Abstract
All the processes described in this textbook require energy. Ample clinical evidence indicates that the brain is exquisitely sensitive to perturbations of energy metabolism. This chapter, adapted from Magistretti PJ (2008) Brain energy metabolism. In: Squire LR, Berg D, Bloom FE, du Lac S, Ghosh A, Spitzer NC (eds) Fundamental neuroscience. Academic, San Diego, pp 271–293, covers the topics of energy delivery, production, and utilization by the brain. Careful consideration of the basic mechanisms of brain energy metabolism is an essential prerequisite to a full understanding of the physiology and pathophysiology of brain function. Abnormalities in brain energy metabolism are observed in a variety of pathological conditions such as neurodegenerative diseases, stroke, epilepsy, and migraine. The chapter reviews the features of brain energy metabolism at the global, regional, and cellular levels and extensively describes recent advances in the understanding of neuroglial metabolic cooperation. A particular focus is the cellular and molecular mechanisms that tightly couple neuronal activity to energy consumption. This tight coupling is at the basis of functional brain-imaging techniques, such as positron emission tomography (PET) and functional magnetic resonance imaging.
Parts of this chapter were originally published in Magistretti P.J. (2008). Brain Energy Metabolism. In Fundamental Neuroscience, 3rd edition, L.R. Squire, D. Berg, F.E. Bloom, S. du Lac, A. Ghosh, and N.C. Spitzer, eds. (San Diego: Academic Press), pp. 271–293, Copyright Elsevier, used with permission.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Astrocyte-neuron metabolic unit
- Blood flow
- Brain
- Brain energy metabolism
- Cell-specific glucose uptake and metabolism
- Glial cells
- Glucose
- Glucose metabolism
- Glucose transporters (GLUTs)
- Glutamate
- Glutamate-stimulated uptake
- Glycogen
- Glycogen metabolism
- Glycolysis
- Ketone bodies
- Lactate
- Lactate and pyruvate serve
- NADPH
- Neurotransmitters
- Pentose phosphate pathway
- Tight coupling
- Tricarboxylic acid cycle
- Vascular endothelial cells
Introduction
Ample clinical evidence indicates that the brain is exquisitely sensitive to perturbations of energy metabolism. This chapter, adapted from Magistretti (2008), covers the topics of energy delivery, production, and utilization by the brain. Careful consideration of the basic mechanisms of brain energy metabolism is an essential prerequisite to a full understanding of the physiology and pathophysiology of brain function. Abnormalities in brain energy metabolism are observed in a variety of pathological conditions such as neurodegenerative diseases, stroke, epilepsy, and migraine. The chapter reviews the features of brain energy metabolism at the global, regional, and cellular levels and extensively describes recent advances in the understanding of neuroglial metabolic cooperation. A particular focus is the cellular and molecular mechanisms that tightly couple neuronal activity to energy consumption. This tight coupling is at the basis of functional brain-imaging techniques, such as positron emission tomography (PET) and functional magnetic resonance imaging.
Energy Metabolism of the Brain as a Whole Organ
Glucose Is the Main Energy Substrate for the Brain
The human brain constitutes only 2% of the body weight, yet the energy-consuming processes that ensure proper brain function account for approximately 25% of total body glucose utilization. With a few exceptions that will be reviewed later, glucose is the obligatory energy substrate of the brain. In any tissue, glucose can follow various metabolic pathways; in the brain, glucose is almost entirely oxidized to CO2 and water through its sequential processing by glycolysis (Fig. 1), the tricarboxylic acid (TCA) cycle (Fig. 2), and the associated oxidative phosphorylation, which yield, on a molar basis, between 30 and 36 ATPs per glucose, depending on the coupling efficiency of oxidative phosphorylation. Indeed, the oxygen consumption of the brain, which accounts for almost 20% of the oxygen consumption of the whole organism, is 160 mmol per 100 g of brain weight per minute and roughly corresponds to the value determined for CO2 production. This O2/CO2 relation corresponds to what is known in metabolic physiology as a respiratory quotient of nearly 1 and demonstrates that carbohydrates, and glucose in particular, are the exclusive substrates for oxidative metabolism. While detailed calculations of the precise rates of glucose utilization can be done, the central point here is that glucose is an essential constituent of macromolecules such as glycolipids and glycoproteins present in neural cells. Also, note that glucose enters the metabolic pathways that result in the synthesis of three key neurotransmitters of the brain: glutamate, GABA, and acetylcholine.
Glycolysis (Embden-Meyerhof pathway). Glucose phosphorylation is regulated by hexokinase, an enzyme inhibited by glucose 6-phosphate. Glucose must be phosphorylated to glucose 6-phosphate to enter glycolysis or to be stored as glycogen. Two other important steps in the regulation of glycolysis are catalyzed by phosphofructokinase and pyruvate kinase. Their activity is controlled by the levels of high-energy phosphates, as well as of citrate and acetyl-CoA. Pyruvate, through lactate dehydrogenase, is in dynamic equilibrium with lactate. This reaction is essential to regenerate NAD+ residues necessary to sustain glycolysis downstream of glyceraldehyde 3-phosphate. PCr phosphocreatine (Redrawn based on Magistretti 2008)
Tricarboxylic acid cycle (TCA cycle) and oxidative phosphorylation. Pyruvate entry into the cycle is controlled by pyruvate dehydrogenase activity that is inhibited by ATP and NADH. Two other regulatory steps in the cycle are controlled by isocitrate and α-ketoglutarate dehydrogenases, whose activities are controlled by the levels of high-energy phosphates (Redrawn based on Magistretti 2008)
Ketone Bodies Become Energy Substrates for the Brain in Particular Circumstances
In particular circumstances, substrates other than glucose can be utilized by the brain. For example, breast-fed neonates have the capacity to utilize the ketone bodies acetoacetate (AcAc) and D-3-hydroxybutyrate (3-HB), in addition to glucose, as energy substrates for the brain. This capacity is an interesting example of a developmentally regulated adaptive mechanism because maternal milk is highly enriched in lipids, resulting in a lipid-to-carbohydrate ratio much higher than that present in postweaning nutrients. Indeed, lipids account for approximately 55% of the total calories contained in human milk, in contrast with 30–35% for a balanced postweaning diet. Another consideration regarding the lipid-rich diet provided during the suckling period relates to its contribution to the process of myelination. The question is whether the polar lipids and cholesterol that make up myelin are derived from dietary sources or are synthesized within the brain. Evidence shows that brain lipids can be synthesized from blood-borne precursors such as ketone bodies. In addition, when suckling rats are fed a diet low in ketones, carbon atoms for lipogenesis can also be provided by glucose. To summarize, ketone bodies and AcAc are energy substrates, as well as precursors for lipogenesis during the suckling period; however, the developing brain appears to be metabolically quite flexible because glucose, in addition to its energetic function, can be metabolized to generate substrates for lipid synthesis.
Starvation and diabetes are two situations in which the availability of glucose to tissues is inadequate and in which plasma ketone bodies are elevated because of enhanced lipid catabolism. Under these conditions, the adaptive mechanisms described for breast-fed neonates become operative in the brain, allowing it to utilize AcAc or 3-HB as energy substrates.
