in 20 fractions over the course of 4 weeks. BC2001 found a significant improvement in 2-year locoregional DFS with the addition of concurrent chemotherapy (67% vs. 54%, p = 0.03) with trends toward improved 5-year OS (48% vs. 35%, p = 0.16; though the study was underpowered to show a difference in survival), reduced 2-year cystectomy rate (11.4% vs. 16.8%, p = 0.07), and higher grades 3–4 acute treatment-related toxicity (36% vs. 28%, p = 0.07) [61]. An exploratory analysis demonstrated a 2-year relapse rate of 18% for patients receiving concurrent chemoradiotherapy vs. 32% for patients receiving RT alone (p = 0.01) [61]. Subgroup analysis indicated no significant differences based on patients receiving whole-bladder RT (n = 63) vs. “modified volume” RT (n = 58) vs. elective whole-bladder RT (n = 239) (p = 0.66) or 64 Gy in 32 fractions (n = 217) vs. 55 Gy in 20 fractions (n = 142) (p = 0.59) [61, 93]. Two important distinctions in this study come to light when comparing patients treated on BC2001 to those treated on RTOG protocols: (1) patients were not required to receive maximal TURBT for enrollment (over a third of patients had biopsy only or incomplete TURBT); (2) no interim cystoscopic assessment or re-biopsy was performed following an induction treatment phase; treatment proceeded continuously, and first post-treatment cystoscopy was performed 6 months following completion of definitive therapy [61]. This trial validated numerous facets of modern-day treatment: use of the 5-FU/mitomycin-C regimen for radiosensitization, use of hypofractionated RT, and continuous treatment without a mid-treatment break.
Evolving Considerations
There has been great evolution of numerous considerations over time when considering the treatment paradigm for MIBC; these are depicted in Fig. 18.1 and include utilization of NAC, alterations in fractionation for RT delivery, hypoxia modification, use of molecular stratification in treatment selection, use of immunotherapy, and response evaluation. Each of these will be briefly addressed in turn.
Neoadjuvant Chemotherapy
From the initial conception of SBP using TMT in the early 1980s, NAC emerged as a treatment of interest due to the potential for tumor downstaging at the time of TURBT and the opportunity for early assessment of response to systemic therapy (with the caveat that administration of NAC would postpone initiation of definitive local treatment). While many of the largest phase III studies aimed at assessing the role of NAC prior to definitive local therapy have been performed on patients receiving RC (e.g., the Nordic 1 Cooperative Bladder Cancer Study Group, Spanish CUETO, Italian GUONE, and SWOG 8710/Intergroup 0080 trials) [14, 94,95,96], there are some prospective, randomized data assessing the role of NAC for patients receiving radical EBRT. A pooled analysis by the West Midlands Urological Research Group and the Australian Bladder Cancer Study Group compiled data from two pilot studies comparing radical RT to radical RT with induction cisplatin; with a total of 255 patients analyzed, no significant difference in OS was noted [97]. The MRC-EORTC published results of a large trial studying 485 patients undergoing either RC or EBRT monotherapy and randomly assigned to receive neoadjuvant MVAC (n = 491) or no NAC (n = 485); while results demonstrated that NAC was associated with higher rates of pathological CR, the 10% absolute improvement in OS at 3 years required to establish NAC as standard of care was not found [98].
With regard to NAC prior to administration of concurrent chemoradiotherapy, RTOG 8903 assessed the addition of neoadjuvant MCV to the standard of concurrent cisplatin with RT and found no impact on CR rate, freedom from distant metastasis, or OS but noted significant hematologic toxicity that led to only a 67% protocol completion rate for patients receiving MCV [80]. The outcomes for patients treated on BC2001 who received NAC were recently published: 117 patients of the initial cohort of 360 (33%) received platinum-based NAC prior to receiving RT +/− concurrent 5-FU/mitomycin-C, and no differences in local control or OS were noted between the two arms among this subgroup of patients receiving NAC [99]. However, patients receiving NAC and concurrent chemoradiotherapy were noted to have a 33% rate of grades 3–4 toxicity as compared to 22% for patients receiving NAC followed by RT alone [99]. Based on findings suggesting no significant benefit in oncologic outcomes and elevated rates of grades 3–4 toxicity, NAC has not continued to evolve as a paradigm-shifting treatment consideration.
Variations in Fractionation
Another unique aspect of TMT for MIBC has been the implementation of multiple fractionation schemes in delivering RT. Accelerated RT with BID fractionation was studied in the early TMT experience out of the University of Paris and subsequently implemented in multiple RTOG trials, including RTOG 9506, RTOG 9706, RTOG 9906, RTOG 0233, and RTOG 0712 [75, 82, 91]. Using accelerated fractionation for these studies – all of which entailed mid-treatment response assessment with cystoscopy to evaluate for CR prior to consolidation chemoradiation or cystectomy – carried the advantage of decreasing time to definitive local treatment. Unlike hyperfractionation, which utilizes a lower dose per fraction in combination with an increased number of daily fractions to ultimately yield a higher cumulative dose, accelerated fractionation yields a similar total dose to conventional fractionation (e.g., 64 Gy) with a shorter treatment package time.
There is limited existing data comparing accelerated fractionation and conventional fractionation. A prospective, randomized trial out of the Royal Marsden Hospital randomized 229 patients with cT2-T3N0-1M0 urothelial carcinoma treated from 1988 to 1998 to one of two EBRT monotherapy regimens: (1) an accelerated fractionation regimen of 60.8 Gy in 32 fractions over 26 days (n = 129) or (2) a conventional fractionation regimen of 64 Gy in 32 fractions over 45 days (n = 100) [100]. The accelerated fractionation RT was delivered using BID RT (first fraction, 1.8 Gy; second fraction, 2.0 Gy) with 6 hours between fractions and a 1-week treatment gap following the first 12 fractions. The primary endpoint was local control, and the trial was powered to detect a 20% difference. While no significant difference was noted between the two arms in terms of local control, OS, or DFS, a significantly higher rate of grades 2–3 bowel toxicity was noted in the accelerated fractionation arm when compared to the conventional fraction arm (44% vs. 26%, p = 0.001) [100]. While not directly comparing these two fractionation schemes in the setting of systemic therapy administration, these results indicate a greater likelihood of toxicity from accelerated fractionation without a well-established oncologic benefit.
With regard to hyperfractionation, a prospective, randomized trial conducted in Sweden randomized 168 patients with cT2-T4N0M0 MIBC to a hyperfractionated regimen of thrice daily RT (1 Gy per fraction, three times per day) to a total dose of 84 Gy or a conventional fractionation regimen of 64 Gy in 32 fractions administered once daily [101]. Both treatments were administered over the course of 8 weeks with a 2-week “rest period” in the middle of treatment. At 10-year follow up, the authors found improved rates of local control and OS for patients receiving hyperfractionation [101]. Similarly, meta-analysis data comparing hyperfractionation to conventional fractionation for bladder cancer (2 trials, 345 patients) has suggested improved OS with hyperfractionation [102]. However, findings of these data may largely be explained by the higher dose achieved with hyperfractionated RT; data from the Netherlands have underscored that dose escalation leads to improved local control, with logistic modeling calculations predicting that an increase in total dose by 10 Gy is associated with 3-year improved local control by an odds ratio of 1.44 [103].
Hypofractionation has also been utilized for SBP in the management of MIBC, especially following results of the BC2001 study, which incorporated a partial 2-by-2 factorial design in which patients could be treated with either conventional fractionation or a hypofractionated regimen of 55 Gy in 20 fractions [61]. Long-term outcomes from BC2001 never demonstrated a significant difference between conventional fractionation RT and hypofractionated RT for any trial endpoint; however, until recently, there was no high-quality data for direct comparison [93]. A recently published, individual patient data meta-analysis of BC2001 and BCON (a phase III trial assessing use of hypoxia-modifying agents, discussed in detail in the next section) analyzed 782 patients between the two trials and aimed to establish non-inferiority of 55 Gy in 20 fractions as compared to 64 Gy in 32 fractions with regard to both locoregional control and late toxicity [104]. While the meta-analysis found comparable toxicity profiles between the two fractionation regimens (2-year late rectal toxicity, 3–6%; 2-year late bladder toxicity, 24–25%), at 10-year median follow-up, it was found that patients receiving 55 Gy in 20 fractions had a lower risk of LRR at 3 years than those treated with conventional fractionation (adjusted HR: 0.71, 95% CI: 0.52–0.96) when controlling for pre-specified prognostic factors for local control including age, sex, tumor stage, use of NAC, and extent of resection at TURBT.
Hypoxia Modification
Following data published in the late 1990s regarding modification of hypoxia-induced radioresistance using entities such as high oxygen-content gas breathing, hemoglobin oxygen affinity modifiers, and nicotinamide suggesting improvement in local control for bladder tumors [105], hypoxia modification became an area of active exploration in the 2000s, especially in the United Kingdom. Hypoxia-modifying agents such as carbogen, a mixture of carbon dioxide and oxygen, and nicotinamide, an oxidoreductase coenzyme, were investigated in phase II trials in combination with radical RT to a dose of 52.5 Gy [106]. Following demonstration of good outcomes with carbogen and nicotinamide (CON) for hypoxia modification, a phase III randomized trial (BCON) comparing RT alone to RT with CON was undertaken in patients with locally advanced bladder cancer [107]. BCON randomized 333 patients (to either RT +/− CON) while permitting fractionation schedules of either 64 Gy in 32 fractions or 55 Gy in 20 fractions. The study found no significant difference between RT and RT with CON for its primary endpoint of cystoscopic control at 6 months (76% for RT alone vs. 81% for RT with CON, p = 0.30) [107]; however, at 10-year follow-up, RT with CON was associated with significantly improved recurrence-free survival (27% vs. 20%, p = 0.04) with a trend toward improved OS (32% vs. 24%, p = 0.07; initially significant at 3-year follow-up) [108]. Further analysis of hypoxia modification has demonstrated that tumor necrosis on pathologic specimen obtained by TURBT predicts for better survival outcomes [109]. Researchers have additionally developed a 24-gene signature predicting for benefit from CON (HR: 0.47, 95% CI: 0.26–0.86; p = 0.015) with both prognostic (p = 0.017) and predictive (p = 0.058) significance [110]. While currently utilized predominantly in the United Kingdom, hypoxia modification remains an active area of interest and further study.
