Abstract
The toxic effects of different forms of nanomaterials comprise a series of biological effects such as oxidative stress; DNA damage; inflammatory response; activation of nuclear transcription factors. Some of these are key characteristics of human carcinogens and have been considered for hazard identification of nanomaterials. In addition, epigenetic changes also play a key role in the multi-step sequential process of carcinogenesis. Epigenetic modifications may constitute changes in DNA methylation, histone modifications (methylation, acetylation etc), and changes in non-coding RNA, leading to an altered gene expression profile. In this chapter, we describe the state-of-the-art of epigenetic modifications induced by different nanomaterials, from a limited number of in vitro- in vivo and human studies, a majority of which is primarily focused on DNA methylation. We also highlight the potential challenges and future directions in the field of epigenetics research in nanomaterial toxicology.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Different aspects of nanotoxicology has been discussed in the previous chapters, and it has become abundantly clear that nanoparticles (NPs) can induce oxidative stress [30, 53, 60, 75], DNA damage [30, 59, 76], alter DNA repair efficiency [11, 91], induce an inflammatory response [75, 76], can potentially be immunomodulatory/immunosuppressive [13, 42, 62, 66] and can affect cell death/cell proliferation [1, 21, 48, 72, 79, 95]. While, it is also clear that the field of nanotoxicology research is quickly emerging and there are significant knowledge gaps, some of the observed effects reported for one or more nanoparticle align with the “Key Characteristics of Carcinogens as a Basis for Organizing Data on Mechanisms of Carcinogenesis” described by Smith et al. [92]. In addition, the “10 key characteristics” identified by Smith et al. [92] towards organization of mechanistic data, also included epigenetic alterations.
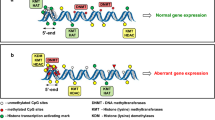
Epigenetic alterations are defined (by NCI) as changes “in the chemical structure of DNA that does not change the DNA coding sequence” [19]. In other words, epigenetic alterations are broadly considered heritable changes that do not involve a change in the DNA sequence itself. Epigenetic alterations, such as DNA/RNA methylation, histone modifications (methylation, acetylation), non-coding RNA regulate gene expression by regulating chromatin structure and accessibility. Such alterations/signatures play a crucial role in proper cellular function, differentiation, development and are influenced by a wide range of endogenous and exogenous factors. One of the most studied epigenetic alteration is DNA methylation (and demethylation), where a methyl-group (CH3) is covalently added to position 5 of the cytosine pyrimidine ring, and a family of DNA methyltransferase (DNMT) and ten-eleven translocation (TET) enzymes play important role in the maintenance of methylation pattern [40, 46, 100]. It is generally accepted that CpG islands (regions with a high frequency of CpG sites) in promoter regions of genes are predominantly unmethylated which activate gene transcription [4, 5]. Besides, DNA methylation, histone modifications play crucial role in maintaining chromatin conformation, and these processes are tightly coupled [12, 27, 40]. These post-translational covalent modifications of histone mainly studied in the tail domains of H3 and H4, include modifications such as acetylation and methylation of lysine and arginine among other [12, 27, 40]; and are regulated by enzyme families such as histone acetyl transferase (HAT), histone deacetylase (HDAC), Histone methyltransferase (HMT). Another group of key regulators are the non-coding RNAs and regulate gene expression at the transcriptional and posttranscriptional levels [26, 99], and closely interact with the DNA methylation and histone modification machinery [41, 102].
From the standpoint of environmental and occupational diseases and that of chemical induced carcinogenesis, it is considerably well established that epigenetic modifications play a crucial role. Epigenetic changes induced by genotoxic environmental carcinogens have been systematically reviewed by Chappell et al. [14]. While epigenetic changes can be used as biomarker of exposure, or as marker of disease and disease progression [52] they can also be used as potential therapeutic targets [50]. This is particularly relevant for NP toxicology, where rate of NP production far exceeds the hazard identification and risk assessment, while an increasing number of workers and consumers are being exposed to these diverse group of materials [8, 38].
Despite considerable progress in the field of epigenetics and disease biology and cancer epigenetics, major gaps remain in understanding of chemical induced epigenetic alterations. This is also true for the field of nanotoxicology. This chapter will therefore summarize the state-of-the-art and developments in understanding NP induced epigenetic changes and comment briefly on future direction. While the NPs discussed in the chapter is not exhaustive, it is representative of some of the most produced and studied particles in relation to toxicology. We have identified studies that have evaluated one or more epigenetic endpoints, however no mechanistic conclusion for individual particle or NP as a group has been made in the chapter due to the limited number of comparable studies (differences in particle properties, experimental design, test system and cell types).
2 Epigenetic Changes Induced by Metal and Metal Oxide Nanoparticles
While the evidence stream on epigenetic changes induced by metal and metal oxide nanoparticle are relatively scarce, some studies have observed epigenetic modifications induced by TiO2-NPs, ZnO-NPs, CuO-NPs, SiO2 NPs, silver and gold nanoparticles among others. In this section we discuss briefly, the distinct mechanistic evidence exhibited by each of these NPs and at the same time try to identify the commonality exhibited by these particles.
2.1 TiO2 Nanoparticles
TiO2-NPs are one of the most abundantly produced nanomaterials, with considerable evidence regarding oxidative stress, cyto-genotoxicity, impaired DNA repair efficiency and limited evidence on immunotoxicity, in vitro and in vivo [20, 39, 74, 88, 10, 63]. While limited by number, in this section (Table 9.1), we discuss the growing number of studies that have identified some of the underlying epigenetic mechanism.
Pogribna et al. [80], studied global (5-mC) and gene specific (EpiTect array) changes in DNA methylation, induced by TiO2-NP (Aeroxide TiO2 P25; 100 μg/mL) in Caco-2, HepG2, NL20 and A-431 cell lines. The authors observed global hypomethylation after 72 h of exposure, for A-431, HepG2 and Caco-2 cells and promoter specific methylation changes in several genes associated with apoptosis and cell cycle modulatory effects. Changes were observed in all cell line for CDKN1A and SCARA3, while genes like GADD45A, DNAJC15, TP53 were also differentially methylated in one or more of the cell lines [80]. In the same study, while the expression of the epigenetic regulators (DNMT1, DNMT3A, DNMT3B, MBD2, UHRF1) were altered, it was not consistent among the cell types. Given that there is epigenetic heterogeneity among tissues and cell types, such studies using multiple cell line (of relevance to the exposure route, and target) can be very important.
Despite evidences of the influence of TiO2 crystal phase on toxicity, the effect of crystal phase on epigenetic changes is not well studied. In our study we reported significant global DNA hypomethylation (5-mC), and a decrease in global hydroxymethylation (5-hmC) induced by different crystal phase of TiO2-NP (NM-102/Anatase, NM-104/Rutile, and NM-105/Anatase-rutile) at sub cytotoxic concentrations in 16-HBE cells [32] however such changes were not mechanistically linked to other toxicity endpoints. In another study the authors used surfaced coated TiO2-NP, to study the effect on DNA methylation, in A549 cells, in addition to other cyto-genotoxic endpoints [93]. The authors observed significant decrease in Long Interspersed Nuclear Element-1 (LINE-1) methylation after 72 h of exposure to silica and citrate coated TiO2-NP, while no significant changes were observed after 48 h of exposure.
