Abstract
Proteins represent the dominant biomass of aquatic animals; consequently, proteins are significant nutrients and energy sources with digestive efficiencies between 60 and almost 100%. For most aquatic animals, the quantity of prey available is typically the nutritional bottleneck. A deficiency of dietary protein or amino acids has long been known to impair immune function and increase the susceptibility of animals to infectious disease. In addition to function as energy source, free amino acids can act as osmolytes. The average dietary protein requirement of fishes is 42%; that of invertebrates appears to be below this value. Protein requirement depends on environmental factors, such as salinity and temperature, as well as trophic level and content of the other macronutrients. Interactions with other macronutrients, however, are not yet adequately considered. Adverse effects occur in animals fed deficient or excess proteinaceous diets. Biomolecular modes of action of hyperproteic diets are beginning to be understood; impairment of the immune system is central. Finally, this chapter points out gaps of protein nutrition in aquatic animals.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Protein requirement
- Protein quality
- Amino acid composition
- Impairment of immunity
- Complement system
- Nutrient-specific foraging
From a quantitative perspective, amino acids (AAs), either free or combined in macromolecules (peptides, proteins), represent the dominant dry mass component of most living systems. In general, aquatic animals consume nearly every type of food ranging from sediment, detritus, fruits, algae, or wood to slime, scales, blood, mollusks, echinoderms, arthropods, mammals, and other fishes in order to meet their needs.
Fishes efficiently digest protein from most feedstuffs with high efficiency of some 60% to almost 100% (Table 2.1). Considering that 65–75% of their carcass dry mass is protein, fishes need to absorb protein efficiently from food, if for nothing else, tissue maintenance and growth (although many carnivorous fishes metabolize AAs for energy). Noteworthy, the dietary protein requirement of aquatic invertebrates lies in the same range (Table 2.2).
Animal prey is richer in proteins and more digestible than plant or detrital materials (Fig. 2.1). However, the quantity of prey available is typically the bottleneck. It is common for fishes to ingest well below 5% of body mass per day when feeding on invertebrate prey. This is consistent with the notion that morphology and behavior of fishes feeding extensively on invertebrates are adapted to optimize energy gain (Bowen et al. 1995). Algae, aquatic macrophytes, and detritus are all inferior to fishes and aquatic invertebrates as sources of protein and energy. Furthermore, protein supply is the major constraint for detritivores.
Dietary protein is the major and most expensive component of formulated aquafeeds; fishmeal is generally considered to be the most ideal protein source for aquatic animals (Li et al. 2009). A deficiency of dietary protein or AAs has long been known to impair immune function and increase the susceptibility of animals to infectious disease (Li et al. 2007). Aquatic invertebrates, fish eggs, embryos, and larvae are no exception, and several studies point out the importance of AAs for their catabolic and anabolic energy metabolism. To demonstrate this importance, we refer to hybrid striped bass and zebrafish: glutamate (Glu) and glutamine (Gln) are more actively oxidized in the proximal intestine, liver, and kidney of fish than glucose and palmitate. Glu provides more energy than Gln in all tissues except in the liver where Gln serves as the main metabolic fuel. In the skeletal muscles, Glu is the preferred nutrient to generate adenosine triphosphate (ATP). Together, Glu plus Gln (plus leucine, Leu) contributes to about 80% of ATP production in the fish tissues (Jia et al. 2017).
In addition to the function of AAs as energy source, free AAs (fAAs), individually or in concert, can act as osmolytes in habitats of varying salinity as recently shown in Amazon river prawn Macrobrachium amazonicum (Mazzarelli et al. 2015) and San Francisco brine shrimp Artemia franciscana (Zadehmohseni et al. 2020).
2.1 Requirement
Estimates of fish dietary protein requirements from many taxa are 2–4 times greater than the amount needed by other vertebrates (German 2011). Most fish species are carnivorous and are adapted to use protein as a preferred energy source over carbohydrate, and thus require high levels of dietary protein (20→70%: Cebidichthys violaceus→Scophthalmus maximus, Pleuronectes platessa) with an average of 42% (Oliva Teles et al. 2020). Figure 2.2 shows schematically the steps of protein digestion.
Protein and the endogenous digestive enzymes fishes produce to hydrolyze them. The digestion of proteins requires multiple steps, beginning with initial hydrolysis in the lumen by extracellular enzymes and followed by membrane digestion by intestinal mucosal enzymes. The enzyme pepsin is listed in parentheses because agastric species (i.e., those that lack an acidic stomach) generally lack this. (From German (2011), with permission from Elsevier)
Some invertebrates appear to have lower protein requirements. The few papers, which studied protein requirement during the ontogenetic development, indicate that it decreases with increasing age (Table 2.2). In Litopenaeus vannamei, Smith et al. (1985) detected that the growth of small individuals is influenced more by protein level than protein source whereas growth of medium and large ones is more influenced by protein source than protein level. Various decapod crustaceans have a wide range of quantitative optimal dietary protein requirements strongly depending on the protein quality.
Early life stages of fishes can acquire exogenous proteinaceous nutrients as AAs or peptides, rather than intact proteins. In fact, the composition of the dietary proteins triggers life history traits. Nevertheless, even recent studies address gross protein requirements of fishes or aquatic invertebrates (Table 2.2) and continue to disclose that protein requirements follow an optimum dose-response curve.
Traditional in the experimental approach, Guy et al. (2018) evaluated the effects of dietary protein levels on growth and body composition of juveniles of the threatened omnivorous black buffalo (Ictiobus niger). Therefore, artificial propagation and culture are frequently a part of native species recovery plans, and developing formulated diets is a critical component of these plans. This study shows that a diet with 41% crude protein produces optimal growth for juvenile black buffalo.
Several data about protein requirement from different papers mutually agree well, others deviate considerably. Such discrepancies within one species are not astonishing, if one considers
-
The high diversity of feed qualities applied (→Chap. 1, Hua and Bureau (2012))
-
The missing standardization of larval age or developmental stages of juveniles used
-
The feeding history of offspring and parental individuals (→AAN III “Nutritional Programming”)
-
The genetic and epigenetic diversity within one species (→Chap. 40)
Abalone species are generally characterized by slow and heterogeneous growth rates. Therefore, artificial feed for abalone must contain sufficient protein and essential AAs (EAAs) in order to satisfy their nutrient requirements. To optimize aquaculture of the intrinsically herbivorous animals, artificial diets have to be amended with animal proteins. Bautista-Teruel et al. (2003) fed Haliotis asinina with fishmeal, shrimp meal, defatted soybean meal, and Arthrospira (Spirulina) sp. Highest weight gain (WG) is attained with a combination of fishmeal, shrimp meal, and defatted soybean meal. Astonishingly, abalone on pure plant protein diets (soybean, spirulina) shows lower WG than on mixed protein sources: The plant diets have relatively low methionine contents; therefore, the AA pattern is not appropriate and, obviously, the main reason for the low abalone growth on the pure plant diet.
Several papers demonstrate adverse effects in animals, if diets are deficient or in excess of proteins. Such effects are well documented in Babylonia areolate, Eriocheir sinensis, Haliotis discus hannai, H. midae, H. iris, Jasus edwardsii, Penaeus monodon, Procambarus clarkia, Scylla serrata, Acipenser baerii ♀× A. gueldenstaedtii ♂, Anguilla anguilla, Barbonymus altus, Gibelion catla, Diplodus vulgaris, Epinephelus malabaricus, Hippoglossus hippoglossus, Hypophthalmichthys nobilis, Labeo fimbriatus, L. rohita, Mystus nemurus, Oreochromis niloticus, Platichthys stellatus, Protonibea diacanthus, Barbonymus gonionotus, Scophthalmus maximus, Coptodon zillii, or Totoaba macdonaldi (references in Table 2.2).
The mechanism by which excess of dietary protein adversely affects life history traits has to be discussed. Due to the limited capacity of digestive enzymes, excess dietary protein can be excreted as nitrogenous waste into the environment (Burford and Williams 2001). In an elaborate study, it has been demonstrated that particularly trypsin sets the physiological limit on growth rate and feed conversion (Torrissen et al. 1994; Lemieux et al. 1999). This is one of the classical modes of action.
In addition to limited trypsin capacity, hyperproteic diet proteins can cause oxidative damage on pancreas functions (Gu and Xu 2010) and acute hepatocellular injury in small mammals (Oarada et al. 2012). Furthermore, hyperproteic diet can reduce weight (Andriamihaja et al. 2010; Camiletti-Móiron et al. 2015). Whether this weight reduction is combined with oxidative stress is discussed controversially, since several studies report decreased internal oxidative stress upon hyperproteic diet (Lacroix et al. 2004; Machín et al. 2004). Therefore, more than one mechanism appears to be responsible for reduced growth or WG upon excess dietary protein supply. Illustrative biomolecular studies are sketched below.
Since many fishes are usually cultured outside their optimal temperature range, it is important to identify the nutritional requirements under these non-optimal thermal conditions. A few years ago, Bowyer et al. (2013) showed that the protein level can be reduced in diets for most marine species, but it is the quality and digestibility of nutrients in the diet that matters when fishes are cultured at non-optimal temperatures. At cooler temperatures, fish metabolism is reduced that, in turn, lowers the gut-transit time, digestibility, digestive enzyme activity and affects the uptake and absorption of nutrients required for energy and growth. Therefore, high-quality protein and low-lipid diets are necessary during cool-water periods.
In the same line of evidence, Oliva Teles et al. (2020) show in a meta-analysis that dietary protein requirements are directly related to fish trophic position and water salinity and slightly, but significantly inversely related to rearing temperature (Fig. 2.3).
Dietary protein requirement (%) of fishes as function of trophic levels (a), temperatures (b), and salinities (c). (From Oliva Teles et al. (2020), with permission from Wiley)
Recent biomolecular studies shed some more light on underlying mechanisms of increased immunity. In juvenile big head carps, consumption of optimal dietary protein amounts improves liver immune responses (lysozyme, immunoglobulin heavy chain, alkaline phosphatase activity) (Sun et al. 2019). Transcriptome analysis identifies differentially expressed genes (DEGs) in the liver in response to different dietary protein levels, and bioinformatics links many DEGs to immune responses, inflammatory responses, and energy metabolism (Fig. 2.4). Moreover, abnormal serum biochemical indices are apparent in the high protein group, indicating that consuming excess protein aggravates liver metabolic burden.
