Abstract
Animal husbandry, poultry farming in particular, accounts for the major proportion of global antimicrobial use. Administration of antimicrobials through feed and drinking water is a common practice in poultry to improve growth performance and to prevent and treat several poultry diseases. Increased use of antimicrobials poses serious threat of product safety (arising from antimicrobial residue in poultry products). Additionally, the possibility of antimicrobial resistance increases with increased use of antimicrobials. As poultry products are one of the most highly consumed products globally, the haphazard use of antimicrobials and emergence of antimicrobial resistance are a major concern that must be addressed sooner rather than later. Resulting treatment failure due to antimicrobial resistance, economic loss, and transmission of that resistance to human would be disastrous. Several European countries have already banned the use of antimicrobials for prophylactic purposes; however, antimicrobial use is still rising in many developing and underdeveloped countries. This chapter discusses the usage of antimicrobials in poultry production and economic effects of using and not using them and also presents the state of antimicrobial resistance resulting from it along with the potential future changes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Antimicrobials are natural, seminatural (semisynthetic), or synthetic substances that kill or inhibit the growth of microorganisms (Page and Gautier 2012). Antimicrobials are effective against various classes of microorganisms such as virus, bacteria, protozoa, and fungi; however, the antimicrobials of common interest are those that are effective against bacteria. This is because bacteria can mutate to variants that are resistant to the antimicrobials used against them. These bacteria, after the mutation, can become a public health concern and jeopardize livestock and human health. The coexisting nature of livestock and human and their dependency on each other provide a greater host range to these resistant bacteria. Fear is growing among the scientific community that such resistance could result in another costly pandemic. Therefore, this chapter focuses on the use of antimicrobials in livestock production; dynamics of antimicrobial flow between humans, animals, and the environment; antimicrobial resistance; and potential alternatives to antimicrobials. This chapter also discusses One Health approach to antimicrobial resistance and provides information on antimicrobial stewardship to provide guidelines to stakeholders involved in the use of antimicrobials.
2 Antimicrobial Use in Livestock
2.1 History
Tracing the date when antimicrobials started to have a dramatic impact on livestock farming can be daunting. Kirchhelle (2018), in their review, mentioned that antimicrobials started to play a bigger role in food production since the 1930s when synthetic sulfonamides came into existence. Sulfonamides were found to be effective against streptococcal infection providing a therapeutic effect on agricultural animals. Food products coming from livestock became even more important during World War II when there was a need to optimize livestock production to meet the increasing demand for food products. Researchers started to study alternatives in the form of antimicrobials to produce more meat at a cheaper cost; however, the practice started to come under scrutiny with the emergence of antibiotic residues in food products and antibiotic resistance (Table 1.1).
2.2 Numbers Behind Antimicrobial Use in Livestock
Globally, more than 27 billion chickens, 1.7 billion cattle and buffaloes, 850 million pigs, and 2.3 billion sheep and goats are farmed (FAOSTAT 2020). Also, other groups of livestock share a significant proportion among the total livestock population. This suggests that a significant portion of the global population relies on some forms of livestock farming directly or indirectly. For those directly dependent on livestock, poor health and productivity of their animals could be devastating with a serious negative impact on their economy for years. Thus, many of them knowingly or unknowingly use substances such as antimicrobials that enhance the productivity of their livestock and prevent/protect their livestock from diseases.
In recent years, the use of antimicrobials is growing at an unprecedented rate in food animals. This is expected to grow similarly for some time as demand for animal protein is growing rapidly (Tilman et al. 2011). An estimated 63 thousand tons of antimicrobials were used in 2010 in livestock, which doubled in 2013 (131,109 tons). This use is further expected to rise up to 67% by 2030 (Van Boeckel et al. 2015). Figures are even more alarming in Asia, where antimicrobial use in chicken and pigs are expected to rise by 129% and 124%, respectively, by 2030 (Van Boeckel et al. 2015). In India, industrial poultry production is expected to grow by 312% by 2030, further increasing the demand for antimicrobials. Developed countries such as Denmark, Sweden, and Norway have been cautious in using antimicrobials; however, the developing countries have not shown any signs of reducing the use of antimicrobials for agricultural purposes (for instance, 8 mg/PCU of antimicrobial use in Norway compared to 318 mg/PCU in China) (Van Boeckel et al. 2017).
In 2010, the top five countries that shared the largest proportion of global antimicrobial use in livestock were China, the United States, Brazil, India, and Germany (Van Boeckel et al. 2015). The more alarming data from BRICS (Brazil, Russia, India, China, and South Africa) suggest that the use of antimicrobials is expected to grow by more than 99% in those countries in the next 10 years, making them future hotspot of global antimicrobial use. The global rise in the use of antibiotics is attributed to the shift toward more intensified livestock farming where a large group of animals is kept in an enclosed environment, increasing antimicrobial pressure to maintain and improve health and productivity. Also, livestock farming has seen significant changes in the last few decades owing mainly to the genetic advancements. Genetic selection has been practiced heavily, and the focus is mostly laid on improving productivity, which has unintentionally given rise to undesirable side effects such as increased frequency of rare recessive alleles. As a result, immune incompetence is more common leading to increased occurrence of pathologies and compromised animal welfare (Rauw et al. 1998; Hocking 2014). Compromised immune system is also one of the reasons that has caused increase in prophylactic and therapeutic use of antimicrobials, possibly giving rise to increased antimicrobial resistance.