Patil et al. [77] also reported global DNA (5-mC) hypomethylation, in MRC-5 cells after 24 and 48 h exposure to TiO2-NP (<100 nm, mixture of rutile and anatase, 1 and 8 μg/mL). They also observed reduced mRNA expression of DNA methyltransferases DNMT1, DNMT3A, and DNMT3B [77]. These changes in DNA methylation were associated with induction of oxidative stress. The influence of TiO2-NP (<25 nm, 24 h) induced oxidative stress on DNA methylation was also reported by Bai et al. [2], in A549 cells, where they observed significant DNA hypermethylation in the promoter region of PARP-1 (encoding poly (ADP-ribose) polymerase 1, involved in DNA repair). Based on the other endpoints (cyto-genotoxic and oxidative stress), Stoccoro et al. [93] suggested that oxidative stress could be a primary event in inducing genotoxicity and epigenotoxicity. While most studies have evaluated DNA methylation as a primary epigenetic endpoint, Jayaram and Payne [45], studied the effect of TiO2-NP induced intracellular superoxide on HDAC9 (histone deacetylase 9) expression, they used SOD-TiO2-NPs (SOD corona), and passivated TiO2-NP. Both passivated and SOD particles did not result in intracellular ROS production. SOD-TiO2-NPs were able to scavenge superoxide and the expression of HDAC9 were comparable to control. Similar results were observed for the passivated TiO2-NPs [45]. In a study by the same group, a decrease in HDAC9 expression, and an increase in HDAC10 expression was observed; induced by food-grade TiO2 particles [44]. While food grade TiO2 particle, more specifically E171 may not be primarily a nanoparticle, electron microscopy analysis shows that at least 36% of the particles (by number) are below 100 nm [29, 97]. In another study [57], in addition to oxidative stress, the authors observed significant global DNA hypomethylation in A549 and 16HBE cells at different concentrations (0.1–100 μg mL − 1, 48 h) of TiO2-N25 and TiO2-A60. Both TiO2-N25 and TiO2-A60 exposure resulted in significant changes in the mRNA and protein expression levels of methylation related genes (DNMT3B, TET1, TET2 and TET3) in A549 and 16HBE cells. While, most studies have observed oxidative stress and changes in DNA methylation induced by TiO2-NP, in one study [56] the authors did not observe significant changes in global DNA methylation/hydroxymethylation or LINE1 methylation in TiO2-NP (21 nm) exposed THP-1, SAEC, RAW264.7 cells at subtoxic concentrations (0.5 and 30 μg/mL for 24 h). Studies reporting the effect of long-term exposure to TiO2-NP on epigenetic changes are limited. Sierra et al. [90] observed changes in DNA methylation (21 CpG sites- corresponding to 22 genes), in TiO2-NP (NM-101; 20.99 ± 6.4 nm) exposed BEAS-2B cells after a period of up to 4 weeks. Since the number of differentially methylated genes were few, further enrichment analysis was not performed on the set.
In an in vivo study [58], the effect of anatase TiO2-NP (25 nm), after 30 day-intranasal instillations in young (5-week) and adult (10-week) mice was investigated and observed that the lung of young mice were more susceptible, compared to the adult mice. While, inflammatory markers were upregulated in both young and adult mice, significant global DNA hypomethylation and decrease in DNA hydroxymethylation were observed in young mice. Significant changes in sequence specific methylation of TNF-α promoter (2 positions) and Thy-1 (cell surface antigen) were also observed. Expression of DNMT mRNAs (DNMT1, DNMT3A and DNMT3B) were significantly upregulated in the young mice as well. Changes in DNA methylation, DNMT expression, and changes in expression of genes associated with pathways in cancer indicated the importance of epigenetic regulation and the potential to be used as biomarker for exposure and disease [58]. Additionally, in a cross-sectional study, Liou et al. [54], investigated the effect of TiO2 exposure in workers handling (n = 26) TiO2-NP and observed an increase in oxidative stress, but no changes in global DNA methylation were observed.
Overall, the studies reporting epigenetic alterations induced by TiO2-NP, primarily investigated DNA methylation changes. Despite the heterogeneity in particle and cell types used is such studies, evidence indicate towards disruption of DNA methyltransferase (DNMT) activity and DNA hypomethylation, often associated with increased oxidative stress. Since oxidative stress and oxidative DNA damage has been shown to interfere with the ability of DNMTs to efficiently interact with DNA [104], resulting in DNA hypomethylation and genomic instability, oxidative stress induced epigenetic mechanisms warrant further investigation.
2.2 Zinc and ZnO Nanoparticles
Previous studies have identified ZnO-NP induced cyto-genotoxicity and oxidative stress, in vitro and in vivo, and a major attribute of ZnO-NP toxicity is ionization/dissolution [96, 101]. However, only a handful of studies have investigated epigenetic alteration. In a study (Table 9.2) which also evaluated epigenetic effect of TiO2-NP, Patil et al. [77] reported DNA hypomethylation and changes in mRNA expression of DNMT1, DNMT3A, and DNMT3B, in MRC-5 cells for ZnO-NP (<100 nm, 1 and 8 μg/mL, 24 and 48 h exposure). Choudhury et al. [84] investigated the effect of ZnO-NP (90 ± 2 nm) in human embryonic kidney cells (HEK-293) on several key endpoints and observed a significant increase in oxidative stress and apoptosis. The authors observed a significant DNA hypomethylation, sequence specific demethylation of LINE1 and an increase in DNA hydroxymethylation, and a significant increase in expression of Ten-Eleven Translocation (TET) [84]. Another in vitro study in HaCaT cells, exposed to ZnO-NP revealed significant increase in H3K9 methylation and a decrease in H4K5 acetylation [28]. These changes were associated with an increase in expression of pro-apoptotic genes and increased oxidative stress and DNA damage and G2/M cell cycle arrest. While, the limited number of studies provide some evidence regarding oxidative stress induced epigenetic alteration, further studies (both in vitro and in vivo) are required to evaluate the epigenetic changes induced by ZnO-NP taking into account physicochemical attributes such as dissolution.
