Abstract
Inorganic nanoparticles impose huge opportunities to clinicians in field of theranostics. Currently FDA has approved hundreds of inorganic nanoparticles for their clinical investigation in different fields of nanomedicine, cosmetics, nutraceuticals, diagnostics, and so on. Potential properties like tunable size, tailorability of surface, targeting at cellular level, and penetrability entail them as system of choice. Despite the tremendous applications, clinical translation of these nanosystems is still a big challenge. The major debatable concern is their non-acceptable toxicity due to accumulation in RES organs. Interestingly in the last few decades, researchers are quite focused on nanoengineering to optimize physiochemical properties and their impact on PK of these nanosystems. The current chapter provides an overview of pharmacokinetic considerations, effect of various factors, physiologically based pharmacokinetic models, and fate and toxicity of inorganic nanoparticles. The chapter highlights over diverse factors optimized while designing these nanoparticulate systems to achieve desired pharmacokinetics.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Recent advances in formulation and development have led to the emergence of nanoparticles as a novel drug delivery system. Nanoparticles constitute the involvement of matter at the atomic levels. When we can see these particles with our naked eye, the bulk properties dominate which are the aggregate of the quantum forces. However, as we go on decreasing the size and reach a particular level like nanoscale, these bulk properties no longer dominate, and, instead, what we see is the control of individual quantum effects like melting point, fluorescence, magnetic behavior, etc. Taking an example, we see that even though large-scale gold particles appear yellow, at nanoscale, the electrons of the atoms are confined, making them interact differently with light. This is why we see a red- to purple-colored solution of gold nanoparticles rather than a yellow one. Conventionally, nanoparticles have a range of 1–100 nm.
1.1 Classification of Nanoparticles
Nanoparticles can be classified into two major broad categories based on composition as given in Fig. 10.1 [1]:
-
(a)
Organic
-
(b)
Inorganic
They can be classified into different types according to their size, morphology, and physical and chemical properties. Some of them are carbon-based nanoparticles (carbon nanotubes (CNTs) and fullerenes, graphene, nanodiamond), ceramic nanoparticles (hydroxyapatite (HA), zirconia (ZrO2), silica (SiO2), titanium oxide (TiO2), and alumina (Al2O3)), metal nanoparticles, (silver, gold, and iron), semiconductor nanoparticles (ZnS, CdS, CdS GaN, GaP), polymeric nanoparticles (biodegradable and nonbiodegradable), and, lipid-based nanoparticles (liposomes, solid lipid nanoparticles, and nanostructured lipid carriers).
Organic nanoparticles are biodegradable and nontoxic in nature and include liposomes, dendrimers, micelles, etc. They are also called polymeric nanoparticles.
Inorganic nanoparticles can be classified into metallic nanoparticles, ceramic nanoparticles, carbon nanotubes, and quantum dots. The consideration of carbon-based nanoparticles as inorganic is justified as carbon nanotubes and graphene are entirely made of carbon atoms and not bound to any hydrogen atom to be considered as organic compounds [2].
Metallic nanoparticles have gained popularity in recent times as therapeutics and diagnostic agents in a myriad of sectors ranging from medical, biomedical, engineering, to agriculture. The adaptability of these metallic nanoparticles to be modified with various functional groups opens the pathway to utilize them for targeted drug delivery and as contrast agents for diagnostic purposes. At nanometer level, there is a vast change in the physical and chemical behavior of these metallic elements owing to their large size to volume ratio and quantum effects.
Metallic nanoparticles can be further divided into magnetic that includes iron oxide nanoparticles and nonmagnetic that includes gold, silver, and zinc nanoparticles. We’ll now look into each of these in brief.
All of the metals mentioned above have a partially filled d-orbital that is responsible for their higher oxidation states and, thus, higher reactivity [3, 4]. The transition elements of the periodic table presently used as nanoparticles include gold [gold nanoparticles (GNPs)], silver [silver nanoparticles (SNPs)], iron [iron oxide nanoparticles (IONPs)], and zinc (zinc oxide nanoparticles).
Gold Nanoparticles (GNPs)
The most widely discussed and utilized include the gold nanoparticles. They have applications in cancer therapy as well as diagnostics. Such agents are called theranostics (by combining the words therapeutic and diagnostic) [5, 6]. GNPs have a size range of 1–100 nm. Those in the range of 20–50 nm have been shown to be most promising for cellular uptake and treatment of tumors [7, 8]. The synthesis of GNPs can be done by chemical means as well as through newer sustainable method known as “green synthesis.” One of the chemical method is the citrate reduction method utilizing trisodium citrate to reduce gold chloride [9, 10]. Green synthesis, on the other hand, is a sustainable and environment-friendly technique of enzymatic reduction of gold particles using plants and microorganisms. When using plants, the plant extract is added to the auric solution resulting in formation of nanoparticles. The concentration of auric solution depends on the species and plant part used [11].
Silver Nanoparticles (SNPs)
Silver is a noble element of the transition metal series known for its healing ability. Silver as nanoparticles is useful in the destruction of cancer cells. It does so by acting as a ROS that kills the mitochondria of the tumor cells [12, 13]. Various methods are used for the synthesis of SNPs. The most widely used is the chemical reduction method.
Iron Oxide Nanoparticles (IONPs)
Iron (II) oxide is paramagnetic in nature, whereas iron (II, III) oxide/Fe3O4 is super paramagnetic in nature. These properties enable them to be successfully used as contrast agents in magnetic resonance imaging (MRI) and magnetic separation technologies like DNA sequencing [8].
Quantum dots are small-sized semiconductor crystals having the ability to showcase intensive fluorescence. They are routinely used as agents in drug delivery and imaging techniques [14].
Ceramic nanoparticles have a porous structure owing to which they can be easily fabricated to suit various needs of sizes and shapes. Few examples of ceramic nanoparticles include calcium phosphate, silica, calcium carbonate, etc. Since these have strong covalent bonds between them, they are relatively stable than metal nanoparticles and, thus, show low thermal and electrical conductivity and low corrosiveness [1].
Carbon nanotubes , as the name suggests, consist of small hollow tubes made entirely of hexagonally arranged carbon atoms. The hollow interior allows it to act as a drug carrier. They are mainly produced through laser ablation method [9, 15].
1.2 Formulation and Development
The synthesis of nanoparticles can be achieved using a wide variety of methods. Traditionally, there are mainly two approaches that are utilized in the synthesis of nanoparticles (Fig. 10.2):
-
1.
