Abstract
Declining kidney function results in mineral and hormonal imbalances that trigger a maladaptive feedback loop favoring the oversecretion of parathyroid hormone (PTH). As kidney disease progresses, chronic hyperphosphatemia, elevated parathyroid hormone and fibroblast grown factor-23, low 1,25-dihydroxyvitamin D, hypocalcemia, and metabolic acidosis can collectively result in disordered bone turnover and vascular calcifications. The term chronic kidney disease – mineral and bone disorder (CKD-MBD) – is used to describe these imbalances and the increased risk of fracture, cardiovascular events, and mortality they confer. Preventing or delaying the progressive metabolic, vascular, and bone complications associated with CKD-MBD is essential. We provide a summary of how to assess and manage the mineral and bone disorders associated with advanced CKD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic kidney disease with mineral and bone disorder (CKD-MBD)
- Secondary hyperparathyroidism
- Hyperphosphatemia
- Renal osteodystrophy
- High bone turnover
- Adynamic bone disorder
Case Presentation
A 62-year-old woman with chronic kidney disease stage 4 (CKD 4) due to uncontrolled hypertension and diabetes mellitus type 2 is referred for evaluation of elevated parathyroid hormone (PTH). She has had mild diffuse skin itching over the last few months but otherwise feels well without history of bone pain or fractures. On physical exam, she has full range of motion and strength in her joints and extremities. There is no joint inflammation or bony abnormalities. Her skin is well perfused and without rash. Her laboratories are pertinent for serum creatinine 3.2 mg/dL, estimated glomerular filtration rate (eGFR) 20 mL/min, bicarbonate (HCO3)– 19 mmol/L (22–31 mmol/L), phosphate 6.2 mg/dL (2.5–4.5 mg/dL), 25-hydroxyvitamin D 18 ng/mL (30–80 ng/mL), 1,25-dihydroxyvitamin D (calcitriol or [1,25-(OH)2-D]) 20 pg/mL (19.9–79.3 pg/mL), calcium (Ca2+) 8.2 mg/dL (8.4–10.5 mg/dL), albumin 4.0 g/dL, intact PTH 375 pg/mL (15–65 pg/mL), and bone-specific alkaline phosphatase (BALP) 75 μg/L (4–36 μg/L). A CT scan performed 2 months previously showed coronary artery calcifications.
Assessment and Diagnosis
Chronic kidney disease with mineral and bone disorder (CKD-MBD) describes the systemic alterations in mineral, metabolic, hormonal, and bone homeostasis that can increase the risk of fractures, vascular calcification, cardiovascular morbidity, and mortality in patients with eGFR <60 mL/min. Patients with CKD are 2–17 times more likely to experience bone fracture than the general population. This risk increases proportionately as kidney function declines, with most CKD patients stages three to five showing signs of high bone turnover and increased PTH [1, 2]. Rates of hip fracture in end-stage kidney disease (ESKD) have increased over the last 30 years. CKD patients with bone fractures have decreased quality of life, longer hospitalizations, incur higher healthcare costs, and experience a 16–60% increase in morbidity and mortality compared to patients who fracture with normal kidney function [3]. CKD-MBD affects bone and mineral metabolism in three main areas: serum mineral and hormone imbalance, decreased bone quality and strength, and increased extraskeletal calcifications.
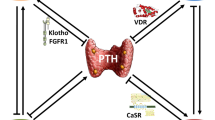
Serum biomarkers used to assess CKD-MBD include phosphate, 1,25-dihydroxyvitamin D, calcium, bicarbonate, PTH, and fibroblast growth factor-23 (FGF-23) (Table 10.1). As eGFR declines, phosphate clearance is reduced, triggering the rise in phosphaturic hormones PTH and FGF-23 in a feedback loop to increase kidney excretion of phosphate. Hyperphosphatemia and elevated FGF-23 along with reduced kidney function result in low 1,25-dihydroxyvitamin D which causes hypocalcemia, yet another trigger for PTH release. Chronic overproduction of parathyroid hormone results in the typical high turnover bone disease seen in these patients (Fig. 10.1). Additionally, metabolic acidosis is frequent in CKD and directly causes bone loss, impaired bone mineralization, and increased FGF-23 (Table 10.1) [4].