Molecular Stratification
Adding further nuance and complexity to the management of MIBC is the idea that molecular stratification of patients’ tumors may both predict for treatment response and guide optimal management [31]. MIBC carries a heterogeneous mutational profile and is considered one of the most highly mutated cancers along with non-small cell lung cancer and squamous cell carcinoma of the head and neck; consequently, efforts to associate molecular subtypes of MIBC with patients’ baseline characteristics and treatment response are underway [111]. With regard to systemic therapy administration, tumors with mutations in genes associated with DNA damage repair (ERCC2, ERBB2, ATM, and RB1) have been shown to demonstrate greater sensitivity to cisplatin [112, 113]. Similarly, increased BCL2 expression has been found to be associated with poorer outcomes for patients receiving concurrent chemoradiotherapy and serves as a marker for patients who may benefit from NAC [114]. For predicting response to RT, existing data has demonstrated that patients with tumors highly expressing MRE11 demonstrate better response to radical RT than those with tumors demonstrating low expression [115]. With significant heterogeneity at the molecular level, investigators at centers worldwide set out to create an international consensus on MIBC molecular subtypes and relate these classes to clinical behavior and treatment response; the authors used 1750 MIBC transcriptomic profiles from 16 published datasets as well as two additional cohorts to develop the following 6 classes [116]:
-
1.
Luminal papillary (LumP)
-
2.
Luminal non-specific (LumNS)
-
3.
Luminal unstable (LumU)
-
4.
Stroma-rich
-
5.
Basal/squamous (Ba/Sq)
-
6.
Neuroendocrine-like (NE-like)
The six molecular classes represented (as follows, percentage of the samples) LumP, 24%; LumNS, 8%; LumU, 15%; stroma-rich, 15%; Ba/Sq, 35%; and NE-like, 3% [116]. mRNA data were utilized to assess for associations with molecular gene signatures for bladder cancer pathways and tumor microenvironment infiltration. The consensus molecular classes were found to be associated with certain genomic alterations: LumP tumors were found to be predominantly associated with mutations in FGFR3 and KDM6A as well as deletions of CDKN2A, whereas LumNS was largely associated with mutations in ELF3 and alterations in PPARG (which were also noted in LumU tumors) [116]. Targeted sequencing data revealed that 58% and 20% of Ba/Sq tumors were associated with TP53 and RB1, respectively, and 49% of Ba/Sq tumors were associated with genomic deletions of 3p14.2 [116].
Of greatest interest to clinicians was description of the association of the six molecular classes with clinical characteristics, OS, and response to treatment. These are briefly summarized in Figure 18.2 [116]. With regard to sociodemographic characteristics, patients with LumP and LumU tumors were found to be more likely to have cT2 (p = 0.009) or cT3-T4 (p < 0.001) disease compared to other molecular classes. Patients with age < 60 were more likely to have LumP tumors (p = 0.001), whereas patients age > 80 were more likely to have LumNS tumors (p = 0.03). Ba/Sq tumors were far more likely to be found among females (p < 0.001) and those with higher clinical stage (p < 0.001) [116]. The association of the six molecular classes with overall survival was analyzed using a multivariable Cox regression model accounting for patients’ age and clinical T, N, and M staging as covariates with the LumP class serving as a reference for comparison. While patients with LumU (HR: 1.49, 95% CI: 0.93–2.39), LumNS (HR: 1.07, 95% CI: 0.63–1.82), and stroma-rich (HR: 0.98, 95% CI: 0.65–1.49) tumors demonstrated similar OS to patients with LumP tumors, patients with Ba/Sq (HR: 1.83, 95% CI: 1.30–2.58, p < 0.001) and NE-like (HR: 2.34, 95% CI: 1.09–5.05, p < 0.03) tumors were associated with significantly worse prognosis [116].
In terms of response to different types of therapy, both LumU and NE-like tumors were felt to be associated with greater response to RT based on demonstrating significantly elevated cell cycle activity and low hypoxia signals when compared to the other classes [116]. Given that the FGFR3 signature was both strongly and specifically activated for patients with LumP tumors, therapies targeting FGFR3 are being investigated. Ba/Sq tumors were found to demonstrate high levels of EGFR and EGFR ligand as well as immune checkpoint markers and genes involved in the mechanisms underlying antigen presentation, all of which would suggest response to immunotherapy; however, none of the molecular classes demonstrated a profile clearly suggesting better or worse response to anti-PD1/PDL1 therapy [116]. While class-based analysis of patients receiving NAC demonstrated no significant association of consensus class with outcome, comparison of the survival curves suggested that patients with LumNS or Ba/Sq tumors may derive greater benefit from patients with NAC, whereas patients with stroma-rich tumors may not [116]. When specifically analyzing patients treated with the anti-PDL1 monoclonal antibody atezolizumab [117], patients were more likely to respond to atezolizumab if they had LumNS (p = 0.05), LumU (p = 0.0044), or NE-like (p = 0.012) tumors [116].
While still an area of growing investigation with need for prospective validation, association of molecular classes with treatment response has the potential to provide considerable guidance in both determination of appropriate therapy of existing options and design of clinical trials ahead. There are multiple ongoing phase II clinical trials aimed at evaluating different treatments based on genetic alterations in DNA damage response; an ongoing phase II trial looking at Risk Enabled Therapy After Initiating Chemotherapy for Bladder Cancer (RETAIN BLADDER; NCT02710734) endeavors to utilize genomic profiles (obtained from sequencing patients’ TURBT specimens while they are receiving cisplatin-based NAC) as well as response to post-chemotherapy TURBT findings to risk-stratify patients [118]. The ALLIANCE trial A03171 (NCT 03609216) is an open phase II trial evaluating for potential bladder preservation in patients receiving dose-dense cisplatin/gemcitabine and has primary and secondary endpoints assessing outcomes based on presence or absence of known genetic alterations [118]. Yet another trial is assessing cisplatin/gemcitabine but with the addition of nivolumab (NCT03558087) for patients with MIBC undergoing SBP [118].
Immunotherapy
Immune checkpoint inhibition (ICI) has been established as a crucial aspect of treatment for non-bladder malignancies and is an area of ongoing investigation for treating MIBC as well. As discussed previously with utilization of molecular stratification, a role for ICI is emerging based on enhanced treatment response in certain tumor types over others. As treatment using ICI has thus far been largely explored in the locally advanced and metastatic settings, treatment paradigms incorporating ICI for patients with MIBC receiving SBP are not yet well established. There are multiple ongoing trials assessing use of ICI for patients receiving SBP followed by RT alone or concurrent chemoradiation. For patients receiving RT alone following TURBT, there are two ongoing phase II trials assessing use of RT with concurrent ICI: NCT03747419 and IMMUNOPRESERVE [119]. NCT03747419 is assessing use of the anti-PDL1 monoclonal antibody avelumab for patients ineligible to receive cisplatin. IMMUNOPRESERVE (Durvalumab Plus Tremelimumab with Concurrent Radiotherapy for Localized Muscle Invasive Bladder Cancer Treated with a Selective Bladder Preservation Approach; NCT 03702179) is a phase II trial sponsored by the Spanish Oncology Genito-Urinary Group (SOGUG) studying joint inhibition of PD1 and CTLA4 concurrently with RT (administered as 46 Gy to the pelvis with 64–66 Gy to the bladder) with the primary endpoint of pathological response at post-treatment biopsy [120].
For patients receiving concurrent chemoradiotherapy +/− ICI following TURBT, there are two phase II trials (NCT03617913 and NCT02621151) and two phase III trials (NCT03775265 [INTACT, NRG/SWOG S1806] and NCT04241185 [KEYNOTE 992]) currently open [119, 121]. NCT03617913 aims to study the CR rate with the addition of avelumab to concurrent chemoradiotherapy using either cisplatin or 5-FU/mitomycin-C, and NCT02621151 is a study investigating lead-in pembrolizumab, maximal TURBT, and adjuvant concurrent chemoradiotherapy with gemcitabine and pembrolizumab using hypofractionated RT of 52 Gy in 20 fractions [119].
NCT03775265 (INTACT, NRG/SWOG S1806) is a phase III RCT randomizing patients with MIBC status post TURBT to concurrent chemoradiotherapy with or without atezolizumab [122, 123]. Patients on S1806 are allowed to receive single-agent cisplatin, single-agent gemcitabine, or 5-FU/mitomycin-C for systemic therapy; patients randomized to the experimental arm additionally receive concurrent and adjuvant atezolizumab 1200 mg every 3 weeks for nine cycles [123]. With regard to RT administration, enrolled patients may be treated with 3DCRT or IMRT, and treatment of pelvic lymph nodes is optional; however, all patients must receive conventionally fractionated treatment to 64–64.8 Gy, as hypofractionation is not permitted on this trial [123]. For the volume to be irradiated, clinicians have the option of treating the small pelvis to 40–50 Gy (or 41.4–50.4 Gy, if treating at 1.8 Gy/fraction) followed by sequential boost(s) to either the (1) bladder tumor alone, (2) the whole bladder alone, or (3) the whole bladder with a secondary sequential boost to the bladder tumor [123]. For patients not receiving nodal RT, the treatment step involving irradiating the small pelvis would be omitted. In addition to having a primary endpoint of bladder-intact event free survival, S1806 has translational objectives of testing that nuclear MRE11, impaired DNA damage response genes, or tumor subtyping are prognostic [123].
