Abstract
For those of us who have special interest in osteoporosis and disease prevention, the battle seems very challenging. This is proposed based on the consistent finding that osteoporosis is both underdiagnosed and undertreated disease even though it is common and causes serious problems and even though effective treatments are available. On the other hand, there seems to be a misunderstanding as several clinicians adopt the concept that bone density is the gauge for assessing bone strength and the response to anti-osteoporotic treatment. In recent years, however, the concept of bone strength has moved beyond density alone and has expanded to include a number of bone characteristics of that collectively are called quality. This chapter will discuss the evidence relating to fracture risk in the population who are classified in the osteopenia range. It will then expand to include current levels of case-finding and appropriate osteopenia management. Where available, analysis of published work describing models of care to implement best practice is presented. Finally, it will present an algorithm for osteopenia treatment-selected examples of clinical recommendations regarding pharmacotherapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The terms “osteoporosis” and “osteopenia” were originally coined to convey the notion that an individual is susceptible to sustaining a fracture following minimal trauma because there is “not enough bone” [1,2,3]. In the absence of a true gold standard, the WHO proposed that the reference standard should be based on BMD measurement made at the femoral neck with dual-energy X-ray absorptiometry (DXA). This site has been the most extensively validated and provides a gradient of fracture risk as high as or higher than that of many other techniques [4]. The recommended reference range was the National Health and Nutrition Examination Survey (NHANES) III reference database for femoral neck measurements in Caucasian women aged 20–29 years [5]. This proposal has been endorsed by many international agencies including the International Osteoporosis Foundation (IOF), the International Society for Clinical Densitometry, and the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). More controversially, a similar threshold value for femoral neck BMD that is used to define osteoporosis in women was proposed for the diagnosis of osteoporosis in men—namely, a value for BMD 2.5 SD or more below the average for young adult women [6].
Although it is well established that the risk of fracture is increased in women with the BMD levels in the osteoporosis range (i.e., BMD: T-score < −2.5), women with higher BMD levels, such as those in the osteopenia range (BMD: T-score < −1 to −2.49), have also been reported at increased risk for fracture. In a previous analysis of 200, 160 postmenopausal women in the National Osteoporosis Risk Assessment (NORA) study, women with osteoporosis had 2.74 times higher 1-year risk of fracture, and women with osteopenia had 1.73 times higher risk of fracture, compared with women with normal BMD, independent of demographic and clinical factors [7].
The BMD level appropriate for intervention with pharmacological treatment in postmenopausal women at increased fracture risk is a critical issue when assessing the potential for reducing the overall fracture rate in the population. Several medications have been shown to prevent bone loss or reduce the risk of fracture in postmenopausal women with low bone mass or osteoporosis [8,9,10,11,12,13,14,15]. However, there is no agreement on the ideal BMD measurement at which to initiate pharmacological therapy. The lack of consensus on treatment intervention thresholds reflects the trade-offs between the known potential benefits versus risks of these treatments, the willingness of patients to initiate and continue therapy, as well as the available resources to pay for medications.
Treatment threshold levels available for consideration in clinical practice emerge principally from two sources. The first is derived from reports developed by the World Health Organization (WHO), and the second is from the National Osteoporosis Foundation (NOF). The WHO provided an operational definition of osteopenia and osteoporosis in 1994 [16]. A postmenopausal woman with a BMD 2.5 SDs or more below the young adult mean (i.e., T-score ≤ −2.5) at any site (spine, hip, or mid radius) is considered to have osteoporosis, and a woman with a BMD between −2.49 and − 1.0 is considered to have osteopenia. Although the WHO cutoff points were designed as diagnostic thresholds and were not developed to provide criteria for selecting patients in whom to initiate therapy, many clinicians and reimbursement sources use the WHO level for osteoporosis (T-score ≤ −2.5) as the treatment intervention threshold.
The NOF developed treatment thresholds by combining BMD measured at the hip with clinical risk factors for fracture (e.g., prior fracture as an adult, family history of fracture, BMI <18, cigarette smoking, excessive alcohol intake) [17, 18]. According to NOF recommendations, women with a T-score of −2.0 or less or − 1.5 or less with at least one risk factor should be considered for treatment. The rationale for these particular threshold levels was evidence-based and influenced by cost-effectiveness considerations [19].
The observation that more than half (52%) of the NORA women experiencing an incident osteoporotic fracture within 1 year had a BMD T-score of −1.0 to −2.5 underscores the unmet need to identify those subjects who are most likely to fracture and might benefit from targeted pharmacological intervention. This chapter will discuss the evidence relating to fracture risk in the population who are classified in the osteopenia range. It will then expand to include current levels of case-finding and appropriate osteopenia management. Where available, analysis of published work describing models of care to implement best practice is presented. Finally, it will present an algorithm for osteopenia treatment-selected examples of clinical recommendations regarding pharmacotherapy.
From T-score to Bone Health
Trabecular bone loss and vertebral fractures are historical hallmarks of osteoporosis. However, 80% of the skeleton is cortical; 80% of all fractures are nonvertebral; and 30% of these are forearm fractures. Moreover, about 70% of all the appendicular bone lost during aging is cortical and results from intracortical remodeling which occurs throughout the cortex but is particularly vigorous in the cortico-trabecular junctional (transitional) zone where the cortical and trabecular compartments merge (Fig. 12.1) [20]. Remodeling during advancing age becomes unbalanced and removes more bone than it deposits leaving residual cortical porosity, which increases bone fragility exponentially and is a quantifiable “footprint” of bone loss [21,22,23].
Originally, the T-score concept was developed to assess for the probability of fragility fractures in postmenopausal white women in their mid to late 60s and older [21]. It has been useful because, in this age group, the disease prevalence is high. The T-score was endorsed as a surrogate marker for the histologic changes in aged bone that render it weak and susceptible to fractures from low loading forces: the lower the score, the worse the fracture risk. It followed intuitively that a low T-score determined the diagnosis of primary osteoporosis. Consequently, today’s bone health specialists appreciate the importance of the T-score in diagnosing osteoporosis [24].
But the T-score has its problems when used outside this intended population. Practitioners have assumed that all patients with abnormally low scores have primary osteoporosis. However, this number alone is insufficient to accurately make such a diagnosis in patients outside the demographic group in which it was developed, simply because the low disease prevalence in younger groups makes the score less accurate as a predictive tool. Furthermore, it has long been apparent that T-scores use is associated with issues, including different T-score values at various skeletal sites (lumbar spine, hip, distal 1/3 radius) [25].
Moreover, re-evaluation of data from pivotal clinical trials has brought into question the long-held idea that increases in bone density parallel increases in bone strength and reduction in fractures and that therapeutic improvement in bone density is the mark of success. Bone strength or resistance to fracture is more complex than density alone. Into this arena enters the concept of bone quality [26].
Bone Loss Is a Continuum, Not a T score
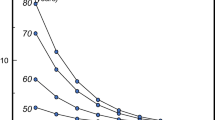
Another limitation of the term osteopenia is that there is a big distance under the curve from −1 to −2.49 standard deviations. Therefore, when it comes to risk assessment, it is important to remember that loss of bone mass is a continuum. And because the risk of fracture is directly related to bone mass, fracture risk is a continuum, too. For every standard deviation of bone mass lost, the relative risk of fracture doubles, but absolute fracture risk is highly age-dependent (Fig. 12.2). In younger women, the relative risk of fracture is quite low, and it remains low even when doubled. On the other hand, the absolute fracture risk of a 50-year-old with a T-score of −3 (a score most clinicians would be very concerned about) is exactly the same as the absolute fracture risk of an 80-year-old woman with a T-score of −1 (a score many clinicians might consider excellent for a woman that age). Thus, the T-score is only part of the story.
Risk of fracture increases with advancing age and continuous loss of bone. (Adapted from Kanis et al. [118]. (Springer publisher/how to get the permission))
Bone mineral density (BMD) measures bone mass, which is simply one component of bone strength. BMD does not assess bone microarchitecture, although it can facilitate a diagnosis of osteopenia or osteoporosis using the WHO definitions. Similarly, BMD is used to monitor risk of fracture, much as blood pressure predicts the risk of cardiovascular disease. Many patients with high blood pressure never have a heart attack or stroke, and many patients with normal blood pressure do—but overall, rising blood pressure and rising risk of cardiovascular disease go together. In concordance, BMD is used to monitor response to treatment, but it is accurate only if the concept of least-specific change (LSC) is taken into account: LSC = 2.77 × the precision error of the machine. Thus, in a good center, BMD measurement of the spine will be ±3%, and measurement of the hip will be ±5% [27].
