Abstract
Despite best efforts to prevent or slow down diabetic kidney disease (DKD), many cases progress to advanced stages and require kidney replacement therapy. Diabetes mellitus is the most common cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD). Kidney transplantation (KT) with or without a pancreas transplant is the preferred treatment in both type 1 and type 2 diabetes patients with advanced kidney disease. Kidney transplantation combined with or without pancreas transplant is associated with better survival outcomes than either peritoneal or hemodialysis.
Transplant options for diabetic patients include kidney transplant alone in ESRD patients with type 2 diabetes mellitus (DM) and combined kidney-pancreas transplantation (simultaneous or sequential) predominantly in type 1 DM patients or rarely in some insulin-dependent type 2 DM patients with ESRD. Despite increased short-term morbidity and mortality, pancreas transplantation yields better long-term outcomes, including overall survival. Preemptive pancreas transplant in type 1 DM may help prevent or ameliorate diabetic kidney disease (DKD). Either simultaneous pancreas-kidney transplantation or pancreas after KT compared to KT alone (either living donor or deceased donor) has a similar benefit on the course of DKD. Simultaneous pancreas-kidney transplantation may be appropriate for selected type 2 DM patients when a living kidney donor is not an option. Pancreas and kidney transplantation seems to be the treatment of choice. For most type 1 and selected type 2 DKD patients in the advanced stages, a kidney transplant with or without pancreas transplant is the best renal replacement option. Pancreatic islet cell transplantation, an emerging alternative, is a nonsurgical procedure not yet widely available due to the limited supply of donor human islet cells and inefficient islet engraftment. Ongoing research may help broaden its availability and may prevent the development of kidney disease and other microvascular complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Kidney transplant
- Pancreas transplant
- Pancreatic islet cell transplant
- Diabetic kidney disease
- Posttransplant diabetes mellitus
Introduction
Diabetes is a global health emergency, with 425 million people affected in 2017 and a projection for 629 million by 2045. Nearly half develop diabetic kidney disease, and its prevalence is rising progressively in parallel with the overall diabetes epidemic, primarily driven by type 2 diabetes [1]. In a recent report based on data from 142 countries, the global percentage of the prevalent end-stage renal disease (ESRD) patients with diabetes increased from 19.0% in 2000 to 29.7% in 2015 worldwide, while the percentage of incident ESRD patients due to diabetes increased from 22.1% to 31.3% [2]. Type 2 DM is now the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide [3] and accounts for nearly 95% of all cases of DKD [4]. According to the 2020 United States Renal Data System (USRDS), prevalent ESRD among all patients with a diagnosis of DM exceeded 300,000 in 2018 in the USA, representing ~38% of all patients on dialysis [5]. Similarly, data from the Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) show that nearly 40% of patients on transplant waiting list in the USA had DM in 2019 [6] (see Fig. 19.1).
Distribution of adults waiting for a kidney transplant by diagnosis. Candidates waiting for transplant at any time in the given year. Candidates listed at more than one center are counted once per listing. Active and inactive patients are included [6]
Type 2 DM is a major risk factor for the development of cardiovascular (CV) and kidney disease and is responsible for a significant number of hospitalizations, morbidity, and mortality. Kidney transplant has emerged as the preferred mode of renal replacement for ESRD, including patients with diabetic kidney disease. Transplant provides both better quality of life and survival advantage compared to dialysis [7, 8]. For example, receiving a deceased donor kidney increases a patient’s chances of survival by twofold and a living-donor graft by fourfold compared to those who remain on the waiting list [9]. In an earlier analysis, transplant increased the projected life expectancy in kidney transplant recipients by 10 years compared with those who remained on dialysis [8].
Kidney and/or pancreatic transplantation has now proved to be the treatment of choice for those patients. Kidney and pancreas transplantation not only solves the problem of organ failure but also achieves insulin independence and reverses the metabolic complications of diabetes. Combined kidney and pancreas transplantation has the best long-term outcome in patients with advanced or end-stage kidney disease [7].
In the past, pancreatic transplant was not offered to type 2 DM patients. However, as later data showed that simultaneous pancreas-kidney (SPK) transplant has resulted in similar outcomes in both type 1 and type 2 DM patients, there is increasing acceptance of type 2 DKD patients for this modality. Still, pancreas transplant is rarely offered to type 2 DM patients; the rate of pancreas transplant in type 2 DM patients increased from 2% in 1995 to only 7% in 2010 [10]. There was some further modest increase after the 2014 revision in the pancreas allocation system (PAS) (see Fig. 19.2). According to the Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients (OPTN/SRTR) 2019 annual report, the total number of pancreas transplants in 2019 was 1015, slightly lower than the previous year, but remained somewhat flat for the past 5 years [11]. Most of these involved simultaneous pancreas-kidney (SPK) transplants followed by pancreas-after-kidney (PAK) and pancreas transplant alone (PTA) [11].
