Abstract
Taurine is an amino acid that differs from the more familiar AAs in possessing a sulfonic rather than a carboxylic group. It is central in membrane stabilization, antioxidation, detoxification, immune response, calcium transport, myocardial contractility, retina development, bile acid metabolism, and endocrine functions. It comprises up to 50% of the free AA pool and is central in osmoregulation. Tau is an essential nutrient in many aquatic animals. Appropriate Tau supplementation sustains many health and metabolism aspects and endurance of farmed fish, while excess Tau seems to be “toxic” to shrimps and fishes. Dietary Tau increases phagocytic activity as well as innate immunity by upregulation of immunity gene transcription. Many details of the metabolism of dietary normal and excess Tau have been identified only phenotypically; details remain to be proven by innovative biomolecular approaches. Furthermore, the role of the intestine microbiota in Tau metabolism has not yet been considered appropriately.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
In addition to the proteinaceous amino acids (AAs) described in previous chapters, another major AA, taurine (Tau, Fig. 11.1a), plays an overwhelming controlling role in animals (Laidlaw et al. 1990). Tau is an AA that differs from the more familiar ones in being a sulfonic rather than a carboxylic AA and in being a β-AA rather than an α-AA. Compared with carboxylate groups, the sulfonate group is a strong acid (Huxtable 1992).
(a) Taurine (=2-aminoethanesulfonic acid). (b) Conjugated bile acids detected in gallbladder bile of juvenile Korean rockfish (Sebastes schlegelii) at the end of the 4-month feeding study. T-0 to T-2.0: diets with increasing Tau content (0.7 to 17 mg g−1 dry matter). Different superscripts indicate significant differences (P < 0.05). (From Kim et al. (2015), with permission from Wiley). (c) Relative mRNA expression of genes related to bile acid and cholesterol metabolism in the liver of tiger puffer (Takifugu rubripes). Different superscripts indicate significant differences (P < 0.05). (From Xu et al. (2020), with permission from the Cambridge University Press; images credit Naturalis Biodiversity Center). L-TAU 8 g kg−1 diet; H-TAU 14 g kg−1 diet; cyp7a1 cholesterol 7α-hydroxylase; hmgcr 3-hydroxy-3-methylglutaryl-CoA reductase; abcg ATP binding cassette subfamily G; fxr farnesoid X receptor; lxra liver X receptor α; hnf4a hepatocyte nuclear factor 4 α; lrh-1 liver receptor homolog-1
The replacement of the carboxyl group by the sulfonate group disables the molecule to form peptide bonds; hence, Tau can usually not be part of peptide chains. Consequently, Tau is the most abundant free AA in animal tissues, accounting for up to 25% of the free AAs pool in the liver, 50% in the kidney, 53% in the muscle, and 19% in the brain (Salze and Davis 2015). Tau plays significant roles in membrane stabilization, antioxidation, detoxification, immune response, calcium transport, myocardial contractility, retina development, bile acid metabolism, and endocrine functions (El-Sayed 2014)—at least in mammals.
Since Tau comprises up to 50% of the free AA pool, it plays a role in osmoregulation as shown in:
-
Marine fishes: European flounder (Platichthys flesus), winter flounder (Pseudopleuronectes americanus), and little skate (Leucoraja erinacea).
-
Brackish fishes: mangrove killifish.
-
Freshwater fishes: walking catfish, tilapia, and common carp (Fugelli and Zachariassen 1976; Frick and Wright 2002; Chara et al. 2011).
The osmoregulatory function of Tau applies also to euryhaline invertebrates in intertidal ecosystems, such as penaeid shrimps Marsupenaeus japonicus, Penaeus monodon (Richard et al. 2011), and Litopenaeus vannamei (Li et al. 2017), prawns Palaemon elegans, bivalves Mytilus galloprovincialis (Ji et al. 2013) and Ruditapes philippinarum (Liu et al. 2013), and the freshwater giant shrimp Macrobrachium rosenbergii (El-Sayed 2014). Another proof of the osmoregulatory activity of Tau is the increase in Tau transporter (tauT) mRNA in carp and tilapia tissues during salinity-induced stress (Takeuchi et al. 2000a, b). Consequently, in maintaining the osmotic homeostasis, the sufficient dietary supply with Tau or its precursors is central (Chara et al. 2011).
Except of spontaneous basic physiological functions, such as antioxidation or osmoregulation, the functions of Tau in fishes and aquatic invertebrates are much less well documented than in mammals. In teleost species where Tau has been identified as an essential nutrient, poor growth and reduced survival are consistently observed during Tau deficiency. However, such phenotypic symptoms are uninformative to the roles of Tau (Salze and Davis 2015) and still subject to extensive studies. As effective antioxidant in aquatic animals (Zhao et al. 2017), Tau counteracts oxidative stresses by scavenging reactive oxygen species (ROS) that are produced as multipurpose tools after the impact of different internal as well as external stressors (Steinberg 2012).
Tau accelerates growth and improves immunity and survival of fish larvae (Kim et al. 2016). Furthermore, it is involved with several important biological functions including fat digestion as a conjugator with bile acids, such as cholic acid or chenodeoxycholic acid in the liver (Fig. 11.1b), and stimulates the hepatic biosynthesis of both bile acid and cholesterol (Fig. 11.1c).
11.1 Taurine Biosynthesis and Physiology
Over the past decade, it has become obvious that Tau is an essential nutrient in many aquatic animals, as shown in octopus (Lopes et al. 2016), sea cucumber (Liu et al. 2016), shrimps (Yue et al. 2013; El-Sayed 2014), and fishes (El-Sayed 2014; Salze and Davis 2015; Magalhães et al. 2019). Meanwhile, several endogenous biosynthesis pathways have been discussed (see below); however, numerous fish species benefit from dietary Tau supplementation, thus showing that the two endogenous pathways are insufficient to provide the necessary amounts of Tau for maximal growth (Salze and Davis 2015). In this review, the authors summarize the recommended Tau supplement for various fish species from dietary 0.2% (common dentex, Dentex dentex; European seabass; rainbow trout) to 1.7% (Japanese flounder).
In larval cobia, Salze et al. (2012) found that Tau supplementation increases specific amylase and trypsin activities in early stages, later also lipase and pepsin. These increased enzymatic activities lead to enhanced nutrient availability, thus providing some explanation to the improved development, growth, and post-weaning survival observed in Tau-supplemented larvae.
Moreover, Tau is an important neurochemical factor in the animal visual system. Abundant Tau is localized in the retinal photoreceptor and neural layers of, for instance, the anadromous ayu (Plecoglossus altivelis), juvenile Japanese flounders, glass eel, and young goldfish (Omura et al. 1997; Omura and Yoshimura 1999; Nusetti et al. 2006, 2010), demonstrating that Tau is involved in the protection of the photoreceptor outer segment, the regulation of neural transmission, and photoreceptor differentiation.