Potential dietary protein-regulated pathways related to liver immune functions in juvenile bighead carp. Blue upregulated; Red downregulated. (From Sun et al. (2019), with permission from Elsevier)
Box 2.1 The Complement System
The complement system, also known as complement cascade, is a part of the immune system that enhances (complements) the ability of antibodies and phagocytic cells to clear microbes and damaged cells from an organism, promote inflammation, and attack the pathogen’s cell membrane. It is part of the innate immune system (Janeway et al. 2001), which is not adaptable and does not change during an individual’s lifetime. The complement system can be recruited and brought into action by antibodies generated by the adaptive immune system.
The complement system consists of a number of small proteins that are synthesized by the liver and circulate in the blood as inactive precursors. When stimulated by one of several triggers, proteases in the system cleave specific proteins to release cytokines and initiate an amplifying cascade of further cleavages. The result of this complement activation (or complement fixation cascade) is stimulation of phagocytes to clear foreign and damaged material, inflammation to attract additional phagocytes, and activation of the cell-killing membrane attack complex. Over 30 proteins and protein fragments make up the complement system, including serum proteins and cell membrane receptors.
In brief, three biochemical pathways activate the complement system: the classical complement pathway, the alternative complement pathway, and the lectin pathway (Ricklin et al. 2017) Box Fig. 1.
Scheme of the complement system. The complement system is made up of about 30 proteins that work together to “complement” the action of antibodies in destroying bacteria. The term “complement” was coined by Nobel Laureate Paul Ehrlich (Chaplin 2005). Complement proteins circulate in the blood in an inactive form. When the first protein in the complement series is activated—typically by antibody that has locked onto an antigen—it sets in motion a domino effect. Each component takes its turn in a precise chain of steps known as the complement cascade. The product is a cylinder inserted into—and puncturing a hole in—the cell’s wall. With fluids and molecules flowing in and out, the cell swells and bursts (credit: DO11.10, Wikimedia)
The most closely linked metabolic pathways include glycolysis/gluconeogenesis, followed by pyruvate metabolism, the citrate cycle, and nitrogen metabolism. Some pathways associated with the immune system are also identified, including cell adhesion molecules, the PI3K-Akt signaling pathway,Footnote 1 complement and coagulation cascades, the Toll-like receptor signaling pathway,Footnote 2 and the NF-κB signaling pathway. Many genes associated with the biosynthesis of immunity-related signaling pathways are detected in the LP vs. OP and HP vs. OP comparisons, including immune responses (lysozyme, immunoglobulin, alkaline phosphatase, etc.), inflammatory reactions (nuclear factor kappa B, interleukin-8, interleukin-10, etc.), and antioxidant ability (catalase, glutathione transferase, glutathione peroxidase, etc.). The discovery of immune-related pathways and unigenes provides one significant theoretical basis for understanding the molecular mechanisms of dietary protein regulation of liver function.
In one invertebrate equivalent Macrobrachium nipponense, Lv et al. (2021) showed that the interaction of dietary protein supply and rearing temperature affects alanine aminotransferase (ALT) and complement component 4 (C4). In contrast to bighead carp, immunity of this shrimp does not significantly improve solely due to modulation of dietary protein contents. In a marine counterpart, Ma et al. (2020) identified that deficient or excessive dietary protein levels depress the growth, health, and anti-stress capacity of abalone (H. discus hannai). 17.6% or 43.3% (but 30%, Table 2.2) of dietary protein contents are not recommended (Fig. 2.5): on non-appropriate diet, pro-inflammatory cytokine tnf-α is upregulated (Fig. 2.5a), and nrf2, a major transcription factor of cytoprotective responses to oxidative stress is downregulated (Fig. 2.5b). Consequently, animals are increasingly susceptible to heat stress (Fig. 2.5c).
Expressions of the immune-related genes (tnf-α (a) and nrf2 (b)) in the hepatopancreas and accumulative mortalities (c) of abalone H. discus hannai after the heat challenge test. Abalones were fed diets with different protein levels. All data are mean ± SE of three replicates. Different letters indicate significant differences (P<0.05). (From Ma et al. (2020), with permission from Elsevier)
2.2 Amino Acid Landscapes
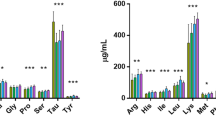
Proteins differ from each another. It can easily be predicted that different AA patterns (“landscapes”) translate into higher trophic levels along the food chain. Consequently, a central challenge is to understand how alterations of community structure transmit into food web processes (Hooper et al. 2005). Herein, macroinvertebrates are a critical trophic link between basal energy sources and higher-order consumers. If macroinvertebrate taxa differ in the quality and quantity of nutrients they contain, then alteration of macroinvertebrate community composition will affect predator fitness (Dwyer et al. 2018). These authors showed that AA composition vary significantly among taxa; simulated deterioration of macroinvertebrate communities changes the AA landscape, resulting in lower availability of essential AAs, particularly threonine, phenylalanine, proline, and tyrosine to carnivores.
In a succeeding paper, Dwyer et al. (2020) verified that changes in AA landscapes affect the growth of individuals of higher trophic levels and the success of these populations. They determined whether AA composition of animal prey alone affects protein synthesis efficiency and N wastage of a freshwater carnivore. River blackfish (Gadopsis marmoratus) were fed two diets differing only in AA composition: the first diet matches the composition of the fish themselves, representing a balanced “ideal protein,” whereas the second diet matches the composition of a major prey (Macrobrachium australiense). By measuring the postprandial increase in metabolic rate and ammonia excretion, it turns out that the AA composition of the fish diet is associated with an increase in protein synthesis, whereas the shrimp diet doubles the amount of dietary AAs directed to catabolic energy production and N wastage.
In a subsequent study, Shakya et al. (2021) show that the AA profile of freshwater macroinvertebrates is not only taxon-specific but can vary temporally (between seasons) and spatially between sites depending on taxa. A change in the community composition of macroinvertebrates can therefore potentially alter the nutritional landscape available to higher-order consumers within riverine environments. How the influence of seasonal and spatial variation in AAs due to inter- and intraspecies differences translates into the growth and metabolism of top predators and across the food web deserves future studies and may be almost as significant as the biochemical bottom-up effect of dietary polyunsaturated fatty acids (→Chap. 30). Therefore, in the following chapters, the controlling effects of individual AAs in aquafeeds will be demonstrated.
2.3 Nutrient-Specific Foraging
How does an animal identify which macronutrients are essential and which may have been deficient in previous feedings? Furthermore, how does it understand or feel that compensatory feeding is overdue? This is an ultimate goal in nutritional ecology.
In nature, many herbivores and omnivores adjust their food selection behavior to regulate the intake of multiple nutrients. Carnivores, however, are generally assumed to optimize the rate of prey capture rather than select prey according to nutrient composition. In an intriguing study, however, Mayntz et al. (2005) showed experimentally that invertebrate predators can forage selectively for protein and lipids to redress specific nutritional imbalances. This selection can take place at different stages of prey handling: The predator may select among foods of different nutritional composition, eat more of a prey if it is rich in nutrients that the predator is deficient in, or extract specific nutrients from a single prey item. This applies particularly to an environment where there is a high probability of encountering nutritionally heterogeneous foods.
There is a growing awareness that nutritional phenotypes are best understood in a multidimensional context, where foraging is viewed as a process of balancing the intake and use of multiple nutrients to satisfy complex and dynamic nutrient needs (Raubenheimer et al. 2009). Simpson and Raubenheimer (1993) coined the term “geometry of nutritional decisions” of an animal, and Mayntz et al. (2005), for instance, reported about nutrient-specific foraging in invertebrate predators.
In details, a common approach has been to assume that a single component dominates these interactions, with the focus usually on energy, nitrogen, or allelochemicals. However, a substantial body of data exists for a number of systems showing that this simplification is not in general warranted because consumer–food relations are usually dominated by the simultaneous effects of several dietary components. Numerous laboratory studies have shown that this view can yield novel insights into unresolved questions and provide a framework for generating new hypotheses (Raubenheimer 2011). Comparably controlled conditions as in laboratory studies do also exist in aquaculture so that this approach is feasible for application in this discipline, since it has already been successfully applied to companion animals (Raubenheimer et al. 2015). Raubenheimer (2011) proposed mixture triangles, which enable an n-dimensional problem to be visualized in an n–1 dimensional space. A recently developed tool from nutritional geometry is the Right-Angled Mixture Triangle (RMT) (Fig. 2.6). Each point represents a mixture of protein (P), lipid (L), and carbohydrate (C). %P and %L increase along the x- and y-axes, respectively, and the P:L ratio of a mixture is given by the slope of the radial that connects the point to the origin. %C of a point is determined as the difference between 100% and the value at which a negatively sloped diagonal through the point intersects with the two axes (Fig. 2.6).
Right-Angled Mixture Triangles (RMTs) provide a platform to plot three components in 2D graphs using proportional data (Raubenheimer et al. 2009). Here Machovsky-Capuska et al. (2016b) model foods, each with a different mixture of protein (%P), lipid (%L), and carbohydrate (%C). Carbohydrate concentration increases with density of blue shading (i.e., across the negative diagonal carbohydrate isolines). Hypothetical food i contains 10% P, 50% L, and 40% C, whereas food ii contains 50% P, 10% L, and 40% C. The model also illustrates that if an animal mixes its intake of two foods (e.g., i and ii), the resulting diet composition is constrained to lie on the line connecting the foods (e.g., green star). By mixing its intake of three foods (e.g., i, ii, and s), the accessible space expands to a triangle connecting these foods (e.g., blue star). (From Machovsky-Capuska et al. (2016b), with permission from Elsevier)
The application of RMTs is shown in a recent example. To understand the nutritional requirements of species and predict their response to environmental changes, Rowe et al. (2018) combined nutritional geometry and metabolic performance. The authors provide evidence that the Indo-Pacific damselfishes (Abudefduf vaigiensis) adjust their energy intake and select specific macronutrients in their diets, thereby reducing the effects of important environmental conditions such as thermal variation on critical metrics of performance when stressed.
