Abstract
Consumption of alcohol is the third leading cause of preventable deaths worldwide making it a major public health issue. After a brief account of the history and current situation of alcohol use and abuse, we describe the clinical pictures of the most severe neuropsychiatric consequences of pathological alcohol consumption, namely, alcohol addiction, Wernicke’s encephalopathy, and fetal alcohol syndrome. We then explain the neurobiological and pharmacological mechanisms of alcohol’s action in the brain that underlie its rewarding effects in humans and other animals. We point out how genetic factors in interaction with the environment influence the risk for heavy drinking, which ultimately may lead to alcohol addiction, a chronic relapsing disorder where relapse is characterized by compulsive alcohol seeking, and the loss of control in limiting alcohol intake. We account for some influential theories that try to explain the dysregulation in brain circuits along the development into an addicted state, and based on these concepts we point to pharmacological strategies that can reduce the risk for relapse and thus will be helpful in managing alcohol-addicted patients.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Alcohol addiction
- Alcohol consumption
- Alcohol dependence
- Alcohol metabolism
- Alcohol-addicted brain
- Alcoholism
- Alcohol-related disorders
- Fetal alcohol syndrome–related disorders
- Glutamate theory, of alcoholism
- Long-lasting synaptic changes
- Mesolimbic dopamine (DA) system
Brief History
Alcohol has been part of human culture since the beginning of recorded history, and thus, alcohol’s harmful effects on human behavior and health are well known. Alcohol is a daily incentive, and in addition to coffee and tea, alcoholic beverages are the most important commodities worldwide. Why do we drink alcohol if it can have a negative impact on our behavior and health? An evolutionary perspective gives at least two explanations. One hypothesis suggests that moderate alcohol consumption is evolutionarily maintained by positive effects on evolutionary fitness – e.g., consumption of alcohol can produce positive mood states and has stress- and tension-relieving effects and may thereby reduce the risk of coronary heart disease, type 2 diabetes, and some other diseases. On a global scale, these positive effects may outweigh any associated health risks of alcohol use and abuse. Unfortunately, this hypothesis is almost impossible to test, since this would require the comparison of two large and very similar populations with and without alcohol consumption over several decades. More likely, however, is a scenario in which the traits that predispose humans to maladaptive alcohol-related behaviors evolved under a long selective regime of low alcohol availability in fruit-eating human and prehuman ancestors. Thus, occasional and even chronic intake of alcohol through sugar-rich plant products susceptible to fermentation, such as nectar, sap, and fruit, might be a behavioral feature that has been shaped over millions of years, from the fruit fly to numerous mammals, including primates and humans. Alcohol in ripening fruits may have indicated a valuable source of nutrients to our ancestors that led to an inherent alcohol preference. Thus, with harmful alcohol doses generally prevented by natural availability, genetic traits for increased intake came under positive selection. No similar pressure worked on genes protecting against harmful effects. As time elapsed, a better understanding of fermentation techniques resulted in the development of beverages that allowed the consumption of enough alcohol to reach intoxication. Historical evidence indicates that as early as 9,000 years ago, beer brewing was commonplace in the late Stone Age village of Jiahu in Northern China. The various brewing procedures occurring then in different ancient cultures probably resulted in a reversal of selective pressures. However, this period was too short to induce adequate evolutionary counterresponses. In this sense, modern alcoholism has been called an evolutionary hangover.
In favor of the evolutionary hangover hypothesis is a very recent discovery in a primary tropical rainforest in West Malaysia, where pen-tailed tree shrews (Ptilocercus lowii) consume intoxicating amounts of alcohol on a daily basis. Pen-tailed tree shrews are mammals that closely resemble modern primates’ early ancestors who lived more than 50 million years ago, and their major daily food source is the nectar from the bertram palm Eugeissona tristis. This indigenous plant bears flowers that actively produce alcohol by means of a number of currently unknown yeast species, in concentrations of up to 3.8%, which is comparable to that of common beer. In this million-year-old ecosystem, the pen-tailed tree shrew has adapted to a daily intake of intoxicating amounts of alcohol by means of metabolic tolerance, without suffering from any obvious negative consequences. This new discovery favors the hypothesis that from an evolutionary perspective, alcohol intake behavior has been shaped over millions of years and should be considered a part of our normal behavioral repertoire, embedded today in traditional and sociocultural contexts.
Alcohol abuse and addictive behavior as a prevalent phenomenon has undoubtedly intensified with the invention of distillation and the wide availability of high-percentage liquors. Wine rarely develops alcohol content over 15% alcohol by volume, since a higher concentration makes it impossible for most yeast species to reproduce. Only by distillation can stronger alcoholic beverages be obtained; the invention of alcohol distillation was made in Salerno in Southern Italy and is dated back to the twelfth century. Since this invention, several gin epidemics have spread throughout old Europe, highlighting for the first time the disruptive forces of highly concentrated liquor. Today, this phenomenon is still observed in Russia, where Vodka drinking is prevalent. Russia has the highest prevalence of alcohol liver cirrhosis and addiction. Interestingly, it was not until the eighteenth century that the idea of distinct adverse effects of alcohol comprising a medical disorder was conceptualized. In 1819, German physician Christoph W. Hufeland (1762–1836) termed the preoccupation with alcohol “dipsomania,” and in 1849 Swedish physician Magnus Huss (1807–1890) differentiated between acute alcohol intoxication and “alcoholismus chronicus,” which he described as a disorder of the body and mind. Such early views had a difficult standing within the medical profession. Only when Elvin Morton Jellinek (1890–1963) developed in the middle of the twentieth century a disease concept of alcoholism with a categorization of different drinker types, a wider acceptance among medical doctors was attained. Today, addiction to alcohol is widely recognized as a distinct medical syndrome, although its definition is still a matter of debate and the pathophysiological mechanisms remain largely unknown, despite an enormous growth of information about how alcohol affects our organ and brain functions. Many neurobiologists working in the field of alcohol addiction believe that it is a disease of the brain reinforcement system. However, alcohol is effectively distributed in all body compartments, thereby affecting the function of most organs. As a consequence, only a holistic approach that takes all organic system levels into consideration will finally give a more complete understanding of the pathophysiological processes involved in alcohol addiction.
Psychological and Neurobiological Aspects of Alcohol Addiction
Epidemiology of Alcohol Consumption and Alcohol-Related Disorders
Today, alcohol consumption is common in many parts of the world, as an estimated two billion people consume alcohol. According to the WHO’s atlas of alcohol exposure, adult alcohol consumption is highest in industrialized countries with a western culture (Fig. 1 WORLD atlas). Alcohol use and abuse account for a tremendous burden of disease and injury and economic costs worldwide. Excessive alcohol drinking is linked to more than 60 diseases, including cancers, cardiovascular diseases, liver cirrhosis, neuropsychiatric disorders, injuries, and fetal alcohol syndrome. A recent report by Jürgen Rehm and colleagues working for the WHO estimates that 4% of the total mortality and between 4% and 5% of the disability-adjusted life-years (DALYs) worldwide are attributable to alcohol. In these calculations, the potential health benefits of regular low-level drinking on, for example, coronary heart disease and type 2 diabetes were also considered but are largely overshadowed by the detrimental effects of alcohol consumption. Importantly, while the major share of the disease burden occurs in developed countries, the burden inflicted per unit of alcohol consumption is highest in low-income countries and poorer populations, setting a major obstacle for further developments in these countries. The study also confirmed that men are much more affected by alcohol than women but that this gender difference seems to be strongly affected by cultural factors (approximate male-to-female ratios in Nigeria 2.5:1, USA and Germany 3:1, Russia 4:1, Brazil 6:1, China and India 9:1). An additional perspective to the dangers associated with alcohol was provided by David Nutt and colleagues from the UK, who examined a variety of individual and social factors and found that when considering the combined harm inflicted upon both the individual and others, alcohol is the most dangerous of all abused drugs. Consequently, alcohol use and abuse bring considerable costs to society; on a global scale, the annual costs are estimated to be 760 billion Euros.