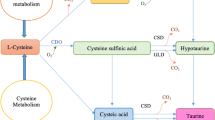
The Tau biosynthetic pathways have been the subject of research for several decades, in spite of which some parts remain poorly characterized. The cysteinesulfinate-decarboxylase (CSD) pathway is the main pathway in mammals. CSD activity occurs in freshwater fishes, such as rainbow trout, common carp, tilapia, bluegill (Lepomis macrochirus) as well as Atlantic salmon (El-Sayed 2014); however, the activity remains an order of magnitude lower than in small mammals. Furthermore, studies with common carp indicate that this species relies on a different pathway than the CSD pathway for Tau production, possibly the pathway using cysteic acid decarboxylase; the precise pathway, however, remains to be ascertained (Salze and Davis 2015). Consequently, one cannot systematically assume that all teleost rely on the CSD pathway for the biosynthesis of Tau. Recently, Ma et al. (2021) found that golden pompano (Trachinotus ovatus) possesses the key enzyme for Tau biosynthesis through two main pathways, and exogenous Tau intake directly affects the expression of synthesis-related genes in the liver. Tau biosynthesis alone, however, is insufficient to satisfy the demands of T. ovatus; the results indicate that approximately 10 g kg−1 of Tau still needs to be provided via diet.
Tau is mainly synthesized in the fish liver. Consequently, the liver is the most-impacted organ in the event of deficiencies. Green liver syndrome is arguably the most specific symptom of Tau deficiency. The green liver originates from the accumulation of biliverdin and reduced excretion of bile pigment from the liver to the bile (Salze and Davis 2015). Tau supplementation effectively reduces the severity of green liver disease in yellowtail fed a diet devoid of fishmeal and containing less than 0.1% Tau (Takagi et al. 2005). This indicates that Tau deficiency can be at the origin of this nutritional disease.
As mentioned, Tau is a major compound of bile and central in lipid metabolism via bile acids. Bile acids are steroids derived from cholesterol, synthesized in the liver, stored in the gallbladder, and released into the intestinal lumen to emulsify fats and help in the absorption of lipids and fat-soluble vitamins. Bile acids are conjugated mainly with Tau and—to a lesser extent—with Gly. Therefore, Tau enhances lipid metabolism in fishes, through increase in the bile salt-activated hepatic lipase activity. Therefore, Tau as well as bile salts can be used as olfactory stimuli to increase feed uptake as documented in European glass eel, European seabass fry, gilthead seabream fry as well as rainbow trout, and channel catfish (El-Sayed 2014; Jirsa et al. 2014).
Less well understood is the Tau biosynthesis in invertebrates. The enhanced growth of P. monodon after dietary addition of Tau indicates limited Tau endogenous synthesis in this shrimp (Shiau and Chou 1994). More recently, Richard et al. (2011) showed that P. monodon has a capacity to regulate Tau synthesis in relation to dietary Cys2 levels. In contrast to P. monodon, the giant river shrimp (M. rosenbergii) appears to cover its Tau requirement by biosynthesis (Smith et al. 1987). However, in both shrimps, details of the biosynthesis pathway remain to be discovered.
Tau is absorbed by the intestinal epithelium through a specific, Na+/Cl−-dependent Tau transporter (TauT). In mammals, compounds are identified to inhibit specifically the uptake of Tau in the intestine, particularly β-alanine. These substances can be classified as anti-nutritional factors (Salze and Davis (2015). TauT is found, for instance, in common carp and tilapia, Senegal sole, Mediterranean blue mussel, and Pacific oyster (Hosoi et al. 2005, 2007). However, information of anti-nutritional substances is lacking; but a deeper understanding of the interactions between these antinutrients (alone or in combination) with Tau—and other nutrients—in respect to bioavailability and metabolism would be invaluable to refine requirement estimates and necessary supplementation levels (Salze and Davis 2015). Furthermore, it has to be evaluated which other factors affect the Tau transport. In addition, it is also unexplored whether the transcription of transporter genes underlies a diurnal rhythmicity itself as it is well understood from genes encoding detoxification enzymes, such as cytochrome P-450 monooxygenases or glutathione transferases (Hooven et al. 2009; Brager et al. 2011) (for more detail,→AAN I “Chrononutrition” (Steinberg 2018)).
In Nile tilapia muscle, Shen et al. (2018) visualized the metabolic trajectory and revealed the metabolic mechanisms of dietary Tau supplementation on growth. Nineteen Tau-induced metabolic changes are involved in carbohydrate, AA, lipid, and nucleotide metabolism; Tau has a central metabolic position (Fig. 11.2). This paper presents pathways and supports the notion that Tau supplementation significantly regulates growth and development.
Metabolic pathways affected by dietary Tau in tilapia muscle extracts. Metabolites in red and blue represent higher or lower levels in tilapia muscle extracts of the Tau-supplemented groups, when compared with the control group. Metabolites in green frames represent nonsignificant change and were detected by 1H NMR, and metabolites with no color marking were not detected. (From Shen et al. (2018), with permission from the American Chemical Society)
In a series of papers, Pinto and coworkers demonstrated the role of Tau in gilthead seabream larvae. The essentiality for Tau appears not to exist; this species can grow on Tau-free diets, since it biosynthesizes Tau from Met. However, Tau dietary supplementation may beneficially affect larval metabolism by increasing Met availability for several other important physiological purposes in the one-carbon metabolism (Pinto et al. 2013a).
In contrast to gilthead seabream, flatfishes, such as the Senegalese sole, require dietary Tau. Flatfishes undergo a visibly marked metamorphosis. Pinto et al. (2013b) assessed the effect of Tau supplementation in sole larvae and juveniles via growth, metamorphosis success, AA metabolism, and transcription of tauT. The transcription increases during larval development, increasing at the onset of metamorphosis (12 days after hatching, DAH) and achieving the highest values at post-metamorphosis (30 DAH; Fig. 11.3). In Senegalese sole juveniles, tauT mRNA is ubiquitously expressed in all analyzed tissues, with high expression in the brain, heart, and eye. In the digestive tract, high tauT expression occurs in hindgut and stomach tissues, indicating that dietary Tau is readily absorbed when the digestive process begins. Furthermore, Tau endogenously used for bile salt conjugation can be reabsorbed at the posterior end of the digestive tract pointing out the presence of an enterohepatic recycling pathway for Tau in Senegalese sole, at least in the juvenile stage. This process appears to be important for the maintenance of the Tau pool in the body—likely not only in this but also in other flatfish species.