2.3 Silica Nanoparticles
Only few studies have reported epigenetic alterations induced by silica nanoparticles (Table 9.3). One such evidence comes from the study by Seidel et al. [86] in Bhas 42 cell, exposed to Min-U-Sil® 5 (crystalline silica, 15 and 25 μg/cm2) or NM-203 (pyrogenic amorphous silica, 2 and 5 μg/cm2). The authors observed significant global DNA hypomethylation and significant changes in the DNMT expression (increased DNMT3A and DNMT3B) [86] and an increase in Histone H4 acetylation and significant changes in HDAC expression by Min-U-Sil® 5, but not for NM-203. For Min-U-Sil® 5, the authors also observed increased acetylated histone H3 lysine 4 (H3K4Ac), histone H3 lysine 9 (H3K9Ac) and acetylated histone H3 lysine 27 (H3K27Ac) on c-Myc promoter, which plays important role in cellular metabolism and proliferation and in cell transformation [86]. Epigenetic alterations were also studied in HaCaT cells, exposed to Sio2 NP (15 nm) [36]; where the authors observed significant increase in promoter methylation of Poly [ADP-ribose] polymerase 1 (PARP-1), which plays key role in chromatin remodelling and multiple DNA damage repair pathways [82]. Using a DNMT1 knockdown, the authors confirmed the role of DNMT1 in the observed Sio2 NP mediated effect. Sio2 NP exposure (5 μg/mL, 30 passage) in BEAS-2B cells also induced significant changes in DNA methylation including PI3K-Akt and sequence specific hypermethylation of CREB3L1 and Bcl-2 promoters indicating the role of mitochondria mediated apoptosis [105]. In a cross-sectional study Liou et al. [54], observed an increase in oxidative stress markers in a group of workers handling SiO2 (n = 31), but changes in DNA methylation was not observed. Based on these studies, it can be suggested that Sio2 NP can induce epigenetic changes to the DNA and histone, and these changes are primarily associated with cell cycle regulation and proliferation. However, important aspect of SiO2 NP toxicity such as the crystal structure and surface silanols have not been addressed by these studies.
2.4 Copper and Copper Oxide Nanoparticles
In an in vitro study [56], the authors evaluated effect of CuO NP (58.7 nm, 0.5 and 30 μg/m, 24 h) in different cell lines (THP-1, SAEC, RAW264.7) and observed small but significant changes in LINE 1 methylation, while no changes in global DNA methylation/hydroxymethylation were observed (Table 9.4). In a study aimed at identifying anticancer effect of green synthesized CuO NP, the authors observed significant inhibition of HDAC family [47] in A549 cells, however description of exposure was not very well defined for further interpretation. Most evidence regarding epigenetic changes induced by copper and copper oxide nanoparticles come from in vivo studies. While several key endpoints were evaluated, here we discuss the epigenetic endpoints of interest. In an in vivo study [55] on BALB/c male mice, using the same CuO NP (58.7 nm) particle as mentioned above [56], the authors observed an Increase in 5-mC levels and 5-hmC levels in lung tissue, hypermethylation of SINE B1 elements in the alveolar macrophages; and methylation of LINE-1 remained unchanged. In their study on female ICR (Institute of Cancer Research) mice, Rosner et al. [83] observed no significant change in global DNA methylation upon whole-body inhalation exposure to CuO NPs (3 days, 6 weeks, and 3 months). Enriched pathways, based on results of miRNA -mRNA expression changes were associated with lysosomal function, adherens and tight junction, pathways in cancer among others. In another study, Ognik et al. [67] observed an increase in oxidative stress markers (lipid peroxides, MDA), a decrease in catalase, total glutathione and global DNA hypomethylation for animals receiving Cu, CuNP through their diet. Through their results the authors suggested that Cu deficiency could impair oxidative defence, and that CuNP supplementation in the diet reduces protein oxidation, nitration, DNA oxidation and methylation [67].
2.5 Silver Nanoparticles
While silver nanoparticles are one of the most studied, both for its biomedical application and to identify its toxicity, the studies reporting epigenetic alterations are limited (Table 9.5). Gonzalez-Palomo et al.[37] in a study on EA.hy926 endothelial cells, observed global hypermethylation, downregulation of some miRNAs (miRNA -126, −155, and − 146) and increase in some inflammatory markers and levels of vascular cell adhesion molecule 1 (VCAM-1). Very few changes in DNA methylation were observed in BEAS-2B cells, exposed for a period of up to 6 weeks to Ag-NP (1 μg/mL), while significant changes in the transcriptome was observed [35]. Brzóska et al. [9] reported changes in miRNA expression (miR-499a, miR-1-3p), however DNA methylation were not altered in HepG2 cells, exposed to Ag-NP (20 nm, 10 μg/mL) for 24 h. In mouse hippocampal neuronal cell line (HT22), Mytych et al. [64] observed a significant increase in DNMT activity and global DNA methylation after 48 h of Ag-NP exposure and also after a recovery period of 96 h. In another study the authors observed significant H3 deacetylation and global DNA hypermethylation in A549 cells, exposed to higher concentration of PVP-coated Ag-NP [7]. While only a small percentage of PVP was part of particle preparation (99.9% Ag, 0.3% PVP), like in most epigenetic studies the influence of surface coating was not very clear [7]. Another study addressed the effect of Ag-NP on phosphorylation of histone H3 [103] in different cell lines (HaCaT, A549, MCF-7), where significant p-H3S10 (phosphorylation of histone H3 at serine 10) was observed, and was associated with cellular uptake of the particles. In contrast to other metal (oxide) nanoparticles, most studies on Ag-NP reported DNA hypermethylation, either at a global or sequence specific levels. However, the mechanistic explanation behind such changes have not been reported. Additionally, toxicologically relevant properties for silver nanoparticles such as effect of shape/size and ionization have also not been reported.
2.6 Gold Nanoparticles
Among metal nanoparticles, gold (Au-NP) has been studied rather extensively for epigenetic mechanism (Table 9.6), however with a focus, primarily on therapeutic properties for biomedical application. Majority of the particles used in such studies are biosynthesized, and are surface coated with citrate, PLGA etc. for specific application. In this section, we try to discuss the evidence on such epigenetic changes, focusing on the toxicological aspect where possible. However, it would be important to remember that surface coating of particle has an influence on uptake and thereby on the observed effects. Therefore, these results cannot be generalized for Au-NP as a group. Patil et al. [78] reported DNA methylation changes in human normal skin fibroblast (hypermethylation after 24 h, hypomethylation after 48 h) and A375 cells (hypomethylation after 24 and 48 h) and aberrant DNMT expression (DNMT1, DNMT3A and DNMT3B) exposed to biosynthesized Au-NP [78]. In another study the authors reported the reduction of cell cycle related protein expression and histone deacetylase activity in HepG2 cells treated with “gold-quercetin loaded into poly(DL-lactide-co-glycolide)” nanoparticles, however due to presence of multiple chemicals [6] such effect cannot be directly attributed to Au-NP. In another in vitro study, in human embryonic stem cells (hESCs), the authors investigated several cyto-genotoxicity and epigenetic endpoints for coated Au-NPs of different size (mercaptosuccinic acid coated-1.5, 4 nm, and citrate coated 14 nm) [87]. These nanoparticles exhibited global DNA hypomethylation (5-mC) and an increase in DNA hydroxymethylation (5-hmC) at sublethal concentrations. This effect was most prominent for Au-NP (4 nm) with 31,000 CpG sites showing demethylation [87]. However, no further interpretation on pathways were provided. In HepG2 cells, exposed to citrate coated Au-NP (10 μg/mL) for 24 h, Brzóska et al. reported changes in miRNA expression (miR-499a, miR-491-5p etc) however DNA methylation were not altered [9]. In a separate study, Ng et al. [65] reported that Au-Np (21 nm, citrate reduction) downregulated the expression of PROS1 (Vitamin K-dependent protein S) gene, mediated by the up-regulation of microRNA-155 (miR-155) but no change in methylation was observed for PROS1.