In short, BMD measurement is used to reflect the bone remodeling continuum and degree of bone loss (Fig. 12.3). In turn, this would raise the question of which women should have their bone mass tested and who of them would require therapy? Various organizations have issued guidelines for measuring BMD in women to assess risk of fracture.
The Burden of Fragility Fractures
There is limited information concerning how many of all the fractures seen in postmenopausal women originate from the larger portion of the population with normal BMD or osteopenia. This information is important because it identifies the number of fractures that are likely to be averted by programs targeted at the whole community or only those with osteoporosis. In a study carried out to determine the age- and BMD-specific burden of fractures in the community and the cost-effectiveness of targeted drug therapy, 1224 women over 50 years of age sustaining fractures over 2 years’ duration were assessed. Of the women sustaining fractures, 80% of 50–59 years olds did not have osteoporosis, 50% of 60–79 year old did not have osteoporosis, and even among those 80+ years old, 30% did not have osteoporosis [28].
Thus, referring to these fragility fractures as “osteoporotic” is misleading because it implies that the fractures come from a group of women identifiable by measurement of BMD. Although women with osteoporosis have an increased risk of fracture and the prevalence of osteoporosis in women with fractures is twice that observed in the population, most fractures in the population occur in women without osteoporosis. It is only in the oldest sectors of the population (80+ years) that a majority of fractures occur in women with osteoporosis.
These observations have important implications in deciding who, when, and how to treat. If a drug halves fracture risk, for each fracture averted, three times more women must be exposed to treatment when treatment is aimed at 50–59-year-olds than 80 + −year-olds. In addition, to identify osteoporosis in women over 50 years, a mass screening program would be required. This can be done by questionnaires to assess for fracture probability, e.g., FRAX. Adding other risk factors, such as bone remodeling status or prevalent fracture, may increase sensitivity and cost-effectiveness because it identifies the highest risk individuals who are most likely to benefit by actually averting the event they are likely to sustain.
These results as well as the outcomes of earlier studies such as the National Osteoporosis Risk Assessment (NORA) study [29]. This was a longitudinal observation study that included over 200,000 postemenopausal women who range in agre from 50 to 104 years and had baseline peripheral BMD measurements. The study assessed the frequency of low bone mass and its association with fracture in women 50–64 years of age in comparison to women ≥65 of age. NORA enrolled 200,160 postmenopausal women ≥50 years of age who had no prior diagnosis of osteoporosis. Baseline BMD was measured at the heel, forearm, or finger. A 1-year follow-up survey requesting incident fractures since baseline was completed by 163,935 women, 87,594 (53%) of whom were 50–64 years of age. Results revealed that more than half (52%) of the NORA women included in that work, who experienced an incident osteoporotic fracture, had a BMD T-score of −1.0 to −2.5.
Both results revealed a consistent pattern of a higher fracture incidence and lower peripheral BMD T-score in both the younger and the older women for all fracture sites, findings which support the suggestion that the definition of osteoporosis and the criteria for subsidized drug therapy would be better served by a gradient-of-risk model using a combination of several risk factors incorporating age and BMD with absolute fracture risk rather than being defined as a single BMD threshold [33,34,35,33].
The Problem of Osteopenia
The “Geoffrey Rose Prevention Paradox” applies to many chronic diseases, including osteopenia: “a large number of people at small risk give rise to more cases than the small number who are at high risk.” In most countries less than half of women and men who sustain a fragility fracture have osteoporosis as diagnosed by DEXA measurements of BMD. The majority have osteopenia. The outcomes of the NORA (Nordic Research on Aging) study were the first one to raise the attention to the “osteopenia challenge.” Out of the 149,562 postmenopausal women aged 50–104 years (mean 64.5 years), only 6.4% of women had a BMD of <−2.5 SD (associated with 18% of all fractures and 26% of hip fractures), but 45.3% of women had a BMD of <−1.0 SD (associated with 70% of all fractures and 77% of hip fractures) [19, 29]. In the Rotterdam study of 4878 women who had DEXA measurements of the femoral neck and were followed up for a mean 6.8 years, the rate of self-reported nonvertebral fractures was 44% with osteoporosis, 43.3% with osteopenia, and 12.6% with normal BMD [34]. Similarly, in an Australian community study of 616 women who had DEXA measurements of the total femur, 124 women had one or more fractures. Of the women with fractures, only 26.9% had osteoporosis, 56.5% had osteopenia, and 16.6% had a normal BMD [35]. Most women and men who suffer from a fragility fracture do not have osteoporosis as defined by the WHO. Therefore, assessment of fracture risk and diagnosis and treatment should not be limited to those with osteoporosis but should include all patients with osteopenia and all patients with clinical risk factors for fracture.
Another work carried out by the Study of Osteoporotic Fractures Research Group in the USA has related the estimated time interval for 10% of women with different degrees of osteopenia to make the transition from osteopenia to osteoporosis. Normal BMD was defined as a T-score at the femoral neck and total hip of −1.00 or higher and osteopenia as a T-score of −1.01 to −2.49. Mild, moderate, and advanced osteopenia were defined as T-scores of −1.10 to −1.49, −1.50 to −1.99, and − 2.0 to −2.49, respectively. The intervals between baseline testing and development of osteoporosis in 4957 women aged 67 years and older (adjusted for BMI, current estrogen use and smoking, current or past use of oral glucocorticoids, and rheumatoid arthritis) in years with 95% confidence limits were normal BMD 16.8 (11.5–24.6), mild osteopenia 17.3 (13.9–21.5), moderate osteopenia 4.7 (4.2–5.2), and advanced osteopenia 1.1 (1.0–1.3). Accordingly, it is clear that the degree of osteopenia is a major factor in predicting the development of osteoporosis and of consequent fracture risk and the degree of osteopenia should be taken into account in arriving at all treatment decisions [36].
The Challenge of Case Finding: Mind the Gap
Among the large group of subjects with osteopenia, there exists a substantial subgroup with bone fragility contributing to the burden of fractures. If an aBMD measurement alone is used in an osteoporosis screening program, women with osteopenia will be excluded from further investigation and so will not be offered treatment [40,41,39]. Challenges of case finding of osteopenic patients are multifaceted (Table 12.1) including the healthcare professionals’ awareness and interest in bone health, identifying specific subjects at high fracture risk as well as adopting appropriate management algorithms. One important approach to case finding—identifying those at risk for fracture in need of treatment, that is, applicable in standard clinical practice—is the use of the fracture risk assessment tool (FRAX) [38]. Another approach is to identify the structural basis of the bone fragility not captured by the aBMD measurement and thereby to quantify “microarchitectural deterioration of bone tissue,” the descriptive component of the definition of “osteoporosis.” Getting the right treatment to the right patient at the right time is of paramount importance if fracture rates are to be significantly reduced as the world’s population ages and lifestyles change.
At the service setup, a good case can be made for the establishment of local groups, including generalists and specialists who are especially interested in osteoporosis, to agree on referral practices and treatment based on local resources. In large hospitals, an “osteoporosis clinic” including different disciplines may facilitate diagnosis and management. There is little doubt that the care of women and men with osteoporosis or osteopenia and those with fragility fractures, particularly the very elderly, can be enormously improved.
At the case finding level, strategies to ensure that individuals who are at high risk of sustaining fragility fractures in general, and hip fractures in particular, have been reliably identified by health systems and best practice guidance for treatment have been published [40].
Case Finding Strategies
While bone density remains one of the most valid and reliable measures of fracture risk, a better delineation of risk factors has led to renewed interest in absolute risk models such as FRAX. New imaging approaches, including vertebral morphometry, have been added to the diagnostic armamentarium and facilitate identification of fractures both early in the disease course (if properly identified) and with less radiation exposure to the patient. This is important because of the severe consequences of prevalent fractures in osteopenia as well as osteoporosis, not only of the hip but also of the much more common spine fractures.
