Abstract
Among all metal nanoparticles, gold nanoparticle has emerged as a better drug delivery system, with higher efficiency and less side effects due to its unique physical, chemical, optical, and electrical properties, higher dug loading, and target transportation of drug. Due to its unique fluorescent quenching, surface-enhanced Raman spectroscopy, surface plasmon resonance properties, good binding capacity, and tunable property, gold nanoparticles have been widely used in target therapy, in vivo molecule imaging, and various sensor and molecular probe manufacturing. The most commonly used approaches for the formulation of gold nanoparticles are categorized as physical, chemical, and biological approaches. Controlling gold nanoparticles’ size, shape, and morphology plays a critical role in its in vitro analysis, pharmacokinetic study, and biomedical application. In vivo, the pharmacokinetics of gold nanoparticle depends on particle size, shape, surface charge, surface modification, and route of exposure. The generally used animal models for the pharmacokinetic studies of gold nanoparticle are rat and mice. Before checking for its pharmacokinetic study, it has to be analyzed for various in vitro studies using different analytical techniques. Gold nanoparticles act as potential X-ray contrast imaging agents with potent X-ray absorption, low toxicity with potential biocompatibility, and high absorption coefficient.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The engineered nanoparticles have attracted strong interest among researchers in the last two decades due to their excellent physicochemical, optical, and electrical properties. The hybridization with organic material makes them diverse and more valid than large-sized materials. With the use in many imaging and therapeutic meanings, nanoparticles (NPs) have proven to be a promising multi-functional platform delivery. For synthesis of NPs, different organic-inorganic or mixture of organic and inorganic resources are used, but among all these, inorganic platforms have succeeded in diagnosis and simultaneous therapy due to their flexibility in alteration, high drug loading capacity, and stability. In biological system, nanoparticle’s interaction with cell and organelles varies depending on the type of the cell, targeting organelles, and routes for uptake employed, yet, surface functionalities, particle size, shape, and accumulation affect largely.
The metal nanoparticles especially gold nanoparticles (AuNPs) in recent year have drawn enormous attention and interests from diverse fields of science, due to their specific features, viz., exceptional tunable optical properties; great X-ray absorption coefficient; distinct unique electronic properties; easy and manageable synthesis; strong binding affinity to amines; disulfides, and thiols; and possible control of its physicochemical properties. The electric, magnetic, optical, and catalytic properties of metal nanostructures are influenced by their shape and size. AuNPs have abundant use in the field of biotechnology and biomedicine to deliver therapeutics because they have large surface bioconjugation with molecular probes and the variation in optical properties which are mainly concerned with localized plasmon resonance (PR).
The bulk gold is inert in nature and yellow in color, while AuNPs are having antioxidant properties and are wine red in color. The data shows that the property of AuNPs significantly depends on the particle-particle interaction and agglomeration. Depending on the shape, the size also varies from 1 nm to 5 μm. The shapes may be spherical, octahedral, decahedral, sub-octahedral, multiple twined, icosahedral multiple twined, tetrahedral, nanotriangles, nanoprisms, nanorods, hexagonal platelets, and irregular shape. Among all these shapes, nanoprisms and triangular-shaped NPs show significant optical properties as compared to the spherical-shaped NPs. Based on morphology, AuNPs are classified into two classes: isotropic AuNPs and anisotropic AuNPs. The isotropic AuNPs due to their uniform surface characteristics exhibit just one plasmon absorption band. The anisotropic AuNPs depending on variations in morphology show multiple plasmon absorption bands, and their morphology can be tuned easily to provide better scanning deep within biological tissues. Several studies have conformed not only optical but also structural, catalytic, magnetic, and electronic properties better in of anisotropic AuNPs compared to spherical AuNPs. The limitations of Raman spectroscopy in mapping any solid liquid and gasses and even cells due to Raman scattering now become possible with the use of metal nanomaterials. The phenomenon is well known as surface-enhanced Raman spectroscopy.
1.1 Characteristics and Application of Gold Nanoparticles
Surface area to volume ratio is another important characteristic where nanoparticle simply permits them to interact with other particles and possible to make diffusion faster with increasing surface area to volume ratio. The field is becoming more interesting as the treatment to affected tissue and cell targeting is possible without damaging healthy tissue and cells. The broad working range of wavelengths and high quantity factor (HQF) of AuNPs make them special for enhanced fluorescence and for therapeutic purposes. Because of small-sized particles with HQF of AuNPs, while used as targeted drug delivery respond significantly to the magnetic field that varies with time, they transfer enough toxic thermal energy to the tumor cells as hyperthermic agents. This property has enabled AuNPs in the field of radiation therapy to enhance radiation. Due to HQF of AuNPs, enhanced electromagnetic field and fluorescence is generated. The AuNPs as fluorescent NPs show good biocompatibilities for molecular imaging of many metabolites and enzymes during identification of cellular functions in cancer (Fig. 11.1). The refractive index detection nanoparticles similarly have added application in the improvement of field-sensitive optical method. AuNPs have also gained advantages over traditional iodine-based X-ray CT molecular probes in CT imaging. The use of AuNPs in molecular probe has shown enhanced absorption coefficient than iodine because gold has higher atomic number as well as electron density that drastically increases CT contrast than in iodine-based molecular probes. All the above AuNPs have one more characteristic advantage that they are non-cytotoxic. Due to such characteristic properties of gold in NPs, the role of AuNPs in biological sciences has become very important. The tunable optical properties, small size with large surface, configuration, and crystallinity, nanoparticles have proven to be unique therapeutic agents with high penetration, drug loading, and cell targeting capacity. In biomedical science, the use of AuNPs in drug therapy, cell or tissue imaging for tumor, photo-thermal therapy, and identification of pathogen in samples has become possible because of surface plasmon resonance (Fig. 11.1). The unique property of NPs in conjugation with gold to deliver has also made gene delivery possible along with its morphological characterization, protein structure elucidation, and identification of strategy for conjugation (Fig. 11.1).