In particular, the proportional macronutrient intakes by A. vaigiensis in different diet and temperature treatments are shown in Fig. 2.7, together with the macronutrient composition of the experimental foods and an estimate of the natural diet. A first point to note is that the fishes feed non-randomly. This is evident in Fig. 2.7 as the proportions of macronutrients eaten by fishes differed from the null hypothesis that A. vaigiensis consume equal proportions of foods.
Right-Angled Mixture Triangle showing foraging choices of damselfish. Each diet represents a proportional mixture of protein (P), lipid (L) and carbohydrate (C) (by energy). To geometrically define diets in an RMT, % P is plotted against % L. Considering that the three macronutrients in the mixture sum to 100%, plotting % P (first axis) and % L (second axis) will automatically reflect the value of % C in the third axis (Raubenheimer 2011). Diets are presented as mean (± SE) for each free choice (PLC) and restricted treatments (CL, PC, and PL) and compared with a null hypothesis that damselfish consume equal proportions of foods (solid black) at low (hollow black) and high temperatures (solid grey). Rowe et al. (2018) also included in the RMT the 98% energetic dietary intake (black square with white cross) of A. vaigiensis in the wild (estimated from Frédérich et al. (2009)). The grey dotted lines given by the slope of the radial that connects the point to the origin represent the P:L ratios of the highest and lowest P:L treatments offered. (From Rowe et al. (2018), with permission from Springer Nature; image credit FAO)
There is considerable spread in macronutrient intake because of the different nutritional treatments, and there is a strong effect of temperature, but it varied depending on the treatment (Fig. 2.7). Specifically, high temperatures increase the proportional energy intake on the different nutritional treatments. The animals tend to adjust the macronutrient intake closer to their natural diet, especially by increasing protein consumption in the high-temperature treatment (Fig. 2.7). Such nutrient-specific diet selection is consistent with the ecology of marine organisms, which forage in nutritionally complex and fluctuating marine environments that vary spatially and temporally (Tait et al. 2014; Machovsky-Capuska et al. 2016a, 2018).
In nature, dietary choices can have profound effects on growth, survival, and reproduction, and the mechanisms that determine what fish choose to eat have been strongly molded by natural selection. Fish bring these mechanisms with them into culture, where they can cause problems, in terms of production, welfare, and environmental impact (Raubenheimer et al. 2012). Long-established cultured species that are farmed intensively, such as salmonids, are usually provided with feeds formulated to contain sufficient nutrients to meet all their known requirements. In theory, fish cultured in this way have neither the need nor the opportunity to be selective about their food. However, there are reasons why an ideal situation is rarely achieved. In the first place, even for well-established species, knowledge about requirements is incomplete. Second, devising optimal feeds for fish of all ages raised in different environments (with respect to temperature, light regimes, and water quality, for example) and that differ in status with respect to maturation, and disease may simply not be possible. In addition, the capacity to provide what is known to be ideal may be compromised by problems of the sustainable supply of potential feed ingredients. For all these reasons, cultured fish may well be given food that is imperfect, with the feed formulation representing a compromise involving nutritional requirements, processing and economic and environmental constraints (Raubenheimer et al. (2012) and references therein). Choosing to feed on smaller conspecifics is a natural aspect of diet selection and a means to overcome protein deficiencies, since smaller conspecifics represent high-quality prey for piscivores, at least in terms of provision of all nutrients needed for growth; cannibalism is therefore a natural feeding strategy in some fishes that are cultured (Jobling et al. 2012). Moreover, elevated cannibalism in farmed carnivores should be considered as inappropriate provision of proteinaceous feeds.
2.4 Concluding Remarks
There is an increasing catalog of studies figuring out the dietary protein requirement in farmed aquatic animals. It is obvious that proteins are significant nutrients and energy sources. However, most approaches follow the traditional format of production studies: feeding → weighing → calculating losses by excretion. Since most often the protein sources vary, the resulting productive traits become almost incomparable. This concern applies to studies of most macro- and micronutrients and is an immanent weakness of studies of productive traits. Furthermore, interactions of macronutrients and their influence on protein requirements are well documented from ecological studies, but considered rather scarcely in aquaculture. It can be predicted that the application of the sketched RMT approach has the potential to improve aquafeeds’ quality. Moreover, in the age of highly developed biomolecular and bioinformatics techniques, the weakness of the classical aquaculture production approach has to be overcome. In brief:
-
The studied strains of aquatic species have to be characterized including hints of potential specific life history traits as discussed in depth in Chap. 40. This information should be deposited in central aquaculture data base.
-
The effect of proteinaceous nutrients on various traits has to be traced back to the “omics” levels to identify general regulatory pathways and to provide a means to translate from studied population to the other one and from one species to the other one. One encouraging example is shown above with the affected immunity traits in bighead carp.
-
Feedback mechanisms of the intestinal microbiota have to be elucidated.
-
Of particular interest is further the identification of biomolecular (genetic, epigenetic) pathways of excess nutrition: Which traits are affected when aquatic animals are fed proteinaceous feeds in excess, and how does this interact with other macro- and micronutrients?
-
Almost all papers neglect the circadian rhythmicity of the animals. They consider creatures more or less as simple cybernetic systems. However, in AAN I “Chrononutrition” (Steinberg 2018), we have seen that all animals studied so far, from invertebrates up to mammals, are subject to a circadian rhythmicity with distinct ups and lows of metabolic activity. The unstudied aquatic animals are no exception to this rule.
Notes
- 1.
Intracellular signaling pathway is important in regulating the cell cycle, directly related to cellular quiescence, proliferation, cancer, and longevity. PI3K activation phosphorylates and activates AKT (protein kinase B), localizing it in the plasma membrane (King et al. 2015).
- 2.
Toll-like receptor signaling pathways play crucial roles in the innate immune system by recognizing pathogen-associated molecular patterns derived from various microbes. Toll-like receptors signal through the recruitment of specific adaptor molecules, leading to activation of the transcription factors NF-κB and IRFs, which dictate the outcome of innate immune responses (Kawasaki and Kawai 2014).
References
Abbas G, Siddiqui PJA, Jamil K (2011) The optimal protein requirements of juvenile mangrove red snapper, Lutjanus argentimaculatus fed isoenergetic diets. Pak J Zool 44(2):469–480
Akpinar Z, Sevgili H, Özgen T, Demir A, Emre Y (2012) Dietary protein requirement of juvenile shi drum, Umbrina cirrosa (L.). Aquac Res 43(3):421–429. https://doi.org/10.1111/j.1365-2109.2011.02845.x
Alava VR, Lim C (1983) The quantitative dietary protein requirements of Penaeus monodon juveniles in a controlled environment. Aquaculture 30(1):53–61. https://doi.org/10.1016/0044-8486(83)90151-5
Andrews JW, Sick LV, Baptist GJ (1972) The influence of dietary protein and energy levels on growth and survival of penaeid shrimp. Aquaculture 1(C):341–347. https://doi.org/10.1016/0044-8486(72)90037-3
Andriamihaja M, Davila AM, Eklou-Lawson M, Petit N, Delpal S, Allek F, Blais A, Delteil C, Tomé D, Blachier F (2010) Colon luminal content and epithelial cell morphology are markedly modified in rats fed with a high-protein diet. Am J Physiol Gastrointest Liver Physiol 299(5):G1030–G1037. https://doi.org/10.1152/ajpgi.00149.2010
Árnason J, Björnsdottir R, Arnarsson I, Árnadottir GS, Thorarensen H (2010) Protein requirements of Atlantic cod Gadus morhua L. Aquac Res 41(3):385–393. https://doi.org/10.1111/j.1365-2109.2009.02439.x