Metamorphosis pattern and tauT expression in Solea senegalensis larvae. DAH days after hatching (=dph). Results for metamorphosis pattern are expressed as percentage of each metamorphic stage (Pre- ; Early
; Early ; Middle
; Middle ,
, , and Late
, and Late ). Results for tauT expression (
). Results for tauT expression ( ) are given as means ± SD. Asterisks represent significant differences for the mean metamorphic stage at a certain age. Different letters represent significant differences for the expression of the tauT throughout larval development. (From Pinto et al. (2013b), with permission from Springer Nature)
) are given as means ± SD. Asterisks represent significant differences for the mean metamorphic stage at a certain age. Different letters represent significant differences for the expression of the tauT throughout larval development. (From Pinto et al. (2013b), with permission from Springer Nature)
Furthermore, Tau induces important protective biochemical mechanisms. In the liver and anterior intestine of European seabass, stress protein levels and the MAPKFootnote 1 pathway as well as lactate dehydrogenase are upregulated (Feidantsis et al. 2014). Furthermore, dietary Tau increases immunity and resistance against hyperammonemia in juvenile yellow catfish (Li et al. 2016) pointing out that appropriate Tau supplementation sustains health and endurance of farmed fish.
In many fishes, replacement of marine proteins with plant proteins leads to reduced growth (Espe et al. 2012) and development of enteritis. Plant ingredients are low in Tau (Liaset and Espe 2008); and thus Tau is conditionally indispensable if the plant ingredients become the main protein source in aquafeeds. Several studies exist in which either voluntary feed intake or growth performance improves following exogenous Tau addition to diets based on non-marine protein ingredients. However, in juvenile Atlantic salmon, the addition of 0.1% Tau to high plant protein diets has an adverse effect on growth (Espe et al. 2012). It decreases the lipid content in the entire fish, without affecting protein accretion, and thus reduces the body lipid-to-protein ratio and increases the liver pool of free AAs. A decreased lipid deposition is also observed in Japanese flounder after Tau supplementation (Han et al. 2014). Taken together, the partitioning of growth and nutrient deposition pattern following the availability of sulfur AAs, including Tau, has to be studied in more detail because such knowledge can facilitate the production of slimmer and healthier fish during the growth period, in which the majority of fat deposition occurs.
Some more studies of dietary Tau effects in farmed animals are listed in Table 11.1. The results span from inconspicuous to beneficial effects. Many studies replace fishmeal by plant-based proteins and supplement Tau.
Supplementing 3.5% Tau in an all-plant protein diet, Hu et al. (2018) showed that this AA is a phagostimulant in large yellow croaker (Larimichthys crocea) and upregulates a variety of olfactory receptor genes in the olfactory epithelium (Fig. 11.4). The phagostimulation aids the development of an all-plant protein feed for this fish. Moreover, Tau-enriched rotifers and Artemia nauplii fed to gilthead seabream larvae have beneficial effects on retinal opsin abundance and gene expression of five main opsins. The Tau effect on the pattern of gene expression of selected opsins in the larvae is age-dependent and potentially improved vision leading to increased hunting success and weight gain (Gaon et al. 2020).
Upregulation of eight olfactory receptor genes of large yellow croaker fed 3.5% dietary Tau. Relative expression levels were calculated according to the 2–ΔΔCT using β-actin as an internal reference gene. *P < 0.05 and **P < 0.01. (From Hu et al. (2018), with permission from Wiley)
Dietary Tau mitigates oxidative stress and apoptosis at low temperatures as shown in obscure pufferfish (Takifugu obscurus) (Fig. 11.5). Apoptosis is a form of programmed cell death that occurs in multicellular organisms (Elmore 2007), and caspases are its executioners. Caspase-3 is the major player responsible for the proteolytic cleavage of many critical cellular proteins (Elmore 2007). Dietary Tau exerts anti-apoptotic action by reduction of caspase-3 transcription at low temperatures (Fig. 11.5).
Effects of Tau on relative expression levels of caspase-3 under low-temperature stress in obscure pufferfish (Takifugu obscurus). Note: Data are expressed as mean + SD (n = 6). Diverse small letters show significant differences (P < 0.05) in different groups at the same time point in Duncan’s test. (From Cheng et al. (2018), with permission from Elsevier)
The effect of dietary Tau supplementation to aquatic broodstock animals and their offspring is scarcely studied. One of the few papers shows that parental 2.7% dietary Tau increases the yolk sac volume and, thus, survival of newly hatched larvae of the California yellowtail (Salze et al. 2019).
Because Tau is that central in many physiological processes, the question arises whether animals can decide to feed on Tau-rich diets if they have the choice. Angell et al. (2012) offered this choice to ass’s-ear abalone (Haliotis asinina) in the form of eight species of brown, red, and green macroalgae varying in nutrient composition. The abalone consistently prefers Hypnea pannosa (red alga) and Ulva flexuosa (green alga). H. pannosa is rich in Tau, but U. flexuosa lacks this AA. Furthermore, the richest alga in Tau is Jania crassa (red alga) which is less preferred by H. asinina than the Tau-free U. flexuosa. This shows that no direct link exists between algal Tau content and the preference of H. asinina.
Similarly in planktonic freshwater cladocerans: in feeding experiments with three different coccal green freshwater algae, differing in Tau contents, it occurs that the Tau content does not steer life history traits in Daphnia magna, Moina macrocopa, and M. micrura (Bouchnak and Steinberg 2013, 2014). Instead, the contents of the AAs, Arg and His, influence the life span of Daphnia, whereas the traits of the Moina sp. appear to be independent of individual AAs.
Only a few trials are dedicated to the effects of dietary Tau on physiology and growth in shrimps. These studies indicate that penaeids (P. monodon, L. vannamei) require this AA for optimal performance during larval and grow-out stages, accelerated larval molting rates, and improved survival rates. However, excess Tau appears to be toxic to L. vannamei larvae (El-Sayed 2014). This statement is in good compliance with several recent fish studies showing that excess Tau has adverse effects on the development of fish larvae. Studying the survival rates of larval yellowtail kingfish (Seriola lalandi), Partridge and Woolley (2016) assume toxic action of Tau excess.
Also in Persian sturgeon (Acipenser persicus), adverse effects of dietary Tau are reported. Increased dietary Tau results in decreased feed intake and reduced growth (Hoseini et al. 2017). The authors assume that the tested Tau levels (0.25–1.6%) exceed the actual demand (Hoseini et al. 2018). Studies with lower dietary Tau levels are pending.