Renal osteodystrophy is a broad term describing the various effects of CKD-MBD on bone formation and resorption. Bone morphology in CKD is mainly impacted by the rate of bone turnover; degree of bone mineralization and bone volume play a lesser role. The net effect of the abovementioned mineral/hormone imbalance is a PTH-induced increase in bone resorption (coupled with increased bone formation) leading to higher rates of bone turnover. The type and degree of renal osteodystrophy depend on the combination of these factors and the guideline-derived medical interventions initiated in response to abnormal serum biomarkers (Fig. 10.2). In early CKD, bone turnover rates tend to be high, driven by secondary hyperparathyroidism. As CKD progresses to ESKD, low bone turnover is more prevalent [1]. This may partially be iatrogenic, due to over-suppression of PTH by medications such as calcium-based phosphate binders, activated vitamin D, and calcimimetics.
The third component of CKD-MBD is extraskeletal calcification. Accelerated vascular calcification is one of the strongest predictors of cardiovascular events and mortality in CKD [5]. Hyperphosphatemia has been consistently shown to increase mortality, likely due to its direct calcifying effect on coronary vessels and valves [6]. Many other mineral and hormonal alterations in CKD-MBD and their treatments have been associated with increased vascular calcification, cardiovascular events, and mortality, including hypercalcemia, the use of calcium-based phosphate binders, high FGF-23, and both high and low PTH [7,8,9]. Calcimimetics such as cinacalcet that reduce PTH and calcium levels may possibly reduce vascular calcification risk in ESKD [10]. Calciphylaxis is a rare but often deadly condition associated with CKD-MBD seen almost exclusively in advanced CKD/ESKD. It is thought to be caused by hydroxyapatite deposition within vessel layers and surrounding subcutaneous adipose tissue resulting in painful skin ulcerations that turn necrotic and are easily infected. These patients need immediate referral to a multidisciplinary care team including a nephrologist, vascular surgeon, and dermatologist, as the 1-year mortality rate is greater than 50% [11].
Management
The management of CKD-MBD is centered on preventing the adverse consequences associated with secondary hyperparathyroidism.
Hyperphosphatemia
-
Low phosphate diet
-
Phosphate binders
Hyperphosphatemia drives secondary hyperparathyroidism, increased serum FGF-23, and inhibits vitamin D activation in CKD. As such, an important early intervention in CKD-MBD is counseling the patient on dietary phosphate restriction, less than 800–1000 mg/day, to keep phosphate “toward normal range” [12]. Controlling phosphate intake can be challenging for patients. The typical American diet includes highly processed foods with large amounts of inorganic phosphate additives. These foods contain nearly 60% more phosphate than similar organic sources without additives, and inorganic phosphate is 100% bioavailable [13]. Two recent trials have shown that dietary guidance on removing foods containing phosphate additives in ESKD patients led to significantly lower serum phosphate levels [14, 15]. Plant-based sources of dietary phosphate, such as grains and legumes, are less bioavailable than natural animal-based sources like dairy, and both of these natural phosphate sources are less bioavailable than foods with inorganic phosphate additives [16, 17].
While dietary control is imperative in hyperphosphatemia, inorganic phosphate additives are ubiquitous and difficult to avoid. Patients will often need medication to help reduce their serum phosphate. Calcium-containing phosphate binders (calcium carbonate and calcium acetate) are the most frequently used worldwide as they are readily available and affordable. However, guidelines now suggest “restricting the dose” of calcium-based binders due to their association with adynamic bone disease, vascular calcification, and mortality, likely attributable to the calcium component [12, 16]. Non-calcium-based binders (sevelamer carbonate and sevelamer hydrochloride) are the most common alternatives; however, like calcium-based binders, their use is limited by pill burden, compliance, and gastrointestinal side effects. Newer phosphate binders contain iron (sucroferric oxyhydroxide and ferric citrate) which have the additional benefit of improving anemia which is common in CKD patients. A recent trial showed that sucroferric oxyhydroxide slowed vascular calcification in dialysis patients [18]. Intestinal phosphate transport inhibitors, tenapanor and nicotinamide, are currently in preclinical testing. Rather than binding gut phosphorous each meal, these agents block paracellular transport of phosphate, reducing intestinal absorption [17]. How these agents will compare to phosphate binders in terms of efficacy, gastrointestinal side effects, and decreased pill burden remains to be determined.