Arshad Hossain M, Almatar SM, James CM (2010) Optimum dietary protein level for juvenile silver pomfret, Pampus argenteus (Euphrasen). J World Aquacult Soc 41(5):710–720. https://doi.org/10.1111/j.1749-7345.2010.00413.x
Bautista-Teruel MN, Fermin AC, Koshio SS (2003) Diet development and evaluation for juvenile abalone, Haliotis asinina: animal and plant protein sources. Aquaculture 219(1–4):645–653. https://doi.org/10.1016/S0044-8486(02)00410-6
Bermudes M, Glencross B, Austen K, Hawkins W (2010) The effects of temperature and size on the growth, energy budget and waste outputs of barramundi (Lates calcarifer). Aquaculture 306(1-4):160–166. https://doi.org/10.1016/j.aquaculture.2010.05.031
Bowen SH, Lutz EV, Ahlgren MO (1995) Dietary protein and energy as determinants of food quality: trophic strategies compared. Ecology 76(3):899–907. https://doi.org/10.2307/1939355
Bowyer JN, Qin JG, Stone DAJ (2013) Protein, lipid and energy requirements of cultured marine fish in cold, temperate and warm water. Rev Aquacult 5(1):10–32. https://doi.org/10.1111/j.1753-5131.2012.01078.x
Burford MA, Williams KC (2001) The fate of nitrogenous waste from shrimp feeding. Aquaculture 198(1–2):79–93. https://doi.org/10.1016/S0044-8486(00)00589-5
Caceres-Martinez C, Cadena-Roa M, Métailler R (1984) Nutritional requirements of turbot (Scophthalmus maximus): I. A preliminary study of protein and lipid utilization. J World Maricul Soc 15(1-4):191–202. https://doi.org/10.1111/j.1749-7345.1984.tb00153.x
Camiletti-Móiron D, Arianna Aparicio V, Nebot E, Medina G, Martínez R, Kapravelou G, Andrade A, Porres JM, López-Jurado M, Aranda P (2015) High-protein diet induces oxidative stress in rat brain: protective action of high-intensity exercise against lipid peroxidation. Nutricion Hospitalaria 31(2):866–874. https://doi.org/10.3305/nh.2015.31.2.8182
Catacutan MR (2002) Growth and body composition of juvenile mud crab, Scylla serrata, fed different dietary protein and lipid levels and protein to energy ratios. Aquaculture 208(1):113–123. https://doi.org/10.1016/S0044-8486(01)00709-8
Chaplin H Jr (2005) Review: the burgeoning history of the complement system 1888–2005. Immunohematology 21(3):85–93
Chen HY, Tsai JC (1994) Optimal dietary protein level for the growth of juvenile grouper, Epinephelus malabaricus, fed semipurified diets. Aquaculture 119(2-3):265–271. https://doi.org/10.1016/0044-8486(94)90181-3
Chen JM, Ye JY, Pan Q, Shen BQ, Wang YH (2010) Effect of dietary protein levels on growth performance and whole body composition of summerling and winterling spotted barbel (Hemibarbus maculates Bleeker). Aquac Nutr 16(4):412–418. https://doi.org/10.1111/j.1365-2095.2009.00680.x
Cho SH, Kim HS, Myung SH, Jung WG, Choi J, Lee SM (2015) Optimum dietary protein and lipid levels for juvenile rockfish (Sebastes schlegeli, Hilgendorf 1880). Aquac Res 46(12):2954–2961. https://doi.org/10.1111/are.12450
Chong ASC, Hashim R, Alt AB (2000) Dietary protein requirements for discus (Symphysodon spp.). Aquac Nutr 6(4):275–278
Dabrowski K (1977) Protein requirements of grass carp fry (Ctenopharyngodon idella Val.). Aquaculture 12(1):63–73. https://doi.org/10.1016/0044-8486(77)90047-3
De Borba MR, Fracalossi DM, Pezzato LE, Menoyo D, Bautista JM (2003) Growth, lipogenesis and body composition of piracanjuba (Brycon orbignyanus) fingerlings fed different dietary protein and lipid concentrations. Aquat Living Resour 16(4):362–369. https://doi.org/10.1016/S0990-7440(03)00061-5
De La Higuera M, García Gallego M, Sanz A, Hidalgo MC, Suárez MD (1989) Utilization of dietary protein by the eel (Anguilla anguilla): optimum dietary protein levels. Aquaculture 79(1-4):53–61. https://doi.org/10.1016/0044-8486(89)90445-6
De D, Ghoshal TK, Kundu J (2012) Effect of feeding different levels of protein on growth performance, feed utilization and digestive enzyme of grey mullet (Mugil cephalus L). Anim Nutr Feed Technol 12(2):179–186
Deng J, Zhang X, Bi B, Kong L, Kang B (2011) Dietary protein requirement of juvenile Asian red-tailed catfish Hemibagrus wyckioides. Anim Feed Sci Technol 170(3-4):231–238. https://doi.org/10.1016/j.anifeedsci.2011.08.014
Deng J, Zhang X, Tao L, Rong H, Bi B, Kang B (2013) Dietary protein requirement of juvenile Fuxian minnow, Anabarilius grahami. J World Aquacult Soc 44(2):220–228. https://doi.org/10.1111/jwas.12022
Deng J, Zhang X, Han X, Tao L, Bi B, Kang B (2014) Dietary protein requirement of juvenile Dianchi golden-line barbell, Sinocyclocheilus grahami. J World Aquacult Soc 45(4):421–429. https://doi.org/10.1111/jwas.12137
Deshimaru O, Yone Y (1978) Optimum level of dietary protein for prawn. Bull Japan Soc Sci Fish 44(12):1395–1397. https://doi.org/10.2331/suisan.44.1395
Dwyer GK, Stoffels RJ, Rees GN, Shackleton ME, Silvester E (2018) A predicted change in the amino acid landscapes available to freshwater carnivores. Freshw Sci 37(1):108–120. https://doi.org/10.1086/696128
Dwyer GK, Stoffels RJ, Silvester E, Rees GN (2020) Prey amino acid composition affects rates of protein synthesis and N wastage of a freshwater carnivore. Mar Freshw Res 71(2):229–237. https://doi.org/10.1071/MF18410
Ebrahimi G, Ouraji H (2012) Growth performance and body composition of kutum fingerlings, Rutilus frisii kutum (Kamenskii 1901), in response to dietary protein levels. Turk J Zool 36(4):551–558. https://doi.org/10.3906/zoo-1008-139
Elangovan A, Shim KF (1997) Growth response of juvenile Barbodes altus fed isocaloric diets with variable protein levels. Aquaculture 158(3-4):321–329. https://doi.org/10.1016/S0044-8486(97)00199-3
El-Dakar AY, Shalaby SM, Saoud IP (2011) Dietary protein requirement of juvenile marbled spinefoot rabbitfish Siganus rivulatus. Aquac Res 42(7):1050–1055. https://doi.org/10.1111/j.1365-2109.2010.02694.x
Felix N, Prince Jeyaseelan MJ (2006) Effects of different protein diets on growth and food conversion ratio of postlarvae of Macrobrachium rosenbergii (de Man). Indian J Fish 53(2):175–180
Frédérich B, Fabri G, Lepoint G, Vandewalle P, Parmentier E (2009) Trophic niches of thirteen damselfishes (Pomacentridae) at the Grand Récif of Toliara, Madagascar. Ichthyol Res 56(1):10–17. https://doi.org/10.1007/s10228-008-0053-2
Fris MB, Horn MH (1993) Effects of diets of different protein content on food consumption, gut retention, protein conversion, and growth of Cebidichthys violaceus (Girard), an herbivorous fish of temperate zone marine waters. J Exp Mar Biol Ecol 166(2):185–202. https://doi.org/10.1016/0022-0981(93)90218-D
Garling DL Jr, Wilson RP (1976) Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus. J Nutr 106(9):1368–1375
German DP (2011) Food acquisition and digestion | Digestive efficiency. In: Farrell AP (ed) Encyclopedia of fish physiology, vol 3. Elsevier, Amsterdam, pp 1596–1607. https://doi.org/10.1016/B978-0-12-374553-8.00142-8
Ghiasvand Z, Matinfar A, Valipour A, Soltani M, Kamali A (2012) Evaluation of different dietary protein and energy levels on growth performance and body composition of narrow clawed crayfish (Astacus leptodactylus). Iran J Fish Sci 11(1):63–77
Gomes Cornélio FH, da Cunha DA, Silveira J, Alexandre D, Silva CP, Fracalossi DM (2014) Dietary protein requirement of juvenile cachara catfish, Pseudoplatystoma reticulatum. J World Aquacult Soc 45(1):45–54. https://doi.org/10.1111/jwas.12090
Gomez GD, Nakagawa H, Kasahara S (1988) Effect of dietary protein/starch ratio and energy level on growth of the giant freshwater prawn Macrobrachium rosenbergii. Bull Japan Soc Sci Fish 54(8):1401–1407. https://doi.org/10.2331/suisan.54.1401
Gómez-Montes L, Garcı́a-Esquivel Z, D’Abramo LR, Shimada A, Vásquez-Peláez C, Viana Ma T (2003) Effect of dietary protein:energy ratio on intake, growth and metabolism of juvenile green abalone Haliotis fulgens. Aquaculture 220(1–4):769–780. https://doi.org/10.1016/S0044-8486(02)00533-1
González S, Craig SR, McLean E, Schwarz MH, Flick GJ (2005) Dietary protein requirement of southern flounder, Paralichthys lethostigma. J Appl Aquacult 17(3):37–50. https://doi.org/10.1300/J028v17n03_03
González-Félix ML, Minjarez-Osorio C, Perez-Velazquez M, Suárez-Jiménez GM, Ibarra-Garcíaparra GE (2014) The Cortez flounder Paralichthys aestuarius as a candidate species for aquaculture: first report on growth in captivity in response to varying dietary protein levels. Aquaculture 420–421:225–230. https://doi.org/10.1016/j.aquaculture.2013.11.006
Green AJ, Jones CLW, Britz PJ (2011) The protein and energy requirements of farmed South African abalone Haliotis midae L. cultured at optimal and elevated water temperatures. Aquac Res 42(11):1653–1663. https://doi.org/10.1111/j.1365-2109.2010.02759.x
Grisdale-Helland B, Helland SJ (1998) Macronutrient utilization by Atlantic halibut (Hippoglossus hippoglossus): diet digestibility and growth of 1 kg fish. Aquaculture 166(1-2):57–65. https://doi.org/10.1016/S0044-8486(98)00274-9
