Abstract
The chapter describes minor components present in vegetable oils and their amphiphilic properties. The formation of association colloids in bulk oils and their interactions with antioxidants are then discussed. We describe the effects of association colloids on the lipid autoxidation process and the role of water in micelle formation and oxidation of oils. Finally, methods for detecting and characterizing association colloids in vegetable oils are described.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
1.1 Minor Components of Vegetable Oils
Vegetable oils are food products obtained from raw materials of plant origin, usually from various oilseeds and fruits, such as the fruits of the olive tree Olea europacea and of the oil palm Elaeis guineensis (Belitz et al. 2009). The three most commonly produced vegetable oils in the world are palm oil, soybean oil, and rapeseed oil (Siger et al. 2015).
The main component of oils are triacylglycerols, also called triacylglycerides (TAG). The TAG content of most vegetable oils is in the 95–98% (w/w) range (Xenakis et al. 2010). In general, the TAG percentage is lower in crude oils and cold-pressed oils, and increases as a result of the refining process. For example, the content of triacylglycerols in crude soybean oil is 95–97%, while in refined, bleached, deodorized oil it increases to over 99% (Xenakis et al. 2010). According to Fine et al. (Fine et al. 2016), TAG concentration in refined rapeseed and sunflower oils is 98%. In turn, Xenakis et al. (2010) indicated that refined cottonseed, canola, palm, and sunflower oils are more than 99% TAG, while virgin olive oil is over 98.5% TAG.
In addition to TAG, oils also contain so-called minor oil components, such as monoacylglycerols and diacylglycerols, free fatty acids, phospholipids, sterols, tocopherols, tocotrienols, chlorophyll, carotenoids, phenolic compounds, ubiquinone, proteins, trace metals, products of lipid autoxidation, and water (Xenakis et al. 2010), (Fine et al. 2016), (Ghazani and Marangoni 2013). There is a greater percentage of minor components in crude oils and cold-pressed oils, and this decreases as a result of the refining process.
Monoacylglycerols are present in oils in smaller quantities (less than 0.2% w/w) than diacylglycerols (0.8–5.8% (w/w)). Crude palm and olive oils are the richest in diacylglycerols (Xenakis et al. 2010).
Free fatty acids are commonly considered to reduce oil quality and are therefore removed during neutralization. According to Ghazani and Marangoni (2013), refining, bleaching, and deodorization can decrease the free fatty acid content of canola oil from 0.3–1.2% to 0.03%.
As reported by Xenakis et al. (2010), the phospholipids content of crude oils falls within the range of 1.5–2.5% for soybean oil, 0.7–0.9% for cottonseed oil, 2.7–3.5% for canola oil, 0.006–0.013% for palm oil, 0.5–1.0% for sunflower oil, and 0.004–0.014% for virgin olive oil. As a result of refining the levels of these compounds decrease to 0.003–0.045% in the case of soybean oil and 0.012% in the case of palm oil. Phospholipids are removed mainly during the degumming process.
Sterols are one group of compounds present in oils that are beneficial from the nutritional point of view, but some of them are removed during refining. The richest sources of sterols are crude corn oil (924.3 mg/100 g) and crude rapeseed oil (823.8 mg/100 g). For rapeseed oil, it has been found that the total sterol content decreases from 823.8 mg/100 g in the crude oil to 767.1 mg/100 g after refining. It should be noted that free sterols are mostly removed—being reduced from 336.2 mg/100 g to 278.3 mg/100 g—while esters remain practically unchanged at 475.4 mg/100 g in crude oil and 484.7 mg/100 g in the refined oil (Verleyen et al. 2002).
Other important nutritional components include tocopherols and tocotrienols. As these possess antioxidant properties, they play an important role in ensuring oxidative stability of oil. The concentration of total tocopherols usually differs in the crude and refined oils. In the case of soybean oil, which is rich in these compounds, the total tocopherol level in the crude oil falls in the range 1094–2484 mg/kg, while in the refined product the range is 200–3327 mg/kg. In turn, sunflower oil contains 725–1892 mg/kg tocopherols in the crude product and 451–1289 mg/kg in the refined oil. Rapeseed oil has 464–1458 mg/kg tocopherols in the crude product and 227–1234 mg/kg in the refined product (Fine et al. 2016).
Other antioxidants present in vegetable oils include phenolic compounds (polyphenols). Rapeseeds contains about ten times greater levels of phenolic compounds (mainly sinapic acid and its derivatives) than other oilseeds (Siger et al. 2015). However, due to their relative hydrophilicity, only a small fraction of the phenolic compounds present in rapeseed is transferred to the oil during the pressing and extraction processes, and a significant part is also removed during refining. Rapeseeds contain as much as 2514–17,693 mg/kg of polyphenols, whereas the crude oil has only 113–629 mg/kg, and refined rapeseed oil contains only 2.1 mg/kg (Fine et al. 2016). Olive oil has a relatively high concentration of polyphenols in the range of 48–145 mg/kg for extra virgin olive oil, 11 mg/kg for extra light olive oil, and 10 mg/kg for cold-pressed olive oil (Xenakis et al. 2010).
The minor oil components also include pigments such as chlorophyll pigments and carotenoids. Although carotenoids are antioxidants, chlorophylls may act pro-oxidatively through photosensitized oxidation. For this reason, chlorophyll dyes are removed during refining in the bleaching process. In the case of crude canola oil the concentration of these pigments lies in the range 4–30 mg/kg, but after refining, bleaching, and deodorization this decreases to less than 0.025 mg/kg (Ghazani and Marangoni 2013). Crude canola oil contains about 130 ppm of carotenoids, mostly xanthophylls (90%) and carotenes (10%) (Ghazani and Marangoni 2013). In turn, in commercially available refined rapeseed oil, the content of β-carotene has been measured at 2.02 ppm (Rokosik et al. 2019).
Vegetable oils contain traces of metal ions (iron, copper, zinc, lead), which act as prooxidants; as such they should be present in lower concentration in the refined oil. In crude canola oil, the quantities of iron and copper present are 0.5–1.5 mg/kg and less than 0.2 mg/kg, respectively, whereas the refined oil contains less than 0.2 mg/kg of iron and less than 0.02 mg/kg of copper (Ghazani and Marangoni 2013).
Despite their hydrophobicity, oils also contain traces of water. Water in oil originates from the extraction and refining processes. The concentration of water in oils can change during storage, once the container has been opened, via absorption from the environment or loss from the oil. In commercially available refined vegetable oils, the water content usually ranges from 0.02% to 0.03% (200–300 ppm) (Xenakis et al. 2010; Budilarto and Kamal-Eldin 2015a). In unfiltered Greek olive oil samples, a higher concentration of 0.09–0.31% has been noted (Xenakis et al. 2010). Higher water content is observed predominantly in cold-pressed, unrefined oils. According to Siger et al. (2017), the concentration of water in cold-pressed common beech, chia, milk thistle, black cumin, white poppy, and black poppy oils was 911 ppm, 437 ppm, 779 ppm, 358 ppm, 583 ppm, and 831 ppm, respectively.