AuNPs specially rod-shaped NPs have several applications in the area of in vivo imaging due to the absorption and scattering of light in the near-IR region due to its characteristics surface plasmon resonance. Due to the very small size of colloidal AuNPs , they have also expanded their potential application through chemical methods. These NPs easily penetrated to the target cells because of their characteristic small size similar to the biological molecules like proteins and DNA.
The low toxicity, good capacity to bind with a widespread choice of organic molecules, and tunable physicochemical properties make them excellent for use as therapeutic agents or drug delivery system at the target site that can provide improvised efficiency of active moiety. The use of gene gun fabricated using gold nanoparticles is very well known for providing excellent delivery of gene. It is being widely used for epidermal delivery of DNA vaccines, and this method is one of the finest approaches in medicine delivery.
AuNPs’ strong affinity for alkynes as compared to other transition metal catalysts makes them superior to homogeneous formulations. The homogeneous systems are more costly and not environmentally friendly. Compared to that, due to quick reduction of active gold complexes into inert metallic gold all through the C-H alkyne activation, they are considered the system of choice. Due to the unique optical and electronic properties of gold nanoparticles, they have become the choice in color-detecting probes in the progress of analytical techniques.
2 Synthesis Strategies
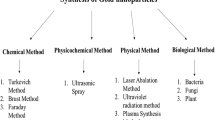
The methods for the development of AuNPs follow the few common technique and same as the development of NPs in general. A variety of methods for the synthesis of AuNPs include chemical, physical, thermal, electrothermal, and biological methods.
2.1 Chemical Methods
In chemical methods , one of the approaches is reduction by agents, viz., hydrazine, thiols, borohydrides, amino boranes, formaldehyde, polyols, hydroxylamine, oxalic and citric acids, hydrogen peroxide, sugars, carbon monoxide, hydrogen, sulfites, acetylene, and several other reducing agents.
Another method in chemical method for the synthesis of AuNPs is the prevention of aggregation of particles using stabilizing agents, viz., sulfur ligands (thiolates), trisodium citrate dihydrate, oxygen and nitrogen-based ligands (including heterocyclic compounds), dendrimers, polymers and surfactants, and phosphorus ligands.
2.2 Turkevich Method
This method for formation of AuNPs is by reduction of hydrogen tetrachloroaurate HAuCl4 in boiling water using trisodium citrate (Fig. 11.2a). Citrate here is acting as stabilizing as well as reducing agent. Several modifications have been given thereafter by different researchers to provide improved and better stability, particle size, and narrow size distribution [34].
2.3 The Brust-Schiffrin Method
This method suggests the synthesis of controlled-sized AuNPs with low dispersibility. The method served an easy way to prepare AuNPs with high thermal and air stability. The method involves transfer of AuCl4 from water to toluene phase using phase-transfer agent. Tetraoctylammonium bromide was used as the phase-transfer agent in this method along with HAuCl4 and reduced by NaBH4, in the presence of dodecanethiol (Fig. 11.2b). Immediately after addition of NaBH4 for reduction of AuCl4, the color changes from orange to deep brown. That clearly specifies the formation of AuNPs [7].
2.4 Reetz and Helbig Method
The method is chemical method to produce confined-sized AuNPs with the aid of cathode and anode charges and additional stabilizers. The method is also known as electrochemical method to produce AuNPs [30]. The method is based on the simple use of anode and cathode electrode cells to perform oxidation and reduction, respectively, as schematically depicted in Fig. 11.3.
The method since then has become an interest of researchers as the yield can be tuned using this simple equipment, with high precision, no requirement of high temperature, and low cost. Several modifications have been experimented and with successful results from time to time on this method to obtain AuNPs with the desired properties.
2.5 Growth Method
The method is simple and quick based on seed growth process. Narrow size distribution can be achieved by this method. Seeds AuNPs are prepared by conventional method and then capping done by suitable metal or capping agent. The reduction method has been used for the preparation of dendrimers/Au nanoparticles. Jana et al. [19] prepared dendrimer AuNPs by the reduction of aqueous solution of HAuCl4 and dilute solution of dendrimers by sodium borohydride (Fig. 11.2c).
2.6 Physical Methods
The physical method uses radiation to synthesize AuNPs. The γ-irradiation, UV radiation, microwave irradiation, and even direct sunlight are also used for the synthesis of gold NPs. Among them, preparation of AuNPs using γ-irradiation is a good method that gives controlled-sized NPs with high purity. This method can provide 5–40 nm size distribution of AuNPs. Different stabilizers can be used in this method, viz., natural polysaccharide alginate and bovine serum albumin protein.
