Abstract
The orders Balbianiales and Thoreales of the subclass Nemaliophycidae are composed of exclusively freshwater members, whereas the Acrochaetiales have marine or freshwater representatives, with the two families (Audouinellaceae and Ottiaceae) having exclusively freshwater representatives. Balbianiales has two genera (Balbiania and Rhododraparnaldia) each one with a single species. Audouinellaceae and Ottiaceae are monotypic with the genera Audouinella and Ottia, respectively. Five species are recognized within the genus Audouinella, whereas Ottia has a single species. Thoreales has two genera: Nemalionopsis with two species and Thorea with 12 species.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acrochaetiales
- Balbianiales
- Eurhodophytina
- Florideophyceae
- Freshwater
- Nemaliophycidae
- Rhodophyta
- Taxonomy
- Thoreales
Subclass Nemaliophycidae T Christensen, Bot Tidsskr 73:66 (1978)
The characteristics of the class are as follows (adapted from Kamiya 2017): thalli epilithic, epiphytic, endophytic, or parasitic; pit plugs with two cap layers, with or without cap membrane, outer cap layer dome-shaped in Balbianiales, Balliales, and Batrachospermales and plate-shaped in other orders; life cycle variable: standard triphasic pattern with alternation of heteromorphic or isomorphic generations, triphasic pattern producing haploid gametophytes from diploid sporophyte through vegetative meiosis (lack of meiospore production) or biphasic pattern lacking carposporophyte stage; carpogonia sessile on vegetative cells or on 1–2-celled stalks, rarely intercalary, occurring as single cells or with 3–8-celled carpogonial branches; fertilized carpogonia giving rise either directly, or after dividing transversely or longitudinally, to small diploid filamentous carposporophytes with terminal carposporangia; auxiliary cells absent; tetrasporangia (where known) cruciate or irregularly cruciate.
The subclass currently has ten orders: Acrochaetiales, Balbianiales, Balliales, Batrachospermales, Colaconematales, Entwisleales, Nemaliales, Palmariales, Rhodachlyales, and Thoreales. The association among five orders within the Nemaliophycidae (Acrochaetiales, Colaconematales, Entwisleales, Nemaliales, and Palmariales) is well resolved, whereas for the remaining orders it is not well established (Lam et al. 2016). Freshwater members are represented in the Acrochaetiales (primarily marine, except for the genera Audouinella and Ottia) and the exclusively freshwater orders Balbianiales, Batrachospermales, and Thoreales.
Order Acrochaetiales Feldmann, Proc Int Seaweed Symp 1:11 (1953)
Members of Acrochaetiales are epilithic, epiphytic or endophytic, marine or freshwater. Thallus is heterotrichous, monosiphonous simple or branched filaments, attached by a single cell or a multicellular prostrate system. Erect filaments are often tufted, with cylindrical to moniliform or irregular in shape, uninucleate cells. Plastids are parietal or axial, discoid, lobed or stellate in shape, single or multiple per cell, without or with one to several pyrenoids. Pit plugs have two cap layers and cap membranes; outer cap layer is thin, plate-like. Monophasic members have reproduction by monospores, biphasic have reduced gametophytes, and triphasic have morphologically similar or dissimilar gametophytes and tetrasporophytes. Carpogonia are sessile on vegetative cells or on one to two-celled stalks, rarely intercalary, without carpogonial branch; fertilized carpogonia giving rise either directly, or after dividing transversely or longitudinally, to small diploid filamentous carposporophytes with terminal carposporangia; auxiliary cells are absent. Spermatangia are borne singly or in clusters on the erect filaments. Tetrasporangia are cruciate or irregularly cruciate.
Freshwater red algae are exclusively in two families, Audouinellaceae Woelkerling and Ottiaceae JR Evans, ML Vis, and GW Saunders. The Audouinellaceae has a single genus Audouinella containing all freshwater species and is characterized by having parietal, band-shaped or laminate plastids, triphasic life history, gametophyte consisting of multicellular base, isomorphic with tetrasporophyte and producing monosporangia (Saunders et al. 2017). The Ottiaceae has a single monospecific genus, Ottia and is characterized by having 1–2 reddish-brown parietal plastids without pyrenoids and gametophyte consisting of heterotrichous filaments growing intertwined on species of Nothocladus s. lat. (Entwisle et al. 2018).
Audouinella Bory, Dictionnaire Classique d’Histoire Naturelle vol 3:341 (1823)
-
Type species: Audouinella hermannii (Roth) Duby, Aug Pyrami de Candolle Botanicon Gallicum:972 (1830).
-
Description: thalli filamentous, heterotrichous, uniaxial, reddish, growing typically in dense tufts; basal portion composed of rhizoidal outgrowth, simple or parenchymatous disc; erect filaments consisting of cylindrical cells with unilateral, opposite, or alternate branches; cells contain one to several reddish plastids without pyrenoids; sexual reproduction by spermatangia and carpogonia; spermatangia colorless, arising in clusters on branch tips; carpogonia sessile or stalked, with a cylindrical base and thin trichogyne; distinct carpogonial filament absent; fertilized carpogonia giving rise directly to gonimoblast filaments; carposporophytes spherical to sub-spherical, consisting of a compact mass of short gonimoblast filaments with terminal carposporangia; carposporangia obovoidal to sub-spherical; tetrasporangia single or in clusters, cruciate, formed at branch tips; asexual reproduction by monosporangia are the only known mode of propagation; monosporangia obovoidal, elliptical, spherical, or sub-spherical, arising from short branches on gametophytes or tetrasporophytes.
-
Diagnostic characters: species of Audouinella are easily misidentified with the “Chantransia” stage of members of the Batrachospermales and Thoreales (Skuja 1934; Starmach 1985; Necchi and Zucchi 1997; Zucchi and Necchi 2003), which makes the identification of the true Audouinella species problematic. Some criteria based on vegetative morphology were proposed to distinguish Audouinella from “Chantransia” stage: color (reddish vs bluish), branching type (regular vs irregular), and monosporangia abundance (abundant vs rare). Species of Audouinella are reddish and tend to have a more regular branching, but it is variable and monosporangia can also be abundant in “Chantransia” stages. Among these characters, the only one that has proven to be more generally applicable in studies based on field or cultured specimens is the thallus color with all species of Audouinella being reddish (Necchi et al. 1993a) and all “Chantransia” being bluish (Necchi et al. 1993b; Necchi and Zucchi 1997; Zucchi and Necchi 2003). However, “Chantransia” stages of Thoreales can be brownish (Chiasson et al. 2007), and some species of Sheathia can be brownish to reddish in addition to bluish (Han et al. 2020; Vis et al. 2020), which could raise some difficulties in identification. Reproductive characters are also important and when present are more reliable to recognize true Audouinella species because in addition to monosporangia they can produce gametangia (carpogonia and spermatangia), carposporophytes, and tetrasporangia, whereas in “Chantransia” stage only monosporangia are produced. However, these reproductive structures are not often observed in field specimens and thus not applicable in many cases. In the absence of reproductive structures other than monosporangia, only reddish populations of freshwater acrochaetioid algae should be interpreted as Audouinella and we follow this scheme.
-
Habitat: species of Audouinella occur in a wide range of environmental variables (Necchi et al. 1993a; Carmona and Necchi 2001; Eloranta et al. 2016): A. huastecana (temperature 26–27 °C, conductivity 900–1128 μS cm−1, pH 7.0–7.6, current velocity 90–130 cm s−1); A. eugenea [temperature 19–27(–29) °C, conductivity (130–)310–900(–1237) μS cm−1, pH (7.0–)7.2–8.3(–8.6), current velocity (9–)21–60 cm s−1]; A. hermannii [temperature 2–16(–26) °C, conductivity (10–)45–200(–380) μS cm−1, pH (4.7–)7.4–8.1(–8.7), current velocity (4–)30–81(–130) cm s−1]; A. tenella (temperature 8 °C, conductivity 110 μS cm−1, pH 7.6, current velocity 27 cm s−1).
-
Distribution: some species have a very restricted distribution (Fig. 4.1) being reported only from the type localities (A. amahatana from Japan and A. huastecana from Mexico), whereas others are widely distributed on two or three continents (A. eugenea from Asia and North and South America, A. hermannii from Europe and North America and A. tenella from North and South America).
Phylogenetic relationships among species: A phylogeny among the species is not feasible because DNA sequences are available only for A. hermannii. The three sequences of A. hermannii differ in 1.2–2.3% for rbcL, with the smallest difference between samples from Canada (New Brunswick) and Ireland and the largest for samples of the USA (North Carolina) and Ireland. Five species are recognized on the basis of morphological characters.
Key to the species of the genus Audouinella
1a | Erect system differentiated into proximal and distal parts; proximal cells cylindrical, distal cells barrel-shaped | A. huastecana |
1b | Erect system undifferentiated into proximal and distal parts, composed exclusively of cylindrical cells | 2 |
2a | Diameter of vegetative cells ≤7 μm | 3 |
2b | Diameter of vegetative cells >7 μm | 4 |
3a | Tetrasporangia obovoidal, 11–14 μm in length, known from Asia (Japan) | A. amahatana |
3b | Tetrasporangia spherical or obovoidal, 8–12 μm in length, known from North and South America | A. tenella |
4a | Branch angles ≤25°, spermatangia ≥6 μm in diameter | A. eugenea |
4b | Branch angles ≥25°, spermatangia ≤6 μm in diameter | A. hermannii |
Audouinella amahatana (Kumano) Garbary, Bibl Phycol 77:19 (1987) (Fig. 4.2a, b)
-
Basionym: Acrochaetium amahatanum Kumano, Jap J Phycol 26:105 (1978).
-
Type : Kobe University, S Kumano, 1.xi.1973 (Holotype, not found).
-
Type locality: Japan, Yamanashi, Okusawa-dani, tributary of Amahata River, 35.408611° N, 138.331667° E (estimated).
-
Description: thalli microscopic, ≤500 μm in height; basal system composed of filaments with fusiform cells, loosely arranged, 5–12 μm in length, 4–6 μm in diameter; erect system with alternate, rarely unilateral branches, branch angles ≤25°, composed of filaments with cylindrical cells, 5–15 μm in length, 4–7 μm in diameter; 1 plastid per cell; monosporangia single or in small groups on short lateral branches, obovoidal, 7–10 μm in length, 5–8 μm diameter; tetrasporangia single or in small groups, mixed with monosporangia, on short lateral branches, obovoidal, 11–14 μm in length, 7–9 μm diameter; gametangia and carposporophytes not observed.
-
Diagnostic characters: this species is distinct from others in the genera by the thinner cells of erect filaments (≤7 μm in diameter), which is similar to A. tenella. However, it differs by the shape and size of tetrasporangia: obovoidal and longer (11–14 μm in length), whereas in A. tenella they are spherical or obovoidal and shorter (8–12 μm in length). In addition, A. amahatana is only known from Japan and A. tenella occurs in North and South America.
-
Representative sequences in GenBank: no sequences available.
-
Distribution: Asia: Japan, known only from the type locality (Fig. 4.1).
Audouinella eugenea (Skuja) Jao, Sinensia 11:362 (1940) (Fig. 4.2c, d)
-
Basionym: Chantransia eugenea Skuja, Beih Bot Centralbl 52:177 (1934).