Top-down approach
-
2.
Bottom-up approach
Top-Down Approach
It involves the breakdown of larger particles into smaller ones (miniaturization) using processes like milling and attrition. Methods that utilize these processes include laser ablation, mechanical milling, and arc discharge. It requires a lot of energy and effort which is one of the disadvantages of the technique [16, 17].
Bottom-Up Approach
This technique involves the formation of nanoparticles starting from smallest of particles like atoms or molecules and builds on further to form nuclei via process of nucleation. The nucleation can take place in any medium. The size of the particles can be carefully controlled. Small-size particles can be obtained when almost every particle enters into the nuclei stage simultaneously. It is a simple, fast, and low-cost technique. Methods like sol-gel, microemulsion, and co-precipitation are included in this [16] [17].
One method cannot be applied to all the elements as the choice of method depends on the physical and chemical properties of the element.
Green Synthesis
Abundant research is being carried on newer methods like “green synthesis” to provide an environment-friendly and sustainable approach to nanoparticle synthesis. Traditional techniques led to the excessive use of noxious and hazardous chemicals and even produced harmful by-products at times.
Green synthesis is a comparatively safer alternative to synthesize nanoparticles that uses plants, bacteria, fungi, and algae to synthesize nanoparticles (Fig. 10.2). The phytochemicals present in plant extracts, namely, alkaloids, flavonoids, etc., obtained from plant waste act as reducing agents, thereby reducing the costs of reducing agents used in chemical synthesis methods [18]. Several studies have been carried out showcasing the success of this technique. To take an example, Latha and Gowri, in 2014, synthesized the ferric (II, III) oxide nanoparticles using Carica papaya leaf extract [4]. The synthesis of metallic nanoparticles inside the bacteria, fungi, algae, and yeast takes place through several mechanisms that reduce them to their elemental form. These mechanisms majorly include enzymatic reactions and other reactions like precipitations, complexation, and efflux transporters [19]. One recent study even found that biogenic silver nanoparticles were similarly effective but less toxic in eukaryotes compared to chemically synthesized silver nanoparticles [20]. The use of green technology in mass scale production of nanoparticles seems promising in coming years given the large amount of research carried on it.
1.3 Evaluation of Inorganic Nanoparticles
Inorganic nanoparticles can be evaluated by several methods as mentioned in Fig. 10.3 based on the particle and drug characteristics. These parameters ultimately affect the performance of these nanoparticles inside our body, i.e., in vivo [8]. Evaluation of nanoparticles in vivo depends on the disease and the tissue or organ it is affecting.
1.4 Applications of Inorganic Nanoparticle
Owing to their low toxicity, targeted drug delivery, and, significant role in imaging, inorganic nanoparticles have found use in numerous sectors. Few applications of inorganic nanoparticles are summarized in Table 10.1. Some inorganic nanoparticle formulations which are presently marketed are given in Table 10.2.
There are numerous nanoparticulate formulations that have received approval from various regulatory agencies for its use in therapeutics and imaging. Some marketed formulations and formulations of inorganic nanoparticles under clinical trials along with its use are given in Table 10.2 [39].
2 Pharmacokinetic Considerations for Inorganic Nanoparticles
In the fascinating era of nanotechnology, inorganic nanoparticles (both ceramic and metallic) offer various exciting approaches to diagnostics and therapeutics to the scientific community. Among various classes of nanosystems, inorganic nanoparticles exhibit distinctive features and biological effect over conventional organic counterparts. For targeted drug delivery, inorganic NPDDSs should reach to target tissues in optimum concentrations and show appropriate release kinetics, illustrating their efficient therapeutic potency. Also, it is very important that inorganic NPDDS should be nontoxic and easily eliminated from the body. Despite of several demerits, inorganic nanoparticles are system of choice for theranostics due to ease of their formulation-development, easier detection in biological fluids and accurate monitoring of their kinetics. In contrast, though organic nanoparticles exhibit simple formulation and biodegradability, it is quite difficult to identify them in biological system. Also, their characterization and traceability are problematic. Some exemplary inorganic nanomaterials include quantum dots, fullerenes, carbon nanotubes, graphene, inorganic nanotubes and metallic nanoparticles (NPs). Among all inorganic nanomaterials, highly exploited ones are gold, silver iron oxide, titanium dioxide (TiO2), and zinc oxide nanoparticles which are also approved by the FDA as theranostics [39]. Gold nanoparticles have been extensively studied in treatment of tumors or rheumatoid arthritis, also adopted as carriers for drug and gene delivery; however silver nanoparticles are employed as antibacterial agents for coating catheters, orthopedic implants, wound dressings, and scaffolds. Major utilization of iron oxide (FeO) NPs is for diagnostics as bioimaging and biosensing, whereas titanium oxide (TiO2) and zinc oxide (ZnO) NPs are highly consumed in cosmetics and toiletries [40].
It is the bitter truth that very few inorganic nanomaterials have been made from bench to bedside translation due to their potential toxicities to major organs. Thus, it is a major challenge to exercise more efforts investigating the toxicity mechanisms to ensure their clinical safety.
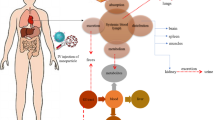
In this regard, a systematic understanding of fundamental pharmacokinetics is crucial for proper risk assessment. The pharmacokinetics of these long circulating inorganic NPDDS is strongly dependent on various physiochemical attributes including shape, size, charge, and coating material. Furthermore, biopharmaceutical factors like protein binding, route of administration, dose and dosage regimen, patient-related factors, and genetic factors also contribute in their cellular uptake and biodistribution. Literature reveals that oral, dermal, or inhalational absorption of these inorganic nanoparticles is comparatively low, i.e., up to 5% or less. However effective cellular uptake can be improved by tuning of their size, charge, and surface modulation. Nanosize of these inorganic nanoparticles facilitates their distribution throughout the body along with accumulation in various organs like the liver, lung, spleen, kidney, and lymph node. It has been reported that inorganic metallic nanoparticles are capable of crossing the blood-brain barrier as well as placental barrier coated with biocompatible polymers. Due to rapid clearance from the blood stream via the mononuclear phagocyte system (MPS) and reticuloendothelial system (RES), inorganic nanoparticles bypass first pass metabolism and undergo longer circulation in the body (could remain in the body up to 6 months or more) and thus high accumulation in vital organs leading to their chronic toxicity over time. Also chances of malignancy may arise in people more exposed to nanomaterials, especially those working on nanomaterial production. Therefore, huge data on in vivo and in vitro kinetics along with computational predictive toxicity is requisite for understanding the implications on long-term usage/exposure (Fig. 10.4).