Secondary Hyperparathyroidism
-
Decrease serum phosphate
-
Increase 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D
-
Decrease PTH with activated vitamin D and/or calcimimetics
The optimal PTH level in CKD is not known. KDIGO suggests reducing “progressively rising or persistently elevated” PTH [12]. Lowering PTH in secondary hyperparathyroidism involves normalizing serum phosphate and repleting both nutritional 25-hydroxyvitamin D deficiency and acquired 1,25-dihydroxyvitamin D deficiency. Activated vitamin D (calcitriol or its analogs doxercalciferol, paricalcitol, and alfacalcidol) is frequently used to effectively lower PTH. These medications can result in hypercalcemia, increased intestinal phosphate absorption, and over-suppression of PTH, so careful attention to dosing and monitoring of serum calcium, phosphate, and PTH levels is prudent [19, 20]. Current guidelines suggest using activated vitamin D only in patients with CKD 4, 5, and ESKD who have “severe and progressive hyperparathyroidism” [12].
Calcimimetics such as cinacalcet or etelcalcetide can be used as an alternative to or in conjunction with activated vitamin D to treat secondary hyperparathyroidism, but they are currently only approved for use in ESKD. Calcimimetics suppress PTH secretion by binding the calcium-sensing receptor on the parathyroid gland. They effectively control PTH in dialysis patients and are especially useful in patients who have hypercalcemia and hyperphosphatemia which limit the use of activated vitamin D. However, despite cinacalcet effectively lowering PTH in a large trial of dialysis patients with severe hyperparathyroidism, there was no reduction in fracture risk or cardiovascular events [21]. Multiple gland parathyroidectomy is ultimately recommended for those with severe hyperparathyroidism who do not respond to or who develop contraindications to medical therapy [12].
Chronic Metabolic Acidosis
Metabolic acidosis is extremely common among CKD patients and can directly contribute to bone loss. Acutely, the bone buffers acid, releasing calcium. Chronic metabolic acidosis tilts the bone turnover scale toward increased resorption and decreased bone formation. It also increases osteoblast production of FGF-23 which further decreases bone formation as well as mineralization [4]. There have been accumulating data that treating metabolic acidosis may slow the progression of kidney disease [22], so CKD guidelines recommend administering base when the serum bicarbonate is less than 22 meq/L. [23] Whether treating metabolic acidosis in CKD improves bone disease has not yet been specifically studied. We treat metabolic acidosis in CKD with a reduced acid diet (lower animal protein) and oral sodium bicarbonate. A new agent, veverimer, that acts as a hydrogen and chloride binder in the gastrointestinal tract, is under investigation for treating metabolic acidosis in CKD and has shown efficacy in early studies [24], although bone effects have not been evaluated.
Osteoporosis
-
Assess bone mineral density in CKD 3–5 and ESRD with DXA
-
Assess bone turnover, using PTH and bone-specific alkaline phosphatase
-
If high bone turnover, consider these agents:
-
Bisphosphonates (dose caution, nephrotoxicity of intravenous forms)
-
Denosumab (monitoring for hypocalcemia)
-
-
If low bone turnover, consider osteoanabolic agents
It is suggested that any CKD patient with evidence of renal osteodystrophy and fracture should be considered as having osteoporosis, as their bone quality and strength are similarly impaired [1]. New to KDIGO guidelines in 2017, the use of DXA to determine BMD is now recommended in patients with CKD 3 and up, who have “evidence of CKD-MBD or risk factors for osteoporosis” [12]. Treating low BMD in CKD first involves assessing the degree of bone turnover. While a bone biopsy is the gold standard in assessing bone turnover and quality, it is an invasive and lengthy process that is rarely performed clinically [25, 26]. Measuring bone turnover markers such as PTH and BALP is a more practical way to assess bone turnover rates, though these have limitations [1, 27, 28]. Significantly high PTH and BALP levels in CKD patients correlate with high bone turnover, and significantly low levels correlate to low bone turnover states. Unfortunately, intermediate levels are difficult to interpret, and a bone biopsy would be ideal in such patients [1].