-
Type: not found.
-
Type locality: Pakistan , Lahore, 31.519904° N, 74.357873° E (estimated).
-
Description: thalli macroscopic, ≥1 mm in height; basal system composed of irregular prostrate mass with densely aggregated filaments of cylindrical cells; erect system with alternate or opposite branches, branch angles ≤25°, composed of filaments with cylindrical cells, (11.5–)28–63 μm in length, (7.5–)9–15(–18.5) μm in diameter; 1 plastid per cell; monosporangia single or in clusters on short lateral branches, obovoidal, 12–18 μm in length, (7.5–)9–15(–18) μm in diameter; spermatangia in groups of 2–3, hyaline, ellipsoidal or obovoidal, 8–12 μm in length, 6–12 μm in diameter; carpogonia, carposporophytes, and tetrasporangia not observed; putative propagules consisting of 1–3 cells, pear-shaped, clavate or irregular, 22–48 μm in length, 16–38 μm in diameter.
-
Diagnostic characters: this species is distinct from others in the genus by the thicker cells of erect filaments (>7 μm in diameter), which is similar to A. hermannii. However, A. eugenea has branching with narrower branch angles (≤25°) and larger spermatangia (≥6 μm in diameter) than A. hermannii.
-
Representative sequences in GenBank: no sequences available.
-
Distribution: Asia: China, Pakistan; North America: Dominican Republic, Jamaica, Mexico; South America: Brazil (Fig. 4.1).
-
Key references: Skuja (1934), Jao (1940), Necchi et al. (1993a), Carmona and Necchi (2001).
Audouinella hermannii (Roth) Duby, Aug Pyrami de Candolle Botanicon Gallicum:972 (1830) (Fig 4.2e–i)
-
Basionym: Conferva hermannii Roth, Catalecta Botanica 3:180 (1806).
-
Type: B 28528, L Treviranus (Neotype, designated by Necchi et al. 1993a).
-
Type locality: Germany, near Bremen, 53.079296° N, 8.801694° E (estimated).
-
Description: thalli macroscopic, ≥1 mm in height; basal system composed of irregular prostrate mass with densely aggregated filaments of cylindrical cells; erect system with alternate or opposite branches, branch angles ≥25°, composed of filaments with cylindrical cells, 35–67 μm in length, (7.5–)9–15(–17.9) μm in diameter; several plastids per cell; monosporangia single or in pairs on short lateral branches, 1–3(–4) cells, obovoidal or sub-spherical, 8–13(–16) μm in length, 7–11 μm in diameter; spermatangia in groups of 2–4, terminal, hyaline, ellipsoidal or obovoidal, 4–6 μm in length, 2.8–6 μm in diameter; carpogonia lanceolate or bottle-shaped, with a filiform trichogyne, slightly broader at the distal end, 23–47 μm in length, 3.5–7 μm in diameter; carposporophytes spherical or semi-spherical, dense, (33–)40–80 μm in diameter; carposporangia obovoidal, ellipsoidal, or pear-shaped, (7.5–)9–15(–20) μm in length, (6–)8–13.5(–16) μm in diameter; tetrasporangia single or in pairs, terminal on short branches, ellipsoidal or obovoidal, 9.5–14(–17) μm in length, (6.5–)8–12(–14.5) μm in diameter.
-
Diagnostic characters: this species is distinct from others in the genus by the thicker cells of erect filaments (>7 μm in diameter), which is similar to A. eugenea . However, A. hermannii has branching with wider branch angles (≥25°) and smaller spermatangia (≤6 μm in diameter) than A. eugenea.
-
Representative sequences in GenBank: MH638330 (COI-5P); VU04033, KC134346, MH638328 (rbcL).
-
Distribution: Europe: Austria, Britain, Belgium, Croatia, Finland, France, Germany, Ireland, Latvia, Lithuania, Poland, Russia, Slovenia, Spain; North America: the USA and Canada (Fig. 4.1).
-
Key references: Drew (1935), Israelson (1942), Starmach (1985), Necchi et al. (1993a).
-
Remarks: several records of this species from regions other than Europe and North America, where it has been reported with the typical morphology and DNA sequence data, were not considered in the geographic distribution, particularly when the description lacked distinct morphological characters. These records include Africa (Chad, Szinte et al. 2020), Asia (China, Shi et al. 2006; India, Ganesan et al. 2018; Iraq, Maulood et al. 2013; Japan, Hirose and Yamagishi 1977), Australasia (Australia and New Zealand, Skinner and Entwisle 2001), and South America (Brazil, Necchi and Zucchi 1995). Further investigations based on molecular and morphological data are required to reevaluate the records of A. hermannii in these regions.
Audouinella huastecana JJ Carmona and Necchi, Eur J Phycol 36:221 (2001) (Fig. 4.2j–l)
-
Type: FCME PA3261, J Carmona and G Montejano, 9.ix.1989 (Holotype).
-
Type locality : Mexico, San Luis Potosí, Ciudad Valles, Choy, 22.326111° N, 99.086389° W.
-
Description: thalli macroscopic, ≥1 mm in height; basal system composed of irregular prostrate mass with densely aggregated filaments of cylindrical cells; erect system differentiated into proximal and distal parts; proximal filaments with cylindrical vegetative cells, 16–36 μm in length, 10–16 μm in diameter, unbranched or sparsely branched; distal filaments with barrel-shaped cells, 6–20 μm in length, 6–12 μm in diameter, abundantly branched to form dense fascicles; erect system with alternate or dichotomous branches, branch angles ≤25°, rarely at wider angles; 1 plastid per cell; monosporangia in clusters on short lateral branches, 1–3 cells, ellipsoidal or obovoidal, 12–22 μm in length, 10–14 μm in diameter. Gametangia, carposporophytes, and tetrasporangia not observed.
-
Diagnostic characters: this species is unique among species of the genus by having the erect system differentiated into proximal and distal parts: proximal unbranched and with filaments of cylindrical cells, distal branched and with filaments of barrel-shaped cells.
-
Representative sequences in GenBank: no sequences available.
-
Distribution: North America: Mexico (Fig. 4.1).
-
Key references: Carmona and Necchi (2001).
Audouinella tenella (Skuja) Papenfuss, Univ Calif Publ Bot 18:326 (1945) (Fig. 4.2m)
-
Basionym: Chantransia tenella Skuja, Beih Bot Centralbl 52:177 (1934).
-
Type : UC 395493, NL Gardner 3309, v.1916 (Isotype).
-
Type locality: the USA, California, Marin County, Mt. Tamalpais, 37.923544° N, 122.596471° W.
-
Description: thalli macroscopic, ≥1 mm in height; basal system composed of irregular prostrate mass with densely aggregated filaments of cylindrical cells; erect system with alternate or opposite branches, branch angles ≥25°, composed of filaments with cylindrical cells, 14–25.7 μm in length, 3.9–6.1 μm in diameter; 1 plastid per cell; tetrasporangia single or in pairs, spherical (undivided) or obovoidal (after first division), 8–12 μm in length, 7–10.5 μm in diameter; carpogonia, carposporophytes, and monosporangia not observed.
-
Diagnostic characters: this species is distinct from others in the genus by the thinner cells of erect filaments (≤7 μm in diameter), which is similar to A. amahatana . However, A. tenella differs by having spherical (undivided) or obovoidal (after first division) and shorter tetrasporangia (8–12 μm in length) than A. amahatana that has obovoidal and longer tetrasporangia (11–14 μm in length). Geographic distribution is also helpful in recognizing these two species: A. amahatana is only known from Japan, whereas A. tenella is reported from North and South America.
-
Representative sequences in GenBank: no sequences available.
-
Distribution: North America: the USA (California); South America: Brazil (Amazonas) (Fig. 4.1).
-
Key references: Skuja (1934), Necchi et al. (1993a), Necchi and Zucchi (1995).
Doubtful Species
-
The reddish species listed below could not be unequivocally recognized as good species in the genus Audouinella. Original descriptions were mostly superficial, allowing them to be associated with several species of the genus, and type specimens were not available for checking the diagnostic characters. Some are probably synonyms of species described in the previous section.
-
Audouinella desikacharyi Ganesan and JA West, Algae 28:46 (2013). Based on a literature search, there are no illustrations available for this species and the description given by Desikachary et al. (1990, as A. pulvinata) is very wide and could be associated with several species. As stated by Ganesan and West (2013), the treatment of A. desikacharyi as a distinct species is made only tentatively, pending critical morphometric and molecular studies on Audouinella species known from India. It could be synonymized with A. eugenea .
-
Audouinella keralaiensis (L Jose and RJ Patel) Ganesan and JA West, Algae 28:46 (2013). Based on the protologue (Jose and Patel 1990), this species is within the circumscription of A. eugenea, also occurring in Asia (China and Pakistan) although no reference was made to that species. While no morphological analysis of diagnostic characters has been made here, there is no basis to recognize it as a distinct species or propose it as a synonym.
-
Audouinella lanosa Jao, Sinensia 12:256 (1941). Based on the protologue (Jao 1941), this species is within the circumscription of A. eugenea , also occurring in China, although no reference was made to that species. The character (monosporangia size) applied by Kumano (2002) to distinguish it from other species showed substantial overlap, particularly A. eugenea. While no morphological analysis of diagnostic characters has been made here, there is no basis to recognize it as a distinct species or propose it as a synonym.
-
All other species referred to Audouinella (see Kumano 2002 for descriptions) are bluish forms that most probably represent “Chantransia” stages of Batrachospermales and Thoreales.
Ottia Entwisle, JR Evans, ML Vis and GW Saunders, J Phycol 54:82 (2017 ‘2018’)
-
Type species: Ottia meiospora (Skuja) Entwisle, JR Evans, ML Vis and GW Saunders, J Phycol 54:82 (2017 ‘2018’).
-
Description: thalli filamentous, heterotrichous, uniaxial, reddish-brown to brown, growing endophytic/epiphytic on species of Nothocladus s. lat.; basal portion composed of irregularly shaped cells, fusiform or inflated in the middle or ends; erect filaments consisting of obovoid to elongate obpyriform cells with unilateral, opposite, or alternate branches; cells contain one and sometimes two reddish-brown parietal plastids without pyrenoids; sexual reproduction putatively with spermatangia and carpogonia; spermatangia spherical, colorless, on branch tips; carpogonia sessile with a cylindrical base and filiform trichogyne; no carposporophytes observed; monosporangia obovoidal, or sub-spherical, arising in clusters on short branches.
-
Diagnostic characters: Ottia like Audouinella may be easily misidentified with the “Chantransia” stage of members of the Batrachospermales and Thoreales. It can be distinguished from “Chantransia” based on having gametangia (carpogonia and spermatangia), whereas “Chantransia” only has monosporangia. In the absence of reproductive structures other than monosporangia, its habit of only growing epiphytic/endophytic on Nothocladus s. lat. can also aid in identification.
-
Habitat: no environmental data have been reported.
-
Distribution: this genus is currently known only from Australia and New Zealand (Fig. 4.1).
-
Ottia meiospora (Skuja) Entwisle, JR Evans, ML Vis, and GW Saunders, J Phycol 54:82 (2017 ‘2018’) (Fig. 4.3a–d).
-
Basionym: Balbiania meiospora Skuja, Acta Hort Bot Univ 14:10 (1944).