Most of the abovementioned data come from poultry, cattle, and pig. There is hardly any accurate data available on antimicrobial use from fish farming. However, fish farming may already be contributing to the major proportion of antimicrobial use globally. The data from South Asia and South America already suggest an extremely high rate of antimicrobial use up to 1400 mg/kg (Van Boeckel et al. 2015) in fish farming. Like any other livestock farming, aquaculture is also shifting toward more efficient and intensive farming with the potential to become a major shareholder of global antimicrobial use.
3 Why Are Antimicrobials Used in Livestock?
3.1 Antimicrobials as a Growth Promoter
The single most controversial use of antimicrobials in livestock is its use as a growth promoter, which dates to the 1950s in the United States, Australia, and some European countries (Dibner and Richards 2005). Studies have reported improved feed conversion and growth in cattle, pigs, poultry, and other animals (Gallo and Berg 1995; Cromwell 2002; Castanon 2007; Chattopadhyay 2014) with some of these studies reporting productivity improvement of up to 10% after the use of antimicrobial growth promoters. The interaction between gut, microbiota, and antimicrobials is thought to be the reason behind growth-promoting effects of antimicrobials, more specifically the reduction of microbial metabolites that cause growth reduction in animals (Visek 1978; Anderson et al. 1999). Antimicrobials reduce the population of opportunistic pathogens and subclinical infections, limiting competition for food and thereby improving growth (Visek 1978). Antimicrobials also increase nutrient availability and absorption by maintaining gut microflora compositions, thereby thinning the barrier in the small intestine, and assisting in the digestion of high-energy diets (Peng et al. 2014).
Although antimicrobials are being used as a growth promoter for decades, there is a lack of reliable recent data on the effect of antimicrobials as growth promoters. Most of the studies on antimicrobials as growth promoter were conducted in the decades of the 1980s and 2000s (Teillant 2015). With the readily available antimicrobials to be used for growth promotion, it is often ignored by farmers that similar results could be achieved by selecting high growing lines, good hygiene, nutrition, and health management. Focusing on these things rather than just relying on antimicrobials for growth-promoting effects could dramatically reduce the use of antimicrobials in livestock.
Additionally, it is important to understand the economic aspects of using antimicrobials as a growth promoter and the potential economic effect of banning antimicrobials as growth promoters. There is limited knowledge on these; however, studies from the countries such as Denmark and Sweden, where antimicrobials have already been banned as a growth promoter, suggest that there is minimal impact on economy (Graham et al. 2007; Sneeringer et al. 2015). The most likely cost after banning antimicrobials as a growth promoter will be to improve hygiene and management, which is significant, but with a long-term positive effect both on animals and humans. Developing countries are a major concern where the production is less controlled, and the impact of the ban is likely higher compared to that of developed countries. The ban could become counterproductive if not handled properly as it could lead to more therapeutic use of antimicrobials to keep animals healthier and more productive. Additionally, to meet the demand of increasing world population, more animals need to be raised if growth-promoting antimicrobials are prohibited, which may subsequently lead to negative impacts on environment and other areas (Hao et al. 2014). Therefore, this is an extraordinarily complex issue requiring intervention from each country to make a common alliance with common goal.
Compared to very few positive effects (such as improved growth and improved feed efficiency) of antimicrobials used as growth promoters, there are numerous negative effects (Edqvist and Pedersen 2001; Hao et al. 2014). They are summarized below:
-
Increases the pool of antimicrobial-resistant genes.
-
Camouflages bad feed, subsequently discouraging improvement in feed development and its alternatives.
-
Helps to hide the subclinical diseases and associated stress.
-
Promotes intensive farming that is less animal-friendly.
-
Disrupts disease treatment by increasing antimicrobial resistance.
-
Provides the best possible environment to bacteria that are mutating to become antimicrobial-resistant.
-
Indirectly impacts human health due to the transfer of antimicrobial resistance.
Based on the above, it is of utmost importance to identify alternatives of growth-promoting antimicrobials and implement those alternatives as soon as possible. Some of the alternatives to antimicrobials are discussed later in this chapter.
3.2 Prophylactic Use of Antimicrobials in Livestock
Farmers do not have any other choices but to use antimicrobials when animals are sick. The use depends on the animal species, stage of production, and disease risk. Similarly, when only a few individuals are sick, farmers choose to use antimicrobials to prevent the spread of disease to other animals. Usually, such antimicrobials are administered at critical points during the livestock production cycle to prevent diseases.
When antimicrobials are used as a prophylactic agent against certain diseases, they are generally used for a short duration and administered via feed or water to a group of animals. For example, most feedlot cattle in the United States (∼83%) are administered with at least one antimicrobial in feed and water to control different disease outbreaks such as diarrhea and pneumonia (Animal and Plant Health Inspection Service 1999). Similarly, broilers are usually administered with bacitracin and sulfonamides via feed to prevent necrotic enteritis and coccidiosis, respectively (McEwen and Fedorka-Cray 2002). In pigs, several antimicrobials such as tiamulin, sulfonamides, tetracyclines, and ceftiofur are used to prevent pneumonia. Additionally, most pigs receive antimicrobials during weaning to prevent them from infectious disease as weaning is one of the most stressful periods in a pig’s life (McEwen and Fedorka-Cray 2002).