Official recorded alcohol consumption plus unrecorded consumption per adult (15 years +) in liters of pure alcohol per year (tourist consumption was not added) and selected prevalence rates for alcohol addiction (Consumption data were taken from Shield et al. (2011); prevalence rates were taken from the WHO report 2008). Note: In some countries like Russia, there is a very high unreported rate of alcohol consumption due to illegal distillation and other sources (4.73 L/adult). Interestingly, population-wide, there is no linear relationship between alcohol consumption per capita and prevalence rates for alcohol addiction (R2 = 0.185) indicating that alcohol policy aiming for the mere reduction of overall alcohol consumption in a country does not necessarily lead to less alcohol-related disorders. The fact that there is no linear relationship between alcohol consumption and alcohol addiction has several reasons. First, alcohol consumption is a necessary precondition to get addicted to alcohol but is not sufficient; many other factors such as genetic predisposition and environmental influences (e.g., stressful adverse life events) contribute to the progression into an addicted behavior. Second, cultural differences in drinking patterns and the use of alcoholic beverages are also important contributing factors. For example, Vodka drinking in Russia produces high rates of alcohol-related disorders. Binge drinking on weekends, typical for Scandinavian countries, produces also high prevalence rates of alcoholism despite very low alcohol consumption per adult. In contrast, in France, as a typical wine drinking country with very high alcohol consumption per adult, comparable low prevalence rates are observed
Even though alcohol drinking inflicts damage in varying ways, the core of the health problem becomes clear when considering that a large portion of the population estimates shown in Fig. 1 is consumed by a relatively small proportion of a given population. These individuals are drawn by the central effects of alcohol into a vicious cycle that alternates between escalating phases of preoccupation and intoxication with alcohol and progressively worsened withdrawal symptoms and negative mood states. Ultimately, the victim is unable to break free from this behavioral pattern that characterizes addiction. Alcohol addiction is typically a slowly progressing disorder. It may take 5–10 years until an affected individual requires medical attention. The clinical picture of fully developed alcohol addiction is easily recognizable by a few key symptoms arising from either a medical-physiological, social, or psychological perspective as to what may comprise the core problem of the disorder.
Key Symptoms of Alcohol Addiction
Symptoms related to the medical-physiological perspective are tolerance and withdrawal. Alcohol tolerance is defined as a reduction in response after intake or the requirement to increase consumption in order to maintain the initial level of response. Withdrawal occurs after abrupt discontinuation of chronic drinking. When the drug leaves the system, characteristic withdrawal symptoms emerge. Although tolerance and withdrawal are objectively measurable criteria, these phenomena are insufficient to describe an addictive behavior. For example, withdrawal symptoms can be easily alleviated by benzodiazepines, yet such treatment does not seem to have any effect on addictive behavior (e.g., relapse to heavy alcohol drinking). Furthermore, tolerance may or may not develop in addicted individuals. Importantly, many pharmacological agents without any abuse liability can cause tolerance with chronic use or withdrawal symptoms after discontinuation.
Because a narrow physiological perspective is insufficient to describe the phenomena associated with addiction, alternative concepts have emerged, most importantly those that classify as addictive every substance that with nonmedical use can cause negative consequences for the individual and society. This view focuses on social functioning, such as that within the family or at work. Thus, an individual may be too preoccupied with alcohol-related activities that normal daily activities become to play a minor role. However, as social functioning depends highly on cultural norms and attitudes (e.g., criminalization, work ethics, etc.), these criteria are difficult to objectify, especially across cultures. Furthermore, it is difficult to translate these aspects on social functioning into animal work.
The psychological perspective recognizes craving and loss of control as the core symptoms of addictive disorders. Craving is easily recognizable both clinically and by the individual. In addition to the impregnable appetite for alcohol, there is an apparently inexplicable irritability and restlessness, which ultimately leads to relapse to drinking. Loss of control refers to the inability to limit drinking to small restricted amounts and can be described both subjectively as an individual experience and objectively as an observable behavior.
Separately, none of these views can sufficiently describe the medical problem. Thus, modern psychiatric diagnostic evaluation uses an integrated approach. A diagnosis of alcohol addiction is typically obtained using a set of criteria laid out in two widely used diagnostic schemes: WHO’s International Classification of Diseases (ICD-10) and the Diagnostic and Statistical Manual-IV (DSM-IV) of the American Psychiatric Association. For alcohol dependence (equated with alcoholism or alcohol addiction), seven criteria are evaluated (Table 1). Harmful alcohol consumption and addiction is defined as the occurrence of at least three of these criteria during the last year.
With a pool of criteria, none of which are necessary or sufficient to confirm a diagnosis, the clinical picture of patients diagnosed with alcohol dependence can vary widely. For example, a young patient may engage in frequent binge drinking, trying various drugs and repeatedly getting into trouble with the authorities. Another person may have consumed high levels of alcohol over many years, is well socially integrated, but starts to feel bad every morning, experiences relief after an early drink, and despite his physician telling him his liver is beginning to fail and counseling him to reduce alcohol, just cannot quit. Each of these patients may fulfill only three to four diagnostic criteria for alcoholism. They may look very different and yet in fact represent different subtypes of the disorder. Hence, it is still being debated as to whether the contributions of individual DSM-IV criteria to the diagnosis represent a single continuum of the disorder, probably related to heavy drinking, or whether the alcohol addiction diagnosis combines multiple phenotypes with a multifactorial structure of underlying risks. The answer to this question will dramatically impact future research and treatment strategies. However, clinical experience suggests that with further disease progression and the development of increasing or total symptom criteria, the clinical picture becomes increasingly uniform, such that apart from the addiction, very little remains of an individual’s personality.
In such severe cases of alcoholism, one acute and life-threatening complication of severe alcoholism is Wernicke’s encephalopathy, a potentially reversible neurological disorder that is caused by a critical lack of thiamine (vitamin B1). A very low lifetime incidence in the general population contrasts with 12.5% in patients with alcoholism. The clinical diagnosis requires the presence of two of the following four signs: dietary deficiencies, abnormalities in the ocular motoric system, cerebellar dysfunction, and either altered mental state or mild memory impairment. Parenteral treatment with thiamine can rapidly improve Wernicke’s encephalopathy. Without treatment, about 80% of the patients develop the Korsakoff’s syndrome, which includes anterograde or global amnesia and severe neuronal loss. Characteristic neuropathological changes observed in both pathological conditions are lesions in periventricular regions around the third and fourth ventricles and in the mamillary bodies. These alterations can easily be detected in vivo by magnetic resonance imaging (MRI). On a cellular level, cerebellar cell density of Purkinje cells is reduced in parallel to an atrophy of the molecular layer. Similar changes are observed in cortical and subcortical regions. Apart from these neurological diseases in severe alcoholics, alcohol-induced brain damage also afflicts the offspring during pregnancy, resulting in fetal alcohol syndrome.
Fetal Alcohol Syndrome–Related Disorders Represent the Most Common Form of Acquired Mental Disabilities, Affecting up to 7 per 1,000 Infants
Passive exposure to alcohol during pregnancy may exert long-lasting toxic effects to the offspring. This has been known for thousands of years and might have influenced nutritional advice given in the book Judges 13:3–4: “You are barren and childless, but you are going to become pregnant and give birth to a son. Now see to it that you drink no wine or other fermented drink.” Unfortunately, some women continue to ignore the well-known harmful effects of alcohol during pregnancy, and thus, alcohol is the most frequent and avoidable cause of mental retardation, due its toxic disturbance on child development. Maternal risk factors of drinking during pregnancy are poverty and low educational levels, and thus, fetal alcohol syndrome is currently a major problem in developing countries. Perinatal exposure to alcohol induces general developmental and specific neuropsychiatric deficits. Phenotypical differences in the syndrome are attributed to genetic disposition and maternal age, as well as general maternal nutrition, the simultaneous abuse of nicotine, and, most importantly, the time window and amount of alcohol exposure. The classical triad of fetal alcohol syndrome was first described by French researcher P. Lemoine in 1968 and comprises growth retardation, craniofacial malformations, and neurocognitive deficits. Since then, several subsyndromes have been systematically classified, and the spectrum of fetal alcohol syndrome–related disorders can be found in as many as 0.7% of children and is characterized according to a four-digit code. The examination quantifies four key diagnostic features, including growth deficiency, the FAS facial phenotype, brain dysfunction, and gestational alcohol exposure (for details see http://depts.washington.edu/fasdpn/htmls/4-digit-code.htm). Within the phenotypic spectrum, an exclusive manifestation in the central nervous system is named alcohol-related neurodevelopmental disorder (ARND), in which exposure during the last trimester of human pregnancy leads to abnormal brain development. The clinical manifestation includes impaired general intelligence, with deficits in learning and memory, attention, executive functioning, emotional instability, and hyperactivity, resulting in low success in school and vocational training. The pathomechanisms involved in ARND have been addressed by translational research involving molecular, cellular, and behavioral animal studies, as well as clinical research using MRI techniques and genetics (Table 2).