-
Homotypic synonym: Audouinella meiospora (Skuja) Garbary, Biblio Phycol 77:112 (1987).
-
Type: NSW 288111–288,115, V Lindauer, 1.xii.1937 (Isolectotypes).
-
Type locality: New Zealand, North Island, Waitangi River near Russell, 35.277744° S, 174.051701° E (estimated).
-
Description: thalli macroscopic, throughout thallus of host; basal system composed of irregular cells, 10–20 μm in length, 2–4 μm in diameter; erect systems with 4–6-celled branches, cells 5–8 μm in length, 4–5 μm in diameter, terminal hairs sometimes present, up to 150 μm in length; spermatangia single or in groups of 2(–3), 4–5 μm in diameter; carpogonium with filiform trichogyne, 40 μm in length; monosporangia (6–)8–11 μm in length, (5–)7–8 μm in diameter.
-
Representative sequences in GenBank: KY806745 (rbcL).
-
Distribution: Australasia: Australia (Victoria, New South Wales), New Zealand (North Island) (Fig. 4.1).
-
Remarks: there are records of Audouinella (Balbiania) meiospora from Brazil, but not included here since they are from a different continent and on a different host (Compsopogon) (Necchi and Zucchi 1995); sequence data are needed to confirm if they belong to this taxon.
(a, b) Audouinella amahatana : (a) filament with wide branch angle (arrow) and monosporangia at the tips (arrowheads); (b) filament with wide branch angle (arrow) and tetrasporangia at the tip (arrowhead); (c, d) A. eugenea : (c) filament with narrow branch angle (arrow) and monosporangium at the tip (arrowhead); (d) filament with a putative propagule (arrow) and spermatangia at the tip (arrowhead); (e–i) A. hermannii : (e) filament with wide branch angles (arrows); (f) tetrasporangium (arrow) at the tip of a branch; (g) spermatangia (arrows) in cluster; (h) trichogyne (arrow) with attached spermatium (arrowhead) and carpogonium (double arrowhead); (i) carposporophyte with carposporangia (arrows); (j–l) A. huastecana : (j) general view of a thallus differentiated in proximal (arrow) and distal (arrowhead) parts; (k) filaments of distal part with barrel-shaped cells; (l) monosporangium (arrow) at the tip of a branch; (m) A. tenella : (m) filament with wide branch angle (arrow) and tetrasporangia (arrowheads). Scale bars: (j) = 500 μm; (e, k) = 20 μm; (a, c, d, g–i) = 10 μm; (b, f, l, m) = 5 μm (Fig. (c, d) reprinted with permission by Taylor and Francis from Carmona and Necchi (2001); Fig. (f–h) reprinted with permission by E. Schweizerbartsche Verlagsbuchhandlung from Necchi et al. (1993a); Fig. (i) reprinted with permission by Academic Press from Sheath and Vis (2015))
(a–d) Ottia meiospora: (a) general view showing filaments intertwined (arrows) with host (arrowhead) whorl; (b) monosporangium (arrow) at the tip of short branch; (c) spermatangium (arrow) at the tip of a branch; (d) putative carpogonium (arrow) with filiform trichogyne (arrowhead); (e–h) Balbiania investiens: (e) general view showing filaments (arrows) growing out of host thallus (arrowheads); (f) monosporangia (arrows) at the tip of branches; (g) spermatangia (arrows) in cluster of three at the tip of elongate cell; (h) carpogonium (arrow) with filiform trichogyne (arrowhead); (i–l) Rhododraparnaldia oregonica: (i) general view of thallus with few lateral branches near the base (arrow) and dense branching (arrowhead) in the distal parts; (j) thallus differentiated into large cells (arrow) of main axis and smaller cells (arrowhead) of lateral branches; (k) carpogonium having a filiform trichogyne (arrow) with attached spermatium (arrowhead) and spermatangia at the tips of elongate cells (double arrowheads); (l) carposporophyte with carposporangia (arrows) at the tips; Scale bars: (a, d, f, j) = 25 μm; (b, c, g, h, k, l) = 10 μm; (e) = 150 μm; (i) = 500 μm (Fig. (a–d) reprinted with permission by Wiley from Entwisle et al. (2018). Image author: Fig. (e–h) C Carter; Fig. (i–l) reprinted with permission by Taylor and Francis from Sheath et al. (1994))
Order Balbianiales Sheath and KM Müller, J Phycol 35:863 (1999)
Members of Balbianiales are freshwater and either epiphytic on Batrachospermum-like genera of the Batrachospermales or growing on rock. Thallus is heterotrichous, branched filaments, attached to the substratum by a “Chantransia” stage or unattached intertwining among branches of host. Plastids are parietal, one or two per cell. Pit plugs have two cap layers with no membrane; outer cap layer is domed. A diploid phase of either a tetrasporophyte or “Chantransia.” Carpogonia cylindrical with fusiform trichogyne, sometimes on a short carpogonial branch; fertilized carpogonia giving rise to small diploid filamentous carposporophytes with terminal carposporangia; auxiliary cells are absent. Spermatangia and similar starch-filled cells on a specialized stalk cell. Sporophyte stage either a tetrasporophyte or “Chantransia.” There is only one family Balbianiaceae Sheath and KM Müller with the same characteristics as the order.
Balbiania Sirodot, Ann Sci Nat Bot sér 6 3:146 (1876)
-
Type species: Balbiania investiens (Lenormand ex Kütz.) Sirodot, Ann Sci Nat Bot sér 6 3:146 (1876).
-
Description: thalli filamentous, heterotrichous, uniaxial, red, monoecious, growing epiphytic on Batrachospermum-like genera of the Batrachospermales; filaments consisting of elongate-cylindrical cells with alternate branches; cells contain one and sometimes two parietal band-shaped or discoid plastids; spherical spermatangia on specialized stalked cells; carpogonia on a short lateral branch and having a cylindrical base and filiform trichogynes; carposporophytes composed of gonimoblast filaments with spherical, obovoidal carposporangia at tips; monosporangia obovoidal, arising singly or in pairs on short branches. Tetrasporophyte with cruciate tetrasporangia.
-
Diagnostic characters: this genus can be distinguished from Rhododraparnadia based on habit (epiphytic), formation of a typical tetrasporophyte (instead of a “Chantransia” stage) and geographic distribution in Europe. It is also similar to Ottia in morphology and habit (epiphytic) but the geographic distributions differ (Europe and Australasia, respectively).
-
Habitat: this species has only been collected as an epiphyte on Batrachospermum-like genera of the Batrachospermales; environmental variables for streams have been reported as follows: pH 6.3–8.1, conductivity 69–206 μS cm−1, water temperature 4–17 °C, mean current velocity 37–43 cm s−1 (Kronborg 1992; Leukart and Knappe 1995; Sheath and Sherwood 2002).
-
Distribution: this genus is currently known only from Europe (Fig. 4.1).
Balbiania investiens (Lenormand ex Kütz.) Sirodot, Ann Sci Nat Bot sér 6 3:146 (1876) (Fig. 4.3e–h)
-
Basionym: Chantransia investiens Lenormand ex Kützing: Species algarum 431 (1849).
-
Type : PC, Lenormand, 1841, 1843 (numerous specimens attributed to Lenormand—PC0511687, PC0511688, PC0511692-PC0511695).
-
Type locality: France, near Vire, 48.842° N, 0.890° W (estimated).
-
Description: thalli macroscopic , intertwined with thallus of host; branched filaments composed of cells, 30–90 μm in length and 4–9 μm in diameter, terminal hairs sometimes present, up to 150 μm in length; spermatangia on specialized stalk, in clusters of 3–5 cells, 4–5 μm in diameter; carpogonium 4–7 μm in diameter with filiform trichogyne, 15–30 μm in length; carposporophyte with 4–6 celled gonimoblast filaments with apical carposporangia, 14–17 μm in length, 8–9 μm in diameter; monosporangia 15–22 μm in length, 7–11 μm in diameter. Filamentous tetrasporophyte rarely observed, cells cylindrical, 42–45 μm in length, 4.5–6 μm in diameter.
-
Representative sequences in GenBank: KM055323 (COI-5P), KF944666 (rbcL).
-
Distribution: Europe: France , Germany, Portugal, Sweden, the UK (Fig. 4.1).
-
Key references: Sirodot (1876), Swale and Belcher (1963), Leukart and Knappe (1995), Sheath and Sherwood (2002).
-
Remarks: Balbiania is treated here as monospecific ; however, there are specimens from Brazil (Necchi and Zucchi 1995) and China (Xie and Shi 2004) attributed to Balbiania meiospora but not included in Ottia meiospora; further investigation is needed to determine which genus these specimens belong or if they represent a new taxon.
Rhododraparnaldia Sheath, Whittick and KM Cole, Phycologia 31:1 (1994)
-
Type species: Rhododraparnaldia oregonica Sheath, Whittick, and KM Cole, Phycologia 31:1 (1994).
-
Description: thalli macroscopic, crimson red, monoecious, opposite branching with branch cells smaller than main axis cells; attached to substratum by filamentous “Chantransia” phase; spermatangia on long colorless stalks; carpogonia cylindrical at base with a filiform trichogyne sometimes inflated at tip; carposporophyte with short gonimoblast filaments and spherical carposporangia at tips.
-
Diagnostic characters: this taxon can be distinguished from Balbiania based on habit (epilithic), the production of a “Chantransia” stage and geographic distribution in Northwest North America.
-
Habitat: it has been found in the following environmental conditions from two locations: pH 8.3, conductivity 30 μS cm−1, water temperature 8–11 °C, mean current velocity 35–61 cm s−1 (Sheath et al. 1994).
-
Distribution: this genus is currently known only from western North America (Fig. 4.1).
Rhododraparnaldia oregonica Sheath, Whittick, and KM Cole, Phycologia 31:1 (1994) (Fig. 4.3i–l)
-
Type: UBC A80770, RG Sheath, 31.iii.1992 (Holotype).
-
Type locality: the USA, Oregon, HJ Andrews Experimental Forest, Watershed 3 gaging station, 44.218627° N, 122.240995° W (estimated).
-
Description: thalli 6.4–15.1 mm in height, 171–383 μm in diameter; branching opposite with main axis cells 15.1–38.7 μm in length, 17.3–30.1 μm in diameter and branch cells 12.9–21.0 μm in length, 4.3–8.5 μm in diameter; spermatangia stalks 24.2–43.7 μm in length; spermatangia 2.0–4.3 μm in diameter; carpogonium 5.2–7.9 μm in diameter with filiform trichogyne, 19.4–29.3 μm in length; carposporangia 7.4–10.8 μm in length, 5.5–7.9 μm in diameter.
-
Representative sequences in GenBank: AF029156 (rbcL).
-
Distribution: North America: the USA, Oregon (two locations) (Fig. 4.1).
-
Key references: Sheath et al. (1994), Sheath and Müller (1999).
-
Remarks: although this taxon was originally collected in two locations in Oregon, subsequent attempts to recollect it in both locations have been unsuccessful.