2 Amphiphilic Properties of Minor Oil Components
The minor components of vegetable oils may be hydrophobic (as with carotenoids), hydrophilic (such as phenolic compounds and proteins), or amphiphilic (monoacylglycerols, diacylglycerols, free fatty acids, phospholipids, sterols, and products of lipid autoxidation such as hydroperoxides, aldehydes, ketones, and epoxides) (Xenakis et al. 2010; Budilarto and Kamal-Eldin 2015a). Amphiphilic molecules possess both hydrophilic and hydrophobic parts and when dispersed in water, their hydrophilic parts preferably interact with water, whereas their hydrophobic parts come into contact with air or a nonpolar solvent. Amphiphiles thus undergo self-assembly and aggregate to form various structures. These assemblies are based on the repellant and coordinating forces between the hydrophobic and hydrophilic moieties of the molecules that constitute them and the surrounding medium (Wang et al. 2012). The type of structures that are formed depends on the hydrophilic–lipophilic balance (HLB) of the molecules. The HLB is calculated from the weight percentage of the molecule’s hydrophilic to hydrophobic groups, and ranges from 1 to 20 (Kralova and Sjöblom 2009). Amphiphilic molecules with a relatively low HLB (3.0–6.0) preferably form water-in-oil emulsions, while a high HLB value (8.0–18.0) facilitates the formation of oil-in-water emulsions. Those minor oil components that have low HLBs—such as free fatty acids, monoacylglycerols, and diacylglycerols, with HLB values of 1.0, 3.4–3.8, and 1.8 respectively—favor the formation and stabilization of reverse micelles in water-in-oil emulsions. Phospholipids, which have an intermediate HLB of about 8.0 are able to form various structures: spherical reverse micelles in bulk oil containing a small amount of water (less than 300 ppm), and lamellar structures, combined with other surface-active compounds (Budilarto and Kamal-Eldin 2015a).
3 Formation of Association Colloids in Bulk Oils
Amphiphilic minor oil components are surface-active compounds (surfactants) and are therefore able to accumulate at the oil–water interface and lower surface tension. At concentrations above their critical micelle concentration (CMC), they self-aggregate and form association colloids (Kittipongpittaya et al. 2014). Association colloids are physical structures formed by amphiphilic compounds, which self-aggregate in a nonpolar environment (bulk oil) with a low water content. Because of the presence of small amounts of water in bulk oil, minor amphiphilic components may concentrate at the oil–water interface and form micellar structures. When association colloids are formed, they can change the physical and chemical properties of the bulk oil (Kittipongpittaya et al. 2014; Rokosik et al. 2020a). As mentioned earlier, vegetable bulk oils contain a lower concentration of water. They can thus be considered a type of water-in-oil nanoemulsion, and the formation of association colloids is in this case possible.
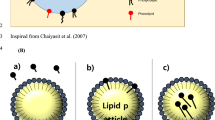
The two main types of association colloids found in vegetable oils are reverse micelles and lamellar structures (Fig. 1). Reverse micelles are micelles in which the polar groups are directed towards the center—a nanoscale hydrophilic core called the “water pool”, which is stabilized by the monolayer of surface-active molecules. It was observed that the size of water droplets increases with the increasing concentration of water in the oil. Reverse micelles are dynamic structures that move by Brownian motion and which exchange water between each other. They have a large interface between their oil and water phases, which ensures that there is contact between the polar and nonpolar compounds. For this reason, reverse micelles are considered to be effective nanoreactors. Reverse micelles are mainly formed by amphiphiles with low HLB values, such as free fatty acids, monoacylglycerols, diacylglycerols, and phospholipids (Xenakis et al. 2010).
Lamellar structures consist of alternating layers of lamellae. In crude vegetable oils, layers of water and oil are separated by layers of appropriately oriented surface-active compounds. The HLB value means that such lamellar structures are mainly formed by phospholipids, which can be accompanied by sterols. These structures in vegetable oils are so far relatively poorly studied and understood (Xenakis et al. 2010).
A necessary condition for the formation of association colloids is that the critical micelle concentrations (CMC) of amphiphilic compounds is exceeded. The CMC values of many minor oil components have already been established, though the values can vary in different oils. The CMC of a specific amphiphile may also be affected by other amphiphiles present in the oil, whether derived from raw materials or from products of lipid oxidation. For this reason, it is significant whether crude oil, refined oil, or refined oil additionally purified of minor components (stripped oil) is used.
In the case of phospholipids, the CMC is affected by the chain length of fatty acyl residues and by the polarity of the head group. It has been demonstrated that DOPC is able to form physical structures at much lower concentrations than phosphatidylcholine with short-chain fatty acyl residues (1,2-dibutyryl-sn-glycero-3-phosphocholine), on account of its lower critical micelle concentration (Chen et al. 2011a). The CMCs of phospholipid 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) is in the range 40–51.13 μmol/kg of oil (Rokosik et al. 2020a; Cui et al. 2014), whereas DOPE (1,2-dioleoyl-sn-glycero-3-phosphoethanolamine) had 200 μmol/kg (Kittipongpittaya et al. 2014). These differences have been explained by the different chemical structures of the compounds. The choline head group of DOPC has a greater polarity than the ethanolamine moiety found in DOPE. As a result, DOPC has a stronger tendency to accumulate at the oil–water interface. Additionally, the critical micelle concentration of phospholipids depends on their hydration index—the extent to which the molecule has imbibed water, expressed on an arbitrary scale from 0 to 100. DOPC, with a hydration index of 100, has a lower CMC than DOPE with its hydration index of 16.
Commercial bulk oils contain a mixture of different minor components, which reduces the CMC in comparison to the CMC values that individual compounds would hold. Some of these components, such as phosphatidylcholine, are necessary for the formation of colloids, while others (like DOPE, stigmasterol, oleic acid, diacylglycerols, and other minor oil compounds) act as cosurfactants, reducing the CMC of phospholipids (Kittipongpittaya et al. 2014; Kim et al. 2019). However, according to Cui et al. (2014) the addition of DOPE alone increases the CMC of DOPC. The amount of water is a critical factor in the formation of association colloids in bulk oil. However, the minimum concentration of water needed to form colloids is difficult to determine, as it depends on the type of amphiphilic substance forming the micelles. For example, aggregation of stigmasterol in stripped corn oil was not observed with 400 ppm of water (Chen et al. 2010), while Cui et al. (2014) reported formation of DOPC micelles at 200–300 ppm of water. In refined commercially available vegetable oils, the water content usually ranges from 200 to 300 ppm, and these oils also contain various amphiphiles. It may be concluded that association colloids are present in practically all types of vegetable oil.