2.7 Green Method
Usually, the chemical methods are providing expensive NPs. The methods require reducing reagents for stabilization of NPs but they are toxic. The disadvantage limits the applications of NPs. The green methods for the synthesis of NPs are the methods that develop cost-effective and eco-friendly nanoparticles that do not use any toxic chemicals. The green method using plants or plant extracts is receiving importance, because of non-toxicity, source availability, low cost, and biodegradable nature. Aloe vera, Medicago sativa, Cinnamomum camphora, Coriandrum sativum, Pelargonium graveolens, Terminalia catappa, Azadirachta indica, Zingiber officinale, Allium cepa, and lemongrass are the different plants that have been reported [27]. Apart from that in green method, NPs are synthesized from enzymes, animal resources, and microorganism. High-power ultrasounds and solar energy (sunlight) both can act as reducing agents for the synthesis of AuNPs.
3 In Vitro Analysis of Gold Nanoparticle
Before going for pharmacokinetic study of AuNP, it should be necessary to evaluate for particle size and measurement of gold content. Size and morphology are very important factors for therapeutic effect and efficiency of contrast, and they also affect cellular uptake. Optical properties of AuNP are highly affected by changing shape. Dynamic light scattering (DLS) is used to measure hydrodynamic diameter, and transmission electron microscopy (TEM) is used to determine their core diameter. Zeta potential is used to measure nanoparticle surface charge. Gold concentration is measured by inductively coupled plasma optical emission spectrometry.
3.1 Dynamic Light Scattering (DLS)
Gold nanoparticles consist of two phases like gold core and coating where both phases have different refractive index. Dynamic light scattering is used to determine particle size of AuNP [20]. DLS measures the light scattering from gold core, its coating and also from adsorbed water molecules. So DLS measurement is better for gold nanoparticle compared to TEM for complex shape determination. DLS also give determination of particle size in cell culture media to mimic particle size in biological system. Generally, it was seen that there is decrease in light scattering intensity with decrease in AuNP concentration. DLS measures fluctuation in intensity of light which are scattered from particle in suspension which undergoes Brownian motion with respect to time. Diffusion coefficient of particle is determined by measuring fluctuation intensity, and it ultimately gives particle size.
3.2 Zeta Potential
Surface charge on AuNP is determined by measuring its zeta potential. By measurement of zeta potential, properties of nanoparticle can be predicted in biological media. It was observed that highly positively charged nanoparticles tend to aggregate in serum, with less time of retention and circulation. So, it will be rapidly engulfed by reticuloendothelial system, but charge on AuNP is negative or near to neutral which makes it more stable in serum and avoid phagocytosis.
3.3 Transmission Electron Microscopy (TEM)
Size and shape of AuNP can be determined by TEM also. Generally, TEM use sample droplet on carbon-coated copper grid and then analyzed for particle size, but for complex type of structural analysis, dried droplet of sample is analyzed by TEM tomography in which sample is analyzed by many angles by rotating the grid. Then images are processed computationally which create three-dimensional image of complex AuNP which is entrapped in polymer matrix.
3.4 ICP-MS
Inductively coupled plasma is a novel technique for more precise dosing measurement of AuNP. Previously, ICP-OES technique was used for quantitative analysis of gold nanoparticle, but when more dilution of gold nanoparticle injected in animal, the sensitivity of ICS-OES is reduced. So, it requires ICP-mass spectrometry (ICP-MS). The analysis of the optimized ICP-MS method offers a quantification limit for Au (III) in colloid samples of 0.15 lg/L that corresponds to 4.40 9109 AuNPs/L considering spherical AuNPs 15 nm sized [3].
3.5 Determination of Gold Nanoparticle Uptake
Cellular uptake of AuNP is very important in determining biomedical application. This helps to check the ability of stealth gold nanoparticle to avoid cellular uptake and also to confirm the cellular uptake by targeted cell. Study of cellular uptake is required to get information about expected therapeutic effect; less therapeutic effect is expected with minimum AuNP uptake. The effect of size and shape of gold nanoparticle on mammalian cell uptake was studied by Chithrani et al. [10]. It was observed that rod-shaped gold nanoparticle showed less uptake than spherical shape. There is a quantitative comparison of gold nanoparticle which is surface modified by citrate and transferrin; it was found that citrate-coated gold nanoparticle showed greater cellular uptake. ICP-OES is more sensitive than CT for determining gold nanoparticle uptake.
4 In Vivo Pharmacokinetics
Before supplying any therapeutic formulation to the patient, thorough pharmacokinetic studies are required to perform. In the case of gold nanoparticles, there are several scientific literature on manufacturing rather than their pharmacokinetic study showing the reason of limited widespread application. Attention is required in pharmacokinetic studies also to explore their therapeutic purpose.
AuNPs are having large surface area so that they can be easily surface modified. Gold nanoparticle showed required sufficient physical electrical, chemical, and optical properties. So, it is widely used for various drug deliveries. The main drawback of AuNP is its safety concerning in vivo, so it requires a clear concept and understanding of gold nanoparticle pharmacokinetics and its risk assessment. Generally pharmacokinetic analysis is carried out based on physiologically based pharmacokinetic (PBPK) modeling as per literature is concerned. It involves the study of absorption, distribution, metabolism, and excretion of gold nanoparticle when given by i.v. or orally. There are various types of surface-modified gold nanoparticle studied, but the most common is PEG-coated gold nanoparticle as PEGylation is carried out to increase circulation time in blood. In one of the efforts to develop PEG-coated hollow gold nanoparticle, blood circulation was found to be 8 h in mice with a particle size of 43 um [37]. It was also found that gold nanoparticle with smaller particle size up to 20 μm has longer half-life compared to larger size up to 80 nm.