Order Thoreales KM Müller, Sheath, AR Sherwood and Pueschel, J Phycol 38:819 (2002)
Members of Thoreales are epilithic or epiphytic macroalgae in freshwaters. Thallus is multiaxial with an inner medulla and outer assimilatory filaments, branched, attached to surfaces by the filamentous “Chantransia” sporophyte. Plastids are parietal, discoid, or lobed multiple per cell. Pit plugs have two plate-like cap layers and no cap membranes; outer cap layer is thin plate-like. Sexual reproductive structures consisting of spermatangia and carpogonia. Spermatangia are borne singly or in clusters on the erect filaments. Carpogonial branches are few-celled and have apical carpogonia that have a sub-spherical or ovoidal base and thin elongate trichogynes. Fertilized carpogonia give rise directly to gonimoblast filaments. Carposporophytes consist of a compact mass of short gonimoblast filaments with terminal carposporangia. Asexual reproduction via monosporangia arising from long branches on gametophytes or at filament apex for “Chantransia.” There is a single family, Thoreaceae Hassall, with the same characteristics as the order, containing two genera Nemalionopsis and Thorea.
Key to the genera
1a | Thallus with assimilatory filaments contained in a mucilaginous matrix, reproductive structures near the tips of assimilatory filaments | Nemalionopsis |
1b | Thallus with assimilatory filaments not contained in a mucilaginous matrix, reproductive structures at the base of assimilatory filaments | Thorea |
Nemalionopsis Skuja, Beih Bot Centralb 52:188 (1934)
-
Type species: Nemalionopsis shawii Skuja Beih Bot Centralb 52:191 (1934).
-
Description: thalli multiaxial, red, reddish-brown, sometimes branched, resembling fuzzy string; assimilatory filaments embedded in a mucilaginous matrix. Sexual reproduction by spermatangia and carpogonia; spermatangia colorless, sub-spherical to ellipsoidal, terminal or sub-terminal on assimilatory filaments; carpogonia sessile with an ovoidal base and thin trichogynes; carposporangia obovoidal to sub-spherical; monosporangia obovoidal, ellipsoidal, spherical or sub-spherical, arising from long branches on gametophytes or at filament apex for “Chantransia.”
-
Diagnostic characters: this genus is distinct from Thorea in having assimilatory filaments embedded in a mucilaginous matrix and reproductive structures are near the tips of the assimilatory filaments.
-
Habitat: species of Nemalionopsis occur in tropical and temperate regions, but there are few measurements for stream characteristics. N. parkeri has been reported from streams with current velocity 29 cm s−1, temperature 13–22 °C, pH 7.1–8.3, and conductivity 220 μS cm−1 (Howard and Parker 1979; Sheath et al. 1993). N. shawii has been collected from streams with current velocity 15–20 cm s−1, temperature 12–28 °C, pH 8.5, conductivity 60 μS cm−1 (Migita and Takasaki 1991; Johnston et al. 2014; Necchi et al. 2016).
-
Distribution: species have been reported from a wide geographic distribution (Fig. 4.4). Some reports are based on DNA sequence data from “Chantransia” collected in locations without macroscopic gametophytic thalli and others are from culture collections (Chiasson et al. 2007; Johnston et al. 2018).
World map showing the distribution of the two species of Nemalionopsis and 12 species of Thorea. Open symbols represent the type localities. Thorea spp. refers to locations where the genus has been reported but the species designation is uncertain by the current criteria; these records do not represent the same species but show the wider geographic distribution of the genus
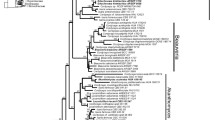
Phylogenetic relationships among species: there are two recognized species of Nemalionopsis (N. parkeri and N. shawii) and in a rbcL phylogeny with Thorea, these two species form a well-supported clade (Fig. 4.5). Although there are sequence data for multiple specimens of each species, intraspecific variation is low (<0.7% rbcL).
Key to the species of the genus Nemalionopsis
1a | Assimilatory filaments composed of ≤12 cells <144 μm in length; geographic occurrence in North America and Hawaiian Islands | N. parkeri |
1b | Assimilatory filaments composed of ≥ 13 cells and ≥145 μm in length, geographic occurrence in Asia, including southeast Asian islands | N. shawii |
Nemalionopsis parkeri ET Johnston and ML Vis, J Phycol 54:167 (2018) (Fig. 4.6a–c)
-
Type: US 60570 (barcode 00165890), RV Howard, 28.ix.1975 (Holotype).
-
Type locality : the USA, North Carolina, Wake County, Lower Barton Creek, Route 1005, 35.959106° N, 78.647677° W (estimated).
-
Description: thalli , reddish-brown, moderately mucilaginous, branched, 10–15 cm in length, 300–400 μm in diameter; medulla 111–454 μm in diameter; assimilatory filaments up to 144 μm in length, composed of 7–12 cells, 7.2–11 μm in length, 3.5–6.5 in μm diameter; spermatangia, carpogonia, and carposporophytes not observed; monosporangia obovoidal, single or in pairs, at the tips of assimilatory branches, 8–14 μm in length, 7–9 μm in diameter.
-
Diagnostic characters: this species has shorter assimilatory filaments with fewer cells than N. shawii.
-
Representative sequences in GenBank: KC596318, KM055242 (COI-5P), KM005142 (rbcL).
-
Distribution: North America: the USA (Florida, North Carolina) and Pacific Islands: Hawaii (Hawai’i, Mau’i) (Fig. 4.4).
-
Key references: Howard and Parker (1979), Sheath et al. (1993), Johnston et al. (2018).
-
Remarks: in the protologue, the rbcL sequence KM005141 from UTEX LB 2737 is stated, but the correct sequence is KM005142 From UTEX LB 2740 (as presented in the supplemental table in Sheath et al. 1993). Sheath et al. (1993) reported N. tortuosa from Florida, but this specimen is interpreted to represent N. parkeri based on morphology and geographic location.
Nemalionopsis shawii Skuja Beih Bot Centralb 52:191 (1934) (Fig. 4.6d–g)
-
Type: NY 945474, WR Shaw and Day #490, 28.iv.1907 (Isotype).
-
Type locality: Philippines: Luzon, Bataan Province, Lamao Forest Reserve, 14.66° N, 120.38° E (estimated).
-
Description: thalli monoecious, dark brown, moderately mucilaginous, branched, 15–50(–100) cm in length, 316–865 μm in diameter; medulla 52–325 μm in diameter; assimilatory filaments 145–390 μm in length, composed of 13–20 cells, 8.5–20 μm in length, 3.5–5.5 μm in diameter; proximal cells cylindrical short to elongate or ellipsoidal; distal cells cylindrical, ellipsoidal, or barrel-shaped; spermatangia 7.0–10.5 μm in length, 4.5–8.0 μm in diameter; carpogonia 2.8–7 μm in diameter, arising from the basal cell of assimilatory filaments or on a one-celled branch with trichogynes 33.2–110 μm in length, 1.4–4 μm in diameter; gonimoblast filaments radially arranged, composed of cylindrical or elongate-cylindrical cells, 10.5–24.5 μm in length, 5.0–7.5 μm in diameter; carposporangia in clusters, obovoidal, 12.5–17.0 μm in length, 9.5–12.0 μm in diameter; monosporangia not observed for gametophyte stage.
-
Diagnostic characters: this species has longer assimilatory filaments with more cells than N. parkeri .
-
Representative sequences in GenBank: KX958099, KX958100 (COI-5P), AB159659, KF557550, KU508674 (rbcL).
-
Distribution: Asia: China, Indonesia, Japan, Nepal, Philippines, Taiwan, Thailand (Fig. 4.4).
-
Key references: Skuja (1934), Sheath et al. (1993), Necchi et al. (2016), Johnston et al. (2018).
-
Remarks: another species epithet, N. tortuosa is common in the literature but currently is regarded as a synonym of N. shawii and all records of that species were treated as N. shawii here.
Thorea Bory, Ann Mus Hist Nat 12:127 (1808)
-
Type (Lectotype) species: Thorea ramosissima Bory, Ann Mus d’Hist Nat 12:128 (1808) nom. illeg. (currently accepted name: T. hispida).
-
Description: thalli multiaxial , red, reddish-brown, sometimes branched, resembling fuzzy string; assimilatory filaments loose, not embedded in a mucilaginous matrix. Sexual reproduction by spermatangia and carpogonia that are close to the base of the assimilatory filaments; spermatangia colorless, terminal or sub-terminal on assimilatory filaments. Carpogonial branches near the base of the assimilatory filaments, 0–2(–3) short-cylindrical or barrel-shaped cells; carpogonia sessile with an ovoidal or bottle-shaped base and thin, filiform trichogynes; fertilized carpogonia giving rise directly to gonimoblast filaments; carposporophytes spherical to sub-spherical, consisting of a compact mass of short gonimoblast filaments with terminal carposporangia; carposporangia obovoidal to sub-spherical; monosporangia obovoidal, ellipsoidal, spherical or sub-spherical, arising from long branches on gametophytes or at filament apex for “Chantransia.”
-
Diagnostic characters: this genus is distinct from Nemalionopsis in having assimilatory filaments loose, not embedded in a mucilaginous matrix and reproductive structures are near the base of the assimilatory filaments.
-
Habitat: in North America, this genus has been collected from streams with alkaline pH (7.0–8.3), warm temperature (18–28 °C), current velocity 24–43 cm s−1, and a wide range of conductivity 180–2140 μS cm−1 (Sheath et al. 1993; Carmona and Necchi 2001). Thorea hispida has been reported from rivers with alkaline pH (7.1–8.6), varying temperature (5.1–26 °C), current velocity 40–70 cm s−1 and high conductivity 314–6300 μS cm−1 in Europe (Bolpagni et al. 2015 and references there in). For South America, there are a few records from Brazil for T. bachmannii (as T. hispida or T. violacea) with stream pH (7.5–8.1), water temperature (18–21 °C), current velocity 17–93 cm s−1, and conductivity 79–970 μS cm−1 (Necchi and Zucchi 1997; Carmona and Necchi 2001; Johnston et al. 2018). Thorea gaudichaudii has been collected from Guam in a stream with pH 8.0, water temperature 28 °C, conductivity 50 μS cm−1 (Johnston et al. 2018). In Japan, Thorea species have been observed in streams with pH 6.6–7.5, water temperature 9.5–28 °C and current velocity 8.1–35.6 cm s−1 (Higa et al. 2007; Terada et al. 2016; Kozono et al. 2018).
-
Distribution: most species have a very restricted distribution (Fig. 4.4) being reported only from the type localities and a few other locales, whereas two species (T. hispida and T. gaudichaudii ) are widely distributed on two or three continents. There are numerous records of Thorea from locations for which the species was not identified or the species to which it should be attributed to is unclear in the current taxonomic scheme; these reports are noted on the map (Fig. 4.4).
-
Remarks: similar to many freshwater red algae, this genus has a simple morphology that is very distinctive at the generic level but differentiating among species using morphological characters has been more difficult. There has been a complicated history of species being placed in synonym, sometimes with more than one species and also being recognized as distinct (Johnston et al. 2018). Characteristics such as thallus size, amount of branching and number of monosporangia per cluster have been used to differentiate species, but these characters could be influenced by environmental conditions and stage in thallus development as well as not being clearly defined. More research on the reproductive characters is needed, especially carposporangia and monosporangia; these structures are similar in size, shape, and cell density and have been variously interpreted by researchers (Sheath et al. 1993; Johnston et al. 2018). Carmona and Necchi (2001) studying specimens with both structures have shown that carposporangia are in fascicles or clusters whereas monosporangia are not. DNA sequence data have distinguished species that share a similar morphology (Johnston et al. 2018).