The classical therapeutic approaches are prevention, early recognition, and interventions on several levels. Public campaigns and individual counseling for alcohol abstinence during pregnancy can be successful, if all professional subgroups are involved. The defining of at-risk constellation helps to prevent perinatal complications by providing sufficient gynecological and pediatric aids, including the treatment of withdrawal syndromes and the prevention of pertained intoxication via breast feeding. Early recognition using the four-digit code establishes the detailed diagnosis in order to begin treatment that involves physiotherapy, speech correction, cognitive training, training in suitable schools, and social and vocational rehabilitation. Secondary problems, such as affective syndromes, can be effectively addressed with psychotropic agents. Secondary psychopathological problems due to excessive alcohol consumption occur not only in the context of fetal alcohol syndrome but are also frequently observed in people suffering from excessive alcohol abuse.
Excessive Alcohol Drinking and Addictive Behavior Often Comes with Other Psychiatric Conditions: Comorbidities of Alcohol Addiction
A diagnostic problem in alcoholism treatment and research is to distinguish the condition from other psychiatric comorbidity that could either be the cause of or caused by or occur separately of alcohol addiction. For example, anxiety or low mood are core symptoms of anxiety disorders or depression but are also invariantly induced by alcohol withdrawal. Thus, it may appear that these two disorders are induced by alcoholism and patients may rather seek help for their depression as for their alcohol drinking problem. Alternatively, as anxious individuals may try to relief their symptoms by alcohol, this could indeed lead into addictive behavior. Generally, it is difficult to establish any of these proposed causalities. First of all, acceptable diagnosis can only be obtained when patients are not intoxicated and acute withdrawal symptoms have subsided. When applying these principles, it is commonly found that depression and anxiety disorders among alcoholic patients are not more common as in the general population speaking against a causal relationship with alcohol addiction. However, clear overrepresentation of alcoholism is found with antisocial personality disorder. Alcohol use also coincides very heavily with tobacco use; comorbidity rates of addiction to both substances can be above 50%. Common biological mechanisms such as interactions on the mesolimbic dopamine (DA) system may underlie this phenomenon.
Drinking Alcohol Is Reinforcing by Activating the Mesolimbic Dopamine (DA) System
Reinforcement is a psychological term describing the process by which the rate or probability of a behavior is increased. The brain regions that play an important role in mediating the reinforcing effects of drugs of abuse, including alcohol, have been identified by a variety of neuropharmacological studies that include lesion, microinjection, and microdialysis experiments. However, the groundbreaking work was performed by the outstanding experimental psychologist James Olds in 1954. His electrical brain stimulation experiments made it apparent that the brain must have specialized sites for reinforcement functions. In these experiments, brain sites in which electrical stimulation was reinforcing were identified, such that a rat self-stimulated these areas frequently and regularly for long periods of time if permitted. Alcohol leads to an increase in sensitivity of the animal to the electrical stimulation. The midbrain DA system, in particular, is sensitive to electrical self-stimulation and has been characterized as a neurochemical substrate of reinforcement. Midbrain DA neurons involved in the initiation of reinforcement processes originate in the ventral tegmental area (VTA) and project to structures closely associated with the limbic system, most prominently the nucleus accumbens (NAC) shell region and prefrontal cortex (PFC). Activation of the midbrain DA system by all kinds of reinforcers has been demonstrated in animals and humans. For example, by means of neuroimaging methods in humans, it has been shown that social attractiveness, sex, and orgasm, and even classical music (but only in musicians) can induce enhanced activity in the NAC. Also, a variety of drugs abused by humans, including alcohol, leads to enhanced mesolimbic DAergic activity, preferentially in the NAC shell region. Importantly, the role of DA in mediating alcohol reinforcement has also been demonstrated in the human brain. Using positron emission tomography (PET) measurements, it could be demonstrated that alcohol ingestion produced a significant reduction in binding of the radioactive-labeled dopamine D2 receptor ligand [11C] raclopride in the NAC. The magnitude of change in [11C] raclopride binding correlated with the psychostimulant effects of alcohol. This indicates that enhanced DA release occurs in response to alcohol drinking and that the degree of psychostimulation is mediated, at least in part, by augmented extracellular DA levels.
Several mechanisms have been suggested how alcohol increases extracellular DA within the NAC. Alcohol decreases the activity of the GABAergic feedback loop from the NAC to and of interneurons within the VTA leading to a disinhibition of the DA neurons. These feedback and interneurons are rich in μ-opioid receptors which negatively control GABA release. Many animal studies demonstrated that alcohol activates these μ-opioid receptors via an indirect mechanism, probably by release of the endogenous ligand beta-endorphin. Importantly, this mechanism has recently also been established for DA release in the human brain. Using a similar PET approach as described above, an NIAAA team led by Markus Heilig could conclusively demonstrate that a functional genetic variant in the human μ-opioid receptors, which alters an asparagine into an aspartic acid residue in the ligand binding domain of the receptor, explains differences in the magnitude of alcohol-evoked DA release in the nucleus accumbens/ventral striatum. Thus, genetic variants of the μ-opioid receptor seem to be involved in an individual’s response to alcohol at the neurochemical and likely also at the behavioral level.
Besides this opioid/GABA interaction, DAergic activity within the VTA is regulated by a variety of other systems. Glutamatergic neurotransmission also controls the mesolimbic DAergic pathway. Projections from the prefrontal cortex, bed nucleus of the stria terminalis, laterodorsal tegmental nucleus, and lateral hypothalamus feed into the VTA, and in addition, there are glutamatergic inputs innervating the NAC. Glutamate release from any one of these projection terminals can act on ionotropic glutamate receptors in the VTA and/or NAC shell to induce DA release. The repeated activation of ionotropic glutamate receptors on DA neurons leads to alcohol-induced synaptic strengthening and thereby alters the reinforcement system.
Alcohol Induces Long-Lasting Synaptic Changes Within the Reinforcement System
A ubiquitous property of all synapses is their ability to undergo activity-dependent changes in synaptic plasticity that can be studied most effectively using electrophysiological methods in brain slices. It has been shown that synaptic plasticity within the reinforcement system becomes manifest following alcohol exposure. Some key studies on drug-induced adaptations in the mesolimbic system have revealed that glutamatergic synapses on DA neurons in the VTA, in particular, undergo plastic changes following intake of drugs of abuse including alcohol. By increasing excitatory synaptic strength, alcohol augments the responsiveness of DA neurons and medium spiny neurons (this is a special cell type of inhibitory GABA neurons representing approximately 90% of the neurons within the nucleus accumbens and striatum) within the VTA and NAC to glutamate and, ultimately, promotes enhanced DA release in brain areas such as the NAC and the prefrontal cortex. Alcohol-induced synaptic strengthening in DA neurons in the VTA or medium spiny neurons within the NAC is associated with changes in AMPA receptor subunit composition. Incorporation of the AMPA receptor subunit GluA1 promotes alcohol-induced synaptic strengthening, probably through the formation of highly conductive, Ca2+-permeable GluA1 homomeric AMPA receptors (Fig. 2), while insertion of GluA2-containing receptors reverts it. Synaptic recruitment of GluA1 subunits and the resultant synaptic potentiation requires the activation of NMDA receptors.
Alcohol consumption increases extracellular DA within the NAC. DArgic neurons originating in the VTA project to the NAC and receive various glutamatergic inputs. Repeated alcohol consumption leads to synaptic strengthening on the level of the VTA which then propagates to medium spiny neurons within the NAC. Activation of NMDA receptors following recovery from alcohol consumption facilitates incorporation of the AMPA receptor subunit GluA1 into the synaptic spines and the formation of highly conductive homomeric GluA1 receptors. This alcohol-induced synaptic strengthening is indicated by enhanced AMPA-driven excitatory postsynaptic currents (EPSCs). As a consequence, an increase in the AMPA/NMDA ratio up to 1 is seen following repeated alcohol consumption (Redrawn from Spanagel and Weiss (1999))
The effects of alcohol on long-term synaptic plasticity have also been studied in the dorsomedial striatum, a striatal subregion that plays a central role in the acquisition and selection of goal-directed actions. Furthermore, the dorsomedial striatum becomes progressively recruited by compulsive alcohol consumption. Alcohol has been found to impair NMDA receptor-dependent long-term potentiation (LTP) in a dose-dependent manner. At the relatively low concentration of 10 mM – a concentration comparable to mildly intoxicating blood alcohol concentrations (BACs) – LTP is abolished in the dorsomedial striatum. It has further been shown that the loss of LTP in the presence of alcohol is not due to a decrease in AMPA receptor-mediated glutamatergic transmission, a finding which is in accordance with another report showing that alcohol has only a weak effect on AMPA receptor-mediated synaptic currents in striatum. These results suggest that alcohol can reverse the direction of synaptic plasticity in a brain area that is critically involved in goal-directed and addictive behavior. Compensatory engagement of the alternative habit system may occur as a result of this impaired goal-directed behavior. Acute alcohol exposure, even at relatively low doses, may thus promote habit formation.