Gu C, Xu H (2010) Effect of oxidative damage due to excessive protein ingestion on pancreas function in mice. Int J Mol Sci 11(11):4591–4600. https://doi.org/10.3390/ijms11114591
Guo Z, Zhu X, Liu J, Han D, Yang Y, Lan Z, Xie S (2012) Effects of dietary protein level on growth performance, nitrogen and energy budget of juvenile hybrid sturgeon, Acipenser baerii ♀× A. gueldenstaedtii ♂. Aquaculture 338–341:89–95. https://doi.org/10.1016/j.aquaculture.2012.01.008
Guy EL, Li MH, Allen PJ (2018) Effects of dietary protein levels on growth and body composition of juvenile (age-1) black buffalo Ictiobus niger. Aquaculture 492:67–72. https://doi.org/10.1016/j.aquaculture.2018.04.002
Guzman C, Gaxiola G, Rosa C, Torre-Blanco A (2001) The effect of dietary protein and total energy content on digestive enzyme activities, growth and survival of Litopenaeus setiferus (Linnaeus 1767) postlarvae. Aquac Nutr 7(2):113–122. https://doi.org/10.1046/j.1365-2095.2001.00161.x
Hammer H, Watts S, Lawrence A, Lawrence J, Desmond R (2006) The effect of dietary protein on consumption, survival, growth and production of the sea urchin Lytechinus variegatus. Aquaculture 254(1-4):483–495. https://doi.org/10.1016/j.aquaculture.2005.10.047
Hooper DU, Chapin FS III, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75(1):3–35. https://doi.org/10.1890/04-0922
Hossain MA, Sultana Z, Kibria ASM, Azimuddin KM (2012) Optimum dietary protein requirement of a thai strain of climbing perch, Anabas testudineus (Bloch, 1792) fry. Turk J Fish Aquat Sci 12(2):4
Hua K, Bureau DP (2012) Exploring the possibility of quantifying the effects of plant protein ingredients in fish feeds using meta-analysis and nutritional model simulation-based approaches. Aquaculture 356–357:284–301. https://doi.org/10.1016/j.aquaculture.2012.05.003
Huang YS, Wen XB, Li SK, Xuan XZ, Zhu DS (2017) Effects of protein levels on growth, feed utilization, body composition, amino acid composition and physiology indices of juvenile Chu's croaker, Nibea coibor. Aquac Nutr 23(3):594–602. https://doi.org/10.1111/anu.12426
Hubbard DM, Robinson EH, Brown PB, Daniels WH (1986) Optimum ratio of dietary protein to energy for red crayfish (Procambarus clarkii). Prog Fish-Cult 48(4):233–237. https://doi.org/10.1577/1548-8640(1986)48<233:ORODPT>2.0.CO;2
Ibarz A, Beltrán M, Fernández-Borràs J, Gallardo MA, Sánchez J, Blasco J (2007) Alterations in lipid metabolism and use of energy depots of gilthead sea bream (Sparus aurata) at low temperatures. Aquaculture 262(2-4):470–480. https://doi.org/10.1016/j.aquaculture.2006.11.008
Islam MS, Tanaka M (2004) Optimization of dietary protein requirement for pond-reared mahseer Tor putitora Hamilton (Cypriniformes: Cyprinidae). Aquac Res 35(13):1270–1276. https://doi.org/10.1111/j.1365-2109.2004.01149.x
Jana SN, Garg SK, Barman UK, Arasu ART, Patra BC (2006) Effect of varying dietary protein levels on growth and production of Chanos chanos (Forsskal) in inland saline groundwater: laboratory and field studies. Aquac Int 14(5):479–498. https://doi.org/10.1007/s10499-006-9050-5
Janeway CAJ, Travers P, Walport M, Shlomchik MJ (2001) Immunobiology: the immune system in health and disease – the complement system and innate immunity, 5th edn. Garland Science, New York. Accessed March 31, 2020
Jauncey K (1982) The effects of varying dietary protein level on the growth, food conversion, protein utilization and body composition of juvenile tilapias (Sarotherodon mossambicus). Aquaculture 27(1):43–54
Jena JK, Mitra G, Biswal S (2012) Effect of dietary protein levels on growth and nutrient utilization of fringe-lipped carp, Labeo fimbriatus (Bloch) fingerlings. Aquac Nutr 18(6):628–639. https://doi.org/10.1111/j.1365-2095.2011.00920.x
Jia S, Li X, Zheng S, Wu G (2017) Amino acids are major energy substrates for tissues of hybrid striped bass and zebrafish. Amino Acids 49(12):2053–2063. https://doi.org/10.1007/s00726-017-2481-7
Jiang S, Wu X, Li W, Wu M, Luo Y, Lu S, Lin H (2015) Effects of dietary protein and lipid levels on growth, feed utilization, body and plasma biochemical compositions of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) juveniles. Aquaculture 446:148–155. https://doi.org/10.1016/j.aquaculture.2015.04.034
Jin M, Zhou QC, Zhang W, Xie FJ, ShenTu JK, Huang XL (2013) Dietary protein requirements of the juvenile swimming crab, Portunus trituberculatus. Aquaculture 414–415:303–308. https://doi.org/10.1016/j.aquaculture.2013.08.028
Jindal M (2011) Protein requirements of catfish Clarias batrachus for sustainable aquaculture. Indian J Fish 58(2):95–100
Jobling M, Alanärä A, Kadri S, Huntingford F (2012) Feeding biology and foraging. In: Huntingford F, Jobling M, Kadri S (eds) Aquaculture and Behavior. Wiley-Blackwell, Chichester, pp 121–149. https://doi.org/10.1002/9781444354614.ch5
Kaushik SJ (1995) Nutrient requirements, supply and utilization in the context of carp culture. Aquaculture 129(1-4):225–241. https://doi.org/10.1016/0044-8486(94)00274-R
Kawasaki T, Kawai T (2014) Toll-like receptor signaling pathways. Front Immunol 5:461–461. https://doi.org/10.3389/fimmu.2014.00461
Khan MA, Abidi SF (2012) Effect of varying protein-to-energy ratios on growth, nutrient retention, somatic indices, and digestive enzyme activities of singhi, Heteropneustes fossilis (Bloch). J World Aquacult Soc 43(4):490–501. https://doi.org/10.1111/j.1749-7345.2012.00587.x
Khan MS, Ang KJ, Ambak MA (1996) The effect of varying dietary protein level on the growth, food conversion, protein utilization and body composition of tropical catfish Mystus nemurus (C. and V.) cultured in static pond water system. Aquac Res 27(11):823–829. https://doi.org/10.1111/j.1365-2109.1996.tb01241.x
Kim KI (1997) Re-evaluation of protein and amino acid requirements of rainbow trout (Oncorhynchus mykiss). Aquaculture 151(1-4):3–7. https://doi.org/10.1016/S0044-8486(96)01483-4
Kim JD, Lall SP (2001) Effects of dietary protein level on growth and utilization of protein and energy by juvenile haddock (Melanogrammus aeglefinus). Aquaculture 195(3–4):311–319. https://doi.org/10.1016/S0044-8486(00)00562-7
Kim S-S, Lee K-J (2009) Dietary protein requirement of juvenile tiger puffer (Takifugu rubripes). Aquaculture 287(1):219–222. https://doi.org/10.1016/j.aquaculture.2008.10.021
Kim KW, Wang XJ, Bai SC (2002) Optimum dietary protein level for maximum growth of juvenile olive flounder Paralichthys olivaceus (Temminck et Schlegel). Aquac Res 33(9):673–679. https://doi.org/10.1046/j.1365-2109.2002.00704.x
Kim KD, Kim KW, Lee BJ, Son MH, Han HS, Kim JD (2014) Dietary protein requirement for young far eastern catfish Silurus asotus. Fish Aquat Sci 17(4):455–459. https://doi.org/10.5657/FAS.2014.0455
Kim KW, Kim KD, Han HS, Moniruzzaman M, Yun H, Lee S, Bai SC (2017) Optimum dietary protein level and protein-to-energy ratio for growth of juvenile parrot fish, Oplegnathus fasciatus. J World Aquacult Soc 48(3):467–477. https://doi.org/10.1111/jwas.12337
King D, Yeomanson D, Bryant HE (2015) PI3King the lock: targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol 37(4):245–251. https://doi.org/10.1097/mph.0000000000000329
Kiriratnikom S, Kiriratnikom A (2012) Growth, feed utilization, survival and body composition of fingerlings of slender walking catfish, Clarias nieuhofii, fed diets containing different protein levels. Songklanakarin J Sci Technol 34(1):37–43
Kithsiri HMP, Sharma P, Zaidi SGS, Pal AK, Venkateshwarlu G (2010) Growth and reproductive performance of female guppy, Poecilia reticulata (Peters) fed diets with different nutrient levels. Indian J Fish 57(1):65–71
Koshio S, Si T, Kanazawa A, Watase T (1993) The effect of dietary protein content on growth, digestion efficiency and nitrogen excretion of juvenile kuruma prawns, Penaeus japonicus. Aquaculture 113(1-2):101–114. https://doi.org/10.1016/0044-8486(93)90344-X
Kpogue D, Gangbazo H, Fiogbe E (2013) A preliminary study on the dietary protein requirement of Parachanna obscura (Günther, 1861) larvae. Turk J Fish Aquat Sci 13:111–117. https://doi.org/10.4194/1303-2712-v13_1_14
Kpundeh MD, Qiang J, He J, Yang H, Xu P (2015) Effects of dietary protein levels on growth performance and haemato-immunological parameters of juvenile genetically improved farmed tilapia (GIFT), Oreochromis niloticus. Aquac Int 23(5):1189–1201. https://doi.org/10.1007/s10499-014-9876-1
Lacroix M, Gaudichon C, Martin A, Morens C, Mathé V, Tomé D, Huneau JF (2004) A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol 287(4):R934–R942. https://doi.org/10.1152/ajpregu.00100.2004
Lazo JP, Davis DA, Arnold CR (1998) The effects of dietary protein level on growth, feed efficiency and survival of juvenile Florida pompano (Trachinotus carolinus). Aquaculture 169(3–4):225–232. https://doi.org/10.1016/S0044-8486(98)00384-6