NCT04241185 (KEYNOTE-992) is a phase III global, multicenter, double-blinded, placebo-controlled RCT randomizing patients with MIBC status post maximal TURBT to concurrent chemoradiotherapy with or without pembrolizumab for SBP [121]. Similar to SWOG S1806, the trial is allowing for cisplatin monotherapy, 5-FU/mitomycin-C, or gemcitabine monotherapy; however, the trial accepts both conventional fractionation (whole bladder +/− pelvic node) and hypofractionation (whole bladder only) [121]. Patients randomized to the experimental arm receive concurrent and adjuvant pembrolizumab for up to nine doses, and those on the control arm will receive a placebo. Similarly, tissue will undergo biomarker analysis. The study aims to assess the primary endpoint of bladder-intact free survival with secondary endpoints of safety, time to occurrence of NMIBC, OS, and metastasis-free survival [121].
Response Evaluation
One of the most pertinent considerations in the evolution of SBP treatment paradigms is that of mid-treatment response evaluation. Among the pioneering single institution experiences establishing use of TMT for SBP, the University of Erlangen appeared distinct from the University of Paris and MGH in that concurrent chemoradiotherapy was completed continuously without a treatment break for response evaluation. The RTOG approach has involved a treatment break following the induction phase of treatment for early response assessment (following delivery of approximately 40–42 Gy) with repeat cystoscopy and tumor site biopsy. Patients complete consolidation chemoradiation only if a complete response or superficial residual disease is noted, whereas the remaining patients are encouraged to pursue cystectomy in the event of residual/persistent disease. One merit of a mid-treatment response assessment includes early identification of non-responders with the hope that, by avoiding treatment that is not working, they may maintain excellent outcomes following receipt of RC. Further, as full-dose RT has not yet been administered at the mid-treatment point, the surgical morbidity associated with operating on previously irradiated tissue could be less/better. On the other hand, it is arguable that patients may unnecessarily be deemed “non-responders” who receive RC before the treatment has taken effect (and would otherwise have demonstrated a clinical CR following completion of planned concurrent chemoradiotherapy). Critics of a treatment break for response assessment also point to the potential for accelerated tumor clonogen repopulation with prolonging treatment package time, with data demonstrating a trend toward inferior local control with longer treatment package time [124, 125].
In contrast to the RTOG approach, patients treated on phase III trials such as BC2001 and BCON did not receive a mid-treatment break for response assessment and demonstrated comparable outcomes; in these patients, the first opportunity for repeat cystoscopy is often at 3 months post-treatment. In the absence of prospective data directly comparing outcomes for patients receiving mid-treatment cystoscopy and tumor site re-biopsy vs. those receiving continuous concurrent chemoradiotherapy following TURBT, there is no clear answer as to which approach is better. However, on the basis of several existing studies demonstrating excellent outcomes for patients treated without treatment break for response assessment, in addition to ongoing RCTs (e.g., SWOG S1806) enrolling patients treated continuously without a treatment break, it is now considered common practice to forego a mid-treatment response assessment.
Post-Treatment Follow-Up
It is essential that patients treated with SBP return for regular cancer surveillance, which is comprised of a thorough history and physical examination, cystoscopy +/− biopsy of tumor site, and urine cytology. This is completed at regular intervals; based on the National Comprehensive Cancer Network (NCCN) guidelines utilized in the United States, MIBC patients treated with SBP should undergo cystoscopy every 3 months for the first 2 years following completion of definitive intent treatment with the following additional testing to be completed every 3–6 months: CT/MR abdomen/pelvis (A/P), chest imaging (e.g., CT chest), renal function testing, complete blood count, comprehensive metabolic panel, and liver function tests [126]. Every 6–12 months, patients should additionally receive urine cytology [126].
Once the first 2 years have passed, for years 3–4, patients may undergo cystoscopy every 6 months and receive CT/MR A/P and CT chest annually, with laboratory evaluation and urine cytology to be performed only as clinically indicated [126]. At year 5, patients may receive cystoscopy annually and should continue to receive CT/MR A/P and CT chest annually. From 5 to 10 years patients are out from treatment, patients are allowed to receive cystoscopy annually with imaging and blood tests only as clinically indicated, and finally, if >10 years out from treatment, patients may elect to discontinue surveillance if they have remained disease-free in that time [126].
Management of Recurrent Disease
Locoregional Recurrence
Continuous surveillance is important for management of potential locoregional or distant recurrences. Cystoscopy with biopsy of the tumor site allows for detection of a local recurrence within the bladder, which may manifest as a superficial, non-muscle-invasive recurrence (which may then be managed with transurethral resection or intravesical therapy) or a muscle-invasive recurrence that would then require salvage cystectomy with pelvic lymphadenectomy. Regularly spaced, frequent cystoscopy allows for early detection and implementation of salvage treatment. As discussed previously with review of outcomes, patients receiving salvage treatment with RC have the potential to maintain similar survival outcomes if the local recurrence is detected and acted upon early. While most LRRs manifest within the first 2 years following completion of definitive therapy, late can recurrences even up to 5 years following treatment [127].
If patients are suspected to have recurrent disease outside the bladder that has remained contained within the pelvis, in addition to evaluation with CT/MR A/P, they may receive a positron emission tomography (PET)-CT for further assessment, which may help aid in identifying nodal involvement. Unfortunately, given the great likelihood of metastatic disease outside of the pelvis in the event of nodal involvement at the time of relapse, the rate of isolated pelvic relapse is small and estimated to range from 5% to 7% [128]. Management of nodal disease in the setting of a tumor identified within the bladder would be addressed with salvage RC with extended PLND with the option of adjuvant PORT depending on postoperative findings. Isolated nodal relapse is an uncommon scenario: management would begin with multidisciplinary input among clinicians with expertise in management of complex urologic cases. Local therapy options could include surgery (depending on multiple determining factors including size, location, local symptoms, and patient candidacy) or RT. Stereotactic body radiotherapy (SBRT) is a consideration for managing patients with oligometastatic disease, with prescribed doses up to 24–32 Gy administered up to 4–5 fractions depending on the dose constraints of adjacent OARs [129]. Strong consideration would additionally be given to administration of systemic therapy to address sites of subclinical disease not visualized on imaging at the time of diagnosis of recurrence. Patients with pelvic recurrences have a poor prognosis; even with efforts at effective salvage treatment, reported median survival ranges from 4 to 8 months [127].
Distant Recurrence
Management of metastatic disease in MIBC is quite complex; as such, extensive discussions regarding the myriad of systemic therapy options available for management in this scenario comprise a separate chapter. For patients with metastatic disease, goals of care should be identified early, and appropriate palliation should be provided when needed to sites of disease yielding local symptoms (e.g., lungs, bone) to promote improved quality of life. Distant recurrence accounts for 30–40% of relapses – with the most common sites being the lungs, liver, and bone – and carries a very poor prognosis [78, 130].
Node-Positive Disease
SBP for patients with clinically involved nodes at the time of diagnosis is an understudied area of clinical practice with no existing randomized data to guide management, as studies evaluating SBP have largely limited enrollment to patients with clinical N0 disease. While RTOG 8802 included a handful of patients with clinically involved nodes, the small size of the patient population limits meaningful interpretation [31, 87]. An ongoing ECOG/NRG study (NCT04216290, EA8185/INSPIRE) is a phase II trial randomizing patients with stage III urothelial carcinoma (any cT, cN1-2, cM0) status post three cycles or more of NAC to concurrent chemoradiotherapy with or without durvalumab, with the primary endpoint being clinical CR [131]. Patients noting no clinical benefit at post-treatment re-staging 8 weeks following completion of treatment are planned to receive salvage RC, and planned stratifications include extent of TURBT (presence of residual disease vs. no residual disease), size of lymph nodes (1–2 cm vs. >2 cm), chemotherapy administered (cisplatin vs. non-cisplatin regimen), whether NAC was administered pre- or post-randomization, and response to pre-randomization NAC [131]. Performance of this trial marks an important step toward establishing concurrent chemoradiotherapy as a primary treatment option for management of clinically node positive MIBC, as data from population-based analyses suggest that nearly 80% of patients with node-positive nonmetastatic are managed with chemotherapy alone as opposed to SBP [132].
Quality of Life Considerations
Health-related quality of life (HRQOL) embodies a multitude of domains including physical (incorporating urinary and sexual function), social, emotional, and psychological well-being, with a diagnosis of MIBC in itself having been shown to significantly impact physical and social function based on population-based analyses on registry patients from the Surveillance, Epidemiology, and End Results (SEER) Program [133]. An important consideration for patients electing to undergo SBP is the impact on HRQOL that undergoing RC would impart. In addition to impact on urinary and sexual function, the associated urinary diversion significantly impacts daily life and body image [134]. While often not initially suspected as a HRQOL culprit, sexual dysfunction secondary to RC is one of the most significant detriments to quality of life. Along with physical changes/altered anatomy accounting for organic etiologies underlying sexual dysfunction (e.g., up to 80% of men may develop erectile dysfunction), negative psychosocial influences such as the stigma associated with urinary diversion may strain intimacy and lead to impaired sexual expression or satisfaction [135]. Existing data also suggests that urinary function and bowel habits are consistently compromised in patients undergoing RC with urinary diversion. The first validated bladder cancer-specific instrument studying HRQOL was the Bladder Cancer Index (BCI), developed in 2007; a pilot study (n = 315) using the BCI found that patients who had received RC scored lower than patients maintaining their native bladder in both function and bother scores across all domains (sexual, urinary, bowel) [136]. Of great interest with potential surprise, patients who had received an orthotopic neobladder demonstrated significantly lower urinary function scores as compared to patients who had received incontinent diversions [136]. The impact of RC with urinary diversion on HRQOL should be strongly considered when determining a treatment course for patients eligible for SBP which may allow them to circumvent issues related to compromised urinary, bowel, and sexual functioning.