Discussing on biotransformation of nanomaterials, it is well established that organic nanoparticles inclusive synthetic polymers or biopolymers exhibit higher biocompatibility as compared to inorganic one. The prime reason behind this is their hydrodynamic behavior in vivo and tailorability of their surface with specific targeting ligands. On the other hand, due to the poor water solubility, stability issues, immunogenicity, and potential toxicity, inorganic nanomaterials are desperately required to modify their surface with a biocompatible organic coating. Thus regardless of compositions, either metallic or ceramic, mandatory requirements for designing of all types of inorganic NPDDS are targeting efficiency, half-life of drug/formulation in blood, and complete elimination from the body without any toxicity.
Various biopharmaceutical approaches are already known to estimate the pharmacokinetics of nanomaterials. Both experimental and mathematical models are utilized to determine pharmacokinetic parameters, i.e., volume of distribution (Vd), clearance (Cl), half-life (T1/2), mean residence time (MRT), maximum or peak concentration (Cmax), area under the time concentration curve (AUC), and bioavailability (F). Pharmacokinetic data provide insights to clinicians to optimize dose and design a dose regimen within the therapeutic window of drug. Consequently modulations are done to achieve desired pharmacokinetic profile of drug in NPDDS. However in case of inorganic NPDDS, estimation of accurate pharmacokinetic parameters and establishment of safety profile of nanomaterials are tough jobs due to unavailability of pharmacokinetic models for assessment. Due to these limitations, inorganic NPDDS are still in infancy stage in the market. Besides traditional approaches of compartment modeling and non-compartmental analysis, physiological models have emerged as newer approach for pharmacokinetic assessment of inorganic NPDDS. These physiological models are purely based on mathematical equations providing a more realistic picture of ADME process in vivo. However, such models have not been explored a lot (limited number of models available); therefore efforts are needed to fill the gap between theoretical assumptions and accurate data gap.
Thus it is quite clear that in order to attain desired pharmacokinetic parameters of inorganic NPDDS, it is necessary to modulate these systems by virtue of modifications in their physiochemical attributes, i.e., hydrodynamic diameter (HD), shape, composition, and surface characteristics [41].
2.1 Impact of Particle Size and Shape
Nanosize of inorganic material has tremendously solved the problem of their cellular uptake across biological membranes despite of their poor solubility and lipophilicity. Due to high surface area and low volume, these particles can easily penetrate various biomembranes for ease of targeting. Generally, particles in size range of 10–15 nm are easily permeated through biomembranes with limited accumulation in nontarget tissues. However, selection of appropriate particle size is crucial for its biotransformation which decides the fate of these nanosystems upon pharmacokinetic translation. It has been reported that nanoparticles having diameter around 5 nm or less prefer renal clearance, whereas particles with little larger hydrodynamic diameter undergo biliary excretion [42,43,44]. Nanoparticles exceeding 100 nm in size are reported to have extremely different pharmacokinetics and biodistribution properties, and their chances of accumulation in vital organs like the spleen, lungs, liver, and kidney arise. Therefore, optimized fabrication methods are adopted to control the mean particle diameter of these nanosystems along with adjustment in various physiochemical attributes of composition. Also, the tuning of size is done as per the requirements of target organ. For instance, in case of brain targeted drug delivery systems, to cross the tight endothelium junctions, the particles should have diameter < 20 nm. In general, the ideal size range of these inorganic NPDDS is 10–150 nm for longer circulation time and desired accumulation at target site [45]. Importantly, the smaller size of these nanosystems simulates with plasma proteins that leads to their longer circulation time and thus enhanced permeation and retention in solid tumors as well as atherosclerosis. Furthermore, it has been reported that both accumulation and penetration are required in tumors; therefore to achieve this, a wide range of nanoparticles, varying in size, are targeted to strike the tumor. Generally larger NPs show their accumulation within the tumor cells toward periphery; however the smaller ones penetrate quite deeper inside the tumor cells.
Inorganic nanoparticles could be of various shapes like spherical, cubic, rod-like, or worm-like which directly influence their cellular uptake. Different absorption patterns have been observed in terms of shape of these NPDDS. In an example of gold nanoparticles, it was observed that among different shapes, i.e., cubic, spherical, and rod-like nanoparticles, the net quantity of nanoparticles absorbed (weight) was higher for spherical particles, whereas total count of nanoparticles was comparatively more for rod-shaped ones [46].
2.2 Impact of Surface Modulation
In the current scenario of precision medicine, huge research is going on for tailoring of inorganic nanoparticles by surface modulation to obtain intelligent nanoparticles with improved targeting efficiency and low toxicity. Functionalization of nanoparticles significantly affects the physical, chemical, and biological nature of the entire formulation and thereby impacts on its pharmacokinetics too. Surface modulation can be done in terms of attachment of ligands, ions, antibodies, coating with proteins, genetic material, polymers, etc., and currently a number of FDA-approved metallic nanoparticles are available as nanomedicines for treatment of tumors, osteoporosis, and rheumatoid arthritis [47].
The surface charge of these inorganic NPDDS interacts with surface proteins of biomembranes and thereby affects absorption and distribution across the membrane. It is well established that positively charged NPDDS show better absorption in comparison to negative ones due to electrostatic interactions. However NPs surface coated with neutral non-ionic polymer imparts stability and hence reduced immunogenicity. Furthermore, surface attributes of these inorganic NPDDS have a crucial role when come in contact with biological fluids. Generally protein-coated inorganic NPDDS show different affinities toward different proteins of biological fluid which ultimately decides their fate of biodistribution.
2.3 Impact of Route of Administration
Administration route of NPDDS impacts biodistribution imperatively due to differential interaction with enzymes, hormones, proteins, ions at the site of administration, as well as site of absorption, and thus a significant change in PK parameters is obtained. Huge research has already been done to elucidate the fate of inorganic nanoparticles in vivo upon administering them through different routes. For instance, it has been reported that when PLGA NPs are administered through intravenous route, they get accumulated in the liver and spleen, but upon subcutaneous or intranodal administration, they are found to get accumulated in the lymph nodes specifically. Therefore one can easily target lymph nodes by administering drug via subcutaneous route, which could be beneficial in certain immunotherapeutic applications [48].