4.1 Absorption
Pulmonary Absorption
It was observed that the translocation of gold nanoparticle is highly affected by particle size when passing through air-blood barrier. In one study, it was seen that by decreasing particle size of gold particle, the translocation of particles is increased in rats. To find the relationship between particle characteristics and particle size, the study of biokinetics of inhaled NPs and distribution of nanoparticle by PBPK model found that translocation was inversely related to particle size [4]. When given in vivo, higher translocation of gold nanoparticle was found with anionic surface when compared to cationic surfaces.
Gastrointestinal Absorption
The study about in vivo gastrointestinal absorption of positively and negatively charged AuNPs from gastrointestinal tract (GIT) has shown characteristic behavior of AuNPS [31]. After instillation of radiolabelled negatively (1.4–200 nm) or positively (2.8 nm) charged AuNPs intraesophageally in rats, AuNPs were able to cross the GIT, but the absorption was unfinished even after 24 h (i.e., 17.2–74.1% endured in the GIT and internal feces), and absorption competence was very low, ranging from 0.37% for small sizes (1.4–2.8 nm) to 0.01% for large size (200 nm). Few such in vivo observation shows size- and surface charge-dependent absorption of AuNPs.
Absorption and Penetration from Skin
Several factors affect any formulation to be penetrated into the skin, including physicochemical property; dose size; surface charge; lesion on the skin; age; skin disease such as irritant dermatitis, atopic eczema, and psoriasis; receptor fluid composition; ultraviolet light exposure; surfactants; and solvents. The same factor also affects penetration and absorption of AuNPs from skin. Due to metallic material during evaluation, analysis with TEM makes it easy to trace the progression of AuNPs beneath the skin.
4.2 Distribution
The factors affecting distribution of AuNPs in biological system rely on several factors including morphology with size, charges on surface, opsonization, surface characteristics, and route of administration. Looking at the effect of morphological characteristics, irrespective of size of AuNPs, distribution occurs predominant in the liver, lymph nodes, and spleen. With reduction in the size of AuNPs, more distribution, including in the liver, spleen, blood, kidney, lymph, brain, and spinal cord, occurs. Depending on the route of administration, AuNPs are distributed in the liver spleen, kidneys, and hepato-biliary system. The administration in lungs usually retains (99%) at the site only. The i.v. administration of small-sized (18 nm) AuNPs accumulated in the liver, spleen, kidneys, and hepatobiliary system, predominantly (90–95%) in the liver, while it completely gets cleared from the blood. To prolong circulation of AuNPs in blood and decrease random uptake by RES, surface coating of NPs is a very well-known phenomenon. PEG is an inert and a compatible component that have shown success in surface coating of NPs to prolong blood circulation time when the target is the tumor tissue rather than the liver, spleen, or kidneys. One more study has shown that the biodistribution of AuNPs depends on the dose of administration [23]. The biodistribution of AuNPs in several studies shows that particle size <200 nm can pass through BBB although penetration depends on size and higher in smaller NPs. The suggested mechanism of BBB penetration is to be passive translocation but limited by the pore size of tight junction or by receptor-mediated transcytosis . The pore size of a tight junction in the BBB is about 20 nm, and thus <20 nm NPs will have easy transportation capacity through this space. Tumor tissue shows good penetration due to its characteristic composition (both capillary permeability and vascular density are high) and absence of lymphatic defense system. The high penetration rate raises issue of rapid clearance and AuNPs do not remain or accumulate in tumor tissue.
4.3 Metabolism
The surface coating with PEG is biocompatible and has greatly enhanced capacity of any NPs not only to penetrate but also to retain in every desired biological site. At the time of metabolism, the polymer or similar coating component cleaved off and then degrade. This is a general metabolic pathway for bioconjugate NPs designed as drug carriers. In the case of AuNPs coated with peptides in a wide range of mammalian cells (including adherent and non-adherent cells, mouse and human cell lines), the in vitro studies have shown that upon internalization of biological molecules attached to AuNP surface, they are degraded within the endosomal compartments through peptide cleavage by the protease cathepsin L. Yet studies are still required further on metabolism of AuNPs.
4.4 Elimination
The AuNPs can be excreted from the body via the usual route, i.e., renal and hepatobiliary clearance. Though several factors may affect clearance of AuNPs, including size and surface chemistry, elimination of AuNPs is significantly low due to opsonization with persistent and main build-up in the body’s metabolism hub liver, spleen, and mesenteric lymph node, even for smaller AuNPs.
5 Gold Nanoparticle in X-Ray Contrast Imaging
Gold nanoparticles (AuNPs) and silver nanoparticles are the regularly used in biomedical application as a nanostructure. Silver nanoparticles have minimum structural stability and maximum cellular toxicity compared to gold nanoparticle. When gold nanoparticle is compared to bulk gold, it has completely different chemical and physical properties. Due to exclusive properties of gold nanoparticle, they have various applications in biological imaging, X-ray contrast imaging and plasmonic biosensing, and contrast enhancement of X-ray computed tomography. Iodinated molecules are having low molecular with high water solubility which shows low toxicity, but blood circulation time is short and easily eliminated from the kidney. So, it requires multiple injection with risk of thyroid dysfunction. In X-ray contrast imaging, AuNP has gained maximum consideration because of many advantages like high absorption coefficient, easy to handle using synthetic process, nontoxic, surface modification for stability, and targeted drug delivery.