From the limited in vivo evidence, it is also clear that Au-NPs can induce epigenetic alterations in different tissue/cell types. In a study by our group [94], in BALB/c mice exposed to different sizes of citrate coated Au-NP (5, 60, 250 nm), while no changes were observed on the level of global DNA methylation (5-mC) and hydroxymethylation (5-hmC), Au-NP (60 nm) induced CpG hypermethylation in ATM, CDK and GSR genes and hypomethylation in GPX, GSR and TRP53. These changes were also associated with deregulations in immune markers. In another study, transplacental miRNA expression changes associated with cell proliferation, apoptosis, inflammation etc. were also observed in liver and lung of mice foetus, for Au-NP (40, 100 nm) administered intraperitonially (3.3 mg/Kg) [3].
3 Epigenetic Changes Induced by Carbon Nanotubes
Due to the growing evidence of toxicity induced by carbon nanotubes (CNTs), and the increasing emphasis on high aspect ratio, and the asbestos analogy [49, 81], there has been considerable research on epigenetic changes compared to other NPs. Classification of “MWCNT-7” as “Possibly carcinogenic to humans” (International Agency for Research on Cancer- IARC Group 2B) [43] and growing emphasis on mechanistic evidence for potential carcinogenicity, has also highlighted the need of epigenetic study. In this section we discuss the studies (Table 9.7) that have used different types of CNTs (MWCNT- Multi wall, SWCNT- Single wall) to evaluate epigenetic alterations, and where possible try to draw parallels with that of evidence available for asbestos.
From our study, in human bronchial epithelial cells (16HBE), we observed significant changes in DNA methylation induced by MWCNTs (JRC NM400; diameter 11 nm, length 846 nm) and SWCNTs (NIST SRM:2483; diameter 0.8 nm, length 8000 nm) [69]. While we did not observe global DNA methylation changes [33, 69] and sequence specific changes in LINE1 methylation [33], we observed significant changes in individual CpG sites for MWCNT (2398 genes were hypomethylated at gene promoters), and SWCNTs (589 CpG sites hypo- or hypermethylated), associated with pathways such as p53 signaling, DNA damage repair and cell cycle. We also were able to identify sequence specific changes in DNA methylation of several important genes such as NPAT/ATM, PIK3R2, DNMT1, HDAC4, SKI and GSTP1 [33, 69]. We did not observe significant changes in miRNA expression, which could however be due to the smaller number of replicates [33]. In a subsequent study we compared the epigenetic alterations observed for CNTs to that induced by asbestos in 16 HBE cells (Esra [24]), and mostly observed no significant changes in global DNA, however sequence specific methylation was altered for ATM (chrysotile, SWCNT, and MWCNT), CDKN1A (SWCNT, MWCNT, and amosite) and TRAF2 (only SWCNT) (Esra [24]) all of which play important role in pathways associated with DNA damage and apoptosis. In our study we also did not observed significant changes in global RNA methylation, which is a post-a translational modification with important role in gene regulatory processes. Our analysis of genome wide methylation changes induced by asbestos fibers (amosite, crocidolite and chrysotile) in 16HBE cells [70] also revealed significant changes in methylation pattern (associated with MAPK signaling pathway, pathways in cancer, WNT gene family and homeobox group) and some of the altered pathways were similar to that observed for CNTs. Differentially methylated regions including HOX genes have also been reported by Kettunen et al. [51] for asbestos-exposed patients. In a recent study, we reported the effect of long-term CNT and amosite exposure and recovery on DNA methylation, and we found that SWCNTs/amosite induced hypermethylation at CpG sites and MWCNT induced hypomethylation at CpG sites [71]. Moreover, spontaneous DNA methylation changes were observed during the recovery period [71]. However, the induction of DNA methylation changes for the chronic exposure period was less pronounced compared to our acute exposure experiment and such difference could be due to both the lower concentration of exposure for the chronic phase and possibly due to tolerance and adaptation. While in our study we observed fewer DNA methylation changes, in a long-term study in BEAS-2B cells, with MWCNT (NM401) exposure period of up to 4 weeks, Sierra et al. [90] observed a significant hypomethylation of 697 CpG, primarily associated with functions such as cell adhesion. The difference between the two long terms studies could primarily be due to the difference in lower exposure concentration (0.25 μg/ml) used in our study [71] compared to that reported by Sierra et al. [90] (20 μg/ml), but also due to the difference in cell lines used.
From one of our study, in BALB/c mice, intratracheal administration of MWCNT and SWCNT resulted in a sequence specific DNA methylation in ATM [94]. Such changes have been observed in other cell types as well. For instance, in one of our studies in THP1 monocytes, while we observed no changes in global methylation or hydroxymethylation, significant gene specific methylation associated with pathways such as platelet activation, VEGF receptor signaling pathway, chromatin organization, pathways in cancer, MAPK signaling pathway, PI3K-Akt signaling pathway were observed [68]. In another study, Saarimäki et al. [85] reported significant changes in transcriptome and epigenome (DNA methylation) associated with pathways related to immune system, cell cycle and signal transduction, in differentiated THP1 monocyte exposed to rigid MWCNT.
In an in vitro study, Chatterjee et al. [15] critically evaluated the effect of MWCNT diameter and length in BEAS-2B and HepG2 cells. In the study, the authors observed a global DNA hypermethylation in HepG2 cells while a global DNA hypomethylation was observed for BEAS-2B cells [15]. These changes were in line with DNMT1 and DNMT3B expression in both cell lines. Of the different forms of MWCNT studied, long-narrow and short-narrow forms induced the most significant epigenetic changes, associated with other oxidative stress and inflammatory markers. In another study, significant global hypomethylation was observed in lung tissue of C57BL/6 mice, exposed to MWCNTs of different length and diameter by oropharyngeal aspiration [18]. In the study narrow diameter and long length MWCNT induced the most significant effect [18], in line with the observation made by Chatterjee et al. [15]. The authors also reported significant sequence specific promoter hypomethylation of IL-1ß, IL-6, and TNF-α at 24 h, associated with increase in the inflammatory markers, followed by a reversal of the trend 7 days post exposure [18]. These two studies [15, 18] taken together, provide significant evidence regarding the influence of diameter and aspect ratio on CNT induced toxicity and epigenotoxicity. Another evidence in support of the asbestos analogy and high aspect ratio fiber toxicity comes from the in vivo study of Chernova et al. [16], where the authors used long and short forms of Amosite and carbon nanotubes. In the study the authors observed induction of mesothelioma, by pleural injection of long CNT and long amosite asbestos fibers, preceded by hypermethylation of overlapping genes p16/Ink4a and p19/Arf [16], which are often inactivated in different forms of cancer.