It is well established that altering route of administration can avoid several demerits related to both dosage form and undesirable first pass metabolism. For instance, in case of lung targeting, NPDDS are preferably administered as inhalation rather by parenteral route for maximal accumulation in the lungs before reaching to systemic circulation. In one more example, comparison between different modes of pulmonary administration was studied in mouse. For this, intratracheal instillation, intratracheal spraying, and intranasal instillation were selected to deliver PLGA nanoparticles. The report on accumulation and bioavailability of drug revealed heterogeneous biodistribution and accumulation of NPDDS in lung tissues [49].
Huang X el al. [7] studied over impact of different parenteral routes on biodistribution of carbon dots. They reported that upon administering them via intravenous, intramuscular, and subcutaneous individually, different values of PK parameters were obtained. They drew a comparison in clearance and urinary accumulation/excretion rate of NPDDS and concluded that the particles exhibit following order for above mentioned PK parameters: intravenous > intramuscular > subcutaneous injections. However accumulation and selective uptake of carbon dots in tumor cells was found to be comparatively higher by subcutaneous and intravenous route in comparison to intramuscular injection. Hence such examples potentiate route-dependent changes in ADME of theranostics and thus their bioavailability. Although tremendous preclinical studies are continuously being done to identify the best routes for a specific target, biodistribution of these inorganic NPDDS is still a big challenge.
2.4 Impact of Composition of Nanomaterial
Most of the inorganic theranostic NPs are composed of either metallic (transition metals) or ceramic compounds having specific configuration and geometry (linear or branched). They exhibit larger size, greater hydrophobicity, and poor biodegradability as compared to organic NPDDS. Such physiochemical features of inorganic NPDDS impact their in vivo performance and affect absorption, biodistribution, elimination, as well as targeting ability. Also due to nonbiodegradability, inorganic NPs remain in the body for a relatively long period of time compared to small molecules, and thus concerns have been raised for their potential toxicities. Due to their long circulation time, abrupt changes in PK parameters have been observed (increased t1/2, reduced clearance, increased volume of distribution, lesser excretion rate, increased mean residence time).
2.5 Impact of the Dose
The relationship between dose and pharmacokinetic process depends upon the order of kinetics followed by inorganic nanoparticles. It could be linear (dose dependent), nonlinear (dose independent), or mixed order, decided by physiological and anatomical attributes of the body during ADME process [50]. In a study by Reeves, L et al. [51], gold nanoparticles (size range of 12–15 nm) were administered in consecutive three doses of 40, 200, and 400 μg/kg to mice via intraperitoneal route up to 8 days. The biodistribution of nanoparticles was monitored at regular intervals. The initial results did not indicate any major difference in blood plasma concentration of drug at 24 hrs. However after progression of time and examination of vital organs like the liver, spleen, lungs, kidneys, and brain, significant accumulation of gold nanoparticles was diagnosed. Also the reports revealed that the accumulation of nanoparticles was linearly related to dose administered. Thus the study confirmed about efficient and non-saturable cellular uptake of gold nanoparticles in a dose-dependent manner. Also Kim Ys et al. [52] confirmed dose-dependent kinetics of PEG-coated AuNPs (12–13 nm size) injected intravenously in mice. They compared two doses, 0.85 mg/kg and 4.26 mg/kg of nanoparticles, and reported a comparative increase in their cellular uptake in a dose-dependent manner in various organs like the liver, spleen, and kidneys. Therefore selection of dose for inorganic NPDDS is very important in terms of their accumulation toxicology.
3 Pharmacokinetic Models for Inorganic Nanoparticles
Physiologically based pharmacokinetic models (PBPK) are gaining importance day by day to provide very realistic description of ADME of various nanosystems using mathematical equations. These models are highly versatile and well adopted to include different doses, routes, and species to predict target tissue dosimetry and thus quite helpful to clinicians. However, only limited PBPK models have been developed for NPDDS yet. The major reason behind unavailability of these models is sophistication in nanoparticle synthesis process which limits the application of one PBPK to another as the NPs prepared by one technique are entirely different from another in their PK aspects. Therefore one PBPK model for a specific nanosystem cannot be a generalized model for another and requires new algorithms for modeling. Furthermore developments of PBPK models require thorough in vivo studies on mechanisms of cellular uptake, biodistribution in tissues, and elimination pathways. PBPK models are generally classified into two classes: blood flow rate-limited and permeation rate-limited models. Blood flow rate-limited models are based on the assumption that there always exists equilibrium between blood and tissue compartments and transfer of nanoparticles from one compartment to another is a function of blood flow rate. However in permeation rate-limited models, it is assumed that rate of diffusion of nanoparticles from one compartment to another is dependent on tissue permeability of concerned compartments. While designing a particular PBPK model, all physiochemical attributes of nanosystems are well evaluated. For instance, size and shape of nanosystems decides their fate of accumulation, biodistribution, and elimination (hepatic or renal). Therefore such important factors are taken into account in PBPK models by inclusion of certain algorithms. In addition to this, physiological conditions (e.g., endothelium pore size in different tissues) are also integrated in PBPK models. Importantly, feasibility of manipulations in PBPK models is there so that additional approaches, like active targeting of nanoparticles, can be integrated by inclusion of selective transport mechanism and kinetics which is a virtue of affinity of the nanoformulation toward its target cells or tissues. Furthermore, other important factors including shape, surface modulation, charge, functional group, etc. can also be considered by clinicians in PBPK models. Despite of interaction of drugs with lymphatic systems, generally they are excluded in PBPK models due to their lower fluid movement rate as well as lesser absorption of drugs in comparison to blood. However, in some exceptional cases, they are incorporated in PBPK models where nanosystems are directly interacting with the lymphatic system. For instance, a nanosystem surface coated with antigen/antibody or some immunogenic ligands will definitely be impacted by lymphatic system for its deposition; in such cases inclusion of lymphatic system becomes mandatory to estimate accurate PK parameters.
Elimination kinetics is evaluated in all PBPK models by simulating with major excreting organs, i.e., liver, kidneys, lungs, and gut. However for nanosystems, elimination is little more complex due to involvement of other organs/tissues in clearance besides the former ones. Among the various identified pathways of elimination for nanosystem, the most important ones are mononuclear phagocyte system (MPS) and reticuloendothelial system (RES). Generally PBPK models developed for animal studies are utilized as surrogate models for nanomedicines in humans which is a matter of concern since physiological parameters are not the same among different species. The most applicable PBPK animal models developed to simulate humans are based on species such as rodents, dogs, and monkeys. A sample PBPK model is illustrated in Fig. 10.5.