Lactate and Pyruvate Serve as Instructive Cases
Apart from ketone bodies, lactate and pyruvate represent other alternative energy substrates to glucose for the brain. These two monocarboxylates can readily enter the cells through specialized monocarboxylate transporters (MCTs) and can be metabolized in the mitochondria through the TCA cycle and the oxidative phosphorylation to produce energy in the form of ATP (Fig. 2). In vitro and ex vivo data have provided extensive demonstration that lactate and pyruvate can sustain neuronal function, i.e., synaptic activity, even in the absence of glucose. In line with this, both monocarboxylates can protect neuron from cell damage/death in conditions of energy deprivation such as in hypoglycemia or during ischemia.
It was initially thought that the permeability of the blood–brain barrier to monocarboxylates was limited suggesting that circulating lactate or pyruvate could not represent important alternative substrates to glucose for the brain in vivo. The demonstration of the expression of MCTs on intraparenchymal brain capillaries along with magnetic resonance spectroscopy (MRS) experiments has led to the reevaluation of the importance of circulating monocarboxylates usage in brain energy metabolism. It is now estimated that under basal plasma lactate condition (≈1.0 mM), lactate is taken up by the human brain where it is fully oxidized accounting to up to 8–10% of its energy requirements. This contribution could even be greater at supraphysiological lactate concentrations (i.e., following intravenous lactate infusion) with a near linear relationship. Increases in blood lactate concentration are observed in different physiological conditions such as during physical exercise, implying that lactate can readily be used by the brain as an alternative energy substrate in such specific conditions. In particular, during moderate-to-vigorous exercises, which results in blood lactate concentration from about 3 mM to up to at least 10 mM, the human brain takes up and oxidizes even more lactate than in normal conditions (which could cover up to 20–25% of the total brain energy demand), and this at the expense of blood glucose utilization. Overall these data demonstrate that plasma lactate can be an energy substrate for the human brain. In addition, when formed within the brain parenchyma from glucose that has crossed the blood–brain barrier, lactate may become the preferential energy substrates for activated neurons (see below).
Summary
Glucose is the obligatory energy substrate for brain, and it is almost entirely oxidized to CO2 and H2O. This simple statement summarizes, with few exceptions, over four decades of careful studies of brain energy metabolism at organ and regional levels. Under ketogenic conditions, such as starvation and diabetes and during breast-feeding, ketone bodies may provide an energy source for the brain. Lactate, formed from glucose within the brain parenchyma or imported from the circulation, is an adequate energy substrate as well.
Tight Coupling of Neuronal Activity, Blood Flow, and Energy Metabolism
A striking characteristic of the brain is its high degree of structural and functional specialization. Thus, when we move an arm, motor areas and their related pathways are activated selectively; intuitively, one can predict that as “brain work” increases locally (e.g., in motor areas), the energy requirements of the activated regions will increase in a temporally and spatially coordinated manner. Because energy substrates are provided through the circulation, blood flow should increase in the modality-specific activated area. More than a century ago, the British neurophysiologist Charles Sherrington showed, in experimental animals, increases in blood flow localized to the parietal cortex in response to sensory stimulation. He postulated that “the brain possesses intrinsic mechanisms by which its vascular supply can be varied locally in correspondence with local variations of functional activity.” With remarkable insight, he also proposed that “chemical products of cerebral metabolism” produced in the course of neuronal activation could provide the mechanism to couple activity with increased blood flow.
Some Mechanisms Couple Neuronal Activity to Blood Flow
Since Sherrington’s seminal work, the search for the identification of chemical mediators that can couple neuronal activity with local increases in blood flow has been intense. These signals can be broadly grouped into two categories: (1) molecules or ions that transiently accumulate in the extracellular space after neuronal activity and (2) specific neurotransmitters that mediate the coupling in anticipation or at least in parallel with local activation (neurogenic mechanisms). The increases in extracellular K+, adenosine, and lactate and the related changes in pH are all a consequence of increased neuronal activity, and all have been considered mediators of neurovascular coupling because of their vasoactive effects. However, the spatial and temporal resolution achieved by these mediators may not be sufficient to entirely account for the activity-dependent coupling between neuronal activity and blood flow. Indeed, these vasoactive agents are formed with a certain delay (seconds) after the initiation of neuronal activity and can diffuse at considerable distance. In this respect, neurogenic mechanisms appear to be better fitted. An attractive addition to the list of potential mediators for coupling neuronal activity to blood flow is nitric oxide (NO). Indeed, NO is an ideal candidate; it is formed locally by neurons and glial cells under the action of a variety of neurotransmitters likely to be released by depolarized afferents to an activated brain area. Nitric oxide is a diffusible and potent vasodilator whose short half-life spatially and temporally restricts its domain of action. However, in several experimental models in which the activity of NO synthase, the enzyme responsible for NO synthesis, was inhibited, a certain degree of coupling was still observed, indicating that NO is probably only one of the regulators of local blood flow acting in synergy with others.
Several products of activity-dependent neuronal and glial metabolism such as lactate, H+, adenosine, prostanoids, and K+ have vasoactive effects and are therefore putative mediators of coupling, although the kinetics and spatial resolution of this mode do not account for all the observed phenomena. As attractive as it is, an exclusively neurogenic mode of coupling neuronal activity to blood flow is unlikely and, moreover, still awaits firm functional confirmation in vivo. Nitric oxide is undoubtedly a key element in coupling, particularly in view of the fact that glutamate, the principal excitatory neurotransmitter, triggers a receptor-mediated NO formation in neurons and glia; this is consistent with the view that whenever a functionally defined brain area is activated and glutamate is released by the depolarized afferents, NO may be formed, thus providing a direct mechanism contributing to the coupling between activity and local increases in blood flow. Astrocytes appear to function as intermediary processor in neurovascular coupling.
Through the activity-linked increase in blood flow, more substrates, namely, glucose and oxygen, necessary to meet the additional energy demands are delivered to the activated area per unit time. The cellular and molecular mechanisms involved in oxygen consumption and glucose utilization are treated in a later section.
Blood Flow and Energy Metabolism Can Be Visualized in Humans
Modern functional brain-imaging techniques enable the in vivo monitoring of human blood flow and the two indices of energy metabolism: glucose utilization and oxygen consumption. For instance, with the use of PET and appropriate positron-emitting isotopes such as 18F and 15O, basal rates, as well as activity-related changes in local blood flow or oxygen consumption, can be studied using 15O-labeled water or 15O2, respectively. Local rates of glucose utilization [also defined as local cerebral metabolic rates for glucose (LCMRglu)] can be determined with 18F-labeled 2-deoxyglucose (2-DG). The use of 2-DG as a marker of LCMRglu was pioneered by Louis Sokoloff and associates at the National Institutes of Health, first in laboratory animals. The method is based on the fact that 2-DG crosses the blood–brain barrier, is taken up by brain cells, and is phosphorylated by hexokinase with kinetics similar to that for glucose; however, unlike glucose 6-phosphate, 2-deoxyglucose 6-phosphate cannot be metabolized further and therefore accumulates intracellularly (Fig. 3).