Identification of Osteopenic Patients with High Fracture Risk
While BMD is used to reflect bone strength and, consequently, low BMD has been considered as a major risk factor for fractures, most patients presenting with a fracture do not have BMD-based osteoporosis, defined according to the World Health Organization (WHO) definition (T-score of −2.5 or below). The best example is hip fracture, where only half of the patients exhibit T-scores below −2.5 [41, 42]. In addition, and independent of bone-related risks, extra-skeletal risk factors such as falls contribute to fracture risk and are present in the majority of patients older than 50 years presenting with a clinical fracture, and falls are the dominant event leading to forearm and hip fracture [43]. Therefore, it is important to consider BMD screening for subjects who present with risk factors for bone loss as well as subjects older than 50 years old presenting with loss of balance and/or recurrent falls.
Identification of Patients with Prevalent Fractures
The primary risk factor for subsequent fracture is a prevalent low-energy fracture, irrespective of whether it is clinically apparent or not. Thus, most guidelines for treatment consider the presence of a low-energy fracture in an osteopenic patient a clear indication for specific osteoporosis therapy [44, 45]. A history of nonvertebral fracture is associated with a doubling of the risk of a subsequent fracture, and the subsequent fracture risk is even quadrupled after a vertebral fracture. The re-fracture risk is, however, not constant over time. It is highest (2–3X) in the years immediately after a first fracture, followed by a gradual waning later on [46]. Forty to 50% of all subsequent fractures occur within 3–5 years after a first fracture, and the presence of such fractures demands rapid intervention with specific osteoporosis drugs to reduce the risk of a subsequent fracture. Prevalent hip, spine, and several other nonvertebral fractures are all associated with increased morbidity and mortality [15], which is higher immediately after fracture than later on. Hip, vertebral, and non-hip, nonvertebral fractures were each associated with approximately one-third of deaths. The major causes of death were related to cardiovascular and respiratory comorbidities [47].
Unfortunately subsequent follow-up of fracture patients after orthopedic fracture repair to identify patients in need of specific osteoporosis treatment is still very limited. Most studies show that only 10–15% of fracture patients treated at orthopedic departments are offered a DXA evaluation and even less patients are offered supplementation with vitamins D and Ca or specific osteoporosis treatment. Fortunately a lot of centers are recognizing this dilemma and have established initiatives for post-fracture care (e.g., fracture liaison service) [48]. Such interventions have the potential to reduce subsequent fractures, morbidity, mortality, and readmissions to hospital.
While hip and other nonvertebral fractures are clinically obvious, the detection of vertebral fractures constitutes a significant problem. Morphometric vertebral fractures are the most frequent fractures in women and men older than 50 years [49], and their presence is a strong predictor of future vertebral, nonvertebral, and hip fracture risk [50, 51]. Clinical vertebral fractures are characterized by back pain lasting for 2–3 months, depending on fracture severity, but they represent only a small subgroup of all vertebral fractures. In large-scale trials, symptomatic vertebral fractures constitute less than 10% of all morphometric fractures [52, 53]. Most morphometric vertebral fractures therefore remain undiagnosed, which results in many patients developing severe osteoporosis with multiple fractures and chronic pain, before effective treatment is initiated. Only when clinical suspicion, e.g., significant height loss, increasing kyphosis, protruding abdomen, rib-iliac crest distance of less than 2 cm, and acute or chronic back pain, is raised, a spine X-ray is performed. But even when lateral X-rays of the spine are available, vertebral fractures are often missed [54, 55].
Thus, detection of prevalent fractures is very important when making decisions on treatment in osteopenic women. This has been further facilitated by accessory software for DXA scanners yielding lateral X-rays of the spine, which permit assessment of vertebral fracture status. This procedure has been given many names: (vertebral morphometry, lateral vertebral assessment (LVA), vertebral fracture assessment (VFA)) (Fig. 12.4). The images are usually of good quality, albeit less detailed than conventional X-rays, and in most cases a good evaluation of compression fractures in the range Dorsal 4-Lumbar 4 is possible. Advantages are low radiation dose, the availability of semiautomatic image analysis tools to assist in measuring vertebral shapes of the individual vertebrae, its plan-parallel projection, and its high negative predictive value. The disadvantage is the inability to study upper thoracic vertebrae, but only a minority of fractures are found there.
If pathology outside this region of interest (ROI) is suspected, other imaging techniques will have to be used. The experience in most centers employing this methodology is, however, that such referrals are needed in less than 10% of cases. According to the International Society for Clinical Densitometry (ISCD), additional X-ray imaging is needed in cases of two or more mild (grade 1) deformities without any moderate or severe (grade 2 or 3) deformities, when lesions in vertebrae cannot be ascribed to benign causes, or when vertebral deformities are found in a patient with a known history of a relevant malignancy [54]. The methodology also permits assessment of spondylosis, and even arteriosclerosis of the abdominal aorta can be evaluated. Differential diagnosis of radiologic osteopenia is shown in Table 12.2.
The prevalence of previously unknown morphometric vertebral fractures has been studied in various at-risk populations. In a study of women and men presenting with a nonvertebral fracture, one out of four had a prevalent morphometric vertebral fracture on vertebral morphometry that was not recognized previously [56]. In another study, the prevalence of morphometric vertebral fractures was 21% in postmenopausal women with osteopenia [68/25].
In patients with BMD-diagnosed osteoporosis, a baseline vertebral fracture assessment (vertebral morphometry) is not necessary for treatment decisions but is helpful in detecting lack of treatment efficacy during follow-up. Fractures occurring in L1–L4 will increase apparent BMD and may be difficult to see on the standard AP image provided by a routine scan.
Identification of High-Risk Individuals Without History of Fracture
The vast majority of osteoporotic fractures take place in osteopenic patients without prevalent fractures. While on one hand many aspects of osteoporosis and fracture risk are clinically recognizable (such as age, gender, and body weight), even before a first fracture has occurred, on the other hand, relative risk estimates are difficult to apply in daily clinical practice. This has been attributed to the finding that their clinical significance depends on the prevalence of fractures in the general population. In order to better delineate individuals at high risk of osteoporotic fracture, the WHO developed the Fracture Risk Assessment (FRAX) tool (www.shef.ac.uk./ FRAX). FRAX is an internet-based clinical tool for calculation of fracture risk in the individual patient based on assessment of significant risk factors for osteoporotic fracture. The FRAX algorithm is based on large-scale prospective population-based studies which isolated the following risk factors as significant determinants of fracture risk: age, gender, body weight and body mass index, a history of fracture, hip fracture in parents, current smoking, excessive alcohol intake, rheumatoid arthritis, glucocorticoid use, and other forms of secondary osteoporosis (Table 12.3) [44].
The National Osteoporosis Foundation (NOF) in the USA and the National Osteoporosis Society (NOS) in the UK have integrated FRAX and BMD for case finding of individuals at high risk for fracture and for treatment decisions in their new guidelines. Treatment thresholds were put at 10-year fracture risk estimates from the FRAX algorithm, at which fracture prevention became cost-effective. Generally, FRAX-based 10-year fracture risk probability of 20% or higher for all osteoporotic fractures and 3% or higher for hip fracture are considered reasonable intervention thresholds [45].
FRAX identifies patients at increased risk of osteoporotic fracture based on some of the dominant risk factors but cannot be used in isolation. However, several known determinant of fracture risk are not included in FRAX. The algorithm does not take into account well-known “dose effects” like glucocorticoid dose. Also, FRAX does not differentiate between having history of one or more osteoporotic fracture and when this fracture(s) has happened, hence miscalculation of the imminent fracture risk. Incorporation of BMD results is limited to results of BMD in the femoral neck. However, total hip BMD is a more precise measure and can be used interchangeably with femoral neck BMD in women, but not in men. Vitamin D deficiency, a well-established risk factor for falls and hip fracture, is not included. The same holds for bone markers, which have been shown to independently affect fracture risk. FRAX may also underestimate fracture risk in individuals with increased propensity for falls. More than 80% of women and men presenting with a clinical fracture to the emergency unit have one or more fall-related risks and exhibit a fourfold increased risk of falls in the year leading up to admission. In another study on 5- and 10-year absolute risks for fractures in patients using glucocorticoids, a history of falls had a greater impact on fracture risk than any other evaluated risk [57]. Finally, it is important to remember that FRAX is only applicable in untreated patients. It cannot be used as a helper in decision-making in patients, who already received specific osteoporosis treatment. However, recent studies revealed the applicability of FRAX in patients who received osteoporosis therapy [58, 59]. A recent study from Switzerland used FRAX to identify patient profiles with increased probability of fracture beyond currently accepted reimbursement thresholds for BMD and osteoporosis. The study found that in particular age, BMI, and parental history of fracture increased the risk for fracture substantially [60].