(a–c) Nemalionopsis parkeri : (a) thallus habit; (b) thallus cross section showing outer photosynthetic, compact assimilatory filaments (arrows) and inner medulla (arrowhead); (c) monosporangia (arrows) at the tips of assimilatory filaments; (d–g) Nemalionopsis shawii : (d) thallus apex with compact assimilatory filaments (arrow) and narrow inner medulla (arrowhead); (e) cross section assimilatory filaments in a mucilaginous matrix stained blue (arrow) and inner unstained medulla (arrowhead); (f) ovoidal carpogonium base (arrow) with long thin trichogyne (arrowhead) extending past the assimilatory filaments; (g) carposporangia (arrows) at the tips of many-celled gonimoblast filaments. Scale bars: (a) = 10 mm; (b) = 50 μm; (c, f, g) = 20 μm; (d) = 500 μm; (e) = 100 μm (Fig. (a–c) reprinted with permission by Taylor and Francis from Howard and Parker (1979))
Phylogenetic relationships among species: rbcL sequence data show high support for the genus and one clade of four species, but less support for the relationship among the remaining five species and one unnamed specimen (Fig. 4.5). Within the clade of four species, T. mauitukitukii sister to T. gaudichaudii and T. quisqueyana sister to T. riekei are well supported. Thorea hispida, T. okadae, and T. indica are closely related in a well-supported clade, whereas T. kokosinga-pueschelii , T. bachmannii , and Thorea sp. (Hawaii) are on longer branches. The sequence recognized as Thorea sp. is genetically distinct, but it was derived from “Chantransia” and no physical specimen exist such that it cannot be formally described according to the nomenclatural rules. Thorea gaudichaudii has considerable intraspecific genetic variation relative to the other species (1.2% versus 0.2% T. hispida, 0.4% T. riekei , and 0.9% T. bachmannii ) but still within the range to be recognized as a single species. There is a total of 12 species in the genus with nine recognized based on DNA sequence data and three based on morphological characters or geographic distribution.
Key to the species of the genus Thorea
1a | Thalli small (no longer than a few millimeters), reproductive structures in middle of assimilatory filaments (somewhat intermediate between Thorea and Nemalionopsis) | T. conturba |
1b | Thalli large (≥4 cm in length), reproductive structures at the base of the assimilatory filaments | 2 |
2a | Assimilatory filaments clavate (greater cell diameter in the upper part) | T. clavata |
2b | Assimilatory filaments non-clavate (equal cell diameter in the upper and basal parts) | 3 |
3a | Geographic distribution in North America | 4 |
3b | Geographic distribution in other continents | 6 |
4a | Occurrence restricted to springs of Texas, the USA, and central Mexico | T. reikei |
4b | Occurrence in rivers from temperate regions or tropical streams of Caribbean Islands | 5 |
5a | Widely occurring from midwestern and eastern USA | T. kokosinga-pueschelii and T. hispida (in part)a |
5b | Restricted to Caribbean Islands | T. quisqueyana |
6a | Geographic distribution in South America | T. bachmannii |
6b | Geographic distribution in Asia, Southeast Asia, and Pacific Islands | 7 |
7a | Geographic distribution restricted to India | T. indica |
7b | Geographic distribution restricted to Japan | T. okadae |
7c | Geographic distribution restricted to Vanuatu | T. mauitukitukii |
7d | Geographic distribution widely occurring throughout Southeast Asia and Pacific Islands | T. gaudichaudii and T. hispida (in part)a |
Thorea bachmannii Pujals ex Sheath, ML Vis, and KM Cole, Eur J Phycol 28:232 (1993) (Fig 4.7a, b)
-
Type: BA-C 12709, A Bachman, 27.x.1965 (Lectotype).
-
Type locality: Argentina, Buenos Aires, Arroyo del Gato, La Plata, 34.928226° S, 57.944067° W (estimated).
-
Description: thalli dioecious, abundantly branched, 10–50 cm in length, 400–1300 μm in diameter; medulla 70–320 μm in diameter; assimilatory filaments 140–550 μm in length, composed of 10–18(–21) cells, 15–30 μm in length, 6–11 μm in diameter; proximal cells cylindrical or barrel-shaped; distal cells elongate-cylindrical; male thalli slender compared to female thalli, spermatangia in pairs terminal or sub-terminal on short branches near base of assimilatory filaments, spherical or obovoidal, 8–10 μm in length, 4–7 μm in diameter; carpogonia 5–7 μm in diameter with trichogynes 100–300 μm in length, 2–4 μm in diameter; carposporangia obovoidal, 17–25 μm in length, 8.5–13 μm in diameter.
-
Diagnostic characters: this species is similar in morphology to numerous Thorea species; it may be distinguished currently on its geographic distribution in South America which does not overlap any other species and DNA sequence data.
-
Representative sequences in GenBank: KX958092 (COI-5P), KX958138, KX958139 (rbcL).
-
Distribution: South America: Argentina, Brazil (Fig. 4.4).
-
Key references: Pujals (1967), Necchi (1987), Necchi and Zucchi (1997), Necchi et al. (2010).
Thorea clavata Seto and Ratnas in Ratnas and Seto, Jap J Phycol 29:248 (1981) (Fig. 4.7c)
-
Type: Private herbarium, M Ratnasabapathy 1218, 6.v.1978 (Holotype), Kobe Coll. Herb. (Isotype, not found).
-
Type locality: Malaysia, Selangor State, Gombak River (19 milestone), 3.322099° N, 101.747846° E (estimated).
-
Description: thalli dull brown, sparsely to moderately branched, length 4.5–10(–12) cm, 480–1425 μm in diameter; medulla 115–420 μm in diameter; assimilatory filaments 130–840 μm in length, composed of (8–)12–25(–40) cells, tapering towards the basal part; cells 10–50 μm in length, 2–9 μm in diameter; spermatangia, carpogonia, carposporangia not observed; monosporangia ovoidal, obovoidal, pear-shaped, 8–20 μm in length, 5.5–14 μm in diameter.
-
Diagnostic characters: this species can be distinguished from all other species except T. zollingeri based on clavate apical cells of the assimilatory filaments and from T. zollingeri by having few monosporangia per clusters.
-
Representative sequences in GenBank: no sequences available.
-
Distribution: Asia: Malaysia, Thailand (Fig. 4.4).
-
Key references: Ratnasabapathy and Seto (1981), Johnston et al. (2018).
-
Remarks: new collections of specimens with the salient morphological characters are needed to determine if these characters are phylogenetically informative.
Thorea conturba Entwisle and Foard, Phycologia 38:49 (1999) (Fig 4.7d–f)
-
Type: MEL 2045617, TJ Entwisle 2832a, 15.vii.1997 (Holotype); NSW 423887, (Isotype).
-
Type locality: Australia , New South Wales, 25 km northeast of Lismore, tributary of Coopers Creek, Byrangery Creek, 28.62° S, 153.42° E.
-
Description: thalli dioecious, dark brown to green, moderately to abundantly branched, no longer than a few mm, 180–400(–460) μm in diameter; medulla 48–69 μm in diameter; assimilatory filaments 65–160(–260) μm in length, composed of 8–18(–30) cylindrical cells, 4–10(–15) μm in length, 4–7 μm in diameter; spermatangia, usually developing in clusters, ellipsoidal, 10–13 μm in length, 3.5–4 μm in diameter; carpogonia 3–5 μm in diameter, inserted directly on the basal cell of assimilatory filaments or on one discoid or barrel-shaped cell with trichogynes 60–140 μm in length, 2–3 μm in diameter; carposporangia obovoidal, 11–15 μm in length, 6–9 μm in diameter.
-
Diagnostic characters: this species has the reproductive structures in the middle of the assimilatory filaments and appears to be a morphology between Nothocladus with reproductive structures near the outer potion of the thallus and Thorea with structures close to the base of the assimilatory filaments.
-
Representative sequences in GenBank: no sequences available.
-
Distribution: Australasia: Australia, known only from the type locality (Fig. 4.4).
-
Key references: Entwisle and Foard (1999), Johnston et al. (2018).
-
Remarks: the distinguishing characteristics of this species may be due to its smaller size; T. hispida has been confirmed from Australia using DNA sequence data and these data are needed to confirm its unique morphology.
Thorea gaudichaudii C Agardh, Systema algarum:56 (1824) (Fig 4.7g, h)
-
Type: LD 17811, no collector or date (Lectotype designated by Sheath et al. 1993, not found).
-
Type locality: Marianas Islands, no GPS possible with the data provided.
-
Description: thalli reddish-brown, moderately branched, ≥ 5 cm in length, 624–1325 μm in diameter; medulla 161–256 μm in diameter; assimilatory filaments 178–628 μm in length, composed of 26–33 cylindrical cells, 14.7–27.7 μm in length, 3.1–6.8 μm in diameter; spermatangia and carpogonia not observed; carposporangia obovoidal, 14.2–23.1 μm in length, 6.8–11.8 μm in diameter; monosporangia obovoidal, 15–20.5 μm in length, 8.9–13.5 μm in diameter.
-
Diagnostic characters: this species has no characteristics to distinguish it from most species of the genus. Its geographic distribution overlaps with T. mauitukitukii , T. okadae , and T. hispida but not with the other species; it cannot be reliably distinguished from species within its geographic range without DNA sequence data.
-
Representative sequences in GenBank: KM055235, KM055236, KX958106 (COI-5P), AB159649, AB159650, KX958156 (rbcL).
-
Distribution: Asia: Japan, Philippines; Pacific Islands: Chuuk, Guam, Vanuatu (Fig. 4.4).
-
Key references: Sheath et al. (1993), Johnston et al. (2018).
-
Remarks: morphometric data include only the measurements from Sheath et al. (1993) (lectotype) and Johnston et al. (2018) (specimens with DNA sequence data). Sheath et al. (1993) reported monosporangia whereas Johnston et al. (2018) reported carposporangia.
Thorea hispida (Thore) Desvaux, Observations sur les plantes des environs d’Angers, pour servir de supplément a la flore Maine et Loire, et de suite à l’histoire naturelle et critique des plantes de France:16 (1818) (Fig. 4.7i–k)
-
Basionym: Conferva hispida Thore, Magasin Encycl 6:398 (1800).
-
Type: P-JU Herbier d’Antoine Laurent de Jussieu cat. no, 375-D, J Thore “1801” (Lectotype), BM, L, and MICH673302 (Isolectotypes).
-
Type locality: France, Landes, Dax, Adour River, 43.708608° N, 1.051945° W (estimated).
-
Description: thalli reddish-brown, abundantly branched, up to 100 cm in length, 500–2000 μm in diameter; medulla 88–611 μm in diameter; assimilatory filaments (350–)500–1000(–1400) μm in length, composed of 15–27 cylindrical cells, 28-49 μm in length, 4.8–8.8 μm in diameter; spermatangia and carpogonia not observed; monosporangia obovoidal, 16.2–21.1 μm in length, 8.0–13.6 μm in diameter.
-
Diagnostic characters: this species has no morphological characteristics to distinguish it from most species of the genus. It is the only species known from Europe; its geographic distribution overlaps with T. mauitukitukii , T. okadae , and T. kokosinga-pueschelii , but not with the other species; it cannot be reliably distinguished from species within its geographic range without DNA sequence data.