In addition, studies have identified considerable evidence of occupational exposure to CNTs, and has been presented in a recent review by Canu et al. [38]. In a study by our group [31], while we did not observe any significant difference in global methylation (5-mC), hydroxymethylation (5-hmC) and LINE1 methylation; we observed significant changes in CpG methylation for DNMT1, ATM, SKI, and HDAC4 in MWCNT exposed workers. These sequence specific methylation changes were also observed in our in vitro studies as discussed above. In another cross-sectional study, Shvedova et al. [89] observed significant enrichment of miRNA-lncRNA-mRNA changes in inflammation, fibrosis, tumor promotion and cancer progression pathways in MWCNT exposed workers. While these studies do not allow for a definitive interpretation regarding the cause or dose response relationship, some other cross-sectional studies have shown significant changes in inflammation and effect on cardiovascular and lung health [25, 34, 98]. These studies provided the first set of evidence of epigenetic alterations induced by occupational exposure to CNTs, but larger longitudinal population studies are needed to provide more detailed inside.
4 Summary and Perspectives
From the studies presented above, it is clear that NPs can induce epigenetic changes both at toxic and sub- cytotoxic concentrations. In the studies discussed above, with the exception of Ag-NP, most of the particles induced global DNA hypomethylation. DNA hypomethylation is observed in carcinogenesis and is highly correlated with methylation of DNA repeat elements [22]. Methylation of repeat elements such as LINE 1 and Alu represent approximately 50% of global methylation levels and hypomethylation has been associated with genomic instability and therefore can be used to study epigenetic alterations induced by NPs. Additionally, it has been established that global DNA methylation levels are modulated by oxidative stress[61, 73], and these mechanisms share one-carbon metabolism as the common denominator. This was observed for several of the NPs, but mostly for TiO2-NP, as discussed above. In a relatively small number of studies and primarily in case of Ag-NP DNA hypermethylation has been observed as well. DNA hypermethylation at global level and at specific loci has been implicated in large number of diseases including cancer [23]. Sequence specific DNA hypermethylation of GSTP1, TP53, p16, p21, ATM among other important genes, increased phosphorylation of H3 and concurrent mRNA dysregulation has been observed for the nanoparticles, similar to that observed for other genotoxic carcinogens such as formaldehyde, benzene [14]. While these findings are extremely interesting, it limits further mechanistic interpretation due to certain study limitations discussed further. While most epigenetic changes have been observed at a sub cytotoxic concentration, the observed results are representative of a single time window. Given that epigenetic changes are highly dynamic, and most of the nanoparticles were studied for shorter duration of exposure, the results cannot provide information of a chronic exposure scenario or that resulting from a change in exposure conditions. Therefore, to understand the dynamic nature of such changes, studies need to be designed taking into account- chronic low exposure, different time windows and an inclusion of recovery period. Another important aspect of a study design, aimed at understanding epigenetic effect should be the selection of appropriate cell type, representative of the exposure and elimination route. Despite the limited evidence, this is something that has been observed for NPs [80] and is extremely important as epigenetic changes are cell type specific. Additional experimental conditions, relevant for nanotoxicological experiments in general, such as physico-chemical properties, dispersion condition, shape of material, aspect ratio of fibres, surface coating and functionalization are equally important in the interpretation of epigenetic changes. Furthermore, these changes observed in mostly in vitro studies must be validated in vivo and if possible, in exposed human population. Epigenetic alterations can be valuable in providing key mechanistic insight which can play important role in risk assessment, regulation, and classification based on mechanistic evidences by agencies such as the IARC [17, 92].
References
Asweto CO et al (2017) Cellular pathways involved in silica nanoparticles induced apoptosis: a systematic review of in vitro studies. Environ Toxicol Pharmacol:191–197. https://doi.org/10.1016/j.etap.2017.09.012
Bai W, Chen Y, Gao A (2015) Cross talk between poly(ADP-ribose) polymerase 1 methylation and oxidative stress involved in the toxic effect of anatase titanium dioxide nanoparticles. Int J Nanomedicine 10:5561–5569. https://doi.org/10.2147/IJN.S88059
Balansky R et al (2013) Transplacental clastogenic and epigenetic effects of gold nanoparticles in mice. Mutat Res 751–752(1):42–48. https://doi.org/10.1016/j.mrfmmm.2013.08.006
Baylin SB (2005) DNA methylation and gene silencing in cancer. Nat Clin Pract Oncol:S4–S11. https://doi.org/10.1038/ncponc0354
Baylin SB et al (2001) Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet 10(7):687–692. https://doi.org/10.1093/hmg/10.7.687
Bishayee K, Khuda-Bukhsh AR, Huh SO (2015) PLGA-loaded gold-nanoparticles precipitated with quercetin downregulate HDAC-Akt activities controlling proliferation and activate p53-ROS crosstalk to induce apoptosis in hepatocarcinoma cells. Mol Cells 38(6):518–528. https://doi.org/10.14348/molcells.2015.2339
Blanco J et al (2017) Polyvinyl pyrrolidone-coated silver nanoparticles in a human lung cancer cells: time- and dose-dependent influence over p53 and caspase-3 protein expression and epigenetic effects. Arch Toxicol 91(2):651–666. https://doi.org/10.1007/s00204-016-1773-0
Boccuni F et al (2020) Occupational exposure to graphene and silica nanoparticles. Part I: workplace measurements and samplings. Nanotoxicology 14(9):1280–1300. https://doi.org/10.1080/17435390.2020.1834634
Brzóska K, Grądzka I, Kruszewski M (2019) Silver, gold, and iron oxide nanoparticles Alter miRNA expression but do not affect DNA methylation in HepG2 cells. Materials 12(7):1038. https://doi.org/10.3390/ma12071038