In patients with BMD-based osteoporosis or presenting with a clinical fracture or both, diagnostic evaluation is necessary to exclude secondary osteoporosis. Such evaluations should include hematologic parameters (Hb, WBC), serum 25-(OH)D3, calcium, creatinine, thyroid-stimulating hormone, parathyroid hormone (PTH), serum/urine electrophoresis, testosterone, and prolactin (in me). According to the clinical picture and suspicion, other serum measurements such as plasma cortisol, tests for celiac disease, and selected other evaluations looking for secondary causes are indicated [61]. It is generally considered that secondary causes of osteoporosis are more common in men than women. Among secondary causes, hypogonadism, which results from the treatment of breast cancer with aromatase inhibitors or the use of androgen deprivation therapies for prostate cancer is considered an emerging clinical challenge [76/29].
There is general consensus on the need for specific osteoporosis treatment in patients with spine or hip fractures and low BMD. For other nonvertebral fractures, different societies advocate different strategies. The NOS recommends drug treatment in all postmenopausal women with a history of any fragility fracture [12], while the NOF advocates performing a dual-energy X-ray absorptiometry (DXA) on patients after nonvertebral fractures to decide, whether specific osteoporotic therapy is indicated. Drug treatment should then be considered in patients having osteoporosis and in patients with osteopenia when FRAX indicates a 10-year fracture probability of at least 3% for hip or at least 20% for major fractures [41].
Thresholds for Intervention
Critically, none of the fracture risk assessment tools currently available directly yield an indication for treatment. Thus, the probability of fracture risk generated needs to be interpreted, and thresholds set, above which pharmaceutical intervention is judged to be warranted. The cost-effectiveness of a therapeutic approach is often a key consideration in threshold setting.
There are two major approaches to the health economic assessment in a particular condition [62, 63]. First, one can assess the cost-effectiveness of the intervention and set the threshold for intervention, for example, FRAX probability, accordingly. Alternatively, one can derive a clinically informed and appropriate intervention threshold and use cost-effectiveness analysis to validate a threshold. The 2017 National Institute for Health and Care Excellence (NICE) updated Multiple Technology Appraisal (MTA) on bisphosphonate use in osteoporosis [64] serves as an example of how, for a common disorder, the strict application of cost-effectiveness thresholds for relatively inexpensive drugs may lead to counterintuitive and potentially harmful guidance [62, 65]. The widespread availability of low-cost generic forms of the main oral and intravenous bisphosphonates resulted in oral treatments being deemed cost-effective above a 1% risk of major osteoporotic fracture. Unfortunately, these were initially interpreted by some payers as clinical intervention thresholds, but, in fact, NICE directs practitioners to the UK National Osteoporosis Guideline Group (NOGG) guidance, which provides an illustration of the alternative approach to threshold setting. NOGG developed its guidance on the basis of clinical appropriateness, setting the threshold at the age-specific 10-year FRAX probability of fracture equivalent to women having already sustained a fracture. This approach, which avoids inappropriate over-treatment of older individuals and under-treatment of younger individuals, has been shown to be cost-effective [44] and has been adopted in many countries [66].
The approach to threshold setting varies substantially across the world, with guidelines using either fixed or variable age-dependent threshold and, sometimes, combining a probability threshold with the requirement for BMD in the osteoporotic range [67]. Even between the USA and UK guidance, there is marked heterogeneity. The National Osteoporosis Foundation in the USA suggests BMD assessment in women and men aged ≥ 65 years or 70 years, respectively, or at younger ages if they have had a prior fracture, and treatment for those with either a history of vertebral or hip fracture, osteoporosis on BMD assessment, or osteopenia and a 10-year FRAX-calculated probability of a hip fracture probability of ≥ 3% or major osteoporotic fracture probability of ≥ 20% [68]. Conversely, as mentioned above, the UK National Osteoporosis Guideline Group (NOGG) recommends the use of FRAX with or without BMD as the first step in risk assessment, with prior fragility fractures at older ages usually a sufficient basis for treatment regardless of other risk factors. Where a 10-year probability has been generated by FRAX, threshold graphs are subsequently used to guide appropriate intervention. The possible outcomes include patient reassurance with further risk calculation at a later date (low risk), BMD assessment (intermediate risk), or immediate treatment without the need for BMD assessment (high risk) [69]. Once BMD has been performed, the 10-year probability of fracture is plotted by age, either above or below a single treatment threshold, which is set at the 10-year fracture probability conferred by having had a previous fragility fracture, corresponding to older UK national guidance. The treatment threshold, thus, increases with age, but even so, the proportion of women potentially eligible for treatment rises from 20 to 40% across the age range assessed. A key message is that it should not be assumed that one size will fit all countries. For example, intervention in China at a threshold of 20% for FRAX major osteoporotic fracture, a threshold used in the USA, would lead to only a very tiny proportion of the population treated [67]. Accordingly, the International Osteoporosis Foundation has published guidance relating to osteoporosis and corticosteroid-induced osteoporosis, which can be readily modified to reflect national priorities and subsequent treatment thresholds [75,76,72].
Treatment Decisions
Criteria for diagnosis are not the same as those for treating osteoporosis and osteopenia. Treatment must be based on assessing future fracture risk and on the medical state/risk factors of each individual. Therefore, authorities agree that decisions about treatment must be individualized and based on good clinical judgment, taking into account patient preferences, comorbidities, previous drug use and risk factors not captured in FRAX, and possible under- or overestimation of fracture risk by FRAX [73, 74]. Treatment of osteopenia was reviewed in an article published by Erickson [40].
Lifestyle Changes General
Changes in lifestyle like smoking cessation, regular exercise, and optimization of nutrition should be implemented in all osteopenic patients. Patient compliance with these measures is, however, poor, and very few prospective data on the anti-fracture efficacy of such measures exist. Smoking has emerged as a significant risk factor for fracture in many epidemiological studies [80,81,77], albeit the influence of dose and duration is less well defined. The same holds for exercise [78, 79], but exercise can slow down bone loss after menopause and is important for muscular strength and coordination in the elderly [77]. The impact of poor nutrition on skeletal health is apparent in its most extreme form in anorexia nervosa, where significant improvement of skeletal mass is important without a reversal of caloric intake in these young women [80, 81].
Calcium and Vitamin Supplement Therapy
In recent years, vitamin D deficiency has emerged as a very important risk factor for osteoporotic fracture, especially at the hip. High turnover bone loss due to secondary hyperparathyroidism due to vitamin D deficiency is considered a major pathogenetic factor in senile osteoporosis [82]. Vitamin D deficiency is endemic worldwide [83], and patients with hip fracture generally have the lowest vitamin D levels among all patient groups studied [100, 101/39, 40]. Vitamin D deficiency does cause not only weaker bones due to osteomalacia but also severe myopathy with loss of muscle strength, selective loss of the rapid type 2 fibers, dyscoordination, and consequently increased propensity for falls [84]. It is therefore not surprising that meta-analyses indicate that correction of vitamin D deficiency results in a decreased fall and fracture risk [85, 86], but the effects depend on the dose of vitamin D and the target population [87]. It is still a matter of debate which doses of vitamin D3 or D2 supplementation are necessary/optimal, taking into account baseline vitamin D status and the desired serum levels to be achieved by supplementation. Daily intake of 400 IU/day is not sufficient, while 800 IU/day reduce falls and fractures significantly [85, 86]. In their controlled clinical trial, Bischoff-Ferrari et al. demonstrated that in a population of post hip fracture patients maybe even higher doses are warranted. In this study, a dose of 2000 IU/day of D3 was superior to 800 IU/day in a cohort of 176 patients all undergoing moderate physiotherapy. Over a 1-year period, the dose of 2000 IU resulted in 25% less falls, 39% less readmissions to hospital, and a staggering 90% reduction in all cause infections, when compared to 800 IU per day [88].