5.1 Why Gold Particle in X-Ray Imaging
Gold solution in colloidal range has been used for various applications. One of the beneficial physical properties of Au is its high capacity of X-ray absorption when it is subjected to X-ray imaging. Compared to other contrast agent like barium sulfate and iodine, gold shows a reasonably high X-ray attenuation coefficient specially used for clinical CT at specified energy level. Contrast imaging window of AuNP is higher compared to iodinated molecules because it has longer vascular retention time and higher molecular weight. AuNPs can be easily surface modified to increase colloidal stability and targeted delivery. One of the first AuNPs developed as an X-ray contrast agent was a 1.9 nm spherical formulation and was shown to provide strong enhancement of the major vessels. However, due to the small size of the agent, the particles were rapidly washed out from renal route, so demonstration by high attenuation in the bladder is taken after 15 min of injection as an image. Increasing the size and/or modifying the surface functionality of AuNP has been shown to improve circulation time, as found in studies of AuNP whose core size was around 10 nm. After coating with PEG, the nanoparticles had an overall size of 38 nm. These nanoparticles were not renally cleared and provided vascular contrast over a period of 12–24 h [8].
Nanoparticle-based CT contrast agents and the in vivo studies can be divided into three main categories:
-
(a)
Blood pool CT contrast agents : Blood pool contrast agents are used to increase the retention time of nanoparticle in circulation by restricting the diffusion by the vascular membrane to allow a longer imaging time period and window.
-
(b)
Passive targeting gives broad-spectrum accumulation of AuNPs within a interested site by getting maximum advantages of enhancement of permeability and retention effect. Passive targeting allows accumulation of appropriate size nanoparticle in tumor tissue compared to normal surrounding tissues.
-
(c)
Active targeting : By active targeting, delivery of contrast agent at specific site of interest can be done, and retention of contrast agent can be increased by surface functionalization with biomolecules like peptides or antibodies which reveal a specific affinity for that site of action.
5.2 Design of AuNPs as X-Ray Contrast Agents
Gold nanoparticles are specifically designed to observe the necessary functional requirements for a contrast agent in biomedical application.
The different types of functional requirements include its mode of delivery because contrast agent should be easily delivered in vivo and also able to transport at the site of action. Secondly, the contrast agent should be non-toxic; it should not give any type of adverse effect at the time of delivery and clearance. The contrasting agent can be able to retain and accumulate at targeted site or organ like blood and cancer cell (Table 11.1). Specifically, AuNP has the capacity to increase contrast enhancement of targeted organ by increasing X-ray attenuation. In designing AuNP, it is necessary to achieve functional requirement of gold nanoparticle, and its specific properties can be controlled by its structural modification. Many properties of gold nanoparticle like its X-ray attenuation coefficient, stability of colloidal Au in body fluid and during storage, retention time in blood circulation, and its biodistribution and cytotoxicity are major concern. Many structural properties size, shape, morphology, molecular functional group, mass concentration, and composition of AuNP are considered for governing the physical, chemical, and biological properties of nanoparticle [21]. AuNP when used as contrasting agent can accommodate higher payload compared to small molecules contrast agent. And it is widely used in specific biomedical applications due to their shape, size, and surface chemistry. AuNP showed long blood circulation time in terms of hours, while iodinated contrast agent media has less circulation time of few minutes. Higher attenuation properties of AuNP are due to its high density and higher atomic number, i.e., 79. Additionally, it is inert and biocompatible with other moieties for surface modification using different ligands. They have strong affinity to interact with molecules which contains sulfur which generally includes thiols, disulfides, and amino acids. And there is a formation of self-assembled monolayers formed by capping of AuNP with sulfur-containing molecules [5]. This binding is used to modify surface properties of particle, e.g., stability of AuNP is increased by surface modification of AuNP using tri-n-octylphosphine oxide (TOPO), oleylamine, and octadecy. Many key properties required for designing of AuNp are presented below.
5.2.1 Composition
Composition plays a very important role in X-ray attenuation and hence its ability to enhance contrast effect, is directly related to the X-ray attenuation, its density in bulk, atomic number, spectrum of x ray source energy and presence of x ray absorption edges.
5.2.2 Size
X-ray imaging ability of AuNP is highly affected by particle size; it finally affects biocompatibility and therapeutic application. Many scientists have focused their research on the effect of size, shape, and concentration of AuNP on cellular uptake of gold nanoparticle for the cellular contrasting application. AuNP when used in size range of 3–50 nm has greater enhancement in contrast topography imaging and also radiotherapy. It was found that among the given range of size of gold nanoparticle, specifically 13 nm particles have excellent contrast ability and significant radioactive disruption [18]. Ultimately requirement for greater attenuation is small size with higher concentration exhibit greater effect.
5.2.3 X-Ray Attenuation
Capacity of substance or tissue to absorb X-rays’ energy is dependent on its atomic number and density. Higher atomic number and density of tissue can absorb more X-rays. So, elements like barium, iodine, and gold exhibit higher atomic number, revealing a high mass attenuation coefficient (μ/ρ). Thus, gold can be excellent candidates for X-ray contrast agents specifically in soft tissues. Among all different elements used for contrast imaging, gold has an atomic number of 79 which is higher compared to iodine, i.e., 53, and barium, i.e., 56, and also a density of 19.3 g/cm3.. So it can able to absorb more X-rays at specific energy levels. Initial intensity (I0) of X-ray photon energy autonomously influences the X-ray attenuation coefficient. As the increase of incident photon energy from the X-ray source, it decreases the mass attenuation coefficient. Gold can be able to give improved contrast enhancement due to high X-ray attenuation coefficient compared with both iodine and barium. The photon attenuation coefficients of gold at 100 KeV is 5.16 and for iodine at same energy of radiation is 1.94. It means that gold has a tendency to give 2.7 times higher contrast X-ray imaging per unit mass than iodine. Various sizes and shapes of AuNP affect the scattering of visible light and its absorption of visible light.