Structure and metabolism of glucose and 2-deoxyglucose (2-DG). 2-DG is transported into cells through glucose transporters and phosphorylated by hexokinase to 2-DG-6-phosphate without significant further processing or dephosphorylation back to glucose. Therefore, when labeled radioactively, 2-DG used in tracer concentrations is a valuable marker of glucose uptake and phosphorylation, which directly indicates glucose utilization (Redrawn based on Magistretti 2008)
We must note that with the use of specific stimulation paradigms, the combined PET analysis of local cerebral blood flow (LCBF) and local oxygen consumption (LCMRO2), in addition to LCMRglu, has revealed a unique and unexpected feature of human brain energy metabolism regulation. The canonical view was that the three metabolic parameters were tightly coupled, implying that, if, for example, CBF increased locally during physiological activation, LCMRglu and LCMRO2 would increase in parallel. In what is now referred to as the phenomenon of “uncoupling,” physiological stimulation of the visual system increases LCBF and LCMRglu in the primary visual cortex without a commensurate increase in LCMRO2, indicating that the additional glucose utilized during neuronal activation might be processed through glycolysis rather than through the tricarboxylic acid (TCA) cycle and oxidative phosphorylation, yielding lactate. Consistent with this prediction, a transient increase in the lactate signal is detected with 1H magnetic resonance imaging spectroscopy in the human primary visual cortex during appropriate visual stimulation. The phenomenon of uncoupling has been confirmed in other cortical areas as well, although its magnitude may differ depending on the modality, and may actually be absent in certain cases.
Summary
Under normal conditions, glucose is virtually the sole energy substrate for the brain and that it is entirely oxidized. New techniques that allow imaging of the three fundamental parameters of brain energy metabolism, namely, blood flow, oxygen consumption, and glucose utilization, provide a more refined level of spatial resolution and demonstrate that brain energy metabolism is regionally heterogeneous and is coupled tightly to the functional activation of specific neuronal pathways.
Energy-Producing and Energy-Consuming Processes in the Brain
What are the cellular and molecular mechanisms that underlie the regulation of brain energy metabolism revealed by the foregoing studies at global and regional levels? In particular, what are the metabolic events taking place in the cell types that make up the brain parenchyma? How is it possible to reconcile whole organ studies indicating complete oxidation of glucose with transient activation-induced glycolysis at the regional level? These and other related questions will be addressed here and in the next sections.
Glucose Metabolism Produces Energy
Before moving on to an analysis of the cell-specific mechanisms of brain energy metabolism, it seems appropriate to briefly review some basic aspects of the energy balance of the brain. Because glucose, in normal circumstances, is the main energy substrate of the brain, the overview will be restricted to its metabolic pathways. Glucose metabolism in the brain is similar to that in other tissues and includes the following principal metabolic pathways: (1) glycolysis occurring in the cytosol, which is associated with the pentose phosphate pathway and glucose storage in the form of glycogen (in astrocytes only, see below); (2) the TCA cycle and oxidative phosphorylation, which take place into the mitochondria.
Glycolysis Is the First Step
Glycolysis (Embden-Meyerhof pathway) is the metabolism of glucose to pyruvate (Fig. 1). It results in the net production of only two molecules of ATP per glucose molecule; indeed, four ATPs are formed in the processing of glucose to pyruvate, but two ATPs are consumed as well (Fig. 1). Under anaerobic conditions, pyruvate is converted into lactate, allowing the regeneration of nicotinamide adenine dinucleotide (NAD+), which is essential to maintain a continued glycolytic flux. Indeed, if NAD+ were not regenerated, glycolysis could not proceed beyond glyceraldehyde 3-phosphate (Fig. 1). Another situation in which the end product of glycolysis is lactate rather than pyruvate is when oxygen consumption does not match glucose utilization, implying that the rate of pyruvate production through glycolysis exceeds pyruvate oxidation by the TCA cycle (see Fig. 2). This condition has been well described in skeletal muscle during intense exercise.
The Tricarboxylic Acid Cycle Produces ATP
Under aerobic conditions, pyruvate is oxidatively decarboxylated to yield acetyl-CoA in a reaction catalyzed by the enzyme pyruvate dehydrogenase (PDH). Acetyl-coenzyme A condenses with oxaloacetate to produce citrate (Fig. 2). This is the first step of the tricarboxylic acid cycle, in which three pairs of electrons are transferred from NAD+ to NADH and one pair from flavin adenine dinucleotide (FAD) to its reduced form (FADH2) – through four oxidation-reduction steps (Fig. 2). NADH and FADH2 transfer their electrons to molecular O2 through the mitochondrial electron transfer chain to produce ATP in the process of oxidative phosphorylation.
The Pentose Phosphate Pathway Produces NADPH
Glycolysis, the TCA cycle, and oxidative phosphorylation produce ATP using glucose as a fuel, but ATP is not the only form of metabolic energy. In the cells of the brain, as in other organs, NADPH provides the reducing power for several biosynthetic reactions in which the precursors are in a more oxidated state than the products. This is the case for the reductive synthesis of free fatty acids from acetyl-CoA, which are components of myelin and of other structural elements of neural cells, such as the plasma membrane.
The processing of glucose through the pentose phosphate pathway produces NADPH. The first reaction in the pentose phosphate pathway is the conversion of glucose 6-phosphate into ribulose 5-phosphate (Fig. 4). This dehydrogenation, in which two molecules of NADPH are generated per molecule of glucose 6-phosphate, is the rate-limiting step of the pentose phosphate pathway. The NADP/NADPH ratio is the single most important factor regulating the entry of glucose 6-phosphate into the pentose phosphate pathway. Thus, if a high reducing power is needed, NADPH levels decrease and the pentose phosphate pathway is activated to generate new reducing equivalents.
The pentose phosphate pathway. In the oxidative branch of the pentose phosphate pathway, two NADPH are generated per glucose 6-phosphate. The first rate-limiting reaction of the pathway is catalyzed by glucose-6-phosphate dehydrogenase; the second NADPH is generated through the oxidative decarboxylation of 6-phosphogluconate, a reaction catalyzed by glucose-6-phosphogluconate dehydrogenase. The nonoxidative branch of the pentose phosphate pathway provides a reversible link with glycolysis by regenerating the two glycolytic intermediates glyceraldehyde 3-phosphate and fructose 6-phosphate. This regeneration is achieved through three sequential reactions. In the first, catalyzed by transketolase, xylulose 5-phosphate and ribose 5-phosphate (which originate from ribulose 5-phosphate, the end product of the oxidative branch) yield glyceraldehyde 3-phosphate and sedoheptulose 7-phosphate. Under the action of transaldolase, these two intermediates yield fructose 6-phosphate and erythrose 4-phosphate. This latter intermediate combines with xylulose 5-phosphate, in a reaction catalyzed by transketolase, to yield fructose 6-phosphate and glyceraldehyde 3-phosphate. Thus, through the nonoxidative branch of the pentose phosphate pathway, two hexoses (fructose 6-phosphate) and one triose (glyceraldehyde 3-phosphate) of the glycolytic pathway are regenerated from three pentoses (ribulose 5-phosphate) (Redrawn based on Magistretti 2008)
NADPH and Glutathione Protect Against Oxidative Damage by Reactive Oxygen Species
Oxidative metabolism, which is so essential to cell viability because of its generation of large amounts of the cellular fuel ATP, also creates potentially harmful by-products called reactive oxygen species (ROS). The superoxide radical anion (O2 − •), hydrogen peroxide (H2O2), and the hydroxy radical (HO•) are three ROS, generated by the transfer of single electrons to molecular oxygen during the oxidative metabolism of glucose taking place in the mitochondrial electron transfer chain. Reactive oxygen species are highly damaging to cells because they can cause DNA disruption and mutations, as well as activation of enzymatic cascades, including proteases and lipases that can eventually lead to cell death.