In conclusion, alcohol-induced excitatory synaptic plasticity has been found in the VTA-NAC projection as well as in the striatum and other brain areas such as the extended amygdala. What are then the behavioral consequences of these synaptic alterations and is alcohol reinforcement mediated by enhanced DA release within the NAC? It was indeed shown that postsynaptic AMPA receptor function in VTA neurons is enhanced after alcohol self-administration. As increased VTA AMPA receptor function can significantly regulate firing of DA neurons, the increased AMPA receptor activity following alcohol consumption may drive the reinforcement process given that enhanced mesolimbic DA release is indeed crucial for the reinforcing properties of alcohol. Several experimental findings argue that this is the case; thus, in intracranial alcohol self-administration experiments, rats will directly self-administer alcohol into the VTA. Further, when comparing different genetically selected alcohol-preferring and nonpreferring rat lines (Table 3) for their basal and alcohol-stimulated release of DA in the NAC by means of microdialysis, it was found that extracellular DA and the percent of baseline increase in DA due to alcohol were significant predictors of the degree of alcohol consumption.
Different genetically selected alcohol-preferring and nonpreferring rat lines have been developed within the last 50 years. The large variability in alcohol preference among individual animals and strains has allowed researchers to selectively breed rats for differential alcohol preference, generating pairs of animal strains that are characterized by particularly low or high alcohol consumption levels. The best-studied pairs of lines were generated in Finland, the United States, and Sardinia. For example, the Finish model – called Alko alcohol (AA) and Alko nonalcohol (ANA) rats – comprises two strains of albino rats based on their selection or rejection of a 10% alcohol solution and water. The AA rats were selectively bred starting in 1963 and voluntarily consume more than 5 g alcohol per kilogram body weight per day (g/kg/day), attaining high BACs, whereas the nonpreferring ANA rats consume less than 0.5 g/kg/day alcohol. Alcohol-preferring rat lines are extremely helpful in obtaining a solid knowledge about the genetic factors modulating initial alcohol consumption and the neurobiological underpinnings of primary alcohol reinforcement processes.
In summary, animal and human research has demonstrated that systemic alcohol has multiple actions on mesolimbic DA neurons and several modulatory neurochemical access points, including ionotropic glutamate receptors, GABAA, serotonin type 3 receptors (5-HT3), neuronal acetycholine (nAch) receptors, growth hormone secretagogue receptors (GHSR-1A), and glycine receptors affecting the NAC, the VTA, and their afferents. Thus, mesolimbic DA activation is a property of alcohol and mediates its reinforcing effects. However, it must be emphasized that reinforcement processes do not necessarily reflect the emotional hedonic components of alcohol; it seems more probable that an enhanced DA signal highlights important stimuli and functions as a neurochemical learning signal for reinforcing stimuli. Whether DA also plays a role in mediating hedonic aspects of alcohol intake is not known. So do people drink alcohol because it is reinforcing and stimulates DA release? If people are interviewed for their motivation to drink alcohol, they will never say “I drink alcohol because of its reinforcing properties.” Although the psychological construct of reinforcement is extremely helpful for the field of neuropsychopharmacology, it does not tell about the drug-taking motives and motivation of an individual.
People Like to Drink Alcohol Because of Its Ability to Alter Emotional States and Many Other Reasons
A new theoretical concept provided by Christian Müller from the University of Erlangen-Nürnberg in Germany proposes that alcohol is an instrument to alter emotional states. Dr. Müller has formulated the drug instrumentalization theory to attempt to explain individual nonaddictive psychoactive drug use. Drug instrumentalization is a learned behavior designed to change the mental state and thereby improve the current quality of life by taking a psychoactive drug. He defines an extensive list of drug instrumentalization goals, such as improved social interaction, the feeling of well-being, tension reduction, and many others. The definition and validation of drug instrumentalization goals will help to understand individual drug-taking profiles that may change over the life course of an individual. To use alcohol as an instrument to improve the current quality of life builds on the multiple effects of alcohol on subjective experience, autonomic activity, motor and cognitive performance, and behavior. Depending on the dose, alcohol usually produces dualistic effects. On the subjective level, alcohol can induce both stimulation and sedation. Stimulation is typically experienced at low blood alcohol levels soon after intake, while sedation develops slowly and gradually, specifically during the descending limb of the blood alcohol elimination curve (Fig. 3). Generally, stimulant effects are experienced as positive and are identified as an alcohol instrumentalization goal by many social drinkers. However, some sedative effects, such as reduced anxiety, are also positively labeled; other effects, such as motor impairment, are generally regarded as unpleasant.
Approximate time course of stimulation and sedation after a single dose of alcohol. Alcohol effects were assessed by the biphasic alcohol effects scale and are given as differences from baseline (left y-axis). Breath alcohol concentration (solid thin line) is plotted on the right y-axis. Stimulation scale scores (red dashed line) peak early and decline slightly below baseline within 90 min after intake. Sedation scale scores (blue dashed line) rise slower and return to baseline after about 3 h. The experiment was conducted in 44 healthy social drinkers by Dr. Vijay Ramchandani from NIAAA (Adapted from Hendler et al. (2012))
Only recently has it become possible to investigate the neurocircuitry that underlies these subjective effects in humans, using in vivo neuroimaging methods. NIAAA researchers led by Dan Hommer found using functional magnetic resonance imaging (fMRI) that alcohol in healthy social drinkers induces activation of the NAC. In the same experiment, the researchers found a blunting of the amygdala response to threatening stimuli, which may underlie the reduction of anxiety seen following alcohol intake and also motivate drinking behavior, in this case via negative reinforcement. In general, these findings are consistent with available data from a long line of research on alcohol-motivated behaviors in experimental animals. The neurobiological mechanisms mediating alcohol’s stimulant effects are generally attributed to activation of the brain reinforcement system. Mechanisms involved in alcohol sedation are less clear but are related to the GABA system. Furthermore, biphasic effects are also observed on the level of autonomic, motor, and cognitive responses, although these are not as clearly associated with rising and falling BACs as stimulation and sedation.
Individuals Vary Highly in Their Responses to Alcohol, Which May Allow Some People to Drink Excessively
Why do some people drink excessively, whereas most people have a very controlled and low alcohol consumption profile? According to Mark Schuckit from the University of California in San Diego, individuals that initially show a low level of response to alcohol may drink more and be at an increased risk for alcoholism. The low-level response hypothesis has been criticized because it focuses largely on the sedative effects of alcohol, and individuals at increased risk for alcoholism often demonstrate increased alcohol-induced stimulation.
Importantly, part of the variability in the response to alcohol can be attributed to genetic factors. This is exemplified by the above-discussed alcohol-evoked DA release in the ventral striatum, which is mediated by genetic variants at μ-opioid receptor gene and likely underlies the increased stimulant response presented by carriers of this variant. It has been suggested that the same genes that mediate an individual’s response to alcohol may also predict the risk for alcoholism. Although a number of candidate genes have been tested under this hypothesis, to date, none have been clearly associated with a distinct response to alcohol and increased risk for alcoholism.
With repeated or chronic intake of alcohol, the subjective experience of its effects changes, i.e., tolerance develops. Tolerance, one of the seven diagnostic criteria for alcoholism according to the DSM-IV, is defined as reduction of response after intake or as the requirement to raise the dose in order to maintain the initial level of response. Typical questions to asses increased tolerance according to DSM-IV are as follows: Have you ever found that you needed to drink more in order to get the same effect as you did when you first started drinking or have you ever found that when you drank the same amount it had much less effect than before? There should be at least a doubling in amount of alcohol required to be counted as clinically significant. Two different mechanisms can account for this phenomenon. In alcoholism, the response to a certain amount of drug has changed. This aspect of tolerance is called functional tolerance and is based on the pharmacodynamic interaction of the drug with the target tissue. Given that the behavioral effects of alcohol originate in the brain, after the development of tolerance, certain neuronal receptors may signal differently. Functional tolerance develops fast and can be long-lasting. Alternatively, tolerance can be based on pharmacokinetic mechanisms, i.e., absorption into the bloodstream, distribution to various body compartments including the brain, and ethanol metabolism. Increased activity of metabolic pathways can significantly reduce the amount of alcohol and thus lead to increased tolerance. Alcohol is predominantly metabolized to acetaldehyde, a reaction that occurs mainly in the liver and is catalyzed by alcohol dehydrogenase (ADH) (Fig. 4). A small fraction of ingested alcohol is also metabolized by other enzymes such as cytochrome P450 or catalase. In a second reaction, the toxic metabolite acetaldehyde is rapidly converted by aldehyde dehydrogenase (ALDH) into acetate (Fig. 4). Interestingly, the brain is protected from circulating acetaldehyde. While alcohol can freely cross the blood-brain barrier, acetaldehyde cannot. However, because a small amount of alcohol is metabolized in the brain (e.g., by some ADH isoforms and catalase), acetaldehyde is generated locally. It was found that under these relatively low concentrations, acetaldehyde can in fact have positive reinforcing properties. These observations have led some researchers to conclude that alcohol acts as a prodrug and only when metabolized in the brain produces its behavioral effects.