Deceased donor pancreas transplant rates among adult wait-list candidates by diagnosis. Transplant rates are computed as the number of deceased donor transplants per 100 patient-years of wait time in a given year. Individual listings are counted separately [11]
Although the recently introduced agents, mainly the SGLT2 inhibitors, raise the expectations that they will further slow the progression of DKD to advanced stages, there will still be a need to implement renal replacement for many patients. The purpose of this chapter is to briefly outline the transplant options for patients with advanced or end-stage diabetic kidney disease.
Transplant Options for Patients with Diabetic Kidney Disease
Diabetic kidney disease represents an increasing percentage of chronic kidney disease populations worldwide. The demand for renal replacement therapy is also on the rise as cases of diabetes have reached epidemic proportions [1, 2, 6]. Kidney transplantation has emerged as the clearly superior alternative for all ESRD of any etiology, especially for DKD, which carries a higher CVD risk and other comorbidities [1, 12,13,14]. Kidney transplantation is now an established modality and becoming increasingly available, with nearly 300,000 transplants performed since 1970 [9]. However, demand remains high such that barely a quarter of patients on the wait list receive a deceased donor kidney transplant within 5 years [6]. Although there is a recent trend toward a slightly increased availability of living-related donor kidneys, only a small fraction of patients benefit from this alternative [6].
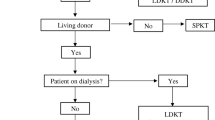
Transplant options (Fig. 19.3) include kidney transplant alone, living or deceased donor (KT), simultaneous pancreas and kidney (SPK) transplant, and pancreas-after-kidney (PAK) transplant [7, 15, 16]. These treatment options generally offer markedly superior survival benefits for ESRD patients, including those with diabetic kidney disease. One-year KT survival in diabetic patients is now near 90% for deceased donors (DD) and 96% for living donors (LD) [7]. Pancreas transplantation has become increasingly successful in recent years due to advances in surgical outcomes and immunosuppression protocols [16,17,18,19]. One-year pancreas graft survival is now nearly 95% when performed as a simultaneous pancreas-kidney (SPK) transplant and 86% when performed as a pancreas after KT (PAK). In one single center, mortality risk for diabetic patients was two- to threefold lower in those who received a pancreas transplant [6, 7, 20]. Other centers report similarly successful long-term outcomes [21, 22].
Options for kidney transplant for patients with diabetic kidney disease. Adapted from A.C. Wiseman [16]. (KT kidney transplantation, DD deceased donor, LRD living-related donor, SPKT simultaneous pancreas-kidney transplantation, LDKT living-related kidney transplant, PAKT pancreas after kidney transplant)
During the early years of transplantation, DKD was considered a relative contraindication for transplant because of the higher cardiovascular risk and obesity. This attitude has now reversed since Wolfe et al. demonstrated that renal transplantation provided a marked survival advantage for diabetic ESRD patients and reduced mortality by 73% compared with patients remaining on the wait list. The projected life expectancy was more pronounced for younger diabetics (presumed type 1 DM) with a gain of 17 years, and the gain was also significant even for patients older than 60 years (presumed type 2 DM) [8, 13]. Long-term follow-up analyses confirm superior outcomes and significant survival benefit for ESRD patients associated with type 1 DM [23].
The evidence supports that preemptive transplant is superior to dialysis or to transplant after initiation of dialysis and results in improved recipient survival [1, 3, 24]. Patients in the USA qualify for kidney transplant listing when their glomerular filtration rate (GFR) is <20 ml/min or when they have initiated maintenance dialysis. Despite the distinct advantages of preemptive transplant, the rates remain disappointingly low in the USA [25]. Lack of clear guidelines on the timing of referral, pre-dialysis patient education, and socioeconomic factors are among the key barriers [25].
Transplant programs have considered obesity [body mass index (BMI) >30–35 kg/m2] as a relative contraindication for transplantation in diabetic patients because of inferior outcomes for both KT and SPK transplant, mainly due to surgical complications. Some centers view only morbid obesity (BMI >40 kg/m2) as an absolute contraindication. Recent advances in bariatric surgery can ameliorate this contraindication and make even obese type 2 DM patients eligible for transplantation [13].