3.3 Therapeutic Use of Antimicrobials in Livestock
Antimicrobial use as a therapeutic agent is a common practice throughout the world and is the least controversial among the three uses of antimicrobials. Usually, antimicrobials are administered to a targeted individual(s) via feed and water or through direct injection. During disease outbreaks, especially in large pig and poultry farms, antimicrobials are administered through the water as a disease can depress feed intake in animals and it is usually believed that animals continue to drink water despite reducing the feed intake during sickness.
Gentamicin, apramycin, and neomycin are used to treat bacterial diarrhea in pigs caused by E. coli and C. perfringens (McEwen and Fedorka-Cray 2002). Similarly, nearly all weaned piglets have access to some form of antimicrobials to control disease outbreaks because of stress during weaning (Dewey et al. 1999). Fluoroquinolones are used to treat E. coli infections in poultry, and it is a common practice to use ionophores and sulfonamides to control coccidiosis. Hatchery use of antimicrobials is also common to control omphalitis in day-old chicks (Ouckema and Phillipe 2009). In dairy cattle, antimicrobials such as penicillins, cephalosporins, and erythromycins are used to treat mastitis (Erskine 2000). Such drugs are a routine practice in cattle, which are usually administered to the entire herd during nonlactating periods (Erskine 2000).
4 Antimicrobial Resistance
Bacteria are referred to as resistant to antimicrobials when they become non-susceptible to one or more antimicrobials. When they become resistant to three or more antimicrobials, they become multidrug-resistant bacteria and then called pan drug-resistant if they are immune to any antimicrobials (Magiorakos et al. 2011).
Many antimicrobials (especially antibiotics) that are used in livestock are also essential for human use. When antimicrobials are used in livestock to prevent disease, it suppresses and eliminates bacteria that are susceptible to the antimicrobials. However, such antimicrobials cannot eliminate those bacteria that are resistant to them. Bacteria have an extraordinary potential to be adaptive to the new environment including the environment with antimicrobials. Those bacterial that are tolerant to antibiotics can multiply within the host and likely become the dominant bacterial population. Such bacteria are also able to transfer the resistant genes to other bacteria. When humans consume food products coming from animals, such bacteria can enter human being and subsequently colonize in the intestine. Once these tolerant bacteria are widespread within the human population and the antibiotics stop working against those bacteria, the treatment strategies can fail and lead to the devastating outcome (Hall et al. 2011; Marshall and Levy 2011).
5 How Antimicrobials and Antimicrobial Resistance Flow Between Humans, Livestock, and the Environment?
The dynamics behind the movement of antimicrobials and antimicrobial resistance from food animals to humans and vice versa is a complex phenomenon. The emergence of antimicrobial resistance and dissemination across and within different species has been summarized below:
5.1 Agricultural Production Method
Housing is one of the major drivers increasing the rate of emergence and dissemination of antimicrobials and antimicrobial resistance. In modern housing systems, a large group of animals is confined within a building or closed space (e.g., battery housing, feedlot cattle, pig barns, etc.). Hundreds of animals share food, water, air, and bedding for a long period. Animals are exposed to their own and other wastes containing antimicrobials and resistant bacteria (Gormaz et al. 2014). Additionally, workers get exposed to a large group of animals and resistant pathogens, who further transmit these pathogens to communities through contaminated clothing, shoes, and surfaces (Fey et al. 2000; Rinsky et al. 2013). Humans are not only exposed to these pathogens in farms but can also get these pathogens from a slaughterhouse. In slaughterhouses, workers are in close contact with animal bodies and equipment used in slaughtering, handling, cutting, processing, and storage of carcasses (Madden 1994; Sammarco et al. 1997). Besides, cross-contamination of pathogens is also linked to the trucks and other vehicles, when such vehicles are not thoroughly cleaned and decontaminated after their use in transporting other animals and food products (Hennessy et al. 1996; Pell 1997). Overcrowding, lack of appropriate sanitary measures, and cross-contamination during handling, transport, and slaughterhouse operations can amplify the dissemination of resistant pathogens, further worsening the situation.
Housing (especially intensive) is the major stressors to animals compromising their immune function. A compromised immune system leads to increased shedding of different kinds of pathogens. Animals are exposed to a series of stressors throughout their life from housing, handling, transport, and lairage at a slaughterhouse. Studies have suggested that stress can result in an increased prevalence of infections (Hayes et al. 2004; Verbrugghe et al. 2012), leading to an increase in the demand for antimicrobials.
5.2 Livestock Waste
Livestock farming results in a large volume of waste products often bigger than the carrying capacity of the environment. Livestock waste may contain resistant pathogens and genes, feed wastes, and spilled antimicrobials (from the feed, water, and excreta). In many countries, these waste products are largely unregulated, which means they are not treated before going into solid and water. This can lead to the release of a large number of antimicrobials and antimicrobial-resistant pathogens to the environment. From the environment, other animals and humans can get exposed to it, which might create an uncontrolled and widespread transfer of resistant pathogens across different species.
5.3 Exposure to Other Animals and Insects
Often livestock buildings are intruded by rodents, birds, insects, and other animals, mostly due to poor biosecurity. Nazni et al. (2005) reported similar pathogens to that found in poultry houses in the flies found in the poultry barn. Rodents in poultry and swine barn have been found to carry antimicrobial-resistant pathogens and disseminate them to the environment (Backhans and Fellstrom 2012). There is a potential transfer of such pathogens and antimicrobials from domesticated animals to wild animals.
