Abstract
-
Introduction: Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has recently and rapidly emerged and developed into a global pandemic. Through the renin–angiotensin system, the virus may impact the lung circulation, but the expression on endothelium may conduct to its activation and further systemic damage. While precise mechanisms underlying these phenomena remain to be further clarified, the understanding of the disease, its clinical course, as well as its immunological and hematological implications is of paramount importance in this phase of the pandemic.
-
Methods: This review summarizes the evidence gathered until 12 June; electronic databases were screened for pertinent reports on coronavirus and inflammatory and hematological changes. Search was conducted by two independent investigators; keywords used were “SARS-CoV-2,” “COVID-19,” “inflammation,” “immunological,” and “therapy.”
-
Results: The viral infection is able to trigger an excessive immune response in predisposed individuals, which can result in a “cytokine storm” that presents an hyperinflammation state able to determine tissue damage and vascular damage. An explosive production of proinflammatory cytokines such as TNF-α IL-1β and others occurs, greatly exaggerating the generation of molecule-damaging reactive oxygen species. These changes are often followed by alterations in hematological parameters. Elucidating those changes in SARS-CoV-2-infected patients could help to understand the pathophysiology of disease and may provide early clues to diagnosis. Several studies have shown that hematological parameters are markers of disease severity and suggest that they mediate disease progression. According to the available literature, the primary hematological symptoms-associated COVID-19, and which distinguish patients with severe disease from patients with nonsevere disease, are lymphocytopenia, thrombocytopenia, and a significant increase in D-dimer levels.
-
Conclusions: SARS-CoV-2 infection triggers a complex response altering inflammatory, hematological, and coagulation parameters. Measuring these alterations at certain time points may help identify patients at high risk of disease progression and monitor the disease severity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
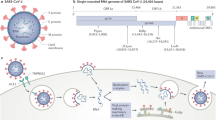
During this winter season, the novel coronavirus [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)], an enveloped virus with nonsegmented single-stranded positive-sense RNA genome, is causing a pandemic of unprecedented magnitude (Huang et al. 2020; Chen et al. 2020a; Zhu et al. 2020). Many researchers are focusing on elucidating the mechanisms of infection and developing a drug or vaccine; however, much uncertainty still presides over many aspects of disease manifestation (Wang et al. 2020a). The spectrum of disease (called COVID-19) ranges from asymptomatic/mild and self-limiting respiratory tract infections (RTIs) – as far as we know this is the majority of infected people – to severe sepsis and ARDS with an hyperinflammatory phenotype often associated with multiorgan failure, especially in elderly patients and with comorbidities. SARS-CoV-2 enters target cells via the angiotensin-converting enzyme 2 (ACE2) by a receptor-mediated endocytosis (Fig. 5.1) (Guan et al. 2020).
ACE 2 is a type I integral membrane protein with several physiological functions, well expressed in lungs (overexpressed in smokers), heart, kidney, and gastrointestinal tract (Zhang et al. 2020a; Tang et al. 2020a). Through the renin–angiotensin system (RAS), the virus may impact the lung circulation, but the expression on endothelium may conduct to its activation and further systemic damage (Wang et al. 2020b; Tang et al. 2020b). Increasing evidence suggests that lymphocytopenia, thrombocytopenia, and disturbances in blood coagulation system, such as elevated levels of D-dimer, are the most common hematological abnormalities observed among coronavirus disease 2019 (COVID-19) patients, especially in the severe stage of infection, and may serve as diagnostic and prognostic tools for COVID-19 (Tan et al. 2020a; Li et al. 2004; Ludvigsson 2020; Wu et al. 2020).
While precise mechanisms underlying these phenomena remain to be further clarified, the understanding of the disease, its clinical course, as well as its immunological and hematological implications is paramount in this phase of the pandemic since new trials and new therapeutic approach should be based on the most precise medicine and knowledge (Shrestha et al. 2020).
5.2 Response to Viral Infection
5.2.1 Initial Response in SARS-CoV-2 Infection vs. V-V ECMO
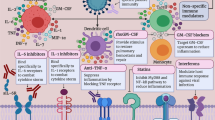
The viral infection is able to trigger an excessive immune response in predisposed individuals, which can result in a “cytokine storm” that presents an hyperinflammation state able to determine tissue damage and vascular damage revealing as fluid leakage and vasodilation responsible of the very profound hemodynamic impairment and also of the exposure of large amounts of tissue factor exposure with coagulation factors activation and consumption (Cummings et al. 2020; Chousterman et al. 2017; van der Poll and Opal 2008; Mehta et al. 2020) (Fig. 5.2). An explosive production of proinflammatory cytokines such as TNF-α IL-1β and others occurs, greatly exaggerating the generation of molecule-damaging reactive oxygen species (ROS). One of the causes of the hyperinflammatory state is the ability of immune cells to dramatically change their metabolism. Similar to cancer cells in many solid tumors, immune cells such as macrophages/monocytes under inflammatory conditions abandon mitochondrial oxidative phosphorylation for ATP production in favor of cytosolic aerobic glycolysis (also known as the Warburg effect) (Bar-Or et al. 2018). The change to aerobic glycolysis allows immune cells to become highly phagocytic, accelerate ATP production, intensify their oxidative burst, and to provide the abundant metabolic precursors required for enhanced cellular proliferation and increased synthesis and release of cytokines. Melatonin, an endogenous molecule, may be useful in this regard (Reiter et al. 2020). Melatonin has been found produced in mitochondria and consequently in every human cell specifically in lung monocytes/macrophage (Muxel et al. 2012). Melatonin has been proposed to reduce the highly proinflammatory cytokine storm and neutralize the generated ROS, thereby preserving cellular integrity and preventing lung damage (Martín Giménez et al. 2020). A similar and combined role may be hypothesized also for vitamin D (Amrein et al. 2020; Martucci et al. 2019). In the current limited health resources scenario, it would be important to adopt any adjuvant treatment that may contribute to a better outcome if it is inexpensive and with few or unimportant side effects at tested doses: vitamin D, vitamin C, as well as melatonin and other potential adjuvant of innate immune system seems to respond to this need.
As suggested by several authors, the main response to the SARS-CoV-2 is the innate immune system (Nasab et al. 2020; Birra et al. 2020). As a key player in this puzzle, there is for sure the complement system. It has, in fact, a relevant role as a bridge in both coagulation and inflammatory system by a continuous cross-talk of mediators (Piacente et al. 2020). Early in COVID-19 disease, Diao et al. have recognized complement deposits in case of renal insufficiency (Diao et al. 2020). In a study from Italy, the plasma levels of sC5b-9 and C5a were significantly higher in COVID patients associated with a high level of acute phase protein release. This is relevant to understand the pathogenesis of pulmonary disease and potentially to recognize new therapeutic targets. In fact, it is known that C5a increase is able to promote the lung sequestration of leukocytes and pulmonary dysfunction, and that sC5b-9 has similar effects by causing transendothelial leukocyte migration and vascular leakage (Cugno et al. 2020; Gralinski et al. 2018).
Such involvement of the complement justifies the use of drugs impacting on this system. First of all, immunoglobulins have entered several protocols of treatment worldwide since their role to enhance specific immunity guided by antibodies (not only on the viral infections but also for the prolonged nosocomial and frequent bacterial superinfections) but also for its immunomodulant role able to decrease C5 activation and deposition of the membrane attack complex (Basta and Dalakas 1994). Moreover, following the pathogenesis of immune-mediated diseases with microangiopathy, the block of complement may be obtained by specific drugs targeting C5 like the humanized monoclonal antibody eculizumab or the mannan-binding lectin-associated serine protease-2 (MASP-2) by the human monoclonal antibody narsoplimab (Patriquin and Kuo 2019).
Interleukin-6 (IL-6), as part of the nonspecific innate immune response, is produced by activated leukocytes and endothelial cells and has as effectors many tissues and cells (Kruttgen and Rose-John 2012). In the cytokine release syndrome characterized by fever and multiorgan dysfunction, it plays a relevant pathogenetic role, and in COVID-19 has been recognized (accompanied by low lymphocyte count) to be associated to poor outcomes (Mehta et al. 2020). IL-6 is well known being linked to the trans-signaling pathway, which causes vascular leakage as the first step of a cascade followed by tissue edema, hypoxia, and finally necrosis.
Tocilizumab is a monoclonal antibody against IL-6 mainly used for the treatment of rheumatoid arthritis. It has recently emerged as an alternative treatment for COVID-19 patients with a documented cytokine storm (Fig. 5.3). Reports and single-center experiences have been documented, and its actual efficacy is going to be assessed by dedicated investigations [NCT04317092] (Luo et al. 2020; Michot et al. 2020; Zhang et al. 2020b).
5.2.2 Respiratory Distress vs. ARDS?
The majority of patients with severe manifestations of COVID-19 meet the criteria for the ARDS according to the Berlin definition (Arabi et al. 2020; Bellani et al. 2016; ARDS Definition Task Force et al. 2012). From the physiological side, the ARDS is represented by the so-called baby lung postulated by Gattinoni and collaborators (Gattinoni et al. 1987). Considering that ARDS is a syndrome, and consequently has a specific development even though with different causes (pulmonary and extrapulmonary), the respiratory impairment of COVID-19 probably is a different syndrome or at least should be classified as an atypical ARDS (Gattinoni et al. 2020a, b). In fact, at least initially (so before the ventilator associated lung injury, the self-induced lung injury or severe bacterial pneumonia does not reveal) the disease does not couple with the baby lung theory or classical CT scan images or physiological respiratory dynamics characteristics (Gattinoni et al. 2020c). In particular, the relevant finding since the beginning of the outbreak spread was a relevant hypoxia associated with almost normal compliance (Marini and Gattinoni 2020). In these patients, the hypoxemia is primarily due to the VA/Q maldistribution caused by the loss of the lung perfusion regulation. High tidal volume follows (increased strain) in association with higher transpulmonary pressure (stress) to assure oxygenation (Gattinoni et al. 2020d). If this increase in stress and strain remains without correction, patient self-inflicted lung injury develops, causing overt lung edema, inflammation, and lymphocyte sequestration (Fig. 5.4).
5.2.3 Other Organ Manifestations
Although COVID-19 is a new disease and much of its pathomechanism remains unknown, it is widely believed that COVID-19 is not a respiratory-only disease, but is a cardinal challenge for many medical disciplines. This is due to the fact that expression of ACE2, a receptor for SARS-CoV-2, is not limited to respiratory track only, but the high ACE2 gene expression is observed in many tissue types (Li et al. 2020a; Nicin et al. 2020; Song et al. 2020). In addition, cytokine storm associated with severe COVID-19 is a systemic disease with serious consequences for patients, including ARDS and multiple-organ failure (Ye et al. 2020). Moreover, an intensified inflammatory reaction, hypercoagulability and endothelial and cardiomyocytes damage are all factors that predispose COVID-19 patients to myocardial infarction (MI) (Atri et al. 2020). SARS-CoV-2-infected patients are at high risk of ischemic stroke; however, the mechanism underlying this phenomenon is unclear, but it has been proposed that high incidence of stroke is associated, similarly to MI, with inflammation, endothelial dysfunction, and blood coagulation imbalance (Oxley et al. 2020; Hess et al. 2020; Avula et al. 2020; Markus and Brainin 2020). Possible role of SARS-CoV-2 in liver failure has also been suggested by the finding that aminotransferases (ALT, AST), lactate dehydrogenase (LDH), creatinine kinase (CK), or myoglobin levels are increased in COVID-19 patients (Zhang et al. 2020c; Bangash et al. 2020). Five sophisticated mechanisms are engaged in liver injury, including inflammation, cytotoxicity of the virus, anoxia, drug-induced liver injury, and reactivation of pre-existing liver disease (Sun et al. 2020). Kidney is another organ that may be damaged in COVID-19 via the cytopathic effects of SARS-CoV-2 on podocytes and proximal straight tubule cells (Cheng et al. 2020; Pan et al. 2020). For the reasons set out above, COVID-19 is a highly contagious disease involving, especially in severe form, many systems, and requiring an interdisciplinary approach.
5.3 COVID and Hematological Changes
5.3.1 Hematological Symptoms of COVID-19
The clinical manifestations of COVID-19 are not consistent and may evolve depending on disease progression. Most cases are asymptomatic or have mild or moderate symptoms, while fewer are characterized by a severe or critical form of SARS-CoV-2 infection (Yuki et al. 2020; Lai et al. 2020; Fung et al. 2020). The purpose of this chapter is to familiarize readers with the primary hematological symptoms associated with SARS-CoV-2 infection and COVID-19 disease manifestation. An increasing number of studies have shown correlations between changes in the blood system and unfavorable rates of disease progression, which are also discussed in this chapter. We focus on discussing the results of original papers, excluding case reports concerning single or small patient presentation. Intentionally, this part has been divided into subsections describing changes in white blood cells (WBCs), red blood cells (RBCs), platelets (PLTs), and plasma coagulation parameters, which have become associated with infection with this novel pathogen.
5.3.2 WBC Count
Due to the nature of inflammatory reactions in response to viral infections, the leukocyte (WBC) system is highly affected in response to SARS-CoV-2 infection. Most of the articles cited here supply patient data relevant at the time of admission to a hospital. Therefore, the long-term effect of infection on the leukocyte population is unknown. Among cases with laboratory-confirmed SARS-CoV-2 infection, the majority of cases present with lymphocytopenia, defined as lymphocyte counts below 1 × 109 cells/L (Huang et al. 2020; Guan et al. 2020; Wang et al. 2020b; Liu et al. 2020a).
In a preliminary report involving 41 COVID-19 patients (median age 49 years) published by Huang et al (2020), it is reported that 45% of patients exhibited a normal number of WBC (4−10 × 109 cells/L); however, patients with severe disease were found to have twofold higher WBCs than those with nonsevere disease. Overall, 40% of patients exhibited elevated number of WBCs (above 10 × 109 cells/L), whilst 25% subjects exhibited leukopenia (WBC count over 4 × 109 cells/L) (Tripodi 2011). Lymphocytopenia was the most common change, observed in 63% of patients. In addition, blood from severely ill patients contains more neutrophils and a lower number of lymphocytes compared with patients with nonsevere disease (Huang et al. 2020). Among the clinical characteristics that the authors identified in 138 infected patients (median age 56 years), elevated WBC and neutrophils counts were more prevalent in patients with severe COVID-19 disease manifestation. In turn, lymphocyte counts in this group were lower (median = 0.8 × 109 cells/L) than in nonsevere patients (median = 0.9 × 109 cells/L) (Wang et al. 2020b). The authors concluded that nonsurvivors exhibited more advanced lymphocytopenia compared with survivors. Low lymphocyte number was further confirmed as a primary hematological symptom of COVID-19 by a study of 137 patients (median age 57 years), of whom 72.3% had lymphocyte counts lower than 1 × 109 cells/L of blood (Tripodi 2011). Finally, Guan et al. reported on a study analyzing 1099 patients (median age 47 years) whereby they demonstrated that 33.7% of these were diagnosed with leukopenia, which inflated to over 61% of patients suffering from severe disease symptoms (Guan et al. 2020). This subgroup was also characterized by lymphocytopenia, which was reported in 96.1% of patients, confirming previous observations by other studies.
The research reviewed thus far concerned patients mostly from Wuhan (China). In contrast to those data, a study involving a small group of 13 Chinese patients (median age 34 years) located outside of Wuhan reported no changes in any of the leukocyte cell types and all the patients recovered (Chang et al. 2020). It may be relevant, however, that these patients were mostly adults without comorbidities and were much younger than those evaluated by other studies.
A further study conducted outside Wuhan reported that of 62 patients (median age 41 years) with mild to moderate clinical symptoms, the majority (62%) had normal WBC counts, while lymphocytopenia was diagnosed in 42% of patients. (Xu et al. 2020) Patient age and stage of the disease appear to be the key factors determining the development of lymphocytopenia and its progression in association with COVID-19. Eosinopenia was reported by Zhang et al. in more than half (52.9%) of 138 cases (median age 57 years) and in 78.8% of 52 COVID-19 patients by Li et al. (Zhang et al. 2020d; Li et al. 2020b).
5.3.3 Lymphocyte Populations
Preliminary studies have investigated the potential impact on lymphocyte subpopulations in response to SARS-CoV-2 infection. A study conducted by Liu et al. involving 40 COVID-19 patients (mean age 48.7 years), concluded that patients with severe disease manifestation had significantly lower numbers of CD3+ and CD8+ T cells both at time of admission and one week post admission. (Liu et al. 2020b) Chen et al. also reported below-normal CD4+ and CD8+ T cells in 21 COVID-19 patients (mean age 56 years), especially in those with severe disease manifestation. In addition, expression of interferon gamma (IFNγ) by CD4+ T cells was reduced in seriously ill patients, which plays a crucial role in the antiviral responses (Chen et al. 2020b). The precise mechanisms responsible for the decrease in lymphocyte number and activity are not known; however, this change seems to be crucial in the pathophysiology of the disease and is directly related to the severity of clinical symptoms. Lymphocyte pathways would therefore be a priority area for further research.
5.3.3.1 Predicting Severity of COVID-19: The Roles of NLR and N8R
Recent studies under review (Liu et al. 2020c; Zhang et al. 2020e) have proposed the possibility of predicting severity of disease based on the neutrophil to lymphocyte ratio (NLR). According to these studies, a high NLR is associated with severe presentation of COVID-19. More specifically, patients over 50 years of age and with an NLR ≥ 3.13 were characterized by severe disease symptoms, and these patients should be monitored intensively due to their vulnerability toward unfavorable disease progression (Liu et al. 2020c). In addition to NLR, Liu et al. observed that the neutrophil to CD8+ T cell ratio (N8R) correlated very well with disease severity, with an area under the curve (AUC) equal to 0.94 (Liu et al. 2020b).
5.3.3.2 Mechanism of Lymphocytopenia: Hypothetical Pathways
There appears to be extensive evidence that the majority of adults presenting with severe COVID-19 symptoms exhibit low lymphocyte counts (Cao 2020; Tan et al. 2020b). The diminished number of lymphocytes is also associated with other coronavirus diseases, including SARS and MERS (Li et al. 2004; Ko et al. 2016). Although the mechanisms of lymphocytopenia are not fully understood, three potential hypotheses are beginning to form. The first of these asserts that lymphocytopenia is associated with intensification of the inflammatory process. During COVID-19 disease, progression cytokine storm syndrome may occur, characterized by increased production of potent pro-inflammatory cytokines. The second proposes that SARS-CoV-2 may directly infect lymphocytes and lead to destruction of lymphoid organs (Cao 2020; Tan et al. 2020b; Lin et al. 2020). Third, glucocorticosteroids used to treat COVID-19 patients are known to cause lymphocytopenia (Yao et al. 2008). These are three extremely diverse hypotheses, but all concur that low lymphocyte count is directly related to the severity of COVID-19 disease, highlighting the importance of elucidating the precise mechanisms responsible for this hematological phenomenon for the accurate diagnosis and prognosis of COVID-19.
5.3.4 Red Blood Cells
No analyses performed to date showed any differences in hemoglobin levels between patients exhibiting severe COVID-19 symptoms and patients with mild/moderate disease manifestation (Huang et al. 2020; Li et al. 2020b; Liu et al. 2020c). However, using a systematic review and meta-analytical approach Lippi and Mattiuzzi have suggested that severely ill COVID-19 patients may have decreased hemoglobin levels (Lippi and Mattiuzzi 2020). These conclusions should be tentatively interpreted as only four studies were included in the statistical analysis and the featured high levels of heterogeneity in their reported findings. Notable, however, levels of iron-containing ferritin were elevated in patients with severe COVID-19 symptoms (Liu et al. 2020b; Chen et al. 2020b). This is associated with the intensification of inflammation, rather than with disturbances in iron metabolism (Northrop-Clewes 2008). Furthermore, Zhang et al. reported the interesting observation that erythrocyte sedimentation rate (ESR) is significantly higher in patients with severe disease manifestation. Increased ESR, as a marker to monitor, may be applicable in clinical practice for predicting the severity of COVID-19, with a very high AUC = 0.95 (Zhang et al. 2020f).
5.3.5 Platelets and Coagulation Markers
Most of the research reviewed in this chapter did not observe thrombocytopenia or platelet count differences between patients diagnosed with serious disease and patients with mild disease. One report involving the largest study group (n = 1099) observed reduced platelet counts in more than half (57.5%) of patients in the intensive care unit, with a median of 137,500 platelets/μL. A meta-analysis of nine studies covering 1779 COVID-19 cases showed that low platelet count is associated with increased severity and mortality of SARS-CoV-2-infected patients (Lippi et al. 2020). These diverse findings indicate a need for further investigation into the involvement of thrombocytopenia and the role of platelets in COVID-19 disease progression.
5.4 Coagulation Disorders in SARS-CoV-2 Infection
Coagulopathy, and more precisely hypercoagulability, is one of the most significant prognostic factors in COVID-19 and a number of definitions have flourished starting from the evaluation of the altered coagulation parameters. COVID-19-associated coagulopathy (CAC) has been proposed as well as the definition of MicroCLOTS (microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome) for the severe cases of pulmonary disease to underline the role in the pathogenesis of the most severe cases of the microvascular pulmonary thrombosis (Ciceri et al. 2020). The thromboinflammatory syndrome initially localized to the lungs, giving the widely availability of the ACE-2 receptors, in case of viremia, may involve other organs like the brain and the liver with very severe consequences largely illustrated by a number of series published on COVID-19 that highlighted the high rate of ischemic stroke and liver impairment (Gandhi and Goerlinger n.d.).
A relevant potential pathogenetic mechanism is the upregulation of tissue factor expression to circulating monocytes, thrombopoietin, and fibrinogen, as well as the downregulation of plasminogen activator inhibitor type 1 (PAI-1) by IL-6. Downregulation of PAI-1 is responsible of more stable and diffused resistance of thrombi with clot formation also in undamaged vessels contributing, in particular in the small pulmonary vessels to the characteristics of the early stage disease that has the pulmonary shunt as the main cause of hypoxia associated to a high elevated compliance (Gattinoni et al. 2020b, d)
Interestingly, the hypercoagulable state has been confirmed by viscoelastic tests exploration. In a thorough evaluation of coagulation parameters in 24 COVID-19 patients comparing standard but wide results associated with thromboelastography, Panigada and collaborators have demonstrated a decreased (considering the mean reference range value) R and K value [respectively 6.3 (3.0–11.9) and 1.5 (0.8–2.9)] and an increase of angle K and maximal amplitude MA [respectively 69.4 (51.1–78.5), and 79.1 (58.0–92.0)]. Such values are not deranged in absolute way and are accompanied by a mild reduction in platelets and antithrombin and probably should be interpreted in the light of the multiorgan impairment and population differences. We are far away from the understanding of the contribution of the endothelial activation, factor consumptions, and liver impairment in the single patient (Panigada et al. 2020).
Fibrinogen may be increased in case of mild and in the early phase of severe disease since it is an acute response protein and may be a contributing marker of hyperinflammation. But it should be considered that in late disease (usually after 7–10 days in the ICU) fibrinogen may be reduced due to large consumption and degradation as well as due to lower production.
Coagulation parameters used in routine practice appeared to be within normal range, although prolonged prothrombin time (PT) (Huang et al. 2020) and shortening of activated partial thromboplastin time (aPTT) (Wu et al. 2020) in severely ill COVID-19 patients were also described. Changes in D-dimer levels are the most commonly observed anomaly of the hemostasis system in patients with COVID-19. Most studies have shown a significant increase in D-dimer levels in patients with severe disease manifestation (Huang et al. 2020; Guan et al. 2020; Wang et al. 2020b; Zhang et al. 2020d, f; Liu et al. 2020b; Chen et al. 2020b). D-dimers are very sensitive and very specific laboratory indicator of the activation of coagulation and fibrinolysis. It is also well known that they are helpful in early diagnosis of acute disseminated intravascular coagulation (DIC) (Tripodi 2011; Bates 2012). Patients with SARS-CoV-2 infection are at high risk of developing this complication. Tang et al. observed that over 71% of nonsurvivor cases met the criteria of DIC (Tang et al. 2020c). It has also been speculated that these patients have an elevation in blood plasmin(ogen) activity, which may enhance the virulence of SARS-CoV-2 and play a cardinal role in hyperfibrinolysis during DIC (Ji et al. 2020). This evidence indicates that special attention must be directed toward thrombotic and hemorrhagic complications in patients with COVID-19.
A state of acute disseminated intravascular coagulation (DIC) very similar to the hemostasis derangement observed in sepsis is frequently described in COVID-19. Using the several available scores to evaluate such conditions all are able to contribute to establish the prognosis in COVID-19 (Taylor Jr et al. 2001).
All this is also associated to a higher frequency of pulmonary embolism that probably was the initial cause of the sudden deaths seen in Chinese outbreak and also the cause of the frequent initial secondary cardiac involvements like ischemia and arrhythmias as well as VA-ECMO need.
5.5 Recommendation from Scientific Societies
The extent to which blood cells and coagulation system represent important diagnostic and prognostic markers for the severity of COVID-19 disease manifestation has led to international and national scientific societies to recommend that these be evaluated in clinical practice. These recommendations endorse, above all, monitoring of patients with particular attention to changes in D-dimer levels to assess the risk of pulmonary embolism and DIC (Thachil et al. 2020; Flisiak et al. 2020).
5.6 Conclusions
Infection with SARS-CoV-2 has presently become a rapidly spreading and devastating global pandemic Although most COVID-19 patients have moderate symptoms and recover quickly, some patients develop severe respiratory failure and acute respiratory compromise often requiring intensive care unit admission and mechanical ventilation. The above is often a result of immunological and hematological response rather than virus infiltration of human cells itself. According to the available literature, the primary hematological symptoms associated with COVID-19, and which distinguish patients with severe disease from patients with nonsevere disease, are lymphocytopenia, thrombocytopenia, and a significant increase in D-dimer levels. In this context, however, there is a shortage of research that would explain the mechanisms responsible for the observed changes.
Abbreviations
- ACE2:
-
angiotensin-converting enzyme 2
- aPTT:
-
activated partial thromboplastin time
- ARDS:
-
acute respiratory distress syndrome
- AT:
-
antithrombin
- AUC:
-
area under curve
- CAC:
-
COVID-19-associated coagulopathy
- CRRT:
-
continuous renal replacement therapy
- COVID-19:
-
Coronavirus Disease 2019
- DIC:
-
disseminated intravascular coagulation
- DFPP:
-
double filtration plasmapheresis
- ESR:
-
erythrocyte sedimentation rate
- FDP:
-
fibrin degradation products
- GAGs:
-
glycosaminoglycans
- ICU:
-
intensive care unit
- MASP-2:
-
mannan-binding lectin-associated serine protease-2
- MERS:
-
Middle East respiratory syndrome
- NLR:
-
neutrophil to lymphocyte ratio
- PAI-1:
-
plasminogen activator inhibitor-1
- SARS-CoV-2:
-
severe acute respiratory syndrome coronavirus 2
- TNF:
-
tumor necrosis factor
- TPE:
-
therapeutic plasma exchange
- UFH:
-
unfractionated heparin
- VA-ECMO:
-
veno-arterial extracorporeal membrane oxygenation
- VTE:
-
venous thromboembolism
References
Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Kostenberger M, Tmava Berisha A, Martucci G, Pilz S, Malle O (2020) Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr
Arabi YM, Murthy S, Webb S (2020) COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307(23):2526–2533. https://doi.org/10.1001/jama.2012.5669
Atri D, Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA (2020) COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. [Published online ahead of print, 2020 Apr 10]. JACC Basic Transl Sci 5(5):518–536
Avula A, Nalleballe K, Narula N et al (2020) COVID-19 presenting as stroke. Brain Behav Immun. S0889-1591(20)30685-1
Bangash MN, Patel J, Parekh D (2020) COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol 5(6):529–530
Bar-Or D, Carrick M, Tanner A, Lieser MJ, Rael LT, Brody E (2018) Overcoming the Warburg effect: is it the key to survival in sepsis? J Crit Care 43:197–201
Basta M, Dalakas MC (1994) High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest 94:1729–1735
Bates SM (2012) D-dimer assays in diagnosis and management of thrombotic and bleeding disorders. Semin Thromb Hemost 38(7):673–682
Bellani G et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315(8):788–800. https://doi.org/10.1001/jama.2016.0291
Birra D, Benucci M, Landolfi L, Merchionda A, Loi G, Amato P, Licata G, Quartuccio L, Triggiani M, Moscato P (2020) COVID 19: a clue from innate immunity. Immunol Res 10:1–8. https://doi.org/10.1007/s12026-020-09137-5
Cao X (2020) COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20(5):269–270
Chang D, Lin M, Wei L, Xie L, Zhu G, Dela Cruz CS, Sharma L (2020) Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA 323(11):1092–1093
Chen N et al (2020a) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223):507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q (2020b) Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J Clin Invest. https://doi.org/10.1172/JCI137244
Cheng Y, Luo R, Wang K et al (2020) Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97(5):829–838
Chousterman BG, Swirski FK, Weber GF (2017) Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol 39:517–528
Ciceri F et al (2020) Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. Online ahead of print
Cugno M et al (2020) Complement activation in patients with COVID-19: a novel therapeutic target. J Allergy Clin Immunol. https://doi.org/10.1016/j.jaci.2020.05.006. S0091-6749(20)30650-3
Cummings MJ et al (2020) Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. medRxiv. Apr 20:2020.04.15.20067157
Diao B, Wang C, Wang R, Feng Z, Tan Y, Wang H et al (2020) Human kidney is a target for novel severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020.03.04.20031120
Flisiak R, Horban A, Jaroszewicz J, Kozielewicz D, Pawłowska M, Parczewski M, Piekarska A, Simon K, Tomasiewicz K, Zarębska-Michaluk D (2020) Management of SARS-CoV-2 infection: recommendations of the Polish Association of Epidemiologists and Infectiologists as of March 31, 2020. Pol Arch Intern Med 130(4):352–357
Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY (2020) A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect 9(1):558–570
Gandhi A, Goerlinger K (n.d.) Coagulopathy in COVID-19: connecting the dots together. J Card Crit Care:TSS 2020. Online ahead of print
Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M (1987) Pressure–volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 136(3):730–736
Gattinoni L, Meissner K, Marini JJ (2020a) The baby lung and the COVID-19 era. Intensive Care Med 25:1–3. https://doi.org/10.1007/s00134-020-06103-5
Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D (2020b) COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 201(10):1299–1300. https://doi.org/10.1164/rccm.202003-0817LE
Gattinoni L, Chiumello D, Rossi S (2020c) COVID-19 pneumonia: ARDS or not? Crit Care 24(1):154. https://doi.org/10.1186/s13054-020-02880-z
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L (2020d) COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 46(6):1099–1102. https://doi.org/10.1007/s00134-020-06033-2
Gralinski LE, Sheahan TP, Morrison TE, Menachery VD, Jensen K, Leist SR et al (2018) Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio 9:e01753–e01718
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS (2020) China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. https://doi.org/10.1056/NEJMoa2002032
Hess DC, Eldahshan W, Rutkowski E (2020) COVID-19-related stroke. Transl Stroke Res 11(3):322–325
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223):497–506
Ji HL, Zhao R, Matalon S, Matthay MA (2020) Elevated plasmin(ogen) as a common risk factor for COVID-19 susceptibility. Physiol Rev 100(3):1065–1075
Ko JH, Park GE, Lee JY, Lee JY, Cho SY, Ha YE, Kang CI, Kang JM, Kim YJ, Huh HJ, Ki CS, Jeong BH, Park J, Chung CR, Chung DR, Song JH, Peck KR (2016) Predictive factors for pneumonia development and progression to respiratory failure in MERS-CoV infected patients. J Infect 73(5):468–475
Kruttgen A, Rose-John S (2012) Interleukin-6 in sepsis and capillary leakage syndrome. J Interferon Cytokine Res 32:60–65
Lai CC, Liu YH, Wang CY, Wang YH, Hsueh SC, Yen MY, Ko WC, Hsueh PR (2020) Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. https://doi.org/10.1016/j.jmii.2020.02.012
Li T, Qiu Z, Zhang L, Han Y, He W, Liu Z, Ma X, Fan H, Lu W, Xie J, Wang H, Deng G, Wang A (2004) Significant changes of peripheral t lymphocyte subsets in patients with severe acute respiratory syndrome. J Infect Dis 189:648–651
Li MY, Li L, Zhang Y, Wang XS (2020a) Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9(1):45
Li Q, Ding X, Xia G, Geng Z, Chen F, Wang L, Wang Z (2020b) A simple laboratory parameter facilitates early identification of COVID-19 patients. medRxiv. https://doi.org/10.1101/2020.02.13.20022830
Lin L, Lu L, Cao W, Li T (2020) Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect:1–14. https://doi.org/10.1080/22221751.2020.1746199
Lippi G, Mattiuzzi C (2020) Hemoglobin value may be decreased in patients with severe coronavirus disease 2019. Hematol Transfus Cell Ther. https://doi.org/10.1016/j.htct.2020.03.001
Lippi G, Plebani M, Henry BM (2020) Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta 506:145–148
Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG (2020a) Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). https://doi.org/10.1097/CM9.0000000000000744
Liu J, Li S, Liu J, Liang B, Wang X, Wang H et al (2020b) Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. https://doi.org/10.1101/2020.02.16.20023671
Liu J, Liu Y, Xiang P, Pu L, Xiong H, Li C et al (2020c) Neutrophil-to-lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. https://doi.org/10.1101/2020.02.10.20021584
Ludvigsson JF (2020 Jun) Systematic review of covid-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109(6):1088–1095
Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J (2020) Tocilizumab treatment in covid-19: a single center experience. J Med Virol
Marini JJ, Gattinoni L (2020) Management of COVID-19 respiratory distress. JAMA. https://doi.org/10.1001/jama.2020.6825
Markus HS, Brainin M (2020) COVID-19 and stroke – a global World Stroke Organization perspective. Int J Stroke 15(4):361–364
Martín Giménez VM, Inserra F, Tajer CD, Mariani J, Ferder L, Reiter RJ, Manucha W (2020) Lungs as target of COVID-19 infection: protective common molecular mechanisms of vitamin D and melatonin as a new potential synergistic treatment. Life Sci 254:117808. https://doi.org/10.1016/j.lfs.2020.117808
Martucci G, McNally D, Parekh D et al (2019) Trying to identify who may benefit most from future vitamin D intervention trials: a post hoc analysis from the VITDAL-ICU study excluding the early deaths. 23:200
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) Hlh across speciality collaboration UK. Covid-19: consider cytokine storm syndromes and immunosuppression. Lancet 395:1033–1034
Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, Balleyguier C, Besse B, Marabelle A, Netzer F, Merad M, Robert C, Barlesi F, Gachot B, Stoclin A (2020) Tocilizumab, an anti-il6 receptor antibody, to treat covid-19-related respiratory failure: a case report. Ann Oncol
Muxel JM, Pires-Lapa MA, Morteiro AW, Cecon E, Tamura EK, Flaeten-Winter LM, Markus RP (2012) NF-kB drives the synthesis of melatonin in RAW 264.7 macrophages by inducing the transcription of the arylalkylamine-N-acetyltransferase (AA-NAT) gene. PLoS One
Nasab MG, Saghazadeh A, Rezaei N (2020) SARS-CoV-2-A tough opponent for the immune system. Arch Med Res. https://doi.org/10.1016/j.arcmed.2020.05.020. May 30:S0188-4409(20)30749-9
Nicin L, Abplanalp WT, Mellentin H et al (2020) Cell type-specific expression of the putative SARS-CoV-2 receptor ACE2 in human hearts. Eur Heart J 41(19):1804–1806
Northrop-Clewes CA (2008) Interpreting indicators of iron status during an acute phase response-lessons from malaria and human immunodeficiency virus. Ann Clin Biochem 45(1):18–32