Retrospective data reporting on HRQOL for patients receiving SBP demonstrates better outcomes for patients receiving TMT as compared to RC. A cross-sectional bi-institutional study in the United States analyzing 226 patients with MIBC eligible for RC who were disease-free for 2 years or longer administered six validated HRQOL instruments and found that TMT was associated with better HRQOL by nearly 10 points out of 100 compared to patients receiving RC (p = 0.001) with greater physical, social, emotional, and cognitive functioning (p < 0.04) [137]. As compared to RC, patients receiving TMT reported better bowel function by 4.5 points (p = 0.02), fewer bowel symptoms by 3–7 points (p < 0.05), better sexual function by 9–32 points (p < 0.02), and better body image by 15 points (p < 0.001) [137]. A cross-sectional questionnaire study performed in Sweden comparing treatment-related side effects from pelvic EBRT monotherapy (n = 58) to RC (n = 251) and population controls (n = 310) found that 74% of irradiated patients reported normal urinary function, and there was no statistically significant difference in distress secondary to gastrointestinal symptoms between EBRT monotherapy and RC (32% vs. 24%) [138]. A similarly high rate of preserved urinary function was reported by MGH, with 75% of patients from their retrospective cohort study demonstrating normal bladder function by urodynamic study [139]. With regard to sexual function, data from MGH suggests that the majority of male patients maintained erectile function with only 8% of male patients expressing dissatisfaction, and separate data reporting on female patients has found that over 70% of women receiving SBP maintained pre-treatment levels of sexual satisfaction [139, 140]. Overall, data from a pooled analysis of multiple RTOG studies (RTOG 8903, 9506, 9706, and 9906) has shown a late grade 3 genitourinary toxicity rate of 5.7% and late grade 3 gastrointestinal toxicity rate of 1.9% among patients retaining their native bladder, with no grades 4–5 toxicities, indicating relatively favorable late term toxicity outcomes for those receiving EBRT with concurrent chemotherapy [141].
Radiation Techniques
Simulation
CT simulation is best performed with the patient having been instructed to present to the radiation oncology clinic with an empty bladder and rectum; treating the patient with a maximally empty bladder allows for greater reproducibility by evading the perils of daily inconsistency in bladder filling. Once the patient has completely voided and made efforts to minimize rectal distension, the patient is then simulated in the supine position using a custom-made device such as a Vac-Lok (Civco, Kalona, Iowa, USA) for immobilization of the pelvis. A CT scan typically without contrast is performed from L1 through mid-femur. Depending on institutional practice, considerations may be made for using IV contrast to aid in identification of vasculature (particularly for clinicians who utilize pelvic nodal irradiation); however, it is critical that renal function be assessed prior to administration of IV contrast due to concern for contrast-induced nephropathy in patients with high risk of compromised renal function secondary to their disease. In lieu of contrast administration to aid in visualization at the time of simulation, any diagnostic imaging obtained as part of pre-treatment workup may be coregistered to guide planning. For clinicians planning on administering a bladder tumor boost, certain institutions have utilized liquid, radio-opaque markers such as lipiodol to aid in identification [142]. Based on institutional practice, some clinicians may opt to treat the patient using an adaptive RT technique with a “plan-of-the-day”; such a practice may involve simulating the patient with a maximally empty bladder, a comfortably/reproducibly full bladder, and the bladder in an intermediary state [143]. At the authors’ institution, patients are simulated and treated with a maximally empty bladder and rectum for every day of treatment, and neither IV/oral contrast nor fiducial markers are utilized for treatment.
Target Volume
There is great variability in practice across the world with regard to target volume delineation, and the optimal RT target volume in TMT for SBP is considered an area of controversy. This is reflected in the protocol of ongoing phase III RCT SWOG S1806, which allows for patients to be treated with or without nodal irradiation as well as treatment of the whole bladder with or without a tumor boost or omission of a whole bladder field and treatment of the tumor only [123]. The patient’s target volumes may then be defined and delineated as follows, as described per protocol of SWOG S1806 [123]:
-
Gross tumor volume (GTV): Macroscopic visible tumor on imaging/cystoscopy
-
Clinical target volume (CTV): Comprised of multiple entities, as stated below
-
CTV_bladder tumor: Defined by diagnostic imaging/site of TURBT (as per findings documented in operative report as well as multidisciplinary discussion with urologic surgeon at time of treatment planning)
-
CTV_prostate: Target volume encompassing prostate and prostatic urethra in male patients
-
CTV_whole bladder: Target volume encompassing the entire bladder, including the CTV_bladder tumor
-
CTV_nodal: Target volume encompassing the pelvic nodes below the common iliac bifurcation, including the presacral nodes, external iliac nodes, internal iliac nodes, and obturator nodes. These nodal regions are anatomically defined as follows:
-
Pre-sacral nodes: This lymphatic area extends from the superior aspect of S1 to the superior aspect of S3 and includes the 1 cm thickness anterior to the sacrum
-
External iliac nodes: This lymph node group is contoured inferiorly up to the superior aspect of the femoral heads and is delineated by creating a 7 mm circumferential expansion around the external iliac vessels.
-
Internal iliac nodes: This lymph node group is contoured inferiorly until no longer visualized or until exiting the pelvis via the greater sciatic notch and is delineated by creating a 7 mm circumferential expansion around the internal iliac vessels.
-
Obturator nodes: This lymphatic area is contoured superiorly where the iliac vessel contours stop and extends inferiorly to the superior aspect of the symphysis pubis; it encompasses the 1 cm width of tissue situated medially to the obturator internus muscles from the anterior to posterior borders of the ilium.
-
All nodal target volumes must be trimmed so that they do not extend outside the pelvis or into adjacent OARs such as the rectum, bowel, or bone.
-
-
-
Planning target volume (PTV) : Comprised of symmetric expansions of CTV to account for inter-fraction variability from internal organ motion or daily set-up. The following are recent trial-utilized values; optimal PTV expansions could be determined based on institutional preferences or analyses determining set-up/positional error.
-
PTV_bladder tumor: 5 mm to 1 cm expansion if using IMRT; 1.0–1.5 cm expansion if using 3DCRT with margin to be determined based on image-guided radiotherapy (IGRT) options available at treating institution.
-
PTV_whole bladder: 5 mm to 1 cm expansion if using IMRT; 1.0–1.5 cm expansion if using 3DCRT (except in the region of the bladder tumor, with margin of 2 cm permissible) with margin to be determined based on image-guided radiotherapy (IGRT) options available at treating institution.
-
PTV_prostate: 5 mm expansion of CTV_prostate for male patients.
-
PTV_nodal: 5 mm expansion of CTV_nodal.
-
Anisotropic expansions may be utilized to achieve dose constraints to adjacent OARs.
-
A treatment plan for a patient treated with 41.4 Gy to the pelvis and nodes followed by a sequential bladder boost to 64.8 Gy is portrayed in Fig. 18.3. Acceptable planning metrics ideally yield coverage V100 ≥ 95% with a hotspot no greater than 110%. The OARs to delineate include the bilateral femoral heads , rectum, and small bowel, with corresponding dose constraints to be listed in the following section on dosing.
A treatment plan for a patient treated with definitive concurrent chemoradiotherapy using IMRT for muscle-invasive bladder cancer. A prescription of 41.4 Gy was used for PTV_pelvis and nodes (lime green volume encompassed by teal isodose line) and was followed by a sequential boost of 23.4 Gy to the PTV_bladder for a cumulative dose of 64.8 Gy (red volume encompassed by dark blue isodose line). The low dose spread from IMRT is additionally depicted (pearl, 20.0 Gy; dark green, 25.0 Gy; brown, 30.0 Gy; teal, 41.4 Gy; yellow, 45.0 Gy; orange, 54.0 Gy; green, 60.0 Gy)
There is no clear consensus regarding treatment of pelvic nodal regions, other than that pelvic nodal irradiation can be administered using conventional fractionation, whereas hypofractionated treatments typically target the whole (or partial) bladder only. Randomized data from a trial performed in Pakistan comparing patients receiving RT to the whole pelvis (n = 120) to those receiving treatment to bladder only (n = 110) found, at 5-year median follow-up, no significant difference in DFS (47.1% vs. 46.9%, p = 0.5), bladder preservation rate (58.9% vs. 57.1%, p = 0.8), or OS (52.9% vs. 51%, p = 0.8) [144].
Therefore, the following are all accepted treatment volumes for irradiating MIBC:
-
Whole pelvis irradiation encompassing bladder and lymph nodes (e.g., to 41.4 Gy), followed by cone down to whole bladder (e.g., to 55.8 Gy), followed by boost to tumor site (e.g., to 64.8 Gy)
-
Whole pelvis irradiation encompassing bladder and lymph nodes (e.g., to 41.4 Gy), followed by cone down to whole bladder (e.g., to 64.8 Gy)
-
Treatment of either whole bladder or tumor site only, with or without a boost
-
This is the most common method of treatment delivery in Europe, where the PTV often incorporates the CTV with a 1.5 cm expansion. In BC2001, the PTV1 consisted of the outer bladder wall with a 1.5 cm expansion, and the PTV2 consisted of the tumor site with a 1.5 cm expansion [61].
-
Dose
Multiple dosing schemes have been utilized in TMT for SBP. Conventionally fractionated RT is administered in 1.8–2.0 Gy per fraction once daily. Daily, conventionally fractionated RT delivers a cumulative dose of 64–64.8 Gy to either (1) the whole bladder or (2) the tumor site only (with a margin), depending on planned treatment delivery method. For patients who are receiving pelvic nodal irradiation, dose delivered to this area typically ranges from 41.4 to 50.4 Gy when administering treatment at 1.8 Gy per fraction or 40 to 50 Gy when treating with 2 Gy daily fractions. If utilizing an accelerated fractionation approach, such as in the RTOG trials (e.g., RTOG 0712), treatment may be administered in 1.5 Gy fractions twice daily (separated by at least 6 hours) [75]. For patients receiving hypofractionated RT, treatment is administered at 2.75 Gy per fraction to a cumulative dose of 55 Gy to either (1) the whole bladder or (2) the tumor site with a margin (partial bladder).