The coordinated activity of two molecules is essential in protecting cells against ROS-mediated damage, or oxidative stress: NADPH and glutathione. Scavenging of ROS is ensured by the sequential action of superoxide dismutase (SOD) and glutathione peroxidase (Fig. 5b). Two superoxide radical anions (O2 − •) are converted by SOD into the less reactive H2O2• Glutathione peroxidase then converts H2O2 into H2O and O2 at the expense of reduced glutathione, which is regenerated by glutathione reductase in the presence of NADPH. Interestingly, glucose metabolism provides ATP and NAD(P)H, the latter contributing to the neutralization of ROS, the harmful by-products of the process (oxidative phosphorylation) which produces the former.
(a) Metabolic interaction between astrocytes and neurons in the synthesis of glutathione. In astrocytes, glutathione (GSH) is synthesized from cysteine (Cys) (produced from cystine), glycine (Gly), and glutamate (Glu). GSH is released from astrocytes into the extracellular space; the membrane-bound astrocytic ectoenzyme, gamma-glutamyl transpeptidase (gamma-GT) releases the dipeptide CysGly, which along with glutamine (Gln also released by astrocytes and taken up by neurons to yield Glu) provides the precursors for neuronal GSH synthesis. (b) Enzymatic reactions for scavenging reactive oxygen species (ROS). The toxic superoxide anion (O2 − •) formed by a variety of physiological reactions, including oxidative phosphorylation (i.e., respiratory chain), is scavenged by superoxide dismutase (SOD), which converts the superoxide anion into hydrogen peroxide (H2O2) and molecular oxygen. Glutathione peroxidase (GPx) converts the still toxic hydrogen peroxide into water; reduced glutathione (GSH) is required for this reaction, in which it is converted into its oxidized form (GSSG). GSH is regenerated through the action of glutathione reductase (GR), a reaction requiring NADPH (Redrawn based on Magistretti 2008)
The metabolism of glutathione is tightly regulated and implies yet another example of neuron-astrocyte cooperation. Glutathione is a tripeptide (GSH; gamma-L-glutamyl-L-cysteinylglycine) synthesized through the concerted action of two enzymes, gammaGluCys synthase, which combines glutamate and cysteine to yield the dipeptide gammaGluCys, and glutathione synthase which adds a glycine to the dipeptide to yield GSH (Fig. 5a).
The glutathione content and reducing potential are considerably higher in astrocytes compared to neurons; this fact, combined with the much higher oxidative activity of neurons versus astrocytes, makes neurons more vulnerable to oxidative stress as well as highly dependent on astrocytes for their protection. Indeed, a cooperativity between astrocytes and neurons appears to exist for glutathione metabolism; astrocytes release GSH which is cleaved by the ectoenzyme gamma-GlutamylTranspeptidase (gamma-GT) which releases CysGly. The dipeptide is transported into neurons (note that neurons cannot take up GSH), providing two precursors for GSH synthesis glutamate; the third precursor of GSH is also provided by astrocytes to neurons under the form of glutamine, from which glutamate is produced through the action of glutaminase (Fig. 5a).
Several neurodegenerative disorders appear to involve a dysfunction in the ability of neural cells to control oxidative stress. For example, a familial form of Amyotrophic Lateral Sclerosis is due to a SOD mutation; evidence for a decrease in GSH content in the substantia nigra has been described in Parkinson’s disease.
Processes Linked to Neuronal Function Consume Energy
The main energy-consuming process of the brain is the maintenance of ionic gradients across the plasma membrane, a condition that is crucial for excitability. Maintenance of these gradients is achieved predominantly through the activity of ionic pumps fueled by ATP, particularly Na+, K+-ATPase, localized in neurons as well as in other cell types such as glia. Activity of these pumps accounts for approximately 50% of basal glucose oxidation in the nervous system. It is estimated that 80–85% of total energy consumed reflects the activity of glutamate-mediated neurotransmission and 10–15% reflects the energy requirements of resting potential maintenance. This value is in remarkable agreement with estimates made in vivo using MRS. When looking at the cellular energetic contribution to these processes, neurons represent by far the major energy-demanding cell type (80–85% of the brain energy requirements) whereas glia account for only 5–15% of the brain’s energy expenditure (Fig. 6).
Energy budget for the rodent central cortex. Relative rates of ATP consumption by resting neurons and glia (Adapted from Frackowiak et al. 2001)
In addition to the maintenance of ionic gradients that are disrupted during activity, other energy-consuming processes exist in neurons. Thus, the permanent synthesis of molecules needed for communications, such as neurotransmitters, or for general cellular purposes consumes energy. Axonal transport of molecules synthesized in the nucleus to their final destination along the axon or at the axon terminal is yet another process fueled by cellular energy metabolism.
Summary
Exactly as in other tissues, the metabolism of glucose, the main energy substrate of the brain, produces two forms of energy: ATP and NADPH. Glycolysis and the TCA cycle produce ATP, whereas energy in the form of reducing equivalents stored in the NADPH molecule is produced predominantly through the pentose phosphate pathway. Reduced glutathione provides a major defense against oxidative stress. Maintenance of the electrochemical gradients, particularly for Na+ and K+, needed for electrical signaling via the action potential and for chemical signaling through synaptic transmission is the main energy-consuming process of neural cells.
Brain Energy Metabolism at the Cellular Level
Glia and Vascular Endothelial Cells, in Addition to Neurons, Contribute to Brain Energy Metabolism
Neurons are the most energy-consuming cell type in the nervous system. However, it is now clear that other neural cells – astrocytes and vascular endothelial cells – not only consume energy but also play a crucial role in the flux of energy substrates to neurons. Arguments for such an active role for nonneuronal cells – in particular, astrocytes – are both quantitative and qualitative. Glial cells make up approximately half of the brain volume and astrocytes even outnumber neurons in the human brain. More compelling for the realization of the key role that astrocytes play in providing energy substrates to active neurons are the cytological relations that exist among brain capillaries, astrocytes, and neurons. These relations, which are illustrated in Fig. 7, are as follows. First, through specialized processes, called end feet, astrocytes surround brain capillaries. This implies that astrocytes form the first cellular barrier that glucose entering the brain parenchyma encounters and make them a likely site of prevalent glucose uptake and energy substrate distribution. More than a century ago, the Italian histologist Camillo Golgi and his pupil Luigi Sala sketched such a principle. In addition to perivascular end feet, astrocytes bear processes that ensheathe synaptic contacts. Astrocytes also express receptors and uptake sites with which neurotransmitters released during synaptic activity can interact. These features endow astrocytes with an exquisite sensitivity to detect increases in synaptic activity. In summary, because of the foregoing structural and unctional characteristics, astrocytes are ideally suited to couple local changes in neuronal activity with coordinated adaptations in energy metabolism (see Fig. 7).
Schematic representation of cytological relations existing among intraparenchymal capillaries, astrocytes, and the neuropil. Astrocyte processes surround capillaries (end feet) and ensheathe synapses. In addition, receptors and uptake sites for neurotransmitters are present on astrocytes. These features make astrocytes ideally suited to sense synaptic activity (a) and to couple it with uptake and metabolism of energy substrates originating from the circulation (b) (Redrawn based on Magistretti 2008)
A Tightly Regulated Glucose Metabolism Occurs in All Cell Types of the Brain, Neuronal and Nonneuronal
Given the high degree of cellular heterogeneity of the brain, understanding the relative role played by each cell type in the flux of energy substrates has largely depended on the availability of purified preparations, such as primary cultures enriched in neurons, astrocytes, or vascular endothelial cells. Such preparations have some drawbacks because they may not necessarily express all the properties of the cells in situ. In addition, one of the parameters of energy metabolism in vivo – namely, blood flow – cannot be examined in cultures. Despite these limitations, in vitro studies in primary cultures have proved very useful in identifying the cellular sites of glucose uptake and its subsequent metabolic fate, particularly, glycolysis and oxidative phosphorylation, thus providing illuminating correlations of two parameters of brain energy metabolism that are monitored in vivo: (1) glucose utilization and (2) oxygen consumption.