Carmo TLL et al (2019) Overview of the toxic effects of titanium dioxide nanoparticles in blood, liver, muscles, and brain of a Neotropical detritivorous fish. Environ Toxicol 34(4): 457–468. https://doi.org/10.1002/tox.22699 LK - http://limo.libis.be/resolver?&sid=EMBASE&issn=15227278&id=doi:10.1002%2Ftox.22699&atitle=Overview+of+the+toxic+effects+of+titanium+dioxide+nanoparticles+in+blood%2C+liver%2C+muscles%2C+and+brain+of+a+Neotropical+detritivorous+fish&stitle=Environ.+Toxicol.&title=Environmental+Toxicology&volume=34&issue=4&spage=457&epage=468&aulast=Carmo&aufirst=Talita+L.+L.&auinit=T.L.L.&aufull=Carmo+T.L.L.&coden=ETOXF&isbn=&pages=457-468&date=2019&auinit1=T&auinitm=L.L
Carriere M et al (2017) Impact of nanoparticles on DNA repair processes: current knowledge and working hypotheses. Mutagenesis 32(1):203–213. https://doi.org/10.1093/mutage/gew052
Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10(5):295–304. https://doi.org/10.1038/nrg2540
Chakraborty B et al (2016) Immunomodulatory properties of silver nanoparticles contribute to anticancer strategy for murine fibrosarcoma. Cell Mol Immunol 13(2):191–205. https://doi.org/10.1038/cmi.2015.05
Chappell G et al (2016) Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: a systematic literature review. Elsevier. https://doi.org/10.1016/j.mrrev.2016.03.004
Chatterjee N et al (2016) Diameter size and aspect ratio as critical determinants of uptake, stress response, global metabolomics and epigenetic alterations in multi-wall carbon nanotubes. Carbon 108:529–540
Chernova T et al (2017) Long-fiber carbon nanotubes replicate asbestos-induced mesothelioma with disruption of the tumor suppressor gene Cdkn2a (Ink4a/Arf). Curr Biol 27(21): 3302–3314.e6. https://doi.org/10.1016/j.cub.2017.09.007
Cogliano VJ et al (2008) Use of mechanistic data in IARC evaluations. Environ Mol Mutagen:100–109. https://doi.org/10.1002/em.20370
Cole E et al (2019) Multiwalled carbon nanotubes of varying size lead to dna methylation changes that correspond to lung inflammation and injury in a mouse model. Chem Res Toxicol 32(8):1545–1553. https://doi.org/10.1021/acs.chemrestox.9b00075
Definition of epigenetic alteration – NCI Dictionary of Cancer Terms – National Cancer Institute (n.d.). Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/epigenetic-alteration. Accessed 4 Feb 2021
Dhupal M et al (2018) Immunotoxicity of titanium dioxide nanoparticles via simultaneous induction of apoptosis and multiple toll-like receptors signaling through ROS-dependent SAPK/JNK and p38 MAPK activation. Int J Nanomedicine 13:6735–6750. https://doi.org/10.2147/IJN.S176087
Dinicola S et al (2015) Multiwalled carbon nanotube buckypaper induces cell cycle arrest and apoptosis in human leukemia cell lines through modulation of AKT and MAPK signaling pathways. Toxicol In Vitro 29(7):1298–1308. https://doi.org/10.1016/j.tiv.2015.05.006
Ehrlich M (2009) DNA hypomethylation in cancer cells. Epigenomics:239–259. https://doi.org/10.2217/EPI.09.33
Ehrlich M (2019) DNA hypermethylation in disease: mechanisms and clinical relevance. Epigenetics:1141–1163. https://doi.org/10.1080/15592294.2019.1638701
Emerce E et al (2019) Carbon nanotube- and Asbestos-induced DNA and RNA methylation changes in bronchial epithelial cells. Chem Res Toxicol 32(5). https://doi.org/10.1021/acs.chemrestox.8b00406
Fatkhutdinova LM et al (2016) Fibrosis biomarkers in workers exposed to MWCNTs. Toxicol Appl Pharmacol 299:125–131. https://doi.org/10.1016/j.taap.2016.02.016
Ferreira HJ, Esteller M (2018) Non-coding RNAs, epigenetics, and cancer: tying it all together. Cancer Metastasis Rev 37(1):55–73. https://doi.org/10.1007/s10555-017-9715-8
Fuks F (2005) DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev:490–495. https://doi.org/10.1016/j.gde.2005.08.002
Gao F et al (2016) Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int J Nanomedicine 11:3859–3874. https://doi.org/10.2147/IJN.S107021
Geiss O et al (2020) Characterisation of food grade titania with respect to nanoparticle content in pristine additives and in their related food products. Food Addit Contam Part A 37(2):239–253. https://doi.org/10.1080/19440049.2019.1695067
Ghosh M et al (2012) In vitro and in vivo genotoxicity of silver nanoparticles. Mutat Res 749(1–2). https://doi.org/10.1016/j.mrgentox.2012.08.007
Ghosh M, Öner D, Poels K, Tabish AM et al (2017a) Changes in DNA methylation induced by multi-walled carbon nanotube exposure in the workplace. Nanotoxicology 11(9–10). https://doi.org/10.1080/17435390.2017.1406169
Ghosh M, Öner D, Duca RC et al (2017b) Cyto-genotoxic and DNA methylation changes induced by different crystal phases of TiO2-np in bronchial epithelial (16-HBE) cells. Mutat Res 796:1–12. https://doi.org/10.1016/j.mrfmmm.2017.01.003
Ghosh M et al (2018) Single-walled and multi-walled carbon nanotubes induce sequence-specific epigenetic alterations in 16 HBE cells. Oncotarget 9(29):20351–20365. https://doi.org/10.18632/oncotarget.24866
Ghosh M et al (2020) Increased telomere length and mtDNA copy number induced by multi-walled carbon nanotube exposure in the workplace. J Hazard Mater 394:122569. https://doi.org/10.1016/j.jhazmat.2020.122569
Gliga AR et al (2018) RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Sci Rep 8(1):1–14. https://doi.org/10.1038/s41598-018-25085-5
Gong C et al (2012) Methylation of PARP-1 promoter involved in the regulation of nano-SiO 2-induced decrease of PARP-1 mRNA expression. Toxicol Lett 209(3):264–269. https://doi.org/10.1016/j.toxlet.2012.01.007
González-Palomo AK et al (2021) Effect of silver nanoparticles (AgNPs) exposure on microRNA expression and global DNA methylation in endothelial cells EA.hy926. Environ Toxicol Pharmacol 81:103543. https://doi.org/10.1016/j.etap.2020.103543
Guseva Canu I et al (2020) State of knowledge on the occupational exposure to carbon nanotube. Int J Hyg Environ Health 225:113472. https://doi.org/10.1016/j.ijheh.2020.113472
Halappanavar S et al (2011) Pulmonary response to surface-coated nanotitanium dioxide particles includes induction of acute phase response genes, inflammatory cascades, and changes in microRNAs: a toxicogenomic study. Environ Mol Mutagen:425–439. https://doi.org/10.1002/em.20639
Handy DE, Castro R, Loscalzo J (2011) Epigenetic modifications: basic mechanisms and role in cardiovascular disease. Circulation 123(19):2145–2156. https://doi.org/10.1161/CIRCULATIONAHA.110.956839
Hanly DJ, Esteller M, Berdasco M (2018) Interplay between long non-coding RNAs and epigenetic machinery: emerging targets in cancer? Philos Trans R Soc B Biol Sci. https://doi.org/10.1098/rstb.2017.0074