-
Representative sequences in GenBank: KC596320, KX9558095, KX958103 (COI-5P), AB159652, AB159653, KX958142 (rbcL).
-
Distribution: Asia: China, Japan; Australasia: Australia; Europe: Austria, Belgium, Croatia, Germany, Hungary, Italy, Lithuania, Netherlands, Poland, Rumania, Russia, Serbia, Slovakia, Spain, the UK; North America: the USA (Ohio), Pacific Islands: Hawaii (Fig. 4.4).
-
Key references: Eloranta et al. (2011), Johnston et al. (2018).
-
Remarks: Johnston et al. (2018) attributed numerous collections to this species using DNA sequence data, but most were “Chantransia,” and no measurements were provided for gametophytes. The distribution shown is based only on records with DNA sequence data or in Europe where only T. hispida occurs. Other records such as those from the Caribbean islands in Sheath et al. (1993) are labeled as Thorea sp. on the map because they may represent T. quisqueyana that is known from the Dominican Republic.
Thorea indica Necchi, Ganesan and JA West, Algae 30:268 (2015) (Fig 4.7l, m)
-
Type: MEL2389295, K Toppo, 13.iii.2014 (Holotype), SJRP 31508 (Isotype).
-
Type locality : India, Uttar Pradesh, Sai River, 26.650194° N, 80.793972° E.
-
Description: thalli dioecious, dark brown to greenish brown, 4–12 cm in length, moderately to abundantly branched; male thalli slender and abundantly branched, 500–900 μm in diameter; female thalli larger and moderately branched, 700–1250 μm in diameter; medulla 150–400 μm in diameter; short assimilatory filaments 25–45 μm in length, composed of 4–8, short barrel-shaped to cylindrical cells, 5–8 μm in diameter; long assimilatory filaments, sparsely branched, 380–500 μm in length, composed of 10–22 cylindrical cells, 4.5–7.5 μm in diameter; spermatangia arising from the short assimilatory filaments, usually developing in clusters or less often in two, obovoidal or ellipsoidal, 8.5–12.0 μm in length, 5–7.5 μm in diameter; carpogonia 5–9.5 μm in diameter with trichogynes 150–225 μm in length, 2.5–4.5 μm in diameter; monosporangia and carposporangia not observed.
-
Diagnostic characters: this species has no morphological characteristics to distinguish it from most species of the genus. It may be distinguished from other species based on geographic distribution or DNA sequence data.
-
Representative sequences in GenBank: KU351644 (COI-5P), KU351645 (rbcL).
-
Distribution: Asia: India, known only from the type locality (Fig. 4.4).
-
Key references: Necchi et al. (2015).
-
Remarks: Thorea has been reported from other location in India, but it is not yet been determined if specimens from other locations represent this or another species (Ganesan et al. 2018).
Thorea kokosinga-pueschelii ET Johnston and ML Vis, J Phycol 54:167 (2018) (Fig. 4.8a–c)
-
Type: MICH 1210810, R.G. Verb, 12.ix.2011 (Holotype), BHO A-1110 (Isotype).
-
Type locality: the USA, Ohio, Knox County, Kokosing River, 40.372617° N, 82.200531° W.
-
Description: thalli reddish-brown, 8–30 cm in length, abundantly branched, 1130–1453 μm in diameter; medulla 130–310 μm in diameter; assimilatory filaments 441–616.7 μm in length, composed of 12–22(–33) cylindrical cells, 23.3–31.9 μm in length and 5.5–8.3 μm in diameter; spermatangia not observed; carpogonia 5.1–5.5 μm in diameter with trichogynes 65.2–269.7 μm in length, 3.1–5.6 μm in diameter; carposporangia obovoidal, 17–22 μm in length; 10–16 μm in diameter.
-
Diagnostic characters: this species has no characteristics to distinguish it from most species of the genus. Its geographic distribution overlaps with T. hispida, but not with the other species but it cannot be reliably distinguished from this species without DNA sequence data.
-
Representative sequences in GenBank: AF506268, KX958150 (rbcL).
-
Distribution: North America: the USA (Ohio, New York) (Fig. 4.4).
-
Key references: Johnston et al. (2018).
Thorea mauitukitukii ET Johnston, KR Dixon, JA West and ML Vis, J Phycol 54:168 (2018) (Fig. 4.8d, e)
-
Type: MICH 1210809, R Dixon and K Dixon Kumano, xii.2005 (Holotype).
-
Type locality: Vanuatu , Efate, stream near Eton Beach, 17.738222° N, 168.562283° E (estimated).
-
Description: thalli reddish, 700–958 μm in diameter; medulla 354–362 μm in diameter; assimilatory filaments 177–305 μm in length, composed of up to 17–32 cylindrical cells, 11–15.5 μm in length, 4–5.7 μm in diameter; spermatangia and carpogonia not observed; carposporangia obovoidal, 15–23.9 μm in length, 9.4–11 μm in diameter.
-
Diagnostic characters: this species has no morphological characteristics to distinguish it from most species of the genus. Its geographic distribution overlaps with T. gaudichaudii , but not with the other species; it cannot be reliably distinguished from this species without DNA sequence data.
-
Representative sequences in GenBank: KX958113 (COI-5P), KX9558154, KX9558155 (rbcL).
-
Distribution: Pacific Islands: Vanuatu, known only from the type locality (Fig. 4.4).
-
Key references: Johnston et al. (2018).
Thorea okadae Yamada, Jap J Bot 24:158 (1949) (Fig 4.8f, g)
-
Type: SAP 046883, H. Hirose, 28iii.1939 (Holotype).
-
Type locality: Japan, Kagoshima, Hishikari along Sendai River, 32.018319° N, 130.587706° E (estimated).
-
Description: thalli reddish-brown, up to 1.5 m in length, abundantly branched, 800–1500(–4000) μm in diameter; medulla 242–395 μm in diameter; short assimilatory filaments up to 150 μm in length, composed of 3–6 cells, 5–18 μm diameter; long assimilatory filaments 150–400 μm in length, composed of 10–15 cylindrical cells, 20–42 μm in length, and 8–14 μm in diameter; spermatangia ovoidal, 10–13 μm in length, 5–6 μm in diameter; carpogonia 6–7 μm in diameter with trichogynes up to 350 μm in length, 3–4 μm in diameter; carposporangia obovoidal, 20–26 μm in length; 13–16 μm in diameter; monosporangia 10 μm in diameter.
-
Diagnostic characters: this species has no morphological characteristics to distinguish it from most species of the genus. Its geographic distribution overlaps with T. gaudichaudii and T. hispida but not with the other species; it cannot be reliably distinguished from species within its geographic range without DNA sequence data.
-
Representative sequences in GenBank: KM055238, KX958104 (COI-5P), AB159654, AB159654, KM005139 (rbcL).
-
Distribution: Asia: Japan (Fig. 4.4).
-
Key references: Yamada (1949), Yoshizaki (1986), Johnston et al. (2018).
Thorea quisqueyana ET Johnston and ML Vis, J Phycol 54:168 (2018)
-
Type: BHO A-0957, R. Thompson, xii.1968, “Chantransia” material from UTEX LB 2743 (Holotype).
-
Type locality : Dominican Republic, La Toma freshwater spring near San Cristobal, 18.457132° N, 70.123626° W (estimated).
-
Description: only known from the “Chantransia” in culture; no gametophytes observed.
-
Representative sequences in GenBank: KM055234 (COI-5P), KM005135 (rbcL).
-
Distribution: North America: Dominican Republic, known only from the type locality (Fig. 4.4).
-
Key reference: Johnston et al. (2018).
Thorea riekei Bischoff, J Phycol 1:111 (1965) (Fig 4.8h–j)
-
Type: UC1498150, HW Bischoff and GF Papenfuss, 13.xi.1975 (Lectotype here designated).
-
Type locality: the USA , Texas, Comal Co., New Braunfels, 29.709148° N, 98.135284° W (estimated).
-
Description: thalli dark red, up to 2 m in length, sparsely branched, 500–900 μm in diameter; medulla 195–271 μm in diameter; assimilatory filaments, unbranched, 186–408(–775) μm in length, composed of 25–45 cylindrical cells, 12–40 μm in length, 3.9–7 μm in diameter; spermatangia and carpogonia not observed; carposporangia obovoidal, 11.5–19.8 μm in length, 8.3–12 μm in diameter; monosporangia single or in clusters, obovoidal, spherical, ellipsoidal, 12–15 μm in length, 8–9 μm in diameter.
-
Diagnostic characters: this species has no morphological characteristics to distinguish it from most species of the genus, but its geographic distribution does not overlap with the other species and can be useful to distinguish it.
-
Representative sequences in GenBank: KM055239, KX958107, KX958109 (COI-5P), AB159656, KX958149, KX958151(rbcL).
-
Distribution: North America: the USA, Mexico (Fig. 4.4).
-
Remarks: sporangia were interpreted as monosporangia in Bischoff (1965) and carposporangia in Johnston et al. (2018). Records of T. hispida from Carmona and Necchi (2001) have been included here as T. riekei based on morphological similarity and geographic distribution of species given in Johnston et al. (2018).
Thorea violacea Bory, Ann Mus Hist Nat 12:133 (1808) (Fig. 4.8k, l)
-
Type : BM000770128, JB Bory, 1801–1802, (Lectotype designated in Sheath et al. 1993), PC0602800, PC0602801 (Isotypes).
-
Type locality: Réunion, riviere des Ramparts, 21.313831° S, 55.623500° E (estimated).
-
Description: thalli reddish-brown , sparsely branched, 993–1878 μm in diameter; medulla 218–304 μm in diameter; assimilatory filaments, 291–533 μm in length, composed of up to 60 cylindrical cells, 24.9–32.9 μm in length, 3.5–7.2 μm in diameter; spermatangia not observed; carpogonia 2.4–6.7 μm in diameter with trichogynes 14.7–56.1 μm in length; carposporangia obovoidal, 7.4–19.3 (mean 17.4) μm in length, 5.4–18.1 (mean 8.4) μm in diameter; monosporangia 16.2–24.5 μm in length 7.4–14.1 μm in diameter.
-
Diagnostic characters: this species has no characteristics to distinguish it from most species of the genus. However, its geographic distribution does not overlap with the other species and can be helpful in distinguishing it.
-
Representative sequences in GenBank: no sequences available.
-
Distribution: Africa: Réunion, known only from the type locality (Fig. 4.4).
-
Key references: Sheath et al. (1993), Johnston et al. (2018).
-
Remarks: numerous species and specimens from distant geographic locations have been attributed to this species, but DNA sequence data have shown at least some of those are unique. For example, T. okadae, T. riekei , T. bachmannii, and T. gaudichaudii had been previously been placed in synonym with T. violacea (Sheath et al. 1993), but more recently they have been showed to be genetically distinct from each other (Johnston et al. 2018). Those researchers suggested that trapezoidal basal cells are unique to this species, but that has yet to be established photographically; the type location is geographically distant from other species and DNA sequence data are needed to confirm whether it should continue to be recognized as a distinct species. Note that sporangia were interpreted as monosporangia by Sheath et al. (1993) and as carposporangia by Johnston et al. (2018).