There Are Multiple Glucose Transporters in the Brain
Glucose enters the cell through specific glucose transporters (GLUTs). Until now as far as 12 different GLUTs have been identified (named GLUT1 to GLUT12), which exhibit different substrates affinities and kinetic properties. In the brain, multiple GLUTs are expressed in a predominantly cell-specific manner.
GLUT1 and 3 represent two major cerebral GLUTs. In the mammalian brain, GLUT1 is found in two different molecular weight forms (45 and 55 kDa) depending on its degree of glycosylation. The 55-kDa form of GLUT1 is essentially localized in brain microvessels, choroid plexus, and ependymal cells, thus representing the first barrier for the transport of circulating glucose into the brain parenchyma. In contrast to the 55-kDa form, the 45-kDa form of GLUT1 is localized predominantly in astrocytes (but also in other glials cells). As opposed to GLUT1, GLUT3 is the specific glucose transporter for neurons. It is widely expressed across the different neuronal populations with a cellular distribution predominating in the neuropil.
Among other GLUTs present in the brain, GLUT4 and 8 represent two other neuronal glucose transporters whose expression pattern, in contrast to GLUT3, is restricted to specific subpopulation of neurons.
Another glucose transporter, GLUT2, is expressed in both neurons and astrocytes in discrete brain areas, such as certain hypothalamic and brain stem nuclei, which participate in the regulation of feeding behavior and in the central control of insulin release. Considering its role a “glucose sensor” in the periphery, GLUT2-expressing cells may “sense” glucose levels and thus participate in the regulation of the above-described cerebral functions.
GLUT5 is localized to microglial cells, the resident macrophages of the brain, taking part in the immune and inflammatory responses of the nervous system. Noteworthily, considering its different substrates affinities and its role in peripheral tissues GLUT5 may function as a better transporter for fructose than for glucose.
Glucose uptake into the brain parenchyma and its use by different neural cells is a highly specified process regulated in a cell-specific manner by different glucose transporter subtypes (Fig. 8). Glucose enters the brain through 55-kDa GLUT1 transporters localized on endothelial cells of the blood–brain barrier. Uptake into astrocytes is mediated by the 45-kDa GLUT1 transporters, whereas GLUT3 transporters mediate this process in neurons. In parallel to this, glucose can be taken up by defined cell populations through the cell-specific expression of particular glucose transporters including GLUT2, 4, and 8. The implication in cerebral glucose homeostasis of other glucose transporters indentified in the brain, GLUT6 and 10, but whose cellular and regional distribution has not been clearly defined, remains to be established.
Cellular distribution of the principal glucose transporters in the nervous system (Redrawn based on Magistretti 2008)
Cell-Specific Glucose Uptake and Metabolism Has Been Studied Extensively
As we have seen, glucose utilization can be assessed with radioactively labeled 2-DG. To determine the cellular site of basal and activity-related glucose utilization, this technique has been applied to homogeneous cultures of astrocytes or neurons. For quantitative purposes and to allow comparisons with in vivo studies, these in vitro experiments, in which radioactive 2-DG is used as a tracer, must be conducted in a medium containing a concentration of glucose near that measured in vivo in the extracellular space of the brain (0.5–2 mM). The basal rate of glucose utilization is higher in astrocytes than in neurons, with values of about 20 and 6 nmol per milligram of protein per minute, respectively. These values are of the same order as those determined in vivo for cortical gray matter (10–20 nmol mg−1 min−1) with the 2-DG autoradiographic technique. In view of this difference and of the quantitative preponderance of astrocytes compared with neurons in the gray matter, these data reveal a significant contribution by astrocytes to basal glucose utilization as determined by 2-DG autoradiography or PET in vivo. Recent high-resolution microautoradiographic imaging ex vivo has indicated and approximately even distribution of 2-DG in neurons and astrocytes.
The contribution of astrocytes to glucose utilization during activation appears to be even more striking. In vitro activation can be mimicked by exposure of the cells to glutamate, the principal excitatory neurotransmitter, because, during activation of a given cortical area, the concentration of glutamate in the extracellular space increases considerably due to its release from the axon terminals of activated pathways. As shown in Fig. 9a, L-glutamate stimulates 2-DG uptake and phosphorylation by astrocytes in a concentration-dependent manner, with an EC50 of 60–80 μM. Unlike other actions of glutamate, stimulation of glucose utilization in astrocytes is mediated not by specific glutamate receptors, but by glutamate transporters. Indeed, in addition to the maintenance of extracellular K+ homeostasis, one of the well-established functions of astrocytes is to ensure the reuptake of certain neurotransmitters, particularly, that of glutamate at excitatory synapses. Five glutamate transporter subtypes have been cloned in various species, including humans. The EAAT-1 and EAAT-2 subtypes (GLAST and GLT-1 in rodents) are localized exclusively in astrocytes, whereas the EAAT-3 and −4 are predominantly localized in neurons, with a widespread distribution for EAAT-3 and a localized distribution to Purkinje neurons for EAAT-4. EAAT-5 is localized in rod photoreceptors and in bipolar cells of the retina. The density of EAAT-1 and −2 is particularly high on astrocytes that surround nerve terminals and dendritic spines, consistent with the prominent role of these transporters in the reuptake of synaptically released glutamate. The driving force for glutamate uptake through the specific transporters is the transmembrane Na+ gradient; indeed, glutamate is cotransported with Na+ in a ratio of one glutamate for every two or three Na+ ions. The selective loss of EAAT-2, the astrocyte-selective glutamate transporter, has been demonstrated in the motor cortex and spinal cord of patients who died of Amyotrophic Lateral Sclerosis, a neurodegenerative disease affecting motor neurons.
(a) Stimulation by glutamate of glucose uptake and phosphorylation in astrocytes. This effect is concentration dependent with an EC50 of ~80 μM. This process is dependent on sodium signaling associated with glutamate uptake and is energy consuming as the increase in sodium activates the Na+, K+-ATPase (see also Fig. 10). (b) Temporal coincidence in the increase in sodium concentration and ATP consumption triggered by glutamate in astrocytes. These processes, which are dependent on the activity of the Na+, K+-ATPase as they are ouabain sensitive, are the effectors of the glutamate-stimulated glycolysis in astrocytes (see also Fig. 10) (Taken from Magistretti and Chatton 2005)
Glutamate-Stimulated Uptake of Glucose by Astrocytes Is a Source of Insight into the Cellular Bases of 18F-2-DG PET In Vivo
The glutamate-stimulated uptake of glucose by astrocytes is a source of insight into the cellular bases of the activation-induced local increase in glucose utilization visualized with 18F-2-DG PET in vivo. As we have seen, focal physiological activation of specific brain areas is accompanied by increases in glucose utilization; because glutamate is released from excitatory synapses when neuronal pathways subserving specific modalities are activated, the stimulation by glutamate of glucose utilization in astrocytes provides a direct mechanism for coupling neuronal activity to glucose utilization in the brain (Fig. 9a). The intracellular molecular mechanism of this coupling requires Na+, K+-ATPase because ouabain, a powerful inhibitor of this pump, completely inhibits the glutamate-evoked 2-DG uptake by astrocytes. Because Na+, K+-ATPase responds to increases in intracellular Na+ (Nai+), the Na+, K+-ATPase is set to be activated when Nai+ rises concomitantly with glutamate uptake (as glutamate is cotransported with Na+, see previous section) (Fig. 9b). These observations indicate that a major determinant of glucose utilization is the activity of Na+, K+-ATPase. How does activation of Na+, K+-ATPase cause increased glucose utilization? The increase in pump activity consumes ATP (Fig. 9b), which is a negative modulator glycolysis (see Fig. 1). Thus, when ATP concentration is low, glycolysis is stimulated, resulting in increased glucose utilization. The activity of hexokinase, the enzyme responsible for glucose and 2-DG phosphorylation (see Fig. 3), is also increased under these conditions. This explains why the increase in glucose utilization, associated with the stimulation of Na+, K+-ATPase, can be monitored with 2-DG, which is not processed beyond the hexokinase step.