Bachler et al. [53] developed a PBPK model for comparative analysis of ionic silver and nanoparticles of silver on the basis of data generated in their toxicological studies. They evaluated various routes of administration for both formulations (i.v., dermal, oral, and inhalation) and compared their plasma/tissue concentration of drug as well as urinary excretion data. Furthermore the model was adopted to predict the absorption and distribution kinetics of both ionic silver and silver nanoparticles.
Various PBPK models have been designed to study the impact on pharmacodynamics of nanomedicines in treatment of several diseases like cancer, diabetes, autoimmune disorders, etc. [54, 55].
4 Possible Degradation Mechanisms of Inorganic Nanoparticles
Determination of degradation mechanism of NPDDS is quite complex and variable since it depends on several parameters. Once administered, inorganic NPDDS are exposed to several physiochemical changes that may lead to their agglomeration and yielding microscopic particle, or they may end up to unstable systems. Furthermore such unstable systems may undergo corrosion and get dissolved or may suffer structural damage. Such nanoparticles may act as reservoir for release of toxic ions produced by corrosive attacks. Chances of undesirable surface modulation are also there which may impact their targeting efficiency and bioactivity. Generally, in such cases, the nanoparticle surface adsorbs the macromolecules (proteins/reactive molecules/ions) from surrounding media which may hamper its absorption and alter entire biodistribution pattern. Conclusively the pharmacokinetic fate of nanomaterials is just a virtue of physiological environment, it is exposed, and its estimation is still a challenge [56].
4.1 Agglomeration
Generally, NPDDS show some unusual behavior in vivo; they have strong tendency to agglomerate in biological fluid due to surface charge, coating, hyphenation, and ligands. It has been observed that the biological fluid or medium in which they are dispersed contributes in agglomeration. Various factors like ionic strength, pH as well as presence of biomolecules like proteins, enzymes and other cellular components generally interact with these nanosystems, and a dramatic change in their state of aggregation, dispersibility, and charge may occur. This leads to destabilization of NPDDS in biological fluid, and thus their agglomeration may take place which further entails several other changes in the properties of these nanosystems, i.e., specific surface area, concentration, mobility, and so forth. Such changes occur at nanoscale leading to loss of their stability and huge changes in PK parameters leading to their undesirable uptake in nontarget tissue, accumulation, and toxicity. In case of nanopowders, it is very common phenomena.
4.2 Adsorption of Macromolecules
Concept of surface chemistry confirms that inorganic hydrophobic powders have electrostatic charges on surface and have a strong tendency to adsorb various biomolecules when come in contact of biological fluid in vivo or dispersed in physiological medium. It is a serious matter of concern for inorganic NPDDS because they have also been reported to adsorb blood serum proteins to become more hydrophilic and attain stability which further leads to changes in mechanism of their attachment and interaction with biomolecules and ultimately results in PK changes of nanosystems.
4.3 Corrosive Degradation of Nanoparticles
Metallic nanoparticles are prone to release ions upon corrosion and thus induce toxicity to organ systems exposed. It has been noted that inorganic NPs upon exposure to certain pH of media, ions, or absorbing biomolecules undergo catalytic reactions and follow a disintegration pathway. A little exposure to oxygen, chlorine, free radicals, and enzymes in surrounding media is favorable for such thermodynamic processes.
Most common examples of corrosion of these metallic nanomaterials include corrosion of gold and silver nanoparticles due to presence of cysteine and chlorine in biological fluids. Several other metals like iron, zinc, and cadmium are reported to be disintegrated in vivo. Consequently, the corrosion leads to both morphological and chemical changes in these nanosystems and thus alters their PK parameters which ultimately results into toxicity due to metal cations.
5 Pharmacokinetics of Long Circulating Inorganic Nanoparticles
The main aim behind designing NPDDS is to achieve targeting and deliver the minimal dose in efficient manner to avoid toxicity. Also, PK parameters are programmed in designing of these nanosystems. Therefore, formulation strategies are based on thorough understanding of interaction between nanoformulations and biological systems. Additionally, the entire journey of NPDDS (ADME process) from entry to exit from the body is evaluated for both therapeutic and toxic potentials.
5.1 Absorption and Cellular Uptake [57, 58]
Inorganic NPPDS are well adapted to be delivered through different routes of administration (e.g., inhalation, oral, skin, and non-IV parenteral), but their absorption into systemic circulation and final cellular uptake is quite variable. Also absorption of these nanosystems is further dependent on several other factors like surface charge, size, shape, pH, PKa, lipophilicity, etc. It has been noted that absorption of cationic nanosystems is more efficient in comparison to anions and neutral ions from the GI tract. However in case of inhalational nanosystems, size ranging 100 nm or less, 80% of inhaled particles get trapped into respiratory tract, and their absorption takes place via olfactory nerve pathway. Furthermore, upon dermal/subcutaneous administration (50–100 nm), penetration of anionic nanosystems is found to be more efficient. Dermal penetration is also dependent upon shape and lipophilicity of NPDDS. Although injectables cause extravasations, they exhibit maximum bioavailability, reaching directly to systemic circulation.
Two basic mechanisms involved in absorption and uptake efficiency of NPDDS are opsonization and phagocytosis. Generally opsonization is responsible for phagocytic clearance in vascular system and involves attachment of opsonin proteins to foreign bodies in blood circulation, stimulation of immune systems, followed by phagocytosis. Importantly inorganic NPDDS which are nonbiodegradable bypass this opsonization rather sequestered in organs where macronuclear phagocytic system or RES systems are there, i.e., the spleen, liver, and kidney, leading to hepatotoxicity or nephrotoxicity.
5.2 Biodistribution in Tissues [55, 57]
Biodistribution of nanoformulation is an important PK parameter that decides its circulation time in the body, duration of action, and toxicity. Upon reaching to systemic circulation, NPDDS partition between blood and adjacent tissue compartments which is basically dependent upon physicochemical properties of these nanosystems (shape, size, partition coefficient, lipophilicity, permeability) and physiological conditions of the body (organ size, body fat index, etc.). One more phenomenon, “protein-corona formation ” of nanoformulations, interferes with their biodistribution [59, 60]. It involves binding of plasma proteins on the surface of nanoformulations which further influences its cellular uptake and degradation pathways of these nanosystems. The most common plasma proteins involved in protein corona formation are serum albumin, immunoglobulins, fibrinogen, and apolipoproteins, which binds to surface of nanosystems by various mechanisms like Van der Waals interactions, hydrogen bonding, and salvation. This ultimately results in drastic changes in surface characteristics and alteration in shape and size and most importantly hampers the stability of these nanosystems. Thus protein corona formation decides the PK fate of biodistribution of these NPDDS.