According to Chen et al. (2010), CMC differences in various media (oils) are due to differences in the fatty acid chain lengths and the presence of other minor components, which are surface active and were not completely removed during stripping. Critical micelle concentrations of amphiphilic minor oil components derived from the raw material (such as phospholipids) may be affected by lipid oxidation products. Many of these products (such as aldehydes) are amphiphilic. For this reason, CMC may change during oil storage, due to the lipid autoxidation process (Jo and Lee 2021).
Crude and commercial vegetable oils usually contain small amounts of water, which produces an oil–water interface in bulk oils. In such an environment, amphiphilic minor components migrate and preferably concentrate on the interface, forming the energetically favored association colloids (Xenakis et al. 2010). Although association colloids arise spontaneously in vegetable oils, in most studies concerning their properties, they are formed by stripping the oil of minor components or using a mixture of triacylglycerols and adding selected surface-active compounds (amphiphilic minor components) in a controlled manner.
The structure of aggregates formed in bulk oils from phospholipids depends on their type and concentration. In stripped soybean oil, 1,2-dibutyryl-sn-glycero-3-phosphocholine (DC4PC) is able to form cylindrical structures, whereas DOPC there forms spherical structures. Both phospholipids have the same hydrophilic headgroups, but DOPC has cis-oleic fatty acids on the sn-1 and sn-2 glycerol positions, whereas DC4PC possess two butyl fatty acids. Amphiphilic compounds such as phospholipids have a large tail area and a small headgroup area, and for this reason they assemble in oils into spherical reverse micelles, because of the critical packing parameter (CPP) being greater than unity. The CPP of DC4PC in stripped soybean oil is approximately less than half lower than that of DOPC, assuming no difference in the effective area of the headgroup. In this case, CPP depends mainly on hydrocarbon tail length. A low CPP value often results in the formation of cylindrical structures (Chen et al. 2010). The self-assembly of DOPC into association colloids has also been confirmed by Rokosik et al. (2020a, b). Cui et al. (2014) observed that DOPE forms reverse micelles in stripped soybean oil, but DHPE (1,2-dihexanoyl-sn-glycero-3-phosphoethanolamine) does not aggregate into association colloids at concentrations up to 10,000 μmol. This indicates that the length of the phospholipid fatty acid residue that affects HLB has a significant effect on the ability of specific compounds to form association colloids. The differences between DOPE and DOPC CMC probably result from phosphatidylcholine interacting more strongly with water than does DOPE. Phosphatidylethanolamine is less hydratable than phosphatidylcholine due to the DOPE amine group, which tends to form hydrogen bonds directly with adjacent phosphate molecules. For this reason, DOPE’s lipid–lipid interactions are stronger than its lipid–water interactions (Cui et al. 2014; Kanamoto et al. 1981; Franks 2009).
Subramanian et al. (2001) observed the formation of mixed micelles from phospholipids present in crude soybean oil. The micellar structure included phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylethanolamine (PE), phosphatidic acid (PA), and phytosphingolipids. The CMC of micelles decreased with increasing degree of phospholipid hydration. Because the hydration indices of PC, PI, PE, and PA were 100, 44, 16, and 8.5 (in arbitrary units with 100 as the maximum), Subramanian et al. believe that the CMC of mixed micelles is greater than that formed from PC. The qualitative and quantitative composition of phospholipids in the oil thus affects the formation of mixed micelles. As this results from the hydration index, PA can significantly affect the assembly of these structures.
Stigmasterol has been found to be capable of forming aggregates in stripped corn oil (Kittipongpittaya et al. 2016). Oleic acid, in turn, does not assemble, probably because of the repulsion of its charged head groups (Kittipongpittaya et al. 2016).
According to Lehtinen et al. (2017), temperature, water, and free fatty acid content strongly affect the self-assembly of lecithin in rapeseed oil. Those authors noted the formation of lecithin cylindrical reverse micelles. An increase in temperature from room temperature to 70 °C resulted in a decrease in the CMC value. CMC decreasing with increasing temperature is a general tendency observed in many experiments. Because of the assembly of oleic acid molecules around polar groups of lecithin, suppression of reverse micelle formation was observed. This resulted from the increased solubility of lecithin in oil. This phenomenon has been observed at a very low concentration of water in oil. At slightly higher water levels, oleic acid acts as a cosurfactant, stabilizing reverse micelles. At the concentration of lecithin above the CMC, the addition of the critical amount of water causes the formation of lamellar structures. Assembly of these structures leads to phase separation, with the consequent removal of the reverse micelles from the oil phase. Phase separation begins at a significantly higher water level in the presence of oleic acid. The behavior of phospholipids in oil may be substantially altered by a very small change in water concentration or the presence of other surface-active compounds (Lehtinen et al. 2017).
4 Interactions of Antioxidants with Association Colloids
Antioxidants in vegetable oils may interact with association colloids, altering their structure and properties, as well as the kinetics of the lipid autoxidation process and the effectiveness of the antioxidants themselves. Rokosik et al. investigated interactions of phenolic compounds (sinapic and ferulic acids) with DOPC association colloids in stripped rapeseed oil (Rokosik et al. 2020a). Measurement of fluorescent probe emission demonstrated the incorporation of phenolic acids into the micelle structure. Another possibility is that the antioxidants were present at the water–oil interface, in the vicinity of the fluorescence probe. The observation of the static mechanism of probe fluorescence quenching confirmed the formation of complexes from phenolics and NBD-PE embedded in the reverse micelles. NBD-PE anisotropy measurements showed that interactions of sinapic and ferulic acid with DOPC reverse micelles resulted in changes in their structure (in particular reduced rigidity). In another study, Rokosik et al. (2020b) found that canolol (common in the rapeseed oil product of sinapic acid decarboxylation) may be incorporated into the structure of DOPC reverse micelles, but is not present in their hydrophilic part. In the case of α-tocopherol, a hydrophobic antioxidant, no interactions with DOPC association colloids were recorded. However, in stripped rapeseed oil without the addition of DOPC, the formation of micellar structures consisting of α-tocopherol was observed. A mixture of canolol and α-tocopherol at a relatively high concentration (500 μmol of each antioxidant/kg of oil) led to changes in the hydrophilic part of the reverse micelles (Rokosik et al. 2020b). Fadel et al. (2017) produced micellar aggregates composed of polyglyceryl-3-diisostearate in vegetable oil. The resulting reverse micelles allow efficient solubilization of polar antioxidants in the oil medium.