Treatment of osteoporosis in CKD can be challenging. Bisphosphonates are cleared by the kidney resulting in a prolonged blood half-life, although this is dwarfed by the bone half-life so the clinical implications are unclear [29]. Nephrotoxicity has been reported, particularly with intravenous agents. Pamidronate is associated with focal glomerular sclerosis (especially with multiple high doses), and zoledronic acid has been reported to cause acute tubular injury even at the 4 mg dose [29, 30]. However, recent data suggest that with careful attention to dosing, infusion rates, and treatment frequency in CKD, nephrotoxicity is rare [1]. Denosumab is not cleared by the kidney; however, serum calcium needs to be monitored frequently posttreatment as it can cause severe hypocalcemia in CKD [31]. While bisphosphonates should be avoided in low bone turnover disease, osteoanabolic agents are likely useful in this setting [1]. Teriparatide has been studied in small numbers of patients with ESKD and low bone turnover [32, 33]. Abaloparatide may have an advantage over teriparatide in that it is less likely to cause hypercalcemia, but this has not been tested in CKD [34].
Outcome
We educated our patient to avoid highly bioavailable dietary phosphate loads, such as processed foods with inorganic phosphate additives (e.g., canned food, dark sodas/colas, deli meat), as well as animal-derived phosphate sources including dairy. At her follow-up visit, her serum phosphate remained above normal, and she admitted to struggling with limiting her milk consumption. Sevelamer carbonate was initiated at 800 mg three times daily with meals to bind dietary phosphate in the gut and limit its absorption, with the goal of returning serum phosphate toward normal levels.
We started cholecalciferol 5000 IU daily, achieving a 25-hydroxyvitamin D level of 40 ng/mL. On follow-up, her 1,25-dihydroxyvitamin D and calcium levels remained in the low normal range; however, her PTH was still quite elevated at 288 pg/mL. Calcitriol 0.25 μg 3× weekly was started, which increased her 1,25-dihydroxyvitamin D and calcium levels to the mid-normal range, and reduced PTH to 109 pg/mL.
We added sodium bicarbonate tablets of 650 mg three times daily to our patient’s regimen, with improvement in serum HCO3- level to 23 mEq/L.
Clinical Pearls/Pitfalls
-
Most patients with chronic kidney disease (CKD) 3 through end-stage kidney disease have some degree of chronic kidney disease-mineral and bone disorder (CKD-MBD).
-
Preventing or delaying progressive metabolic and bone complications is essential to reducing the high morbidity and mortality rates associated with CKD-MBD.
-
In patients with CKD, careful monitoring of bone and mineral abnormalities can represent opportunities to reduce risk of fractures, cardiovascular events, and mortality.
References
Damasiewicz MJ, Nickolas TL. Rethinking bone disease in kidney disease. JBMR Plus. 2018;2(6):309–22.
Pimentel A, Ureña-Torres P, Zillikens MC, Bover J, Cohen-Solal M. Fractures in patients with CKD—diagnosis, treatment, and prevention: a review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017;92:1343–55.
Floege J, Drüeke TB. Mineral and bone disorder in chronic kidney disease: pioneering studies. Kidney Int. 2020;98:807–11.
Bushinsky DA. Acidosis and renal bone disease. In: Olgaard K, Salusk IB, Silver J, editors. The spectrum of mineral and bone disorders in chronic kidney disease. 2nd ed. Oxford University Press; 2010. p. 253–65.
Liu M, Li XC, Lu L, et al. Cardiovascular disease and its relationship with chronic kidney disease. Eur Rev Med Pharmacol Sci. 2014;19:2918–26.
Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71(5):438–41.
Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease a systematic review and meta-analysis. JAMA. 2011;305(11):1119–27.
Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22(1):124–36.
Shantouf R, Kovesdy CP, Kim Y, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1106–14.
Ureña-Torres PA, Floege J, Hawley CM, et al. Protocol adherence and the progression of cardiovascular calcification in the ADVANCE study. Nephrol Dial Transplant. 2013;28(1):146–52.
Nigwekar SU, Zhao S, Wenger J, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421–9.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder CKD-MBD. Kidney Int Suppl. 2017;7(1):1–59. https://kdigo.org/wp-content/uploads/2017/02/2017-KDIGO-CKD-MBD-GL-Update.pdf. Published 2017. Accessed March 20, 2021.