It has been reported that nanoformulations’ distribution mechanism is strongly dependent on their size and concentration in particular body compartment. Therefore their transport across different compartments or their accumulation in certain cells is dependent upon their diffusion rate, permeation rate, and pore size of biomembranes. Interestingly, in case of prodrugs, NPDSS and high molecular weight metallic NPs, their targeting efficiency and biodistribution are entirely dependent on their penetrating ability and retention potential. For instance, such systems are designed to accumulate in tumor cells or inflammatory sites, having high vasculature, so that large pores of tumor cells (above 100 nm) may facilitate their easier transport and thus accumulation in tumor cells. Therefore various factors are evaluated while designing PBPK models for tumor cells.
Furthermore, several physiological barriers (BBB, blood-placental barrier, blood-testis barrier etc.) play crucial roles in rate and extent of biodistribution of these nanosystems across various compartments. In literature, contribution of anatomical features of these barriers in biodistribution is well established. However a thorough understanding of transport mechanism across these barriers entails bioengineering of nanosystems for better penetration and higher permeation to deliver the drug.
5.3 Elimination of Inorganic Nanoparticles [55, 57]
Despite of usual routes of elimination (hepatic and renal), nanosystems can be excreted via various other routes depending upon their route of administration. For instance, in case of inhalation, dermal, and parenteral routes of administration, elimination may take place from the lungs, skin, as well as circulatory system too. Degradation and elimination of inorganic nanoparticles is still a big challenge to clinicians. In general, the elimination of these systems involves several enzymatic and chemical degradation pathways which ultimately result in renal or biliary clearance of nanomedicines. However various PBPK models confirm that these nanoformulations do not follow usual elimination and clearance pathway; besides this, they accumulate in tissues/organs, get released slowly, and remain in blood circulation for longer durations.
Elimination kinetics of these nanosystems involves their clearance from blood, renal clearance, and biliary excretion. In blood, opsonization and phagocytosis (kind of immunogenic response produced for foreign bodies) is a major process of clearance. However bioengineered NPDDS can avoid this mechanism by surface modulation, so that they can reach to their target cells before being cleared from circulatory systems.
In the liver, macrophages produced by Kupffer cells contribute in biotransformation of nanomedicines. The concentration of these macrophages is extremely high in the liver and acts as pool for phagocytic clearance of NPs. This phagocytic clearance also involves opsonization, by virtue of binding of NPDDS with immunoglobulins. Furthermore it has been reported that extremely fine inorganic NPs engulfed by Kupffer cell produce free radicals, tumor necrosis factors, interleukins, and other inflammatory mediators which later on lead to hepatotoxicity. Spleen also contributes in phagocytic elimination of NPDDS.
Among various routes of excretion, renal route is extremely important for clearance of nanomedicines; however due to several limitations posed in various parts of nephron (from glomerulus till distal end), their clearance becomes compromised in the kidney. The fine pore size of fenestrations in glomerular endothelium (50–100 nm) and thin basement membrane of blood capillaries restrict passage of nanoparticles and filter them out which leads to accumulation in the kidney and hence nephrotoxicity.
6 Pharmacokinetic Fate of Inorganic Nanoparticles
Inorganic nanoparticles including gold, silver, iron, cadmium, zinc, silica, phosphate, and other inorganic compounds are utilized for several biomedical applications, but their pharmacokinetic fate in vivo is quite variable in terms of their blood circulation time, retention in different organs, and mechanism of biodegradation and excretion.
It has been reported that NPs made of Ag, ZnO, CdSe, and FeO2 undergo catalytic reaction and corrode slowly, which ultimately releases metal ions in biosystem. It is assumed that gold and silver are quite inert and thus stable against corrosion; however attachment of certain ligands like thiols, available in amino acids (glutathione) inside cells, pulls out gold ions at surface of NP, ultimately leading to its dissolution.
Balfourier et al. [61] studied over pharmacokinetic fate of gold nanoparticles in fibroblast cells up to 6 months. Their study reveals that inertness of gold is just a myth and it also undergoes degradation likewise other inorganic nanoparticles. Utilizing electron microscopy imaging and transcriptomics, they deduced that biotransformation of gold nanoparticles is a two-step mechanism. The former step involves generation of ROS in lysosomes (entrapping fine gold NPs) catalyzed by NADPH oxidase and nuclear factor erythroid. However, the latter step is recrystallization of gold nanoparticles, which results into self-assembled nanoforms/nanoleaves of biomineralized crystals of gold. The recrystallization is favored by biological chelating agent naming metallothioneins present in cytosol. Thus they confirmed over ionic degradation of gold NPs.
Bailly et al. [62] prepared gold NPs utilizing a new laser-based technique with merits of least contaminants and better surface chemistry. They conjugated the gold NPs with dextran polymer to enhance its biocompatibility and studied the PK of NPs. In results they reported that coated gold nanoparticles prepared are highly biocompatible and nonimmunogenic (confirmed by IL-6 levels) and do not accumulate in the liver (confirmed by ALAT and ASAT activities) and kidney (creatinine clearance). Furthermore, the NPs are capable of rapid clearance from systemic circulation. However, their limited accumulation in the liver and spleen was confirmed.
Pandey .S et al. [63] prepared and evaluated calcium phosphate nanoparticles encapsulating methotrexate (MTX-CAP-NP). During pharmacokinetic evaluation, they reported selective targeting of NPDDS specifically in arthritic bones and higher concentration in blood besides entering in other organs. They disclosed that due to simulation of calcium phosphate with biominerals, they accumulate and degrade in tissues like bones and teeth like other biominerals.
Superparamagnetic iron oxide (FeOx) NPs are extremely reactive, and corrosion is their prime mechanism of disintegration, which can further be confirmed by elemental analysis using coupled plasma mass spectrometry (ICP-MS). However elemental analysis results do not portray the accurate results due to presence of higher concentration of endogenous iron inside the body. Besides this, sophisticated techniques like electron paramagnetic resonance (EPR) can be employed to differentiate between iron nanoparticles and endogenous iron, and temperature-dependent susceptibility method can be adopted. Such evaluations provide insights to biotransformation iron NPs in short and long terms. Lysosomal degradation is their prime mechanism of elimination.