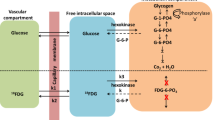
A compartmentalization of glucose uptake during activation has also been unequivocably found by Marco Tsacopoulos and colleagues in the honeybee drone retina. In this highly organized, crystal-like nervous tissue preparation, photoreceptor cells form rosette-like structures that are surrounded by glial cells. In addition, mitochondria are exclusively present in the photoreceptor neurons. Light activation reveals an increase in radioactive 2-DG uptake in the glial cells surrounding the rosettes but not in the photoreceptor neurons. An increase in O2 consumption is nevertheless measured in photoreceptor neurons. After activation of photoreceptors by light, glucose is probably taken up predominantly by glial cells, which then release a metabolic substrate to be oxidized by photoreceptor neurons.
In summary, as indicated in the operational model described in Fig. 10, upon activation of a particular brain area, glutamate released from excitatory terminals is taken up by a Na+-dependent transporter located on astrocytes. The ensuing local increase in intracellular Na+ concentration activates Na+, K+-ATPase, which in turn stimulates glucose uptake by astrocytes. The key role of glial glutamate transporters in activity-dependent glucose uptake by the brain has been demonstrated in vivo. This model delineates a simple mechanism for coupling synaptic activity to glucose utilization; in addition, it is consistent with the notion that the signals detected during physiological activation in humans with 18F-2-DG PET and autoradiography in laboratory animals may predominantly reflect uptake of the tracer into astrocytes. This conclusion does not question the validity of the 2-DG-based techniques; rather, it provides a cellular and molecular basis for these functional brain-imaging techniques.
Schematic representation of the mechanism for glutamate-induced glycolysis in astrocytes during physiological activation. At glutamatergic synapses, presynaptically released glutamate depolarizes postsynaptic neurons by acting at specific receptor subtypes. The action of glutamate is terminated by an efficient glutamate uptake system located primarily in astrocytes. In fact, glutamate is taken up predominantly into astrocytes. Glutamate is cotransported with Na+, resulting in an increase in the intraastrocytic concentration of Na+, leading to an activation of the astrocyte Na+, K+-ATPase. Activation of Na+, K+-ATPase stimulates glycolysis (i.e., glucose utilization and lactate production). The stoichiometry of this process is such that for one glutamate molecule taken up with three Na+ ions, one glucose molecule enters astrocytes, two ATP molecules are produced through glycolysis, and two lactate molecules are released. Within the astrocyte, one ATP fuels one “turn of the pump,” while the other provides the energy needed to convert glutamate to glutamine by glutamine synthase (see Fig. 12). Once released by astrocytes, lactate can be taken up by neurons and serve as an energy substrate. (For graphic clarity only lactate uptake into presynaptic terminals is indicated. However, this process could also take place at the postsynaptic neuron.) In accord with recent evidence, glutamate receptors are also shown on astrocytes. This model, which summarizes in vitro experimental evidence indicating glutamate-induced glycolysis, is taken to show cellular and molecular events occurring during activation of a given cortical area (arrow labeled A, activation). Direct glucose uptake into neurons under basal conditions is also shown (arrow labeled B, basal conditions). Pyr pyruvate, Lac lactate, Gln glutamine, G G protein (Adapted from Pellerin and Magistretti 1994)
Lactate Released by Astrocytes Is a Metabolic Substrate for Neurons
If glucose uptake during activation can be ascribed predominantly to astrocytes, this suggests that energy substrate(s) must be released by these cells to meet energy needs of neurons. Several lines of evidence have pointed to a particular role of lactate as an astrocytic metabolic substrate for neurons, some of which are as follows:
-
Lactate is quantitatively the main metabolic intermediate released by astrocytes in cultures. Other quantitatively less important intermediates released by astrocytes are pyruvate (approximately 10 times less than lactate) and α-ketoglutarate, citrate, and malate, which are released in marginal amounts.
-
Lactate transporters (MCTs) are expressed in both astrocytes and neurons (in addition to capillaries).
-
Mimicking activation in vitro by exposing cultured astrocytes to glutamate results in a marked release of lactate and, to a lesser degree, pyruvate. This glutamate-evoked lactate release shows the same pharmacology and time course as glutamate-evoked glucose utilization and indicates that glutamate stimulates the processing of glucose through glycolysis.
-
As previously mentioned (section “Lactate and Pyruvate Serve as Instructive Cases”), synaptic activity can be sustained in vitro and ex vivo preparations in the presence of lactate alone.
-
Using isolated guinea pig retina preparations, Tsacopoulos and collaborators have demonstrated that lactate, formed glycolytically from glucose, is released by Mueller (glial) cells to fuel photoreceptor neurons.
-
MRS studies in rat and humans using different neuronal stimulation paradigms demonstrate the presence of a transient peak in lactate concentration in the activated brain areas. These observations are consistent with the notion of an activation-induced glycolysis (i.e., increased glucose utilization coupled to lactate production and release).
Thus, a metabolic compartmentation during neuronal activation whereby glucose taken up by astrocytes and metabolized glycolytically to lactate is then released in the extracellular space to be utilized by neurons is consistent with the above-mentioned biochemical and electrophysiological observations. This array of in vitro and in vivo experimental evidence is summarized in the model of cell-specific metabolic regulation illustrated in Fig. 10.
How lactate formed within the brain parenchyma (e.g., through glutamate-induced glycolysis in astrocytes) or imported from the plasma (i.e., mainly in particular conditions such as vigorous exercise, see Lactate and pyruvate serves as Instructive Case) may provide advantages over glucose to fulfill neuronal energetic needs? Lactate, after its conversion to pyruvate by a reaction catalyzed by lactate dehydrogenase (LDH), can provide, on a molar basis, 15–18 ATPs through the TCA cycle and oxidative phosphorylation (Figs. 1 and 2) in a process that does not require energy. In contrast, the first obligatory step of glycolysis necessitates the use of one molecule of ATP to produce glucose 6-phosphate from glucose (Fig. 1). In this regard, lactate is thus a readily energy provider compared to glucose and can be more energetically favorable; in particular in conditions of high energy needs, when maintenance of high ATP levels is critical, such as during neuronal activation. In addition, lactate may contribute to the redox potential of neurons, since through its conversion to pyruvate it generates NADH, hence providing energy in the form of reducing equivalents useful for a variety of metabolic reactions but also for ROS scavenging after its conversion to NADPH (Fig. 5).