Huaux F et al (n.d.) Mesothelioma response to carbon nanotubes is associated with an early and selective accumulation of immunosuppressive monocytic cells. Part Fibre Toxicol 13(1):46. https://doi.org/10.1186/s12989-016-0158-0
IARC Working Group on the Evaluation of Carcinogenic Risks to Humans (2017) Carbon nanotubes. IARC Monogr 111
Jayaram DT, Payne CK (2020a) Food-grade TiO2Particles generate intracellular superoxide and Alter epigenetic modifiers in human lung cells. Chem Res Toxicol 33(11):2872–2879. https://doi.org/10.1021/acs.chemrestox.0c00331
Jayaram DT, Payne CK (2020b) Intracellular generation of superoxide by TiO2Nanoparticles decreases histone deacetylase 9 (HDAC9), an epigenetic modifier. Bioconjug Chem 31(5):1354–1361. https://doi.org/10.1021/acs.bioconjchem.0c00091
Jin B, Robertson KD (2013) DNA methyltransferases, DNA damage repair, and cancer. Adv Exp Med Biol:3–29. https://doi.org/10.1007/978-1-4419-9967-2_1
Kalaiarasi A et al (2018) Copper oxide nanoparticles induce anticancer activity in A549 lung cancer cells by inhibition of histone deacetylase. Biotechnol Lett 40(2):249–256. https://doi.org/10.1007/s10529-017-2463-6
Kalishwaralal K et al (2009) Silver nanoparticles inhibit VEGF induced cell proliferation and migration in bovine retinal endothelial cells. Colloids Surf B Biointerfaces 73(1):51–57. https://doi.org/10.1016/j.colsurfb.2009.04.025
Kane AB, Hurt RH, Gao H (2018) The asbestos-carbon nanotube analogy: an update. Toxicol Appl Pharmacol 361:68–80
Kelly TK, De Carvalho DD, Jones PA (2010) Epigenetic modifications as therapeutic targets. Nat Biotechnol:1069–1078. https://doi.org/10.1038/nbt.1678
Kettunen E et al (2017) Asbestos-associated genome-wide DNA methylation changes in lung cancer. Int J Cancer 141(10):2014–2029. https://doi.org/10.1002/ijc.30897
Ladd-Acosta C (2015) Epigenetic signatures as biomarkers of exposure. Curr Environ Health Rep 2(2):117–125. https://doi.org/10.1007/s40572-015-0051-2
Li JJ et al (2010) Autophagy and oxidative stress associated with gold nanoparticles. Biomaterials 31(23):5996–6003. https://doi.org/10.1016/j.biomaterials.2010.04.014
Liou S-HH et al (2017) Global DNA methylation and oxidative stress biomarkers in workers exposed to metal oxide nanoparticles. J Hazard Mater 331:329–335. https://doi.org/10.1016/j.jhazmat.2017.02.042
Lu X, Miousse IR, Pirela SV, Moore JK et al (2016a) In vivo epigenetic effects induced by engineered nanomaterials: a case study of copper oxide and laser printer-emitted engineered nanoparticles. Nanotoxicology 10(5):629–639. https://doi.org/10.3109/17435390.2015.1108473
Lu X, Miousse IR, Pirela SV, Melnyk S et al (2016b) Short-term exposure to engineered nanomaterials affects cellular epigenome. Nanotoxicology 10(2):140–150. https://doi.org/10.3109/17435390.2015.1025115
Ma Y et al (2017) Titanium dioxide nanoparticles induce size-dependent cytotoxicity and genomic DNA hypomethylation in human respiratory cells. RSC Adv 7(38):23560–23572. https://doi.org/10.1039/C6RA28272E
Ma Y et al (2019) Different effects of titanium dioxide nanoparticles instillation in young and adult mice on DNA methylation related with lung inflammation and fibrosis. Ecotoxicol Environ Saf 176:1–10. https://doi.org/10.1016/j.ecoenv.2019.03.055
Magdolenova Z et al (2014) Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 8(3):233–278. https://doi.org/10.3109/17435390.2013.773464
Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. Biomed Res Int. https://doi.org/10.1155/2013/942916
Menezo YJR et al (2016) Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reprod Biomed Online:668–683. https://doi.org/10.1016/j.rbmo.2016.09.006
Mitchell LA et al (2009) Mechanisms for how inhaled multiwalled carbon nanotubes suppress systemic immune function in mice. Nat Nanotechnol 4(7):451–456. https://doi.org/10.1038/nnano.2009.151
Murugadoss S et al (2020) Agglomeration of titanium dioxide nanoparticles increases toxicological responses in vitro and in vivo. Part Fibre Toxicol 17(1):10. https://doi.org/10.1186/s12989-020-00341-7
Mytych J et al (2017) Prolonged effects of silver nanoparticles on p53/p21 pathway-mediated proliferation, DNA damage response, and methylation parameters in HT22 hippocampal neuronal cells. Mol Neurobiol 54(2):1285–1300. https://doi.org/10.1007/s12035-016-9688-6
Ng CT et al (2011) The induction of epigenetic regulation of PROS1 gene in lung fibroblasts by gold nanoparticles and implications for potential lung injury. Biomaterials 32(30):7609–7615. https://doi.org/10.1016/j.biomaterials.2011.06.038
Ngobili TA, Daniele MA (2016) Nanoparticles and direct immunosuppression. Exp Biol Med:1064–1073. https://doi.org/10.1177/1535370216650053
Ognik K et al (2019) The effect of copper nanoparticles and copper (II) salt on redox reactions and epigenetic changes in a rat model. J Anim Physiol Anim Nutr 103(2):675–686. https://doi.org/10.1111/jpn.13025
Öner D et al (2017) Epigenetic effects of carbon nanotubes in human monocytic cells. Mutagenesis 32(1):181–191. https://doi.org/10.1093/mutage/gew053
Öner D, Ghosh M, Bové H et al (2018a) Differences in MWCNT- and SWCNT-induced DNA methylation alterations in association with the nuclear deposition. Part Fibre Toxicol 15(1):11. https://doi.org/10.1186/s12989-018-0244-6
Öner D, Ghosh M, Moisse M et al (2018b) Global and gene-specific DNA methylation effects of different asbestos fibres on human bronchial epithelial cells. Environ Int 115:301–311. https://doi.org/10.1016/j.envint.2018.03.031
Öner D et al (2020) Induction and recovery of CpG site specific methylation changes in human bronchial cells after long-term exposure to carbon nanotubes and asbestos. Environ Int 137:105530. https://doi.org/10.1016/j.envint.2020.105530
Ou L et al (2017) The mechanisms of graphene-based materials-induced programmed cell death: a review of apoptosis, autophagy, and programmed necrosis. Int J Nanomedicine:6633–6646. https://doi.org/10.2147/IJN.S140526
Oxidative stress alters global histone modification and DNA methylation (2015) Free Radic Biol Med 82:22–28. https://doi.org/10.1016/J.FREERADBIOMED.2015.01.028
Pakrashi S et al (2014) In vivo genotoxicity assessment of titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLoS One 9(2):e87789. https://doi.org/10.1371/journal.pone.0087789