Patient Selection and Kidney Transplant
For optimal outcomes, a careful pre-transplant evaluation and risk screening are essential. Each modality has its advantages and disadvantages depending on patient selection (Table 19.1). As the waiting list for kidney transplantation continues to grow, the need for selecting appropriate candidates for transplant becomes fundamental. To maximize the success rates of transplant, a careful review and evaluation of coexisting medical and psychosocial comorbidities should be performed to intervene, if possible, before the procedure [26].
It is important to evaluate patients carefully for contraindications including recent or active malignancy, active infection, advanced atherosclerotic cardiac and vascular disease, alcohol-drug dependence, psychiatric disease, and morbid obesity [7, 13]. If significant coronary artery disease is present, transplantation can still proceed after appropriate therapy, which may include coronary artery revascularization. As noted above, severe obesity is no longer an absolute contraindication. Morbidly obese patients can become eligible for transplant after bariatric surgery [13].
The current guidelines suggest that transplant candidates should be evaluated carefully and in an unbiased multidisciplinary setting, involving physicians, surgeons, psychologists, social workers, financial counselors, and dietitians, and sometimes the patients. This process may take considerable resources and time depending on the extent of testing needed for each patient. At the end of the evaluation, patients should be discussed at a multidisciplinary Selection Committee for a consensus agreement on the final listing [27].
As the demand for kidney transplant is rising, there is a shortage of available kidneys [6]. Living donation accounts for one-third of kidney transplants performed in the USA, showing a remarkable increase in the annual number of living donors from 1988 to 2004, although there is a recent trend toward a decline [28,29,30]. Family members have usually been the main source of living donations, although unrelated donations from friends and coworkers have recently increased. Altruistic anonymous donations from strangers are also increasing. Potential living donors need a comprehensive and cautious evaluation to minimize the risks in a healthy altruistic donor who is willing to undertake a major surgical procedure to help another [31]. Kidney paired donation, a national United Network for Organ Sharing (UNOS)-sponsored swapping of incompatible donors, has facilitated multiple living donor transplants, but the impact on the number of transplants has been modest [6, 28, 29].
Pancreas Transplantation
The first human pancreas transplant was performed in 1966 by Dr. Lillehei at the University of Minnesota [32]. The procedure was performed simultaneously with a kidney transplant in a young female with diabetic kidney disease. Unfortunately, the patient could remain insulin-free for only a few weeks. Although other pancreas transplants were performed over the next few years, the success rates were initially low. But later improvements in surgical techniques, immunosuppressive medications, and organ donor management have allowed pancreatic transplantation to become a well-accepted and commonly performed procedure [21].
Pancreas transplantation in conjunction with kidney transplantation, either simultaneously or after kidney transplantation, has proved valuable especially for DKD patients with type 1 DM and for some type 2 patients as well [7, 17, 33]. Pancreas transplant alone in type 1 DM patients before the onset of kidney disease may be particularly helpful in preventing kidney disease and other microvascular complications of diabetes and avoid the need for renal replacement [34, 35]. Based on 2004 to 2015 data, patient survival rates for SPK, PAK, or PTA ranged from 96 to 99% at 1 year, 89 to 91% at 5 years, and 70 to 80% at 10 years postoperatively [20].
Pancreatic transplantation can achieve improvements in metabolic disorders, including glucose and glucagon metabolism. Secondary complications of diabetes also show improvement, including improvement of left ventricular function and reversal of diastolic dysfunction [36]. Improvements in DKD [37], peripheral and autonomic diabetic neuropathy, possible diabetic retinopathy [37, 38], and serum triglyceride and low-density lipoproteins are also among the expected benefits [39].
Simultaneous pancreas-kidney (SPK) transplant initially carries a high mortality risk relative to living donor kidney recipients through 18 months posttransplantation, likely related to the surgical procedure complications. But the risk improved after the early postoperative period with better long-term outcomes [40]. A UNOS database review of all adult pancreas and kidney-pancreas transplants between 1996 and 2012 showed that graft survival was the best in adults 40–49 years of age [40].
Indications for Pancreas Transplants
The most common indication for a pancreatic transplant is insulin-dependent diabetes mellitus (IDDM). In most cases, patients have classic type 1 diabetes mellitus, an autoimmune disease with the presence of anti-insulin or anti-islet cell antibodies. Patients who develop IDDM from previous pancreatic resections or chronic pancreatitis have also received pancreas or islet cell transplants [41,42,43]. Many of these patients will have complications of IDDM, including hypoglycemic unawareness, diabetic ketoacidosis, as well as other organ sequelae such as kidney disease, retinopathy, and neuropathy [38, 39].
In the past, type 2 diabetes mellitus was considered a contraindication for pancreatic transplant, despite its proven success in type 1 diabetics. The presence of considerable overlap of clinical presentation of both types especially in the setting of renal failure, over-reliance on the presence of detectable C peptide, which is no longer considered reliable in determining DM type, and incomplete understanding of the pathogenesis were probably the main barriers [44]. Recently, there has been recognition of adult-onset diabetes that is insulin responsive [45,46,47]. Although these patients may previously have been characterized as type 2 diabetics, they show features of type 1 patients. They often are not obese, and they develop ketoacidosis and retinopathy. Some have even demonstrated late onset of insulin antibody development. Syndromes such as latent autoimmune diabetes in adults (LADA) or maturity-onset diabetes of the young (MODY) fall in this category [1, 45,46,47,48]. Such patients were previously classified as type 1½ diabetics, but recognition of these syndromes would allow these patients to benefit from a pancreas transplant as well. These diabetes variants clinically behave similarly to type 1 diabetes and benefit from pancreas or islet cell transplantation. There is growing evidence that these specific categories of type 2 diabetes patients with overlapping features of type 1 diabetes may benefit from a pancreas and kidney transplant. Increasing numbers of transplants are now offered to such patients [13, 41, 49, 50].
In most instances, pancreas transplants are performed in conjunction with a kidney transplant, either simultaneous (SPK) or pancreas after kidney (PAK), with good success rates [13, 33, 51]. The presence of diabetic renal disease with a GFR of less than 20 mL/min/1.73 m2 or with the need to initiate dialysis is an indication for a kidney transplant as well.
The workup for a transplant candidate is exhaustive and like that of the kidney transplant recipient (see above) may consume considerable time and effort. Identification and management of the various sequelae of diabetes before the planned surgery are essential to minimize the risk of perioperative complications, including graft failure, infection, and death. In most centers, candidates are usually younger (<50 years of age) and non-obese (BMI <30). Results of pancreas transplants have not been as good in older or obese patients [52, 53].
Pancreatic Islet Cell Transplantation
Pancreas alone or islet cell transplant has emerged as another option for type 1 diabetics or MODY or LADA cases without renal disease. Successful pancreas transplant or beta islet cell replacement achieves excellent glycemic control and prevents the microvascular complications of diabetes including retinopathy, neuropathy, and kidney disease [34, 35, 54, 55]. There has been a long-standing interest in islet cell replacement since the turn of the nineteenth century, but the modality has not been clinically feasible until the development of the Edmonton Protocol in 2000 [56]. The harvested cells are transplanted via the portal vein and engraft in the liver and can achieve insulin independency. However, many challenges remain. Often, repeat islet cell infusions are necessary. Harvesting adequate numbers of cells is inefficient and often requires multiple donors. In the case of non-autologous transplants, immune reactivity and the need for anti-rejection treatment may be a problem [11, 56, 57]. There is ongoing active research in multiple fronts, including genetically modified islet cells, encapsulating islet cells in protected lattices, xenotransplants using genetically modified porcine cells, or using pluripotent stem cells [56, 58, 59]. With continued progress in this non-surgical technique, we can imagine a breakthrough in the treatment of diabetes and preventing its devastating complications including kidney disease.
Posttransplant Diabetes Mellitus (PTDM)
A major complication of kidney transplantation is the development of posttransplant posttransplant diabetes mellitus, which poses an important risk factor for cardiovascular disease and other diabetic complications, including kidney disease after transplantation [60, 61]. New onset diabetes mellitus in the posttransplantation setting (PTDM), regardless of the timing of detection or whether it was present undetected prior to transplantation or not, develops in 10–40% of patients [62,63,64] (see Fig. 19.4).
Posttransplant diabetes among adult kidney transplant recipients. Percentage of adult deceased donor kidney recipients who were nondiabetic at transplant and developed diabetes posttransplant. Posttransplant diabetes is reported on the Transplant Recipient Follow-up form. Death and graft failure are treated as competing events [6]
Multiple factors contribute to the increased risk of PTDM. Immunosuppressive medications including steroids, calcineurin inhibitors, and mammalian target of rapamycin inhibitors are the main offenders. Higher doses of steroids have been associated with increased risk of PTDM. Both tacrolimus and cyclosporine also can increase the risk of PTDM, with a higher risk associated with tacrolimus than cyclosporine [64]. Other factors predisposing to PTDM include pre-transplant impaired glucose tolerance [65], obesity [66], hypomagnesemia [67], increased age (≥40 to 45 years), African American race, and deceased donor kidney transplantation [63, 65, 66, 68].
Posttransplantation diabetes mellitus (PTDM) leads to increased rates of cardiovascular disease mortality [68, 69], graft rejection, and decreased survival. Diabetic complications, such as ketoacidosis, hyperosmolar hyperglycemic state, neuropathy, diabetic kidney disease, and infection, can also occur [68, 70]. Often glycemic control can be achieved successfully using oral agents, especially dipeptidyl peptidase-4 (DPP-4) [71, 72]. Similarly, a recent meta-analysis showed that SGLT2 inhibitors effectively lowered HbA1c, reduced body weight, and helped preserve kidney function in transplant patients with PTDM and good kidney function without adverse events [73]. Optimal glycemic control and cardiovascular risk management improved outcomes markedly since 1996 [70, 74].
Summary and Conclusions
When measures to forestall kidney disease fail and patients reach advanced stages requiring renal replacement therapy, transplantation is distinctly superior to either peritoneal or hemodialysis. Transplant options include deceased donor or living donor kidney or combined pancreas and kidney transplant (simultaneous or pancreas after kidney transplantation). Simultaneous pancreas-kidney transplant replaces kidney function and corrects the underlying metabolic disorder and affords the best survival advantage in the long run despite an initial increase in postsurgical mortality. Pancreas transplant alone is an option for type 1 and other forms of insulin-dependent diabetes patients and can prevent serious microvascular complications of diabetes, including kidney disease. Pancreatic islet cell transplantation is a nonsurgical technique with various configurations in experimental stages that promise optimal insulin independence but is not yet widely available clinically.
References
Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–45.
Cheng H-T, Xu X, Lim PS, Hung K-Y. Worldwide epidemiology of diabetes-related end-stage renal disease, 2000-2015. Diabetes Care. 2021;44(1):89–97.
Steddon SC, Cunningham J, Ashman N. Oxford handbook of nephrology and hypertension. 2d ed. Oxford: Oxford University Press; 2014.
Duru OK, Middleton T, Tewari MK, Norris K. The landscape of diabetic kidney disease in the United States. Curr Diab Rep. 2018;18(3):14.
System USRD: 2020 USRDS Annual Data Report: Epidemiology of kidney disease in the United States. In. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
Hart A, Lentine KL, Smith JM, Miller JM, Skeans MA, Prentice M, Robinson A, Foutz J, Booker SE, Israni AK, et al. OPTN/SRTR 2019 annual data report: kidney. Am J Transplant. 2021;21(Suppl 2):21–137.
Perez-Saez MJ, Pascual J. Kidney transplantation in the diabetic patient. J Clin Med. 2015;4(6):1269–80.
Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341(23):1725–30.
Rana A, Godfrey EL. Outcomes in solid-organ transplantation: success and stagnation. Tex Heart Inst J. 2019;46(1):75–6.
Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2011;8(1):6–16.
Kandaswamy R, Stock PG, Miller J, Skeans MA, White J, Wainright J, Kyaw NTT, Niederhaus S, Israni AK, Snyder JJ. OPTN/SRTR 2019 annual data report: pancreas. Am J Transplant. 2021;21(Suppl 2):138–207.
Einollahi B, Rostami Z. Favorable survival rate after kidney transplantation in diabetic patients. Saudi J Kidney Dis Transpl. 2014;25(3):655–6.
Fourtounas C. Transplant options for patients with type 2 diabetes and chronic kidney disease. World J Transplant. 2014;4(2):102–10.
Guerra G, Ilahe A, Ciancio G. Diabetes and kidney transplantation: past, present, and future. Curr Diab Rep. 2012;12(5):597–603.
Wiseman AC. The role of kidney-pancreas transplantation in diabetic kidney disease. Curr Diab Rep. 2010;10(5):385–91.
Wiseman AC. Kidney transplant options for the diabetic patient. Transplant Rev (Orlando). 2013;27(4):112–6.
Thwaites SE, Gurung B, Yao J, Kable K, Robertson P, Ryan BJ, Lam VW, Pleass HC, Chapman JR, Hawthorne WJ, et al. Excellent outcomes of simultaneous pancreas kidney transplantation in patients from rural and urban Australia: a national service experience. Transplantation. 2012;94(12):1230–5.
van Dellen D, Worthington J, Mitu-Pretorian OM, Ghazanfar A, Forgacs B, Pararajasingam R, Campbell B, Parrott NR, Augustine T, Tavakoli A. Mortality in diabetes: pancreas transplantation is associated with significant survival benefit. Nephrol Dial Transplant. 2013;28(5):1315–22.
Wiseman AC. Pancreas transplant options for patients with type 1 diabetes mellitus and chronic kidney disease: simultaneous pancreas kidney or pancreas after kidney? Curr Opin Organ Transplant. 2012;17(1):80–6.
Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Curry MA, Prentice MA, Fox A, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2016 annual data report: pancreas. Am J Transplant. 2018;18(Suppl 1):114–71.
Gruessner AC. Update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR). Rev Diabet Stud. 2011;2011(8):6–16.
Laftavi MR, Pankewycz O, Gruessner A, Brian M, Kohli R, Feng L, Said M, Sharma R, Patel S. Long-term outcomes of pancreas after kidney transplantation in small centers: is it justified? Transplant Proc. 2014;46(6):1920–3.
Esmeijer K, Hoogeveen EK, van den Boog PJM, Konijn C, Mallat MJK, Baranski AG, Dekkers OM, de Fijter JW, Dutch Transplant C. Dutch kidney transplant C: superior long-term survival for simultaneous pancreas-kidney transplantation as renal replacement therapy: 30-year follow-up of a Nationwide cohort. Diabetes Care. 2020;43(2):321–8.
Zelniker TA, Braunwald E. Cardiac and renal effects of sodium-glucose co-transporter 2 inhibitors in diabetes: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(15):1845–55.
Helmick RA, Jay CL, Price BA, Dean PG, Stegall MD. Identifying barriers to preemptive kidney transplantation in a living donor transplant cohort. Transplant Direct. 2018;4(4):e356.
Steinman TI, Becker BN, Frost AE, Olthoff KM, Smart FW, Suki WN, Wilkinson AH. Clinical practice committee ASoT: guidelines for the referral and management of patients eligible for solid organ transplantation. Transplantation. 2001;71(9):1189–204.
optn.transplant.hrsa.gov: Educational Guidance on Patient Referral to Kidney Transplantation. In.; 2015.
Gill J, Joffres Y, Rose C, Lesage J, Landsberg D, Kadatz M, Gill J. The change in living kidney donation in women and men in the United States (2005-2015): a population-based analysis. J Am Soc Nephrol. 2018;29(4):1301–8.
Goldfarb DA. Re: the decline in living kidney donation in the United States: random variation or cause for concern? J Urol. 2014;192(2):499–500.
Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe RD, Baliga P. American society of T: living donor kidney transplantation: overcoming disparities in live kidney donation in the US–recommendations from a consensus conference. Clin J Am Soc Nephrol. 2015;10(9):1687–95.
Wright L, Faith K, Richardson R, Grant D. Joint Centre for Bioethics UoTTO: ethical guidelines for the evaluation of living organ donors. Can J Surg. 2004;47(6):408–13.
Kelly WD, Lillehei RC, Merkel FK, Idezuki Y, Goetz FC. Allotransplantation of the pancreas and duodenum along with the kidney in diabetic nephropathy. Surgery. 1967;61(6):827–37.
Lindahl JP, Hartmann A, Horneland R, Holdaas H, Reisaeter AV, Midtvedt K, Leivestad T, Oyen O, Jenssen T. Improved patient survival with simultaneous pancreas and kidney transplantation in recipients with diabetic end-stage renal disease. Diabetologia. 2013;56(6):1364–71.
Boggi U, Rosati CM, Marchetti P. Follow-up of secondary diabetic complications after pancreas transplantation. Curr Opin Organ Transplant. 2013;18(1):102–10.
Lindahl JP, Jenssen T, Hartmann A. Long-term outcomes after organ transplantation in diabetic end-stage renal disease. Diabetes Res Clin Pract. 2014;105(1):14–21.
Fiorina P, La Rocca E, Astorri E, Lucignani G, Rossetti C, Fazio F, Giudici D, di Carlo V, Cristallo M, Pozza G, et al. Reversal of left ventricular diastolic dysfunction after kidney-pancreas transplantation in type 1 diabetic uremic patients. Diabetes Care. 2000;23(12):1804–10.
Boggi U, Vistoli F, Amorese G, Giannarelli R, Coppelli A, Mariotti R, Rondinini L, Barsotti M, Piaggesi A, Tedeschi A, et al. Results of pancreas transplantation alone with special attention to native kidney function and proteinuria in type 1 diabetes patients. Rev Diabet Stud. 2011;8(2):259–67.
Koznarova R, Saudek F, Sosna T, Adamec M, Jedinakova T, Boucek P, Bartos V, Lanska V. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant. 2000;9(6):903–8.
Biesenbach G, Biesenbach P, Bodlaj G, Pieringer H, Schmekal B, Janko O, Margreiter R. Impact of smoking on progression of vascular diseases and patient survival in type-1 diabetic patients after simultaneous kidney-pancreas transplantation in a single Centre. Transpl Int. 2008;21(4):357–63.
Siskind E, Maloney C, Akerman M, Alex A, Ashburn S, Barlow M, Siskind T, Bhaskaran M, Ali N, Basu A, et al. An analysis of pancreas transplantation outcomes based on age groupings--an update of the UNOS database. Clin Transpl. 2014;28(9):990–4.
Hau HM, Jahn N, Brunotte M, Lederer AA, Sucher E, Rasche FM, Seehofer D, Sucher R. Short and long-term metabolic outcomes in patients with type 1 and type 2 diabetes receiving a simultaneous pancreas kidney allograft. BMC Endocr Disord. 2020;20(1):30.
Stites E, Kennealey P, Wiseman AC. Current status of pancreas transplantation. Curr Opin Nephrol Hypertens. 2016;25(6):563–9.
Wojtusciszyn A, Branchereau J, Esposito L, Badet L, Buron F, Chetboun M, Kessler L, Morelon E, Berney T, Pattou F, et al. Indications for islet or pancreatic transplantation: statement of the TREPID working group on behalf of the Societe francophone du diabete (SFD), Societe francaise d'endocrinologie (SFE), Societe francophone de transplantation (SFT) and Societe francaise de nephrologie - dialyse - transplantation (SFNDT). Diabetes Metab. 2019;45(3):224–37.
Esmatjes E, Fernandez C, Rueda S, Nicolau J, Chiganer G, Ricart MJ, Junca E, Fernandez-Cruz L. The utility of the C-peptide in the phenotyping of patients candidates for pancreas transplantation. Clin Transpl. 2007;21(3):358–62.
Buzzetti R, Zampetti S, Maddaloni E. Adult-onset autoimmune diabetes: current knowledge and implications for management. Nat Rev Endocrinol. 2017;13(11):674–86.
Grill V. LADA: a type of diabetes in its own right? Curr Diabetes Rev. 2019;15(3):174–7.
Hals IK. Treatment of latent autoimmune diabetes in adults: what is best? Curr Diabetes Rev. 2019;15(3):188–93.
Garrahy A, Mijares Zamuner MB, Byrne MM. An evolving spectrum of diabetes in a woman with GCK-MODY. Endocrinol Diabetes Metab Case Rep. 2019;2019.
Orlando G, Stratta RJ, Light J. Pancreas transplantation for type 2 diabetes mellitus. Curr Opin Organ Transplant. 2011;16(1):110–5.
Chan CM, Chim TM, Leung KC, Tong CH, Wong TF, Leung GK. Simultaneous pancreas and kidney transplantation as the standard surgical treatment for diabetes mellitus patients with end-stage renal disease. Hong Kong Med J. 2016;22(1):62–9.
Fouzas I, Antoniadis N, Giakoustidis D, Tatsou N, Mouloudi E, Karapanagiotou A, Sklavos A, Tsitlakidis A, Karakatsanis A, Myserlis G, et al. Simultaneous pancreas-kidney transplantation: initial results from a center in Greece. Transplant Proc. 2012;44(9):2712–4.
Eller K, Kniepeiss D, Rosenkranz AR. Preoperative risk evaluation: where is the limit for recipients of a pancreatic graft? Curr Opin Organ Transplant. 2013;18(1):97–101.
Laurence JM, Marquez MA, Bazerbachi F, Seal JB, Selzner M, Norgate A, McGilvray ID, Schiff J, Cattral MS. Optimizing pancreas transplantation outcomes in obese recipients. Transplantation. 2015;99(6):1282–7.
Guerra J, Melo MJ, Goncalves JA, Nascimento C, Santana A, da Costa AG. Renal transplantation in type 1 diabetes mellitus: an unusual case report. Transplant Proc. 2015;47(4):1042–4.
He Y, Xu Z, Zhou M, Wu M, Chen X, Wang S, Qiu K, Cai Y, Fu H, Chen B, et al. Reversal of early diabetic nephropathy by islet transplantation under the kidney capsule in a rat model. J Diabetes Res. 2016;2016:4157313.
Gamble A, Pepper AR, Bruni A, Shapiro AMJ. The journey of islet cell transplantation and future development. Islets. 2018;10(2):80–94.