5.4 Movement of Animals and Food
There is extensive movement of live animals across the different parts of the world, for example, the movement of poultry breeding stock from Europe to Asia and within Europe and live sheep export from Australia to the Middle East. If the use of antimicrobials is permitted in exporting countries but not in the importing countries, there is a likelihood of antimicrobial-resistant pathogen transfer from the exporting country to the importing one.
In addition to live animals, there is an extensive export and import of food products throughout the world. Major producers of pork, poultry, fish, and beef extensively export these products to other countries (Silbergeld and Dailey 2017). This extensive trading makes it impossible for countries to assess the flow of pathogens through food products between the countries. Food can be contaminated with resistant bacteria through several routes, i.e., from bacteria present in animals, from bacteria added during culture, and from bacterial cross-contamination during the processing of foods (Verraes et al. 2013).
Especially in developing countries, antimicrobials are misused due to poor regulations in the supply chain. Moreover, a large population in such countries is in close contact with the animals. It hence burgeons the chances of transmissions of resistant microorganisms from animals to humans from handling of the animals.
5.5 Environment
The environment is not only a significant reservoir of many pathogens but also facilitates their dissemination by forming a cycle of pathogen contamination. In addition to getting pathogens from livestock wastes, antimicrobials used as crop pesticides also lead to soil and water contamination (Bhandari et al. 2019), subsequently leading to the emergence of resistant bacteria. Moreover, globalization and urbanization have led to environmental pollution, further compromising livestock and human health and increasing the demand for antimicrobials (Balakrishnan et al. 2019). Antimicrobials used in agriculture, human, and veterinary medicine are partially metabolized by animals and humans and end up being released into the environment through sewage systems. Antimicrobials used in aquaculture are directly added into the water, leading to a high antimicrobial concentration in water and the sediments. Studies in various countries have detected a low concentration of antimicrobials in different environmental compartments such as municipal wastewater, sewage plant effluent, and even groundwater (Kümmerer 2004; Kolpin et al. 2002; Sacher et al. 2001). Most of the commonly used antimicrobials are not biodegradable and persist in the aquatic ecosystem (Kümmerer 2003). These antibiotics may have direct effects upon the resident microbial community of sediments in the ecosystem (Nygaard et al. 1992). The presence of active antibiotic compounds in the environment exerts a selective pressure which might create the occurrence of antibiotic-resistant phenotypes that may spread in the environment through the microbial species (Thanner et al. 2016). In addition to the release of antibiotics leading to the development of resistant bacteria, bacteria themselves are also excreted by humans and animals which end up in the ecosystems.
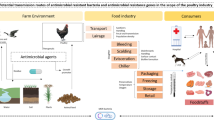
Humans and animals are a part of a complex environmental phenomenon. Several human activities such as traveling, contact with livestock, and contact with wild animals lead to the dissemination of antimicrobials and pathogens that are resistant to antimicrobials. The environment in which both human and animal live completes the cycle of this dissemination. Therefore, we must reduce the release of antimicrobials to the environment to disrupt this cycle and to slow down the zoonotic transmission of antimicrobial resistance (Fig. 1.1).
6 Zoonosis of Antimicrobial Resistance
It is estimated that more than 50% of pathogens that can infect human beings can also infect other animals (Taylor et al. 2001). Therefore, there is a huge potential for transfer of antimicrobial resistance from animals to human beings and vice versa. The earliest documented evidence of animal-human transmission of antimicrobial resistance was in the 1970s during the Salmonella epidemic in a human hospital that was traced back to the calves infected by Salmonella (Labro and Bryskier 2014). Since then, documentation of antimicrobial resistance in livestock and humans and the spread of resistant bacteria between animals and humans is large and readily available (Woolhouse and Ward 2013).
In the United States alone, more than 2.8 million cases of illnesses are due to some form of antimicrobial-resistant infections leading to more than 35,000 deaths per year (Centers for disease control and prevention (CDC) 2019). Antimicrobial resistance contributes to 700,000 deaths annually with estimated 214,000 neonatal deaths attributed to resistant sepsis infections (Pokharel et al. 2020). The data on livestock deaths due to antimicrobial resistance is scarce; however, studies have reported antimicrobial resistance to antibiotics in E. coli, Salmonella spp., Campylobacter spp., and Enterococcus spp. that are responsible for most infections in livestock (McEwen and Fedorka-Cray 2002; WHO 2003; Aarestrup et al. 2008).
Increased resistance to antimicrobials usually coincides with the use of such antimicrobials in livestock that are used for food production. For example, in poultry, fluoroquinolones are heavily used to treat respiratory diseases, and it is no surprise that increased resistance to fluoroquinolones has been heavily documented in humans, mostly linked to poultry consumption (Endtz et al. 1991; Nelson et al. 2007). In a more recent study, approximately 90% of isolates from poultry showed some form of resistance to antimicrobials such as sulfonamides, tetracyclines, fluoroquinolones, and third-generation cephalosporins (Kaesbohrer et al. 2012). Similarly, methicillin-resistant Staphylococcus aureus (MRSA) is a growing concern among people that are in contact with animals, both livestock and pets (Labro and Bryskier 2014). Enterococcus is another commensal bacteria found in both human and animal guts, which are intrinsically resistant to cephalosporins and can also acquire resistance to quinolones, macrolides, and tetracyclines (Murray 1990).