Alcohol metabolism. Alcohol is mainly metabolized by a two-step reaction via acetaldehyde into acetate which can serve as a substrate for ATP production in the Krebs cycle. The main pathway involves the alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) gene families. Important protective genetic variants leading to accumulation of the toxic intermediate acetaldehyde are shown. A similar effect is accomplished by pharmacological blockade of ALDH by disulfiram (Antabus®), which is used as therapeutic approach in alcoholics, although with only moderate success. Although the main site of alcohol metabolism is the liver, small amounts are also metabolized in the brain leading to local production of acetaldehyde which does not penetrate the blood-brain barrier
Although the development of metabolic tolerance (i.e., induction of these enzymes with chronic use leads to faster elimination) is not critical for the progression to alcoholism, individual differences in ethanol metabolizing enzymes impact significantly the risk for developing the disorder. Genetic polymorphisms in ADHs and ALDH result in altered enzyme activities, leading to slow elimination of toxic acetaldehyde, the accumulation of which in the bloodstream causes a highly aversive reaction that includes facial flushing, nausea, dizziness, headache, and tachycardia. Because acetaldehyde accumulation is a deterrent for further alcohol use, these genetic variants protect against the risk of alcoholism. There are different isoforms of ALDH. For example, ALDH2*2 encodes for a nearly inactive isoform, and about 10% of the Japanese population are homozygous for the ALDH2*2. This population does not tolerate alcohol, and individuals are almost never diagnosed with alcoholism. Even in heterozygous individuals, the ALDH2*2 allele exerts a strong protective effect on the risk of developing alcoholism. The ALDH2*2 allele is highly prevalent in East Asian populations where up to 50% may be carriers but is nearly absent in people of Caucasian decent. The reason for this regional enrichment is unclear. Positive selection by an unknown environmental factor is a likely mechanism. However, the apparent advantage of the ALDH2*2 variant comes with a price. If carriers do engage in alcohol drinking or are otherwise exposed to acetaldehyde, they have an increased risk of developing cancer, especially within the gastrointestinal tract.
The finding that some genetic variants in alcohol-metabolizing enzymes strongly protect against the risk of developing alcoholism is one of the most replicated findings in population genetics. As such, it is an exemption within the genetics of complex psychiatric disorders – including other substance dependencies – that commonly deal with very small effect sizes for individual genes. As discussed in the beginning of this chapter, alcohol is set apart from other drugs of abuse by the fact that it is foremost a nutrient of considerable caloric value. Most animals, from invertebrates to mammals, will consume alcohol when available at millimolar concentration, which is about 1,000-fold higher than many other psychotropic agents. To defend the organism from the various dangers arising from the ingestion of alcohol, powerful mechanisms have evolved, likely with particular selective pressure on metabolic pathways.
Gene Variants Contribute to the Risk of Developing Alcoholism
Besides the ADH and ALDH families, other genes contribute to the risk of alcoholism, which likely has an approximately 50% genetic origin. Two general scientific human approaches to identify genes contributing to this risk are genetic linkage analysis and genetic association studies such as genome-wide association studies (GWAS). GWAS employing high numbers (500,000+) of single nucleotide polymorphisms (SNPs) across the genome have now been conducted in a variety of complex disorders and have been shown to be a successful tool in identifying underlying susceptibility genes (for all published GWAS see: www.genome.gov/26525384). Several GWASs have recently been conducted on alcohol-related phenotypes with sample sizes up to 50,000 cases and have implicated a number of novel genes. Interestingly, the most consistent findings have again been seen in genetic variants of alcohol-metabolizing genes. GWASs offer great promise for detecting candidate genes for the development of alcohol addiction. Human genetic data can be further enriched by information from animal studies. A new translational approach for the integration of data sets that derive from forward genetics in animals and genetic association studies including GWAS in humans is called convergent functional genomics. The aim of forward genetics in animals and association studies in humans is to identify mutations (e.g., single nucleotide polymorphisms – SNPs) that produce a certain phenotype, i.e., “from phenotype to genotype.” The repertoire of forward genetics in animals includes the generation of random mutations in an organism, either by radiation or by chemical mutagens such as N-ethyl-N-nitrosourea, and then through a series of breeding of subsequent generations, isolating individuals with a phenotype relevant for addictive behavior. Most powerful, however, in terms of forward genetics is combined quantitative trait loci (QTL) analysis and differential gene expression profiling in recombinant inbreed rodent lines or genetically selected animals for a specific phenotype, e.g., high vs. low alcohol consumption (Table 3). Baysian approaches allow combining such animal genomics data with GWAS information from a similar addiction-relevant human phenotype, thereby enhancing the explanatory power of genetic studies.
In 2009, a convergent functional genomics approach targeting alcohol addiction was successfully applied by a research team from the German Central Institute of Mental Health in Mannheim. In this study, Marcella Rietschel and colleagues conducted a GWAS on a well-characterized case-control sample of alcohol-addicted patients and found approximately 100 SNPs with very low p-values. This information was combined with comparison of global gene expression profiles in alcohol-addicted versus nonaddicted rats which added another 20 SNPs which would not have been considered solely on the basis of the human data. The combined pool of candidate SNPs was genotyped in a replication sample. Fifteen SNPs showed significant association with the same allele as in the original GWAS, eight of these derived from the animal data demonstrating that relevant genetic information had been added by the convergent functional genomics strategy. Nine SNPs were located in genes, including ADH1 that had been reported previously to be associated with alcohol addiction demonstrating the validity of such a hypothesis-free whole genomic approach. One major finding, an SNP in the gene for GATA-binding protein 4 (GATA4), could be replicated several times by independent research teams. Notably, this particular GATA4 SNP was found to be associated with the risk of relapse in abstinent patients. This gene encodes a member of the GATA family of zinc finger transcription factors and controls expression of natriuretic peptide (ANP). A gene dose effect was found in the different GATA4 genotypes explaining the variance of ANP plasma concentration. Hence, genetic variation in GATA4 might influence relapse via modulation of ANP plasma levels. These results will help to identify abstinent patients who are at an increased risk of relapse and further show that gene products acting on peripheral organs – GATA4 and ANP are crucial for myocardial differentiation and function – can impact on addictive behavior.
Another example of how the response to pharmacological treatment may be influenced by genetic polymorphisms in drug target genes is the previously discussed functional SNP in the μ-opioid receptor gene, which predicts naltrexone efficacy as measured in terms of relapse behavior. This is probably the most solid pharmacogenetic finding in psychopharmacology, and a simple genetic test will help to improve the treatment situation with naltrexone dramatically.
The functional relevance of a genetic variant for addictive behaviors and the underlying brain circuits can be studied in humans. This has become possible with vast developments in imaging techniques such as functional MRI (fMRI) and spectroscopy (MRS). With these techniques, neurobiologically relevant endophenotypes can be assessed and correlated with gene variations, an approach termed imaging genetics. For example, IMAGEN is the first multicenter functional and structural imaging genetics study aiming at identifying the genetic and neurobiological basis of alcohol addiction and other psychiatric disorders in 2,000 adolescents. This large sample provides sufficient statistical power to study the contribution of a single gene variant to a specific endophenotype.
However, the most powerful approach for functional validation of candidate genes derived from genomics studies such as the ones discussed above is reverse genetics. This “from genotype to phenotype” experimental strategy allows to test the effect of a candidate gene on a specific behavior, e.g., alcohol relapse. The most common reverse genetic approach is deletion of the gene in question in mice, generally known as knockout, and subsequent behavioral analysis. However, the generation of a conventional knockout model is time consuming and cost-intensive, has no tissue specificity, and, since the gene is ablated early in development, numerous compensatory mechanisms may ensue. Through more advanced techniques such as Cre/loxP and tetracycline-inducible systems, a gene of interest can be expressed or inactivated in a tissue-specific and time-controlled manner. More than 100 genes have by now been studied for alcohol reinforcement and consumption in genetically modified rodent models. Most studies have focused on brain-expressed genes with a wide range of function, including most of the principal neurotransmitter systems, several neurohormones, and a number of signaling molecules. In terms of alcohol intake, about one-fourth of the genetic modifications increased consumption and approximately the same percentage decreased alcohol consumption. Genetically modified rodent models confirm the role of glutamate, GABA, and DA in alcohol reinforcement, and some mouse mutants also produced surprising results. For example, in mice lacking a functional corticotropin-releasing hormone receptor 1 (CRHR1), an astonishing gene × environment interaction was discovered (see below). In summary, more than 50 genes have been identified that influence alcohol consumption and some genes have been found to induce excessive alcohol consumption especially under specific environmental conditions. Far less, however, is known about the genes and molecular networks that lead to addictive behavior. In order to study this, not only genetically manipulated laboratory animals are needed but also appropriate animal models that allow to measure craving (compulsive alcohol seeking) and relapse. These animal models do in fact exist and are extensively used to study potential anticraving and antirelapse compounds (Box 1).