Several reviews have emphasized the need of addition of calcium to vitamin D for fracture prevention, and a dose of 1000 to 1200 mg/day was advocated [89]. Whether the calcium dose can get too high is still a matter of debate, but studies from one center published in 2008 reported that supplements of 1000 mg calcium/day on top of a baseline intake of 800 mg/day increased the risk of vascular events including myocardial infarction in healthy postmenopausal women and men [108, 109/47, 48]. In this context, it is reassuring that, when intake of vitamin D3 is sufficient, the need for calcium intake is considered to be lower [90].
Prevention of Falls and Protection Against Fall Trauma
Over 90% of hip fractures and all Colles fractures are caused by falls, mostly in house. The role of physical exercise is still debated, but exercise interventions together with other measures such as removing loose carpets, reduce use of sleep medicine and other tranquilizers, correct visual impairment, etc. reduce the risk and rate of falls in older people living in the community [91], but no data that fall prevention decreases the risk of fracture are yet available. Similarly, as noted above, vitamin D supplements improve muscle function and decrease the risk of falls. The role of hip protectors remains controversial. They seem to work in nursing homes [92, 93], but less in community-dwelling elderly, mainly due to discomfort and practicality [94, 95].
Pharmacotherapy
Most clinical trials of specific therapies for osteoporosis and osteopenia have focused on patients with osteoporosis and/or the presence of hip or vertebral fracture. Few randomized controlled trials have been performed on patients with osteopenia, but some have included osteopenic patients allowing post hoc analyses.
Alendronate
In the Fracture Intervention Trial (FIT) 1 and FIT 2 trials of patients with osteopenia of the femoral neck with and without vertebral fractures, alendronate decreased the risk of radiological fractures (relative risk (RR) 0.48, 95% confidence interval (CI) 0.41–0.81) and of clinical vertebral fractures (RR 0.41, 95% CI 0.19 - 0.76) [96]. The FOSIT study evaluated the safety and effects on bone mineral density (BMD) of alendronate 10 mg in postmenopausal women with lumbar spine BMD T-score of −2 or more. After 12 months the incidence of nonvertebral fractures was reduced significantly by 47% [97].
Risedronate
Post hoc analysis of data available from four Phase III risedronate trials: BMD Multinational (BMD-MN) [98], BMD-North America (NA) [99], Vertebral Efficacy with Risedronate Therapy-Multinational (VERT-MN) [100], and Vertebral Efficacy with Risedronate Therapy-North America (VERT-NA) [101] (in which efficacy and safety of risedronate in the prevention and treatment of postmenopausal osteoporosis have been demonstrated) were carried out. Using data only from osteopenic women included in these trials, the effect of risedronate in reducing the risk of fragility fractures in women with femoral neck T-scores in the osteopenic range and without prevalent vertebral fracture was evaluated. Six hundred and twenty postmenopausal women with osteopenia were included, receiving either placebo (= 309) or risedronate 5 mg (= 311). Risedronate reduced the risk of fragility fractures by 73% over 3 years versus placebo (= 0.023); cumulative fragility fracture incidence was 6.9% in placebo-treated versus 2.2% in risedronate-treated patients. The magnitude of the effect was similar in the sensitivity analysis subset [102].
Zoledronate
Zoledronate (also known as zoledronic acid) has characteristics that make it attractive for use in women who have osteopenia. It is administered by intravenous injection at intervals of 1 year or longer. Reid et al. [103] conducted a 6-year, double-blind trial involving 2000 women with osteopenia (defined by a T-score of −1.0 to −2.5 at either the total hip or the femoral neck on either side) who were 65 years of age or older. Participants were randomly assigned to receive four infusions of either zoledronate at a dose of 5 mg (zoledronate group) or normal saline (placebo group) at 18-month intervals. A dietary calcium intake of 1 g per day was advised, but calcium supplements were not provided. Participants who were not already taking vitamin D supplements received cholecalciferol before the trial began (a single dose of 2.5 mg) and during the trial (1.25 mg per month). The primary endpoint was the time to first occurrence of a nonvertebral or vertebral fragility fracture. Results revealed that women who received zoledronate had a lower risk of nonvertebral fragility fractures (hazard ratio, 0.66; P = 0.001), symptomatic fractures (hazard ratio, 0.73; P = 0.003), vertebral fractures (odds ratio, 0.45; P = 0.002), and height loss (P < 0.001). The study concluded that the risk of nonvertebral or vertebral fragility fractures was significantly lower in women with osteopenia who received zoledronate than in women who received placebo.
Strontium
In the Spinal Osteoporosis Therapeutic Intervention (SOTI) and TReatment Of Postmenopausal OSteoporosis (TROPOS) trials [104] in women with osteopenia of the lumbar spine, strontium ranelate reduced the risk of vertebral fracture in women with no prevalent fractures (RR 0.41, 95% CI 0.17–0.99) and in women with prevalent fractures (RR 0.62, 95% CI 0.44–0.88).17 In women with osteopenia at both the lumbar spine and femoral neck, treatment with strontium ranelate reduced the risk of fracture (RR 0.48, 95% CI 0.24–0.96). Specific drug treatment appears to be effective and is justified to reduce the risk of further fractures in patients with osteopenia, particularly those with prevalent fractures.
Raloxifene
Selective estrogen receptor modulators (SERMs) are nonsteroidal synthetic agents, which exert estrogen-like properties on the bone and cardiovascular systems but estrogen antagonistic actions in the breast and, in some cases, the endometrium. The first SERM developed both for breast cancer prevention and for osteoporosis, raloxifene, is now approved in many countries for the treatment of osteoporosis.
Multiple Outcomes of Raloxifene Evaluation (MORE) study [105] reported similar rates of vertebral fracture risk reduction in raloxifene-treated women with osteopenia—defined as a total hip T-score > −2.5 without a prevalent vertebral fracture—compared with those with osteoporosis at 3 years. The relative risk reduction for vertebral fractures with raloxifene compared with placebo was 0.53 (0.32–0.88, 95% CI) in osteopenic women; the relative risk for clinical vertebral fractures in osteopenic women was 0.25 (0.04–0.63). Information about reduction of nonvertebral fractures has not been provided raloxifene analyses.
Hormone Replacement Therapy (HRT)
Conjugated equine estrogens significantly reduced the risk of clinical vertebral, hip, and total fractures in postmenopausal women in the Women’s Health Initiative, the vast majority of whom did not have bone density testing but who were not selected based on having diagnosed osteoporosis [106].
Estrogen receptors have been demonstrated on both osteoblasts and osteoclasts [107, 108]. Estrogen replacement therapy (ERT) or combined estrogen/progestin therapy (HRT) reduces bone turnover by about 50% and improves bone balance at each individual BMU in postmenopausal women [109]. The Women’s Health Initiative (WHI), a randomized study comprising over 16,000 postmenopausal women, demonstrated a significant 34% reduction of hip fractures after treatment with combined conjugated equine estrogen and [110] estrogen alone in those women who had undergone hysterectomy [111]. The study, however, also found a nearly 30% increased risk of coronary heart disease, 40% increased risk of stroke, increased risk of thromboembolic events, and 26–35% increased risk of breast cancer. These results led to less enthusiasm for long-term estrogen therapy worldwide. The decision to initiate ERT/HRT should be individualized and based on a balanced assessment of risk and benefits by the physician and patient. Current recommendations support restricting the use of estrogen in most women to 5 years in the perimenopausal period [40], with the aim mainly to reduce hot flushes and other postmenopausal symptoms, and regular mammography should be performed.
Androgen Replacement Therapy in Males
In hypogonadal males, low testosterone levels result in a high turnover state in bone leading to bone loss and increased risk of fracture. The main driver of this turnover increase is low circulating estrogen levels, just as in postmenopausal women [112]. The low estrogen arises from insufficient aromatase conversion from testosterone, either due to low testosterone levels or insufficient aromatase activity [113]. Testosterone replacement therapy in hypogonadism will increase circulating estradiol levels and thereby reduce bone turnover and increase BMD [114]. In hypogonadism, usually defined as total testosterone levels below 8 nmol/l and hypogonadal symptoms [115], testosterone replacement will lead to increases in bone mass similar to those seen after ERT/HRT [115, 116], but randomized controlled studies with fracture endpoints are still lacking. Due to the fear of inducing prostate cancer, clinicians have, however, been quite reluctant to institute testosterone replacement therapy. Recent data suggest, however, that prostate cancers occurring in hypogonadal males have a worse prognosis than cancers occurring in eugonadism [117]. Moreover, 16 population studies were unable to demonstrate any relation between testosterone levels and risk of prostate cancer [50]. Nevertheless, regular controls of prostate-specific antigen (PSA) and digital rectal exploration before and after institution of therapy are still warranted.