Cooke A. Dietary food-additive phosphate and human health outcomes. Compr Rev Food Sci Food Saf. 2014;16(5):906–1021.
Sullivan C, Sayre SS, Leon JB, et al. Effect of food additives on hyperphosphatemia among patients with end-stage renal disease: a randomized controlled trial. JAMA. 2009;301(6):629–35.
de Fornasari MLL, dos Santos Sens YA. Replacing phosphorus-containing food additives with foods without additives reduces phosphatemia in end-stage renal disease patients: a randomized clinical trial. J Ren Nutr. 2017;27(2):97–105.
Barreto FC, Barreto DV, Massy ZA, Drüeke TB. Strategies for phosphate control in patients with CKD. Kidney Int Rep. 2019;4(8):1043–56.
Cozzolino M, Ketteler M, Wagner CA. An expert update on novel therapeutic targets for hyperphosphatemia in chronic kidney disease: preclinical and clinical innovations. Expert Opin Ther Targets. 2020;24(5):477–88. https://doi.org/10.1080/14728222.2020.1743680.
Isaka Y, Hamano T, Fujii H, et al. Optimal phosphate control related to coronary artery calcification in dialysis patients. J Am Soc Nephrol. 2021;32(3):723–35.
Wang AYM, Fang F, Chan J, et al. Effect of paricalcitol on left ventricular mass and function in CKD-the OPERA trial. J Am Soc Nephrol. 2014;25:175–86.
Thadhani R, Appelbaum E, Pritchett Y, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–84.
EVOLVE Trial Investigators, Chertow GM, Block GA, Correa-Rotter R, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–94.
Navaneethan SD, Shao J, Buysse J, Bushinsky D. Effects of treatment of metabolic acidosis in CKD a systematic review and meta-analysis. Clin J Am Soc Nephrol. 2019;14(7):1011–20.
Andrassy KM. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3(1):1–116.
Adrogué HJ, Madias NE. Veverimer: an emerging potential treatment option for managing the metabolic acidosis of CKD. Am J Kidney Dis. 2020;76(6):861–7.
Evenepoel P, Cunningham J, Ferrari S, et al. European Consensus Statement on the diagnosis and management of osteoporosis in chronic kidney disease stages G4-G5D. Nephrol Dial Transplant. 2021;36:42–59.
Nickolas TL. The quest for better biomarkers of bone turnover in CKD. J Am Soc Nephrol. 2018;29:1353–5.
Sprague SM, Bellorin-Font E, Jorgetti V, et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis. 2016;67(4):559–66.
Salam S, Gallagher O, Gossiel F, Paggiosi M, Khwaja A, Eastell R. Diagnostic accuracy of biomarkers and imaging for bone turnover in renal osteodystrophy. J Am Soc Nephrol. 2018;29(5):1557–65.
Damasiewicz MJ, Nickolas TL. Bisphosphonate therapy in CKD: the current state of affairs. Curr Opin Nephrol Hypertens. 2020;29(2):221–6.
Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney Int. 2003;64(1):281–9.
Dave V, Chiang CY, Booth J, Mount PF. Hypocalcemia post denosumab in patients with chronic kidney disease stage 4-5. Am J Nephrol. 2015;41(2):129–37.
Cejka D, Kodras K, Bader T, Haas M. Treatment of hemodialysis-associated adynamic bone disease with teriparatide (PTH1-34): a pilot study. Kidney Blood Press Res. 2010;33:221–6.
Sumida K, Ubara Y, Hoshino J, et al. Once-weekly teriparatide in hemodialysis patients with hypoparathyroidism and low bone mass: a prospective study. Osteoporos Int. 2016;27(4):1441–50.
Miller P, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA. 2017;316(7):722–33.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Barta, V.S., DeVita, M.V., Rosenstock, J.L. (2021). Chronic Kidney Disease – Mineral and Bone Disorder (CKD-MBD). In: Cusano, N.E. (eds) Osteoporosis. Springer, Cham. https://doi.org/10.1007/978-3-030-83951-2_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-83951-2_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83950-5
Online ISBN: 978-3-030-83951-2
eBook Packages: MedicineMedicine (R0)