Box 1 Animal Models for Compulsive Alcohol Seeking (Craving) and Relapse
The core features of alcohol addiction are the progressive loss of control over the amount and the contextual circumstances accompanying alcohol use. This results in a compulsive search (craving) and inability to refrain from its use even after long periods of abstinence (relapse), especially when exposed to stimuli previously associated with alcohol consumption. These core features, i.e., loss of control, craving, and relapse can be mimicked in animal models. The validity of animal models is typically assessed using three evaluation criteria, including face, construct, and predictive validity. Reliability is also a critical issue in complex animal models. At the present time, the reinstatement and alcohol deprivation paradigms are the models for which these issues have been addressed most systematically.
Alcohol seeking (craving) in animals can be studies by the so-called reinstatement paradigm. In this procedure, an animal is trained to self-administer alcohol and is then subjected to extinction, that is, the animal is tested under conditions of nonreinforcement until operant responding appears to be extinguished. When the animal reaches some criterion of unresponsiveness, various stimuli are presented. A stimulus is said to reinstate the alcohol-seeking behavior if it causes renewed responding, i.e., lever pressing, without any further response-contingent drug reward. In humans, alcohol priming, negative mood states, and stress- or drug-associated cues are able to produce an increase of self-reported craving. Similarly, reinstatement of alcohol-seeking behavior in rodents can be induced by a small quantity of alcohol. This phenomenon is consistent with the widely reported description of the “first-drink” phenomenon by which ingestion of a small amount of alcohol may induce a strong subjective state of craving in abstinent alcohol-dependent subjects. This priming effect can even occur in alcoholics who have been abstinent for years. Stress caused by intermittent mild electric shocks to the animals’ feet as well as alcohol-associated olfactory cues can also reinstate previously extinguished responding for alcohol. The neuronal substrates mediating alcohol-, stress-, and cue-induced reinstatement are not identical. For example, the opioid receptor antagonist naltrexone reduces cue-induced reinstatement of alcohol-seeking behavior, whereas stress-induced reinstatement is not affected by this drug but can be blocked by CRHR1 antagonists. Furthermore, foot-shock stress and response-contingent presentation of an alcohol-associated light cue, acting as a conditioned stimulus, also augments reinstated responding. Thus, additive effects of these stimuli on responding are observed showing that more than one neurobiological pathway are involved in provoking alcohol-seeking behavior. In conclusion, the reinstatement model can be used to study the neurobiological and molecular basis of alcohol seeking since there appears to be a good correspondence between the events that induce this behavior in laboratory animals and those that provoke craving in humans. Furthermore, naltrexone reduces craving in alcohol-dependent patients and cue-induced reinstatement of alcohol seeking in animals.
In the alcohol deprivation paradigm, rats have free access to different alcohol solutions (5%, 10%, and 20% reflecting alcoholic beverages consumed by humans such as beer, wine, and spirits). After 2 months of continuous access to alcohol, the rats are deprived of alcohol for 3 days. Following this deprivation phase, all alcohol solutions are presented again. This procedure is repeated monthly for the following 10 months. The introduction of repeated deprivation (withdrawal) phases for several days/weeks is crucial in developing an addictive behavior, as the negative consequences of acute, protracted, and conditioned withdrawal trigger further drinking and induce relapse behavior. Following a deprivation phase, representation of the alcohol solutions leads to a pronounced transient rise in alcohol intake and preference. This is termed the alcohol deprivation effect (ADE). This relapse-like drinking phenomenon is observed across several species including rats, mice, monkeys, and human social drinkers. The increase in alcohol drinking probably reflects an increase in alcohol seeking, which, according to self-reports of some alcohol-addicted subjects, can also increase progressively during abstinence. Following repeated ADEs, alcohol drinking behavior can become uncontrolled and compulsive, which becomes evident by a progressive resistance of the animal’s alcohol consumption to taste adulteration. In this experiment, quinine is added to the alcohol solution. Despite the aversive taste, compulsively drinking rats consume large amounts of the quinine-containing alcohol solution following a deprivation phase. In addition, pronounced changes in the diurnal rhythm of drinking activity are observed following alcohol deprivation in chronic drinking rats. Most of these animals still show high drinking activity during the inactive phase, and some animals even show no differences in drinking activity between the dark and light phases of the daily cycle. Such a level of drinking activity is far beyond the normal controlled behavior seen in the appropriate control animals and points to alterations in circadian rhythmicity. In summary, following a deprivation phase, changes in the alcohol intake patterns of animals are seen. The animals consume not only more alcohol but also large amounts of highly concentrated alcohol solutions at inappropriate times during their daily cycle in an uncontrolled and compulsive manner. The ADE in long-term voluntary alcohol drinking rats is therefore used as a measure of compulsive drinking and as a measure of relapse behavior. Finally, the clinically effective antirelapse drugs acamprosate and naltrexone reduce or even abolish the ADE lending predictive value to this model for the development of novel and improved drugs for the treatment of craving and relapse. Today, this animal model is used as a gold standard for testing novel synthesized antirelapse compounds.
A third approach is called postdependent drinking – a procedure that leads to allostatic dysregulation of the reward system, a process that according to George Koob from the Scripps Research Institute in La Jolla is crucial in the development of alcohol addiction. He proposes that chronic alcohol intake induces counteradaptive processes within the reward system that fail to return within the normal homeostatic range resulting in an allostatic state, i.e., a chronic deviation from the normal reward set point. This state is not only caused by dysregulation of reward circuits, but also by the activation of brain and hormonal stress responses. In order to induce an allostatic dysregulation of reward function in animals, repeated exposure to high amounts of alcohol is used. In one experimental setup, rats or mice are housed for several weeks in alcohol vapor chambers to deliver repeated cycles of alcohol intoxication and withdrawal. This procedure leads to long-lasting neural plasticity and persistently increased alcohol intake. For example, rats are trained to respond for a 10% alcohol solution in an operant situation and then are exposed to chronic intermittent alcohol vapor for 8 weeks. When subsequently tested for operant alcohol responding or reinstatement behavior, persistent augmented alcohol responding will be observed. In conclusion, exposure to repeated cycles of intoxication and withdrawal leads to persistently increased alcohol intake in rodents (called postdependent state). Importantly, the postdependent state is also characterized by an augmented behavioral sensitivity to stress, and a pathological engagement of extrahypothalamic CRH transmission and CRHR1 signaling is the underlying mechanism.
Excessive Alcohol Consumption Is Often a Result of Complex Gene ✕ Environment Interaction
The general role of CRH transmission and CRHR1 activation is to mediate stress-induced behaviors and neuroendocrine responses. CRH and its receptor were first identified by its ability to stimulate adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary, ultimately resulting in glucocorticoid release from the adrenal cortex. Extensive networks of CRH-expressing neurons and CRHR1 are also present in extra-hypothalamic-pituitary-adrenocortical (HPA) structures, including the amygdala. Mice lacking a functional CRHR1 were studied in a free-choice alcohol drinking paradigm. Water and an alcohol solution that was given at increasing concentrations were offered as drinking fluids. In these experiments, the genotypes did not differ in their daily intake of alcohol. All mice were then repeatedly exposed to a social defeat stress and a forced swim stress. During these stress episodes, no differences in alcohol intake compared with baseline drinking were observed in either the wild type or knockouts. After a period of about 3 weeks, however, the alcohol intake of the knockout mice began progressively to increase. This increased alcohol intake in the knockouts persisted and was still present 6 months after exposure to the second set of stressors. In comparison, those knockouts with long-term voluntary access to alcohol that had not been exposed to the two sets of stressors displayed no changes in alcohol intake over time. In summary, knockout mice that lack a functional CRHR1 receptor do not differ from wild-type mice in alcohol intake and preference under stress-free housing conditions. After repeated stress, however, the knockouts increase their alcohol consumption, which is then maintained at an elevated level throughout their life span. In a similar vein, a lowered threshold for stress-induced reinstatement of alcohol seeking in alcohol-preferring msP rats (Table 3) was described. These animals show a genetic variation of the Crhr1 promoter that is accompanied by increased CRH1 receptor density. This shows that Crhr1 genotype and expression interact with environmental stress to reinstate alcohol-seeking behavior. In conclusion, this is one of the first striking gene × environment interactions to have been demonstrated for alcohol seeking and consumption. From these findings, it can be assumed that alterations in the human CRH1 receptor gene (hCRHR1) might constitute a genetic risk factor for alcoholism, particularly when associated with stressful life events; indeed, human genetic studies have been able to establish such a link. In one study, two haplotype SNPs (htSNP – clusters of variants that are in linkage disequilibrium, which means that determining a single SNP, the tagging SNP, gives sufficient information for the entire cluster) within the CRHR1 locus have been associated with risky drinking patterns in two independent population samples. One sample is derived from the Mannheim Study of Children at Risk, an ongoing epidemiological cohort study of the outcome of early risk factors from infancy into adulthood. In this cohort, drinking behavior and stressful life events were assessed. The adverse life event items addressed all areas of young adult life, i.e., transition from school to job, partner, family, parents, living conditions, legal problems, and health problems. In addition, an assessment of all negative life events occurring over the previous 3 years was obtained by means of a standardized interview with the parents. Interactions between the two htSNPs covering the hCRHR1 gene and adverse life events with respect to heavy drinking in adolescence were then studied, and a gene × environment interaction was detected. These findings provide the first evidence in humans that the hCRHR1 gene interacts with exposure to stressful life events and may predict heavy alcohol use in adolescents. In the meantime, this finding has been replicated several times and is one of the best examples of gene × environment interaction in the field of biological psychiatry research.