Dose Constraints (Conventional Fractionation) [75, 123]
Rectum: V30 ≤ 50%, V55 ≤ 10%
Femoral heads: Max 45 Gy
Small bowel: V50 ≤ 15 cc, V45 ≤ 100 cc, V30 ≤ 150 cc
Fields
While 3DCRT has historically been utilized for management of MIBC, with wide margins (1.5 cm as per BC2001 or 2 cm as per RTOG approach) to account for organ motion and variations in set-up prior to advances in IGRT, IMRT is being increasingly utilized with the aid of cone beam CT (CBCT) for daily image guidance. IMRT carries multiple benefits, including the potential for use of simultaneous integrated boost (SIB) technique to the primary tumor site and shorter treatment delivery times with the ability to minimize dose to adjacent OARs by optimizing beam angles/dose entry, thereby providing more conformal treatment [145]. However, 3DCRT still remains frequently utilized. For patients receiving treatment with 3DCRT incorporating pelvic nodal irradiation, treatment has historically encompassed a “small pelvis” that may be treated using a four-field technique (AP/PA and opposed laterals) with the following field borders [75]:
-
Superior border: Mid-sacroiliac joint
-
Inferior border: Bottom of the obturator foramen
-
Lateral borders: 1.5 cm margin on the pelvic brim for AP/PA fields or 2 cm beyond the CTV for the lateral fields
In designing the AP/PA fields, care must be taken to block the femoral heads. In designing the lateral fields, the rectum and small bowel should be blocked.
RTOG Approach
To summarize an example of radiotherapeutic management, the RTOG approach will be utilized as an example. Following a diagnosis of MIBC with decision to pursue SBT with TMT, a patient receives maximal TURBT and is then initiated on induction treatment of concurrent chemoradiotherapy to a dose of 40–45 Gy administered over 20–25 fractions. In the first phase of RT, the whole bladder is treated with a 2 cm margin, and the initial treatment volume incorporates the prostatic urethra in men (or the proximal 2 cm of the urethra in women) as well as the pelvic lymphatics (which may be treated using the previously described “small pelvis” technique if using 3DCRT). Following induction therapy, a repeat cystoscopy with biopsy of the primary tumor site is performed after a 2–3-week treatment break. Patients with residual disease noted on pathology are then referred for RC, whereas patients with CR (or good response with only superficial disease remaining) then proceed with the consolidation phase of treatment. In the consolidation phase of treatment, patients receive the remainder of the prescription dose, which is administered as a cone treatment down to the whole bladder (an additional 10–14 Gy) followed by a final boost to the tumor alone with a 2 cm margin (an additional 10 Gy).
Summary of Treatment Recommendations
For patients considered eligible for SBP who are receiving TMT, we recommend maximal TURBT with adjuvant concurrent chemoradiotherapy. Patients are considered optimal candidates if they present with cT2-T3aN0M0 disease with unifocal disease and without CIS or tumor-associated hydronephrosis, although consideration for bladder sparing is on a case-by-case basis. Options for concurrent systemic therapy include cisplatin monotherapy, gemcitabine monotherapy, 5-FU/mitomycin-C, or a cisplatin-based regimen with 5-FU or paclitaxel. RT regimens vary based on institutional practice but include conventionally fractionated RT of 64.8 Gy in 36 fractions to the bladder +/− pelvic nodes or hypofractionated RT of 55 Gy in 20 fractions to the whole bladder only. It is the authors’ practice to perform conventionally fractionated radiotherapy, delivering 41.4 Gy in 23 fractions to the PTV encompassing the whole bladder and pelvic nodes followed by a 23.4 Gy in 13 fraction boost to the whole bladder PTV for a cumulative dose of 64.8 Gy given over 36 fractions. Consideration may additionally be given to concurrent and adjuvant immunotherapy with patient enrollment on a clinical trial.
Conclusion
SBP is an emerging treatment paradigm for definitive management of MIBC with great promise. The most effective regimen investigated to date incorporates TMT with maximal TURBT followed by RT administered with concurrent radiosensitizing chemotherapy. While currently limited to patients deemed eligible for candidacy based on strict selection criteria, future treatment directions include broadening eligibility and assessing outcomes for patients with MIBC who do not necessarily meet these criteria. Studies on HRQOL suggest comparatively higher quality of life associated with bladder sparing versus RC; while there are no prospective, randomized data to directly compare these two treatments, a steadily increasing body of literature suggests no significant difference in oncologic outcomes, which makes bladder sparing treatment very compelling. In addition to continued assessment of systemic therapy options, ICI is additionally under investigation with ongoing RCTs, and recently designed RCTs are now incorporating molecular subtyping with the ultimate goal of having these be prospectively validated. The results of ongoing RCTs prospectively investigating conventional fractionation vs. hypofractionation and pelvic nodal irradiation vs. treatment of bladder only are eagerly awaited. Further study will additionally be needed to elucidate a better understanding of optimal management for patients with node-positive disease as we approach an era of improved systemic therapy now incorporating ICI.
References
Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel, Switzerland). 2020;8(1):15. https://doi.org/10.3390/medsci8010015.
Magers MJ, Lopez-Beltran A, Montironi R, Williamson SR, Kaimakliotis HZ, Cheng L. Staging of bladder cancer. Histopathology. 2019;74(1):112–34. https://doi.org/10.1111/his.13734.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Mostafa MH, Sheweita SA, O’Connor PJ. Relationship between schistosomiasis and bladder cancer. Clin Microbiol Rev. 1999;12(1):97–111.
Zhu C-Z, Ting H-N, Ng K-H, Ong T-A. A review on the accuracy of bladder cancer detection methods. J Cancer. 2019;10(17):4038–44. https://doi.org/10.7150/jca.28989.
Galgano SJ, Porter KK, Burgan C, Rais-Bahrami S. The role of imaging in bladder cancer diagnosis and staging. Diagnostics (Basel, Switzerland). 2020;10(9). https://doi.org/10.3390/diagnostics10090703.
Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol Off J Am Soc Clin Oncol. 2001;19(3):666–75. https://doi.org/10.1200/JCO.2001.19.3.666.
Muilwijk T, Akand M, Gevaert T, Joniau S. No survival difference between super extended and standard lymph node dissection at radical cystectomy: what can we learn from the first prospective randomized phase III trial? Transl Androl Urol. 2019;8(Suppl 1):S112–5. https://doi.org/10.21037/tau.2018.12.09.
Gschwend JE, Heck MM, Lehmann J, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol. 2019;75(4):604–11. https://doi.org/10.1016/j.eururo.2018.09.047.
Siddiqui KM, Izawa JI. Ileal conduit: standard urinary diversion for elderly patients undergoing radical cystectomy. World J Urol. 2016;34(1):19–24. https://doi.org/10.1007/s00345-015-1706-1.
Monn MF, Kaimakliotis HZ, Cary KC, et al. Short-term morbidity and mortality of Indiana pouch, ileal conduit, and neobladder urinary diversion following radical cystectomy. Urol Oncol. 2014;32(8):1151–7. https://doi.org/10.1016/j.urolonc.2014.04.009.
Lukka H. In favour of bladder preservation using combined modality treatment. Can Urol Assoc J = J l’Association des Urol du Canada. 2009;3(5):412–5. https://doi.org/10.5489/cuaj.1157.
Dalbagni G, Genega E, Hashibe M, et al. Cystectomy for bladder cancer: a contemporary series. J Urol. 2001;165(4):1111–6.
Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66. https://doi.org/10.1056/NEJMoa022148.
Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48(2):202–6. https://doi.org/10.1016/j.eururo.2005.04.006.
Eisenberg MS, Thompson RH, Frank I, et al. Long-term renal function outcomes after radical cystectomy. J Urol. 2014;191(3):619–25. https://doi.org/10.1016/j.juro.2013.09.011.
Zaghloul MS, Awwad HK, Akoush HH, Omar S, Soliman O, el Attar I. Postoperative radiotherapy of carcinoma in bilharzial bladder: improved disease free survival through improving local control. Int J Radiat Oncol Biol Phys. 1992;23(3):511–7. https://doi.org/10.1016/0360-3016(92)90005-3.
Zaghloul MS, Christodouleas JP, Smith A, et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: a randomized phase 2 trial. JAMA Surg. 2018;153(1):e174591. https://doi.org/10.1001/jamasurg.2017.4591.
Christodouleas JP, Hwang W-T, Baumann BC. Adjuvant radiation for locally advanced bladder cancer? A question worth asking. Int J Radiat Oncol Biol Phys. 2016;94(5):1040–2. https://doi.org/10.1016/j.ijrobp.2016.01.015.
Baumann BC, Bosch WR, Bahl A, et al. Development and validation of consensus contouring guidelines for adjuvant radiation therapy for bladder cancer after radical cystectomy. Int J Radiat Oncol Biol Phys. 2016;96(1):78–86. https://doi.org/10.1016/j.ijrobp.2016.04.032.
Solanki AA, Martin B, Korpics M, Small C, Harkenrider MM, Mitin T. Adjuvant radiotherapy use by US radiation oncologists after radical cystectomy for muscle-invasive bladder cancer. Clin Oncol (R Coll Radiol). 2017;29(7):429–35. https://doi.org/10.1016/j.clon.2017.02.005.
Fischer-Valuck BW, Michalski JM, Mitra N, et al. Effectiveness of postoperative radiotherapy after radical cystectomy for locally advanced bladder cancer. Cancer Med. 2019;8(8):3698–709. https://doi.org/10.1002/cam4.2102.
Soulié M, Straub M, Gamé X, et al. A multicenter study of the morbidity of radical cystectomy in select elderly patients with bladder cancer. J Urol. 2002;167(3):1325–8.
Stimson CJ, Chang SS, Barocas DA, et al. Early and late perioperative outcomes following radical cystectomy: 90-day readmissions, morbidity and mortality in a contemporary series. J Urol. 2010;184(4):1296–300. https://doi.org/10.1016/j.juro.2010.06.007.
Novotny V, Hakenberg OW, Wiessner D, et al. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51(2):392–7. https://doi.org/10.1016/j.eururo.2006.06.014.
Donat SM, Shabsigh A, Savage C, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol. 2009;55(1):177–85. https://doi.org/10.1016/j.eururo.2008.07.018.
Novara G, Catto JWF, Wilson T, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol. 2015;67(3):376–401. https://doi.org/10.1016/j.eururo.2014.12.007.