5 The Effects of Association Colloids on Lipid Autoxidation in Bulk Oils
The association colloids found in vegetable oils may affect the lipid autoxidation process and the effectiveness of antioxidant action. Brimberg (1993a, b) postulated that the transition from the initiation to the propagation phase of lipid oxidation is governed by the CMC of hydroperoxides and its modification by other amphiphilic compounds. According to Brimberg and Kamal-Eldin (2003), oxidation of lipids in bulk oils begins as a pseudo-first-order reaction, in which hydroperoxides are formed. When the hydroperoxides exceed their CMC, they begin to aggregate into reverse micelles. At this point, the reaction rate changes to second order and autoxidation passes to the propagation phase. Consequently, the efficiency of pro-oxidants and antioxidants depends on how they modulate the hydroperoxide CMC. However, the presence of both water from the raw material or the refining process and of amphiphilic compounds derived from seeds and fruits means that association colloids are already present at the initiation stage. Certainly, the amphiphilic oxidation products (such as hydroperoxides) affect the structure of reversed micelles.
Budilarto and Kamal-Eldin (2015b) showed that micelle size in sunflower and canola oils increases during and slightly before the end of the induction phase; their size then reaches a maximum and they collapse. Association colloids, though the formation of the oil–water interface, can physically affect the location of lipids and the oxidation process. Both hydroperoxides that are surface-active compounds and metal ions migrate to the oil–water interface. This facilitates the decomposition of hydroperoxides, catalyzed by the presence of transition metal ions, resulting in the formation of free radical products and acceleration of the autoxidation processes (Rokosik et al. 2020a). Free fatty acids and monoacylglycerols also act as prooxidants by concentrating at the oil–water interface and speeding up lipid hydroperoxide decomposition—the micellar effect. In this way, association colloids provide a reaction site for oxidation to take place—a “nanoreactor” (Budilarto and Kamal-Eldin 2015a).
As in oil–water emulsions, oxidation also occurs in interfacial regions. The smaller interface in bulk oil (compared to the emulsion) means the autoxidation is slower and the effective concentration of interfacial antioxidants is higher. As a result of the smaller interface surface, the effect of amphiphilic compounds derived from raw material and of amphiphilic oxidation products on the properties of the interfacial region and the structure of reverse micelles is greater.
According to the hypothesis of Budilarto and Kamal-Eldin (2015a), formation of a nanoemulsion and molecular organization are the most important factors for lipid autoxidation during the initial stage (induction period), with free radical reactions becoming relevant in the propagation and termination phases. Primary antioxidants and synergists stabilize micelles during the initial stage, whereas during propagation they mainly scavenge free radicals (Budilarto and Kamal-Eldin 2015a).
Chen et al. (2010) investigated the influence of DOPC and DC4PC on the oxidation kinetics of soybean oil lipids. These two phospholipids have the same choline hydrophilic group. For this reason, their effect on lipid autoxidation by chemical pathways would be expected to be similar. However, it turned out that the spherical structures formed in stripped soybean oil by DOPC were prooxidative, whereas the cylindrical structures formed by DC4PC had no effect on autoxidation rate. It must therefore be concluded that association colloids formed from phospholipids in bulk oil affect the autoxidation rate of lipids and their impact depends on the form/shape of structures. The prooxidative effect of DOPC reverse micelles has been confirmed by Chen et al. (2011a) and by Rokosik et al. (2020a, b).
The antioxidant properties of phospholipids have however been demonstrated: these include an ability to chelate metal ions, decompose hydroperoxides, scavenge free radicals, and increase the antioxidant potential of tocopherols. Physical structures assembled from phospholipids may increase the antioxidant activity of tocopherols by allowing them to concentrate at the oil–water interface, where lipid oxidation is most intense (Chen et al. 2010; Koga and Terao 1995). In this way a physical proximity of antioxidants and prooxidants is created. According to Chen et al. (2011a), DOPC in stripped soybean oil improves the activity of α-tocopherol and its water-soluble analogue at low concentrations (10 μmol), while at high concentrations of both antioxidants (100 μmol), a decrease in their activity was recorded. Those authors believe that DOPC reverse micelles form a negatively charged interface that attracts prooxidative metal ions. The presence of both antioxidants in the same location as the transition metals allows them to reduce metals into a more prooxidative state, thereby increasing the rate of lipid oxidation. This phenomenon is seen as a decrease in antioxidant efficiency. In this mechanism, we can also observe the embedding of surface-active hydroperoxides into reverse micelles. These substances are the substrate in metal-promoted lipid autoxidation, accelerating the process.
It has also been demonstrated that the hydrophilic Trolox has a higher antioxidant potential than the hydrophobic α-tocopherol. On the basis of NBD-PE fluorescence, it was found that emission of this probe was affected by Trolox much more strongly than in the case of α-tocopherol. This indicates that both antioxidants have different locations in DOPC reverse micelles. This may be due to the better solubility of Trolox in water, which leads it to be located in the aqueous phase, where α-tocopherol is practically insoluble in water, so at best it could be present in the oil–water interface. Such differences in the location of Trolox and α-tocopherol may affect their antioxidant and prooxidant activities.
Kittipongpittaya et al. confirmed that Trolox and α- tocopherol take up different locations in mixed micelles made of DOPC, DOPE, stigmasterol, oleic acid, and diacylglycerols in stripped corn oil (Kittipongpittaya et al. 2014). Those authors noted that α-tocopherol was unlikely to concentrate at the oil–water interface, while Trolox could partition at the same location as NBD-PE at the association colloids’ oil–water interface. Nevertheless, the association structures were not found to have an effect on the antioxidant efficiency of either antioxidant at concentrations of 10 or 50 μmol/kg.
It has however been shown that association colloids assembled from mixed components substantially decrease the oxidative stability of oil. Cui et al. observed the prooxidant action of micelles obtained from a mixture of DOPC and DOPE in stripped soybean oil (Cui et al. 2014). In another study, Cui et al. (2015) explained how DOPC and DOPE reverse micelles affect the activity of antioxidants: it was found that DOPC association colloids decreased the activity of 100 μmol α-tocopherol and Trolox. DOPE reverse micelles increase the antioxidant activity of α-tocopherol by regenerating oxidized α-tocopherol (quinone) by the phosphatidylethanolamine primary amine group. However, no effect of DOPE on the physical location of α-tocopherol was found.
Homa et al. (2015) recorded that hydroperoxide type is related to prooxidant activity. Hydroperoxides with DHA, EPA, and α-linolenic acids have high surface activities, which facilitates their ability to form associates and to accelerate oxidation in fish and soybean oils. In turn, linoleic acid and oleic acid hydroperoxides are less surface active, and micelles formed with them in high linoleic safflower and high oleic safflower oils are not pro-oxidative.