The CRH System Is Involved in the Progression of Addictive Behavior
The alcohol-addicted brain is characterized by long-term neuroadaptations that recruit a negative emotional state which leads to excessive alcohol ingestion motivated by relief of negative emotionality (Fig. 5). Modeling this process by several investigators has allowed validating the augmented CRH signaling in the amygdala as a pathological mechanism that drives negative emotionality and thereby relief drinking. A pathological engagement of extra-HPA CRH/CRHR1 signaling, in particular in the amygdala, has been found in the postdependent state (Box 1). Postdependent escalation can be inhibited by systemic or intra-amygdala application of CRHR1 antagonists. The latter finding is likely to reflect an upregulation of CRHR1 expression within the amygdala in the postdependent state. CRH/CRHR1 signaling also plays a role in mediating stress-related drinking situations. A variety of physical and psychological stressors are important risk factors for the induction of craving and relapse, and it has been shown that stress is a main contributor in reinstating alcohol-seeking behavior in animals. Stress- but not cue-induced alcohol-seeking responses (Box 1) are blocked by systemic administration of CRHR1 antagonists demonstrating an involvement of CRH/CRHR1 signaling also in stress-related drinking behaviors. Thus, recruitment of the amygdala CRH system is key to dependence and stress-induced behavioral phenotypes.
Conceptual framework for the progression of alcohol addiction over time, illustrating the shift in underlying motivational mechanisms adapted and modified from Heilig and Koob (2007). From initial, largely positively reinforcing, pleasurable alcohol effects, the addictive process progresses over time to be maintained by relief from a negative emotional state (negative reinforcement). The positive reinforcing effects of alcohol are mainly DA driven and shift to the negative reinforcing effects of alcohol results from progressive recruitment of the CRH system. Maladaptive interactions of DA and CRH within the amygdala may underlie the shift into compulsive alcohol use as proposed by our colleague Dr. Anita Hansson (Modified from Heilig and Koob (2007))
This all provides convincing evidence that selective CRHR1 blockade may be a treatment option in alcohol-addicted patients. In line with this, several pharmaceutical companies have developed new compounds for translation of these basic findings into clinical applications. However, the use of specific CRHR1 antagonists in humans may face two limitations. First, only a relapse risk that is driven by a high stress load may be efficiently attenuated. Secondly, actions of CRHR1 antagonists on HPA-axis activity might counteract their desired therapeutic effects in alcohol-addicted patients.
The Glutamate Theory of Alcoholism
In a key publication more than 20 years ago, David Lovinger and colleagues from the NIAAA demonstrated that NMDA receptor function is inhibited by alcohol in a concentration-dependent manner over the range of 5–50 mM (for reference, BAC of 50 mg/dL is equivalent to an concentration of 10.6 mM), a range that also produces intoxication. Together with other findings, this suggests that alcohol-induced inhibition of responses to NMDA receptor activation contribute to the neural and cognitive impairments associated with intoxication. But how can the molecule ethanol directly interfere with NMDA receptor function? The NMDA receptor is a ligand-gated ion channel with a heteromeric assembly of NR1, NR2 (A–D), and NR3 subunits. The NR1 subunit is crucial for channel function, the NR2 subunits contain the glutamate-binding site, and the NR3 subunits have some modulatory function on channel activity, especially under pathological conditions. Electrophysiological studies show that alcohol interacts with domains that influence channel activity, suggesting that residues within transmembrane domains may be involved. In the search for a possible binding site of alcohol at the NMDA receptor, several site-directed mutagenesis studies have been performed and putative binding sites in TM3 and 4 of the NR1 and NR2A subunits, respectively, identified. The NMDA receptor is, therefore, a primary molecular interaction site for ethanol molecules.
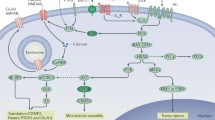
In recent years, the glutamate theory of alcoholism has emerged as a major theory in the addiction research field. In fact, alcohol affects the glutamatergic system on the molecular, synaptic, and cellular levels, and one hypothesis within the framework of the glutamate theory proposes that alcohol consumption may lead to an enhanced activity of the glutamatergic system in alcohol-dependent patients. This glutamate-induced hyperexcitability within the central nervous system (hyperglutamatergic state) becomes uncovered during alcohol withdrawal (Fig. 6).
The glutamate theory of alcohol addiction is the most prominent theory in the alcohol research field. The antiglutamatergic effect of alcohol intake on the NMDA receptor results in an upregulation of glutamatergic activity following chronic exposure. During alcohol withdrawal and conditioned withdrawal responses, this results in an overshooting glutamate response – a hyperglutamatergic state which is associated with craving and relapse behavior (Redrawn from Spanagel and Kiefer (2008))
Numerous microdialysis studies have consistently shown augmented glutamate levels in various brain sites during acute and conditioned withdrawal. Furthermore, it has been suggested that augmented glutamatergic activity during protracted abstinence may contribute to craving and relapse behavior, thus providing the rationale for using antiglutamatergic compounds such as acamprosate for relapse prevention. Hence, the clinically used compound acamprosate dampens a hyperglutamatergic state – a finding which has not only been made in animals but also in humans by means of glutamate spectroscopy. Further, by using this MR-based technology, enhanced glutamate levels in the brain of alcohol-addicted patients during withdrawal have been demonstrated. The mode of action of acamprosate provides a good rationale for the glutamatergic theory of alcoholism and suggests further that the blockade of ionotropic glutamate receptors should also reduce relapse and craving. In fact, memantine and neramexane – both are NMDA receptor channel blockers – can dose-dependently reduce the ADE. Both compounds produce alcohol-like effects in animals and humans and attenuate cue-induced craving in a dose-related fashion in alcohol-addicted patients, providing a new concept of a substitution therapy for alcoholism.
Blockade of AMPA receptors also dose-dependently reduces cue-induced reinstatement behavior and the ADE. Using GluA1, A2, and A3 knockout mice, it was shown that the GluA3 subunit is critical in mediating these behaviors. Interestingly, topiramate – an anticonvulsant compound that blocks AMPA/kainate receptors, in addition to having other sites of action – reduces relapse rates in alcohol-addicted patients and the harm of excessive drinking. Not only ionotropic but also metabotropic glutamate receptors, which modulate the glutamatergic tone, hold a great potential for antirelapse medication. Thus, mGlu5 receptor antagonists are effective in the reinstatement and ADE model and no tolerance to their antirelapse-like effects develops as MPEP, a selective mGlu5 receptor antagonist, exhibits the same effect size in the ADE model even after multiple treatment cycles. This is an important finding as tolerance to repeated acamprosate treatment has been reported; however, considering the cognitive deficits repeatedly reported under mGluR5 blockade, the clinical usability of these compounds has to be clarified in future studies. Activation of group II metabotropic glutamate receptors attenuates both stress- and cue-induced reinstatement behavior and the ADE as well. These are very important findings with respect to the glutamate theory of alcoholism as activation of presynaptic mGlu2/3 receptors may lower a hyperglutamatergic tone and thereby reduce the risk of relapse. How can these preclinical findings be translated into the human situation?