Doubtful Species
-
Thorea brodensis Klas, Hedwigia 75:283 (1936). This species is only known from the type locality in Europe. The protologue is confusing providing morphological measurements for summer and winter forms. This species was included in Kumano (2002) based on the greater measurements for the summer form. Sheath et al. (1993) considered it a synonym of T. violacea but since that species is currently recognized only from the type locality on the island of Réunion, that synonym is unlikely based on geographic distribution. It is most likely a synonym of T. hispida, currently the only species recognized in Europe.
-
Thorea flagelliformis Zanardini, Mem Reale Inst Ven Sci, Let Art 17:148 (1872). There is a very short description provided in the protologue which cannot be used to assess whether it is distinct based on morphology and there has been no study or report of this species since the protologue. The type location in southeast Asia (Sarawak) is a region of high species diversity for this genus and therefore no synonym can be suggested based on biogeography.
-
Thorea prowsei Ratnas and Seto, Jap J Phycol 29:246 (1981). This species is only known from the type locality. In the protologue, it was distinguished from T. gaudichaudii by having thicker clusters of monosporangia. However, the number of monosporangia or carposporangia in a cluster varies considerably within and among species such that it is not a good taxonomic character. DNA sequence data should be obtained to determine if these species are synonymous since there are numerous species in Southeast Asia and Pacific Islands only distinguished by genetic variation.
-
Thorea siamensis S Traichaiyaporn and Kumano, Nat Hist J Chulalongkorn Univ 8:28 (2008). This species is only known from the type locality. In the protologue, it was distinguished from T. gaudichaudii based on smaller carposporangia but measurements of T. gaudichaudii in Johnston et al. (2018) overlap (16–19 × 8–9 μm in T. siamensis and 14.2–23.1 × 6.8–11.8 μm in T. gaudichaudii). DNA sequence data should be obtained to determine if these species are synonymous since there are numerous species in Southeast Asia and Pacific Islands only distinguished by genetic variation.
-
Thorea zollingeri F Schmitz, Ber Deut Bot Ges 10:134 (1892). This species is named from material collected in Java and has not been reported since. Sheath et al. (1993) examined the type specimen and determined that it had clavate assimilatory filaments like T. clavata and differed from that species based on the number of monosporangia per cluster. However, the assimilatory filament shown is not convincingly clavate [Fig. 12 in Sheath et al. (1993)] and the number of monosporangia per cluster varies within and among species. Southeast Asia is a region with numerous species having similar morphology such that it would be difficult to determine to which it might be synonymous.
(a, b) Thorea bachmannii : (a) thallus with loose assimilatory filaments (arrow); (b) young carpogonium with ovoidal base (arrow) and long, thin trichogyne (arrowhead); (c) Thorea clavata : (c) assimilatory filament tapering with wider cell (arrow) near apex and cells narrowing (arrowhead) towards the filament base; (d–f) Thorea conturba : (d) thallus with loose assimilatory filaments (arrow); (e) assimilatory filament (arrow) with carpogonium composed of an ovoidal base (arrowhead) and long, thin trichogyne (double arrowhead); (f) carposporangium (arrow) at the tip of a multi-celled gonimoblast filament; (g, h) Thorea gaudichaudii : (g) thallus with loose assimilatory filaments (arrows) and thin medulla (arrowhead); (h) obovoidal carposporangia (arrows) at the base of the assimilatory filaments; (i–k) Thorea hispida: (i) thallus with loose assimilatory filaments (arrows) and thick medulla (arrowhead); (j) assimilatory filament composed of cylindrical cells (arrows) that do not narrow towards the base; (k) obovoidal carposporangia (arrows) at the base of the assimilatory filaments; (l, m) Thorea indica : (l) thallus with loose assimilatory filaments (arrow) and thick medulla (arrowhead); (m) young carpogonium with ovoidal base (arrow) and long, thin trichogyne (arrowhead). Scale bars: (a) = 500 μm; (b, f, m) = 10 μm; (c, e, h, k) = 20 μm; (d, g) = 250 μm; (i) = 1 mm; (j, l) = 50 μm (Fig (c) reproduced with permission by the publisher (Taylor and Francis) from Sheath et al. (1993). Fig. (d–f) reprinted with permission by Taylor and Francis from Entwisle and Foard (1999); Image author: Fig. (i) C Carter)
(a–c) Thorea kokosinga-pueschelii : (a) thallus habit with few branches; (b) thallus with loose assimilatory filaments (arrows) and thick medulla (arrowhead); (c) obovoidal carposporangia (arrows) at the base of the assimilatory filaments; (d, e) Thorea mauitukitukii : (d) thallus with loose assimilatory filaments (arrows) and thick medulla (arrowhead); (e) carposporangium (arrow) at the tip of a multi-celled gonimoblast filament; (f, g) Thorea okadae : (f) carpogonium with ovoidal base (arrow) and long, thin trichogyne (arrowhead); (g) obovoidal carposporangia (arrows) at the base of the assimilatory filaments; (h–j) Thorea riekei : (h) thallus habit with numerous branches; (i) thallus with loose assimilatory filaments (arrows) and thick medulla (arrowhead); (j) putative obovoidal carposporangium (arrow) at the base of the assimilatory filaments; (k, l) Thorea violacea : (k) thallus with loose assimilatory filaments (arrows) and thick medulla (arrowhead); (l) obovoidal and ellipsoidal carposporangia (arrows) at the base of the assimilatory filaments. Scale bars: (a, h) = 20 mm; (b, d, i, l) = 400 μm; (c, e, j) = 20 μm; (f) = 50 μm; (g) = 25 μm; (k) = 250 μm (Image author: Fig. (f, g) R Terada)
References
Agardh CA (1824) Systema algarum. Literis Berlingianis, Berlin
Bischoff HW (1965) Thorea riekei sp. nov. and related species. J Phycol 1:111–117. https://doi.org/10.1111/j.1529-8817.1965.tb04567.x
Bolpagni R, Amadio C, Johnston E, Racchetti E (2015) New physical and chemical perspectives on the ecology of Thorea hispida (Thoreaceae). J Limnol 74:294–301. https://doi.org/10.4081/jlimnol.2014.1058
Bory de Saint-Vincent JBGM (1808) Sur un genre nouveau de la cryptogamie aquatique, nomme Thorea. Ann Mus Hist Nat 12:126–135
Bory de Saint-Vincent JB (1823) Dictionaire Classique d’Histoire Naturelle, vol 3. Rey and Gravier, Paris
Carmona JJ, Necchi O Jr (2001) A new species and expanded distributions of freshwater Audouinella (Acrochaetiaceae, Rhodophyta) from Central Mexico and south-eastern Brazil. Eur J Phycol 36:217–226. https://doi.org/10.1080/09670260110001735368
Chiasson WB, Johanson KG, Sherwood AR, Vis ML (2007) Phylogenetic affinities of the form taxon Chantransia pygmaea (Rhodophyta) specimens from the Hawaiian islands. Phycologia 46:257–262. https://www.tandfonline.com/doi/abs/10.2216/06-79.1
Christensen T (1978) Annotations to a textbook of phycology. Bot Tidsskr 73:65–70
Desikachary TV, Krishnamurthy V, Balakrishnan MS (1990) Rhodophyta. vol. 1. Madras Science Foundation, Madras
Desvaux NA (1818) Observations sur les plantes des environs d’Angers, pour servir de supplément a la flore Maine et Loire, et de suite à l’histoire naturelle et critique des plantes de France. Angers, Paris
Drew K (1935) The life history of Rhodochorton violaceum (Kütz.) comb. nov. (Chantransia violacea Kütz.). Ann Bot 49:439–450
Duby JE (1830) Botanicon Gallicon, 2, Part 2 edn. Ve Desray, Paris
Eloranta P, Kwandrans J, Kusel-Fetzmann E (2011) Rhodophyceae and Phaeophyceae; Süßwasserflora von Mitteleuropa Band 7. Spectrum Akademischer, Heidelberg
Eloranta P, Eloranta A, Perämäki P (2016) Intensive study of freshwater red algae (Rhodophyta) in Finland. Fottea 16:122–132. https://doi.org/10.5507/fot.2015.025
Entwisle TJ, Foard HJ (1999) Freshwater Rhodophyta in Australia: Ptilothamnion richardsii (Ceramiales) and Thorea conturba sp. nov. (Batrachospermales). Phycologia 38:47–53. https://www.tandfonline.com/doi/abs/10.2216/i0031-8884-38-1-47.1
Entwisle TJ, Evans JR, Vis ML, Saunders GW (2018) Ottia meiospora (Ottiaceae, Rhodophyta), a new genus and family endophytic within the thallus of Nothocladus (Batrachospermales, Rhodophyta) J Phycol 54:79–84. https://doi.org/10.1111/jpy.12603
Feldmann J (1953) L’évolution des organes femelles chez les Floridées. Proc Int Seaweed Symp 1:11–12
Ganesan EK, West JA (2013) Nomenclatural changes for some freshwater red algae from India. Algae 28:45–51. https://doi.org/10.4490/algae.2013.28.1.045
Ganesan EK, West JA, Necchi O Jr (2018) A catalogue and bibliography of non-marine (freshwater and estuarine) Rhodophyta (red algae) of India. Phytotaxa 364:1–48. https://doi.org/10.11646/phytotaxa.364.1.1
Garbary DJ (1987) The Acrochaetiaceae (Rhodophyta): an annotated bibliography. Bibl Phycol 77:1–267
Han J-F, Nan F-R, Feng J, Lv J-P, Liu X-D, Xie S-L (2020) Affinites of four freshwater putative “Chantransia” stages (Rhodophyta) in southern China from molecular and morphological data. Phytotaxa 441:47–59. https://doi.org/10.11646/phytotaxa.441.1.4
Higa A, Kasai F, Kawachi M, Kumano S, Sakayama H, Miyashita M, Watanabe MM (2007) Seasonality of gametophyte occurrence, maturation and fertilization of the freshwater red alga Thorea okadae (Thoreales, Rhodophyta) in the Kikuchi River, Japan. Phycologia 46:160–167. https://www.tandfonline.com/doi/abs/10.2216/05-39.1
Hirose H, Yamagishi T (1977) Illustrations of the Japanese freshwater algae. Uchida Rokakuho, Tokyo
Howard RV, Parker BC (1979) Nemalionopsis shawii forma caroliniana (forma nov.) (Rhodophyta: Nemaliales) from the Southeastern United States. Phycologia 18:330–337. https://www.tandfonline.com/doi/abs/10.2216/i0031-8884-18-4-330.1