As a global public health threat affecting both humans and animals, antimicrobial resistance has warranted several national and international communities to work together on implementing policies to preserve the efficacy of medically important antimicrobials. The concept of critically important antimicrobials was developed in a second workshop held between the WHO, FAO, and OIE in 2004. The WHO classified antimicrobials into five groups based on their importance to human medicine and released a guideline in 2018, which recommended that the highest priority critically important antimicrobials (HPCIA) should not be used in food-producing animals (WHO 2017). The HPCIA includes five classes of antimicrobials: quinolones; third-, fourth-, and fifth-generation cephalosporins; macrolides and ketolides; glycopeptides; and polymyxins. Study in some European countries has shown that it is possible to maintain health and productivity with no use of cephalosporins and fluoroquinolones in livestock; however, total exclusion of macrolides is difficult as they are critically important in managing respiratory disease in pigs, poultry, cattle, and other animals. This makes it more complex as respiratory diseases in livestock are associated with significant economic losses in most countries (Lhermie et al. 2020).
7 One Health and Antimicrobial Resistance
Antimicrobial resistance is a multifaceted global issue (Pokharel et al. 2020). Both human and veterinary medicine are the major contributor to the emergence of antimicrobial resistance. The issue is not going to affect one single species in the world; it could well become the most widespread pandemic in the future, affecting the largest number of species throughout the world. Therefore, there is a need for a multidisciplinary approach involving humans, animals, and the environment, which is referred to as One Health.
The WHO defines One Health as a “concept and approach to designing and implementing programs, policies, legislation and research in which multiple sectors communicate and work together to achieve better public health outcomes” (WHO 2017). The origin of One Health is centuries old and recognizes both human and animal health. More recently, this concept recognizes environmental health too. In summary, there are three domains in this approach: human health, animal health, and environmental health.
Among the three domains, human health takes a major emphasis. Antimicrobial resistance genes have been reported to be highly prevalent in some common pathogens in humans such as E. coli, K. pneumonia, and S. aureus (Robinson et al. 2016). Livestock has played a major role in the transmission of antimicrobial resistance, which was already discussed previously in this chapter. Livestock and associated products will continue to play a significant role in the dissemination of antimicrobial residue and antimicrobial resistance in the future. At present, there is a lack of knowledge transfer between human and veterinary medicine, causing inconsistencies in the use of antimicrobials in humans and animals. The collaborative approach between human and veterinary medicine can mitigate this and provide sustainable solutions (Fig. 1.2).
The third domain, environment, is getting considerable recognition in recent years. As discussed earlier, the environment is a significant transmission reservoir for most of the pathogens in humans and animals, without which the disease cycle cannot be mostly completed (Pornsukarom and Thakur 2017). Soil and water contamination of antimicrobials can lead to the emergence of antimicrobial-resistant bacteria that are already in soil and water (Grenni et al. 2018). Similarly, other aspects of the environment such as air pollution have led to increased infections in humans and animals, subsequently increasing the demand for antimicrobials that further aids in the emergence of antimicrobial resistance.
8 Third-Generation Cephalosporins: A One Health Example
Third-generation cephalosporins are widely used in humans and animals. These are classified as critically important antimicrobials by the WHO (2017). Third-generation cephalosporins have a broad-spectrum activity, and some of their uses include controlling bovine respiratory disease in cow, preventing E. coli infections in chicks, and treating pneumonia, arthritis, and other conditions in humans (Greko et al. 2009). Resistance to third-generation cephalosporins has been reported in E. coli and K. pneumonia (Park 2014; de Kraker et al. 2011). Several studies reported that voluntary withdrawal of third-generation cephalosporin use in chicks was followed by the drop in its resistance in E. coli (Hiki et al. 2015; Dutil et al. 2010). Countries such as Denmark, Australia, and Canada have placed a voluntary ban on the use of these drugs recognizing the resulting human health risk with their use in animals (Collignon and McEwen 2019).
8.1 Antimicrobial Stewardship
Antimicrobial resistance is a one-world issue. Therefore, it is in everyone’s interest to preserve the efficacy of antimicrobials by properly using them, following the guidelines, monitoring their use and resistance, and implementing good stewardship programs. Antimicrobial stewardship is a set of actions that promote the responsible use of antimicrobials and can be summarized with 5Rs: responsibility, review, reduce, refine, and replace (Page et al. 2014). The 5R approach guides livestock farmers, veterinarians, physicians, and other relevant stakeholders who are involved in antimicrobial use to adopt best practice and management of antimicrobial use. With regard to good stewardship, prevention of disease in livestock is more important than the treatment, which means vaccination and good husbandry are critical in putting antimicrobial use in check (Table 1.2).
9 Alternatives to Antimicrobial Use in Livestock
As suggested by good antimicrobial stewardship, we can identify and implement the practices that can either replace or reduce antimicrobial use and also reduce the likelihood of infections in animals. Such practices can include early intervention long time before the infections such as vaccinations. There are several vaccines available that can help prevent several infections in livestock (e.g., cattle, E. coli, Salmonella vaccine; pigs, E. coli vaccine, vaccine against bacterial pneumonia; and poultry, vaccine against pasteurellosis, Salmonella vaccine). Another important strategy that can reduce the antimicrobial load includes good husbandry practices. Good sanitation in and around the farm can reduce bacterial load around the farm, good air and water quality can prevent horizontal transmission of diseases, and good feed can help protect animals against many conditions such as salmonellosis and mycotoxins. Good air quality and appropriate ventilation in the animal farm can help control high gaseous levels (e.g., ammonia level in poultry houses) subsequently reducing several bacterial infections.