5.2.4 Mass Concentration
The X-ray attenuation capacity of element with high atomic number is primarily directed by photoelectric absorption, which depends on variation in mass concentration. The higher is the mass concentration, the greater is its X-ray attenuation. So ultimately, it increases higher pay load at site of delivery which enhance the contrast in imaging. There is a possibility of adverse effect in vivo by giving large dose of exogenous contrast media. Therefore, there is a need to determine exact dose of contrast agent to enhance contrast effect by avoiding cytotoxicity. Relatively higher mass concentration of contrast agent is required in computational topography (CT) compared to other techniques for imaging; it is the main limitation of CT. Thus, a body organ like bone having a high background X-ray attenuation will require a higher mass concentration of gold to produce same enhancement in contrast imaging compared to organ with low-attenuating background like tumor [24].
5.2.5 Contrast Enhancement
Contrast enhancement is directly related to the mass concentration at site of application. Generally, differential contrast of 30 house field unit (HU) is necessary to detect 80 keV. Based on the background signals at the site of application, dose of AuNP is differing depending upon the need of contrast enhancement. Based on mass attenuation coefficient with the change in mass fraction of the contrast agent, the minimum detectable mass fraction can be calculated.
5.3 X-Ray Imaging Technology Using Gold Nanoparticle
5.3.1 Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI) is a non-invasive technique for imaging normally used for diagnosis of disease, molecular imaging, and cell tracing. This is due to its more ability to give high geotemporal resolution with its excellent ability to contrast soft tissue. AuNPs having a diameter of less than 2.5 nm behave like a semiconducting quasimolecules and possess magnetic properties based on their attached ligand for protection. The synthesis and characterization of new derivatives of gold nanoparticles as MRI contrast agents also have been carried out [32]. Gold nanoparticles are stabilized by dimethylaminopyridine (DMAP). Average diameter of nanoparticle was found to be 2.25 nm. The DMAP molecules were then replaced by Gd-DTPA-based chelates along with butanethiol molecules. The 38% higher relaxivity for butanethiol molecules inserted gadolinium-DTPA nanoparticle is highly attributed to the restricted tumbling of ligand molecules at the gold nanoparticle surface.
5.3.2 Computed Tomography (CT) and Nuclear Imaging
Computed tomography (CT) is a commonly used technique for imaging that uses X-rays, and it uses detector array which create cross-sectional images of the body with high geotemporal resolution [28]. This imaging technique gives idea about 3D details of organs for diagnosis of disease and their therapy. Main drawback of this imaging is sensitivity towards soft tissue. And CT contrast agents are used to improve the sensitivity of CT imaging. AuNP is extensively used to investigate the effect of contrast agent to improve imaging in CT. AuNP has a capacity to absorb X-ray due to higher electron density compared to tissue, so that it can produce direct contrast effect at its own position. The investigation shows that the effect of AuNP is threefold higher compared to iodine as contrasting agent in CT at 100 keV [16]. The advancement in CT machine minimizes the movement in objects and is able to get more enhanced imaging of coronary arteries with gold, even in obese patients.
5.3.3 Fluorescence Imaging
AuNP exhibits special optical property of surface plasmon resonance, but when size of particle is reduced to sub-nanometer range, then gold particle exists in the form of nanoclusters and possesses photoluminescence. AuNPs possess optical properties by which they are activated by light and will produce oxygen free radical. Due to these optical properties, it becomes good candidates as photosensitizers for photodynamic therapy of cancers. The synthesis and characterization of highly luminescent folate-functionalized Au22 cluster Au22-FA showed the brightness of 4.77 mM−1 cm−1 [29]. The presence of folate groups on gold particle gives rise to additional luminescence enhancement by energy transfer sensitization.
5.3.4 Photoacoustic Imaging
Photoacoustic imaging (PAI) is also a non-invasive imaging technique which observes the anatomy physiology and functional and molecular signals of diseased tissue with great resolution. Due to the absence of ionizing radiation, photoacoustic imaging is safer than nuclear imaging and fluorescence imaging. AuNPs exhibit surface plasmon resonance (SPR) effect due to its strong and tunable optical absorption; it can be possible by the presence of free charges on the surface of AuNPs which oscillate with the electromagnetic field, which gives optical absorption. Photoacoustic imaging using AuNP is utilized to study brain vasculature and its functionality in small animals. The research demonstrated that the application of PEGylated AuNPs having central core of silica is used for PA imaging given by i.v. [36]. It is analyzed in NIR region to improve contrast of the brain vasculature of a rat compared to background tissue. The images present a gradual enhancement of the optical absorption in the brain vessels by up to 63% after three sequential administrations of AuNPs. The photoacoustic images confirmed the good clarity in vasculature of brain in rat with effective enhancement in absorption in blood. It showed 81% over the intrinsic contrast after 2 h from injection.