Lee SM, Kim KD, Park HG, Kim CH, Hong KE (2001) Protein requirement of juvenile Manchurian trout Brachymystax lenok. Fish Sci 67(1):46–51. https://doi.org/10.1046/j.1444-2906.2001.00197.x
Lee SM, Park CS, Bang IC (2002) Dietary protein requirement of young Japanese flounder Paralichthys olivaceus fed isocaloric diets. Fish Sci 68(1):158–164. https://doi.org/10.1046/j.1444-2906.2002.00402.x
Lee SM, Lee JH, Kim KD, Cho SH (2006) Optimum dietary protein for growth of juvenile starry flounder, Platichthys stellatus. J World Aquacult Soc 37(2):200–203. https://doi.org/10.1111/j.1749-7345.2006.00027.x
Lemieux H, Blier P, Dutil J-D (1999) Do digestive enzymes set a physiological limit on growth rate and food conversion efficiency in the Atlantic cod (Gadus morhua)? Fish Physiol Biochem 20(4):293–303. https://doi.org/10.1023/a:1007791019523
Li P, Yin YL, Li D, Kim WS, Wu G (2007) Amino acids and immune function. Br J Nutr 98(2):237–252. https://doi.org/10.1017/S000711450769936X
Li P, Mai K, Trushenski J, Wu G (2009) New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37(1):43–53. https://doi.org/10.1007/s00726-008-0171-1
Li W, Wen X, Zhao J, Li S, Zhu D (2016) Effects of dietary protein levels on growth, feed utilization, body composition and ammonia–nitrogen excretion in juvenile Nibea diacanthus. Fish Sci 82(1):137–146. https://doi.org/10.1007/s12562-015-0945-9
Liu Y, Feng L, Jiang J, Liu Y, Zhou XQ (2009) Effects of dietary protein levels on the growth performance, digestive capacity and amino acid metabolism of juvenile Jian carp (Cyprinus carpio var. Jian). Aquac Res 40(9):1073–1082. https://doi.org/10.1111/j.1365-2109.2009.02201.x
Liu X, Mai K, Liufu Z, Ai Q (2014) Effects of dietary protein and lipid levels on growth, nutrient utilization, and the whole-body composition of turbot, Scophthalmus maximus, Linnaeus 1758, at different growth stages. J World Aquacult Soc 45(4):355–366. https://doi.org/10.1111/jwas.12135
Liu W, Jiang M, Wu JP, Wu F, Tian J, Yang CG, Wen H (2017) Dietary protein level affects the growth performance of large male Genetically Improved Farmed Tilapia, Oreochromis niloticus, reared in fertilized freshwater cages. J World Aquacult Soc 48(5):718–728. https://doi.org/10.1111/jwas.12384
Lochmann RT, Phillips H (1994) Dietary protein requirement of juvenile golden shiners (Notemigonus crysoleucas) and goldfish (Carassius auratus) in aquaria. Aquaculture 128(3-4):277–285. https://doi.org/10.1016/0044-8486(94)90317-4
Luo Z, Liu YJ, Mai KS, Tian LX, Liu DH, Tan XY (2004) Optimal dietary protein requirement of grouper Epinephelus coioides juveniles fed isoenergetic diets in floating net cages. Aquac Nutr 10(4):247–252. https://doi.org/10.1111/j.1365-2095.2004.00296.x
Lv B, Liu B, Zhou Q, Song C, Sun C, Zhang H, Liu B, Jiang Z, Jiang S, Liu M (2021) Effects of different temperatures and protein levels on growth performance, physiological response and expression of immune-related genes of juvenile oriental river prawn (Macrobrachium nipponense). Aquaculture 536:736435. https://doi.org/10.1016/j.aquaculture.2021.736435
Ma S, Guo Y, Sun L, Fan W, Liu Y, Liu D, Huang D, Li X, Zhang W, Mai K (2020) Over high or low dietary protein levels depressed the growth, TOR signaling, apoptosis, immune and anti-stress of abalone Haliotis discus hannai. Fish Shellfish Immunol 106:241–251. https://doi.org/10.1016/j.fsi.2020.08.004
Machín M, Simoyi MF, Blemings KP, Klandorf H (2004) Increased dietary protein elevates plasma uric acid and is associated with decreased oxidative stress in rapidly-growing broilers. Comp Biochem Physiol B 137(3):383–390. https://doi.org/10.1016/j.cbpc.2004.01.002
Machovsky-Capuska GE, Senior AM, Benn EC, Tait AH, Schuckard R, Stockin KA, Cook W, Ogle M, Barna K, Melville D, Wright B, Purvin C, Raubenheimer D (2016a) Sex-specific macronutrient foraging strategies in a highly successful marine predator: the Australasian gannet. Mar Biol 163(4):75. https://doi.org/10.1007/s00227-016-2841-y
Machovsky-Capuska GE, Senior AM, Simpson SJ, Raubenheimer D (2016b) The multidimensional nutritional niche. Trends Ecol Evol 31(5):355–365. https://doi.org/10.1016/j.tree.2016.02.009
Machovsky-Capuska GE, Miller MGR, Silva FRO, Amiot C, Stockin KA, Senior AM, Schuckard R, Melville D, Raubenheimer D (2018) The nutritional nexus: linking niche, habitat variability and prey composition in a generalist marine predator. J Anim Ecol 87(5):1286–1298. https://doi.org/10.1111/1365-2656.12856
Mahmud S, Chakraborty SC, Das M (1996) Performance of rainbow trout (Oncorhynchus mykiss) Fed on different dietary protein with fixed energy ratio. Asian-Australas J Anim Sci 9(1):31–35
Martinez-Cordova LR, Campaña Torres A, Porchas-Cornejo MA (2003) Dietary protein level and natural food management in the culture of blue (Litopenaeus stylirostris) and white shrimp (Litopenaeus vannamei) in microcosms. Aquac Nutr 9(3):155–160. https://doi.org/10.1046/j.1365-2095.2003.00235.x
Martínez-Palacios CA, Harfush-Melendez M, Chávez-Sánchez C, Ross LG (1996) The optimum dietary protein level for the Mexican cichlid Cichlasoma urophthalmus (Günther): a comparison of estimates derived from experiments using fixed-rate feeding and satiation feeding. Aquac Nutr 2(1):11–20. https://doi.org/10.1111/j.1365-2095.1996.tb00003.x
Martínez-Palacios CA, Ríos-Durán MG, Ambriz-Cervantes L, Jauncey KJ, Ross LG (2007) Dietary protein requirement of juvenile Mexican silverside (Menidia estor Jordan 1879), a stomachless zooplanktophagous fish. Aquac Nutr 13(4):304–310. https://doi.org/10.1111/j.1365-2095.2007.00479.x
Mayntz D, Raubenheimer D, Salomon M, Toft S, Simpson SJ (2005) Nutrient-specific foraging in invertebrate predators. Science 307(5706):111–113. https://doi.org/10.1126/science.1105493
Mazid MA, Tanaka Y, Katayama T, Asadur Rahman M, Simpson KL, Chichester CO (1979) Growth response of Tilapia zillii fingerlings fed isocaloric diets with variable protein levels. Aquaculture 18(2):115–122. https://doi.org/10.1016/0044-8486(79)90024-3
Mazzarelli CCM, Santos MR, Amorim RV, Augusto A (2015) Effect of salinity on the metabolism and osmoregulation of selected ontogenetic stages of an amazon population of Macrobrachium amazonicum shrimp (Decapoda, Palaemonidae). Braz J Biol 75(2):372–379. https://doi.org/10.1590/1519-6984.14413
McGoogan BB, Gatlin DM III (1998) Metabolic requirements of red drum, Sciaenops ocellatus, for protein and energy based on weight gain and body composition. J Nutr 128(1):123–129. https://doi.org/10.1093/jn/128.1.123
Méndez-Martínez Y, Yamasaki-Granados S, García-Guerrero MU, Martínez-Córdova LR, Rivas-Vega ME, Arcos-Ortega FG, Cortés-Jacinto E (2017) Effect of dietary protein content on growth rate, survival and body composition of juvenile cauque river prawn, Macrobrachium americanum (Bate 1868). Aquac Res 48(3):741–751. https://doi.org/10.1111/are.13193
Merola N (1988) Effects of three dietary protein levels on the growth of pacu, Colossoma mitrei Berg, in cages. Aquac Res 19(2):145–150. https://doi.org/10.1111/j.1365-2109.1988.tb00417.x
Miegel RP, Pain SJ, van Wettere WHEJ, Howarth GS, Stone DAJ (2010) Effect of water temperature on gut transit time, digestive enzyme activity and nutrient digestibility in yellowtail kingfish (Seriola lalandi). Aquaculture 308(3-4):145–151. https://doi.org/10.1016/j.aquaculture.2010.07.036
Minjarez-Osorio C, González-Félix ML, Perez-Velazquez M (2012) Biological performance of Totoaba macdonaldi in response to dietary protein level. Aquaculture 362–363:50–54. https://doi.org/10.1016/j.aquaculture.2012.07.028
Mohanta KN, Mohanty SN, Jena JK, Sahu NP (2008) Protein requirement of silver barb, Puntius gonionotus fingerlings. Aquac Nutr 14(2):143–152. https://doi.org/10.1111/j.1365-2095.2007.00514.x
Mohanty SS, Samantaray K (1996) Effect of varying levels of dietary protein on the growth performance and feed conversion efficiency of snakehead Channa striata fry. Aquac Nutr 2(2):89–94. https://doi.org/10.1111/j.1365-2095.1996.tb00013.x
Morimoto Kofuji PY, Hosokawa H, Masumoto T (2006) Effects of dietary supplementation with feeding stimulants on yellowtail Seriola quinqueradiata (Temminck & Schlegel; Carangidae) protein digestion at low water temperatures. Aquac Res 37(4):366–373. https://doi.org/10.1111/j.1365-2109.2005.01435.x
Murai T, Akiyama T, Takeuchi T, Watanabe T, Nose T (1985) Effects of dietary protein and lipid levels on performance and carcass composition of fingerling carp. Bull Japan Soc Sci Fish 51(4):605–608. https://doi.org/10.2331/suisan.51.605
Ng WK, Abdullah N, De Silva SS (2008) The dietary protein requirement of the Malaysian mahseer, Tor tambroides (Bleeker), and the lack of protein-sparing action by dietary lipid. Aquaculture 284(1-4):201–206. https://doi.org/10.1016/j.aquaculture.2008.07.051