Good husbandry practices also involve farmers following appropriate biosecurity measures. Controlling what goes into the farm can help prevent a lot of diseases in animals. For example, the use of appropriate clothing and foot baths, control of vectors, control of birds and rodents, and use of Salmonella free food can be easily practiced on the farm. Another less common practice involves the use of beneficial bacteria in the form of probiotics, which can act as an antibiotic growth promoter in animals (Reid and Friendship 2000); however, more studies are yet to be conducted to understand more about probiotics and their role in the farm as an alternative to antimicrobials. In addition to probiotics, prebiotics and organic acids can also provide health benefits to animals by stimulating growth, metabolism, and composition of beneficial bacteria in the gastrointestinal tract and eliminating the harmful one (Solis-Cruz et al. 2019).
Genetic selection is another avenue that could provide potential solution to the widespread use of antimicrobials. Herds that are resistant to certain diseases can be selected that could possibly eliminate the use of antimicrobials for that disease. Studies are scarce on the use of genetic selection to achieve pathogen-resistant animals but can be food for thought for animal scientists to tackle the issue. More recently, bacteriophages have emerged as a potential alternative to antimicrobials, which works by specifically attacking bacteria; however, lack of regulatory guidance and clinical trials has hindered the possibility of using bacteriophages in large scale (Romero-Calle et al. 2019). Different alternatives to antimicrobials and how they work have been summarized in Table 1.3.
10 Conclusion
Antimicrobials are the most important discoveries of human and animal health, and ironically, antimicrobial resistance is one of the greatest crises to public health. The use of antimicrobials in the livestock sector in different parts of the globe is indiscriminate and unregulated. There is a lack of data about the scale of their use, and more studies are required to understand the fate of these antimicrobials in the environment and their consequences on human health. Livestock farming should urgently be recognized as a major contributor to the development of antimicrobial resistance, and countries need to develop legislation regulating prophylactic use of antimicrobials in farming. The evidence presented across countries indicates that it is possible to reduce antimicrobial use and gain highly intensive and productive production systems (Cogliani et al. 2011). A coordinated effort between governments, industry, and scientists is required for effective action on antimicrobial resistance. An immediate step to tackle the problem would be to develop strategies for improved antimicrobial stewardship involving both human medicine and livestock industry and develop alternative approaches to combat microbial disease and improve livestock production.
Key Notes
-
Antimicrobial is a complex subject.
-
Antimicrobial use in livestock is rising at an alarming rate, driven by increasing demand for animal protein globally.
-
Data on the antimicrobial use is not sufficient, which warrants more study on the topic.
-
Antibiotic resistance is a public health crisis.
-
Livestock farming is a major contributor to the development of antimicrobial resistance.
-
Keeping animals healthy is important in reducing the use of antimicrobials.
-
Antimicrobial resistance is a One Health issue. More than that, it is a one-world issue.
-
5R approach can help become a good antimicrobial steward and help tackle antimicrobial resistance.
-
Strategies that can help reduce the use of antimicrobials include good farm management, vaccination, biosecurity, probiotics, and genetic selection.
References
Aarestrup FM, Wegener HC, Collignon P (2008) Resistance in bacteria of the food chain: epidemiology and control strategies. Expert Rev Anti Infect Ther 6:733–750
Anderson DB, McCracken VJ, Aminovi RI et al (1999) Gut microbiology and growth-promoting antibiotics in swine. Pig News Inform 20:115–122
Animal and Plant Health Inspection Service (1999) Feedlot ‘99. Part 3: health management and biosecurity in US feedlots. US Department of Agriculture, Washington, DC
Backhans A, Fellstrom C (2012) Rodents on pig and chicken farms—a potential threat to human and animal health. Infect Ecol Epidemiol 2:10
Balakrishnan K, Dey S, Gupta T et al (2019) The impact of air pollution on deaths, disease burden, and life expectancy across the states of India: the global burden of disease study. Lancet Planet Health 3:e26–e39
Bhandari G, Zomer P, Atreya K et al (2019) Pesticide residues in Nepalese vegetables and potential health risks. Environ Res 172:511–521
Castanon JIR (2007) History of the use of antibiotic as growth promoters in European poultry feeds. Poult Sci 86:2466–2471
Centers for disease control and prevention (CDC) (2019) 2019 AR Threats Report. https://www.cdc.gov/drugresistance/biggest-threats.html. Accessed 25 Jan 2021
Chattopadhyay MK (2014) Use of antibiotics as feed additives: a burning question. Front Microbiol 5:334
Cogliani C, Goossens H, Greko C (2011) Restricting antimicrobial use in food animals: lessons from Europe. Microbe 6:274–279
Collignon PJ, McEwen SA (2019) One health—its importance in helping to better control antimicrobial resistance. Trop Med Infect Dis 4:22