Glycogen, the Storage Form of Glucose, Is Localized in Astrocytes
Glycogen is the single largest energy reserve of the brain; it is mainly localized in astrocytes, although ependymal and choroid plexus cells, as well as certain large neurons in the brain stem, contain glycogen. When compared to the contents in liver and muscle, glycogen concentration in the brain is exceedingly small, about 100 and 10 times inferior, respectively. Thus, the brain can hardly be considered a glycogen storage organ, and here the function of glycogen should be viewed as that of providing a metabolic buffer during physiological activity.
Glycogen Metabolism Is Coupled to Neuronal Activity
Glycogen turnover in the brain is extremely rapid, and glycogen levels are finely coordinated with synaptic activity. For example, during general anesthesia, a condition in which synaptic activity is markedly attenuated, glycogen levels rise sharply. Interestingly, however, the glycogen content of cultures containing exclusively astrocytes is not increased by general anesthetics; this observation indicates that the in vivo action of general anesthetics on astrocyte glycogen is due to the inhibition of neuronal activity, stressing the existence of a tight coupling between synaptic activity and astrocyte glycogen. Accordingly, reactive astrocytes, which develop in areas where neuronal activity is decreased or absent as a consequence of injury, contain high amounts of glycogen.
Certain Neurotransmitters Regulate Glycogen Metabolism in Astrocytes
Glycogen levels in astrocytes are tightly regulated by various neurotransmitters. Several monoamine neurotransmitters – namely, noradrenaline, serotonin, and histamine – are glycogenolytic in the brain, in addition to certain peptides, such as vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase–activating peptide (PACAP), and adenosine and ATP. The effects of all these neurotransmitters are mediated by their cogent specific receptors coupled to second messenger pathways that are under the control of adenylate cyclase or phospholipase C. The initial rate of glycogenolysis activated by VIP and noradrenaline is remarkably close to the rate of glucose utilization of the gray matter. This correlation indicates that glycosyl units mobilized in response to glycogenolytic neurotransmitters can provide quantitatively adequate substrates for the energy demands of the brain parenchyma. These observations show that neuronal signals (e.g., certain neurotransmitters) can exert receptor-mediated metabolic effects on astrocytes in a manner similar to peripheral hormones on their target cells.
An intriguing question is whether the glycosyl units mobilized through glycogenolysis are used by astrocytes to meet their energy demands during activation or are metabolized to a substrate, such as lactate, which is then released for the use of neurons. While there is evidence that glycogen mobilization may fulfill some astrocytes’ own metabolic needs, glycogen breakdown typically results in lactate production and release in the extracellular space, supporting the view of a role of astrocytic glycogen-derived lactate for supplying neuronal functions. For instance, it was demonstrated that astrocytic glycogen mobilization (likely associated with the transfer of lactate from astrocytes to neurons) is required to sustain neuronal activity during intense stimulation in a mouse optic nerve preparation and to maintain glutamatergic synaptic transmission (i.e., neurotransmitter release) in co-culture models. Recently, the demonstration of the implication of glycogen metabolism in higher brain functions was also obtained by showing the importance of glycogen mobilization for the processes of memory consolidation in both chicken and rodent. In particular, the involvement of astrocytic glycogen-derived lactate in long-term memory formation, and for the in vivo maintenance of long-term potentiation (LTP) of synaptic strength, in the rat brain was demonstrated.
Summary
Under basal conditions, glucose uptake and metabolism occur in every brain cell type. Glucose uptake is mediated by specific transporters that are distributed in a cell-specific manner. Astrocytes play a critical role in the utilization of glucose coupled to excitatory synaptic transmission. The molecular mechanisms of this coupling are stoichiometrically directed: for each synaptically released glutamate molecule taken up with three Na+ ions by an astrocyte, one glucose molecule enters the same astrocyte, two ATP molecules are produced through glycolysis, and two lactate molecules are released and consumed by neurons to yield 15–18 ATPs through oxidative phosphorylation. Neuronal signals, e.g., certain neurotransmitters, can exert receptor-mediated glycogenolysis in astrocytes in a manner similar to peripheral hormones on their target cells. However, this type of effect by neurotransmitters is temporally specified and spatially restricted within activated areas, possibly to provide additional energy substrates in register with local increases in neuronal activity.
Glutamate and Nitrogen Metabolism: A Coordinated Shuttle Between Astrocytes and Neurons
As has been shown, synaptically released glutamate is removed rapidly from the extracellular space by a transporter-mediated reuptake system that is particularly efficient in astrocytes. This mechanism contributes in a crucial manner to the fidelity of glutamate-mediated neurotransmission. Indeed, glutamate levels in the extracellular space are low (<3 μM), allowing for optimal glutamate-mediated signaling after depolarization while preventing overactivation of glutamate receptors, which could eventually result in excitotoxic neuronal damage.
One may wonder how astrocytes dispose of the glutamate that they take up, because, unlike carbohydrates or lipids, amino acids cannot be stored. The predominant pathway in peripheral tissues for disposing of amino acids is the transfer of their a amino group to a corresponding α-keto acid; this reaction is catalyzed by aminotransferases (Fig. 11). In astrocytes, the α amino group of glutamate can be transferred to oxaloacetate to yield α-ketoglutarate (α-KG) and aspartate in a reaction catalyzed by aspartate aminotransferase (AAT). The α-KG generated is an intermediate of the TCA cycle and is therefore oxidized further. Another transamination reaction catalyzed by alanine aminotransferase (ALAT) transfers the α amino group of glutamate to pyruvate, resulting in the formation of alanine and α-KG.
Metabolic fate of glutamate taken up by astrocytes. ALAT alanine aminotransferase, GDH glutamate dehydrogenase, GS glutamine synthase, AAT aspartate aminotransferase, GPT glutamate dehydrogenase, α-KG α-ketoglutarate (Redrawn based on Magistretti 2008)
Two other pathways exist in astrocytes to metabolize glutamate. First, glutamate can be converted directly into α-KG through an NAD-requiring oxidative deamination catalyzed by glutamate dehydrogenase (GDH) (see Fig. 11). Glutamate, by entering the TCA cycle indirectly (through AAT or ALAT) or directly (through GDH), is an energy substrate for astrocytes. Second, the quantitatively predominant metabolic pathway of glutamate in astrocytes is its amidation to glutamine, an ATP-requiring reaction in which an ammonium ion is fixed on glutamate (see Fig. 11). This reaction is catalyzed by glutamine synthase (GS), an enzyme almost exclusively localized in astrocytes, and provides an efficient means of disposing not only of glutamate but also of ammonium (Fig. 12). Glutamine is released by astrocytes and is taken up by neurons, where it is hydrolyzed back to glutamate by the neuron-specific phosphate-dependent mitochondrial enzyme glutaminase. This metabolic pathway, often referred to as the glutamate-glutamine shuttle, is a clear example of cooperation between astrocytes and neurons (Fig. 12). It allows the removal of potentially toxic excess glutamate from the extracellular space, while returning to the neuron a synaptically inert (glutamine does not affect neurotransmission) precursor with which to regenerate the neuronal pool of glutamate.