5.3.5 X-Ray Fluorescence Imaging (XRF)
Generally, X-ray fluorescence imaging technique involves the excitation of XRF photon with the X-rays. XRF photons and scattered photons get identified and analyzed to quantify the distribution of element in tissue. This process is known as X-ray fluorescence computed tomography (XFCT), and this is one of the promising approaches for identification, quantification, and distribution across the space of organ for that element. This technique works better over X-ray imaging and fluorescence imaging. Due to high energy of XRF (30–70 keV), it is easy to penetrate biological tissues for imaging in deeper organ compared to other techniques. In one of the study, the localization of AuNP in early detection of tumor and pharmacokinetic parameters were studied. They demonstrated specific localization to sites of disease by adapting gold nanoparticles with small targeting ligands in murine spinal cord injury models using X-ray fluorescence imaging (XRF) X-ray imaging technology [14].
5.4 Toxicity of Gold Nanoparticles
When any nanomaterial is subjected to in vivo, then potential toxicity is a matter of concern when it is evaluated in vitro and in vivo for safety. There is a variation in interaction of AuNP with biological molecules because of difference in parameters like size, shape, surface charge, and coating material for surface modification. It is generally said that plain gold nanoparticle is toxic compared to surface-modified AuNP. By proper surface modification, toxicity can be reduced or even eliminated. AuNPs of less than 2 nm in size have the ability to bind irreversibly with biomolecules like DNA, so they induce more toxicity compared to particle with size more than ≥3 nm. It was found in the study that AuNPs with more than 3 nm particle size are considered to be nontoxic in the body as well as in vitro. However, depending upon the retention time of AuNP in specific organ, it will have more impact on long-term toxicity. Some laboratories have investigated the cellular toxicity of gold nanoparticles with regard to particle size, shape, and surface group. In one more study, gold nanoparticles with 2 nm diameter were analyzed which were surface modified with cationic and anionic group on surface in three types of cells [13]. It was observed that cationic is more toxic even in less concentration compared to anionic. This is due to the electrostatic interaction between cationic particle and anionic membrane. The study of different sizes and shapes of gold nanoparticle for its cellular uptake in cell line of human cervical cancer cell was done [10]. They found that average particle size around 50 nm spheres was easily taken up by cell line compared to small and larger size than 50 nm, and it was also seen that sphere-shaped particle can be easily taken up than nanorods. AuNP surface modification is required to reduce toxicity, and also its biocompatibility can be increased for biomedical application. The generally used technique for modification is PEGylation. The most commonly used surface modification is PEGylation. It was found that PEG is more likely to enhance the solubility, which reduce nonspecific binding, thus can improve the biocompatibility and circulation half-life of AuNPs by using thiolated polyethylene glycol. Au-S covalent bonding is useful for exchange of surfactant using PEG ligand [25].
References
Ahangari A, Salouti M, Saghatchi F. Gentamicin-gold nanoparticles conjugate: a contrast agent for X-ray imaging of infectious foci due to Staphylococcus aureus. IET Nanobiotechnol. 2016;10(4):190–4.
Al-Neami AQ, Al-Karam LQ, Humadi MD, Alwan MH. Applications and advantages of gold nanoparticles as X-ray contrast agent. J Biomed Eng Med Devic. 2017;2(128):2.
Allabashi R, Stach W, de la Escosura-Muñiz A, Liste-Calleja L, Merkoçi A. ICP-MS: a powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J Nanopart Res. 2009;11(8):2003–11.
Bachler G, Losert S, Umehara Y, von Goetz N, Rodriguez-Lorenzo L, Petri-Fink A, Rothen-Rutishauser B, Hungerbuehler K. Translocation of gold nanoparticles across the lung epithelial tissue barrier: combining in vitro and in silico methods to substitute in vivo experiments. Part Fibre Toxicol. 2015;12(1):1–18.
Boca SC, Astilean S. Detoxification of gold nanorods by conjugation with thiolated poly(ethylene glycol) and their assessment as SERS-active carriers of Raman tags. Nanotechnology. 2010;21(23):235601.
Boote E, Fent G, Kattumuri V, Casteel S, Katti K, Chanda N, Kannan R, Katti K, Churchill R. Gold nanoparticle contrast in a phantom and juvenile swine: models for molecular imaging of human organs using x-ray computed tomography. Acad Radiol. 2010;17(4):410–7.
Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc Chem Commun. 1994;7:801–2.
Cai QY, Kim SH, Choi KS, Kim SY, Byun SJ, Kim KW, Park SH, Juhng SK, Yoon KH. Colloidal gold nanoparticles as a blood-pool contrast agent for X-ray computed tomography in mice. Investig Radiol. 2007;42(12):797–806.
Chien CC, Wang CH, Wang CL, Li ER, Lee KH, Hwu Y, Lin CY, Chang SJ, Yang CS, Petibois C, Margaritondo G. Synchrotron microangiography studies of angiogenesis in mice with microemulsions and gold nanoparticles. Anal Bioanal Chem. 2010;397(6):2109–16.
Chithrani BD, Ghazani AA, Chan WCW. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6(4):662–8.
Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka JP, Fuster V, Fisher EA, Mulder WJ, Proksa R, Fayad ZA. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256(3):774–82.
Eck W, Nicholson AI, Zentgraf H, Semmler W, Bartling S. Anti-CD4-targeted gold nanoparticles induce specific contrast enhancement of peripheral lymph nodes in X-ray computed tomography of live mice. Nano Lett. 2010;10(7):2318–22.
Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15(4):897–900.