11.2 Taurine and Immunity
Table 11.1 includes several hints that dietary Tau increases innate immunity of aquatic animals. Comprehensive surveys of underlying mechanisms, however, are scarce. In one of their biomolecular studies in young grass carps, Yan et al. (2019) identified the multifaceted role of dietary Tau in the immune function—besides improvement of growth. Dietary Tau
-
(a)
Strengthens the ability against enteritis.
-
(b)
Increases antimicrobial compounds.
-
(c)
Downregulates pro-inflammatory cytokines and upregulates anti-inflammatory cytokines.
In zebrafish, dietary malate triggered a metabolic shift via activating the TCA cycle, leading to elevated Tau production (Yang et al. 2020). To prove the efficiency of Tau, this AA is supplemented. In fact, exogenous Tau increases the survival of zebrafish after Vibrio alginolyticus infection (Fig. 11.6a) as malate did as well. Moreover, exogenous Tau and malate regulate the transcription of innate immunity genes (Fig. 11.6b), boost phagocytosis (Fig. 11.6c), and promote the generation of ROS and activated nitrogen oxide. The two metabolites alleviate excessive immune responses to bacterial challenge and protect from bacterial infection. Reprogramming the metabolome with exogenous Tau is also beneficially effective in crucian carp, reared at elevated temperatures (Jiang et al. 2019) (→Chap. 26).
(a) Survival of zebrafish in the presence of graded doses of Tau (in μg), 21 zebrafish each group. (b, c) Expression of innate immunity genes in zebrafish in the presence of 70 μg malate or 12.5 μg Tau (b). Twenty-five zebrafish spleens were collected in each group. Five were pooled as a sample, yielding five biological repeats for analysis of gene expression. Samples were collected in 24 h after the injection of malate or Tau for 3 days. (c) Phagocytosis in the absence or presence of 70 μg malate or 12.5 μg Tau. Macrophages were separated from the head kidney of Nile tilapia and incubated with 20 mM malate or Tau. Then, 1:100 bacterial cells were added. Three biological repeats were performed. Results are displayed as mean ± SEM, and significant differences are identified (*P < 0.05, **P < 0.01) as determined by two-tailed Student’s t-test. (From Yang et al. (2020), credit Taylor and Francis). ptgs-2 prostaglandin-endoperoxide synthase 2 involved in the conversion of arachidonic acid to prostaglandin H2, an important precursor of prostacyclin, which is expressed in inflammation; tnf-α, tumor necrosis factor α, a pro-inflammatory cytokine; c3b, part of complement component 3 (→box in Chap. 2); il-1β pro-inflammatory interleukin 1β; il-10 anti-inflammatory interleukin 10; il-6 interleukin 6 pro-inflammatory cytokine and anti-inflammatory myokine; il-8 pro-inflammatory interleukin 8; il-21 interleukin 21 induces cell division/proliferation; PBS phosphate-buffered saline. Inflammation follows a biphasic stage starting with initiation, where cells are attracted toward the affected side and release pro-inflammatory signal molecules and ROS (Kany et al. 2019). In the resolving phase, anti-inflammatory molecules are released to end the inflammation (Opal and DePalo 2000).
11.3 Interactions with Nutrients
An intensive interplay exists between Tau and other AAs, not only with the S-containing AAs. Recently, Candebat et al. (2020) showed that adequate dietary Met spares dietary Tau; and vice versa, insufficient dietary Met induces increased Tau demand in juvenile yellowtail kingfish (Seriola lalandi).
In larval Nile tilapia, a number of AAs (Trp, Arg, His, Leu, Ile, Val, Ala, Gly, Thr, and Tau) increase with increasing supplemental Tau up to 10 g kg−1. However, with further Tau increase, body AAs decrease (Al-Feky et al. 2015). This indicates that excessive Tau is excreted to keep body Tau at optimal concentrations. This process is energy demanding and therefore reduced growth occurs. This phenomenon is documented in juvenile Pacific white shrimp (Yue et al. 2013), rainbow trout, and gilthead seabream (Al-Feky et al. 2015). At the first glance, the above explanation seems plausible; however, it is unlikely that the pure excretion without any transformation is that energy demanding that growth retardation results (Steinberg 2012). Therefore, an additional or even alternate mechanistic background for the adverse effect must apply. Excessive dietary Tau can also lead to cessation of growth rates through reducing feed intake, as shown in Japanese flounder (Park et al. 2002) or rainbow trout (Gaylord et al. 2006). Furthermore, Glover and Hogstrand (2002) showed that Tau increases subepithelial zinc accumulation in rainbow trout but decreases the passage of zinc to post-intestinal compartments. Since zinc is both a nutrient and a toxicant of importance and since Tau is the most abundant free AA in intestinal mucosa of fishes (Auerswald et al. 1997), this may be an alternate toxicity mode of action of excess Tau.
11.4 Concluding Remark
Tau can be considered essential to most aquatic animals. However, many details of its metabolism of dietary normal and excess doses have been identified only phenotypically. Dietary excess Tau deserves special attention. Details remain to be proven and abstracted from individual levels by innovative biomolecular approaches. Here, innovative approaches means application of omics techniques not only as sophisticated monitoring tools, for instance, some transcription of genes as weak hint of the underlying pathway; rather, it means to apply these techniques so comprehensive that signaling pathways can be proposed. Furthermore, the role of the intestine microbiota in Tau metabolism has not yet been considered. Combining next-generation sequencing with proteomics or metabolomics will possibly identify comprehensive signaling pathways for growth or reproduction. Moreover, beneficial effects have to be observed if, or how, they translate into succeeding generations.
Notes
- 1.
Mitogen-activated protein kinases are involved in directing cellular responses to a diverse array of stimuli, such as mitogens, osmotic stress, heat shock, and pro-inflammatory cytokines. They regulate cell functions including proliferation, gene expression, differentiation, mitosis, cell survival, and apoptosis (Pearson et al. 2001).