Management of Osteoporosis and Osteopenia in the Very Elderly
Very elderly women and men (aged 80 years and over) are the fastest-growing segment of the population. About 25–30% of the population burden of all fragility fractures is in women and men over 80, who are at high risk for fracture, particularly nonvertebral fracture, because of their high prevalence of osteoporosis and osteopenia and high incidence of falls. After a hip fracture, approximately 20% of patients do not survive more than a year, and 50% do not regain their previous level of independence. Vertebral fractures are associated with back pain, height loss, kyphosis, and functional disability. The prevalence of vertebral deformities increases from 5–10% in women in the 50s to 45–55% of those in the 80s. Only a proportion of older women and men with osteoporosis or osteopenia receive specific treatment. Some clinicians may consider that patients over 80 years are too old or that it is too late to significantly alter the course of the disease. Based on pooled data of 1392 women aged 80 or over from the HIP, VERT-MN, and VERT-NA trials [10,11,12,13], risedronate resulted in a 44% reduction in vertebral fractures but not in nonvertebral fractures [102]. In 1488 women between 80 and 100 years of age from the SOTI and TROPOS trials [104] and followed up for 3 years, strontium ranelate reduced the risk of vertebral, nonvertebral, and clinical symptomatic fractures within the first year by 59% (p = 0.002), 41% (p = 0.027), and 37% (p = 0.012), respectively. At the end of 3 years, vertebral, nonvertebral, and symptomatic clinical fractures were reduced by 32% (p = 0.013), 31% (p = 0.011), and 22% (p = 0.040), respectively. Strontium ranelate was reported to be well tolerated and as safe as in younger patients. Women and men are therefore never too old for treatment, and it is never too late to treat those with osteoporosis or osteopenia, particularly when they have a fragility fracture.
Treatment Algorithm for Osteopenia
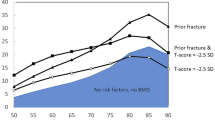
An ever-increasing array of effective treatments is available to protect patients with osteopenia against fractures. While there is general consensus on treating osteopenic individuals with prevalent low-energy fractures, the treatment of osteopenia without fracture is still debatable. However, current evidence indicates that specific pharmacotherapy should be instituted if an osteopenic patients has prevalent fractures or suffers new fractures, be it clinical or asymptomatic. Moreover, a significant accumulation of several significant risk factors, for example, as indicated by the FRAX tool may constitute an indication for pharmacotherapy. Patients without such risk factors should be counseled on a “bone-friendly” lifestyle with nutritional modifications, regular exercise, moderation in alcohol use, and if possible smoking cessation. In patients with low vitamin D levels, Ca and vitamin D supplementation may also be indicated (Fig. 12.5).
Algorithm for osteopenia treatment: intervention thresholds based on the occurrence of fracture and the 10-year risk probability of major osteoporotic fracture/hip fracture calculated by FRAX tool. High fracture risk: major osteoporosis fracture risk >20%/hip fracture risk >3%. Low fracture risk: major osteoporosis fracture risk <20%, hip fracture risk <3%
Bisphosphonates, taken orally or intravenously, remain the dominant treatment modalities for osteopenia. They reduce fracture risk in osteoporotic as well as osteopenic individuals. Questions exist about the very long-term safety of these drugs, but the best data available so far [72] suggest that 10 years with 90% suppression of bone turnover is safe. Denosumab constitutes a possible alternative to bisphosphonates. In younger postmenopausal women with osteopenia, estrogen or estrogen/progestin still has a place as a short-term (up to 5 years) treatment, especially in women with menopausal symptoms. Similarly, SERMs should be considered in younger postmenopausal women, especially those at increased risk of breast cancer. In males with low testosterone levels, testosterone substitution is indicated as it improves skeletal integrity. However, long-term controlled studies on this treatment are still required, but the risk of prostate cancer does not seem to be as big as previously anticipated. Teriparatide would currently rarely be considered in women or men with cheaper anabolics available; however, initial therapy with anabolics to bring osteopenic patients out of the risk zone followed by an antiresorptive would probably be the ideal treatment [40].
In conclusion, osteopenia is not a disease but is a marker for risk of fractures. Older persons are at risk of having unrecognized osteoporosis, which may be discovered only after a fracture (such as a broken hip). The need to establish treatment efficacy in osteopenia has become more pressing, given the clinical trend to base intervention decisions on absolute fracture risk. Many patients at high risk for fracture do not have T-scores of less than −2.5 but rather have osteopenia in combination with other risk factors, such as age. Intervention in such patients currently lacks an adequate evidence base, though several therapeutic options are available. A treatment algorithm has been suggested based on bone mineral density and the fracture risk probability. If the bone density is already abnormal, lifestyle changes can help slow progression of bone loss and reduce the occurrence of fractures. Pharmacotherapy is indicated in patients with osteopenia and low-trauma fractures or at high risk of sustaining a fracture.
References
Albright F. Osteoporosis. Ann Intern Med. 1947;27(6):861–82.
Cooper A, Cooper BB. A treatise on dislocations, and on fractures of the joints. London: Churchill; 1822.
Schapira D, Schapira C. Osteoporosis: the evolution of a scientific term. Osteoporos Int. 1992;2(4):164–7.
Kanis JA, Adachi JD, Cooper C, Clark P, Cummings SR, Diaz-Curiel M, Harvey N, Hiligsmann M, Papaioannou A, Pierroz DD, Silverman SL, Szulc P. Standardising the descriptive epidemiology of osteoporosis: recommendations from the Epidemiology and Quality of Life Working Group of IOF. Osteoporos Int. 2013.
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–89.
Kanis JA, Bianchi G, Bilezikian JP, Kaufman JM, Khosla S, Orwoll E, Seeman E. Towards a diagnostic and therapeutic consensus in male osteoporosis. Osteoporos Int. 2011;22:2789–98.
Siris ES, Miller PD, Barrett-Connor E, et al. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–22.
Black DM, Cummings SR, Karpf DB, et al. Fracture Intervention Trial Research Group, Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41.
Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82.
Ettinger B, Black DM, Mitlak BH, et al. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators, Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene. JAMA. 1999;282:637–45.
Harris ST, Watts NB, Genant HK, et al. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group, Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344–52.
Writing Group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA. 2002;288:321–33.
McClung MR, Geusens P, Miller PD, et al. Hip Intervention Program Study Group, Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–40.
Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
Chesnut CH III, Silverman S, Andriano K, et al. PROOF Study Group, A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis. Am J Med. 2000;109:267–76.
World Health Organization (WHO Study Group). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO Study Group. World Health Organ Tech Rep Ser. 1994;843:1–129.
National Osteoporosis Foundation. Physician's Guide to Prevention and Treatment of Osteoporosis. Belle Mead: Excerpta Medica Inc.; 1999.
National Osteoporosis Foundation. Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis: executive summary. Osteoporos Int. 1998;8(suppl 4):S3–6.
Siris ES, Chen Y, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164(10):1108–12.
Zebaze RM, Ghasem-Zadeh A, Bohte A, et al. Intracortical remodelling and porosity in the distal radius and post-mortem femurs of women: a cross-sectional study. Lancet. 2010;375(9727):1729–36.
Carter DR, Hayes WC. The compressive behavior of bone as a two-phase porous structure. J Bone Joint Surg Am. 1977;59(7):954–62.
Schaffler MB, Burr DB. Stiffness of compact bone: effects of porosity and density. J Biomech. 1988;21(1):13–6.
Miller PD. Guidelines for the diagnosis of osteoporosis: T-scores vs fractures. Rev Endocr Metab Disord. 2006;7:75–89.