Nordgarden U, Hemre GI, Hansen T (2002) Growth and body composition of Atlantic salmon (Salmo salar L.) parr and smolt fed diets varying in protein and lipid contents. Aquaculture 207(1-2):65–78. https://doi.org/10.1016/S0044-8486(01)00750-5
Nose T (1979) Summary report on the requirements of essential amino acids for carp. In: Tiews K, Halver JE (eds) Finfish nutrition and fishfeed technology. Heenemann, Berlin, pp 145–156
Oarada M, Tsuzuki T, Nikawa T, Kohno S, Hirasaka K, Gonoi T (2012) Refeeding with a high-protein diet after a 48 h fast causes acute hepatocellular injury in mice. The British journal of nutrition 107(10):1435–1444. https://doi.org/10.1017/S0007114511004521
Okorie OE, Kim YC, Lee S, Bae JY, Yoo JH, Han K, Bai SC, Park GJ, Choi SM (2007) Reevaluation of the dietary protein requirements and optimum dietary protein to energy ratios in Japanese eel, Anguilla japonica. J World Aquacult Soc 38(3):418–426. https://doi.org/10.1111/j.1749-7345.2007.00113.x
Oliva Teles A, Couto A, Enes P, Peres H (2020) Dietary protein requirements of fish – a meta-analysis. Rev Aquacult 12:1445–1477. https://doi.org/10.1111/raq.12391
Ozório ROA, Valente LMP, Correia S, Pousão-Ferreira P, Damasceno-Oliveira A, Escórcio C, Oliva-Teles A (2009) Protein requirement for maintenance and maximum growth of two-banded seabream (Diplodus vulgaris) juveniles. Aquac Nutr 15(1):85–93. https://doi.org/10.1111/j.1365-2095.2008.00570.x
Pan LQ, Xiao GQ, Zhang HX, Luan ZH (2005) Effects of different dietary protein content on growth and protease activity of Eriocheir sinensis larvae. Aquaculture 246(1-4):313–319. https://doi.org/10.1016/j.aquaculture.2004.12.023
Papaparaskeva-Papoutsoglou E, Alexis MN (1986) Protein requirements of young grey mullet, Mugil capito. Aquaculture 52(2):105–115. https://doi.org/10.1016/0044-8486(86)90030-X
Parazo MM (1990) Effect of dietary protein and energy level on growth, protein utilization and carcass composition of rabbitfish, Siganus guttatus. Aquaculture 86(1):41–49. https://doi.org/10.1016/0044-8486(90)90220-H
Pavasovic A, Anderson AJ, Mather PB, Richardson NA (2007) Influence of dietary protein on digestive enzyme activity, growth and tail muscle composition in redclaw crayfish, Cherax quadricarinatus (von Martens). Aquac Res 38(6):644–652. https://doi.org/10.1111/j.1365-2109.2007.01708.x
Peres H, Oliva-Teles A (1999) Influence of temperature on protein utilization in juvenile European seabass (Dicentrarchus labrax). Aquaculture 170(3-4):337–348. https://doi.org/10.1016/S0044-8486(98)00422-0
Pérez-Casanova JC, Lall SP, Gamperl AK (2009) Effect of feed composition and temperature on food consumption, growth and gastric evacuation of juvenile Atlantic cod (Gadus morhua L.) and haddock (Melanogrammus aeglefinus L.). Aquaculture 294(3-4):228–235. https://doi.org/10.1016/j.aquaculture.2009.06.005
Ramnarine IW (2004) Quantitative protein requirements of the edible snail Pomacea urceus (Müller). J World Aquacult Soc 35(2):253–256. https://doi.org/10.1111/j.1749-7345.2004.tb01082.x
Ramzan Ali M, Afzal M, Farhan Khan M, Naqvi SMHM, Akhtar S (2014) Dietary protein requirement of giant river catfish, Sperata seenghala (Sykes), determined using diets of varying protein level. Pak J Nutr 13(3):151–156. https://doi.org/10.3923/pjn.2014.151.156
Raubenheimer D (2011) Toward a quantitative nutritional ecology: the right-angled mixture triangle. Ecol Monogr 81(3):407–427. https://doi.org/10.1890/10-1707.1
Raubenheimer D, Simpson SJ, Mayntz D (2009) Nutrition, ecology and nutritional ecology: toward an integrated framework. Funct Ecol 23(1):4–16. https://doi.org/10.1111/j.1365-2435.2009.01522.x
Raubenheimer D, Huntingford F, Jobling M, Kadri S, Simpson S, Sánchez-Vázquez J (2012) Nutrition and diet choice. In: Huntingford F, Jobling M, Kadri S (eds) Aquaculture and Behavior. Wiley-Blackwell, Chichester, pp 150–182. https://doi.org/10.1002/9781444354614.ch6
Raubenheimer D, Machovsky-Capuska GE, Gosby AK, Simpson S (2015) Nutritional ecology of obesity: from humans to companion animals. Br J Nutr 113(Suppl):S26–S39. https://doi.org/10.1017/s0007114514002323
Renukaradhya KM, Varghese TJ (1986) Protein requirement of the carps, Catla catla (Hamilton) and Labeo rohita (Hamilton). Proc Anim Sci 95(1):103–107. https://doi.org/10.1007/BF03179363
Ricklin D, Barratt-Due A, Mollnes TE (2017) Complement in clinical medicine: clinical trials, case reports and therapy monitoring. Mol Immunol 89:10–21. https://doi.org/10.1016/j.molimm.2017.05.013
Rodrigues APO, Moro GV, Dos Santos VRV, de Freitas LEL, Fracalossi DM (2019) Apparent digestibility coefficients of selected protein ingredients for pirarucu Arapaima gigas (Teleostei: Osteoglossidae). Lat Am J Aquat Res 47(2):310–317. https://doi.org/10.3856/vol47-issue2-fulltext-11
Rosas C, Cuzon G, Taboada G, Pascual C, Gaxiola G, Van Wormhoudt A (2001) Effect of dietary protein and energy levels on growth, oxygen consumption, haemolymph and digestive gland carbohydrates, nitrogen excretion and osmotic pressure of Litopenaeus vannamei (Boone) and L. setiferus (Linne) juveniles (Crustacea, Decapoda; Penaeidae). Aquac Res 32(7):531–547. https://doi.org/10.1046/j.1365-2109.2001.00573.x
Rowe CE, Figueira W, Raubenheimer D, Solon-Biet SM, Machovsky-Capuska GE (2018) Effects of temperature on macronutrient selection, metabolic and swimming performance of the Indo-Pacific damselfish (Abudefduf vaigiensis). Mar Biol 165(11):178. https://doi.org/10.1007/s00227-018-3435-7
Sa R, Gavilán M, Rioseco MJ, Llancabure A, Vargas-Chacoff L, Augsburger A, Bas F (2014) Dietary protein requirement of Patagonian blennie (Eleginops maclovinus, Cuvier 1830) juveniles. Aquaculture 428–429:125–134. https://doi.org/10.1016/j.aquaculture.2014.02.017
Salhi M, Bessonart M, Chediak G, Bellagamba M, Carnevia D (2004) Growth, feed utilization and body composition of black catfish, Rhamdia quelen, fry fed diets containing different protein and energy levels. Aquaculture 231(1–4):435–444. https://doi.org/10.1016/j.aquaculture.2003.08.006
Santiago CB, Reyes OS (1991) Optimum dietary protein level for growth of bighead carp (Aristichthys nobilis) fry in a static water system. Aquaculture 93(2):155–165. https://doi.org/10.1016/0044-8486(91)90214-R
Sedgwick RW (1979) Influence of dietary protein and energy on growth, food consumption and food conversion efficiency in Penaeus merguiensis de Man. Aquaculture 16(1):7–30. https://doi.org/10.1016/0044-8486(79)90168-6
Sethuramalingam TA, Gideon KJ (2003) Evaluation of some animal and plant protein sources in the diets of juvenile freshwater prawn Macrobrachium idae (Heller). J Adv Zool 24(1-2):13–18
Shah Alam M, Watanabe WO, Carroll PM (2008) Dietary protein requirements of juvenile black sea bass, Centropristis striata. J World Aquacult Soc 39(5):656–663. https://doi.org/10.1111/j.1749-7345.2008.00204.x
Shakya M, Silvester E, Holland A, Rees G (2021) Taxonomic, seasonal and spatial variation in the amino acid profile of freshwater macroinvertebrates. Aquat Sci 83(2):32. https://doi.org/10.1007/s00027-021-00789-5
Shiau S-Y (1998) Nutrient requirements of penaeid shrimps. Aquaculture 164(1–4):77–93. https://doi.org/10.1016/S0044-8486(98)00178-1
Shiau SY, Huang SL (1989) Optimal dietary protein level for hybrid tilapia (Oreochromis niloticus × O. aureus) reared in seawater. Aquaculture 81(2):119–127. https://doi.org/10.1016/0044-8486(89)90237-8
Shyong WJ, Huang CH, Chen HC (1998) Effects of dietary protein concentration on growth and muscle composition of juvenile Zacco barbata. Aquaculture 167(1-2):35–42. https://doi.org/10.1016/S0044-8486(98)00313-5
Siddiqui AQ, Howlader MS, Adam AA (1988) Effects of dietary protein levels on growth, feed conversion and protein utilization in fry and young Nile tilapia, Oreochromis niloticus. Aquaculture 70(1-2):63–73. https://doi.org/10.1016/0044-8486(88)90007-5
Simpson SJ, Raubenheimer D (1993) A multi-level analysis of feeding behaviour: the geometry of nutritional decisions. Phil Trans R Soc B 342(1302):381–402. https://doi.org/10.1098/rstb.1993.0166
Singh PK, Gaur SR, Barik P, Sulochana, Shukla S, Singh S (2005) Effect of protein levels on growth and digestibility in the Indian major carp, Labeo rohita (Hamilton) using slaughter house waste as the protein source. Int J Agri Biol 7 (6):939-941. 1560–8530/2005/07–6–939–941
Singh RK, Vartak VR, Balange AK (2007) Effects of dietary protein and lipid levels on growth and body composition of silver dollar (Metynnis schreitmuelleri) fry. Isr J Aquacult Bamid 59(1):17–22
Singh RK, Chavan SL, Desai AS, Khandagale PA (2008) Influence of dietary protein levels and water temperature on growth, body composition and nutrient utilization of Cirrhinus mrigala (Hamilton, 1822) fry. J Therm Biol 33(1):20–26. https://doi.org/10.1016/j.jtherbio.2007.09.003