References
Abdel-Tawwab M, Monier MN (2018) Stimulatory effect of dietary taurine on growth performance, digestive enzymes activity, antioxidant capacity, and tolerance of common carp, Cyprinus carpio L., fry to salinity stress. Fish Physiol Biochem 44(2):639–649. https://doi.org/10.1007/s10695-017-0459-8
Adeshina I, Abdel-Tawwab M (2020) Dietary taurine incorporation to high plant protein-based diets improved growth, biochemical, immunity, and antioxidants biomarkers of African catfish, Clarias gariepinus (B.). Fish Physiol Biochem 46(4):1323–1335. https://doi.org/10.1007/s10695-020-00791-y
Al-Feky SSA, El-Sayed AFM, Ezzat AA (2015) Dietary taurine enhances growth and feed utilization in larval Nile tilapia (Oreochromis niloticus) fed soybean meal-based diets. Aquac Nutr. https://doi.org/10.1111/anu.12266
Angell AR, Pirozzi I, de Nys R, Paul NA (2012) Feeding preferences and the nutritional value of tropical algae for the abalone Haliotis asinina. PLoS One 7(6):e38857. https://doi.org/10.1371/journal.pone.0038857
Auerswald L, Jürss K, Schiedek D, Bastrop R (1997) The influence of salinity acclimation on free amino acids and enzyme activities in the intestinal mucosa of rainbow trout, Oncorhynchus mykiss (Walbaum). Comp Biochem Physiol A 116(2):149–155. https://doi.org/10.1016/S0300-9629(96)00167-3
Betancor MB, Laurent GR, Ortega A, de la Gándara F, Tocher DR, Mourente G (2019) Taurine metabolism and effects of inclusion levels in rotifer (Brachionus rotundiformis, Tschugunoff, 1921) on Atlantic bluefin tuna (Thunnus thynnus, L.) larvae. Aquaculture 510:353–363. https://doi.org/10.1016/j.aquaculture.2019.05.040
Bouchnak R, Steinberg CEW (2013) Algal diets and natural xenobiotics impact energy allocation in cladocerans. I. Daphnia magna. Limnologica 43(6):434–440. https://doi.org/10.1016/j.limno.2013.01.007
Bouchnak R, Steinberg CEW (2014) Algal diets and natural xenobiotics impact energy allocation in cladocerans. II. Moina macrocopa and M. micrura. Limnologica 44(1):23–31. https://doi.org/10.1016/j.limno.2013.06.002
Brager AJ, Prosser RA, Glass JD (2011) Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiol Int 28(8):664–672. https://doi.org/10.3109/07420528.2011.601968
Brill RW, Horodysky AZ, Place AR, Larkin MEM, Reimschuessel R (2019) Effects of dietary taurine level on visual function in European sea bass (Dicentrarchus labrax). PLoS One 14(6):e0214347. https://doi.org/10.1371/journal.pone.0214347
Candebat CL, Booth M, Codabaccus MB, Pirozzi I (2020) Dietary methionine spares the requirement for taurine in juvenile yellowtail kingfish (Seriola lalandi). Aquaculture 522:735090. https://doi.org/10.1016/j.aquaculture.2020.735090
Ceccotti C, Al-Sulaivany BSA, Al-Habbib OAM, Saroglia M, Rimoldi S, Terova G (2019) Protective effect of dietary taurine from ROS production in European seabass under conditions of forced swimming. Animals 9(9):607. https://doi.org/10.3390/ani9090607
Chara O, Espelt MV, Krumschnabel G, Schwarzbaum PJ (2011) Regulatory volume decrease and P receptor signaling in fish cells: mechanisms, physiology, and modeling approaches. J Exp Zool Part A 315 A(4):175–202. https://doi.org/10.1002/jez.662
Cheng CH, Guo ZX, Wang AL (2018) The protective effects of taurine on oxidative stress, cytoplasmic free-Ca2+ and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol 77:457–464. https://doi.org/10.1016/j.fsi.2018.04.022
Coutinho F, Simões R, Monge-Ortiz R, Furuya WM, Pousão-Ferreira P, Kaushik S, Oliva-Teles A, Peres H (2017) Effects of dietary methionine and taurine supplementation to low-fish meal diets on growth performance and oxidative status of European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 479:447–454. https://doi.org/10.1016/j.aquaculture.2017.06.017
de Moura LB, Diógenes AF, Campelo DAV, Almeida FLAD, Pousão-Ferreira PM, Furuya WM, Oliva-Teles A, Peres H (2018) Taurine and methionine supplementation as a nutritional strategy for growth promotion of meagre (Argyrosomus regius) fed high plant protein diets. Aquaculture 497:389–395. https://doi.org/10.1016/j.aquaculture.2018.07.038
Dehghani R, Oujifard A, Mozanzadeh MT, Morshedi V, Bagheri D (2020) Effects of dietary taurine on growth performance, antioxidant status, digestive enzymes activities and skin mucosal immune responses in yellowfin seabream, Acanthopagrus latus. Aquaculture 517:734795. https://doi.org/10.1016/j.aquaculture.2019.734795
Dong J, Cheng R, Yang Y, Zhao Y, Wu G, Zhang R, Zhu X, Li L, Li X (2018) Effects of dietary taurine on growth, non-specific immunity, anti-oxidative properties and gut immunity in the Chinese mitten crab Eriocheir sinensis. Fish Shellfish Immunol 82:212–219. https://doi.org/10.1016/j.fsi.2018.08.029
El-Sayed AFM (2014) Is dietary taurine supplementation beneficial for farmed fish and shrimp? A comprehensive review. Rev Aquacult 6(4):241–255. https://doi.org/10.1111/raq.12042
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Espe M, Ruohonen K, El-Mowafi A (2012) Effect of taurine supplementation on the metabolism and body lipid-to-protein ratio in juvenile Atlantic salmon (Salmo salar). Aquac Res 43(3):349–360. https://doi.org/10.1111/j.1365-2109.2011.02837.x
Feidantsis K, Kaitetzidou E, Mavrogiannis N, Michaelidis B, Kotzamanis Y, Antonopoulou E (2014) Effect of taurine-enriched diets on the Hsp expression, MAPK activation and the antioxidant defence of the European sea bass (Dicentrarchus labrax). Aquac Nutr 20(4):431–442. https://doi.org/10.1111/anu.12096
Frick NT, Wright PA (2002) Nitrogen metabolism and excretion in the mangrove killifish Rivulus marmoratus. I. the influence of environmental salinity and external ammonia. J Exp Biol 205(1):79–89
Fugelli K, Zachariassen KE (1976) The distribution of taurine, gamma-aminobutyric acid and inorganic ions between plasma and erythrocytes in flounder (Platichthys flesus) at different plasma osmolalities. Comp Biochem Phys A 55(2):173–177. https://doi.org/10.1016/0300-9629(76)90088-8