Translational Approach in Medication Development
In the field of research into medications for alcohol addiction, a roadmap for translational research has recently been provided by Markus Heilig and his research group at the NIAAA. In the absence of suitable pharmacological tools to target the CRH system, they decided to explore the possibility of interacting with a closely related stress-mediating neuropeptide, substance P, which signals through its neurokinin 1 receptor. Following their preclinical finding that mice genetically deficient in neurokinin 1 receptors show a marked decrease in voluntary alcohol consumption, the group performed an explorative randomized study in recently detoxified alcohol-dependent inpatients using the neurokinin 1 receptor antagonist LY686017 and placebo. LY686017 suppressed spontaneous alcohol craving, improved general well-being, blunted craving induced by a stress challenge procedure, and attenuated concomitant cortisol responses. In addition, it was shown that LY686017 reduced fMRI responses elicited by alcohol-related cues. These findings indicate the potential efficacy of this drug as an anticraving and antirelapse medication. Moreover, these series of experiments represents a genuine translational approach to the linking of preclinical work and clinical efficacy, a link which could otherwise only be established through the performance of time-consuming and cost-intensive phase II/III studies. Two pharmaceutical companies are now exploiting these positive results in full-scale clinical trials. This sets the example of how drug development should proceed, i.e., on the basis of the identification of putative target molecules from either a hypothesis-free whole genomic approach or a transcriptomic approach. Functional validation must then be provided in appropriate animal models. Having achieved a positive signal in these animal models, studies in alcohol-addicted subjects need to be performed that include, as a minimum, measures of cue and stress reactivity. If a positive signal is once more obtained, then a randomized clinical trial (RCT) is warranted.
Outlook
In a recent review about neurobiology and addiction, the Canadian alcohol researcher Harold Kalant comes to an important conclusion: “It is inherently impossible to explain addiction by pursuing only the analytical study of drug interactions with the nervous system at ever finer levels of molecular structure and function.” Instead of the classical reductive scientific approach in alcohol addiction research, it is nowadays proposed to take a systems approach for achieving a better understanding of addictive behavior. Thus, only by studying the interaction of drug effects with the different system levels, i.e., from the genetic to the molecular level, from the synaptic to the neuronal network level, and finally from the behavioral level to its interactions with the environment, we will be able to detangle the multidimensional puzzle of addictive behavior.
The different levels can then be studied using new -omics technologies which allow the identification of genetic variations and quantification of molecules at the levels of mRNA, protein, and metabolites on a global scale. Furthermore, the use of multielectrode in vivo recordings enables to learn more about the neuronal ensembles involved in disease progression, while a variety of neuroimaging techniques allow the evaluation of neuronal network activity on a much larger scale. For the first time, therefore, we are in a position to gather comprehensive data systematically on different biological system levels. In such a hypothesis-free approach, we receive bioinformation on all system levels, ranging from the gene to molecules to synaptic plasticity to neuronal network activity. By means of computational neuroscience, this novel information can be combined with what has been learned during the 30 years’ experience of a hypothesis-driven reductionist approach in neurobiological-oriented alcohol research, and this will then hopefully lead to a better understanding of the molecular and physiological processes underlying alcoholism.
Although further breakthroughs in preclinical research on pharmacological anticraving and antirelapse compounds will be attained in the near future, it remains questionable whether these drugs will really enter the market. Preclinical research can validate many good treatment targets; however, unless pharmaceutical industry gets onboard to bring forward molecules with drug-like properties for these targets, clearly no clinical development can happen. As an alternative to pharmacological interventions, deep brain stimulation (DBS) has been already successfully applied in severe cases of alcohol-addicted patients by stimulation in the nucleus accumbens. Clearly, in the next years to come, ethical issues have to be considered by our society and more animal and human research is needed to study the mode of action of DBS, the limitations, the side effect profiles and the long-term consequences of this extremely promising treatment procedure.
Abbreviations
- 5-HT3:
-
Serotonin type 3 receptor
- AA:
-
Alko alcohol (alcohol-preferring rat line)
- ACTH:
-
Adrenocorticotropic hormone
- ADE:
-
Alcohol deprivation effect
- ADH:
-
Alcohol dehydrogenase
- ALDH:
-
Aldehyde dehydrogenase
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (specific agonist for the AMPA receptor mimicking the effects of the neurotransmitter glutamate)
- ANA:
-
Alko nonalcohol (alcohol-nonpreferring rat line)
- ANP:
-
Atrial natriuretic peptide
- ARND:
-
Alcohol-related neurodevelopmental disorder
- ATP:
-
Adenosintriphosphate
- BAC:
-
Blood alcohol concentration
- CRH:
-
Corticotropin-releasing hormone
- CRHR1:
-
Corticotropin-releasing hormone receptor 1
- DA:
-
Dopamine
- DALs:
-
Disability-adjusted life-years
- DBS:
-
Deep brain stimulation
- DSM-IV:
-
Diagnostic and Statistical Manual of Mental Disorders – Version IV
- EPSC:
-
Excitatory postsynaptic current
- FAS:
-
Fetal alcohol syndrome
- fMRI:
-
Functional magnetic resonance imaging
- GABA:
-
Gamma-aminobutyric acid
- GATA4:
-
GATA-binding protein 4
- GHSR-1A:
-
Growth hormone secretagogue receptor
- GluA1:
-
Glutamate receptor 1
- GWAS:
-
Genome-wide association studies
- hCRHR1:
-
Human CRH1 receptor gene
- HPA axis:
-
Hypothalamic-pituitary-adrenocortical axis
- htSNP:
-
Haplotype SNP
- ICD-10:
-
WHO’s International Classification of Diseases – Version 10
- LTP:
-
Long-term potentiation
- LY686017:
-
Neurokinin 1 receptor antagonist
- MPEP:
-
2-Methyl-6-(phenylethynyl)pyridine hydrochloride (a selective mGluR5 receptor antagonist)
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- NAC:
-
Nucleus accumbens
- nAch:
-
Neuronal acetylcholine
- NIAAA:
-
National Institute on Alcohol Abuse and Alcoholism
- NMDA:
-
N-methyl-d-aspartate (specific agonist at the NMDA receptor mimicking the action of glutamate)
- PET:
-
Positron emission tomography
- PFC:
-
Prefrontal cortex
- QTL:
-
Quantitative trait loci
- RCT:
-
Randomized clinical trial
- SNPs:
-
Single nucleotide polymorphisms
- VTA:
-
Ventral tegmental area
- WHO:
-
World Health Organization
References
Björk K, Hansson AC, Sommer WH (2010) Genetic variation and brain gene expression in rodent models of alcoholism implications for medication development. Int Rev Neurobiol 91:129–171
Gass JT, Olive MF (2008) Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol 75:218–265
George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M (2008) Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science 319:1536–1539
Heilig M, Koob GF (2007) A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci 30:399–406
Hendler et al (2012) In: Sommer WH, Spanagel R (eds) Neurobiology of alcoholism (in press)
Koob GF (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–243
Müller CP, Schumann G (2011) Drugs as instruments – a new framework for nonaddictive psychoactive drug use. Behav Brain Sci 34(6):293–310
Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J (2009) Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 373:2223–2233
Shield KD et al (2011) Global and country specific adult per capita consumption of alcohol, 2008. Sucht 57:99–117
Sommer WH, Spanagel R (eds) (2012) Behavioral neurobiology of alcohol addiction. Current topics in behavioral neuroscience (CTBN). Springer (in press)
Spanagel R (2009) Alcoholism – a systems approach from molecular physiology to behavior. Physiol Rev 89:649–705
Spanagel R, Kiefer F (2008) Drugs for relapse prevention of alcoholism – 10 years of progress. Trends Pharmacol Sci 29:109–115
Spanagel R, Mann K (eds) (2005) Drugs for relapse prevention of alcoholism. Milestones in drug therapy. Birkhäuser, Basel
Spanagel R, Weiss F (1999) The dopamine hypothesis of reward: past and current status. Trends Neurosci 22:521–527
Wiens F, Zitzmann A, Lachance MA, Yegeles M, Pragst F, Wurst FM, von Holst D, Guan SL, Spanagel R (2008) Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci U S A 105:10426–10431
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this entry
Cite this entry
Spanagel, R., Zink, M., Sommer, W.H. (2022). Alcohol: Neurobiology of Alcohol Addiction. In: Pfaff, D.W., Volkow, N.D., Rubenstein, J.L. (eds) Neuroscience in the 21st Century. Springer, Cham. https://doi.org/10.1007/978-3-030-88832-9_107
Download citation
DOI: https://doi.org/10.1007/978-3-030-88832-9_107
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-88831-2
Online ISBN: 978-3-030-88832-9
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences