Abstract
High quality evidence for interventions in chronic urticaria is mainly confined to second generation H1 antihistamines, introduced in the 1980s, and omalizumab, approved for chronic spontaneous urticaria in 2014. Other interventions with low evidence have been used by specialists for decades to manage patients who do not respond to H1 antihistamines. Pharmacological interventions include oral corticosteroids, H2 antihistamines, anti-leukotrienes, immunosuppressives and the sulphone anti-inflammatories. Non-pharmacological interventions include diet, phototherapy and psychological assessment. Evidence-based guideline recommendations are based on the highest quality evidence of efficacy and safety available at the time, but low-quality evidence does not necessarily equate to low clinical effectiveness. A case in point is oral corticosteroids that are widely acknowledged by clinicians and patients to be highly effective for severe chronic urticaria, but have not been studied systematically because clinical experience shows they work. On the other hand, well-known adverse effects limit their use to short-term rescue therapy. Low-evidence interventions are still of value today if applied selectively when management with H1 antihistamines, omalizumab or both is not effective or available. Targeted interventions such as immunosuppressives for autoimmune urticaria and sulphones for delayed pressure urticaria can be cost effective and safe when used appropriately, but clinicians need to be familiar with the risks as well as potential benefits of using interventions off-label. Availability of treatment depends on funding and access to specialist care with important regional and geographical differences so the importance of having wide-ranging options to suit individual patients continues.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Core Messages-
In patients not responding to or having contraindications against the treatment options recommended in the guidelines, several other treatment options with low-quality evidence can be tried.
-
Pharmacological interventions include oral corticosteroids, H2 antihistamines, anti-leukotrienes, immunosuppressives and the sulphone anti-inflammatories.
-
Non-pharmacological interventions include diet, phototherapy and psychological assessment.
H1 antihistamines and omalizumab are currently the only licensed drugs for the treatment of chronic spontaneous urticaria. This leaves a therapeutic void for chronic urticaria patients who do not respond adequately to antihistamines or for whom omalizumab is either not available or not effective. Historically, many interventions have been used to treat urticaria off licence, most of which are still available and can be valuable for the right patients in the right circumstances. Some drugs are more likely to be effective for specific subtypes or situations and are known as ‘targeted’ treatments. The evidence base for many of these treatments is based on small studies, case reports, anecdotal reports or clinical experience. Particular care is required when recommending these drugs. Physicians need to be aware of potential side effects, contraindications or interaction with other medications.
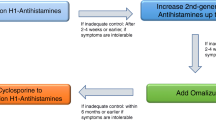
Most of these interventions are summarized in Table 9 of the 2018 EAACI/GA2LEN/EDF/WAO guidelines [1]. This chapter aims to summarize the evidence and practical guidance for using unlicensed drugs and non-drug interventions, i.e. diets, phototherapy, psychotherapy and desensitization, that continue to be valuable in the real world for management of some patients with chronic urticaria in special circumstances. All low-evidence interventions should be used in conjunction with a second generation antihistamine concurrently. They should be considered when antihistamines, omalizumab and ciclosporin, alone or in combination are not available or tolerated.
13.1 Low-Evidence Pharmacological Interventions in Chronic Urticaria
13.1.1 Anti-inflammatory Sulphones
13.1.1.1 Dapsone
Dapsone is an old-fashioned sulphonamide anti-bacterial drug, which has useful properties on inflammation and is still quite widely used in Dermatology for different conditions, including chronic urticaria. It is also used as a treatment of leprosy.
13.1.1.1.1 Evidence for Dapsone in Urticaria
Even though dapsone is widely used for difficult urticaria there have been relatively few publications [2,3,4]. A study comparing a double dose of antihistamine (desloratadine) with or without dapsone 50 mg daily in CSU showed no difference in the overall disease activity when the two groups were compared at 3 months, but a few of the patients treated with dapsone remained in complete remission 3 months after finishing it (while still on the antihistamine) [5]. A chart review suggested that patients with delayed pressure urticaria responded better to dapsone than those with delayed pressure and spontaneous urticaria [6]. Another chart review in 62 CSU patients recorded a complete response in 29 (47%), with a mean time to improvement of 1.1 months and mean time to complete response of 5.2 months. Ten patients remained clear after stopping with a follow-up of 0.3–10.0 months [7]. A modest improvement was found in a placebo-controlled cross-over trial over 6 weeks. Of the 22 patients treated with dapsone, 3 showed complete resolution of hives and itch, while 31% and 41% had ≥50% resolution of hives and itch, respectively [8].
13.1.1.1.2 Dose and Length of Treatment
The usual starting dose is 75–100 mg of dapsone a day. This can be increased up to 150 mg daily if there are no significant side effects.
13.1.1.1.3 Interactions with Other Medicines
Dapsone should not usually be taken with other sulphonamides, e.g. sulphasalazine or a medicine for gout called probenecid. Concentrations in the blood increase if taken with an antibiotic called trimethoprim. It may possibly reduce the contraceptive effect of combined oral contraceptives.
13.1.1.1.4 Checks During Treatment
Screening blood tests for anaemia, liver function and glucose-6-phosphate dehydrogenase should be done before starting dapsone. A blood count and liver function tests should be repeated a week after starting treatment, a month later and then every 3 months on treatment.
13.1.1.1.5 Contraindications to Treatment
Dapsone should not be taken if there is a history of reacting to sulphonamides.
13.1.1.1.6 Possible Side Effects of Treatment
The commonest unwanted effect is anaemia. This is more likely to be a problem at higher doses. Mild anaemia is usually unimportant, but more severe anaemia may result in becoming out of breath and feeling tired. Bluish discolouration of the lips may be apparent at high doses of dapsone due to an increase in methaemoglobin with reduced oxygen carriage and possible shortness of breath. There is a small risk of paraesthesia with long-term use although this is rare. Headache and gastrointestinal side effects may occur. A few people feel unwell 3–6 weeks after starting dapsone with fever, rash and enlarged lymph glands (dapsone hypersensitivity syndrome). The drug should be stopped immediately if this happens.
13.1.1.1.7 Summary
Dapsone is a useful treatment for some patterns of difficult chronic urticaria, including delayed pressure urticaria and CSU not responding to antihistamines, but the published evidence for using it is not strong. It should be taken in addition to an antihistamine. It may allow steroids to be stopped or taken at a lower dose. It is usually well tolerated but anaemia is a risk that must be checked for with blood tests before and during treatment.
13.1.1.2 Sulphasalazine
Is a long acting sulphonamide , called sulphapyridine, coupled to a derivative of salicylic acid called 5-aminosalicylic acid. It is usually used for inflammatory bowel disease, such as ulcerative colitis or Crohn’s disease.
13.1.1.2.1 Evidence for Sulphasalazine in Urticaria
There have been reports of using sulphasalazine for severe CSU that was steroid-dependent in some patients [9, 10] and others with delayed pressure urticaria [11]. Twenty-six patients with CSU (83.9%) showed an improvement in symptoms within the first 3 months, with 51.6% of patients becoming asymptomatic within the first 6 months of starting sulphasalazine in a retrospective record review. Eleven patients (35.4%) achieved complete relief of symptoms after tapering off sulphasalazine therapy although two patients had to stop treatment because of side effects [12].
13.1.1.2.2 Dose and Length of Treatment
The effective dose varies between individuals. A usual starting dose might be 500 mg twice daily, increasing by 500 mg daily at intervals of 2 weeks to a maximum regular dose of 4 g (eight tablets) daily.
13.1.1.2.3 Possible Side Effects of Treatment
Quite a wide range of possible side effects have been described including anaemia, rashes (which may be severe), loss of appetite, dizziness and reduced sperm counts. Treatment should be stopped immediately if there is any suspicion of a serious blood disorder. This may present with bruising, infections or anaemia. Sore throat, fever, malaise or unexpected illness should be reported since these symptoms may result from side effects of the drug. Overall, about 75% of unwanted effects show themselves within 3 months of starting treatment. The urine may be coloured orange and some soft contact lenses may be stained.
13.1.1.2.4 Interactions with Other Medicines
Sulphasalazine should not be taken with methotrexate or azathioprine.
13.1.1.2.5 Checks During Treatment
Bone marrow, kidney and liver function should be checked with a blood test before starting treatment and then monthly for the first 3 months. Checks can be less frequent after this: once every 3 months should be sufficient while treatment continues, provided there are no problems. Glucose-6-phosphate dehydrogenase should also be checked before making a decision to start sulphasalazine .
13.1.1.2.6 Reasons for Avoiding It
Sulphasalazine should be avoided if there is a previous history of adverse reactions to sulphonamides or aspirin. It should only be used during pregnancy and breast feeding if there is no alternative.
13.1.1.2.7 Summary
Sulphasalazine may be useful for CSU and delayed pressure urticaria but does carry some risks of unwanted effects that may be serious and needs to be monitored.
13.1.2 Tranexamic Acid
Tranexamic acid exerts its antifibrinolytic activity by inhibiting plasmin, which breaks down clots. It is mainly used to treat women with heavy periods.
13.1.2.1 Evidence for Tranexamic Acid in Urticaria
Although a trial of tranexamic acid for chronic urticaria patients seemed to show no benefit [13], clinical experience indicates that the treatment may be effective for a few patients with unexplained (‘idiopathic’) nonhistaminergic angioedema, especially those without wheals [13, 14]. Its use has been endorsed in recent guidelines on urticaria [15].
13.1.2.2 Dose and Length of Treatment
The dose of tranexamic acid for angioedema varies between patients. Daily doses range from 0.5 to 4.5 g daily with most patients finding the right balance at 1½–3 g a day. There is no limit to the length of time tranexamic acid can be taken.
13.1.2.3 Possible Side Effects of Treatment
The most likely side effects are nausea, vomiting and diarrhoea at large doses. Treatment should be stopped if changes in colour vision develop or a thrombosis occurs.
13.1.2.4 Interactions with Other Medicines
Tranexamic acid should not be taken at the same time as other medicines that promote clotting, such as epsilon aminocaproic acid.
13.1.2.5 Checks During Treatment
No checks are necessary when tranexamic acid is taken for less than 3 months. Blood testing for liver function and regular eye checks are recommended by the manufacturer for patients on long-term treatment of hereditary angioedema.
13.1.2.6 Contraindications to Treatment
The medicine should not be taken if there is a history of thromboembolic disease and should be used with caution in patients receiving oral contraceptives or on a background of ischaemic heart disease.
13.1.2.7 Summary
Tranexamic acid may be useful for some patients with unexplained recurrent angioedema who have not responded to usual treatments with antihistamines and short courses of steroid tablets. The treatment should not be taken by patients who have had thrombosis and should be stopped immediately if thrombosis or changes in colour vision develop.
13.1.3 Montelukast
Montelukast is a cysteinyl leukotriene (LTD4, E4, C4) inhibitor. It binds with high affinity and selectivity to the CysLT1 receptor. Symptoms of urticaria are mainly due to histamine release from mast cells in the skin. Failure to respond to an antihistamine may be due to other mediators of inflammation, including leukotrienes generated at the time of histamine release. The development of cysteinyl leukotriene receptor antagonists for asthma (also known as antileukotrienes) has provided an opportunity to try these medicines for chronic urticaria that does not respond well to antihistamine treatment alone. Montelukast is now the only antileukotriene available in Europe. By contrast, zileuton is an orally active inhibitor of 5-lipoxygenase that inhibits leukotriene (LTB4, LTC4, LTD4 and LTE4) formation that is only available in the USA. There are no studies of zileuton in chronic urticaria but, in theory, it might be effective. Antileukotrienes, when used, should be given with an antihistamine.
13.1.3.1 Evidence for Using Montelukast in Urticaria
Encouraging results have been seen in patients with aspirin-sensitive urticaria treated [16], delayed pressure urticaria [17] and CSU with predominant angioedema [18, 19], but CSU patients without angioedema may also benefit [20,21,22]. There have been anecdotal reports of patients with autoreactive CSU [23] and cold urticaria [24] improving, but this needs to be confirmed with well-designed clinical studies.
13.1.3.2 Dose and Length of Treatment
The daily adult dose of montelukast is 10 mg. It is usually taken at bedtime. The medicine may start to have a useful effect within a week, but the benefit seems to increase for up to 6 weeks [20]. There is probably no advantage in going beyond this if it has not worked by then. There is no time limit to treatment, but it is always good practice to try stopping medicines periodically to see if they are still needed.
13.1.3.3 Possible Side Effects of Treatment
Bowel symptoms, fever, headache, nausea, vomiting and increased upper respiratory tract infections may occur but there are no predictable unwanted effects from taking montelukast and it is usually well tolerated. A range of other possible side effects has been reported including anxiety, depression, dizziness, dry mouth, muscle and joint complaints and sleep disorders including dream abnormalities and nightmares (especially in children) so it is best not to increase the dose above 10 mg a day in adults and the approved dose in children. Urticaria has been reported as a side effect.
13.1.3.4 Interactions with Other Medicines and Reasons for Avoiding It
There are no important interactions with other medicines. It should not be used in pregnancy or during breastfeeding unless essential. A very rare condition of the lungs, called Churg-Strauss syndrome, may be more likely to develop in asthmatics.
13.1.3.5 Checks During Treatment
No regular checks are recommended by the manufacturer.
13.1.3.6 Summary
Montelukast blocks leukotrienes, which may contribute to the development of signs and symptoms of urticaria in some patients. It appears to work best for aspirin-sensitive chronic urticaria. It may be helpful for some patients with CSU including those with angioedema, delayed pressure urticaria. A single daily dose appears to be safe and well tolerated. It can be taken as long as it helps (with an antihistamine). It will probably not work if it has not done so within 6 weeks.
13.1.4 H2 Antihistamines
Several H2 antihistamines are available. Ranitidine was the most widely used in the context of treating chronic urticaria but is currently not available in the EU and the US because an impurity (NDMA) has been identified that may have pro-carcinogenic properties in humans pending further investigation. Cimetidine was little used until the withdrawal of ranitidine since it may interfere with hepatic metabolism of other drugs (including some H1 antihistamines) and has anti-androgenic effects, including gynaecomastia but famotidine remains available without these risks. Skin testing with H1 and H2 analogues in healthy volunteers showed that H2 receptors in skin cause vasodilatation (erythema) and whealing but not flare [25]. Blockade of H1 and H2 receptors with chlorphenamine and cimetidine, respectively, resulted in significant histamine skin test weal suppression that was non-significantly greater with combined treatment [26].
13.1.4.1 Evidence for Using H2 Antihistamines in Urticaria
Total symptom score was significantly less with cimetidine and chlorphenamine than placebo and chlorphenamine in patients with chronic idiopathic urticaria (syn. CSU) not responding to chlorphenamine alone at 4 and 8 weeks [27]. A similar outcome was found with hydroxyzine and cimetidine [28]. In a study of symptomatic dermographism, the addition of ranitidine to cetirizine raised the threshold for a whealing response, but it did not improve symptoms overall [29]. A Cochrane review of H2 antihistamines in urticaria concluded that it did not allow confident decision-making about the use of H2-receptor antagonists for urticaria. Although some of the studies reported a measure of relief of symptoms of urticaria and rather minimal clinical improvement in some of the participants, the evidence was regarded as weak and unreliable [30]. A subsequent small randomized double-blind placebo-controlled study of patients with CSU found no benefit from adding ranitidine to cetirizine but was underpowered [31]. Clinical experience, nevertheless, suggests that combining H2 antihistamines with a second generation H1 antihistamine may be beneficial in some patients with chronic urticaria, despite the lack of confirmatory large placebo-controlled trials. There are no publications on the use of famotidine in chronic urticaria .
13.1.5 Immunosuppressives
Immunosuppressives have been used successfully as an adjunct to antihistamines for severe CSU since the demonstration of functional histamine releasing autoantibodies in some patients in the late 1980s giving rise to the concept of autoimmune urticaria. Ciclosporin has been the most widely used and studied.
13.1.5.1 Ciclosporin
Ciclosporin was isolated originally from a fungus (Hypocladium inflatum gams). It is a powerful immunosuppressive, inhibiting T cell activation by blocking lymphokines including interleukin-2. It also inhibits histamine release from basophils. This may be one of the reasons it can be useful for severe urticaria even when autoantibodies cannot be demonstrated. It is included in the 2018 EAACI/GA2LENWAO/EDF guideline treatment algorithm for chronic urticaria as a fourth line intervention in patients who fail omalizumab [1].
13.1.5.1.1 Evidence for Using Ciclosporin in Urticaria
Studies of ciclosporin in severe CSU [32,33,34] have shown that about 2/3 of patients clear on treatment but the condition often relapses on stopping. In a recent systematic review of 18 studies including 2 randomized controlled trials, the overall response rate to treatment with ciclosporin (2–5 mg/kg) at 4, 8 and 12 weeks was 54%, 66% and 73%, respectively [35]. About 25% of patients who cleared after treatment with ciclosporin at 4 mg/kg body weight for 4–8 weeks were still clear on an antihistamine 5 months later [34]. Some patients with symptomatic dermographism also benefit [36]. Another systematic review of the literature indicated that a positive baseline autologous serum skin test, basophil histamine release assay or basophil activation test, elevated baseline plasma D-dimer levels and low total IgE predict a good response to treatment [37].
13.1.5.1.2 Dose and Length of Treatment
There is still discussion about the best dose of ciclosporin and how long it should be taken. 38 Starting at 4 mg/kg body weight/day for 4 weeks, reducing to 3 mg/kg/day for 6 weeks and then 2 mg/kg/day for a final 6 weeks works for many patients. Lower doses taken for 5 months may also be effective [38]. More than one course of ciclosporin may be given although it is probably better to look at other therapies if this proves necessary. Long-term treatment with immunosuppressive therapies for over a year should only be undertaken when there is no reasonable alternative because there are potential concerns about encouraging infections, lymphomas and skin cancers. A review of the safety and effectiveness of ciclosporin taken at low doses for up to 10 years in one centre was favourable [39].
13.1.5.1.3 Possible Side Effects of Treatment
Among patients treated with <2 mg/kg, 2–<4 mg/kg and 4–5 mg/kg of ciclosprin, 6%, 23% and 57% experienced one or more adverse event, respectively [35]. The main risks are hypertension, renal impairment and predisposition to infections. Hyperkalaemia and increased lipids may occur. Some side effects of ciclosporin are more unpleasant than dangerous. They include slight tremor, burning sensations of the hands and feet, and swelling of the gums, nausea, muscle weakness, missed periods and increased facial hair growth, which settle on stopping treatment. The effectiveness and safety of some immunizations may be reduced and live vaccines should not be given for 3 months after stopping treatment.
13.1.5.1.4 Checks Before and During Treatment
The most important checks are on renal function, which may go down and blood pressure, which may go up. It is usual to check kidney function with two separate blood tests before starting treatment, every fortnight for the first month and then monthly. Blood pressure should be checked at the same time. Liver function should be checked on blood tests before, and every month on treatment because mild reversible inflammation may occur. Viral hepatitis and HIV infection should be excluded when there is clinical suspicion before starting treatment.
It is important to decide who will make these checks and who will be responsible for acting on any abnormal results. It is common practice to have ‘shared care’ agreements between primary care practitioners and hospitals, or hospital departments with each other. These should be worked out before treatment is started. Women of childbearing age should have a pregnancy test before starting and ensure adequate contraception throughout treatment and for 2 weeks after finishing. Breastfeeding should be avoided.
13.1.5.1.5 Interactions with Other Medicines
Some medicines may increase the level of ciclosporin in the blood including some antibiotics, painkillers (e.g. aspirin, ibuprofen), a treatment for gout (allopurinol) and some blood pressure treatments (e.g. nifedipine, diltiazem). Grapefruit juice can also do this. Other drugs reduce the levels of ciclosporin, such as some anticonvulsants (e.g. phenytoin, carbamazepine). It is recommended that St John’s wort should not be taken at the same time. There may be an increased risk of muscle inflammation with statins.
13.1.5.1.6 Cautions and Contraindications
The main reasons for not using ciclosporin would be reduced kidney function, uncontrolled blood pressure, active serious infections and previous cancers.
13.1.5.1.7 Summary
Ciclosporin is a useful treatment for many patients with severe and disabling chronic urticaria. A decision to start it must only be taken after trying other medicines, including antihistamines, and arrangements for careful monitoring must be in place.
13.1.5.2 Methotrexate
Is used in low doses as an immunosuppressive drug for a number of conditions including psoriasis and rheumatoid arthritis. It has also been found to help patients with severe chronic urticaria, especially when they would otherwise have to take regular steroids to control their symptoms or they are unable to tolerate other immunosuppressive therapies, such as ciclosporin. Methotrexate is a derivative of folic acid, known as an antifolate, which interferes with dihydrofolate reductase and the production of DNA in actively dividing cells.
13.1.5.2.1 Evidence for Using Methotrexate in Urticaria
There have only been a few reports of methotrexate being used successfully for chronic urticaria [40,41,42,43]. One controlled studies comparing it against placebo for 3 months five showed no benefit but the duration of treatment was probably too short since methotrexate is usually administered long term. Clinical experience, however, has shown that it may be valuable for selected urticaria patients who do not respond to antihistamine treatment, including those who would otherwise need steroids.
13.1.5.2.2 Dose and Length of Treatment
A small test dose of methotrexate is usually given to check it is suitable before beginning regular treatment at a higher dose. It is essential to take the medicine only once a week rather than daily to minimize the risk of myelosuppression. The benefit is not immediate. It may take 4–6 weeks to begin working. There is no definite limit on the length of time methotrexate can be taken, provided that there are no complications. It is common practice to recommend that Methotrexate is taken on Mondays and Folic acid on Fridays as a useful ‘aide memoire’ or folic acid on each day that is not the methotrexate day.
13.1.5.2.3 Possible Side effects of Treatment
Sore throats, bad mouth ulcers or unusual bruising may be a sign of reduced bone marrow function, mandating an urgent blood count. Pneumonitis may occur occasionally with prolonged treatment, especially in the rheumatoid disease population. Methotrexate should be stopped if a persistent dry cough or unexplained breathlessness develops until the possibility of pneumonitis due to the drug has been investigated. Alcohol can be more damaging to the liver than usual when on methotrexate. It should be avoided completely if possible during treatment. Nausea may be a problem for a day or two after taking methotrexate in some people but can often be reduced by taking an anti-sickness medicine beforehand, such as prochlorperazine, dividing the dose over 36 h or administering the treatment subcutaneously.
13.1.5.2.4 Checks Before and During Treatment
Blood must be checked for bone marrow, kidney and liver function before starting methotrexate. Viral hepatitis and HIV infection should be excluded. It is good practice to have a baseline chest X-ray. Blood counts and liver function tests must be checked weekly for the first month, fortnightly for a month and then monthly as long as the treatment continues. It is important to decide who will make these checks and who will be responsible for acting on any abnormal results. Women of child-bearing age must check that they are not pregnant with a pregnancy test before starting and ensure adequate contraception throughout treatment. Pregnancy and fathering children should be avoided for 6 months after finishing. Breastfeeding should be avoided during treatment. Keeping a personal booklet for methotrexate monitoring is recommended .
13.1.5.2.5 Interactions with Other Medicines
There are several types of medicine that should be taken with care or avoided:
-
1.
Aspirin and other non-steroidal anti-inflammatory drugs (e.g. ibuprofen, diclofenac): these may reduce elimination of methotrexate by the kidneys and increase the levels of methotrexate in the body.
-
2.
Antibacterials: some antibiotics can increase the risks of methotrexate affecting myelopoiesis. Examples of this include trimethoprim, co-trimoxazole and penicillins.
-
3.
Others: some medicines taken for inflammatory bowel disease (e.g. sulphasalazine), malaria (e.g. pyrimethamine), gout (e.g. probenecid) and epilepsy (e.g. phenytoin).
13.1.5.2.6 Cautions and Contraindications
Methotrexate should not normally be used if there is an underlying blood disorder, reduced kidney function, persistent liver inflammation, peptic ulceration or ulcerative colitis .
13.1.5.2.7 Summary
Methotrexate is a potentially useful treatment for some patients with disabling chronic urticaria who have not responded to guideline treatments and would otherwise need steroids to control it. Its use must be monitored closely with regular blood tests and some medicines should not be taken at the same time. Symptoms of infection, including bad sore throats, may be important and usually mean that the blood should be checked and methotrexate discontinued temporarily .
13.1.5.3 Mycophenolate Mofetil
Is an immune suppressing drug used primarily for the prevention of organ transplant rejection. It is also used for some severe skin diseases, including blistering disorders. It works by reducing the formation of lymphocytes that are involved in autoimmune conditions.
13.1.5.3.1 Evidence for Using Mycophenolate in Urticaria
There have only been two studies published to date. Nine patients with evidence of autoimmune CSU who had not been controlled on antihistamines and courses of steroids were treated with mycophenolate for 12 weeks [44]. Four cleared completely and five improved. The improvement was still present 6 months later. A later study involving chart review looked at the results of a step-up followed by a step-down approach to using mycophenolate for CSU. The average time to achieve disease control was 14 weeks at doses of mycophenolate ranging from 1 to 6 g daily [45]. As is the case for other low level evidence interventions, mycophenolate should only be used in selected patients when other treatments, including antihistamines, omalizumab and ciclosporin, alone or in combination, have failed or have not been well tolerated.
13.1.5.3.2 Dose and Length of Treatment
The starting dose is usually 1g of mycophenolate twice a day, but it may be necessary to increase this up to 1.5 g twice daily (maximum). The initial course of treatment should be for 3 months but longer periods may be appropriate for some patients.
13.1.5.3.3 Possible Side effects of Treatment
Mycophenolate is generally safe and well tolerated when used in short courses for urticaria. A number of important side effects have been reported in patients taking it in combination with other immunosuppressives to prevent transplant rejection, including infections (including pneumonia, cold sores, thrush and shingles), gastrointestinal symptoms (including abdominal pain, diarrhoea and nausea) anxiety, tremor and headache. The effectiveness of some vaccines may be reduced and live vaccines should not be given for 3 months after stopping treatment.
13.1.5.3.4 Interactions with Other Medicines
There are relatively few interactions with other medicines. The blood levels of mycophenolate may be affected by other immune suppressing drugs given at the same time, but this mainly applies to transplant patients. Other drugs that may affect the levels of mycophenolate include cholestyramine and rifampicin. Oral contraceptives are not affected .
13.1.5.3.5 Checks During Treatment
A full blood count should be checked weekly for the first month, fortnightly for the next 2 months and then monthly. Blood must be tested for liver function once every month.
13.1.5.3.6 Contraindications to Treatment
Mycophenolate must be avoided during pregnancy since it can cause birth defects. Women of child-bearing age should be on effective contraception throughout treatment. It should also be avoided during breast feeding. It should be stopped if severe infections, such as pneumonia or chickenpox develop. Immune suppressing drugs, such as mycophenolate, should not be used if there is a past history of cancer.
13.1.5.3.7 Summary
There is only limited evidence that mycophenolate can improve the symptoms of patients with severe CSU. It has a number of important risks when it is used with other immune suppressing treatments in transplant patients, but appears to be safe and well tolerated in chronic urticaria.
13.1.5.4 Azathioprine
Azathioprine is an immune suppressing drug that has been used for serious immune skin conditions for many years including blistering disorders and atopic eczema. Azathioprine is an imidazole derivative of 6-mercaptopurine (6-MP). It is rapidly broken down in vivo into 6-MP. 6-MP readily crosses cell membranes and is converted intracellularly into a number of purine thioanalogues. It reduces the number and function of T and B-cells.
13.1.5.4.1 Evidence for Using Azathioprine in Urticaria
Azathioprine has been used occasionally for patients with difficult chronic urticaria who would otherwise need systemic steroids. Steroids were withdrawn in two patients with chronic urticaria after treatment with azathioprine [46]. It was found to be as effective as ciclosporin for CSU in a recent randomized comparison [47].
13.1.5.4.2 Dose and Length of Treatment
The daily dose is based on body weight. Treatment usually starts at 2 mg/kg body weight per day but may need to go up or down a little from this. The tablets are taken two or three times a day. It is common practice to start them with steroids for the first 3 weeks and then continue without steroids for 3–6 months, but azathioprine can be used in other ways. The benefits of azathioprine seem to continue for months after stopping treatment in many patients with eczema, and the same may be true for urticaria.
13.1.5.4.3 Possible Side Effects of Treatment
The most important side effects are bone marrow suppression and liver inflammation. Malaise, aching, fevers or vomiting may occur rarely in the first week or two of treatment. Azathioprine must be stopped immediately if unexplained bruising, bleeding or serious infections develop. The effectiveness of some vaccines may be reduced and live vaccines should not be given for 3 months after stopping treatment. Total sunblocks should be worn in strong sunlight as a precaution to minimize any risk of skin cancers developing later in life.
13.1.5.4.4 Interactions with Other Medicines
Azathioprine should not be taken at the same time as other medicines that suppress the immune system unless essential, and the treatment must be monitored closely. Concomitant therapy with ACE-inhibitors, trimethoprim/sulphamethoxazole, cimetidine or indomethacin increases the risk of myelosuppression.
13.1.5.4.5 Checks During Treatment
Thiopurine methyltransferase (TPMT) should be checked before starting treatment and blood must be monitored regularly for bone marrow and liver function. The general rule is that blood tests should be done weekly for the first month, fortnightly for a month and then monthly.
13.1.5.4.6 Contraindications to Treatment
In common with all immune-suppressing drugs, azathioprine should be avoided if cancer has been treated in the past, including melanoma. It should also be avoided in pregnancy unless essential and in patients who have had previous bad reactions to it. It should not be used in patients who have had HIV or hepatitis B or C infection without careful assessment.
13.1.5.4.7 Summary
Azathioprine may be used occasionally for very severe CSU that has not responded to antihistamine treatment and would otherwise need regular steroids to control it. The treatment must be monitored carefully with blood tests and is usually given for 3–6 months .
13.1.6 Miscellaneous
13.1.6.1 Doxepin
Doxepin , a tricyclic antidepressant, has been used as a treatment for urticaria since the 1980s. It has potent H1 and H2 antihistaminic properties. It also has anti-cholinergic and anti-serotoninergic effects. The doses of doxepin used for depression are usually much higher than those used for urticaria. There is unlikely to be any mood lifting effect when taken for urticaria although it may be helpful if depression is also a problem. Doxepin may be most valuable when taken at night if sleep is disturbed by itching or swellings.
13.1.6.1.1 Evidence for Using Doxepin in Urticaria
Doxepin was found to be more effective than diphenhydramine at a dose of 10 mg three times a day [48] and as effective as mequitazine at a dose of 5 mg twice daily [49]. It has not been compared against modern non-sedating antihistamines.
13.1.6.1.2 Dose and Length of Treatment
It is best to start at the lowest dose which is 25 mg at night with an option of working up to 75 mg daily. This can either be taken as a single dose at night or split into two or three smaller doses over the day. The highest total daily dose recommended for depression is 300 mg with a maximum single dose of 100 mg but these very high levels are probably never appropriate for urticaria. There is no time limit for which doxepin can be taken.
13.1.6.1.3 Possible Side Effects of Treatment
Sedation is the commonest unwanted effect. A dry mouth and blurring of vision are more likely as the dose increases. Other side effects may include constipation, difficulty in passing water, feeling light headed on standing up quickly, increased appetite, rashes and some rare changes in the blood. There may be heart complications in the elderly with pre-existing cardiac disease.
13.1.6.1.4 Interactions with Other Medicines
One of the disadvantages of doxepin is the high number of possible interactions with other medicines (and alcohol too). These include other antidepressants, certain strong painkillers (e.g. tramadol), some drugs for heart rhythm problems (e.g. amiodarone), drugs for epilepsy and some antihypertensives (e.g. diltiazem). A few treatments that may be used for urticaria, such as epinephrine and cimetidine, should be avoided concurrently if possible.
13.1.6.1.5 Cautions and Contraindications
Doxepin should not be taken after a recent heart attack or in severe liver disease. It should be used with caution in pregnancy and the elderly since it may cause confusion, unwanted falls in blood pressure on standing up, glaucoma of the eyes and difficulty passing water.
13.1.6.1.6 Checks During Treatment
No routine checks are required.
13.1.6.1.7 Summary
Doxepin has been used to treat difficult urticaria for about 30 years. It has a number of side effects, which tend to increase with the dose and interactions with other medicines, which need to be considered carefully before starting.
13.1.6.2 Epinephrine
The use of epinephrine , in chronic urticaria, is limited to the treatment of patients with cold urticaria who develop anaphylaxis or angioedema of the upper airways after cold liquids. Patients with cold urticaria, who are at risk of anaphylaxis or angioedema of the throat should carry two epinephrine autoinjectors but this is rare [50]. Although some patients with severe episodes of CSU describe a feeling of tightness or scratchiness in the throat, they can be reassured that throat closure is not a feature of the illness. By contrast, throat angioedema is a feature of anaphylaxis and may be experienced in very severe acute urticaria.
13.1.6.2.1 Evidence for Using Epinephrine in Histaminergic Angioedema
There are no studies of epinephrine for throat angioedema or anaphylaxis in cold urticaria, but it is known to work well.
13.1.6.2.2 Dose and Method of Administration
Epinephrine injections for self-administration are available on prescription in preloaded syringes that deliver a single dose. The standard adult dose is 300 μg. A 500 μg injector is also available. Junior pens are available for children weighing 15–30 kg, which deliver 150 μg. A second dose may be necessary if the swelling has not started to go down within 5 min.
An over-the-counter epinephrine ‘puffer’ spray for asthma may be used for angioedema of the throat but is currently only available in the USA. The aerosol should be puffed 4–5 times directly onto the swelling in the throat and not inhaled (as directed for asthma attacks) or sprayed underneath the tongue (unless it is too swollen). The same number of puffs can be repeated after 5–10 min. An epinephrine injection can still be given if the swelling worsens despite the inhaler.
13.1.6.2.3 Possible Side Effects of Treatment
Epinephrine can be life-saving in an emergency but may also raise blood pressure, cause anxiety and shaking and make the skin look pale. These effects wear off within an hour and usually present no problems. However, they may be risky for a few people with poorly controlled high blood pressure, angina or those who are at risk of stroke.
13.1.6.2.4 Interactions with Other Medicines
Intramuscular epinephrine should ideally not be used at the same time as taking tricyclic antidepressants (e.g. amitriptyline, doxepin), beta-blockers (e.g. propranolol, atenolol) or angiotensin converting enzyme inhibitors (e.g. ramipril, captopril) but should be always be given in a life-threatening situation if other measures have failed.
13.1.6.2.5 Summary
Epinephrine is a valuable treatment for severe swelling of the tongue or throat and may be life-saving. It is usually given by intramuscular injection but it may be used as a puffer spray for throat or tongue angioedema. There are important potential interactions with tricyclic antidepressants and beta blockers. Patients with pre-existing angina and high blood pressure are at risk of exacerbation.
13.1.7 Steroids
13.1.7.1 Anabolic Steroids
Anabolic steroids are different to corticosteroids (e.g. prednisolone). Danazol is an example of an anabolic steroid. It is a synthetic steroid with properties of a weak androgen. It has complicated effects on sex hormone production and can be used for gynaecological conditions, including endometriosis. It also increases plasma proteins in the blood and may be used to treat hereditary angioedema where there is a deficiency of C1 inhibitor. It may work in cholinergic urticaria in a similar way, since a protease inhibitor called alpha-1-antichymotrypsin was found to be reduced.
13.1.7.2 Danazol
13.1.7.2.1 Evidence for Danazol in Cholinergic Urticaria
The level of alpha-1-antichymotrypsin increased with danazol treatment and wheal counts decreased over 4 weeks in a placebo-controlled study [51]. Several cases of patients with cholinergic urticaria responding to danazol have been reported [51, 52].
13.1.7.2.2 Details and Length of Treatment
Danazol is no longer available in many countries. Treatment can be continued for months or years if necessary at the lowest dose that controls symptoms. Danazol should be taken with an antihistamine. Although adverse effects are common in the HAE population on prolonged treatment, clinical experience shows that it is well tolerated in the short term (3–6 months) at doses between 200 and 600 mg daily in the cholinergic urticaria population and may allow re-establishment of symptom control with an antihistamine alone in some responders.
13.1.7.2.3 Possible Side Effects of Treatment
Up to 80% of patients treated with danazol in an HAE population can be expected to develop side effects in the long term, the most common ones being weight gain, virilization and menstrual disorders as well as headache, myalgia, depression and acne. There is also an increased risk of cardiovascular disease. Because of its androgenizing effects, it should be avoided or given at least doses in females. Pregnancy must be avoided. Abnormalities of liver function and lipoproteins may occur. Benign adenomas, rashes, muscle aches, depression, fatigue and changes in libido have been reported. It is not recommended in children or the elderly.
13.1.7.2.4 Checks Before and During Treatment
A full blood count, liver function tests and lipid profile should be done at baseline with repeat liver profile and cholesterol at 3 months. A blood count, liver profile and cholesterol should be repeated with every 6 months of continuous treatment. A liver ultrasound scan is advised every 2–3 years on long-term treatment.
13.1.7.2.5 Interactions with Other Medicines
Danazol may affect the plasma level of carbamazepine and other anticonvulsants. It can cause insulin resistance, potentiate the action of warfarin and oppose the action of anti-hypertensive agents, possibly through fluid retention. Taking statins metabolized by CYP3A4 (e.g. simvastatin) at the same time increases a risk of myopathy.
13.1.7.2.6 Cautions and Contraindications
Danazol must be avoided in pregnancy and breastfeeding, in patients with significantly impaired hepatic, renal or cardiac function and with active thrombosis.
13.1.7.2.7 Summary
Danazol can be considered in the short term for severe treatment-resistant cholinergic urticaria when high dose antihistamines are not effective. It is generally more suited to men since virilizing side effects in women may be unacceptable. Monitoring with blood tests is mandatory.
13.1.7.3 Corticosteroids (Steroids)
Oral steroids have been used for many years to treat severe urticaria that does not respond to antihistamines. They are useful acutely because they nearly always work if the dose is right. Larger doses given for longer (e.g. 20 mg prednisolone daily for a month) are immunosuppressive. The problem with steroids is the risk of unwanted effects if they are taken for many weeks or months without a break. They may also reduce the ability of the body to produce natural steroid (cortisol), which is essential for good health. Several different types of steroid tablets can be used. The usual one is prednisolone, which comes in plain, coated and soluble forms. Other oral formulations include prednisone (precursor of prednisolone), methyl prednisolone, betamethasone, dexamethasone and hydrocortisone.
13.1.7.3.1 Evidence for Using Corticosteroids in Chronic Urticaria
There is only one prospective study of prednisone for CSU, mainly because steroids were introduced before the era of evidence-based medicine [53]. About 50% of patients with antihistamine-unresponsive CSU responded well to prednisone starting at the relatively low dose of 25 mg for 3 days, 12.5 mg for 3 days, reducing to 6.25 mg/day over 4 days, but many relapsed despite antihistamines after stopping. There is a need for good studies to show how long they should be taken and the best dose.
13.1.7.3.2 Dose and Length of Treatment
Clinical experience has shown that taking prednisolone at around ½ mg per kilogram body weight (usually 25–40 mg daily in adults) for 1–3 days can be very helpful for the most severe attacks of urticaria or bad attacks of angioedema as ‘rescue’ treatment. There is little risk from doing so provided the courses are not repeated too often. Long courses of continuous steroids must generally be avoided although there may be special situations when this might be necessary in some people, such as delayed pressure urticaria that cannot be controlled in other ways. There are many ways of prescribing steroids but it is usually appropriate to reduce the dose slowly after being on them continuously for more than 3 weeks, especially if treatment has been taken for months.
13.1.7.3.3 Possible Side Effects of Treatment
Short courses of steroids can sometimes make people feel more energetic and wakeful, but this does not last for more than a few days. This is why steroids are usually taken in the morning to reduce wakefulness at night. It is common to feel lacking in energy as the dose comes down after a long course of treatment. There is a tendency to gain weight, unless care is taken to prevent this, and to lose muscle strength. The skin and bones may become weaker. Spots, increased body and facial hair growth and bruises may be more likely. Serious infections, such as chickenpox in adults, may be more harmful and measures may be necessary to give protection. Some vaccinations may not ‘take’ well and others may not be safe. Increased blood pressure and sugar diabetes may be promoted. Stopping steroids suddenly after a long period of time can lead to low blood pressure and faintness. They should therefore be reduced cautiously on medical advice after a long course of treatment.
13.1.7.3.4 Interactions with Other Medicines and Reasons for Being Careful
Steroids can usually be taken safely with other medicines except aspirin and other non-steroidal anti-inflammatory drugs (e.g. ibuprofen) because there is an increased risk of gastric bleeding. They should be used with care in diabetics and patients with stomach ulcers, high blood pressure and osteoporosis. Prolonged courses of corticosteroids increase susceptibility to infections and severity of infection. Patients who have never had chickenpox should be regarded as being at risk of severe infection.
13.1.7.3.5 Checks During Treatment
No checks are usually needed when steroids are taken for 10 days or less. It is good practice to check weight, urine (for sugar) and blood pressure in the clinic when steroids are taken regularly for weeks. Bone density scans (DEXA) should be done if steroids have to be taken for at least 6 months. Steroids should only be prescribed in the first trimester of pregnancy if essential because of concerns about abnormalities developing in the baby although they are generally very safe.
13.1.7.3.6 Summary
Oral corticosteroids may be necessary for the most difficult forms of urticaria but should only be taken for the shortest period necessary and at the least dose. Rescue prednisolone for one to three days can be taken in addition to antihistamines for severe urticaria outbreaks, including angioedema. Steroids should not be stopped suddenly after 6 weeks and the dose should be agreed with the specialist or GP.
13.1.8 Anticoagulants
There is a small literature on anticoagulants being effective in CSU, possibly relating to the observed activation of the extrinsic (tissue factor) pathway in chronic urticaria with increased D-Dimer and prothrombin fragment F1+2 formation being related to disease severity [54]. However the risks of anticoagulation are significant.
13.1.8.1 Heparin
A patient with treatment refractory CSU not responding to warfarin cleared completely with subcutaneous heparin given for an unrelated reason [55]. A small cohort of antihistamine-resistant CSU patients with elevated D-dimer improved with heparin and tranexamic acid [56].
13.1.8.2 Warfarin
There have been several case reports of CSU responding to warfarin . A small cross-over study appeared to confirm this [57]. The same patient responded to two different coumarin anticoagulants including warfarin, suggesting a class benefit [58].
13.1.9 Antineutrophilic Drugs
13.1.9.1 Colchicine
Is mainly used in the context of neutrophilic urticaria, normocomplementaemic urticarial vasculitis and neutrophilic urticarial dermatoses but one retrospective review found that it might be helpful in chronic urticaria [59].
13.1.9.2 Biologicals
There is increasing enthusiasm to identify biological drugs to treat patients with chronic urticaria who do not respond to H1 antihistamines and omalizumab but only a few small studies and case reports have been published to date. The evidence is generally insufficient to justify the risks and costs of biological agents other than omalizumab for chronic urticaria at the present time.
13.1.9.3 Anakinra
Benefit has been reported in a case of cold contact urticaria with positive ice cube test and negative NLRP3 mutation [60] and in refractory delayed pressure urticaria [61]. By contrast, anakinra is the treatment of choice for Schnitzler syndrome and may be used in other autoinflammatory disorders presenting with urticarial rash.
13.1.9.4 Anti-TNFs
A case report [62] and open series suggest that etanercept [63], adalimumab [64] or infliximab [65] may be useful for treatment refractory CSU, especially cases that do not respond to omalizumab.
13.1.9.5 Rituximab
Although depletion of B-cells and reduction of functional autoantibodies are theoretically desirable as a way of providing long-term control of autoimmune urticaria rare reports of severe risk including progressive multifocal encephalopathy make this option unattractive. Case reports indicate that it may [66] or may not [67] be effective for CSU.
Treatment with the monoclonal antibodies secukinumab, mepolizumab, benralizumab, reslizumab and dupilumab has also been reported to benefit patients with chronic urticaria anecdotally.
13.1.10 Immunosuppressives (Other than Ciclosporin, Methotrexate, Azathioprine and Mycophenolate Mofetil)
Tacrolimus and cyclophosphamide have been reported in treatment refractory chronic urticaria in addition to the more commonly used immunosuppressive options. The evidence for using them is less and there does not appear to be a clear advantage. Cyclophosphamide carries additional risks of haemorrhagic cystitis, secondary tumours and infertility when given intravenously and should probably be avoided.
13.1.10.1 Tacrolimus
Two open series indicate a similar response rate to ciclosporin with complete resolution in some patients [68, 69].
13.1.10.2 Cyclophosphamide
Case reports have documented good outcomes with oral [70] and intravenous treatment [71].
13.1.11 Immunomodulators
Recognition that some patients have an autoimmune aetiology has promoted the use of immunomodulatory drugs as well as immunosuppressives to optimize safety. Reports to date have been encouraging but do not support the routine use of these products.
13.1.11.1 Hydroxychloroquine
A placebo-controlled study showed an improvement in quality of life of patients with CSU but no overall improvement in disease activity [72] but a later study showed a lower proportion of therapeutic failures [73].
13.1.11.2 Intravenous Immunoglobulins
There have been no double-blind studies to date but open studies suggest benefit [74,75,76]. However, the risk of infusion reactions, including aseptic meningitis, and relative shortage of IVIG in some communities mean that this option should only be used in exceptional cases.
13.1.11.3 Plasmapheresis
Was used as a proof of concept that functional autoantibodies were potentially pathogenic in a small case series [77]. Although it has been adopted successfully in clinical practice since then it is not very practical in the long term as urticaria relapses when autoantibodies recover weeks after treatment cessation.
13.1.12 Vitamin D
Reports of vitamin D deficiency correction in chronic urticaria leading to improvement in disease activity are interesting [78, 79], but a recent systematic review concluded that although high dose vitamin D supplementation for 4–12 weeks might help to decrease the disease activity in some CSU patients, well-designed randomized placebo-controlled studies are now needed to determine the cut-off levels of vitamin D for supplementation and treatment outcomes [80].
13.2 Non-drug Interventions
13.2.1 Diet
Although IgE-mediated food allergy is a rare underlying cause of chronic spontaneous urticaria (CSU), there are some patients in which pseudoallergic reactions to naturally occurring food ingredients or food additives have been observed. When in doubt, a pseudoallergen-free diet should be tried. Diet protocols containing low levels of natural as well as artificial food pseudoallergens are available and have been successfully used in different countries. Also, a low histamine diet may improve symptoms in some patients. This kind of treatment requires very cooperative and adherent patients, since it usually comprises a trial period of at least 2–3 weeks before beneficial effects are observable. Success rates may also vary considerably due to regional and cultural differences in eating and food preparation habits. However, those diets are not yet proven in well-designed double-blind placebo-controlled studies and are therefore still controversial [1].
Diets have been used for many years to manage urticaria but the benefits have been difficult to ascertain due to the lack of blinding in studies (except oral provocation) and the natural history of chronic urticaria to remit. There is an old literature on minimizing dietary salicylates and food additives (including colours, preservatives, stabilizers, anti-oxidants and flavour enhancers) that are incorporated into a low pseudoallergen diet that has been popular in Europe for over 20 years. More recently, low histamine diets have been promoted based on open studies. A systematic review of publications on diet in chronic urticaria divided diets into three main groups: low pseudoallergen, low histamine and fish avoidance, which induced complete remission in 4.8%, 11.7% and 10.6% of patients, with partial remission in 37.0%, 43.9% and 4.3%, respectively [81]. The authors concluded that there is evidence for the benefit of diets in symptomatic CSU patients only. However, the level of evidence is low for the benefit of systematic diets in CSU and double-blind controlled trials of diet are lacking.
13.2.1.1 Low Pseudoallergen Diet
Pseudoallergic food reactions are due to intolerance rather than allergy. This means that conventional skin and blood tests for specific IgE are negative. They resemble allergic reactions (hence the name) since histamine release from mast cells with leukotriene generation is believed to mediate the symptoms of urticaria. Urinary leukotriene levels reduced more in CSU patients responding to a low pseudoallergen diet than non-responders [82]. Dietary pseudoallergens are not restricted to food additives and natural salicylates. They include histamine (found in tuna, bananas, avocado, walnut and well-matured cheeses) and alcohol. They have been found in tomato extracts, white wine and herbs [83]. There are no simple diagnostic tests for dietary pseudoallergens. The best way of showing whether or not they aggravate or even cause urticaria is to go on a strict low pseudoallergen diet for 3 weeks. If the urticaria improves on the diet, it is likely that pseudoallergens in food or beverages were making it worse. It may then be possible to track down which foods should be avoided by reintroducing them at intervals of 3 days. Food intolerance usually settles when urticaria clears so it is often possible to reintroduce the offending foods later, unlike allergies which may be life-long.
13.2.1.1.1 Evidence for Low Pseudoallergen Diets in Urticaria
Over 70% of 64 In-patients with chronic spontaneous urticaria improved over 2 weeks on a strict low pseudoallergen diet after eating only cooked potatoes, rice and water for 3 days before admission [84]. Over 70% of them showed some improvement over the first 2 weeks of the diet as an inpatient but only 19% of the diet responders reacted to challenge capsules containing additives or salicylic acid, suggesting that other substances in food were relevant. Most of the patients were still improved or clear after 6 months, without antihistamines, and about half were back on a normal diet without problems. About 30% of outpatients responded to the same diet in another prospective open study by the same group but less than half of these did very well [85]. A study from Italy found similar results [86]. Patients responding to a pseudoallergen diet for 5 weeks who followed a step-wise incremental build-up challenge protocol found one or more groups of foods triggered a recurrence of their urticaria, often foods containing biogenic amines (such as histamine) and salicylates [87].
13.2.1.1.2 Details and Length of Treatment
The diet should be followed for 3 weeks before foods are reintroduced step-by-step every 3 days if there has been an improvement. There is no specific order for this but it makes sense to add favourite foods first and leave those that are more likely to be responsible for the urticaria (including alcohol) to the end. If, on the other hand, there has been no improvement on the low pseudoallergen diet, a full diet can be restarted.
13.2.1.1.3 Compatibility with Other Diets
A dietician should normally be consulted if the patient requires a diet for other medical reasons, such as diabetes, coeliac disease, high blood fats or weight reduction.
13.2.1.1.4 Summary
A low pseudoallergen diet may be helpful for some patients with chronic spontaneous urticaria. It may be tried instead of antihistamines or as well as them if they do not provide sufficient relief. Failure to improve within 3 weeks indicates that diet is not useful and that food is not the cause.
13.2.1.2 Low Histamine Diet
Histamine rich foods include fermented foods, beer or wine, scombroid fish, cheese, vinegar and pickles. Dietary histamine is metabolized mainly by diamine oxidase (DAO) in the bowel.
13.2.1.2.1 Evidence for a Low Histamine Diet
The weekly urticarial activity scores and plasma histamine levels were significantly lower after a 4 week low histamine diet in a small study of Korean CSU patients although plasma levels of DAO were unchanged [88]. A third of patients with moderate to severe CSU gave a history of histamine intolerance. They were challenged with oral histamine after a low histamine and pseudoallergen diet. During the diet, 46% of patients responded with reduced CSU activity (UAS7 reduction of ≥7). Following double-blind, placebo-controlled oral histamine provocation, 17% of patients gave a positive weal response. There appeared to be little relationship between patient history, response to diet and the weal response to oral histamine provocation. The authors concluded that histamine intolerance as a cause of CSU was rare and could not be diagnosed from the history [89].
13.2.1.2.2 Phototherapy and Photochemotherapy
In addition to a literature on using phototherapy to desensitize patients with solar urticaria, there is a small but increasing literature on narrow-band ultraviolet B phototherapy (NB-UVB) and psoralen with ultraviolet A (PUVA) therapy to treat CSU or symptomatic dermographism when antihistamines and other ‘second line’ medicines have been unsuccessful. The mechanism is unknown but may involve reduction of mast cell ‘releasability’.
13.2.1.2.3 Evidence for Phototherapy in Urticaria
A comparison of NB-UVB with PUVA in steroid-dependent antihistamine unresponsive patients with CSU showed an improvement in average UAS7 over 90–180 days [90]. There was a significant reduction in UAS7 over 8–16 sessions in patients with CSU allocated randomly to loratadine alone or loratadine with phototherapy [91]. An open study showed clearance in just under half of CSU patients and improvement in the remainder with a median number of 31 exposures [92]. Open studies of antihistamine-unresponsive symptomatic dermographism have also show benefit [93, 94].
13.2.1.2.4 Dose and Length of Treatment
Ultraviolet treatments are given two or three times a week for at least 6 weeks but the specific details will depend on protocols in each centre, skin type and the response to treatment.
13.2.1.2.5 Interactions with Other Medicines
Some medicines, such as tetracycline antibiotics, make the skin more sensitive to ultraviolet therapy and should be avoided during treatment if possible. Antihistamines can be taken safely.
13.2.1.2.6 Checks During Treatment
The skin should be checked for cancers before starting treatment and any new moles or lumps that come up during it should be looked at.
13.2.1.2.7 Reasons for Avoiding Ultraviolet Treatment
Ultraviolet should not usually be given after previous skin cancers or radiation therapy. Having heat bumps (polymorphic light eruption) or other light-sensitive conditions, such as lupus erythematosus, would usually be a contraindication. Loss of skin colour (vitiligo) would put the skin at risk of burning very easily.
13.2.1.2.8 Possible Side Effects of Treatment
Burning can usually be avoided by increasing the doses carefully. There are slight concerns about the long-term effects of ultraviolet radiation encouraging skin cancers. These risks are probably very small initially but may increase after around 150 PUVA treatments and 300 NB-UVB exposures.
13.2.1.2.9 Summary
NB-UVB and PUVA treatments may be helpful for troublesome CSU and symptomatic dermographism but should only be tried if antihistamines have not worked well. There are some risks of burning and slight concern about possibly promoting skin cancers with high exposures to ultraviolet. A course of treatment usually involves at least 18 visits to hospital. It is not clear how long any relief from urticaria will last afterwards.
13.2.1.3 Psychological Therapies
There is increasing recognition of the psychological burden of chronic urticaria and its association with mental health disorders. A systematic review and meta-analysis of psychiatric co-morbidity in chronic urticaria patients revealed that almost one out of three CU patients have at least one underlying psychiatric disorder [95]. None of the studies reviewed clarified whether the psychiatric disorders pre-existed the CU onset or not, and no association was found between CU severity and duration and psychological functioning. The review highlights the need for a multidisciplinary therapeutic approach involving prompt recognition and management of any potential psychiatric disorder in addition to urticaria treatment. Another systematic review identified psychosocial factors as having a prevalence of 46% in CSU but their contribution to the development and exacerbation of illness symptoms was not quantifiable [96]. A recent report suggested that disease activity and stress are linked in a subpopulation of chronic spontaneous urticaria patients [97].
13.2.1.3.1 Evidence for Psychotherapy in Chronic Urticaria
There are few reported studies of psychological therapies for patients with CU promoting resolution of urticaria but no reports of pharmacological management of mental health disorders resulting in disease resolution. Four patients (three CSU and one idiopathic angioedema) were recruited into a brief Whole Person Treatment Approach course based on non-dualistic concepts of mind and body connectedness, and utilizing psychotherapy-derived listening skills for up to 10 h long sessions, once per week. Treatment efficacy rating, using Urticaria Activity Score and the Urticaria Severity Score, and reduction of drug usage, showed patients experienced long-term resolution of urticaria and cessation of hospitalization for angioedema and came off regular antihistamine medication [98]. Hypnosis provided relief of pruritus as measured by three self-report parameters in a small study by comparison with baseline and control session values but there was no change in the number of hives. Hypnotizable patients had fewer hives and were more symptomatic during the control session. At review 5–14 months after therapy, six patients were free of hives and an additional seven reported improvement [99].
13.2.1.4 Psychotherapy
Psychosocial situation should be evaluated in all patients with chronic urticaria not only to uncover and solve potential problems for treatment adherence but also to detect stress factors that may cause or contribute to urticaria symptoms.
13.2.1.5 Desensitization
Desensitization protocols do exist for several forms of inducible urticarias. As they consist of frequent re-exposure to the trigger for whealing, they are often met with adherence problems. Protocols exist for solar urticaria in which UV-light exposure is required, for cholinergic urticaria in which sweat-inducing activity is required, cold urticaria which require daily cold baths or showers and for heat urticaria which require the application of heat to the skin at the determined threshold. Desensitization in solar urticaria is not recommended due to any increase of risk for skin cancer development. All other desensitization protocols do require a great amount of commitment from the patient and do not acquire long-term tolerance. All desensitization treatments come with the risk of severe exacerbation of urticaria induced by trigger exposure.
References
Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73(7):1393–414.
Watanabe J, Shimamoto J, Kotani K. The Effects of Antibiotics for Helicobacter pylori Eradication or Dapsone on Chronic Spontaneous Urticaria: A Systematic Review and Meta-Analysis. Antibiotics (Basel). 2021;10(2):156.
Boehm I, Bauer R, Bieber T. Urticaria treated with dapsone. Allergy. 1999;54(7):765–6.
Noda S, Asano Y, Sato S. Long-term complete resolution of severe chronic idiopathic urticaria after dapsone treatment. J Dermatol. 2012;39(5):496–7.
Engin B, Ozdemir M. Prospective randomized non-blinded clinical trial on the use of dapsone plus antihistamine vs. antihistamine in patients with chronic idiopathic urticaria. J Eur Acad Dermatol Venereol. 2008;22(4):481–6.
Grundmann SA, Kiefer S, Luger TA, Brehler R. Delayed pressure urticaria - dapsone heading for first-line therapy? J Dtsch Dermatol Ges. 2011;9(11):908–12.
Liang SE, Hoffmann R, Peterson E, Soter NA. Use of dapsone in the treatment of chronic idiopathic and autoimmune urticaria. JAMA Dermatol. 2019;155(1):90–5.
Morgan M, Cooke A, Rogers L, Adams-Huet B, Khan DA. Double-blind placebo-controlled trial of dapsone in antihistamine refractory chronic idiopathic urticaria. J Allergy Clin Immunol Pract. 2014;2(5):601–6.
Jaffer AM. Sulfasalazine in the treatment of corticosteroid-dependent chronic idiopathic urticaria. J Allergy Clin Immunol. 1991;88(6):964–5.
McGirt LY, Vasagar K, Gober LM, Saini SS, Beck LA. Successful treatment of recalcitrant chronic idiopathic urticaria with sulfasalazine. Arch Dermatol. 2006;142(10):1337–42.
Engler RJ, Squire E, Benson P. Chronic sulfasalazine therapy in the treatment of delayed pressure urticaria and angioedema. Ann Allergy Asthma Immunol. 1995;74(2):155–9.
Orden RA, Timble H, Saini SS. Efficacy and safety of sulfasalazine in patients with chronic idiopathic urticaria. Ann Allergy Asthma Immunol. 2014;112(1):64–70.
Munch EP, Weeke B. Non-hereditary angioedema treated with tranexamic acid. A 6-month placebo controlled trial with follow-up 4 years later. Allergy. 1985;40(2):92–7.
Du-Thanh A, Raison-Peyron N, Drouet C, Guillot B. Efficacy of tranexamic acid in sporadic idiopathic bradykinin angioedema. Allergy. 2010;65(6):793–5.
Powell RJ, Leech SC, Till S, Huber PA, Nasser SM, Clark AT, et al. BSACI guideline for the management of chronic urticaria and angioedema. Clin Exp Allergy. 2015;45(3):547–65.
Pacor ML, Di Lorenzo G, Corrocher R. Efficacy of leukotriene receptor antagonist in chronic urticaria. A double-blind, placebo-controlled comparison of treatment with montelukast and cetirizine in patients with chronic urticaria with intolerance to food additive and/or acetylsalicylic acid. Clin Exp Allergy. 2001;31(10):1607–14.
Nettis E, Pannofino A, Cavallo E, Ferrannini A, Tursi A. Efficacy of montelukast, in combination with loratadine, in the treatment of delayed pressure urticaria. J Allergy Clin Immunol. 2003;112(1):212–3.
Nettis E, Colanardi MC, Soccio AL, Ferrannini A, Vacca A. Desloratadine in combination with montelukast suppresses the dermographometer challenge test papule, and is effective in the treatment of delayed pressure urticaria: a randomized, double-blind, placebo-controlled study. Br J Dermatol. 2006;155(6):1279–82.
Akenroye AT, McEwan C, Saini SS. Montelukast reduces symptom severity and frequency in patients with angioedema-predominant chronic spontaneous urticaria. J Allergy Clin Immunol Pract. 2018;6(4):1403–5.
Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: a single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110(3):484–8.
Wan KS. Efficacy of leukotriene receptor antagonist with an anti-H1 receptor antagonist for treatment of chronic idiopathic urticaria. J Dermatolog Treat. 2009;20(4):194–7.
Sarkar TK, Sil A, Pal S, Ghosh C, Das NK. Effectiveness and safety of levocetirizine 10 mg versus a combination of levocetirizine 5 mg and montelukast 10 mg in chronic urticaria resistant to levocetirizine 5 mg: A double-blind, randomized, controlled trial. Indian J Dermatol Venereol Leprol. 2017;83(5):561–8.
Tedeschi A, Suli C, Lorini M, Airaghi L. Successful treatment of chronic urticaria. Allergy. 2000;55(11):1097–8.
Hani N, Hartmann K, Casper C, Peters T, Schneider LA, Hunzelmann N, et al. Improvement of cold urticaria by treatment with the leukotriene receptor antagonist montelukast. Acta Derm Venereol. 2000;80(3):229.
Robertson I, Greaves MW. Responses of human skin blood vessels to synthetic histamine analogues. Br J Clin Pharmacol. 1978;5:319–22.
Marks R, Greaves MW. Vascular reactions to histamine and compound 48/80 in human skin: suppression by a histamine H2-receptor blocking agent. Br J Clin Pharmacol. 1977;4(3):367–9.
Bleehen SS, Thomas SE, Greaves MW, Newton J, Kennedy CT, Hindley F, et al. Cimetidine and chlorpheniramine in the treatment of chronic idiopathic urticaria: a multi-centre randomized double-blind study. Br J Dermatol. 1987;117(1):81–8.
Minocha YC, Minocha KB, Sood VK, Dogra A. Evaluation of H2 receptor antagonists in chronic idiopathic urticaria. Indian J Dermatol Venereol Leprol. 1995;61(5):265–7.
Sharpe GR, Shuster S. In dermographic urticaria H2 receptor antagonists have a small but therapeutically irrelevant additional effect compared with H1 antagonists alone. Br J Dermatol. 1993;129(5):575–9.
Fedorowicz Z, van Zuuren EJ, Hu N. Histamine H2-receptor antagonists for urticaria. Cochrane Database Syst Rev. 2012;3
Guevara-Gutierrez E, Bonilla-Lopez S, Hernandez-Arana S, Tlacuilo-Parra A. Safety and efficacy of cetirizine versus cetirizine plus ranitidine in chronic urticaria: double-blind randomized placebo-controlled study. J Dermatolog Treat. 2015;26(6):548–50.
Barlow RJ, Kobza Black A, Greaves MW. Treatment of severe chronic urticaria with cyclosporin. Eur J Dermatol. 1993;3:273–5.
Toubi E, Blant A, Kessel A, Golan TD. Low-dose cyclosporin A in the treatment of severe chronic idiopathic urticaria. Allergy. 1997;52(3):312–6.
Grattan CE, O'Donnell BF, Francis DM, Niimi N, Barlow RJ, Seed PT, et al. Randomized double-blind study of cyclosporin in chronic ‘idiopathic’ urticaria. Br J Dermatol. 2000;143(2):365–72.
Kulthanan K, Chaweekulrat P, Komoltri C, Hunnangkul S, Tuchinda P, Chularojanamontri L, et al. Cyclosporine for chronic spontaneous urticaria: a meta-analysis and systematic review. J Allergy Clin Immunol Pract. 2018;6(2):586–99.
Toda S, Takahagi S, Mihara S, Hide M. Six cases of antihistamine-resistant dermographic urticaria treated with oral ciclosporin. Allergol Int. 2011;60(4):547–50.
Kulthanan K, Subchookul C, Hunnangkul S, Chularojanamontri L, Tuchinda P. Factors predicting the response to cyclosporin treatment in patients with chronic spontaneous urticaria: a systematic review. Allergy Asthma Immunol Res. 2019;11(5):736–55.
Boubouka CD, Charissi C, Kouimintzis D, Kalogeromitros D, Stavropoulos PG, Katsarou A. Treatment of autoimmune urticaria with low-dose cyclosporin A: a one-year follow-up. Acta Derm Venereol. 2011;91(1):50–4.
Kessel A, Toubi E. Cyclosporine-A in severe chronic urticaria: the option for long-term therapy. Allergy. 2010;65(11):1478–82.
Weiner MJ. Methotrexate in corticosteroid-resistant urticaria. Ann Intern Med. 1989;110(10):848.
Gach JE, Sabroe RA, Greaves MW, Black AK. Methotrexate-responsive chronic idiopathic urticaria: a report of two cases. Br J Dermatol. 2001;145(2):340–3.
Perez A, Woods A, Grattan CE. Methotrexate: a useful steroid-sparing agent in recalcitrant chronic urticaria. Br J Dermatol. 2010;162(1):191–4.
Saghi L, Solomon M, Baum S, Lyakhovitsky A, Trau H, Barzilai A. Evidence for methotrexate as a useful treatment for steroid-dependent chronic urticaria. Acta Derm Venereol. 2011;91:303–6.
Shahar E, Bergman R, Guttman-Yassky E, Pollack S. Treatment of severe chronic idiopathic urticaria with oral mycophenolate mofetil in patients not responding to antihistamines and/or corticosteroids. Int J Dermatol. 2006;45(10):1224–7.
Zimmerman AB, Berger EM, Elmariah SB, Soter NA. The use of mycophenolate mofetil for the treatment of autoimmune and chronic idiopathic urticaria: experience in 19 patients. J Am Acad Dermatol. 2012;66(5):767–70.
Tal Y, Toker O, Agmon-Levin N, Shalit M. Azathioprine as a therapeutic alternative for refractory chronic urticaria. Int J Dermatol. 2015;54(3):367–9.
Pathania YS, Bishnoi A, Parsad D, Kumar A, Kumaran MS. Comparing azathioprine with cyclosporine in the treatment of antihistamine refractory chronic spontaneous urticaria: a randomized prospective active-controlled non-inferiority study. World Allergy Organ J. 2019;12(5):100033.
Greene SL, Reed CE, Schroeter AL. Double-blind crossover study comparing doxepin with diphenhydramine for the treatment of chronic urticaria. J Am Acad Dermatol. 1985;12(4):669–75.
Harto A, Sendagorta E, Ledo A. Doxepin in the treatment of chronic urticaria. Dermatologica. 1985;170(2):90–3.
Yee CSK, El Khoury K, Albuhairi S, Broyles A, Schneider L, Rachid R. Acquired cold-induced urticaria in pediatric patients: a 22-year experience in a tertiary care center (1996-2017). J Allergy Clin Immunol Pract. 2019;7(3):1024–31.e3.
Wong E, Eftekhari N, Greaves MW, Ward AM. Beneficial effects of danazol on symptoms and laboratory changes in cholinergic urticaria. Br J Dermatol. 1987;116(4):553–6.
Berth-Jones J, Graham-Brown RA. Cholinergic pruritus, erythema and urticaria: a disease spectrum responding to danazol. Br J Dermatol. 1989;121(2):235–7.
Asero R, Tedeschi A. Usefulness of a short course of oral prednisone in antihistamine-resistant chronic urticaria: a retrospective analysis. J Investig Allergol Clin Immunol. 2010;20(5):386–90.
Chua SL, Gibbs S. Chronic urticaria responding to subcutaneous heparin sodium. Br J Dermatol. 2005;153(1):216–7.
Asero R, Tedeschi A, Coppola R, Griffini S, Paparella P, Riboldi P, et al. Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119(3):705–10.
Asero R, Tedeschi A, Cugno M. Heparin and tranexamic acid therapy may be effective in treatment-resistant chronic urticaria with elevated d-dimer: a pilot study. Int Arch Allergy Immunol. 2010;152(4):384–9.
Parslew R, Pryce D, Ashworth J, Friedmann PS. Warfarin treatment of chronic idiopathic urticaria and angio-oedema. Clin Exp Allergy. 2000;30(8):1161–5.
Samarasinghe V, Marsland AM. Class action of oral coumarins in the treatment of a patient with chronic spontaneous urticaria and delayed-pressure urticaria. Clin Exp Dermatol. 2012;37(7):741–3.
Pho LN, Eliason MJ, Regruto M, Hull CM, Powell DL. Treatment of chronic urticaria with colchicine. J Drugs Dermatol. 2011;10(12):1423–8.
Bodar EJ, Simon A, de Visser M, van der Meer JW. Complete remission of severe idiopathic cold urticaria on interleukin-1 receptor antagonist (anakinra). Neth J Med. 2009;67(9):302–5.
Lenormand C, Lipsker D. Efficiency of interleukin-1 blockade in refractory delayed-pressure urticaria. Ann Intern Med. 2012;157(8):599–600.
Magerl M, Philipp S, Manasterski M, Friedrich M, Maurer M. Successful treatment of delayed pressure urticaria with anti-TNF-alpha. J Allergy Clin Immunol. 2007;119(3):752–4.
Sand FL, Thomsen SF. TNF-alpha inhibitors for chronic urticaria: experience in 20 patients. J Allergy (Cairo). 2013;2013:130905.
Bangsgaard N, Skov L, Zachariae C. Treatment of refractory chronic spontaneous urticaria with adalimumab. Acta Derm Venereol. 2017;97(4):524–5.
Wilson LH, Eliason MJ, Leiferman KM, Hull CM, Powell DL. Treatment of refractory chronic urticaria with tumor necrosis factor-alfa inhibitors. J Am Acad Dermatol. 2011;64(6):1221–2.
Chakravarty SD, Yee AF, Paget SA. Rituximab successfully treats refractory chronic autoimmune urticaria caused by IgE receptor autoantibodies. J Allergy Clin Immunol. 2011;128(6):1354–5.
Mallipeddi R, Grattan CE. Lack of response of severe steroid-dependent chronic urticaria to rituximab. Clin Exp Dermatol. 2007;32(3):333–4.
Kessel A, Bamberger E, Toubi E. Tacrolimus in the treatment of severe chronic idiopathic urticaria: an open-label prospective study. J Am Acad Dermatol. 2005;52(1):145–8.
Dorman SM Jr, Regan SB, Khan DA. Effectiveness and safety of oral tacrolimus in refractory chronic urticaria. J Allergy Clin Immunol Pract. 2019;7(6):2033–4.e1.
Asero R. Oral cyclophosphamide in a case of cyclosporin and steroid-resistant chronic urticaria showing autoreactivity on autologous serum skin testing. Clin Exp Dermatol. 2005;30(5):582–3.
Bernstein JA, Garramone SM, Lower EG. Successful treatment of autoimmune chronic idiopathic urticaria with intravenous cyclophosphamide. Ann Allergy Asthma Immunol. 2002;89(2):212–4.
Reeves GE, Boyle MJ, Bonfield J, Dobson P, Loewenthal M. Impact of hydroxychloroquine therapy on chronic urticaria: chronic autoimmune urticaria study and evaluation. Intern Med J. 2004;34(4):182–6.
Boonpiyathad T, Sangasapaviliya A. Hydroxychloroquine in the treatment of anti-histamine refractory chronic spontaneous urticaria, randomized single-blinded placebo-controlled trial and an open label comparison study. Eur Ann Allergy Clin Immunol. 2017;49(5):220–4.
O'Donnell BF, Barr RM, Black AK, Francis DM, Kermani F, Niimi N, et al. Intravenous immunoglobulin in autoimmune chronic urticaria. Br J Dermatol. 1998;138(1):101–6.
Dawn G, Urcelay M, Ah-Weng A, O'Neill SM, Douglas WS. Effect of high-dose intravenous immunoglobulin in delayed pressure urticaria. Br J Dermatol. 2003;149(4):836–40.
Mitzel-Kaoukhov H, Staubach P, Muller-Brenne T. Effect of high-dose intravenous immunoglobulin treatment in therapy-resistant chronic spontaneous urticaria. Ann Allergy Asthma Immunol. 2010;104(3):253–8.
Grattan CE, Francis DM, Slater NG, Barlow RJ, Greaves MW. Plasmapheresis for severe, unremitting, chronic urticaria. Lancet. 1992;339(8801):1078–80.
Sindher SB, Jariwala S, Gilbert J, Rosenstreich D. Resolution of chronic urticaria coincident with vitamin D supplementation. Ann Allergy Asthma Immunol. 2012;109(5):359–60.
Rasool R, Masoodi KZ, Shera IA, Yosuf Q, Bhat IA, Qasim I, et al. Chronic urticaria merits serum vitamin D evaluation and supplementation; a randomized case control study. World Allergy Organ J. 2015;8(1):15.
Tuchinda P, Kulthanan K, Chularojanamontri L, Arunkajohnsak S, Sriussadaporn S. Relationship between vitamin D and chronic spontaneous urticaria: a systematic review. Clin Transl Allergy. 2018;8:51.
Cornillier H, Giraudeau B, Samimi M, Munck S, Hacard F, Jonville-Bera AP, et al. Effect of diet in chronic spontaneous urticaria: a systematic review. Acta Derm Venereol. 2019;99(2):127–32.
Akoglu G, Atakan N, Cakir B, Kalayci O, Hayran M. Effects of low pseudoallergen diet on urticarial activity and leukotriene levels in chronic urticaria. Arch Dermatol Res. 2012;304(4):257–62.
Zuberbier T, Pfrommer C, Specht K, Vieths S, Bastl-Borrmann R, Worm M, et al. Aromatic components of food as novel eliciting factors of pseudoallergic reactions in chronic urticaria. J Allergy Clin Immunol. 2002;109(2):343–8.
Zuberbier T, Chantraine-Hess S, Hartmann K, Czarnetzki BM. Pseudoallergen-free diet in the treatment of chronic urticaria. A prospective study. Acta Derm Venereol. 1995;75(6):484–7.
Magerl M, Pisarevskaja D, Scheufele R, Zuberbier T, Maurer M. Effects of a pseudoallergen-free diet on chronic spontaneous urticaria: a prospective trial. Allergy. 2010;65(1):78–83.
Pigatto PD, Valsecchi RH. Chronic urticaria: a mystery. Allergy. 2000;55(3):306–8.
Bunselmeyer B, Laubach HJ, Schiller M, Stanke M, Luger TA, Brehler R. Incremental build-up food challenge--a new diagnostic approach to evaluate pseudoallergic reactions in chronic urticaria: a pilot study: stepwise food challenge in chronic urticaria. Clin Exp Allergy. 2009;39(1):116–26.
Son JH, Chung BY, Kim HO, Park CW. A histamine-free diet is helpful for treatment of adult patients with chronic spontaneous urticaria. Ann Dermatol. 2018;30(2):164–72.
Siebenhaar F, Melde A, Magerl M, Zuberbier T, Church MK, Maurer M. Histamine intolerance in patients with chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2016;30(10):1774–7.
Bishnoi A, Parsad D, Vinay K, Kumaran MS. Phototherapy using narrowband ultraviolet B and psoralen plus ultraviolet A is beneficial in steroid-dependent antihistamine-refractory chronic urticaria: a randomized, prospective observer-blinded comparative study. Br J Dermatol. 2017;176(1):62–70.
Sheikh G, Latif I, Lone KS, Hassan I, Jabeen Y, Keen A. Role of adjuvant narrow band ultraviolet B phototherapy in the treatment of chronic urticaria. Indian J Dermatol. 2019;64(3):250.
Aydogan K, Karadogan SK, Tunali S, Saricaoglu H. Narrowband ultraviolet B (311 nm, TL01) phototherapy in chronic ordinary urticaria. Int J Dermatol. 2012;51(1):98–103.
Borzova E, Rutherford A, Konstantinou GN, Leslie KS, Grattan CE. Narrowband ultraviolet B phototherapy is beneficial in antihistamine-resistant symptomatic dermographism: a pilot study. J Am Acad Dermatol. 2008;59(5):752–7.
Heelan K, Murphy M. Symptomatic dermatographism treated with narrowband UVB phototherapy. J Dermatolog Treat. 2015;26(4):365–6.
Konstantinou GN, Konstantinou GN. Psychiatric comorbidity in chronic urticaria patients: a systematic review and meta-analysis. Clin Transl Allergy. 2019;9:42.
Ben-Shoshan M, Blinderman I, Raz A. Psychosocial factors and chronic spontaneous urticaria: a systematic review. Allergy. 2013;68(2):131–41.
Schut C, Magerl M, Hawro T, Kupfer J, Rose M, Gieler U, et al. Disease activity and stress are linked in a subpopulation of chronic spontaneous urticaria patients. Allergy. 2020;75(1):224–6.
Lindsay K, Goulding J, Solomon M, Broom B. Treating chronic spontaneous urticaria using a brief ‘whole person’ treatment approach: a proof-of-concept study. Clin Transl Allergy. 2015;5:40.
Shertzer CL, Lookingbill DP. Effects of relaxation therapy and hypnotizability in chronic urticaria. Arch Dermatol. 1987;123(7):913–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Grattan, C., Zuberbier, T., Maurer, M. (2021). Other Interventions for Chronic Urticaria. In: Zuberbier, T., Grattan, C., Maurer, M. (eds) Urticaria and Angioedema. Springer, Cham. https://doi.org/10.1007/978-3-030-84574-2_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-84574-2_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84573-5
Online ISBN: 978-3-030-84574-2
eBook Packages: MedicineMedicine (R0)