Reisinger SA, Mohiuddin M, Mulholland SG. Combined pre- and postoperative adjuvant radiation therapy for bladder cancer--a ten year experience. Int J Radiat Oncol Biol Phys. 1992;24(3):463–8. https://doi.org/10.1016/0360-3016(92)91060-z.
Santos F, Zakaria AS, Kassouf W, Tanguay S, Aprikian A. High hospital and surgeon volume and its impact on overall survival after radical cystectomy among patients with bladder cancer in Quebec. World J Urol. 2015;33(9):1323–30. https://doi.org/10.1007/s00345-014-1457-4.
Bajaj A, Martin B, Bhasin R, et al. The impact of academic facility type and case volume on survival in patients undergoing curative radiation therapy for muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2018;100(4):851–7. https://doi.org/10.1016/j.ijrobp.2017.11.040.
Mirza A, Choudhury A. Bladder preservation for muscle invasive bladder cancer. Bladder cancer (Amsterdam, Netherlands). 2016;2(2):151–63. https://doi.org/10.3233/BLC-150025.
Hamad J, McCloskey H, Milowsky MI, Royce T, Smith A. Bladder preservation in muscle-invasive bladder cancer: a comprehensive review. Int Braz J Urol. 2020;46(2):169–84. https://doi.org/10.1590/S1677-5538.IBJU.2020.99.01.
Winquist E, Booth CM. Trimodality therapy for muscle-invasive bladder cancer: concurrent chemotherapy is not enough. J Clin Oncol. 2020;38(24):2709–11. https://doi.org/10.1200/JCO.19.02959.
Ritch CR, Balise R, Prakash NS, et al. Propensity matched comparative analysis of survival following chemoradiation or radical cystectomy for muscle-invasive bladder cancer. BJU Int. 2018;121(5):745–51. https://doi.org/10.1111/bju.14109.
Hollenbeck BK, Miller DC, Dunn RL, Montie JE, Wei JT. The effects of stage divergence on survival after radical cystectomy for urothelial cancer. Urol Oncol. 2005;23(2):77–81. https://doi.org/10.1016/j.urolonc.2004.08.012.
van Dijk PR, Ploeg M, Aben KKH, et al. Downstaging of TURBT-based muscle-invasive bladder cancer by radical cystectomy predicts better survival. ISRN Urol. 2011;2011:458930. https://doi.org/10.5402/2011/458930.
Marchioni M, Primiceri G, Delli Pizzi A, et al. Could bladder multiparametric MRI be introduced in routine clinical practice? Role of the new VI-RADS score: results from a prospective study. Clin Genitourin Cancer. 2020;18(5):409–415.e1. https://doi.org/10.1016/j.clgc.2020.03.002.
James ND, Pirrie S, Liu W, et al. Replacing TURBT with mpMRI for staging MIBC: pilot data from the BladderPath study. J Clin Oncol. 2020;38(6_suppl):446. https://doi.org/10.1200/JCO.2020.38.6_suppl.446.
Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol Off J Am Soc Clin Oncol. 2017;35(20):2299–305. https://doi.org/10.1200/JCO.2016.69.2327.
Zhong J, Switchenko J, Jegadeesh NK, et al. Comparison of outcomes in patients with muscle-invasive bladder cancer treated with radical cystectomy versus bladder preservation. Am J Clin Oncol. 2019;42(1):36–41. https://doi.org/10.1097/COC.0000000000000471.
Vashistha V, Wang H, Mazzone A, et al. Radical cystectomy compared to combined modality treatment for muscle-invasive bladder cancer: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2017;97(5):1002–20. https://doi.org/10.1016/j.ijrobp.2016.11.056.
Huddart RA, Birtle A, Maynard L, et al. Clinical and patient-reported outcomes of SPARE - a randomised feasibility study of selective bladder preservation versus radical cystectomy. BJU Int. 2017;120(5):639–50. https://doi.org/10.1111/bju.13900.
Herr HW. Conservative management of muscle-infiltrating bladder cancer: prospective experience. J Urol. 1987;138(5):1162–3. https://doi.org/10.1016/S0022-5347(17)43535-X.
Henry K, Miller J, Mori M, Loening S, Fallon B. Comparison of transurethral resection to radical therapies for stage B bladder tumors. J Urol. 1988;140(5):964–7. https://doi.org/10.1016/s0022-5347(17)41899-4.
Herr HW. Transurethral resection of muscle-invasive bladder cancer: 10-year outcome. J Clin Oncol. 2001;19(1):89–93. https://doi.org/10.1200/JCO.2001.19.1.89.
Barnes RW, Dick AL, Hadley HL, Johnston OL. Survival following transurethral resection of bladder carcinoma. Cancer Res. 1977;37(8 Pt 2):2895–7.
Milner WA. The role of conservative surgery in the treatment of bladder tumours. Br J Urol. 1954;26(4):375–86. https://doi.org/10.1111/j.1464-410x.1954.tb04920.x.
Nerli RB, Reddy M, Koura AC, Prabha V, Ravish IR, Amarkhed S. Cystoscopy-assisted laparoscopic partial cystectomy. J Endourol. 2008;22(1):83–6. https://doi.org/10.1089/end.2007.0105.
Park JC, Citrin DE, Agarwal PK, Apolo AB. Multimodal management of muscle-invasive bladder cancer. Curr Probl Cancer. 2014;38(3):80–108. https://doi.org/10.1016/j.currproblcancer.2014.06.001.
Ma B, Li H, Zhang C, et al. Lymphovascular invasion, ureteral reimplantation and prior history of urothelial carcinoma are associated with poor prognosis after partial cystectomy for muscle-invasive bladder cancer with negative pelvic lymph nodes. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2013;39(10):1150–6. https://doi.org/10.1016/j.ejso.2013.04.006.
Kassouf W, Swanson D, Kamat AM, et al. Partial cystectomy for muscle invasive urothelial carcinoma of the bladder: a contemporary review of the M. D. Anderson Cancer Center experience. J Urol. 2006;175(6):2058–62. https://doi.org/10.1016/S0022-5347(06)00322-3.
Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol. 2004;172(3):878–81. https://doi.org/10.1097/01.ju.0000135530.59860.7d.
Smaldone MC, Jacobs BL, Smaldone AM, Hrebinko RLJ. Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology. 2008;72(3):613–6. https://doi.org/10.1016/j.urology.2008.04.052.
Mistretta FA, Cyr S-J, Luzzago S, et al. Partial cystectomy with pelvic lymph node dissection for patients with nonmetastatic stage pT2-T3 urothelial carcinoma of urinary bladder: temporal trends and survival outcomes. Clin Genitourin Cancer. 2020;18(2):129–137.e3. https://doi.org/10.1016/j.clgc.2019.09.008.
Fahmy N, Aprikian A, Tanguay S, et al. Practice patterns and recurrence after partial cystectomy for bladder cancer. World J Urol. 2010;28(4):419–23. https://doi.org/10.1007/s00345-009-0478-x.
Duncan W, Quilty PM. The results of a series of 963 patients with transitional cell carcinoma of the urinary bladder primarily treated by radical megavoltage X-ray therapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1986;7(4):299–310. https://doi.org/10.1016/s0167-8140(86)80059-7.
Quilty PM, Duncan W. The influence of hemoglobin level on the regression and long term local control of transitional cell carcinoma of the bladder following photon irradiation. Int J Radiat Oncol Biol Phys. 1986;12(10):1735–42. https://doi.org/10.1016/0360-3016(86)90313-5.
Davidson SE, Symonds RP, Snee MP, Upadhyay S, Habeshaw T, Robertson AG. Assessment of factors influencing the outcome of radiotherapy for bladder cancer. Br J Urol. 1990;66(3):288–93. https://doi.org/10.1111/j.1464-410x.1990.tb14929.x.
Pollack A, Zagars GK, Swanson DA. Muscle-invasive bladder cancer treated with external beam radiotherapy: prognostic factors. Int J Radiat Oncol Biol Phys. 1994;30(2):267–77. https://doi.org/10.1016/0360-3016(94)90004-3.
Sauer R, Birkenhake S, Kühn R, Wittekind C, Schrott KM, Martus P. Efficacy of radiochemotherapy with platin derivatives compared to radiotherapy alone in organ-sparing treatment of bladder cancer. Int J Radiat Oncol Biol Phys. 1998;40(1):121–7. https://doi.org/10.1016/s0360-3016(97)00579-8.
James ND, Hussain SA, Hall E, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477–88. https://doi.org/10.1056/NEJMoa1106106.
Barringer BS. Radium treatment of cancer of the bladder. Ann Surg. 1935;101(6):1425–8. https://doi.org/10.1097/00000658-193506000-00015.
Barringer BS. Twenty-five years of radon treatment of cancer of the bladder. JAMA. 1947;135(10):616–8.
Lenz M, Cahill GF. The treatment of cancer of the bladder by radium needles. Am J Roentgenol Radium Ther. 1947;58(4):486–92.
Wijnmaalen A, Helle PA, Koper PC, et al. Muscle invasive bladder cancer treated by transurethral resection, followed by external beam radiation and interstitial iridium-192. Int J Radiat Oncol Biol Phys. 1997;39(5):1043–52. https://doi.org/10.1016/s0360-3016(97)00375-1.
Rozan R, Albuisson E, Donnarieix D, et al. Interstitial iridium-192 for bladder cancer (A multicentric survey: 205 patients). Int J Radiat Oncol. 1992;24(3):469–77. https://doi.org/10.1016/0360-3016(92)91061-Q.
Pernot M, Hubert J, Guillemin F, et al. Combined surgery and brachytherapy in the treatment of some cancers of the bladder (partial cystectomy and interstitial iridium-192). Radiother Oncol J Eur Soc Ther Radiol Oncol. 1996;38(2):115–20. https://doi.org/10.1016/0167-8140(96)82354-1.
Bos MK, Marmolejo RO, Rasch CRN, Pieters BR. Bladder preservation with brachytherapy compared to cystectomy for T1-T3 muscle-invasive bladder cancer: a systematic review. J Contemp Brachytherapy. 2014;6(2):191–9. https://doi.org/10.5114/jcb.2014.43777.