Rokosik et al. (2020a) have suggested that the presence of DOPC reverse micelles in stripped rapeseed oil accelerates the decomposition of hydroperoxides to hexanal. They indicate that the antioxidant effect of sinapic acid in bulk rapeseed oil is affected by DOPC reverse micelles. The formation of association colloids from amphiphilic lipid autoxidation products (hydroperoxides, hexanal and others) reduces the effectiveness of sinapic acid in oil without added DOPC. In another study, Rokosik et al. (2020b) noted that DOPC association colloids affect the antioxidant efficiency of canolol and α-tocopherol. Simultaneously, at concentrations of 100 μmol for both substances, a decrease of their antioxidant action effectiveness over time was observed. This effect probably results from the increased concentration of amphiphilic autoxidation products, which affects the structure of DOPC micelles or the formation of mixed micelles with antioxidants, modifying their effectiveness. It has been shown that α-tocopherol affects levels of both lipid hydroperoxides and hexanal, though in canolol only hexanal is affected. These differences may come from the different location of the antioxidants in the association colloids. It was also demonstrated that the antioxidant synergism of canolol and α-tocopherol only occurs in DOPC reverse micelles and is not observed in oil lacking these structures. This indicates the important role of association colloids in the antioxidant synergism phenomenon.
Homma et al. (2016) observed that the charge of association colloids affects lipid oxidation in ethyl oleate. Structures formed from anionic, cationic, and nonionic surfactants respectively retard, accelerate or have no effect on oxidation rates.
Changes in antioxidants efficiency at the interface in bulk oil may also be related to the “cut-off” effect, which is described as the parabolic dependence of antioxidant efficiency on molecular chain length. It is explained by changes in reactant diffusivity, antioxidants’ ability to self-aggregate, and their solubility in the interfacial region. Molecule chain length may also affect reactivity orientation at the interface (Costa et al. 2021). In this context, it should be stated that the presence of amphiphilic minor compounds in bulk oil, the formation of association colloids, changes in their structure and in the ability of antioxidants to aggregate at the interface may affect the cut-off effect.
The effectiveness of antioxidants in the presence of association colloids should also be considered in the context of the polar paradox, according to which polar antioxidants are more efficient than nonpolar antioxidants in bulk oils (Costa et al. 2015). Lipid oxidation in oil-in-water emulsions occur in the interfacial region. Similarly, in bulk oil—which can be considered a nanoemulsion—the oil–water interface plays an important role in autoxidation. Surface-active hydroperoxides and metal ions accumulate at the interface in association colloids and, as a result of the metal-catalyzed decomposition of hydroperoxides, free radicals are formed, accelerating autoxidation. In this sense, association colloids play a catalytic role. The presence of polar antioxidants in colloids can effectively inhibit this process, in line with the polar paradox. The formation of reverse micelles, changes in their structure and composition (such as due to the appearance of amphiphilic oxidation products) may alter the effective interfacial concentration of antioxidants. This decrease in antioxidants’ effective interfacial concentration in an oil–water emulsion was observed as a result of an increase in the molar volume of the surfactant to volume of emulsion ratio (Costa et al. 2020).
The rate and mechanism of lipid oxidation in association colloids depends on many factors. The concentration of the substrates, antioxidants, and prooxidants in association colloids depends on the quantity of surface-active compounds available to form such structures (free fatty acids, phospholipids, antioxidants). The location and orientation of reactants in colloids, as well as changes in their structure at different temperatures, affect lipid oxidation rate. For example, the anionic interface of micelles attracts metals catalyzing the decomposition of hydroperoxides (Chaiyasit et al. 2007).
6 The Role of Water in the Formation of Association Colloids and its Effects on Lipid Autoxidation in Bulk Oil
The water content of an oil changes during prolonged storage after the package has been opening. This happens due to the absorption of water from the environment and its evaporation from the oil, but also due to oxidation (Chen et al. 2011b; Park et al. 2014). Water content negatively affects the quality of oils: it is involved in triacylglycerol hydrolysis to free fatty acids in thermal processes and in the presence of lipase. Water is a solvent for many amphiphilic and hydrophilic substances, which affects their reactivity. This concerns both compounds with antioxidant properties, such as phenolic compounds or ascorbic acid, as well as prooxidative substances, such as lipid hydroperoxides or transition metals (Kittipongpittaya et al. 2016). Additionally, water is one of the basic requirements for the formation of association colloids in the oil. Water activity (which may affect the oxidation rate) may be influenced by amphiphilic compounds and by the presence of association colloids (Chaiyasit et al. 2007).
Due to its hydrophilic structure, water is practically immiscible with oil, with a solubility ranging from 0.05 to 0.3%. Water may have an effect on the critical micelle concentration of minor components in oil. Changes in the CMC of medium chain triacylglycerols and of lecithin in corn oil with water content were described by Kim et al. (2018), who argued that such changes are associated with the available water, which creates sites or areas in bulk oil for packaging amphiphilic compounds, such as phospholipids (PL). The size and number of association colloids are affected by the water-to-phospholipid ratio. If the concentration of PL is greater than the water content, the PL forms a large number of small-sized micelles. Demand for PL is thus high, which is equivalent to high CMC. An increase in humidity will lead to an increase in the area available for binding with the PL. More PL is needed to pack the available space in the water molecules, thus increasing CMC. At a humidity higher than 900 ppm, water molecules have a greater affinity for each other than for PL molecules. Small association colloids merge and form larger structures, reducing the interface and thus decreasing CMC (Kim et al. 2018).
Most studies have focused on the formation of association colloids from phospholipids. The appearance of additional surfactants, and even minor changes in water content, may alter the structure of phospholipid micelles (Rokosik et al. 2020a; Cui et al. 2014; Xu et al. 2019). According to Lehtinen et al. (2017), oleic acid in the presence of a low water concentration will accumulate around lecithin polar groups in rapeseed oil, increasing its solubility in oil and thus increasing the CMC value—that is, the concentration of lecithin necessary to create association colloids. The interaction of oleic acid and lecithin, however, is strictly dependent on the water content in the system: greater water content causes the precipitation of lecithin in the form of laminar structures, while added oleic acid acts as a cosurfactant and decreases the rate of phase separation, stabilizing reverse micelles (Lehtinen et al. 2017).
Although water is only present in trace amounts in oil, it has a significant impact on oxidation and other processes, thanks to its ability to dissolve antioxidants and pro-oxidants. However, water, apart from its interaction with other amphiphilic and hydrophilic substances, has no effect on the rate of autoxidation (Kittipongpittaya et al. 2016; Chen et al. 2011b). In the absence of surfactants (minor components) association colloids, considered as oxidation centers, are not formed, hence the rate of oxidation is similar to that in oils with different moisture contents (Budilarto and Kamal-Eldin 2015a).
Aldehydes such as propanal, hexanal, and nonanal accelerate the lipid oxidation rate in soybean oil and alter its water content (in comparison to soybean oil without aldehydes), but the changes depended on the type of aldehyde and on the initial relative humidity of the oil. Nonanal and hexanal contributed to the removal of water from soybean oil, while propanal stabilized the moisture (Jo and Lee 2021).
In turn, Kim et al. (2014) confirmed that water may function as a substrate for the formation of volatile oxidation products during the autoxidation of bulk oil. Park et al. (2014) found that changes in water content in corn oil and the rate of oxidation depend on the availability of oxygen and on storage temperature.
Water content and mobility could affect the size and number of association colloids, as well as the activity of reactants at the interface or in the water core. The dimensions of inner water core in association colloids may determine the type of molecules incorporated into the colloid structure. Those factors may affect lipid oxidation rate in bulk oil (Chaiyasit et al. 2007).
7 Methods Used in Association Colloids Studies
Studies of the properties of association colloids in vegetable oils and the effects they have on autoxidation usually require the formation of colloidal structures under controlled conditions. It is thus necessary to remove amphiphilic minor components before assembling the micelles or lamellar structures from compounds added to the oil in carefully measured amounts. The removal of minor compounds from oil is generally performed using column chromatography with n-hexane as an eluent. The most common types of chromatographic bed used include silica gel, silicic acid, activated charcoal, and aluminum oxide, which have undergone the appropriate activation. Beds are used individually or in combination. Aluminum oxide mainly removes antioxidants from vegetable oils (Nyström et al. 2007; Khuwijitjaru et al. 2009; Romero et al. 2007). Chromatographic beds containing silica gel are used to purify oil of tocopherols and sterols (Hrádková et al. 2013; Fang et al. 2017). Verleyen et al. (2002) separated free and esterified sterols from vegetable oils using silica gel. Most researchers use two chromatographic methods together: silicic acid and activated charcoal (Kittipongpittaya et al. 2016; Homma et al. 2015; Boon et al. 2008). Activated charcoal is used mainly to remove pigments, especially chlorophyll and carotenoids. It is often employed due to its low cost and strong adsorption properties, but it is not very selective of the compounds it removes, therefore it is often used alongside additional adsorbents (Rajczykowski and Loska 2016). Rokosik et al. (2019) in their research into appropriate chromatographic beds for purifying rapeseed oil from its minor components considered all the types just mentioned. They determined effectiveness of each bed type by measuring the concentration of the residual dyes (chlorophyll, carotenoids), sterols, and tocopherols in the purified oil. As a result, a three-stage optimized method for removing minor components from rapeseed oil was developed. The oil was dissolved in n-hexane (1: 1 v/v) and passed sequentially through the column with silicic acid, activated charcoal, and a second layer of silicic acid; the partially purified oil was then directed to a column with activated charcoal and aluminum oxide. In the last step, a column containing silica gel was used (Rokosik et al. 2019).
Association colloids are formed in bulk oils in the presence of small amounts of water, the concentration of which influences the properties of the micellar structures. It is thus often necessary to determine water content. For this purpose, the thermogravimetric method and the Karl Fischer titration are used. Drying the oil in the oven is straightforward and inexpensive, but is time-consuming and can lead to many errors; for example, volatile substances (volatile fatty acids) are lost at the drying temperature, which may falsify results (Xie et al. 2017). For this reason, the most common methods are the Karl Fischer volumetric (Leito and Jalukse 2019) and coulometric methods, which are fast, accurate, and reproducible. Due to the trace amounts of water in oils, the coulometric method is recommended (Felgner et al. 2008).
Spectroscopy is generally used in determining the CMC of amphiphilic minor oil components, with TCNQ spectroscopy being most common. The absorbance of 7,7,8,8-tetracyanoquinodimethane clearly increases as a result of micelle formation, due to the charge transfer of TCNQ in the presence of aggregates. The critical micelle concentration is determined as an inflexion point in the semilogarithmic plot showing the dependence of TCNQ absorbance (at 480 nm) on the amphiphilic substance concentration (Kittipongpittaya et al. 2014; Cui et al. 2014; Chen et al. 2010; Subramanian et al. 2001). The CMC may be also determined on the basis of NBD-PE fluorescence probe emission intensity, which increases upon formation of reverse micelles in oil as a result of changes in the microenvironment of the probe. As with the TCNQ technique, CMC is read from the plot of fluorescence intensity versus surfactant concentration (Rokosik et al. 2020a, b).
Fluorescence probes may also be used to detect association colloids and to find changes in the environment, structure, and interactions of micelles with antioxidants. DAF (5-dodecanoylaminofluorescein) probes can be used to observe how amphiphilic components like phosphatidylcholine can increase the exposure of surfactants to the aqueous phase of association colloids. DAF may also record changes in pH resulting from the addition of oleic acid to bulk oil. In turn, the ability of DAF to detect free radicals may be employed to determine how amphiphilic compounds impact oxidative reactions in oil (Chaiyasit et al. 2008). Amphiphilic NBD-PE fluorescence probes ((N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt) may detect the formation of association colloids: exposure of the NBD-PE head group to a polar environment leads to a decrease in fluorescence intensity. This probe is preferentially located at the oil–water interface and orients its hydrophilic moiety toward the water core. The imino group or the oxygen molecule on the NBD probe can hydrogen bond with water molecules, leading to fluorescence quenching. The increased NBD-PE fluorescence intensity in the presence of reverse micelles formed from phospholipids may be due to the ability of amphiphilic compounds to compete for the oil–water interface, thus decreasing interactions between NBD-PE and water, and leading to an increase in fluorescence intensity (Kittipongpittaya et al. 2014; Rokosik et al. 2020a).
NBD-PE may be used to investigate the physical location of antioxidants in association colloids. For example, Trolox can partition in the same location as NBD-PE, at the oil–water interface of association colloids. This leads to a measurable decrease in probe fluorescence intensity (Kittipongpittaya et al. 2014). Measurements of NBD-PE fluorescence quenching in combination with fluorescence decay (lifetime) allow it to be determined whether the mechanism of fluorescence quenching is static or dynamic. The static quenching mechanism implies that the ground-state nonfluorescent complex between NBD-PE and an antioxidant has been formed. In this way, the presence of sinapic and ferulic acids in the DOPC reverse micelles structure or at the oil–water interface in the vicinity of fluorescence probe could be determined (Rokosik et al. 2020a). Fluorescence anisotropy measurements are used to study changes in the structure of association colloids. This value reflects the rotational freedom of the fluorescent molecule. The increase in fluorescence anisotropy of NBD-PE molecules embedded in micelles shows the formation of structures with enhanced rigidity. Changes in rigidity were observed as a result of the incorporation of sinapic and ferulic acid into DOPC reverse micelles, and after formation of canolol molecular associations in stripped rapeseed oil (Rokosik et al. 2020a, b).
Small-angle X-ray scattering (SAXS) and cryogenic transmission electron microscopy (cryo-TEM) can determine the structural properties and morphology of reverse micelles; some of the structures detected in this way in vegetable oil include spherical, cylindrical, hexagonal, and lamellar shapes (Fig. 1), (Chen et al. 2010; Lehtinen et al. 2017).