Parfitt AM. Interpretation of bone densitometry measurements: disadvantages of a percentage scale and a discussion of some alternatives. J Bone Miner Res. 1990;5:537–40.
Webber CE. Uncertainties in bone mineral density T-scores. Clin Invest Med. 1998;21:88–93.
Blake GM, Fogelman I. Interpretation of bone densitometry studies. Semin Nucl Med. 1997;27:248–60.
Goldstein S. Osteopenia: when to intervene? OBG Manag. 2006;18:45–55.
Sanders KM, Nicholson GC, Watts JJ, Pasco JA, Henry MJ, Kotowicz MA, Seeman E. Half the burden of fragility fractures in the community occur in women without osteoporosis. When is fracture prevention cost-effective? Bone. 2006;38:694–700.
Siris ES, Brenneman SK, Miller PD, Barrett-Connor E, Chen Y, Sherwood LM, Abbott TA. Predictive value of low BMD for 1-year fracture outcomes is similar for postmenopausal women ages 50-64 and 65 and older: results from the National Osteoporosis Risk Assessment (NORA). J Bone Miner Res. 2004;19:1215–20.
Kanis J, Johnell O, Oden A, De Laet C, Oglesby A, Jonsson B. Intervention thresholds for osteoporosis. Bone. 2002;31:26–31.
Chrischilles E. Outcomes assessment in osteoporosis: strategies for improvement. Med Interface. 1996;9(7):127–33.
Melton L, Atkinson E, O'Fallon WM, Wahner HW, Riggs BL. Long term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res. 1993;8(10):1227–33.
Nevitt MC, Johnell O, Black DM, Ensrud K, Genant HK, Cummings SR, et al. Bone mineral density predicts non-spine fractures in very elderly women. Osteoporos Int. 1994;4:325–31.
Schuit SC, Van der Klift M, de Laet CE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam study. Bone. 2004;34(1):195–202.
Pasco JA, Seeman E, Henry MJ, Merriman EN, Nicholson GC, Kotowicz MA. The population burden of fractures originates in women with osteopenia not osteoporosis. Osteoporos Int. 2006;17(9):1404–9.
Gourlay ML, Fine JP, Preisser JS, et al. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225–33.
Rose G. Sick individuals and sick populations. Int J Epidemiol. 2001;30(3):427–32. discussion 33–4
Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. [PMC free article] [PubMed] [Google Scholar].
Oriwaki K, Komaba H, Noto S, et al. Cost-effectiveness of alendronate for the treatment of osteopenic postmenopausal women in Japan. J Bone Miner Res. 2013;28(2):395–403.
Eriksen EF. Treatment of osteopenia. Rev Endocr Metab Disord. 2012;13(3):209–23.
National osteoporosis Foundation-Clinicians guide to prevention and treatment of osteoporosis. www.nof.org/professionals/Clinicians_Guide.htm [serial online]. Available at: www.nof.org/professionals/Clinicians_Guide.htm.
Lyles KW, Colon-Emeric C, Magaziner J, Adachi J, Pieper CF, Hyldstrup L, Recknor C, Nordsletten L, Moore KA, Lavecchia C, Zhang J, Mesenbrink P, Abrams K, Orloff JJ, Horowitz Z, Eriksen EF, Boonen S. The effect of once yearly zoledronic acid on new fractures and mortality after hip fracture. 2007; In press.
van Helden S, van Geel AC, Geusens PP, Kessels A, Nieuwenhuijzen Kruseman AC, Brink PR. Bone and fall-related fracture risks in women and men with a recent clinical fracture. J Bone Joint Surg Am. 2008;90:241–8.
Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A. Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int. 2008;19:1395–408.
Dawson-Hughes B, Tosteson AN, Melton LJ 3rd, Baim S, Favus MJ, Khosla S, Lindsay RL. Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19(4):449–58.
van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 2009;68:99–102.
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21.
Chevalley T, Hoffmeyer P, Bonjour JP, Rizzoli R. An osteoporosis clinical pathway for the medical management of patients with low-trauma fracture. Osteoporos Int. 2002;13:450–5.
Sambrook P, Cooper C. Osteoporosis. Lancet. 2006;367:2010–8.
Lems WF. Clinical relevance of vertebral fractures. Ann Rheum Dis. 2007;66:2–4.
Delmas PD, Genant HK, Crans GG, Stock JL, Wong M, Siris E, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone. 2003;33:522–32.
Black DM, Boonen S, Cauley J, Delmas P, Eastell R, Reid I, et al. Effect of once-yearly infusion of zoledronic acid 5 mg on spine and hip fracture reduction in postmenopausal women with osteoporosis: the HORIZON pivotal fracture trial. J Bone Miner Res. 2006;21:S16.
Cummings SR, San MJ, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65.
Schousboe JT, Vokes T, Broy SB, Ferrar L, McKiernan F, Roux C, et al. Vertebral fracture assessment: the 2007 ISCD official positions. J Clin Densitom. 2008;11:92–108.
Delmas PD, van de Langerijt L, Watts NB, Eastell R, Genant H, Grauer A, et al. Underdiagnosis of vertebral fractures is a worldwide problem: the IMPACT study. J Bone Miner Res. 2005;20:557–63.
Gallacher SJ, Gallagher AP, McQuillian C, Mitchell PJ, Dixon T. The prevalence of vertebral fracture amongst patients presenting with non-vertebral fractures. Osteoporos Int. 2007;18:185–92.
van Staa TP, Geusens P, Pols HA, de Laet C, Leufkens HG, Cooper C. A simple score for estimating the long-term risk of fracture in patients using oral glucocorticoids. QJM. 2005;98:191–8.
Leslie WD, Lix LM, Johansson H, Oden A, McCloskey E, Kanis JA. Does osteoporosis therapy invalidate FRAX for fracture prediction? J Bone Miner Res. 2012;27:1243–51.
El Miedany Y, El Gaafary M, El Yassaki A, Youssef S, Nasr A, Ahmed I. Monitoring osteoporosis therapy: Can FRAX help assessing success or failure in achieving treatment goals? World J Rheumatol. 2014;4(2):14–21.
Lippuner K, Johansson H, Kanis JA, Rizzoli R. FRAX assessment of osteoporotic fracture probability in Switzerland. Osteoporos Int. 2010;21:381–9.
Tannenbaum C, Clark J, Schwartzman K, Wallenstein S, Lapinski R, Meier D, et al. Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab. 2002;87:4431–7.
Harvey NC, McCloskey E, Kanis JA, Compston J, Cooper C. Bisphosphonates in osteoporosis: NICE and easy? Lancet. 2017;390(10109):2243–4.
Harvey NC, McCloskey E, Kanis JA, Compston J, Cooper C. Cost-effective but clinically inappropriate: new NICE intervention thresholds in osteoporosis (Technology Appraisal 464). Osteoporos Int. 2018;29(7):1511–3.
NICE. TA464: bisphosphonates for treating osteoporosis. London: National Institute for Health and Care Excellence; 2017.
Sims I (2017) Many more eligible for bisphosphonates after NICE lowers threshold to 1%. PULSE. http://www.pulsetoday.co.uk/clinical/more-clinical-areas/musculoskeletal/many-more-eligible-for-bisphosphonates-after-nice-lowers-threshold-to-1/20034787.article. Accessed 26 July 2017.
Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV, Advisory Board of the National Osteoporosis Guideline Group. A systematic review of intervention thresholds based on FRAX : a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11(1):25.
Kanis JA, Harvey NC, Cooper C, Johansson H, Oden A, McCloskey EV. A systematic review of intervention thresholds based on FRAX: a report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos. 2016;11(1):25.
National Osteoporosis Foundation. Clinician’s guide to the prevention and treatment of osteoporosis. Washington, DC: National Osteoporosis Foundation; 2013.
Compston J, Bowring C, Cooper A, Cooper C, Davies C, Francis R, Kanis JA, Marsh D, McCloskey EV, Reid DM, Selby P. Diagnosis and management of osteoporosis in postmenopausal women and older men in the UK: National Osteoporosis Guideline Group (NOGG) update 2013. Maturitas. 2013.
Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2018.
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24(1):23–57.