Smith LL, Lee PG, Lawrence AL, Strawn K (1985) Growth and digestibility by three sizes of Penaeus vannamei Boone: effects of dietary protein level and protein source. Aquaculture 46(2):85–96. https://doi.org/10.1016/0044-8486(85)90193-0
Steinberg CEW (2018) Aquatic Animal Nutrition–A Mechanistic Perspective from Individuals to Generations. Springer Nature Switzerland AG, Cham, Switzerland. https://doi.org/10.1007/978-3-319-91767-2
Stone DAJ, Harris JO, Wang H, Mercer GJ, Schaefer EN, Bansemer MS (2013) Dietary protein level and water temperature interactions for greenlip abalone Haliotis laevigata. J Shellfish Res 32(1):119–130. https://doi.org/10.2983/035.032.0118
Sun S, Wu Y, Yu H, Su Y, Ren M, Zhu J, Ge X (2019) Serum biochemistry, liver histology and transcriptome profiling of bighead carp Aristichthys nobilis following different dietary protein levels. Fish Shellfish Immunol 86:832–839. https://doi.org/10.1016/j.fsi.2018.12.028
Syama Dayal J, Ahamad Ali S, Ambasankar K, Singh P (2003) Effect of dietary protein level on its in vitro and in vivo digestibility in the tiger shrimp Penaeus monodon (Crustacea: Penaeidae). Indian J Mar Sci 32(2):151–155
Tait AH, Raubenheimer D, Stockin KA, Merriman M, Machovsky-Capuska GE (2014) Nutritional geometry and macronutrient variation in the diets of gannets: the challenges in marine field studies. Mar Biol 161(12):2791–2801. https://doi.org/10.1007/s00227-014-2544-1
Thirumurugan R, Subramanian P (2004) Growth of juvenile freshwater prawn Macrobrachium malcolmsonii fed with isonitrogenous diets containing different biowastes. Bangladesh J Fish Res 8(1):1–9
Tibbetts SM, Lall SP, Anderson DM (2000) Dietary protein requirement of juvenile American eel (Anguilla rostrata) fed practical diets. Aquaculture 186(1-2):145–155. https://doi.org/10.1016/S0044-8486(99)00363-4
Torrissen KR, Lied E, Espe M (1994) Differences in digestion and absorption of dietary protein in Atlantic salmon (Salmo salar) with genetically different trypsin isozymes. J Fish Biol 45(6):1087–1104. https://doi.org/10.1006/jfbi.1994.1204
Tung CH, Alfaro AC (2011) Effect of dietary protein and temperature on the growth and health of juvenile New Zealand black-footed abalone (Haliotis iris). Aquac Res 42(3):366–385. https://doi.org/10.1111/j.1365-2109.2010.02631.x
Tzafrir-Prag T, Schreibman MP, Lupatsch I, Zarnoch CB (2010) Preliminary studies of energy and protein requirements of Atlantic horseshoe crabs, Limulus polyphemus, grown in captivity. J World Aquacult Soc 41(6):874–883. https://doi.org/10.1111/j.1749-7345.2010.00430.x
Unnikrishnan U, Paulraj R (2010) Dietary protein requirement of giant mud crab Scylla serrata juveniles fed iso-energetic formulated diets having graded protein levels. Aquac Res 41(2):278–294. https://doi.org/10.1111/j.1365-2109.2009.02330.x
Vijayagopal P, Gopakumar GN, Vijayan KK (2008) Empirical feed formulations for the marine ornamental fish, striped damsel, Dascyllus aruanus (Linné 1758) and their physical, chemical and nutritional evaluation. Aquac Res 39(15):1658–1665. https://doi.org/10.1111/j.1365-2109.2008.02039.x
Wang J, Jiang Y, Li X, Han T, Yang Y, Hu S, Yang M (2016) Dietary protein requirement of juvenile red spotted grouper (Epinephelus akaara). Aquaculture 450:289–294. https://doi.org/10.1016/j.aquaculture.2015.08.007
Ward LR, Carter CG, Crear BJ, Smith DM (2003) Optimal dietary protein level for juvenile southern rock lobster, Jasus edwardsii, at two lipid levels. Aquaculture 217(1-4):483–500. https://doi.org/10.1016/S0044-8486(02)00258-2
Wee KL, Tacon AGJ (1982) A preliminary study on the dietary protein requirement of juvenile snakehead. Bull Japan Soc Sci Fish 48(10):1463–1468. https://doi.org/10.2331/suisan.48.1463
Winfree RA, Stickney RR (1981) Effects of dietary protein and energy on growth, feed conversion efficiency and body composition of Tilapia aurea. J Nutr 111(6):1001–1012. https://doi.org/10.1093/jn/111.6.1001
Wu L, Dong S (2002) Effects of protein restriction with subsequent realimentation on growth performance of juvenile Chinese shrimp (Fenneropenaeus chinensis). Aquaculture 210(1-4):343–358. https://doi.org/10.1016/S0044-8486(01)00860-2
Wu B, Luo S, Xie S, Wang J (2016) Growth of rare minnows (Gobiocypris rarus) fed different amounts of dietary protein and lipids. Lab Animal 45(3):105–111. https://doi.org/10.1038/laban.936
Yan X, Yang J, Dong X, Tan B, Zhang S, Chi S, Yang Q, Liu H, Yang Y (2020a) The optimal dietary protein level of large-size grouper Epinephelus coioides. Aquac Nutr 26(3):705–714. https://doi.org/10.1111/anu.13030
Yan X, Yang J, Dong X, Tan B, Zhang S, Chi S, Yang Q, Liu H, Yang Y (2020b) The protein requirement of grouper Epinephelus coioides at grow-out stage. Aquac Nutr 26(5):1555–1567. https://doi.org/10.1111/anu.13102
Yang SD, Liou CH, Liu FG (2002) Effects of dietary protein level on growth performance, carcass composition and ammonia excretion in juvenile silver perch (Bidyanus bidyanus). Aquaculture 213(1-4):363–372. https://doi.org/10.1016/S0044-8486(02)00120-5
Yang SD, Lin TS, Liou CH, Peng HK (2003) Influence of dietary protein levels on growth performance, carcass composition and liver lipid classes of juvenile Spinibarbus hollandi (Oshima). Aquac Res 34(8):661–666. https://doi.org/10.1046/j.1365-2109.2003.00880.x
Yang M, Wang J, Han T, Yang Y, Li X, Jiang Y (2016) Dietary protein requirement of juvenile bluegill sunfish (Lepomis macrochirus). Aquaculture 459:191–197. https://doi.org/10.1016/j.aquaculture.2016.03.044
Yang M, Wang JT, Han T, Yang YX, Li XY, Tian HL, Zheng PQ (2017) Dietary protein requirement of juvenile triangular bream Megalobrama terminalis (Richardson, 1846). J Appl Ichthyol 33(5):971–977. https://doi.org/10.1111/jai.13405
Ye C, Wu Y, Sun Z, Wang A (2017a) Dietary protein requirement of juvenile obscure puffer, Takifugu obscurus. Aquac Res 48(5):2064–2073. https://doi.org/10.1111/are.13040
Ye W, Han D, Zhu X, Yang Y, Jin J, Xie S (2017b) Comparative study on dietary protein requirements for juvenile and pre-adult gibel carp (Carassius auratus gibelio var. CAS III). Aquac Nutr 23(4):755–765. https://doi.org/10.1111/anu.12442
Yoshimatsu T, Furuichi M, Kitajima C (1992) Optimum level of protein in purified experimental diets for redlip mullet. Bull Japan Soc Sci Fish 58(11):2111–2117. https://doi.org/10.2331/suisan.58.2111
Yousif OM, Osman MF, Anawhi AA, Cherian T (1996) Optimum protein-to-energy ratio for two size groups of rabbitfish, Siganus canaliculatus (Park). Aquac Nutr 2(4):229–233. https://doi.org/10.1111/j.1365-2095.1996.tb00064.x
Zadehmohseni B, Zakeri M, Yavari V, Haghi M (2020) Effects of different salinities on amino acid profile in Artemia franciscana. Aquac Res 51(8):3443–3451. https://doi.org/10.1111/are.14679
Zehra S, Khan MA (2012) Dietary protein requirement for fingerling Channa punctatus (Bloch), based on growth, feed conversion, protein retention and biochemical composition. Aquac Int 20(2):383–395. https://doi.org/10.1007/s10499-011-9470-8
Zhang J, Zhou F, Wang LL, Shao Q, Xu Z, Xu J (2010) Dietary protein requirement of juvenile black sea bream, Sparus macrocephalus. J World Aquacult Soc 41(Suppl 2):151–164. https://doi.org/10.1111/j.1749-7345.2010.00356.x
Zhang Q, Wang Q, Yu H, Mai K, Tong T, Dong L, Xu M (2015) Optimal dietary protein to energy ratio for juvenile peanut worm Sipunculus nudus Linnaeus. Fish Sci 81(4):713–722. https://doi.org/10.1007/s12562-015-0885-4
Zhang YL, Song L, Liu RP, Zhao ZB, He H, Fan QX, Shen ZG (2016) Effects of dietary protein and lipid levels on growth, body composition and flesh quality of juvenile topmouth culter, Culter alburnus Basilewsky. Aquac Res 47(8):2633–2641. https://doi.org/10.1111/are.12712
Zhang NN, Ma QQ, Fan WJ, Xing Q, Zhao YL, Chen LQ, Ye JY, Zhang ML, Du ZY (2017a) Effects of the dietary protein to energy ratio on growth, feed utilization and body composition in Macrobrachium nipponense. Aquac Nutr 23(2):313–321. https://doi.org/10.1111/anu.12395
Zhang Y, Sun Z, Wang A, Ye C, Zhu X (2017b) Effects of dietary protein and lipid levels on growth, body and plasma biochemical composition and selective gene expression in liver of hybrid snakehead (Channa maculata ♀ × Channa argus ♂) fingerlings. Aquaculture 468:1–9. https://doi.org/10.1016/j.aquaculture.2016.09.052
Zhou JB, Zhou QC, Chi SY, Yang QH, Liu CW (2007) Optimal dietary protein requirement for juvenile ivory shell, Babylonia areolate. Aquaculture 270(1–4):186–192. https://doi.org/10.1016/j.aquaculture.2006.07.050
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Steinberg, C.E.W. (2022). Protein Requirement—‘Only Meat Makes You Strong’. In: Aquatic Animal Nutrition. Springer, Cham. https://doi.org/10.1007/978-3-030-87227-4_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-87227-4_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87226-7
Online ISBN: 978-3-030-87227-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)