Sternberg CN, Pansadoro V, Calabrò F, et al. Can patient selection for bladder preservation be based on response to chemotherapy? Cancer. 2003;97(7):1644–52. https://doi.org/10.1002/cncr.11232.
Herr HW, Scher HI. Neoadjuvant chemotherapy and partial cystectomy for invasive bladder cancer. J Clin Oncol Off J Am Soc Clin Oncol. 1994;12(5):975–80. https://doi.org/10.1200/JCO.1994.12.5.975.
Bazzi WM, Kopp RP, Donahue TF, et al. Partial cystectomy after neoadjuvant chemotherapy: Memorial Sloan Kettering Cancer Center contemporary experience. Int Sch Res Not. 2014;2014:702653. https://doi.org/10.1155/2014/702653.
Thomas R, Hall R. Radical transurethral resection and chemotherapy in. BJU Int. 1999;83(4):432–7. https://doi.org/10.1046/j.1464-410x.1999.00970.x.
Solsona E, Climent MA, Iborra I, et al. Bladder preservation in selected patients with muscle-invasive bladder cancer by complete transurethral resection of the bladder plus systemic chemotherapy: long-term follow-up of a phase 2 nonrandomized comparative trial with radical cystectomy. Eur Urol. 2009;55(4):911–9. https://doi.org/10.1016/j.eururo.2008.08.027.
Mitin T, Hunt D, Shipley WU, et al. Transurethral surgery and twice-daily radiation plus paclitaxel-cisplatin or fluorouracil-cisplatin with selective bladder preservation and adjuvant chemotherapy for patients with muscle invasive bladder cancer (RTOG 0233): a randomised multicentre phase. Lancet Oncol. 2013;14(9):863–72. https://doi.org/10.1016/S1470-2045(13)70255-9.
Coen JJ, Zhang P, Saylor PJ, et al. Bladder preservation with twice-a-day radiation plus fluorouracil/cisplatin or once daily radiation plus gemcitabine for muscle-invasive bladder cancer: NRG/RTOG 0712-a randomized phase II trial. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(1):44–51. https://doi.org/10.1200/JCO.18.00537.
Rödel C, Grabenbauer GG, Kühn R, et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol Off J Am Soc Clin Oncol. 2002;20(14):3061–71. https://doi.org/10.1200/JCO.2002.11.027.
Perdonà S, Autorino R, Damiano R, et al. Bladder-sparing, combined-modality approach for muscle-invasive bladder cancer: a multi-institutional, long-term experience. Cancer. 2008;112(1):75–83. https://doi.org/10.1002/cncr.23137.
Efstathiou JA, Spiegel DY, Shipley WU, et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: the MGH experience. Eur Urol. 2012;61(4):705–11. https://doi.org/10.1016/j.eururo.2011.11.010.
Fung CY, Shipley WU, Young RH, et al. Prognostic factors in invasive bladder carcinoma in a prospective trial of preoperative adjuvant chemotherapy and radiotherapy. J Clin Oncol Off J Am Soc Clin Oncol. 1991;9(9):1533–42. https://doi.org/10.1200/JCO.1991.9.9.1533.
Shipley WU, Winter KA, Kaufman DS, et al. Phase III trial of neoadjuvant chemotherapy in patients with invasive bladder cancer treated with selective bladder preservation by combined radiation therapy and chemotherapy: initial results of Radiation Therapy Oncology Group 89-03. J Clin Oncol Off J Am Soc Clin Oncol. 1998;16(11):3576–83. https://doi.org/10.1200/JCO.1998.16.11.3576.
Krasnow RE, Drumm M, Roberts HJ, et al. Clinical outcomes of patients with histologic variants of urothelial cancer treated with trimodality bladder-sparing therapy. Eur Urol. 2017;72(1):54–60. https://doi.org/10.1016/j.eururo.2016.12.002.
Housset M, Maulard C, Chretien Y, et al. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(11):2150–7. https://doi.org/10.1200/JCO.1993.11.11.2150.
Sauer R, Dunst J, Altendorf-Hofmann A, Fischer H, Bornhof C, Schrott KM. Radiotherapy with and without cisplatin in bladder cancer. Int J Radiat Oncol Biol Phys. 1990;19(3):687–91. https://doi.org/10.1016/0360-3016(90)90497-8.
Shipley WU, Prout GRJ, Einstein AB, et al. Treatment of invasive bladder cancer by cisplatin and radiation in patients unsuited for surgery. JAMA. 1987;258(7):931–5.
Kaufman DS, Shipley WU, Griffin PP, Heney NM, Althausen AF, Efird JT. Selective bladder preservation by combination treatment of invasive bladder cancer. N Engl J Med. 1993;329(19):1377–82. https://doi.org/10.1056/NEJM199311043291903.
Tester W, Porter A, Asbell S, et al. Combined modality program with possible organ preservation for invasive bladder carcinoma: results of RTOG protocol 85-12. Int J Radiat Oncol Biol Phys. 1993;25(5):783–90. https://doi.org/10.1016/0360-3016(93)90306-g.
Tester W, Caplan R, Heaney J, et al. Neoadjuvant combined modality program with selective organ preservation for invasive bladder cancer: results of Radiation Therapy Oncology Group phase II trial 8802. J Clin Oncol Off J Am Soc Clin Oncol. 1996;14(1):119–26. https://doi.org/10.1200/JCO.1996.14.1.119.
Kaufman DS, Winter KA, Shipley WU, et al. The initial results in muscle-invading bladder cancer of RTOG 95-06: phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5(6):471–6. https://doi.org/10.1634/theoncologist.5-6-471.
Hagan MP, Winter KA, Kaufman DS, et al. RTOG 97-06: initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int J Radiat Oncol Biol Phys. 2003;57(3):665–72. https://doi.org/10.1016/s0360-3016(03)00718-1.
Kaufman DS, Winter KA, Shipley WU, et al. Phase I-II RTOG study (99-06) of patients with muscle-invasive bladder cancer undergoing transurethral surgery, paclitaxel, cisplatin, and twice-daily radiotherapy followed by selective bladder preservation or radical cystectomy and adjuvant chemotherapy. Urology. 2009;73(4):833–7. https://doi.org/10.1016/j.urology.2008.09.036.
Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol Off J Am Soc Clin Oncol. 2014;32(34):3801–9. https://doi.org/10.1200/JCO.2014.57.5548.
Oh KS, Soto DE, Smith DC, Montie JE, Lee CT, Sandler HM. Combined-modality therapy with gemcitabine and radiation therapy as a bladder preservation strategy: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys. 2009;74(2):511–7. https://doi.org/10.1016/j.ijrobp.2008.08.021.
Hall E, Hussain SA, Porta N, et al. BC2001 long-term outcomes: a phase III randomized trial of chemoradiotherapy versus radiotherapy (RT) alone and standard RT versus reduced high-dose volume RT in muscle-invasive bladder cancer. J Clin Oncol. 2017;35(6_suppl):280. https://doi.org/10.1200/JCO.2017.35.6_suppl.280.
Malmström PU, Rintala E, Wahlqvist R, Hellström P, Hellsten S, Hannisdal E. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial I. The Nordic Cooperative Bladder Cancer Study Group. J Urol. 1996;155(6):1903–6.
Martinez-Piñeiro JA, Gonzalez Martin M, Arocena F, et al. Neoadjuvant cisplatin chemotherapy before radical cystectomy in invasive transitional cell carcinoma of the bladder: a prospective randomized phase III study. J Urol. 1995;153(3 Pt 2):964–73.
Peyton CC, Tang D, Reich RR, et al. Downstaging and survival outcomes associated with neoadjuvant chemotherapy regimens among patients treated with cystectomy for muscle-invasive bladder cancer. JAMA Oncol. 2018;4(11):1535–42. https://doi.org/10.1001/jamaoncol.2018.3542.
Wallace DM, Raghavan D, Kelly KA, et al. Neo-adjuvant (pre-emptive) cisplatin therapy in invasive transitional cell carcinoma of the bladder. Br J Urol. 1991;67(6):608–15. https://doi.org/10.1111/j.1464-410x.1991.tb15225.x.
Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. International collaboration of trialists. Lancet (London, England). 1999;354(9178):533–40.
Hussain SA, Porta N, Hall E, et al. Outcomes in patients with muscle-invasive bladder cancer treated with neoadjuvant chemotherapy followed by (chemo)radiotherapy in the BC2001 trial. Eur Urol. 2021;79(2):307–15. https://doi.org/10.1016/j.eururo.2020.11.036.
Horwich A, Dearnaley D, Huddart R, et al. A randomised trial of accelerated radiotherapy for localised invasive bladder cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2005;75(1):34–43. https://doi.org/10.1016/j.radonc.2004.11.003.
Näslund I, Nilsson B, Littbrand B. Hyperfractionated radiotherapy of bladder cancer. A ten-year follow-up of a randomized clinical trial. Acta Oncol. 1994;33(4):397–402. https://doi.org/10.3109/02841869409098435.
Stuschke M, Thames HD. Hyperfractionated radiotherapy of human tumors: overview of the randomized clinical trials. Int J Radiat Oncol Biol Phys. 1997;37(2):259–67. https://doi.org/10.1016/s0360-3016(96)00511-1.
Pos FJ, Hart G, Schneider C, Sminia P. Radical radiotherapy for invasive bladder cancer: what dose and fractionation schedule to choose? Int J Radiat Oncol Biol Phys. 2006;64(4):1168–73. https://doi.org/10.1016/j.ijrobp.2005.09.023.
Choudhury A, Porta N, Hall E, et al. Hypofractionated radiotherapy in locally advanced bladder cancer: an individual patient data meta-analysis of the BC2001 and BCON trials. Lancet Oncol. 2021;22(2):246–55. https://doi.org/10.1016/S1470-2045(20)30607-0.
Overgaard J, Horsman MR. Modification of hypoxia-induced radioresistance in tumors by the use of oxygen and sensitizers. Semin Radiat Oncol. 1996;6(1):10–21. https://doi.org/10.1053/SRAO0060010.