Grüner F, Blumendorf F, Schmutzler O, Staufer T, Bradbury M, Wiesner U, Rosentreter T, Loers G, Lutz D, Richter B, Fischer M, Schulz F, Steiner S, Warmer M, Burkhardt A, Meents A, Kupinski M, Hoeschen C. Localising functionalised gold-nanoparticles in murine spinal cords by X-ray fluorescence imaging and background-reduction through spatial filtering for human-sized objects. Sci Rep. 2018;8(1):16561.
Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Gold nanoparticles: a new X-ray contrast agent. Br J Radiol. 2006 Mar;79(939):248–53.
Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49(18):N309.
Han S, Bouchard R, Sokolov KV. Molecular photoacoustic imaging with ultra-small gold nanoparticles. Biomed Opt Express. 2019;10(7):3472–83.
Iqbal M, Usanase G, Oulmi K, Aberkane F, Bendaikha T, Fessi H, Zine N, Agusti G, Errachid E-S, Elaissari A. Preparation of gold nanoparticles and determination of their particles size via different methods. Mater Res Bull. 2016;79:97–104.
Jana NR, Gearheart L, Murphy CJ. Seeding growth for size control of 5−40 nm diameter gold nanoparticles. Langmuir. 2001;17(22):6782–6.
Jans H, Liu X, Austin L, Maes G, Huo Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal Chem. 2009;81(22):9425–32.
Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer‐based nanomedicine: from design principles to clinical applications. J Intern Med. 2014;276(6):579–617.
Khademi S, Sarkar S, Shakeri-Zadeh A, Attaran N, Kharrazi S, Ay MR, Azimian H, Ghadiri H. Targeted gold nanoparticles enable molecular CT imaging of head and neck cancer: an in vivo study. Int J Biochem Cell Biol. 2019;1(114):105554.
Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Ramanujam VS, Urayama A, Vergara L, Kogan MJ, Soto C. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393(4):649–55.
Lee N, Choi SH, Hyeon T. Nano-sized CT contrast agents. Adv Mater. 2013;25(19):2641–60.
Liu H, Doane TL, Cheng Y, Lu F, Srinivasan S, Zhu J-J, Burda C. Control of surface ligand density on PEGylated gold nanoparticles for optimized cancer cell uptake. Part Part Syst Charact. 2015;32(2):197–204.
Manohar N, Reynoso FJ, Diagaradjane P, Krishnan S, Cho SH. Quantitative imaging of gold nanoparticle distribution in a tumor-bearing mouse using benchtop x-ray fluorescence computed tomography. Sci Rep. 2016;6:22079.
Parida UK, Bindhani BK, Nayak P. Green synthesis and characterization of gold nanoparticles using onion (Allium cepa) extract. World J Nano Sci Eng. 2011;1(04):93.
Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R. Targeted gold nanoparticles enable molecular CT imaging of cancer. Nano Lett. 2008;8(12):4593–6.
Pyo K, Ly NH, Yoon SY, Shen Y, Choi SY, Lee SY, Joo S-W, Lee D. Highly luminescent folate-functionalized Au22 nanoclusters for bioimaging. Adv Healthc Mater. 2017;6(16):1700203.
Reetz MT, Helbig W. Size-selective synthesis of nanostructured transition metal clusters. J Am Chem Soc. 1994;116(16):7401–2.
Schleh C, Semmler-Behnke M, Lipka J, Wenk A, Hirn S, Schäffler M, Schmid G, Simon U, Kreyling WG. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology. 2012;6(1):36–46.
Shahid M. Water soluble gold nanoparticles based high relaxivity MRI contrast agents. Mater Res Express. 2020;6(12):1250h1251.
Tian A, Yang C, Zhu B, Wang W, Liu K, Jiang Y, Qiao Y, Fu H, Li Z. Polyethylene-glycol-coated gold nanoparticles improve cardiac function after myocardial infarction in mice. Can J Physiol Pharmacol. 2018;96(12):1318–27.
Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75.
Yang J, Wang T, Zhao L, Rajasekhar VK, Joshi S, Andreou C, Pal S, Hsu H-T, Zhang H, Cohen IJ, Huang R, Hendrickson RC, Miele MM, Pei W, Brendel MB, Healey JH, Chiosis G, Kircher MF. Gold/alpha-lactalbumin nanoprobes for the imaging and treatment of breast cancer. Nat Biomed Eng. 2020;4(7):686–703.
Yang X, Skrabalak SE, Li Z-Y, Xia Y, Wang LV. Photoacoustic tomography of a rat cerebral cortex in vivo with au nanocages as an optical contrast agent. Nano Lett. 2007;7(12):3798–802.
You J, Zhou J, Zhou M, Liu Y, Robertson JD, Liang D, Van Pelt C, Li C. Pharmacokinetics, clearance, and biosafety of polyethylene glycol-coated hollow gold nanospheres. Part Fibre Toxicol. 2014;11(1):26.
Zhang S, Li L, Chen J, Chen Z, Zhang W, Lu H. Quantitative imaging of Gd nanoparticles in mice using benchtop cone-beam X-ray fluorescence computed tomography system. Int J Mol Sci. 2019;20(9):2315.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Patel, N., Chaudhary, S., Patel, J.K. (2022). Metallic Gold Nanoparticles: In Vivo Pharmacokinetics and X-Ray Contrast Imaging Studies. In: Patel, J.K., Pathak, Y.V. (eds) Pharmacokinetics and Pharmacodynamics of Nanoparticulate Drug Delivery Systems . Springer, Cham. https://doi.org/10.1007/978-3-030-83395-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-83395-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83394-7
Online ISBN: 978-3-030-83395-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)