Interestingly, macrophages, endothelial cells, or mesenchymal stem cells which are meant for engulfment of these nanosystems exhibit a special mechanism in biotransformation of inorganic nanoparticles. In conditions of starvation, these cells have been reported to expel FeOx NPs, Au NPs, QDs, or CNTs, in extracellular medium in form of tiny microdroplets which are further transported across the body. Thus despite of local degradation, such NPs traverse to different organ systems and may stay there for long. Hence degradation of inorganic NPS is nonlinear and unpredictable, because it is difficult to decide that up to what extent the particles injected will dissolve or will remain unmodified.
Moreover advanced technologies like TEM are quite helpful in determination of NP interaction with cell cultures within biological environment in situ. Furthermore TEM also facilitates the study over various transformations of NPs upon generation of reactive oxygen species by electron.
Conclusively we can say that inorganic NP cores can be degraded in vivo and the following strategies can be adopted to foster the degradation and elimination of inorganic theranostics:
-
1.
Manipulation in elemental composition of inorganic NPDDS without compromising distinctive chemical, physical, and pharmaceutical properties.
-
2.
Tuning of size can be done to harness faster elimination.
-
3.
Hyphenation and surface modulation with biodegradable and biocompatible polymers (PEG), to reduce their uptake from RES system and faster clearance.
7 Toxicity Concerns of Long Circulating Inorganic Nanoparticles [64,65,66]
Huge literature is there over toxicokinetics of nanomaterials. Well, toxicity concerns associated with nanomedicines are definitely so loud that various regulations over the globe have given guidelines for preparations and utilization of these nanosystems in different fields like agrochemicals, nutraceuticals, food, cosmetics, drugs, and so on. Irrespective of novel techniques of bioengineering applied, the core characteristics of nanosystems make them exclusive and entail toxicity concerns. The key features of these inorganic nanoparticles responsible for their toxicity are extremely fine size, penetrability at cellular level, entrapment and accumulation in RES organs, nonbiodegradability, and non-excretable through renal route. Furthermore they can cross various physiological barriers easily like BBB and placental barriers and thus may produce other toxic effects.
Due to their small size, they can penetrate both cellular and nuclear membranes and may interact with organelles and genetic material. Also due to hydrophobicity and high surface to volume ratio, they have higher adsorptive capacity, easily bind to biomolecules, and disturb the homeostasis at cellular level.
Carnovale et al. [67] synthesized and studied over uptake degradation and toxicity of gold NPs in human prostate cancer cells. They developed eight sets of NPs to study their fate in vivo, i.e., role of protein-corona formation in presence of cellular proteins and impact of shape and size in accumulation, circulation, biodistribution, and excretion. In conclusion the study disclosed that rod- and cube-shaped NPs stabilized in presence of cetyltrimethylammonium bromide (CTAB) are minimally toxic and well tolerated as compared to spherical- and prism-shaped nanoparticles. Also study confirms that serum proteins do not participate in any toxicity though they have a crucial role in cellular uptake mechanism.
In a recent review by Sani et al. [68], they contradicted the toxicity of gold nanoparticles. Instead they highlighted that toxicity of gold NPs is not a big concern. Furthermore they also confirmed that gold itself is inert and non-catalytic, so chances of free radical or any electron transfer reactions is minimal. A limited toxicity is reported in case of gold NPs, which can further be avoided through several means like optimization of shape and size and functionalization of surface.
Yaqoob et al. [69] reviewed over PK aspects of gold, silver, and palladium nanoparticles. In studies they also confirmed that cytotoxicity of gold NPs is size dependent and can be minimized by alterations in shape and size. However in case of silver nanoparticles, they disclosed that toxicity of silver NPs is independent of their shape and size and coating thickness. The main reason of toxicity is their catalytic behavior in aqueous medium and interaction with functional groups of amino acids like amines and thiols. Silver NPs release silver cations, which are extremely toxic to cellular components and reported to damage genetic material like DNA at nuclear level. For Pd-NPs, they revealed that like silver NPs, their cytotoxicity is due to their catalytic behavior. However sizes of NPs do have some impact on toxicity too.
Mao BH et al. [70] explored AgNPs and studied their effect at the different levels. Various doses of silver NPs were evaluated in Drosophila melanogaster for its lethal effect as well as cellular and molecular defects. Lethal dose delayed development cycle in embryonic stage and ultimately led to the death of developing as well as young animals; however sublethal dose impacted on tolerance to oxidative stress and shortened the life span. They also reported active participation of silver nanoparticles in surge of ROS-induced immunogenic cycle leading to apoptosis, DNA damage, and autophagy.
In a study by Sambale F et al. [71], toxicity of silver nanoparticles was evaluated on various human and animal cell lines, i.e., human fibroblast cell line (NIH-3 T3), human lung adenocarcinoma epithelial cell line (A-549), human hepatocellular carcinoma cell line (HEP-G2-cells), and rat adrenal pheochromocytoma cell line. In the study, they evaluated the effect of different concentrations of silver nanoparticles on the viability of the cells by MTT assay and compared it with silver ions too. Also the pharmacokinetic degradation was estimated by photometric assays. Furthermore, the data obtained was utilized in preparation of dose-response curves, determination of inhibitory concentration (IC50 value), total lethal concentration, and adverse effect concentration for individual cell lines. They also employed electric-cell-substrate-impedance-sensing (ECIS) approach to visualize and identify cell behavior (mechanism of cell death) in real time. Conclusively, they reported that mechanism of cell death is dependent on concentration of silver nanoparticles or ions. Cell death may be either due to apoptosis mediated via Caspase 3/7 activity (nanoparticles) or necrosis (silver ions) at lethal dose.
Some of the possible mechanisms of toxicity has been discussed in the following section and depicted in Fig. 10.6.
7.1 Reactive Oxygen Species and Free Radicals
Various charges on surface of inorganic NPs are responsible for electrostatic interaction of these molecules with surrounding biomolecules in vivo. These interactions are basically electron transfer reactions which result into generation of reactive oxygen species (ROS). The formed ROS anticipate other catalytic chain reactions in biological medium and thus disturb the entire homeostasis at cellular level. Additionally, an oxidative stress is generated which further results in formation of free radicals. The oxidative stress leads to77 recruitment of inflammatory mediators. Moreover, free radicals generated aggravate the toxicity to next level by causing peroxidation of lipidic biomolecules, destabilization of proteins, and damage to genetic material at nuclear level. This entire cascade leads to cellular nanotoxicity in vivo.