An association of two triacylglycerol molecules forming a backbone (2.5 nm) has been detected in olive oil using dynamic light scattering (DLS) (Xenakis et al. 2010). To measure the hydrodynamic radius and polydispersity index of colloidal structures in oil, modified thin-layer cell dynamic light-scattering instruments with 3D optics can be also applied. The use of this variant of the DLS method avoids the problem of multiple scattering in the undiluted sample and the fluorescence of chlorophylls present in the oil. Static small angle light scattering (SALSA) allows the detection of colloids up to 15 μm. This is a good technique for turbid samples, such as virgin olive oil (Papadimitriou et al. 2013).
The surface activity of amphiphilic compounds can be determined by measuring its effect on the interfacial tension of oil using interfacial tensiometry (with a drop-shape analyzer) (Kittipongpittaya et al. 2014, 2016).
8 Conclusions
Vegetable oils are often thought of as homogeneous products. However, the presence of small amounts of water mean they are better understood as nanoemulsions. Amphiphilic compounds derived from the raw material and amphiphilic oxidation products accumulate at the interface, forming association colloids (spherical reverse micelles, as well as lamellar, cylindrical, and hexagonal structures). Lipid oxidation takes place mainly in the interfacial region. The accumulation of pro-oxidants (such as hydroperoxides decomposed to free radicals in the presence of metal ions) and amphiphilic or hydrophilic antioxidants at the interface can significantly affect this process. In addition, amphiphilic compounds that do not participate in oxidation reactions, due to their presence in the interfacial region, can modify the physical properties of the association colloids. They can thus indirectly affect the effectiveness of pro-oxidants and antioxidants by changing the location and orientation of reactants in the colloid structure. Association colloids hence play an important role in the autoxidation of vegetable oils. Controlling the processes taking place in them—for example by altering the composition of the amphiphilic compounds—may contribute to better ways of managing this unfavorable autoxidation.
References
Belitz H-D, Grosch W, Schieberle P (2009) Food chemistry. Springer, Berlin, Heidelberg. http://springerlink.bibliotecabuap.elogim.com/10.1007/978-3-540-69934-7
Boon CS, Xu Z, Yue X, McClements DJ, Weiss J, Decker EA (2008) Factors affecting lycopene oxidation in oil-in-water emulsions. J Agric Food Chem 4(56):1408–1414
Brimberg UI (1993a) On the kinetics of the autoxidation of fats. J Am Oil Chem Soc 70(3):249–254
Brimberg UI (1993b) On the kinetics of the autoxidation of fats. II. Monounsaturated substrates. J Am Oil Chem Soc 70(11):1063–1067
Brimberg UI, Kamal-Eldin A (2003) On the kinetics of the autoxidation of fats: influence of pro-oxidants, antioxidants and synergists. Eur J Lipid Sci Technol 105(2):83–91
Budilarto ES, Kamal-Eldin A (2015a) The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils: supramolecular chemistry of lipid oxidation. Eur J Lipid Sci Technol 117(8):1095–1137
Budilarto ES, Kamal-Eldin A (2015b) Water content and micelle size change during oxidation of sunflower and canola oils: lipid oxidation: water content & micelle size. Eur J Lipid Sci Technol 117(12):1971–1977
Chaiyasit W, Elias RJ, McClements DJ, Decker EA (2007) Role of physical structures in bulk oils on lipid oxidation. Crit Rev Food Sci Nutr 47(3):299–317
Chaiyasit W, McClements DJ, Weiss J, Decker EA (2008) Impact of surface-active compounds on physicochemical and oxidative properties of edible oil. J Agric Food Chem 56(2):550–556
Chen B, Han A, McClements DJ, Decker EA (2010) Physical structures in soybean oil and their impact on lipid oxidation. J Agric Food Chem 58(22):11993–11999
Chen B, Han A, Laguerre M, McClements DJ, Decker EA (2011a) Role of reverse micelles on lipid oxidation in bulk oils: impact of phospholipids on antioxidant activity of α-tocopherol and Trolox. Food Funct 2(6):302–309
Chen B, McClements DJ, Decker EA (2011b) Minor components in food oils: a critical review of their roles on lipid oxidation Chemistry in bulk oils and emulsions. Crit Rev Food Sci Nutr 51(10):901–916
Costa M, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C, Romsted LS (2015) A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the “cut-off” effect. Food Chem 175:233–242
Costa M, Freiría-Gándara J, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C (2020) Effects of droplet size on the interfacial concentrations of antioxidants in fish and olive oil-in-water emulsions and nanoemulsions and on their oxidative stability. J Colloid Interface Sci 562:352–362
Costa M, Losada-Barreiro S, Paiva-Martins F, Bravo-Díaz C (2021) Polyphenolic antioxidants in lipid emulsions: partitioning effects and interfacial phenomena. Foods 10(3):539
Cui L, Kittipongpittaya K, McClements DJ, Decker EA (2014) Impact of Phosphoethanolamine reverse micelles on lipid oxidation in bulk oils. J Am Oil Chem Soc 91(11):1931–1937
Cui L, McClements DJ, Decker EA (2015) Impact of phosphatidylethanolamine on the antioxidant activity of α-tocopherol and Trolox in bulk oil. J Agric Food Chem 63(12):3288–3294
Fadel O, Girard L, Gomes Rodrigues D, Bauduin P, Le Goff X, Rossignol-Castera A (2017) I in. Micellization in vegetable oils: a structural characterisation. Colloids Surf B Biointerfaces 154:279–286
Fang B, Zhang M, Shen YM (2017) Importance of the higher retention of tocopherols and sterols for the oxidative stability of soybean and rapeseed oils. J Food Sci Technol 54(7):1938–1944
Felgner A, Schlink R, Kirschenbühler P, Faas B, Isengard H-D (2008) Automated Karl Fischer titration for liquid samples – water determination in edible oils. Food Chem 106(4):1379–1384
Fine F, Brochet C, Gaud M, Carre P, Simon N, Ramli F (2016) I in. Micronutrients in vegetable oils: the impact of crushing and refining processes on vitamins and antioxidants in sunflower, rapeseed, and soybean oils: micronutrients in vegetable oils. Eur J Lipid Sci Technol 118(5):680–697
Franks F (2009) Water science reviews. Cambridge University Press, Cambridge
Ghazani SM, Marangoni AG (2013) Minor components in canola oil and effects of refining on these constituents: a review. J Am Oil Chem Soc 90(7):923–932
Homma R, Suzuki K, Cui L, McClements DJ, Decker EA (2015) Impact of association colloids on lipid oxidation in Triacylglycerols and fatty acid ethyl esters. J Agric Food Chem 63(46):10161–10169