Oxley TJ, Mocco J, Majidi S et al (2020) Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med 382(20):e60
Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG (2020) Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 46(6):1114–1116
Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A (2020) Hypercoagulability of COVID-19 patients in intensive care unit. a report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost 17. https://doi.org/10.1111/jth.14850
Patriquin CJ, Kuo KHM (2019) Eculizumab and beyond: the past, present, and future of complement therapeutics. Transfusion Med Rev 33:256–265
Piacente C, Martucci G, Miceli V, Pavone G, Papeo A, Occhipinti G, Panarello G, Lorusso R, Tanaka K, Arcadipane A (2020) A narrative review of antithrombin use during veno-venous extracorporeal membrane oxygenation in adults: rationale, current use, effects on anticoagulation, and outcomes. Perfusion 267659120913803
Reiter RJ et al (2020) Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: a mechanistic analysis. Med Drug Discov 6:100044. https://doi.org/10.1016/j.medidd.2020.100044
Shrestha GS et al (2020) Precision medicine for COVID-19: a call for better clinical trials. Crit Care
Song H, Seddighzadeh B, Cooperberg MR, Huang FW (2020) Expression of ACE2, the SARS-CoV-2 receptor, and TMPRSS2 in prostate epithelial cells [Published online ahead of print, 2020 May 6]. Eur Urol. https://doi.org/10.1016/j.eururo.2020.04.065
Sun J, Aghemo A, Forner A, Valenti L (2020) COVID-19 and liver disease. Liver Int 40(6):1278–1281
Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, Wang Q, Miao H (2020a) Lymphopenia predicts disease severity of covid-19: a descriptive and predictive study. Signal Transduct Target Ther 5:33
Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ et al (2020b) Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 5:33. https://doi.org/10.1038/s41392-020-0148-4
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z (2020a) Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost
Tang X et al (2020b) Comparison of hospitalized patients with acute respiratory distress syndrome caused by covid-19 and h1n1. Chest
Tang N, Li D, Wang X, Sun Z (2020c) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18(4):844–847
Taylor FB Jr, Toh CH, Hoots WK et al (2001) Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation On behalf of the Scientific Subcommittee on disseminated intravascular coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Thromb Haemost 86:1327–1330
Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T (2020) ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost 18(5):1023–1026
Tripodi A (2011) D-dimer testing in laboratory practice. Clin Chem 57(9):1256–1262
van der Poll T, Opal SM (2008) Host-pathogen interactions in sepsis. Lancet Infect Dis 8:32–43
Wang J et al (2020a) Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. https://doi.org/10.1002/JLB.3COVR0520-272R
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z (2020b) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. https://doi.org/10.1001/jama.2020.1585
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y (2020) Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med
Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ (2020) Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. https://doi.org/10.1136/bmj.m606
Yao Z, DuBois DC, Almon RR, Jusko WJ (2008) Pharmacokinetic/pharmacodynamic modeling of corticosterone suppression and lymphocytopenia by methylprednisolone in rats. J Pharm Sci 97(7):2820–2832
Ye Q, Wang B, Mao J (2020) The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect 80(6):607–613
Yuki K, Fujiogi M, Koutsogiannaki S (2020) COVID-19 pathophysiology: A review. Clin Immunol. 215:108427. https://doi.org/10.1016/j.clim.2020.108427
Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS (2020a) Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 46(4):586–590
Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (2020b) The cytokine release syndrome (crs) of severe covid-19 and interleukin-6 receptor (il-6r) antagonist tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents 105954
Zhang C, Shi L, Wang FS (2020c) Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 5(5):428–430
Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD (2020d) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. https://doi.org/10.1111/all.14238
Zhang B, Zhou X, Zhu C, Feng F, Qiu Y, Feng J et al (2020e) Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19. medRxiv. https://doi.org/10.1101/2020.03.12.20035048
Zhang H, Wang X, Fu Z, Luo M, Zhang Z, Zhang K et al (2020f) Potential factors for prediction of disease severity of COVID-19 patients. medRxiv. https://doi.org/10.1101/2020.03.20.20039818
Zhu N et al (2020) A novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382(8):727–733. https://doi.org/10.1056/NEJMoa2001017
Conflicts of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Słomka, A. et al. (2021). Immunological and Hematological Response in COVID-19. In: Asea, A.A.A., Kaur, P. (eds) Coronavirus Therapeutics – Volume I. Advances in Experimental Medicine and Biology, vol 1352. Springer, Cham. https://doi.org/10.1007/978-3-030-85109-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-030-85109-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-85108-8
Online ISBN: 978-3-030-85109-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)