Metabolic intermediates are released by astrocytes to regenerate the glutamate neurotransmitter pool in neurons. Glutamine, formed from glutamate in a reaction catalyzed by glutamine synthase (GS), is released by astrocytes and taken up by neurons, which convert it into glutamate under the action of glutaminase. GS is an enzyme selectively localized in astrocytes. This metabolic cycle is referred to as the glutamate-glutamine shuttle. Other quantitatively less important sources of neuronal glutamate are lactate, alanine (ALA), and α-ketoglutarate (α-KG). In astrocytes, glutamate is synthesized de novo from α-KG in a reaction catalyzed by glutamate dehydrogenase (GDH). The carbon backbone of glutamate is exported by astrocytes after conversion into glutamine under the action of GS; the conversion of leucine into α-ketoisocaproate (α-KIC), catalyzed by leucine transaminase (LT), provides the amino group for the synthesis of glutamine from glutamate. Carbons “lost” from the TCA cycle as α-KG is converted into glutamate are replenished by oxaloacetate (OxA) formed from pyruvate in a reaction catalyzed by pyruvate carboxylase (PC), another astrocyte-specific enzyme aspartate (ASP), pyruvate (PYR) (Redrawn based on Magistretti 2008)
Because some of the glutamate released by neurons enters at the α-KG level, of the TCA cycle in astrocytes, not all glutamate can be regenerated through the glutamate-glutamine shuttle. Hence, de novo synthesis is required to maintain the neuronal glutamate pool. Glutamate can be synthesized through NADPH-dependent reductive amination of α-KG catalyzed by GDH (Figs. 11 and 12). For the synthesis of glutamate, glucose provides the carbon backbone as α-KG through the TCA cycle, whereas an exogenous source of nitrogen is necessary (Fig. 12). Convincing evidence indicates that plasma leucine provides the nitrogen required for net glutamate synthesis from α-KG. Thus, leucine taken up from the circulation at astrocytic end feet provides the amino group to α-KG to generate glutamate in a reaction catalyzed by leucine transaminase (LT) (Fig. 12). Because this reaction takes place in astrocytes, to replenish the neuronal glutamate pool, the astrocytes export glutamate as glutamine.
Note that because α-KG is used for glutamate synthesis, metabolic intermediates downstream of α-KG must be available to maintain a sustained flux through the TCA cycle in astrocytes (Fig. 12). This need is met by the activity of the enzyme pyruvate carboxylase (PC), which fixes CO2 on pyruvate to generate oxaloacetate, which, by condensing with acetyl-CoA, maintains the flux through the TCA cycle. The carboxylation of pyruvate to oxaloacetate is referred to as an anaplerotic (Greek for “fill up”) reaction. Interestingly, like glutamine synthase, PC is selectively localized in astrocytes. The fact that these two enzymes are localized in astrocytes in conjunction with the existence of a glutamate-glutamine shuttle stresses that astrocytes are essential for maintaining the neuronal glutamate pool used for neurotransmission (Fig. 12).
Summary
A key function of astrocytes is to remove synaptically released glutamate. A large proportion of glutamate is transformed to glutamine through an energy-requiring process that also allows for the detoxification of ammonium. Glutamine released by astrocytes regenerates the neuronal glutamate pool. Part of the glutamate is also regenerated through the fixation of the amino group of leucine onto the TCA intermediate α-KG, providing another indication of the tight link existing between glutamate and nitrogen metabolism and of the crucial function that astrocytes play in maintaining the neuronal glutamate pool at levels that ensure the maintenance of synaptic transmission.
The Astrocyte-Neuron Metabolic Unit
From a strictly energetic viewpoint, the brain can be seen as an almost exclusive glucose-processing machine producing H2O and CO2. However, the metabolism of glucose in the brain is specified temporally, spatially, and functionally. Thus, glucose metabolism increases with exquisite spatiotemporal precision in register with neuronal activity. The site of this increase is not the neuronal cell body; rather, it is the neuropil, where presynaptic terminals, postsynaptic elements, and astrocytes ensheathing synaptic contacts are localized. This cytological relation between astrocytes and neurons is also manifested by a functional metabolic partnership: in response to a neuronal signal (glutamate), astrocytes release a glucose-derived metabolic substrate for neurons (lactate). Glucose also provides the carbon backbone for regeneration of the neuronal pool of glutamate. This process results from a close astrocyte-neuron cooperation. Indeed, the selective localization of pyruvate carboxylase in astrocytes, indicating the need to replenish the TCA cycle with carbon backbones, strongly suggests that glucose-derived metabolic intermediates are used for glutamate (and other amino acid) synthesis. The newly synthesized glutamate is not provided as such by astrocytes to neurons; rather, it is converted into glutamine by glutamine synthase, another enzyme localized selectively in astrocytes. Glutamate, taken up by astrocytes during synaptic activity, undergoes the same metabolic process, also being released as glutamine (the glutamate-glutamine shuttle).
In conclusion, the axon terminal of glutamatergic neurons, which are the main communication lines in the nervous system, and the astrocytic processes that surround them should be viewed as a metabolic unit in which the neuron furnishes the activation signal (glutamate) to the astrocyte and the astrocyte provides not only the precursors needed to maintain the neurotransmitter pool, but also the energy substrate (lactate) (Fig. 13). The efficacy of the predominant excitatory synapse in the brain, the glutamatergic synapse, cannot be maintained without a close astrocyte-neuron interaction.
The astrocyte-neuron metabolic unit. Glutamatergic terminals and the astrocytic processes that surround them can be viewed as a highly specialized metabolic unit in which the activation signal (glutamate) is furnished by the neuron to the astrocyte, whereas the astrocyte provides the precursors needed to maintain the neurotransmitter pool (glutamine, lactate, alanine), as well as the energy substrate (lactate). AP astrocyte process (Redrawn based on Magistretti 2008)
Outlook
Brain energy metabolism provides the basis for functional brain imaging. It would therefore be extremely useful to develop metabolic indices of activity that could provide indication of brain function with techniques that would be much easier to implement and to be applied to realistic environments and not limited to high-end imaging facilities such as PET or fMRI. Another important point is to better understand the role of possible dysfunctions of brain energy metabolism in neuropsychiatric disorders.
References
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738
Figley CR, Stroman PW (2011) The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur J Neurosci 33:577–588
Frackowiak RSJ, Magistretti PJ, Shulman RG, Adams M (2001) Neuroenergetics: relevance for functional brain imaging. HFSP, Strasbourg
Gladden LB (2004) Lactate metabolism: a new paradigm for the third millennium. J Physiol 558:5–30
Magistretti PJ (2006) Neuron-glia metabolic coupling and plasticity. J Exp Biol 209:2304–2311
Magistretti PJ (2008) Brain energy metabolism. In: Squire LR, Berg D, Bloom FE, du Lac S, Ghosh A, Spitzer NC (eds) Fundamental neuroscience. Academic, San Diego, pp 271–293
Magistretti PJ, Chatton JY (2005) Relationship between L-glutamate-regulated intracellular Na(+) dynamics and ATP hydrolysis in astrocytes. J Neural Transm 112:77–85
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91:10625–10629
Raichle ME, Mintun MA (2006) Brain work and brain imaging. Annu Rev Neurosci 29:449–476
Schurr A (2006) Lactate: the ultimate cerebral oxidative energy substrate? J Cereb Blood Flow Metab 26:142–152
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM (2011) Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144(5):810–823
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this entry
Cite this entry
J. Magistretti, P., Allaman, I. (2022). Brain Energy and Metabolism. In: Pfaff, D.W., Volkow, N.D., Rubenstein, J.L. (eds) Neuroscience in the 21st Century. Springer, Cham. https://doi.org/10.1007/978-3-030-88832-9_56
Download citation
DOI: https://doi.org/10.1007/978-3-030-88832-9_56
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-88831-2
Online ISBN: 978-3-030-88832-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences