Abstracts
Kinetic features of oxidation and cis-trans isomerization of methyl linoleate in the presence of lipophilic thiol in hydrocarbon solution are considered taking 2-mercaptoethanol (RSH), the simplest thiol, as an example. The effects of hydrophilic endogenous thiol glutathione (GSH) on hydrogen peroxide decomposition, thiol-ene reaction of GSH with unsaturated phenol resveratrol (RVT) and the oxidation of sunflower oil and methyl linoleate in the micellar solution in the presence of GSH are discussed as well. Both RSH and GSH were found to reduce hydroperoxides and H2O2, and these reactions are accompanied by free radical formation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
All living organisms on earth cannot exist without oxygen, yet oxygen is inherently dangerous to their existence. Respiratory chains in living nature and autoxidation of organic substances frequently generate different active radicals and peroxides OH•, O2•−, LO2•, H2O2, LOOH, so called reactive oxygen species (ROS), which appear to be responsible for oxygen toxicity.
Due to the crucial roles played by lipids for structural and signaling activities, the efficiency of the antioxidant network in controlling lipid reactivity and transformations is an interdisciplinary research field extended from chemistry to biology and medicine (Halliwell 2007). In this context, polyunsaturated fatty acid (PUFA) reactivity with free radicals is known to occur via two main processes: (1) the lipid peroxidation (Niki 2012), and (2) the cis–trans isomerization (Ferreri and Chatgilialoglu 2012).
For a long time, almost for all the last century, hydrocarbon and lipid (LH) oxidation was considered as a free radical chain branching process, which can be auto initiated or initiated by extern source (initiators, I, radiation, etc.) (Emanuel et al. 1965; Scott 1965; Emanuel and Gal 1978; Frankel 2005; Kamal-Eldin 2003; Denisov and Afanas’ev 2005). The rate of chain processes is equal to the product of chain initiation rate (Wi) and the length of the chain (ν):
The chain initiation rate depends sufficiently on the hydroperoxide concentration:
Here, w0 is the initiation rate without LOOH participation, kd—apparent rate constant and e—the so called “radical escape” for LOOH decomposition.
So, the promotion of hydroperoxide decomposition into free radicals resulted in accelerated oxidation. On the contrary, the heterolytic nonradical reduction of hydroperoxides leads to the decrease of Wi and the oxidation rate.
The length of the chain is equal to the ratio оf the rates of propagation and termination (Wt):
The most common way to protect products and materials against oxidation is to use chain breaking antioxidants (InH), which react with active radicals:
Inhibitors increase the termination rate Wt, so, the chain length (ν) grows a decrease, and by this reason InH retard the oxidation as a whole (Halliwell 2007; Niki 2012; Ferreri and Chatgilialoglu 2012; Scott 1965; Emanuel and Gal 1978; Frankel 2005; Kamal-Eldin 2003; Denisov and Afanas’ev 2005).
Thio compounds have long been known as peroxide destroyers; they are traditionally used as synergistic antiperoxide additives in antioxidative compositions for lubricants (Hawkiks and Worthikgston 1963). Thiol antioxidants act through a variety of mechanisms, including (1) as components of the general thiol/disulfide redox buffer, (2) as metal chelators, (3) as radical quenchers, (4) as substrates for specific redox reactions (5) as specific reductants of individual protein disulfide bonds (thioredoxin) (Ulrich and Jakob 2019). So, antioxidant effect of thiols includes both reducing the chain initiating rate (Ri) and shortening the length of the chain (ν).
However, there are some circumstances that reduce the antioxidant effect of thiols, and sometimes even lead to an acceleration of lipid spoilage.
-
1.
Thiyl radicals RS• are known to catalyze the cis/trans isomerization of unsaturated fatty acids (LH) (Ferreri and Chatgilialoglu 2012; Chatgilialoglu et al. 2002; Chatgilialoglu and Ferreri 2005; Mengele et al. 2015; Chatgilialoglu and Bowry 2018). Thiyl radicals are generated from thiols under the radical stress in the “radical repair reaction” as well as during the activity of some enzymes (Scheme 1).
In the free radical isomerization, the addition-elimination of a thiyl radical is enough to produce the mono-trans geometrical isomers (Chatgilialoglu and Ferreri 2005). Unsaturated fatty acid molecules present in the living organisms and high-quality natural oils adopt the cis-configuration (Afaf and Min 2008; Sebedio and Christie 1998). Trans-isomers appear usually in the course of hydrogenation and high-temperature treatment of natural oils. They are undesirable components, because trans-lipids incorporate into cell membranes and thus violate the balance of exchange processes.
-
2.
Thiols can add to unsaturated compounds according to Scheme 2, and recently, these reactions are intensively discussed in connection with the so-called thiol-ene click chemistry (Kade et al. 2010; Turunc and Meier 2012; Koo et al. 2010; Vanslambrouck et al. 2021):
The thiol-ene click reactions are mainly used to synthesize linear and branched heterochain polymers (Hoyle and Bowman 2010). The concept of click chemistry was first used by B. Sharpless in 2001 to refer to chemical reactions suitable for the rapid and reliable synthesis of potential drugs by combining individual small elements (Kolb et al. 2001).
-
3.
Thiols can accelerate the oxidation of hydrocarbons and lipids owing to low yield of free radicals in the reactions with hydroperoxides. It has been shown (Mengele et al. 2015; Sies and Jones 2007), that thiols behave as inhibitors in the initial steps of the oxidation of substrates, thoroughly purified from peroxide impurities. However, real systems usually do contain hydroperoxides and/or hydrogen peroxide, so thiols, used as additives, can accelerate the oxidation.
In this work taking 2-mercaptoethanol (RSH), the simplest thiol, as an example, we consider the kinetic features of oxidation and cis-trans isomerization of methyl linoleate in the presence of lipophilic thiol in hydrocarbon solution. The effects of hydrophilic thiol glutathione (GSH) on hydrogen peroxide decomposition, thiol-ene reaction of GSH with unsaturated phenol resveratrol (RVT) and the oxidation of sunflower oil and methyllinoleate in the micellar solution in the presence of GSH are discussed as well.
2-Mercaptoethanol (RSH) is known as an efficient radioprotector and antioxidant. It is widely used in the analysis of proteins and is added to components of enzymatic reactions to inhibit the oxidation of free sulfhydryl residues and maintain the protein activity, as well as to protect readily oxidizable compounds from oxygen (Roy 2005; Aitken et al. 2008).
Figures 1 and 2 illustrate the effect of mercaptoethanol (RSH) and thiyl radicals formed in exchange reactions between RSH and radicals generated during the thermal decomposition of the initiator azo-bis-isobutyronitrile (AIBN) in an atmosphere of N2 and O2 on the formation of trans isomers in non-chain (Fig. 1) consumption and oxygen uptake in the chain oxidation (Fig. 2) cis-cis methyllinoleate (LH). Figure 1 presents the kinetic curves of consumption of cis,cis-methyl linoleate (LH) in reactions with radicals produced in the decomposition of AIBN (cyclohexane was used as solvent) in the presence (curves 3 and 4) and in the absence of mercaptoethanol (RSH) (curves 1,2) in air(curves 4) and in nitrogen (curve 3) atmosphere. At low concentration of LH (5 mM) and at the initiation rate (Wi = 3.8·10−9 M/s), it is the non-chain regime (ν≤1) and cisLH is practically not consumed. In the presence of mercaptoethanol (RSH), the starting cisLH is consumed and trans-isomers are formed. From comparison of the rates of cisLH consumption in the presence of RSH (curves 3 and 4: under N2 1.7·10−7 M/s and under O2 3.7·10−8 M/c) and Wi, it follows that thiyl radicals catalytically accelerate the cis—trans-isomerization and the number of catalytic cycles is of the order of a few dozen (~45). Cis-trans-isomerization occurs in accordance with Scheme 1, supplemented by the reactions: r• + LH → rH + L•; L• + RSH → LH + RS• (under N2) and: r• + O2 → rO2•; rO2• + LH → rOOH + L•; L• + O2 → LO2•; LO2• + RSH → RS• (under O2).
RSH readily reacts with alkyl radicals (kL• ~ 104 M−1· s−1), being relatively inert toward peroxyl ones (kLO2 ~ 10 M−1· s−1). For this reason, the rate of isomerization in oxygen is lower than that in N2 (see curves 3 and 4).
Figure 2 shows the kinetics of oxygen uptake during the oxidation of greater concentration of LH, initiated by AIBN, in the absence (control) or presence of additives. In the presence of 50 mM diphenylamine (AmH), the LH oxidation was inhibited confirming that the aromatic amines are antioxidants (Emanuel et al. 1965; Scott 1965; Emanuel and Gal 1978; Frankel 2005; Kamal-Eldin 2003; Denisov and Afanas’ev 2005) (curve 1). In contrast, 50 mM RSH added alone to the solution of LH induced its oxidation (curve 2) and with the mixture AmH+ RSH the oxidation further increased (curve 3).
These results are summarized in Table 1. The rates of O2 uptake (WO2) are reported along with the percentages of trans-isomers which were determined by GC analyses after work-up and in parallel experiments conducted with the same concentrations of reagents, but in a nitrogen atmosphere (columns 3 and 4). They show that during one hour, RSH catalyzes trans-isomer formation and slightly increases LH oxidation rate. On the other hand, AmH added alone decreased the oxidation rate and gave only traces of trans-isomers formation. However, in the joint presence of RSH and AmH, both LH oxidation rate and cis-trans isomerization rate are substantially increased under aerobic and anaerobic conditions. So, diphenylamine shows a synergistic effect with mercaptoethanol, increasing the rate of oxidation and cis/trans isomerization of LH in the joint presence.
The observed synergism is probably based on the high activity of diphenylaminyl radicals (Am•) in the abstraction of hydrogen. In the presence of oxygen, i.e. in the presence of peroxyl radicals in the system, AmH intercepts rO2• and replaces them with aminyl radicals that are more active in the reaction with RSH:
An increase in the rate of LH oxidation in the presence of mercaptoethanol (control and curve 2 in Fig. 2) may be due to the addition of O2 to the adduct {RS-LH•} as well (see Scheme 1, (ii)):
This reaction affects both the rate of oxidation and the rate of cis/trans isomerization.
The curve 4 in Fig. 2 shows the O2 uptake during LH oxidation initiated with a mixture of acetylcholine (ACh) and tert-butyl-hydroperoxide (t-BuOOH) which generate radicals with the rate equal to Wi initiated by AIBN in the control case. The increase of the oxidation rate in the presence of RSH and relative decrease of the yield of transLH may be associated with interaction of RSH with t-BuOOH resulting in free radical formation.
Glutathione (GSH) is a water soluble thiol. This the most common cytosolic thiol belongs to endogenous biological antioxidants synthesized directly in living organisms. GSH interacts with hydroxyl radicals, reduces hydrogen peroxide, hydroperoxides, and –S–S– disulfide bonds, and prevents the oxidation of proteins (Aitken et al. 2008; Saito and Kawabata 2004; Winterbourn and Metodieva 1995; Sajewicz et al. 2015; Winterbourn 2016; Takashima et al. 2012). There are reports on significant changes in the GSH content during the development of many pathologies, in particular, Alzheimer’s, Parkinson’s, cardiovascular, and oncological diseases (Penninck 2000; Wu et al. 2004; Conway et al. 1987; Townsend et al. 2003; Estrela et al. 2006; Toyokuni 2014; Stavrovskaya 2000; Guo et al. 2018). The redox pair GSH/GSSG and H2O2 are central to the determination of redox homeostasis and intracellular information transmission-cellular signaling. In living organisms, hydroperoxides are reduced by glutathione peroxidases, enzymes specific for organs and tissues that use GSH as a substrate and efficiently reduce H2O2 and organic hydroperoxides, including the hydroperoxides of membrane polyunsaturated fatty acids. But with H2O2, the thiols can react directly. The reaction of reduction of H2O2 by thiols (TSH) is described by the stoichiometric equation (Abedinzadeh et al. 1989; Winterbourn and Hampton 2008; Winterbourn 2015, 2018; Zinatullina et al. 2019):
However, the actual interaction of glutathione with H2O2 proceeds by a complex mechanism, including the formation of intermediate complexes GSH-GSH (Zinatullina et al. 2019, 2021), GSH-H2O2 (Abedinzadeh et al. 1989; Abedinzadeh 2001). It was found that the reaction between GSH and H2O2 is accompanied by the formation of radicals (Zinatullina et al. 2020, 2017a). Using the inhibitor method and employing an original radical acceptor (Zinatullina et al. 2016), it was shown that, in deionized water, the rate of radical formation in the reaction with H2O2 is increased in the following sequence: glutathione ≈ homocysteine < cysteine. Using the spin trap method and employing 5,5-dimethyl-1-pyrroline-N-oxide, it was showed that the interaction between GSH and H2O2 really leads to the formation of thiyl radicals (Zinatullina et al. 2020). The radical yield is low (<1%); however, it may be enough to initiate chain processes.
It was found (Zinatullina et al. 2017b, 2018, 2021) that, in the presence of H2O2 in aqueous solutions, thiol-ene chain reactions of GSH with unsaturated phenols resveratrol (RVT) and caffeic acid are initiated. Resveratrol and caffeic acid are plant polyphenols. They contain an unsaturated bond in the side substituents of the aromatic cycle. Recently, these phenols, in particular RVT (3,5,4′-trihydroxystilbene), have attracted the attention of physicians and biochemists owing to the so-called “French paradox,” i.e. an unusually low level of cardiovascular and oncological diseases despite a high-calorie diet with an abundance of fat that is observed in some regions of France against the background of regular consumption of red wine (Yu et al. 2012; Salehi et al. 2018).
Figure 3 shows that resveratrol (RVT) consumption is observed only in the joint presence of glutathione and H2O2. At the same concentrations of GSH and H2O2 the initial RVT consumption rate (WRVT) increases linearly with an increase of RVT concentration (Fig. 4). It should be noted that the linear dependences in Fig. 4 cut off segments on the ordinate axis that are equal (within the error) to the rate of radical initiation (Wi), measured by the inhibitor method using a polymethine dye (a water-soluble radical acceptor (Zinatullina et al. 2016)). So, the rate of RVT consumption is satisfactorily described by Eq. (6) for chain reactions of oxidation and polymerization with quadratic chain termination on the leading chain radicals:
Kinetic curves of consumption of 0.03 mM RVT in reaction with GSH (1) in the absence and (2–5) in the presence of 4.55 mM H2O2; the GSH concentration (mM): (1) 25 (RVT concentration of 0.033 mM), (2) 0, (3) 2.5, (4) 5, and (5) 10 (Zinatullina et al. 2021)
Dependences of the RVT consumption rates (WRVT) on the RVT concentration in the reaction mixture of 4.55 mM H2O2 with different initial GSH concentrations (mM): 1–10; 2–5; 3–2.5 (Zinatullina et al. 2021)
Here, the parameter a ≅ 1.0 (M·s)−0.5 is similar to the ratio of the rate constants of the propagation (kp) and chain termination reactions (kt) a = kp/(2kt)0.5.
Analysis of the composition of products formed in the reactions of GSH with H2O2 and with RVT, made by electrospray ionization mass spectrometry in the positive-ion measuring mode, showed the following. (1) The initial GSH solution contains sufficiently stable dimers GSH-GSH (ions M’H+ 615,17). (2) The main product of the GSH oxidation in the reaction with H2O2 in accordance with Eq. (5) is the corresponding disulfide GSSG (M”H+ 613,16). (3) In a mixture of GSH, RVT and H2O2 in deionized water, the main products are GSSG disulfide and a product of M”‘H+ 568.16, the mass of which corresponds to hydroperoxide (PO2H), which can be obtained as a result of the sequential addition of the thiyl radical GS• and oxygen to RVT:
Using the experimental data on the kinetics, the product composition and the published data on reactions of GSH with H2O2 and thiyl radicals, in (Zinatullina et al. 2021) a kinetic model of the complex interaction between GSH and RVT in the presence of H2O2 in an aqueous medium at 37 °C is proposed. The model includes 19 quasi-elementary reactions with respective rate constants, in particular, the formation of intermediate GSH–H2O2 and GSH–GSH complexes, the formation of radicals, and their subsequent transformations into final products in reactions with RVT and GSH. A computer simulation based on the developed model adequately describes the kinetics and mechanism of this thiol-ene reaction.
In the last decade, much attention has been paid to the signaling role of glutathione, often in combination with H2O2, in oxidative stress regulating and the response of living organisms to external influences. The GSH molecule contains two carboxyl groups with pKa 2.5 and 3.7. Therefore, in water, GSH forms acidic solutions (pH <<7), and in alkaline solutions, it often shifts the pH to the acidic side.
From the comparison of the rates of GSH consumption and radical formation (Wi) in Table 2 it can be seen that in phosphate buffer solutions, the rate of thiol consumption increases and Wi decreases sharply. One of the reasons for the increase of WGSH in PBS and especially in PB is the additional to H2O2 oxidation of GSH by air oxygen. At initial concentrations of 10 mM of thiol and 2 mM of H2O2, more thiols were consumed in buffer solutions than is required according to the stoichiometry of Eq. (5).
Figure 5 shows a nontrivial dependence of the GSH consumption rate in the reaction with H2O2 on the pH of the reaction mixture in phosphate buffer solutions. It can be seen that in areas close to the physiological pH values of 6.8–7.4, the rate increases exponentially. This means that at pH ≥ 7, GSH consumption occurs mainly in the reactions:
In these reactions, as in an acidic medium, disulfide and water are formed, but the limiting stage is the thiolate-anion reaction.
2 Glutathione in Lipid Oxidation
The vast majority of studies on the biochemistry of GSH and other natural thiols are carried out under conditions close to physiological in animal organisms, i.e. in buffer solutions providing pH = 7.2–7.4. Under such conditions, the rate of reactions of thiols with ROS is largely determined by the contribution of the thiolate anion to these processes and depends on the pKa value of the SH bond. In a large review (Aldini et al. 2018) the data on the antioxidant and regenerative activity of natural thiols were analyzed and it was concluded that the antioxidant activity of thiol is due to the thiolate anion, the relative concentration of which is regulated by the acidity of the thiol.
Glutathione was tested under lipid autoxidation conditions at 80 °C in two concentrations (0.1 mM and 1.0 mM) (Fig. 6) in oxidation of triacylglicerols of sunflower oil (TGSO). It can be seen from this figure that GSH doesn’t show chain-breaking antioxidant activity, i.e. its kinetic curves at both concentrations are almost the same as that for the control sample. Figure 7 demonstrates the effect of truly chain-breaking endogenous phenolic antioxidants adrenaline, tocopherol and epicatechine of different activities on TGSO oxidation under similar conditions. GSH is highly hydrophilic, water soluble thiol. Probably, in the oil medium, glutathione does not dissolve, but forms a transparent dispersion, the particles of which are shielded by TGSO ester groups that prevent the interaction of GSH with peroxyl radicals and hydroperoxides.
Oxidation of methyl linoleate (LH) is widely used as a model reaction for the oxidation of unsaturated lipids (Niki 2012; Frankel 2005; Loshadkin et al. 2020). For testing a variety of water soluble bioantioxidants and their mixtures, water micellar solutions LH are more convenient and successfully used as a kinetic model of the biological process of lipid peroxidation. Recently (Loshadkin et al. 2020), the kinetics of oxygen uptake (WO2) during the chain oxidation of LH in micellar solutions of the nonionic surfactant Triton-100 (TX-100) initiated by the water-soluble initiator 2,2′-azobis (2-methylpropionamide) dihydrochloride (AAPH) has been studied up to the deep stages of LH oxidation. During the oxidation of a freshly prepared LH solution in the TX-100 micellar system, an initial non-stationary stage of increasing the oxygen absorption rate to the Wst value is observed, the duration of which decreases with an increase in the amount of added LH and the initiation rate. These features are due to structural changes over time in the micellar system, which, by the time Wst is reached, consists of mixed TX-100 micelles with formed hydroperoxides (~2% of TX-100), in the hydrophobic interior of which LH is solubilized. Such micelles provide complete interception of the radicals generated by the initiator, and chain oxidation of LH and TX-100 occurs with a quadratic chain termination.
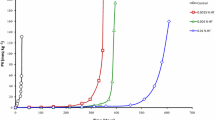
The phosphate buffer was obtained by mixing 0.05 M solutions of NaH2PO4 (Merck) and Na2HPO4 (Merck), purified from traces of metals of variable valence using Chelex-100 resin (Bio-Rad). The rates of O2 absorption during LH oxidation in a micellar solution of TX-100 were measured using a computerized biological oxygen monitor from Yellow Springs Instruments Co. Model 5300A (USA) with Clarke electrode as sensor. The oxidation rate was determined as the slope of the kinetic curves of the [O2] decrease in the reaction mixture. A comparison of the antioxidant effect of glutathione in LH oxidation in a micellar solution of TX-100 initiated by AAPH, in bidistillate (Fig. 8a) and in the phosphate buffer pH 7.4 (Fig. 8b) shows that in a phosphate buffer solution at the same gross concentrations of AAPH, TX-100 and LH, glutathione additives provide a deeper and longer inhibition than that in deionized water, i.e. the antiradical and antioxidant effects of thiols are more and brighter pronounced in the phosphate buffer. However, it should be noted that even in the phosphate buffer, the stoichiometric coefficient of inhibition for GSH is less than 1 and decreases with increasing concentration of GSH: 0.13; 0.085; 0.065 (Fig. 8b).
The effect of GSH on the rate of oxygen uptake (WO2) during the oxidation of AAPH-initiated (4 mM) methyl linoleate (10 mM) in a micellar solution of TX-100 (50 mM) in bidistillate (a) and the phosphate buffer pH 7.4 (b), 37 °C. Concentration of GSH (left) in bidistillate in mM: 1-0; 2-1; 3-2; 4-5; 5-10. Right, in the phosphate buffer pH 7.4 in mM: 1-0; 2-0,2; 3-0,5; 4-1; 5-2
3 Conclusions and Perspective
For the first time, we detected the formation of radicals in the reaction of mercaptoethanol with hydroperoxides, which caused an acceleration of lipid oxidation in an organic medium (Mengele et al. 2015). To find out whether the formation of radicals accompanies the interaction of H2O2 with glutathione, known as the main endogenous bioantioxidant, the kinetics of this reaction was studied in pure deionized water to exclude the influence of transition metal impurities in buffer solutions. The radical formation was discovered and the rates of radical initiation were measured by the inhibitor method using the original radical acceptor. By the spin trap method, the formation of namely thiyl radicals was shown in the reaction of glutathione with H2O2. The radical yield is low (<1%); however, it has been enough to initiate a chain thiol-ene reaction between GSH and resveratrol, plant phenol with unsaturated bond in a side substituent to aromatic ring, in the presence of H2O2 (Zinatullina et al. 2020, 2021). The rates of GSH consumption (WGSH) and radical formation (Wi) in reaction of GSH with H2O2 are sensitive to the pH value and ionic composition of the medium. When the pH ≥ 7, WGSH is increases exponentially, and Wi decreases sharply to 0. Therefore, animals and humans, whose physiological pH value is higher than 7.2, are protected from the formation of radicals, and for them glutathione demonstrates antioxidant properties in the best way. However, the detected reactions involving glutathione in neutral and acidic environments may be important, for example, in plants, or when using glutathione in winemaking, cosmetics or food additives.
References
Abedinzadeh Z (2001) Sulfur-centered reactive intermediates derived from the oxidation of sulfur compounds of biological interest. Can J Physiol Pharmacol 79(2):166–170. https://doi.org/10.1139/y00-085
Abedinzadeh Z, Gardes-Albert M, Ferradini C (1989) Kinetic study of the oxidation mechanism of glutathione by hydrogen peroxide in neutral aqueous medium. Can J Chem 67:1247
Afaf K-E, Min DB (eds) (2008) Lipid oxidation pathways. AOCS Press, Champain
Aitken CE, Marshall RA, Puglisi JD (2008) An oxygen scavenging system for improvement of dye stability in single-molecule fluorescence experiments. Biophys J 94(5):1826–1835. https://doi.org/10.1529/biophysj.107.117689
Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F (2018) N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radical Res 52(7):751–762. https://doi.org/10.1080/10715762.2018.1468564
Chatgilialoglu C, Bowry VW (2018) Why not trans? Inhibited radical isomerization cycles and coupling chains of lipids and alkenes with alkane-thiols. J Org Chem 83(16):9178–9189. https://doi.org/10.1021/acs.joc.8b01216
Chatgilialoglu C, Ferreri C (2005) Trans lipids: the free radical path. Acc Chem Res 36:441. https://doi.org/10.1021/ar0400847
Chatgilialoglu C, Altieri A, Fischer H (2002) The kinetics of Thiyl radical-induced reactions of monounsaturated fatty acid esters. J Am Chem Soc 124:12816–12823
Conway JG, Neptun DA, Garvey LK, Popp JA (1987) Carcinogen treatment increases glutathione hydrolysis by gamma-glutamyltranspeptidase. Carcinogenesis 8:999–1004
Denisov ET, Afanas’ev IF (2005) Oxidation and antioxidants in organic chemistry and biology. CRC Press Taylor & Francis Group
Emanuel NM, Gal D (1978) Oxidation of ethylbenzene. A model reaction. Nauka, Moscow
Emanuel NM, Denisov ET, Maizus ZN (1965) Chain reactions of hydrocarbons oxidation in liquid phase. Nauka, Moscow
Estrela JM, Ortega A, Obrador E (2006) Glutathione critical reviews. Clin Lab Sci 43(2):143–181. https://doi.org/10.1080/10408360500523878
Ferreri C, Chatgilialoglu C (2012) Lipid isomerization in Encyclope-dia of radicals in chemistry. In: Chatgilialoglu C, Studer A (eds) Biology and materials. John Wiley & Sons Ltd, Chichester, UK, pp 1599–1622
Frankel EN (2005) Lipid oxidation. The Oily Press, Glasgow
Guo RW, Yang G, Feng ZJ, Zhu YJ et al (2018) Glutathione-induced amino-activatable micellar photosensitization platform for synergistic redox modulation and photodynamic therapy. Biomater Sci 6(5):1238–1249. https://doi.org/10.1039/c8bm00094h
Halliwell B (2007) Gutteridge, free radicals in biology and medicine, 4th edn. University Press, Oxford
Hawkiks WL, Worthikgston MA (1963) Synergistic antioxidant combinations. Carbon black substitutes. J Polymer Sci Part A 1:3489–3497
Hoyle CE, Bowman CN (2010) Thiol–ene click chemistry. Angew Chem Int Ed 49:1540. https://doi.org/10.1002/anie.200903924
Kade MJ, Burke DJ, Hawker CJ (2010) The power of thiol-ene chemistry. J Polym Sci A Polym Chem 48:743–750. https://doi.org/10.1002/pola.23824
Kamal-Eldin A (2003) Lipid oxidation pathway. AOCS Press, Champaign, IL
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40(11):2004–2021. https://doi.org/10.1002/1521-3773(20010601)40:11<2004::aid-anie2004>3.3.co;2-x
Koo SPS, Stamenović MM, Prasath RA, Inglis AJ et al (2010) Limitations of radical thiol-ene reactions for polymer-polymer conjugation. J Polym Sci A Polym Chem 48:1699–1171
Loshadkin DV, Pliss EM, Kasaikina OT (2020) Features of methyl linoleate oxidation in triton X-100 micellar buffer solutions. Russ J Appl Chem 93(7):1083–1088. https://doi.org/10.1134/S1070427220070216
Mengele EA, Krugovov DA, Kasaikina OT (2015) Effect of mercaptoethanol on the hydrocarbon oxidation and cis—trans-isomerization of unsaturated lipid. Russ Chem Bull 64:846. https://doi.org/10.1007/s11172-015-0943-1
Niki E (2012) Lipid peroxidation in encyclopedia of radicals in chemistry. In: Chatgilialoglu C, Studer A (eds) Biology and materials. John Wiley & Sons Ltd, Chichester, UK, pp 1577–1597
Penninck MJ (2000) A short review on the role of glutathione in the response of yeasts to nutritional, environmental, and oxidative stresses. Enzym Microb Technol 26:737–742
Roy K-M (2005) Thiols and organic sulphides. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim. https://doi.org/10.1002/14356007.a26_767
Saito S, Kawabata J (2004) Synergistic effects of thiols and amines on antiradical efficiency of protocatechuic acid. J Agric Food Chem 52:8163–8168
Sajewicz W, Zalewska M, Milnerowicz H (2015) Comparative study on thiol drugs’ effect on tert-butyl hydroperoxide induced luminol chemiluminescence in human erythrocyte lysate and hemoglobin oxidation. Toxicol In Vitro 29:149
Salehi B, Mishra AP, Nigam M, Sener B, Kilic M, Sharifi-Rad M, Fokou PVT, Martins N, Sharifi-Rad J (2018) Resveratrol: a double-edged sword in health benefits. Biomedicines 6(3):91. https://doi.org/10.3390/biomedicines6030091
Scott G (1965) Atmospheric oxidation and Antioxidation. Elsevier, Amsterdam
Sebedio JL, Christie WW (1998) Trans fatty acids in human nutrition. The Oily Press, Dundee
Sies H, Jones DP (2007) Oxidative stress. In: Fink G (ed) Encyclopedia of stress, vol 3. Elsevier, San Diego, CA, pp 45–48
Stavrovskaya AA (2000) Cellular mechanisms of multidrug resistance of tumor cells. Biochemistry (Mosc) 65:95–106
Takashima M, Shichiri M, Hagihara Y, Yoshida Y, Niki E (2012) Reactivity toward oxygen radicals and antioxidant action of thiol compounds. Biofactors 38(3):240. https://doi.org/10.1002/biof.1014
Townsend DM, Tew KD, Tapiero H (2003) The importance of glutathione in human disease. Biomed Pharmacother 57:145–155
Toyokuni S (2014) Iron and thiols as two major players in carcinogenesis: friends or foes? Front Pharmacol 5:200
Turunc O, Meier AR (2012) The thiol-ene (click) reaction for the synthesis of plant oil derived polymers. Eur J Lipid Sci Technol 115:41–54. https://doi.org/10.1002/ejlt.201200148
Ulrich K, Jakob U (2019) The role of thiols in antioxidant systems. Free Radic Biol Med 140:14–27
Vanslambrouck S, Riva R, Ucakar B, Préat V, Gagliardi M, Molin DGB, Lecomte H, Jérôme C (2021) Thiol-ene reaction: an efficient tool to design lipophilic Polyphosphoesters for drug delivery systems. Molecules 26(6):1750. https://doi.org/10.3390/molecules26061750
Winterbourn CC (2015) Are free radicals involved in thiol-based redox signaling? Free Rad Biol Med 80:164–170
Winterbourn CC (2016) Revisiting the reactions of superoxide with glutathione and other thiols. Arch Biochemistry Biophysics 595:68
Winterbourn CC (2018 Aug 20) Biological production, detection and fate of hydrogen peroxide. Antioxid Redox Signal. 29(6):541–551. https://doi.org/10.1089/ars.2017.7425. Epub 2017 Dec 14
Winterbourn CC, Hampton MB (2008) Thiol chemistry and specificity in redox signaling. Free Radic Biol Med 45(5):549–461. https://doi.org/10.1016/j.freeradbiomed.2008.05.004
Winterbourn CC, Metodieva D (1995) Reaction of superoxide with glutathione and other thiols. Methods Enzymol 252:81–86
Wu G, Fang YZ, Yang S, Lupton JR, Turner ND (2004) Glutathione metabolism and its implications for health. J Nutr 134:489–492
Yu W, Fu Y-C, Wang W (2012) Cellular and molecular effects of resveratrol in health and disease. J Cell Biochem 11(3):752–759. https://doi.org/10.1002/jcb.23431
Zinatullina KM, Kasaikina OT, Kuzmin VA, Khrameeva NP, Shapiro BI (2016) Interaction of polymethine dyes with hydroperoxides and free radicals. Russ Chem Bull 65(12):2825. https://doi.org/10.1007/s11172-016-1663-x
Zinatullina KM, Kasaikina OT, Kuz’min VA, Khrameeva NP, Shapiro BI (2017a) Kinetic characteristics of the interaction of natural thiols with peroxyl radicals and hydrogen peroxide. Russ Chem Bull 66(7):1300. https://doi.org/10.1134/S0023158417050093
Zinatullina KM, Khrameeva NP, Kasaikina OT, Kuzmin VA, Shapiro BI (2017b) Kinetic characteristics of the interaction of resveratrol with peroxyl radicals and natural thiols in an aqueous medium. Russ Chem Bull 66(11):2145. https://doi.org/10.1007/s11172-017-1995-1
Zinatullina KM, Khrameeva NP, Kasaikina OT (2018) Interaction of natural thiols and catecholamines with reactive oxygen species. Bulg Chem Commun 50:25
Zinatullina KM, Kasaikina OT, Kuz’min VA, Khrameeva NP (2019) Interaction of glutathione with hydrogen peroxide. Kinetic Model Kinet Catal 60(3):266. https://doi.org/10.1134/S0023158419030169
Zinatullina KM, Kasaikina OT, Motyakin MV, Ionova IS, Degtyarev EN, Khrameeva NP (2020) Features of radical formation in the reactions of thiols with hydrogen peroxide. Russ Chem Bull 69(10):1865. https://doi.org/10.1007/s11172-020-2971-8
Zinatullina KM, Kasaikina OT, Khrameeva NP, Indeykina MI, Kononikhin AS (2021) Interaction between glutathione and resveratrol in the presence of hydrogen peroxide: a kinetic model. Kinet Catal 62(2):255. https://doi.org/10.1134/S0023158421020130
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
This work was supported by the Russian Foundation for Basic Research (project no. 20-03-00753) and performed under a state task (project no. 0082-2018-0006, registration no. АААА-А18-118020890097-1).
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this manuscript.
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Kasaikina, O.T., Zinatullina, K.M., Kancheva, V.D., Slavova-Kasakova, A.K., Loshadkin, D.V. (2022). Effect of Lipophilic and Hydrophilic Thiols on the Lipid Oxidation. In: Bravo-Diaz, C. (eds) Lipid Oxidation in Food and Biological Systems. Springer, Cham. https://doi.org/10.1007/978-3-030-87222-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-87222-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87221-2
Online ISBN: 978-3-030-87222-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)