Park EJ, Park K (2009) Oxidative stress and pro-inflammatory responses induced by silica nanoparticles in vivo and in vitro. Toxicol Lett 184(1):18–25. https://doi.org/10.1016/j.toxlet.2008.10.012
Park MVDZ et al (2011) The effect of particle size on the cytotoxicity, inflammation, developmental toxicity and genotoxicity of silver nanoparticles. Biomaterials 32(36):9810–9817. https://doi.org/10.1016/j.biomaterials.2011.08.085
Patil NA, Gade WN, Deobagkar DD (2016) Epigenetic modulation upon exposure of lung fibroblasts to TiO2 and ZnO nanoparticles: alterations in DNA methylation. Int J Nanomedicine 11:4509–4519. https://doi.org/10.2147/IJN.S110390
Patil YM, Rajpathak SN, Deobagkar DD (2019) Characterization and DNA methylation modulatory activity of gold nanoparticles synthesized by Pseudoalteromonas strain. J Biosci 44(1):1–13. https://doi.org/10.1007/s12038-018-9842-6
Patlolla A, Patlolla B, Tchounwou P (2010) Evaluation of cell viability, DNA damage, and cell death in normal human dermal fibroblast cells induced by functionalized multiwalled carbon nanotube. Mol Cell Biochem 338(1–2):225–232. https://doi.org/10.1007/s11010-009-0356-2
Pogribna M et al (2020) Effect of titanium dioxide nanoparticles on DNA methylation in multiple human cell lines. Nanotoxicology 14(4):534–553. https://doi.org/10.1080/17435390.2020.1723730
Poland CA et al (2008) Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat Nanotechnol 3(7):423–428. https://doi.org/10.1038/nnano.2008.111
Ray Chaudhuri A, Nussenzweig A (2017) The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol:610–621. https://doi.org/10.1038/nrm.2017.53
Rossner P et al (2020) Gene expression and epigenetic changes in mice following inhalation of copper(II) oxide nanoparticles. Nano 10(3):550. https://doi.org/10.3390/nano10030550
Roy Choudhury S et al (2017) ZnO nanoparticles induced reactive oxygen species promotes multimodal cyto- and epigenetic toxicity. Toxicol Sci 156(1):kfw252. https://doi.org/10.1093/toxsci/kfw252
Saarimäki LA et al (2020) Toxicogenomics analysis of dynamic dose-response in macrophages highlights molecular alterations relevant for multi-walled carbon nanotube-induced lung fibrosis. NanoImpact 20:100274. https://doi.org/10.1016/j.impact.2020.100274
Seidel C et al (2017) Epigenetic changes in the early stage of silica-induced cell transformation. Nanotoxicology 11(7):923–935. https://doi.org/10.1080/17435390.2017.1382599
Senut M-C et al (2016) Size-dependent toxicity of gold nanoparticles on human embryonic stem cells and their neural derivatives. Small 12(5):631–646. https://doi.org/10.1002/smll.201502346
Shukla RK et al (2011) ROS-mediated genotoxicity induced by titanium dioxide nanoparticles in human epidermal cells. Toxicol In Vitro 25(1):231–241. https://doi.org/10.1016/j.tiv.2010.11.008
Shvedova AA et al (2016) Integrated analysis of dysregulated ncRNA and mRNA expression profiles in humans exposed to carbon nanotubes. PLoS One 11(3):e0150628. https://doi.org/10.1371/journal.pone.0150628
Sierra MI et al (2017) DNA methylation changes in human lung epithelia cells exposed to multi-walled carbon nanotubes. Nanotoxicology 11(7):857–870. https://doi.org/10.1080/17435390.2017.1371350
Singh N et al (2017) Exposure to engineered nanomaterials: impact on DNA repair pathways. Int J Mol Sci. https://doi.org/10.3390/ijms18071515
Smith MT et al (2016) Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis
Stoccoro A et al (2017) Multiple endpoints to evaluate pristine and remediated titanium dioxide nanoparticles genotoxicity in lung epithelial A549 cells. Toxicol Lett 276:48–61. https://doi.org/10.1016/j.toxlet.2017.05.016
Tabish AM et al (2017) Changes in DNA methylation in mouse lungs after a single intra-tracheal Administration of Nanomaterials. PLoS One 12(1):e0169886. https://doi.org/10.1371/journal.pone.0169886
Unfried K et al (2008) Carbon nanoparticle-induced lung epithelial cell proliferation is mediated by receptor-dependent Akt activation. Am J Phys Lung Cell Mol Phys 294(2):L358–L367. https://doi.org/10.1152/ajplung.00323.2007
Vandebriel RJ, De Jong WH (2012) A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl:61–71. https://doi.org/10.2147/NSA.S23932
Verleysen E et al (2020) Physicochemical characterization of the pristine E171 food additive by standardized and validated methods. Nano 10(3):592. https://doi.org/10.3390/nano10030592
Vlaanderen J et al (2017) A cross-sectional study of changes in markers of immunological effects and lung health due to exposure to multi-walled carbon nanotubes. Nanotoxicology 11(3):395–404. https://doi.org/10.1080/17435390.2017.1308031
Wei JW et al (2017) Non-coding RNAs as regulators in epigenetics (review). Oncol Rep 37(1):3–9. https://doi.org/10.3892/or.2016.5236
Williams K, Christensen J, Helin K (2012) DNA methylation: TET proteins – guardians of CpG islands? EMBO Rep 13(1):28–35. https://doi.org/10.1038/embor.2011.233
Yu KN et al (2013) Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol In Vitro 27(4):1187–1195. https://doi.org/10.1016/j.tiv.2013.02.010
Zhao Y, Sun H, Wang H (2016) Long noncoding RNAs in DNA methylation: new players stepping into the old game. Cell Biosci:45. https://doi.org/10.1186/s13578-016-0109-3
Zhao X, Toyooka T, Ibuki Y (2017) Silver nanoparticle-induced phosphorylation of histone H3 at serine 10 is due to dynamic changes in actin filaments and the activation of Aurora kinases. Toxicol Lett 276:39–47. https://doi.org/10.1016/j.toxlet.2017.05.009
Ziech D et al (2011) Reactive oxygen species (ROS) – induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res:167–173. https://doi.org/10.1016/j.mrfmmm.2011.02.015
Zou Y et al (2016) DNA Hypermethylation of CREB3L1 and Bcl-2 associated with the mitochondrial-mediated apoptosis via PI3K/Akt pathway in human BEAS-2B cells exposure to silica nanoparticles. PLoS One 11(6):e0158475. https://doi.org/10.1371/journal.pone.0158475
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Ghosh, M., Godderis, L., Hoet, P. (2022). Epigenetic Mechanisms in Understanding Nanomaterial-Induced Toxicity. In: Louro, H., Silva, M.J. (eds) Nanotoxicology in Safety Assessment of Nanomaterials. Advances in Experimental Medicine and Biology, vol 1357. Springer, Cham. https://doi.org/10.1007/978-3-030-88071-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-88071-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-88070-5
Online ISBN: 978-3-030-88071-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)