Abstract
In this chapter, Dr. Khalifeh, Dr. Dibble, Dr. Dy, and Dr. Ray discuss the subspecialty of Peripheral Nerve. Surgery is a mainstay of treatment for common disorders of the peripheral nervous system, including compressive neuropathies, nerve tumors, peripheral nerve sources of pain, and paralyzing nerve injury. The management of peripheral nervous system injury has evolved significantly over the last century, facilitated by the development of macroscopic exploration and repair, microsurgical techniques, neurophysiologic monitoring, and biologic insights into mechanisms of nerve injury and regeneration. Nerve transfers represent a recent innovation in the reconstructive strategy for peripheral nerve injury, resulting in a marked improvement in clinical outcomes. A nerve transfer involves the distal transection and coaptation of a functioning, expendable donor nerve onto a non-functioning recipient nerve. It transforms a proximal nerve injury to a distal repair, taking advantage of the robust regeneration potential of the peripheral nervous system to allow for earlier reinnervation and quicker return of function in a paralyzed target muscle. More recently, nerve transfers can be modified and repurposed to treat neurologic deficits following injury to the central nervous system, including paralysis from spinal cord injury and stroke, with promising results.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Our understanding and management of peripheral nervous system pathology has evolved significantly over the twentieth and twenty-first centuries [1]. The earliest reports of nerve transfers were performed around 1900 as salvage procedures for brachial plexus avulsions. Seddon and Sunderland then moved the field forward with investigations of the pathobiology of nerve injuries in the 1940s and 1950s. The modern era began in the 1990s as lessons from nerve transfers and nerve grafting were combined with technological advances to increase the toolkit of peripheral nerve surgeons. Rational scientific inquiry through anatomical studies, laboratory experiments, and clinical observations, particularly during times of war, paved the way for an improved understanding of the peripheral nervous system [1]. Informed by biological insights and extensive clinical experiences, surgery for peripheral nerve injury has moved from conjectures of futility toward a more hopeful modern era, whereby patients can reasonably expect moderate recovery of function with surgical intervention [2].
The major milestones in the development of peripheral nerve surgery clinical practice include primary exploration, external neurolysis with/without neurorrhaphy, microsurgical interrogation, the use of intraoperative neurophysiologic monitoring, and internal neurolysis and interfascicular grafting [2]. In the last three decades, nerve transfers have emerged as a preferred treatment option for various peripheral nerve and brachial plexus injuries [3] (Fig. 64.1). In many cases, they show cost-effectiveness [4, 5] and improved motor outcomes as compared with in situ nerve repair and grafting techniques [6]. During a nerve transfer, a functional and redundant donor nerve is deliberately transected and transposed distally onto a paralyzed recipient nerve. By placing regenerating axons in proximity to the motor endplates, a proximal nerve injury is transformed to a distal repair, thereby reducing the regenerative distance and time required for muscle reanimation. This “rewiring” approach leverages both the peripheral nervous system’s unique capacity for long-distance regeneration and the central nervous system’s propensity for plasticity and cortical reorganization with rehabilitation to reestablish volitional control of paralyzed muscles.
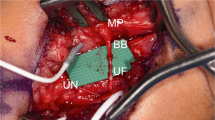
Transferring the distal pronator quadratus branch of the anterior interosseous nerve to the fascicular motor component of the ulnar nerve to reconstruct intrinsic hand muscles following ulnar nerve injury. Figure demonstrates the exposure of the distal median and ulnar nerves. (a) Shows a generous incision marked out to incorporate Guyon’s canal as well as the path of the distal median nerve. (b) Shows the exposure and identification of individual nerves, with the yellow loop on the left side around the nerve to the pronator quadratus, a terminal branch of the median nerve, and the white loop on the right side around the distal ulnar motor fascicle, the target recipient of a nerve transfer. (Clinical photographs copyright Christopher J. Dy, MD MPH (used with permission))
More recently, nerve transfers have been modified and creatively repurposed for applications of functional neurorestoration in the setting of central nervous system pathology [7]. This includes neurologic deficits resulting from chronic cerebral injury (e.g., stroke, traumatic brain injury, or cerebral palsy) [8, 9], traumatic spinal cord injury [10,11,12,13], or other paralyzing disorders affecting the upper and/or lower motor neurons (e.g., acute flaccid myelitis) [14, 15]. We have conducted a single-center prospective trial involving patients with cervical spinal cord injury and tetraplegia who underwent distal upper extremity nerve transfers for muscle reanimation, years and up to decades following the initial injury. In many cases, the patients recovered meaningful use of critical arm and hand movements, such as elbow extension, grasp, thumb opposition, and wrist/finger extension [11]. Our promising results have set the stage for the current, larger multicenter trial (ClinicalTrials.gov Identifier: NCT04023591).
The typical peripheral nerve neurosurgical practice comprises a wide variety of presenting conditions, including compressive neuropathies (e.g., carpal tunnel syndrome, peroneal nerve compression), benign and malignant nerve tumors (e.g., schwannoma, malignant peripheral nerve sheath tumors), peripheral nerve sources of pain (e.g., traumatic neuroma), and paralyzing nerve injuries (e.g., peripheral nerve injury, brachial plexus injury). Surgery is a mainstay of treatment for these conditions, with demonstrable patient benefits to quality of life, distal function, and, in certain conditions, overall survival. Emerging clinical applications targeting functional recovery, as detailed above, present opportunities for insights into neuroanatomy, neurophysiology, and neurorehabilitation. Major multidisciplinary efforts are being made to advance the basic and translational science of the field, and surgeons across the world continue to innovate and refine their surgical techniques. Peripheral nerve surgery will likely play an increasingly central role within paradigms of reconstruction and functional restoration following central and peripheral neurologic injury.
-
1.
Surgery is a mainstay of treatment for common disorders of the peripheral nervous system, including compressive neuropathies, nerve tumors, peripheral nerve sources of pain, and paralyzing nerve injury.
-
2.
The management of peripheral nervous system injury has evolved significantly over the last century, facilitated by the development of macroscopic exploration and repair, microsurgical techniques, neurophysiologic monitoring, and biologic insights into mechanisms of nerve injury and regeneration.
-
3.
Nerve transfers represent a recent innovation in the reconstructive strategy for peripheral nerve injury, resulting in a marked improvement in clinical outcomes.
-
4.
A nerve transfer involves the distal transection and coaptation of a functioning, expendable donor nerve onto a non-functioning recipient nerve. It transforms a proximal nerve injury to a distal repair, taking advantage of the robust regeneration potential of the peripheral nervous system to allow for earlier reinnervation and quicker return of function in a paralyzed target muscle.
-
5.
More recently, nerve transfers can be modified and repurposed to treat neurologic deficits following injury to the central nervous system, including paralysis from spinal cord injury and stroke, with promising results.
References
Friedman AH. An eclectic review of the history of peripheral nerve surgery. Neurosurgery. 2009;65(4 Suppl):A3–8.
Midha R, Grochmal J. Surgery for nerve injury: current and future perspectives. J Neurosurg. 2019;130(3):675–85.
Ray WZ, Chang J, Hawasli A, Wilson TJ, Yang L. Motor nerve transfers: a comprehensive review. Neurosurgery. 2016;78(1):1–26.
Wali AR, Park CC, Brown JM, Mandeville R. Analyzing cost-effectiveness of ulnar and median nerve transfers to regain forearm flexion. Neurosurg Focus. 2017;42(3):E11.
Khalifeh JM, Dibble CF, Dy CJ, Ray WZ. Cost-effectiveness analysis of combined dual motor nerve transfers versus alternative surgical and nonsurgical management strategies to restore shoulder function following upper brachial plexus injury. Neurosurgery. 2019;84(2):362–77.
Garg R, Merrell GA, Hillstrom HJ, Wolfe SW. Comparison of nerve transfers and nerve grafting for traumatic upper plexus palsy: a systematic review and analysis. J Bone Joint Surg Am. 2011;93(9):819–29.
Brown JM, Mahan MA, Mandeville R, Carter BS. Establishing reconstructive neurosurgery as a subspecialty. Neurosurg Focus. 2017;43(1):E7.
Spinner RJ, Shin AY, Bishop AT. Rewiring to regain function in patients with spastic hemiplegia. N Engl J Med. 2018;378(1):83–4.
Zheng MX, Hua XY, Feng JT, et al. Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. N Engl J Med. 2018;378(1):22–34.
Khalifeh JM, Dibble CF, Van Voorhis A, et al. Nerve transfers in the upper extremity following cervical spinal cord injury. Part 2: preliminary results of a prospective clinical trial. J Neurosurg Spine. 2019:1–13. https://doi.org/10.3171/2019.4.SPINE19399. Online ahead of print.PMID: 31299645
Khalifeh JM, Dibble CF, Van Voorhis A, et al. Nerve transfers in the upper extremity following cervical spinal cord injury. Part 1: systematic review of the literature. J Neurosurg Spine. 2019:1–12. https://doi.org/10.3171/2019.4.SPINE19173. Online ahead of print.PMID: 31299644
van Zyl N, Hill B, Cooper C, Hahn J, Galea MP. Expanding traditional tendon-based techniques with nerve transfers for the restoration of upper limb function in tetraplegia: a prospective case series. Lancet. 2019;394(10198):565–75.
Dibble CF, Khalifeh JM, VanVoorhis A, Rich JT, Ray WZ. Novel nerve transfers for motor and sensory restoration in high cervical spinal cord injury. World Neurosurg. 2019;128:611–5. e611.
Pino PA, Intravia J, Kozin SH, Zlotolow DA. Early results of nerve transfers for restoring function in severe cases of acute flaccid myelitis. Ann Neurol. 2019;86(4):607–15.
Wilks AW, Ray WZ, Al-Lozi MT, Bucelli RC. Nerve transfer as a novel treatment for West Nile virus-associated acute flaccid paralysis. J Neurol Sci. 2019;407:116502.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khalifeh, J.M., Dibble, C.F., Dy, C.J., Ray, W.Z. (2021). Rewiring the Peripheral Nervous System. In: Agarwal, N., Reddy, V. (eds) Surviving Neurosurgery. Springer, Cham. https://doi.org/10.1007/978-3-030-86917-5_64
Download citation
DOI: https://doi.org/10.1007/978-3-030-86917-5_64
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86916-8
Online ISBN: 978-3-030-86917-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)