Gaon A, Nixon O, Tandler A, Falcon J, Besseau L, Escande M, El Sadin S, Allon G, Koven W (2020) Dietary taurine improves vision in different age gilthead sea bream (Sparus aurata) larvae potentially contributing to increased prey hunting success and growth. Aquaculture 736129. https://doi.org/10.1016/j.aquaculture.2020.736129
Gaylord TG, Teague AM, Barrows FT (2006) Taurine supplementation of all-plant protein diets for rainbow trout (Oncorhynchus mykiss). J World Aquacult Soc 37(4):509–517. https://doi.org/10.1111/j.1749-7345.2006.00064.x
Glover CN, Hogstrand C (2002) Amino acid modulation of in vivo intestinal zinc absorption in freshwater rainbow trout. J Exp Biol 205(1):151–158
Guimarães IG, Skjærven K, Moren M, Espe M, Hamre K (2018) Dietary taurine supplementation in plant protein based diets do not affect growth and reproductive performance of zebrafish. Aquac Res 49(5):2013–2022. https://doi.org/10.1111/are.13658
Han Y, Koshio S, Jiang Z, Ren T, Ishikawa M, Yokoyama S, Gao J (2014) Interactive effects of dietary taurine and glutamine on growth performance, blood parameters and oxidative status of Japanese flounder Paralichthys olivaceus. Aquaculture 434(0):348–354. https://doi.org/10.1016/j.aquaculture.2014.08.036
Hartati R, Briggs MRP (1993) Effect of feeding attractants on the behaviour and performance of juvenile Penaeus monodon Fabricius. Aquac Res 24(5):613–624. https://doi.org/10.1111/j.1365-2109.1993.tb00637.x
Hooven LA, Sherman KA, Butcher S, Giebultowicz JM (2009) Does the clock make the poison? Circadian variation in response to pesticides. PLoS One 4(7):e6469. https://doi.org/10.1371/journal.pone.0006469
Hoseini SM, Hosseini SA, Eskandari S, Amirahmadi M (2018) Effect of dietary taurine and methionine supplementation on growth performance, body composition, taurine retention and lipid status of Persian sturgeon, Acipenser persicus (Borodin, 1897), fed with plant-based diet. Aquac Nutr 24(1):324–331. https://doi.org/10.1111/anu.12563
Hoseini SM, Hosseini SA, Eskandari S, Amirahmadi M, Soudagar M (2017) The effect of dietary taurine on growth performance and liver histopathology in Persian sturgeon, Acipenser persicus (Borodin, 1897) fed plant-based diet. Aquac Res 48(8):4184–4196. https://doi.org/10.1111/are.13238
Hosoi M, Shinzato C, Takagi M, Hosoi-Tanabe S, Sawada H, Terasawa E, Toyohara H (2007) Taurine transporter from the giant Pacific oyster Crassostrea gigas: function and expression in response to hyper- and hypo-osmotic stress. Fish Sci 73(2):385–394. https://doi.org/10.1111/j.1444-2906.2007.01346.x
Hosoi M, Takeuchi K, Sawada H, Toyohara H (2005) Expression and functional analysis of mussel taurine transporter, as a key molecule in cellular osmoconforming. J Exp Biol 208(22):4203–4211. https://doi.org/10.1242/jeb.01868
Hu J, Wang Y, Le Q, Yu N, Cao X, Zheng H, Kuang S, Zhang M, Zheng J, Wu X, Wang J, Tao S, Yan X (2018) Transcriptomic analysis reveals olfactory-related genes expression in large yellow croaker (Larimichthys crocea) regulated by taurine: may be a good phagostimulant for all-plant protein diets. Aquac Res 49(2):1095–1104. https://doi.org/10.1111/are.13559
Huxtable RJ (1992) Physiological actions of taurine. Physiol Rev 72(1):101–164. https://doi.org/10.1152/physrev.1992.72.1.101
Ji C, Wu H, Wei L, Zhao J, Wang Q, Lu H (2013) Responses of Mytilus galloprovincialis to bacterial challenges by metabolomics and proteomics. Fish Shellfish Immunol 35(2):489–498. https://doi.org/10.1016/j.fsi.2013.05.009
Jiang M, Chen ZG, Zheng J, Peng B (2019) Metabolites-enabled survival of crucian carps infected by Edwardsiella tarda in high water temperature. Front Immunol 10(AUG):1991. https://doi.org/10.3389/fimmu.2019.01991
Jirsa DO, Stuart KR, Salze GP, Rhodes MA, Davis DA, Drawbridge MA (2014) Limiting amino acids in practical diets for California yellowtail, Seriola lalandi. J World Aquacult Soc 45(6):681–690. https://doi.org/10.1111/jwas.12158
Kany S, Vollrath JT, Relja B (2019) Cytokines in inflammatory disease. Int J Mol Sci 20(23):6008. https://doi.org/10.3390/ijms20236008
Kim SK, Kim KG, Kim KD, Kim KW, Son MH, Rust M, Johnson R (2015) Effect of dietary taurine levels on the conjugated bile acid composition and growth of juvenile Korean rockfish Sebastes schlegeli (Hilgendorf). Aquac Res 46(11):2768–2775. https://doi.org/10.1111/are.12431
Kim YS, Sasaki T, Awa M, Inomata M, Honryo T, Agawa Y, Ando M, Sawada Y (2016) Effect of dietary taurine enhancement on growth and development in red sea bream Pagrus major larvae. Aquac Res 47(4):1168–1179. https://doi.org/10.1111/are.12573
Kotzamanis Y, Kumar V, Tsironi T, Grigorakis K, Ilia V, Vatsos I, Brezas A, van Eys J, Gisbert E (2020) Taurine supplementation in high-soy diets affects fillet quality of European sea bass (Dicentrarchus labrax). Aquaculture 520:734655. https://doi.org/10.1016/j.aquaculture.2019.734655
Laidlaw SA, Grosvenor M, Kopple JD (1990) The taurine content of common foodstuffs. J Parenter Enter Nutr 14(2):183–188. https://doi.org/10.1177/0148607190014002183
Li EC, Wang XD, Chen K, Xu C, Qin JG, Chen LQ (2017) Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev Aquacult 9(1):57–75. https://doi.org/10.1111/raq.12104
Li M, Lai H, Li Q, Gong S, Wang R (2016) Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus fulvidraco fed all-plant protein diets. Aquaculture 450:349–355. https://doi.org/10.1016/j.aquaculture.2015.08.013
Liaset B, Espe M (2008) Nutritional composition of soluble and insoluble fractions obtained by enzymatic hydrolysis of fish-raw materials. Process Biochem 43(1):42–48. https://doi.org/10.1016/j.procbio.2007.10.007
Liu S, Zhou Y, Ru X, Zhang M, Cao X, Yang H (2016) Differences in immune function and metabolites between aestivating and non-aestivating Apostichopus japonicus. Aquaculture 459:36–42. https://doi.org/10.1016/j.aquaculture.2016.03.029
Liu X, Ji C, Zhao J, Wu H (2013) Differential metabolic responses of clam Ruditapes philippinarum to vibrio anguillarum and Vibrio splendidus challenges. Fish Shellfish Immunol 35(6):2001–2007. https://doi.org/10.1016/j.fsi.2013.09.014
Lopes VM, Faleiro F, Baptista M, Pimentel MS, Paula JR, Couto A, Bandarra N, Anacleto P, Marques A, Rosa R (2016) Amino and fatty acid dynamics of octopus (Octopus vulgaris) early life stages under ocean warming. J Therm Biol 55:30–38. https://doi.org/10.1016/j.jtherbio.2015.11.006
Ma QW, Guo HY, Zhu KC, Guo L, Liu BS, Zhang N, Liu B, Yang JW, Jiang SG, Zhang DC (2021) Dietary taurine intake affects growth and taurine synthesis regulation in golden pompano, Trachinotus ovatus (Linnaeus 1758). Aquaculture 530:735918. https://doi.org/10.1016/j.aquaculture.2020.735918
Magalhães R, Martins N, Martins S, Lopes T, Diáz-Rosales P, Pousão-Ferreira P, Oliva-Teles A, Peres H (2019) Is dietary taurine required for white seabream (Diplodus sargus) juveniles? Aquaculture 502:296–302. https://doi.org/10.1016/j.aquaculture.2018.12.019
Martins N, Diógenes AF, Magalhães R, Matas I, Oliva-Teles A, Peres H (2021) Dietary taurine supplementation affects lipid metabolism and improves the oxidative status of European seabass (Dicentrarchus labrax) juveniles. Aquaculture 531:735820. https://doi.org/10.1016/j.aquaculture.2020.735820
Martins N, Magalhães R, Castro C, Couto A, Díaz-Rosales P, Oliva-Teles A, Peres H (2019) Taurine modulates hepatic oxidative status and gut inflammatory markers of European seabass (Dicentrarchus labrax) fed plant feedstuffs-based diets. Amino Acids 51(9):1307–1321. https://doi.org/10.1007/s00726-019-02769-4
Michelato M, Furuya WM, Gatlin DMI (2018) Metabolic responses of Nile tilapia Oreochromis niloticus to methionine and taurine supplementation. Aquaculture 485:66–72. https://doi.org/10.1016/j.aquaculture.2017.11.003
Nusetti S, Obregón F, Lima L (2006) Neuritic outgrowth from goldfish retinal explants, interaction of taurine and zinc. In: Oja SS, Saransaari P (eds) Advances in experimental medicine and biology: taurine 6, vol 583. Springer, New York, pp 435–440. https://doi.org/10.1007/978-0-387-33504-9_50
Nusetti S, Urbina M, Lima L (2010) Effects of zinc ex vivo on taurine uptake in goldfish retinal cells. J Biomed Sci 17(SUPPL. 1):S13. https://doi.org/10.1186/1423-0127-17-S1-S13
Omura Y, Hach A, Furukawa E, Ueck M, Lake N (1997) Immunocytochemical localization of taurine in the pineal organ and retina of an anadromous fish, Plecoglossus altivelis. Arch Histol Cytol 60(2):153–162. https://doi.org/10.1679/aohc.60.153
Omura Y, Yoshimura R (1999) Immunocytochemical localization of taurine in the developing retina of the lefteye flounder Paralichthys olivaceus. Arch Histol Cytol 62(5):441–446. https://doi.org/10.1679/aohc.62.441
Opal SM, DePalo VA (2000) Anti-inflammatory cytokines. Chest 117(4):1162–1172. https://doi.org/10.1378/chest.117.4.1162
Park GS, Takeuchi T, Yokoyama M, Seikai T (2002) Optimal dietary taurine level for growth of juvenile Japanese flounder Paralichthys olivaceus. Fish Sci 68(4):824–829. https://doi.org/10.1046/j.1444-2906.2002.00498.x
Partridge GJ, Woolley LD (2016) The performance of larval Seriola lalandi (Valenciennes, 1833) is affected by the taurine content of the Artemia on which they are fed. Aquac Res. https://doi.org/10.1111/are.12967
Pearson G, Robinson F, Beers Gibson T, Xu B-e, Karandikar M, Berman K, Cobb MH (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22(2):153–183. https://doi.org/10.1210/edrv.22.2.0428
Pinto W, Figueira L, Santos A, Barr Y, Helland S, Dinis MT, Aragão C (2013a) Is dietary taurine supplementation beneficial for gilthead seabream (Sparus aurata) larvae? Aquaculture 384–387:1–5. https://doi.org/10.1016/j.aquaculture.2012.12.011
Pinto W, Rønnestad I, Dinis MT, Aragão C (2013b) Taurine and fish development: insights for the aquaculture industry. Adv Exp Med Biol 776. https://doi.org/10.1007/978-1-4614-6093-0_30
Poppi DA, Moore SS, Glencross BD (2018) The effect of taurine supplementation to a plant-based diet for barramundi (Lates calcarifer) with varying methionine content. Aquac Nutr 24(4):1340–1350. https://doi.org/10.1111/anu.12671
Raggi T, Tacon AGJ, Lemos D (2019) Feeding of juvenile cobia, Rachycentron canadum: evaluation of practical feeds, comparison of commercial fishmeal replacers, and estimation of essential amino acid requirements. J World Aquacult Soc 50(2):317–335. https://doi.org/10.1111/jwas.12587
Richard L, Vachot C, Surget A, Rigolet V, Kaushik SJ, Geurden I (2011) The effect of choline and cystine on the utilisation of methionine for protein accretion, remethylation and trans-sulfuration in juvenile shrimp Penaeus monodon. Br J Nutr 106(6):825–835. https://doi.org/10.1017/S0007114511001115
Saleh NE, Wassef EA, Ashry AM (2020) Is a taurine supplement necessary in fishmeal-based feeds for juvenile European sea bass (Dicentrarchus labrax)? Aquac Int 28(1):321–333. https://doi.org/10.1007/s10499-019-00464-5
Salze G, McLean E, Craig SR (2012) Dietary taurine enhances growth and digestive enzyme activities in larval cobia. Aquaculture 362-363:44–49. https://doi.org/10.1016/j.aquaculture.2012.07.021
Salze GP, Davis DA (2015) Taurine: a critical nutrient for future fish feeds. Aquaculture 437:215–229. https://doi.org/10.1016/j.aquaculture.2014.12.006
Salze GP, Davis DA, Stuart K, Drawbridge M (2019) Effect of dietary taurine in the performance of broodstock and larvae of California yellowtail Seriola dorsalis. Aquaculture 511:734262. https://doi.org/10.1016/j.aquaculture.2019.734262
Sarih S, Djellata A, Roo J, Hernández-Cruz CM, Fontanillas R, Rosenlund G, Izquierdo M, Fernández-Palacios H (2019) Effects of increased protein, histidine and taurine dietary levels on egg quality of greater amberjack (Seriola dumerili, Risso, 1810). Aquaculture 499:72–79. https://doi.org/10.1016/j.aquaculture.2018.09.011