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgstrom F, Cooper C, Diez Perez A, Eastell R, Hofbauer LC, Kanis JA, Langdahl BL, Lesnyak O, Lorenc R, McCloskey E, Messina OD, Napoli N, Obermayer-Pietsch B, Ralston SH, Sambrook PN, Silverman S, Sosa M, Stepan J, Suppan G, Wahl DA, Compston JE. A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int. 2012;23(9):2257–76.
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S, Borgstrom F, Cooper C, Perez AD, Eastell R, Hofbauer LC, Kanis JA, Langdahl BL, Lesnyak O, Lorenc R, McCloskey E, Messina OD, Napoli N, Obermayer-Pietsch B, Ralston SH, Sambrook PN, Silverman S, Sosa M, Stepan J, Suppan G, Wahl DA, Compston JE. An appendix to the 2012 IOF-ECTS guidelines for the management of glucocorticoid-induced osteoporosis. Arch Osteoporos. 2012;7(1–2):25–30.
Liu J, Curtis EM, Cooper C, Harvey NC. State of the art in osteoporosis risk assessment and treatment. J Endocrinol Invest. 2019;42(10):1149–64.
Orwoll ES, Bevan L, Phipps KR. Determinants of bone mineral density in older men. Osteoporos Int. 2000;11:815–21.
Bjarnason NH, Christiansen C. The influence of thinness and smoking on bone loss and response to hormone replacement therapy in early postmenopausal women. J Clin Endocrinol Metab. 2000;85:590–6.
Cummings SR. Prevention of hip fractures in older women: a population-based perspective. Osteoporos Int. 1998;8(Suppl 1):S8–12.
Chesnut CH III. Bone mass and exercise. [review] [17 refs]. Am J Med. 1993;95:34S–6.
Turner CH, Robling AG. Exercise as an anabolic stimulus for bone. [Review] [89 refs]. Curr Pharm Des. 2004;10:2629–41.
Davies KM, Pearson PH, Huseman CA, Greger NG, Kimmel DK, Recker RR. Reduced bone mineral in patients with eating disorders. Bone. 1990;11:143–7.
Vestergaard P, Emborg C, Stoving RK, Hagen C, Mosekilde L, Brixen K. Patients with eating disorders. A high-risk group for fractures. Orthop Nurs. 2003;22:325–31.
Riggs BL. Role of the vitamin D-endocrine system in the pathophysiology of postmenopausal osteoporosis. [Review] [41 refs]. J Cell Biochem. 2003;88:209–15.
Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009;24:693–701.
Glerup H, Mikkelsen K, Poulsen L, Hass E, Overbeck S, Andersen H, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–24.
Bischoff-Ferrari HA, Willett WC, Wong JB, Giovannucci E, Dietrich T, Dawson-Hughes B. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–64.
Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, Staehelin HB, Bazemore MG, Zee RY, et al. Effect of vitamin D on falls: a meta-analysis. JAMA. 2004;291:1999–2006.
Izaks GJ. Fracture prevention with vitamin D supplementation: considering the inconsistent results. BMC Musculoskelet Disord. 2007;8:26.
Bischoff-Ferrari H, et al. Effects of extended physiotherapy and high dose vitamin D on falls and morbidity after hip fracture. Arch Intern Med. 2010;170(9):813–20.
Boonen S, Lips P, Bouillon R, Bischoff-Ferrari HA, Vanderschueren D, Haentjens P. Need for additional calcium to reduce the risk of hip fracture with vitamin d supplementation: evidence from a comparative meta-analysis of randomized controlled trials. J Clin Endocrinol Metab. 2007;92:1415–23.
Heaney RP. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97:13–9.
Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;3:CD007146.
Forsen L, Arstad C, Sandvig S, Schuller A, Roed U, Sogaard AJ. Prevention of hip fracture by external hip protectors: an intervention in 17 nursing homes in two municipalities in Norway. Scand J Public Health. 2003;31:261–6.
Meyer G, Warnke A, Bender R, Muhlhauser I. Effect on hip fractures of increased use of hip protectors in nursing homes: cluster randomised controlled trial. BMJ. 2003;326:76.
Cameron ID, Venman J, Kurrle SE, Lockwood K, Birks C, Cumming RG, et al. Hip protectors in aged-care facilities: a randomized trial of use by individual higher-risk residents. Age Ageing. 2001;30:477–81.
O’Halloran PD, Murray LJ, Cran GW, Dunlop L, Kernohan G, Beringer TR. The effect of type of hip protector and resident characteristics on adherence to use of hip protectors in nursing and residential homes–an exploratory study. Int J Nurs Stud. 2005;42:387–97.
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the fracture intervention trial. JAMA. 1998;280:2077–82.
Pols HA, Felsenberg D, Hanley DA, Stepan J, Munoz-Torres M, Wilkin TJ, et al. Multinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Fosamax International Trial Study Group. Osteoporos Int. 1999;9:461–8.
Fogelman I, Ribot C, Smith R, et al. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab. 2000;85:1895–900. [PubMed]
McClung MR, Bensen WG, Bolognese MA, et al. Risedronate increases bone mineral density at the hip, spine and radius in postmenopausal women with low bone mass. Osteoporos Int. 1998;8:111.
Reginster J, Minne HW, Sorensen OH, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. [PubMed]
Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52.
Siris ES, Simon JA, Barton IP, McClung MR, Grauer A. Effects of risedronate on fracture risk in postmenopausal women with osteopenia. Osteoporos Int. 2008;19(5):681–6.
Reid IR, Horne AM, Mihov B, Stewart A, Garratt E, Wong S, Wiessing KR, Bolland MJ, Bastin S, Gamble GD. Fracture prevention with Zoledronate in older women with osteopenia. N Engl J Med. 2018;379:2407–16.
Reginster JY, Seeman E, Vernejoul MC, Adami S, Compston J, Phenekos C, Devogelaer JP, Curiel MD, Sawicki A, Goemaere S, Sorensen OH, Felsenberg D, Meunier PJ. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–22. 124. Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators.[see comment][erratum appears in JAMA 1999 Dec 8;282(22):2124] JAMA. 1999;282:637–45.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33.
Eriksen EF, Colvard DS, Berg NJ, Graham ML, Mann KG, Spelsberg TC, Riggs BL. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988;241:84–6.
Oursler MJ, Osdoby P, Pyfferoen J, Riggs BL, Spelsberg TC. Avian osteoclasts as estrogen target cells. Proc Natl Acad Sci U S A. 1991;88:6613–7.
Eriksen EF, Langdahl B, Vesterby A, Rungby J, Kassem M. Hormone replacement therapy prevents osteoclastic hyperactivity: a histomorphometric study in early postmenopausal women. J Bone Miner Res. 1999;14:1217–21.
The womens health initiative steering committee effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the womens health initiative randomized controlled trial. JAMA.
Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:529–41.
Khosla S, Melton LJ III, Riggs BL. Clinical review 144: estrogen and the male skeleton. [Review] [54 refs]. J Clin Endocrinol Metab. 2002;87:1443–50.
Carlsen CG, Soerensen TH, Eriksen EF. Prevalence of low serum estradiol levels in male osteoporosis. Osteoporos Int. 2000;11:697–701.
Anderson FH, Francis RM, Peaston RT, Wastell HJ. Androgen supplementation in eugonadal men with osteoporosis: effects of 6 months’ treatment on markers of bone formation and resorption. J Bone Miner Res. 1997;12:472–8.
Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in adult men with androgen deficiency syndromes: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2006;91:1995–2010.
Eginster JY, Seeman E, Vernejoul MC, Adami S, Compston J, Phenekos C, Devogelaer JP, Curiel MD, Sawicki A, Goemaere S, Sorensen OH, Felsenberg D, Meunier PJ. Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: Treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab. 2005;90:2816–22.
Morgentaler A. Rapidly shifting concepts regarding androgens and prostate cancer. Sci World J. 2009;9:685–90.
Morgentaler A. Testosterone replacement therapy and prostate risks: where’s the beef? Can J Urol. 2006;13(Suppl 1):40–3.
Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–95.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
El Miedany, Y. (2022). Osteopenia: Mind the Gap. In: El Miedany, Y. (eds) New Horizons in Osteoporosis Management. Springer, Cham. https://doi.org/10.1007/978-3-030-87950-1_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-87950-1_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87949-5
Online ISBN: 978-3-030-87950-1
eBook Packages: MedicineMedicine (R0)