Cromwell GL (2002) Why and how antibiotics are used in swine production. Anim Biotechnol 13:7–27
de Kraker MEA, Wolkewitz M, Davey PG et al (2011) Burden of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay associated with bloodstream infections due to Escherichia coli resistant to third-generation cephalosporins. J Antimicrob Chemother 66:398–407
Dewey CE, Cox BD, Straw BE et al (1999) Use of antimicrobials in swine feeds in the United States. J Swine Health Prod 7:19–25
Dibner JJ, Richards JD (2005) Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci 84:634–643
Dutil L, Irwin R, Finley R et al (2010) Ceftiofur resistance in salmonella enterica serovar Heidelberg from chicken meat and humans, Canada. Emerg Infect Dis 16:48
Edqvist L, Pedersen KB (2001) Antimicrobials as growth promoters: resistance to common sense. In: Harremoës P, Gee D, MacGarvin M (eds) Late lessons from early warnings: the precautionary principle 1896–2000. Environment issue report, no 22. European Environment Agency, Copenhagen, pp 93–100
Endtz HP, Ruijs GJ, van Klingeren B et al (1991) Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Antimicrob Chemother 27:199–208
Erskine RJ (2000) Antimicrobial drug use in bovine mastitis. In: Prescott JF, Baggot JD, Walker RD (eds) Antimicrobial therapy in veterinary medicine, 3rd edn. Iowa State University Press, Ames, pp 712–734
FAOSTAT (2020). http://www.fao.org/faostat/en/#data/QA. Accessed 25 Jan 2021
Fey PD, Safranek TJ, Rupp ME et al (2000) Ceftriaxone-resistant salmonella infection acquired by a child from cattle. N Engl J Med 342:1242–1249
Gallo GF, Berg JL (1995) Efficacy of a feed-additive antibacterial combination for improving feedlot cattle performance and health. Can Vet J 36:223
Gormaz JG, Fry JP, Erazo M et al (2014) Public health perspectives on aquaculture. Curr Environ Health Rep 1:227–238
Graham JP, Boland JJ, Silbergeld E (2007) Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep 122:79–87
Greko C, Badiola JI, Catry B et al (2009) Reflection paper on the use of third and fourth generation cephalosporins in food producing animals in the European Union: development of resistance and impact on human and animal health. J Vet Pharmacol Ther 32:515–533
Grenni P, Ancona V, Caracciolo AB (2018) Ecological effects of antibiotics on natural ecosystems: a review. Microchem J 136:25–39
Hall MAL, Dierikx CM, Stuart JC et al (2011) Dutch patients, retail chicken meat and poultry share the same ESBL genes, plasmids and strains. Clin Microbiol Infect 17:873–880
Hao H, Cheng G, Iqbal Z et al (2014) Benefits and risks of antimicrobial use in food-producing animals. Front Microbiol 5:288
Hayes JR, English LL, Carr LE et al (2004) Multiple-antibiotic resistance of enterococcus spp. isolated from commercial poultry production environments. Appl Environ Microbiol 70:6005–6011
Hennessy TW, Hedberg CW, Slutsker L et al (1996) A national outbreak of salmonella enteritidis infections from ice cream. N Engl J Med 334:1281–1286
Hiki M, Kawanishi M, Abo H et al (2015) Decreased resistance to broad-spectrum cephalosporin in Escherichia coli from healthy broilers at farms in Japan after voluntary withdrawal of ceftiofur. Foodborne Pathog Dis 12:639–643
Hocking PM (2014) Unexpected consequences of genetic selection in broilers and turkeys: problems and solutions. Br Poultry Sci 55:1–12
Kaesbohrer A, Schroeter A, Tenhagen BA et al (2012) Emerging antimicrobial resistance in commensal Escherichia coli with public health relevance. Zoonoses Publ Health 59(Suppl 2):158–165
Kirchhelle C (2016) Toxic confusion: the dilemma of antibiotic regulation in west German food production (1951–1990). Endeavour 40:114–127
Kirchhelle C (2018) Pharming animals: a global history of antibiotics in food production (1935–2017). Palgrave Commun 4:1–13
Kolpin DW, Furlong ET, Meyer MT et al (2002) Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: a national reconnaissance. Environ Sci Technol 36:1202–1211
Kümmerer K (2003) Significance of antibiotics in the environment. J Antimicrobiol Chem 52:5–7
Kümmerer K (2004) Resistance in the environment. J Antimicrobiol Chem 54:311–320
Labro M, Bryskier J (2014) Antibacterial resistance: and emerging “zoonosis”? Expert Rev Anti Infect Ther 12:1441–1461
Lhermie G, La Ragione RM, Weese JS et al (2020) Indications for the use of highest priority critically important antimicrobials in the veterinary sector. J Antimicrobiol Chem 75:1671–1680
Madden RH (1994) Microbial hazards in animal products. Proc Nutr Soc 53:309–316
Magiorakos AP, Srinivasan A, Carey RB et al (2011) Multidrug resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
Marshall BM, Levy SB (2011) Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 24:718–733
McEwen SA, Fedorka-Cray PJ (2002) Antimicrobial use and resistance in animals. Clin Infect Dis 34(Suppl 3):S93–S106
Murray BE (1990) The life and times of the enterococcus. Clin Microbiol Rev 3:46–65
Nazni WA, Seleena B, Lee HL et al (2005) Bacterial fauna from the house fly, Musca domestica (L.). Trop Biomed 22:225–231
Nelson JM, Chiller TM, Powers JH et al (2007) Fluoroquinolone-resistant campylobacter species and the withdrawal of fluoroquinolones from use in poultry: a public health success story. Clin Infect Dis 44:977–980