Shen GP, Huang Y, Dong JY, Wang XX, Cheng KK, Feng JH, Xu JJ, Ye JD (2018) Metabolic effect of dietary taurine supplementation on Nile tilapia (Oreochromis nilotictus) evaluated by NMR-based metabolomics. J Agric Food Chem 66(1):368–377. https://doi.org/10.1021/acs.jafc.7b03182
Shiau S-Y, Chou B-S (1994) Grass shrimp, Penaeus monodon, growth as influenced by dietary taurine supplementation. Comp Biochem Physiol A 108(1):137–142. https://doi.org/10.1016/0300-9629(94)90065-5
Smith BR, Miller GC, Mead RW, Taylor REL (1987) Biosynthesis of asparagine and taurine in the freshwater prawn, Macrobrachium rosenbergii (De man). Comp Biochem Phys B 87(4):827–831. https://doi.org/10.1016/0305-0491(87)90396-8
Steinberg CEW (2012) Stress ecology–environmental stress as ecological driving force and key player in evolution. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-2072-5
Steinberg CEW (2018) Aquatic animal nutrition–a mechanistic perspective from individuals to generations. Springer Nature Switzerland AG, Cham, Switzerland. https://doi.org/10.1007/978-3-319-91767-2
Takagi S, Murata H, Goto T, Ichiki T, Munasinghe DMS, Endo M, Matsumoto T, Sakurai A, Hatate H, Yoshida T, Sakai T, Yamashita H, Ukawa M, Kuramoto T (2005) The green liver syndrome is caused by taurine deficiency in yellowtail, Seriola quinqueradiata fed diets without fishmeal. Aquac Sci 53(3):279–290. https://doi.org/10.11233/aquaculturesci1953.53.279
Takeuchi K, Toyohara H, Kinoshita M, Sakaguchi M (2000a) Ubiquitous increase in taurine transporter mRNA in tissues of tilapia (Oreochromis mossambicus) during high-salinity adaptation. Fish Physiol Biochem 23(2):173–182. https://doi.org/10.1023/A:1007889725718
Takeuchi K, Toyohara H, Sakaguchi M (2000b) A hyperosmotic stress-induced mRNA of carp cell encodes Na+- and cl−- dependent high affinity taurine transporter. BBA-Biomembranes 1464(2):219–230. https://doi.org/10.1016/S0005-2736(00)00158-9
Tong S, Wang L, Kalhoro H, Volatiana JA, Shao Q (2019) Effects of supplementing taurine in all-plant protein diets on growth performance, serum parameters, and cholesterol 7α-hydroxylase gene expression in black sea bream. Acanthopagrus schlegelii. J World Aquacult Soc. https://doi.org/10.1111/jwas.12611
Tong S, Wang L, Kalhoro H, Volatiana JA, Shao Q (2020) Effects of supplementing taurine in all-plant protein diets on growth performance, serum parameters, and cholesterol 7α-hydroxylase gene expression in black sea bream, Acanthopagrus schlegelii. J World Aquacult Soc 51(4):990–1001. https://doi.org/10.1111/jwas.12611
Wei Y, Liang M, Xu H, Zheng K (2019) Taurine alone or in combination with fish protein hydrolysate affects growth performance, taurine transport and metabolism in juvenile turbot (Scophthalmus maximus L.). Aquac Nutr 25(2):396–405. https://doi.org/10.1111/anu.12865
Wei Y, Zhang Q, Xu H, Liang M (2020) Taurine requirement and metabolism response of tiger puffer Takifugu rubripes to graded taurine supplementation. Aquaculture 524:735237. https://doi.org/10.1016/j.aquaculture.2020.735237
Wijerath Wiriduge HAS, Zhang Y, Liu J, Yang M, Zhang W, Mai K (2020) Dietary taurine improves muscle growth and texture characteristics in juvenile turbot (Scophthalmus maximus). Aquac Rep 17:100305. https://doi.org/10.1016/j.aqrep.2020.100305
Xu H, Zhang Q, Kim SK, Liao Z, Wei Y, Sun B, Jia L, Chi S, Liang M (2020) Dietary taurine stimulates the hepatic biosynthesis of both bile acids and cholesterol in the marine teleost, tiger puffer (Takifugu rubripes). Br J Nutr 123(12):1345–1356. https://doi.org/10.1017/S0007114520000161
Yan LC, Feng L, Jiang WD, Wu P, Liu Y, Jiang J, Tang L, Tang WN, Zhang YA, Yang J, Zhou XQ, Kuang SY (2019) Dietary taurine supplementation to a plant protein source-based diet improved the growth and intestinal immune function of young grass carp (Ctenopharyngodon idella). Aquac Nutr 25(4):873–896. https://doi.org/10.1111/anu.12907
Yang MJ, Xu D, Yang DX, Li L, Peng XX, Chen ZG, Li H (2020) Malate enhances survival of zebrafish against vibrio alginolyticus infection in the same manner as taurine. Virulence 11(1):349–364. https://doi.org/10.1080/21505594.2020.1750123
Yokogoshi H, Mochizuki H, Nanami K, Hida Y, Miyachi F, Hiroaki O (1999) Dietary taurine enhances cholesterol degradation and reduces serum and liver cholesterol concentrations in rats fed a high-cholesterol diet. J Nutr 129(9):1705–1712. https://doi.org/10.1093/jn/129.9.1705
Yue YR, Liu YJ, Tian LX, Gan L, Yang HJ, Liang GY, He JY (2013) The effect of dietary taurine supplementation on growth performance, feed utilization and taurine contents in tissues of juvenile white shrimp (Litopenaeus vannamei, Boone, 1931) fed with low-fishmeal diets. Aquac Res 44(8):1317–1325. https://doi.org/10.1111/j.1365-2109.2012.03135.x
Zhang Y, Wei Z, Yang M, Liu D, Pan M, Wu C, Zhang W, Mai K (2021) Dietary taurine modulates hepatic oxidative status, ER stress and inflammation in juvenile turbot (Scophthalmus maximus L.) fed high carbohydrate diets. Fish Shellfish Immunol 109:1–11. https://doi.org/10.1016/j.fsi.2020.11.029
Zhao Y, Zhang Q, Yuan L, Liu X (2017) Effects of dietary taurine on the growth, digestive enzymes, and antioxidant capacity in juvenile sea cucumber, Apostichopus japonicus. J World Aquacult Soc 48(3):478–487. https://doi.org/10.1111/jwas.12338
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Steinberg, C.E.W. (2022). Taurine—‘Controlling Rather than Fueling’. In: Aquatic Animal Nutrition. Springer, Cham. https://doi.org/10.1007/978-3-030-87227-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-87227-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87226-7
Online ISBN: 978-3-030-87227-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)