Homma R, Johnson DR, McClements DJ, Decker EA (2016) Influence of iron solubility and charged surface-active compounds on lipid oxidation in fatty acid ethyl esters containing association colloids. Food Chem 199:862–869
Hrádková I, Merkl R, Šmidrkal J, Kyselka J, Filip V (2013) Antioxidant effect of mono- and dihydroxyphenols in sunflower oil with different levels of naturally present tocopherols. Eur J Lipid Sci Technol 115(7):747–755
Jo S, Lee J (2021) Evaluation of the effects of aldehydes on association colloid properties and oxidative stability in bulk oils. Food Chem 338:127778
Kanamoto R, Wada Y, Miyajima G, Kito M (1981) Phospholipid- phospholipid interaction in soybean oil. J Am Oil Chem Soc 58(12):1050–1053
Khuwijitjaru P, Yuenyong T, Pongsawatmanit R, Adachi S (2009) Degradation kinetics of gamma-Oryzanol in antioxidant-stripped Rice bran oil during thermal oxidation. J Oleo Sci 58(10):491–497
Kim JY, Kim M-J, Lee J (2014) Role of moisture on the lipid oxidation determined by D2O in a linoleic acid model system. Food Chem 146:134–140
Kim J, Kim M-J, Lee J (2018) The critical micelle concentration of lecithin in bulk oils and medium chain triacylglycerol is influenced by moisture content and total polar materials. Food Chem 261:194–200
Kim J, Woo Y, Ryu J, Kim M-J, Lee J (2019) Lecithin near its critical micelle concentration increases oxidative stability of non-stripped corn oil but not stripped corn oil. Eur J Lipid Sci Technol 121:1800219
Kittipongpittaya K, Panya A, Cui L, McClements DJ, Decker EA (2014) Association colloids formed by multiple surface active minor components and their effect on lipid oxidation in bulk oil. J Am Oil Chem Soc 91(11):1955–1965
Kittipongpittaya K, Panya A, Decker EA (2016) Role of water and selected minor components on association colloid formation and lipid oxidation in bulk oil. J Am Oil Chem Soc 93(1):83–91
Koga T, Terao J (1995) Phospholipids increase radical-scavenging activity of vitamin E in a bulk oil model system. J Agric Food Chem 43(6):1450–1454
Kralova I, Sjöblom J (2009) Surfactants used in food industry: a review. J Dispers Sci Technol 30(9):1363–1383
Lehtinen O-P, Nugroho RWN, Lehtimaa T, Vierros S, Hiekkataipale P, Ruokolainen J (2017) I in. Effect of temperature, water content and free fatty acid on reverse micelle formation of phospholipids in vegetable oil. Colloids Surf B Biointerfaces 1(160):355–363
Leito I, Jalukse L (2019) Traceability, validation and measurement uncertainty in chemistry, vol 3 practical examples. https://doi.org/10.1007/978-3-030-20347-4
Nyström L, Achrenius T, Lampi A-M, Moreau RA, Piironen V (2007) A comparison of the antioxidant properties of steryl ferulates with tocopherol at high temperatures. Food Chem 101(3):947–954
Papadimitriou V, Dulle M, Wachter W, Sotiroudis TG, Glatter O, Xenakis A (2013) Structure and dynamics of veiled virgin olive oil: influence of production conditions and relation to its antioxidant capacity. Food Biophys 8(2):112–121
Park JW, Kim JY, Kim M-J, Lee J (2014) Evaluation of oxygen-limitation on lipid oxidation and moisture content in corn oil at elevated temperature. J Am Oil Chem Soc 91(3):439–444
Rajczykowski K, Loska K (2016) Heavy metals adsorption on aluminium oxide and stabilization of spent adsorbent using a SULSTAR technology. Arch Waste Manag Environ Prot 18(2):67–72
Rokosik E, Dwiecki K, Rudzińska M, Siger A, Polewski K (2019) Column chromatography as a method for minor components removal from rapeseed oil. Grasas Aceites 70(3):316
Rokosik E, Siger A, Rudzińska M, Siejak P, Dwiecki K (2020a) Formation of phospholipid association colloids in rapeseed oil and their effect on lipid autoxidation in the presence of Sinapic and Ferulic acid. Eur J Lipid Sci Technol 122(2):1900243
Rokosik E, Siger A, Rudzińska M, Dwiecki K (2020b) Antioxidant activity and synergism of canolol and α-tocopherol in rapeseed oil is affected by the presence of phospholipid association colloids. LWT 133:110095
Romero N, Robert P, Masson L, Ortiz J, González K, Tapia K (2007) Effect of α-tocopherol, α-tocotrienol and Rosa mosqueta shell extract on the performance of antioxidant-stripped canola oil (brassica sp.) at high temperature. Food Chem 104(1):383–389
Siger A, Kaczmarek A, Rudzińska M (2015) Antioxidant activity and phytochemical content of cold-pressed rapeseed oil obtained from roasted seeds: antioxidant activity and phytochemical content. Eur J Lipid Sci Technol 117(8):1225–1237
Siger A, Dwiecki K, Borzyszkowski W, Turski M, Rudzińska M, Nogala-Kałucka M (2017) Physicochemical characteristics of the cold-pressed oil obtained from seeds of Fagus sylvatica L. Food Chem 225:239–245
Subramanian R, Ichikawa S, Nakajima M, Kimura T, Maekawa T (2001) Characterization of phospholipid reverse micelles in relation to membrane processing of vegetable oils. Eur J Lipid Sci Technol 103:93–97
Verleyen T, Forcades M, Verhe R, Dewettinck K, Huyghebaert A, De Greyt W (2002) Analysis of free and esterified sterols in vegetable oils. J Am Oil Chem Soc 79(2):117–122
Wang C, Wang Z, Zhang X (2012) Amphiphilic building blocks for self-assembly: from Amphiphiles to supra-amphiphiles. Acc Chem Res 45(4):608–618
Xenakis A, Papadimitriou V, Sotiroudis TG (2010) Colloidal structures in natural oils. Curr Opin Colloid Interface Sci 15(1–2):55–60
Xie W-Q, Gong Y-X, Yu K-X (2017) Efficient quantification of water content in edible oils by headspace gas chromatography with vapour phase calibration: quantification of water in edible oils. J Sci Food Agric 98(8):3208–3212
Xu N, Shanbhag AG, Li B, Angkuratipakorn T, Decker EA (2019) Impact of phospholipid–tocopherol combinations and enzyme-modified lecithin on the oxidative stability of bulk oil. J Agric Food Chem 67(28):7954–7960
Acknowledgements
The authors very much appreciate the financial support of the National Science Centre, Poland, under grant NCN 2016/23/B/NZ9/02793.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dwiecki, K., Bąkowska, E. (2022). The Effects of Association Colloids on Lipid Autoxidation in Bulk Oils. In: Bravo-Diaz, C. (eds) Lipid Oxidation in Food and Biological Systems. Springer, Cham. https://doi.org/10.1007/978-3-030-87222-9_12
Download citation
DOI: https://doi.org/10.1007/978-3-030-87222-9_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-87221-2
Online ISBN: 978-3-030-87222-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)