Nygaard K, Lunestad BT, Hektoen H et al (1992) Resistance to oxytetracycline, oxolinic acid and furazolidone in bacteria from marine sediments. Aquaculture 104:31–36
Ouckema R, Phillipe C (2009) Salmonella isolations: historical OHSFP trends. In: Proceedings of salmonellosis, antimicrobial use and antimicrobial resistance symposium; May 6, Guelph, Ontario
Page SW, Gautier P (2012) Use of antimicrobial agents in livestock. Rev Sci Tech OIE 31:145–188
Page S, Prescott J, Weese S (2014) The 5Rs approach to antimicrobial stewardship. Vet Rec 175:207–209
Park SH (2014) Third-generation cephalosporin resistance in gram-negative bacteria in the community: a growing public health concern. Korean J Intern Med 29:27
Pell AN (1997) Manure and microbes: public and animal health problem? J Dairy Sci 80:2673–2681
Peng M, Salaheen S, Biswas D (2014) Animal health: global antibiotic issues. Encycl Agric Food Syst 1:346–357
Pokharel S, Shrestha P, Adhikari B (2020) Antimicrobial use in food animals and human health: time to implement ‘one health’ approach. Antimicrob Resist Infect Control 9:181
Pornsukarom S, Thakur S (2017) Horizontal dissemination of antimicrobial resistance determinants in multiple salmonella serotypes following isolation from the commercial swine operation environment after manure application. Appl Environ Microbiol 83:1–14
Rauw WM, Kanis E, Noordhunizen-Stassen EN et al (1998) Undesirable side effects of selection for high production efficiency in farm animals: a review. Livest Prod Sci 56:15–33
Reid G, Friendship R (2000) Alternatives to antibiotic use: microbiological perspective. In: Pork industry conference on addressing issues of antibiotic use in livestock production, 16–17 October 2000, Urbana, Illinois
Rinsky JL, Nadimpalli M, Wing S et al (2013) Livestock-associated methicillin and multidrug resistant Staphylococcus aureus is present among industrial, not antibiotic-free livestock operation workers in North Carolina. PLoS One 8:e67641
Robinson TP, Bu DP, Carrique-Mas J et al (2016) Antibiotic resistance is the quintessential one health issue. Trans R Soc Trop Med Hyg 110:377–380
Romero-Calle D, Benevides RG, Góes-Neto A et al (2019) Bacteriophages as alternatives to antibiotics in clinical care. Antibiotics 8:138
Sacher F, Lange FT, Brauch HJ et al (2001) Pharmaceuticals in groundwaters: analytical methods and results of a monitoring program in Baden-Württemberg, Germany. J Chromatogr A 938:199–210
Sammarco ML, Ripabelli G, Ruberto A et al (1997) Prevalence of salmonellae, listeriae, and Yersiniae in the slaughterhouse environment and on work surfaces, equipment, and workers. J Food Prot 60:367–371
Silbergeld EK, Dailey JL (2017) Biological plausibility of associations between antimicrobial use in food-producing animals and increased risks of human exposures to, and infections by, antimicrobial resistant zoonotic pathogens. In: WHO guidelines on use of medically important antimicrobials in food-producing animals. World Health Organization, Geneva
Sneeringer S, MacDonald J, Key N et al (2015) Economics of antibiotic use in U.S. livestock production. https://www.ers.usda.gov/webdocs/publications/45485/err-200.pdf?v=0. Assessed 24 Jan 2021
Solis-Cruz B, Harnandez-Patlan D, Hargis BM et al (2019) Use of prebiotics as an alternative to antibiotic growth promoters in the poultry industry. In: Franco-Robles E (ed) Prebiotics and probiotics: potential benefits in nutrition and health. IntechOpen, London, p 89053
Taylor LH, Latham SM, Woolhouse ME (2001) Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 356:983–989
Teillant A (2015) Cost and benefits of antimicrobial use in livestock. In: AMR Control, pp 116–112
Thanner S, Drissner D, Walsh F (2016) Antimicrobial resistance in agriculture. MBio 7:e02227–e02215
Tilman D, Balzer C, Hill J et al (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci 108:20260–20264
Van Boeckel TP, Brower C, Gilbert M et al (2015) Global trends in antimicrobial use in food animals. Proc Natl Acad Sci 112:5649–5654
Van Boeckel TP, Glennon EE, Chen D et al (2017) Reducing antimicrobial use in food animals. Science 357:1350–1352
Verbrugghe EM, Boyen F, Gaastra W et al (2012) The complex interplay between stress and bacterial infections in animals. Vet Microbiol 155:2–4
Verraes C, Van Boxstael S, Van Meervenne E et al (2013) Antimicrobial resistance in the food chain: a review. Int J Environ Res Public Health 10:2643–2669
Visek WJ (1978) The mode of growth promotion by antibiotics. J Anim Sci 46:1447–1469
WHO (2003) Impacts of antimicrobial growth promoter termination in Denmark: the WHO international review panel’s evaluation of the termination of the use of antimicrobial growth promoters in Denmark: Foulum, Denmark 6–9 November 2002
WHO (2017) One health. https://www.who.int/news-room/q-a-detail/one-health Assessed 30 Jan 2021
Woolhouse ME, Ward MJ (2013) Sources of antimicrobial resistance. Science 341:1460–1461
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pokharel, B., Karna, S.R. (2022). Antimicrobials in Livestock Production and Its Cross-Domain Dynamics. In: Akhtar, N., Singh, K.S., Prerna, Goyal, D. (eds) Emerging Modalities in Mitigation of Antimicrobial Resistance. Springer, Cham. https://doi.org/10.1007/978-3-030-84126-3_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-84126-3_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-84125-6
Online ISBN: 978-3-030-84126-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)