Hoskin PJ, Saunders MI, Phillips H, et al. Carbogen and nicotinamide in the treatment of bladder cancer with radical radiotherapy. Br J Cancer. 1997;76(2):260–3. https://doi.org/10.1038/bjc.1997.372.
Hoskin PJ, Rojas AM, Bentzen SM, Saunders MI. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol Off J Am Soc Clin Oncol. 2010;28(33):4912–8. https://doi.org/10.1200/JCO.2010.28.4950.
Song YP, Mistry H, Choudhury A, Hoskin P. Long-term outcomes of hypoxia modification in bladder preservation: update from BCON trial. J Clin Oncol. 2019;37(7_suppl):356. https://doi.org/10.1200/JCO.2019.37.7_suppl.356.
Eustace A, Irlam JJ, Taylor J, et al. Necrosis predicts benefit from hypoxia-modifying therapy in patients with high risk bladder cancer enrolled in a phase III randomised trial. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2013;108(1):40–7. https://doi.org/10.1016/j.radonc.2013.05.017.
Yang L, Taylor J, Eustace A, et al. A gene signature for selecting benefit from hypoxia modification of radiotherapy for high-risk bladder cancer patients. Clin cancer Res an Off J Am Assoc Cancer Res. 2017;23(16):4761–8. https://doi.org/10.1158/1078-0432.CCR-17-0038.
Matulay JT, Kamat AM. Advances in risk stratification of bladder cancer to guide personalized medicine. F1000Research. 2018;7. https://doi.org/10.12688/f1000research.14903.1.
Robertson AG, Kim J, Al-Ahmadie H, et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell. 2017;171(3):540–556.e25. https://doi.org/10.1016/j.cell.2017.09.007.
Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152–65. https://doi.org/10.1016/j.ccr.2014.01.009.
Mitra AP. Molecular substratification of bladder cancer: moving towards individualized patient management. Ther Adv Urol. 2016;8(3):215–33. https://doi.org/10.1177/1756287216638981.
Choudhury A, Nelson LD, Teo MTW, et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010;70(18):7017–26. https://doi.org/10.1158/0008-5472.CAN-10-1202.
Kamoun A, de Reyniès A, Allory Y, et al. A consensus molecular classification of muscle-invasive bladder cancer. Eur Urol. 2020;77(4):420–33. https://doi.org/10.1016/j.eururo.2019.09.006.
Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76. https://doi.org/10.1016/S0140-6736(16)32455-2.
Grivas P. DNA damage response gene alterations in urothelial cancer: ready for practice? Clin Cancer Res an Off J Am Assoc Cancer Res. 2019;25(3):907–9. https://doi.org/10.1158/1078-0432.CCR-18-2512.
Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA Cancer J Clin. 2020;70(5):404–23. https://doi.org/10.3322/caac.21631.
Cuellar MA, Medina A, Girones R, et al. Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy as bladder-sparing therapy in patients with localized muscle invasive bladder cancer: a SOGUG study. J Clin Oncol. 2020;38(15_suppl):TPS5097-TPS5097. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS5097.
Balar AV, James ND, Shariat SF, et al. Phase III study of pembrolizumab (pembro) plus chemoradiotherapy (CRT) versus CRT alone for patients (pts) with muscle-invasive bladder cancer (MIBC): KEYNOTE-992. J Clin Oncol. 2020;38(15_suppl):TPS5093-TPS5093. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS5093.
Jiang DM, Chung P, Kulkarni GS, Sridhar SS. Trimodality therapy for muscle-invasive bladder cancer: recent advances and unanswered questions. Curr Oncol Rep. 2020;22(2):14. https://doi.org/10.1007/s11912-020-0880-5.
Singh P, Tangen C, Efstathiou JA, et al. INTACT: phase III randomized trial of concurrent chemoradiotherapy with or without atezolizumab in localized muscle invasive bladder cancer—SWOG/NRG1806. J Clin Oncol. 2020;38(6_suppl):TPS586-TPS586. https://doi.org/10.1200/JCO.2020.38.6_suppl.TPS586.
Maciejewski B, Majewski S. Dose fractionation and tumour repopulation in radiotherapy for bladder cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1991;21(3):163–70. https://doi.org/10.1016/0167-8140(91)90033-d.
Moonen L, vd Voet H, de Nijs R, Horenblas S, Hart AA, Bartelink H. Muscle-invasive bladder cancer treated with external beam radiation: influence of total dose, overall treatment time, and treatment interruption on local control. Int J Radiat Oncol Biol Phys. 1998;42(3):525–30. https://doi.org/10.1016/s0360-3016(98)00263-6.
Flaig TW, Spiess PE, Agarwal N, et al. Bladder cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(3):329–54. https://doi.org/10.6004/jnccn.2020.0011.
Witjes JA, Bruins M, Cathomas R, et al. European Association of Urology guidelines. 2020 Edition. In: Vol presented. European Association of Urology Guidelines Office. 2020. http://uroweb.org/guideline/bladder-cancer-muscle-invasive-and-metastatic/.
Kassam SN, Aziz Z, Hung LS, Sridhar SS. A case of isolated rectal recurrence of muscle invasive bladder cancer. Can Urol Assoc J = J l’Association des Urol du Canada. 2013;7(5–6):E376–80. https://doi.org/10.5489/cuaj.1223.
Reshko LB, Richardson MK, Spencer K, Kersh CR. Stereotactic body radiation therapy (SBRT) in pelvic lymph node oligometastases. Cancer Invest. 2020;38(10):599–607. https://doi.org/10.1080/07357907.2020.1801713.
Wallmeroth A, Wagner U, Moch H, Gasser TC, Sauter G, Mihatsch MJ. Patterns of metastasis in muscle-invasive bladder cancer (pT2-4): an autopsy study on 367 patients. Urol Int. 1999;62(2):69–75. https://doi.org/10.1159/000030361.
Joshi M, Kim S, Solanki AA, et al. EA8185: Phase II study of bladder-sparing chemoradiation (chemoRT) with durvalumab in clinical stage III, node-positive urothelial carcinoma (INSPIRE), ECOG-ACRIN/nrg collaboration. J Clin Oncol. 2021;39(6):TPS500
Haque W, Verma V, Butler EB, Teh BS. Chemotherapy versus chemoradiation for node-positive bladder cancer: practice patterns and outcomes from the National Cancer Data Base. Bladder Cancer (Amsterdam, Netherlands). 2017;3(4):283–91. https://doi.org/10.3233/BLC-170137.
Smith AB, Jaeger B, Pinheiro LC, et al. Impact of bladder cancer on health-related quality of life. BJU Int. 2018;121(4):549–57. https://doi.org/10.1111/bju.14047.
Shih C, Porter MP. Health-related quality of life after cystectomy and urinary diversion for bladder cancer. Meng M V, ed. Adv Urol. 2011;2011:715892. https://doi.org/10.1155/2011/715892.
Modh RA, Mulhall JP, Gilbert SM. Sexual dysfunction after cystectomy and urinary diversion. Nat Rev Urol. 2014;11(8):445–53. https://doi.org/10.1038/nrurol.2014.151.
Gilbert SM, Wood DP, Dunn RL, et al. Measuring health-related quality of life outcomes in bladder cancer patients using the Bladder Cancer Index (BCI). Cancer. 2007;109(9):1756–62. https://doi.org/10.1002/cncr.22556.
Mak KS, Smith AB, Eidelman A, et al. Quality of life in long-term survivors of muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2016;96(5):1028–36. https://doi.org/10.1016/j.ijrobp.2016.08.023.
Henningsohn L, Wijkström H, Dickman PW, Bergmark K, Steineck G. Distressful symptoms after radical radiotherapy for urinary bladder cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2002;62(2):215–25. https://doi.org/10.1016/s0167-8140(01)00455-8.
Zietman AL, Sacco D, Skowronski U, et al. Organ conservation in invasive bladder cancer by transurethral resection, chemotherapy and radiation: results of a urodynamic and quality of life study on long-term survivors. J Urol. 2003;170(5):1772–6. https://doi.org/10.1097/01.ju.0000093721.23249.c3.
Kachnic LA, Shipley WU, Griffin PP, et al. Combined modality treatment with selective bladder conservation for invasive bladder cancer: long-term tolerance in the female patient. Cancer J Sci Am. 1996;2(2):79–84.
Efstathiou JA, Bae K, Shipley WU, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol Off J Am Soc Clin Oncol. 2009;27(25):4055–61. https://doi.org/10.1200/JCO.2008.19.5776.
Freilich JM, Spiess PE, Biagioli MC, et al. Lipiodol as a fiducial marker for image-guided radiation therapy for bladder cancer. Int Braz J Urol. 2014;40(2):190–7. https://doi.org/10.1590/S1677-5538.IBJU.2014.02.08.
Kibrom AZ, Knight KA. Adaptive radiation therapy for bladder cancer: a review of adaptive techniques used in clinical practice. J Med Radiat Sci. 2015;62(4):277–85. https://doi.org/10.1002/jmrs.129.
Tunio MA, Hashmi A, Qayyum A, Mohsin R, Zaeem A. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: single-institution experience. Int J Radiat Oncol Biol Phys. 2012;82(3):e457–62. https://doi.org/10.1016/j.ijrobp.2011.05.051.
Foroudi F, Wilson L, Bressel M, et al. A dosimetric comparison of 3D conformal vs intensity modulated vs volumetric arc radiation therapy for muscle invasive bladder cancer. Radiat Oncol. 2012;7:111. https://doi.org/10.1186/1748-717X-7-111.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bajaj, A., Sachdev, S. (2022). Bladder-Sparing Approaches to Treatment of Muscle-Invasive Bladder Cancer. In: Stratton, K.L., Morgans, A.K. (eds) Urologic Oncology. Springer, Cham. https://doi.org/10.1007/978-3-030-89891-5_18
Download citation
DOI: https://doi.org/10.1007/978-3-030-89891-5_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-89890-8
Online ISBN: 978-3-030-89891-5
eBook Packages: MedicineMedicine (R0)