Aref A, Zayan T, Pararajasingam R, Sharma A, Halawa A. Pancreatic transplantation: brief review of the current evidence. World J Transplant. 2019;9(4):81–93.
Griffin TP, Martin WP, Islam N, O'Brien T, Griffin MD. The promise of mesenchymal stem cell therapy for diabetic kidney disease. Curr Diab Rep. 2016;16(5):42.
Nakamura T, Fujikura J, Inagaki N. Advancements in transplantation therapy for diabetes: pancreas, islet and stem cell. J Diabetes Investig. 2021;12(2):143–5.
Rysz J, Franczyk B, Radek M, Cialkowska-Rysz A, Gluba-Brzozka A. Diabetes and cardiovascular risk in renal transplant patients. Int J Mol Sci. 2021:22(7).
Schachtner T, Stein M, Reinke P. Diabetic kidney transplant recipients: impaired infection control and increased alloreactivity. Clin Transpl. 2017:31(7).
Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4(4):583–95.
Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178–85.
Sharif A, Hecking M, de Vries AP, Porrini E, Hornum M, Rasoul-Rockenschaub S, Berlakovich G, Krebs M, Kautzky-Willer A, Schernthaner G, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14(9):1992–2000.
Klangjareonchai T, Eguchi N, Tantisattamo E, Ferrey AJ, Reddy U, Dafoe DC, Ichii H. Current pharmacological intervention and medical management for diabetic kidney transplant recipients. Pharmaceutics. 2021:13(3).
Nagib AM, Elsayed Matter Y, Gheith OA, Refaie AF, Othman NF, Al-Otaibi T. Diabetic nephropathy following Posttransplant diabetes mellitus. Exp Clin Transplant. 2019;17(2):138–46.
Alamdari A, Asadi G, Minoo FS, Khatami MR, Gatmiri SM, Dashti-Khavidaki S, Heydari Seradj S, Naderi N. Association between pre-transplant Magnesemia and post-transplant Dysglycemia in kidney transplant recipients. Int J Endocrinol Metab. 2020;18(1):e97292.
Miles AM, Sumrani N, Horowitz R, Homel P, Maursky V, Markell MS, Distant DA, Hong JH, Sommer BG, Friedman EA. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998;65(3):380–4.
Boudreaux JP, McHugh L, Canafax DM, Ascher N, Sutherland DE, Payne W, Simmons RL, Najarian JS, Fryd DS. The impact of cyclosporine and combination immunosuppression on the incidence of posttransplant diabetes in renal allograft recipients. Transplantation. 1987;44(3):376–81.
Keddis MT, El Ters M, Rodrigo E, Dean P, Wohlfahrtova M, Kudva YC, Lorenz EC, Cosio FG. Enhanced posttransplant management of patients with diabetes improves patient outcomes. Kidney Int. 2014;86(3):610–8.
Abdelaziz TS, Ali AY, Fatthy M. Efficacy and safety of dipeptidyl Peptidase-4 inhibitors in kidney Transplant recipients with post-transplant diabetes mellitus (PTDM)- a systematic review and meta-analysis. Curr Diabetes Rev. 2020;16(6):580–5.
Haidinger M, Werzowa J, Hecking M, Antlanger M, Stemer G, Pleiner J, Kopecky C, Kovarik JJ, Doller D, Pacini G, et al. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation--a randomized, double-blind, placebo-controlled trial. Am J Transplant. 2014;14(1):115–23.
Chewcharat A, Prasitlumkum N, Thongprayoon C, Bathini T, Medaura J, Vallabhajosyula S, Cheungpasitporn W. Efficacy and safety of SGLT-2 inhibitors for treatment of diabetes mellitus among kidney Transplant patients: a systematic review and meta-analysis. Med Sci (Basel). 2020:8(4).
Kim YC, Shin N, Lee S, Hyuk H, Kim YH, Kim H, Park SK, Cho JH, Kim CD, Ha J, et al. Effect of post-transplant glycemic control on long-term clinical outcomes in kidney transplant recipients with diabetic nephropathy: a multicenter cohort study in Korea. PLoS One. 2018;13(4):e0195566.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Giusti, S., Batuman, V. (2022). Kidney Transplantation and Kidney Pancreas Transplantation. In: Lerma, E.V., Batuman, V. (eds) Diabetes and Kidney Disease. Springer, Cham. https://doi.org/10.1007/978-3-030-86020-2_19
Download citation
DOI: https://doi.org/10.1007/978-3-030-86020-2_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-86019-6
Online ISBN: 978-3-030-86020-2
eBook Packages: MedicineMedicine (R0)