Israelson G (1942) The freshwater Florideae of Sweden. Symbol Bot Upsal 6:1–134
Jao CC (1940) Studies on the freshwater algae of China IV. Subaerial and aquatic algae from Nonyoh, Hunan, part 2. Dermatol Sin 11:241–361
Jao CC (1941) Studies on the freshwater algae of China VII. A preliminary account of the Chinese freshwater Rhodophyceae Sinensia 12:245–290
Johnston ET, Lim P-E, Buhari N, Keil EJ, Djawad I, Vis ML (2014) Diversity of freshwater red algae (Rhodophyta) in Malaysia and Indonesia from morphological and molecular data. Phycologia 53:329–334. https://doi.org/10.2216/13-223.1
Johnston EJ, Dixon KR, West JA, Buhari N, Vis ML (2018) Three gene phylogeny of the Thoreales (Rhodophyta) reveals high species diversity. J Phycol 54:159–170. https://doi.org/10.1111/jpy.12618
Jose L, Patel RJ (1990) A new fresh water species of Acrochaetium (Acrochaetium keralayensis sp. nov.) from India. Indian Bot Contactor (IBC) 7:177–180
Kamiya M (2017) Class Florideophyceae, Subclass Nemaliophycideae, Orders Acrochaetiales, Balbianiales and Thoreales. In: Kamiya M, Lindstrom SC, Nakayama T et al (eds) Syllabus of plant families; Adolf Engler’s Syllabus der Planzenfamilien—Part 2/2—Photoautotrophic eukaryotic algae—Rhodophyta. Borntraeger Science, Stuttgart, pp 33–52
Klas Z (1936) Eine neue Thorea aus Jugoslawien, Thorea brodensis Klas sp. n. Hedwigia 75:273–284
Kozono J, Nishihara GN, Endo H, Terada R (2018) Effect of temperature and PAR on photosynthesis of an endangered freshwater red alga, Thorea okadae, from Kagoshima, Japan. Phycologia 57:619–629. https://doi.org/10.2216/18-26.1
Kronborg L (1992) Studies on the ecology and host specificity of Balbiania investiens (Rhodophyceae). Nord J Bot 12:537–540. https://doi.org/10.1111/j.1756-1051.1992.tb01832.x
Kumano S (1978) Occurrence of a new freshwater species of Acrochaetium, Rhodophyta, in Japan. Jpn J Phycol 26:105–108
Kumano S (2002) Freshwater red algae of the world. Biopress, Bristol
Kützing FT (1849) Species algarum. FA. Brockhaus, Leipzig
Lam DW, Verbruggen H, Saunders GW, Vis ML (2016) Multigene phylogeny of the red algal subclass Nemaliophycidae. Mol Phylog Evol 94:730–736. https://doi.org/10.1016/j.ympev.2015.10.015
Leukart P, Knappe J (1995) Observations on Balbiania investiens (Rhodophyta) from two new locations in Germany and from laboratory culture. Nova Hedwigia 60:527–532
Maulood BK, Hassan FM, Al-Lami AA, Toma JJ, Ismail AM (2013) Checklist of algal flora in Iraq. Ministry of Environment, Baghdad
Migita S, Takasaki M (1991) The freshwater red alga Nemalionopsis tortuosa from a new locality at Amagi City, Fukuoka Prefecture. Jpn Bull Dept Fish Nagasaki Univ 69:1–5
Müller KM, Sherwood AR, Pueschel CM, Gutell RR, Sheath RG (2002) A proposal for a new red algal order, the Thoreales. J Phycol 38:807–820. https://doi.org/10.1046/j.1529-8817.2002.01055.x
Necchi O Jr (1987) Sexual reproduction in Thorea Bory (Rhodophyta, Thoreaceae). Jpn J Phycol 35:106–112
Necchi O Jr, Zucchi MR (1995) Systematics and distribution of freshwater Audouinella (Acrochaetiaceae, Rhodophyta) in Brazil. Eur J Phycol 30:209–218. https://doi.org/10.1080/09670269500650991
Necchi O Jr and Zucchi MR (1997) Audouinella macrospora (Acrochaetiaceae, Rhodophyta) is the Chantransia stage of Batrachospermum (Batrachospermaceae). Phycologia 36:220–224. https://doi.org/10.2216/i0031-8884-36-3-220.1
Necchi O Jr, Sheath RG, Cole KM (1993a) Systematics of the freshwater Audouinella (Acrochaetiaceae, Rhodophyta) in North America. 1. The reddish species. Algol Stud 70:11–28
Necchi O Jr, Sheath RG, Cole KM (1993b) Systematics of the freshwater Audouinella (Acrochaetiaceae, Rhodophyta) in North America. 2. The bluish species. Algol Stud 71:13–21
Necchi O Jr, Oliveira MC, Salles P (2010) Molecular systematics of Thorea (Rhodophyta, Thoreales) species in Brazil. Braz J Bot 33:227–235. https://doi.org/10.1590/S0100-84042010000200004
Necchi O Jr, Paiano MO, West JA, Ganesan EK, Loiseaux-de Goër S (2015) Thorea indica sp. nov. (Thoreales, Rhodophyta) from Uttar Pradesh, India. Algae 30:265–274. https://doi.org/10.11646/phytotaxa.364.1.1
Necchi O Jr, West JA, Rai SK, Ganesan EK, Rossignolo NL, Loiseaux de Goër S (2016) Phylogeny and morphology of the freshwater red alga Nemalionopsis shawii (Rhodophyta, Thoreales) from Nepal. Phycol Res 64:11–18. https://doi.org/10.1111/pre.12116
Papenfuss GF (1945) Review of the Acrochaetium-Rhodochorton complex of the red algae. Univ Calif Publ Bot 18:299–334
Pujals C (1967) Notas sobre Rhodophycophyta de la Argentina. Revista del Museo Argentino de Ciencias Naturales Bernardino Rivadavia. Hydrobiología 2:57–76
Ratnasabapathy M, Seto R (1981) Thorea prowsei sp. nov. and Thorea clavata sp. nov. (Rhodophyta, Nemaliales) from West Malaysia. Jpn J Phycol 29:243–250
Roth AG (1806) Catalecta Botanica, fasc. 3. Bibliopolio Io Fr Gleditschianeipzig, Leipzig
Saunders GW, Jackson C, Salomaki ED (2017) Phylogenetic analyses of transcriptome data resolve familial assignments for genera of the red-algal Acrochaetiales-Palmariales complex (Nemaliophycidae). Mol Phylog Evol 119:151–159. https://doi.org/10.1016/j.ympev.2017.11.002
Schmitz F (1892) Die systematische Stellung der Gattung Thorea Bory. Ber Deut Bot Ges 10:115–142
Sheath RG, Müller KM (1999) Systematic status and phylogenetic relationships of the freshwater genus Balbiania (Rhodophyta). J Phycol 35:855–864. https://doi.org/10.1046/j.1529-8817.1999.3540855.x
Sheath RG, Sherwood AR (2002) Phylum Rhodophyta (red algae). In: John DM, Whitton BA, Brooks AJ (eds) The freshwater algal flora of the British Isles. Cambridge University Press, Cambridge, pp 123–143
Sheath RG, Vis ML (2015) Red Algae. In: Wehr JD, Sheath RG, Kociolek JP (eds) Freshwater algae of North America. Academic Press, San Diego, pp 237–264. https://doi.org/10.1016/B978-0-12-385876-4.00005-0
Sheath RG, Vis ML, Cole KM (1993) Distribution and systematics of the freshwater red algal family Thoreaceae in North America. Eur J Phycol 28:231–241. https://doi.org/10.1080/09670269300650341
Sheath RG, Whittick A, Cole KM (1994) Rhododraparnaldia oregonica, a new freshwater red algal genus and species intermediate between the Acrochaetiales and the Batrachospermales. Phycologia 33:1–7. https://doi.org/10.2216/i0031-8884-33-1-1.1
Shi Z, Xie S, Hua D (2006) Flora Algarum Sinicarum Aquae Dulcis. 12: Rhodophyta. Science Press, Beijing
Sirodot S (1876) Le Balbiania investiens. Étude organogénique et phylsiologique Ann Sci Nat Bot sér 6(3):146–174
Skinner S, Entwisle TJ (2001) Non-marine algae of Australia: 3. Audouinella and Balbiania (Rhodophyta). Telopea 9:713–723
Skuja H (1934) Untersuchungen über die Rhodophyceen des Süsswassers. Beih Bot Centralbl 52:173–192
Skuja H (1944) Untersuchungen über die Rhodophyceen des Süsswassers. [VII-XII]. Acta Hortic Bot Univ Latv 14:3–64
Starmach K (1985) Chantransia hermannii (Roth) Desvaux and the systematic position of the genera Chantransia, Pseudochantransia and Audouinella. Acta Soc Bot Pol 54:273–284
Swale EMF, Belcher JH (1963) Morphological observations on wild and cultured material of Rhodochorton investiens (Lenormand) nov. comb. (Balbiania investiens (Lenorm.) Sirodot). Ann Bot 27:281–290
Szinte AL, Taylor JC, Abosede AT, Vis ML (2020) Current status of freshwater red algal diversity (Rhodophyta) of the African continent including description of new taxa (Batrachospermales). Phycologia 59:187–199. https://doi.org/10.1080/00318884.2020.1732149
Terada R, Watanabe Y, Fujimoto M, Tatamidani I, Kokubu S, Nishihara GN (2016) The effect of PAR and temperature on the photosynthetic performance of a freshwater red alga, Thorea gaudichaudii (Thoreales) from Kagoshima, Japan. J Appl Phycol 28:1225–1263. https://doi.org/10.1007/s10811-015-0660-z
Thore J (1800) Sur la Conferva hispida. Magasin Encycl 6:398–403
Traichaiyaporn S, Khuantrairong T, Kumano S (2008) Thorea siamensis (Thoreaceae, Rhodophyta) from Thailand. Nat Hist J Chulalongkorn Univ 8:27–33
Vis ML, Tiwari S, Evans JR, Stancheva R, Sheath RG, Kennedy B, Lee J, Eloranta P (2020) Revealing hidden diversity in the Sheathia arcuata morphospecies (Batrachospermales, Rhodophyta) including four new species. Algae 35:213–224. https://doi.org/10.4490/algae.2020.35.8.31
Xie S, Shi, Z (2004) Balbiania Sirodot a new record of freshwater red algae from China. Acta Bot Boreal Occident Sin 24:1732–1733
Yamada Y (1949) On the species of Thorea from the far eastern Asia. Jpn J Bot 24:155–159
Yoshizaki M (1986) The morphology and reproduction of Thorea okadai (Rhodophyta). Phycologia 25:476–481. https://www.tandfonline.com/doi/abs/10.2216/i0031-8884-25-4-476.1
Zanardini G (1872) Phycearum indicarum pugillus a Cl. Eduardo Beccari ad Borneum, Sincapoore et Ceylanum annis MDCCCLXV-VI-VII collectarum quas cognitas determinavit, novasque descripsit iconibusque illustrare curavit Joannes Zanardini. Mem Reale Istit Ven Sci Let Art 17:129–170
Zucchi MR, Necchi O Jr (2003) Blue-greenish acrochaetioid algae in freshwater habitats are ‘Chantransia’ stages of Batrachospermales ‘sensu lato’ (Rhodophyta). Cryptogam Algol 24:117–131. https://sciencepress.mnhn.fr/en/periodiques/algologie/24/2/blue-greenish-acrochaetioid-algae-freshwater-habitats-are-chantransia-stages-batrachospermales-sensu-lato-rhodophyta
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vis, M.L., Necchi Jr, O. (2021). Subphylum Eurhodophytina, Class Florideophyceae, Subclass Nemaliophycidae, Orders Acrochaetiales, Balbianiales, and Thoreales. In: Freshwater Red Algae. Springer, Cham. https://doi.org/10.1007/978-3-030-83970-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-030-83970-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83969-7
Online ISBN: 978-3-030-83970-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)