7.2 Disruption of the Cytoskeleton Structure
As discussed earlier, inorganic nanoparticles are hydrophobic and posses surface charges; chances of their interaction with cytoskeleton are quite high. It is well known that cytoskeletal structure is very important in terms of receptor modulation and signaling pathway; therefore any changes of disruption may lead to anomalies in cellular functions. In case of diagnostics, it has been observed that certain dyes, administered as NPs, are cytosolic in nature and diffuse inside the cells by disruption of cytoskeleton. Such cytolytic interactions have also been observed in case of blood cells, for example, hemolysis and thrombosis have been reported when these nanosystems are dispersed in blood or plasma in vitro.
7.3 Genotoxicity and Alteration of Signaling Pathway
In the previous section, penetrability of inorganic NPDDS has already been discussed. Consequently entrance of these nanosystems inside cells and nucleus cannot be denied. Additionally their capability to bind amino acids/proteins/DNA/RNA impacts the intracellular signaling pathways and thus disturbs the homeostasis of cells. This further leads to alteration in cellular mechanisms of protein synthesis, DNA transcription, RNA translation, and other biochemical processes of cell cycle. Ultimately such cellular changes lead to faulty protein/genetic material synthesis and results into genotoxicity.
7.4 Inflammation Mediated Nanotoxicity
As discussed in the previous sections , electrostatic interaction of inorganic nanoparticles induces oxidative stress inside the cells. As per pathophysiology of cellular mechanisms, it is proven fact that oxidative stress, free radicals, and ROS inside the cells are the triggers to induce inflammatory cycle. The initial step involves recruitment of inflammatory mediators (TNF α,-interleukins, β-cells, cytokines, chemokines, etc.) at the site signal induction. Later on a cascade of inflammatory cycle proceeds till cell death or phagocytosis.
For instance, most of the carbon nanoparticles including graphene and carbon nanotubes induce inflammation-mediated toxicity in various cells like keratinocytes, lung’s alveoli, and epithelial cells of bronchus etc.
8 Conclusion
Upon thorough investigation it can be deduced that pharmacokinetics of long circulating inorganic nanoparticles is an outcome of their physiochemical properties and their interaction with surrounding biosystem. Although PK tracking and tracing are difficult, sophisticated technologies of imaging and development of PBPK models have solved these problems up to a great extent. However exact prediction of fate of these nanosystems prepared by different synthetic methods is not possible. The biodistribution and degradation mechanisms are unclear and unusual and require multiple labeling strategies to identify and integrate each individual component in model. However, literature proves that all inorganic (metallic and ceramic) NPDDS undergo degradation mechanism to get dissolved or disintegrated in vivo. Thus, their circulation and retention time may vary, but ultimately they lose their identity inside biosystems.
Abbreviations
- ADME:
-
Absorption-distribution-metabolism-elimination
- NPDDS:
-
Nanoparticulate drug delivery systems
- NPs:
-
Nanoparticles
- PBPK:
-
Physiologically based pharmacokinetic models
- PK:
-
Pharmacokinetics
- RES:
-
Reticuloendothelial system
References
Thomas S, Harshita BSP, Mishra P, Talegaonkar S. Ceramic nanoparticles: fabrication methods and applications in drug delivery. Curr Pharm Des. 2015;21(42):6165–88.
Inorganic compound | chemical compound | Britannica [Internet]. [cited 2021 Aug 23]. Available from: https://www.britannica.com/science/inorganic-compound
Sridharan K. The electromagnetic spectrum. In: Spectral methods in transition metal complexes. Elsevier; 2016.
Drummer S, Madzimbamuto T, Chowdhury M. Green synthesis of transition-metal nanoparticles and their oxides: a review. Materials. 2021;14(11):2700.
Warner S. Diagnostics + therapy = theranostics: strategy requires teamwork, partnering, and tricky regulatory maneuvering. Sci. 2004;18(16):38–9.
Sharma H, Mishra PK, Talegaonkar S, Vaidya B. Metal nanoparticles: a theranostic nanotool against cancer. Drug Discov Today. 2015;20(9):1143–51.
Elsayed I, Huang X, Elsayed M. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–35.
Pandey P, Dahiya M. A brief review on inorganic nanoparticles. 2016.
Li W, Cao Z, Liu R, Liu L, Li H, Li X, et al. AuNPs as an important inorganic nanoparticle applied in drug carrier systems. Artif Cells Nanomed Biotechnol. 2019;47(1):4222–33.
Zabielska-Koczywąs K, Wojtalewicz A, Użarowska E, Klejman A, Wojtkowska A, Dolka I, et al. Distribution of glutathione-stabilized gold nanoparticles in feline fibrosarcomas and their role as a drug delivery system for doxorubicin—preclinical studies in a murine model. Int J Mol Sci. 2018;19(4):1021.
Ahmed S, Annu IS, Yudha SS. Biosynthesis of gold nanoparticles: a green approach. J Photochem Photobiol B Biol. 2016;161:141–53.
Subbaiya R, Saravanan M, Priya AR, Shankar KR, Selvam M, Ovais M, et al. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol. 2017;11(8):965–72.
Wei Q-Y, Xu Y-M, Lau ATY. Recent progress of nanocarrier-based therapy for solid malignancies. Cancers. 2020;12(10):2783.
Matea C, Mocan T, Tabaran F, Pop T, Mosteanu O, Puia C, et al. Quantum dots in imaging, drug delivery and sensor applications. Int J Nanomedicine. 2017;12:5421–31.
Sajid MI, Jamshaid U, Jamshaid T, Zafar N, Fessi H, Elaissari A. Carbon nanotubes from synthesis to in vivo biomedical applications. Int J Pharm. 2016;501(1–2):278–99.
Iqbal P, Preece JA, Mendes PM. Nanotechnology: the “top-down” and “bottom-up” approaches. In: Supramolecular chemistry. Wiley: Chichester, UK; 2012.
Slepička P, Slepičková Kasálková N, Siegel J, Kolská Z, Švorčík V. Methods of gold and silver nanoparticles preparation. Materials. 2019;13(1):1.
Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME, et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6(1):35–44.
Ghosh S, Ahmad R, Banerjee K, AlAjmi MF, Rahman S. Mechanistic aspects of microbe-mediated nanoparticle synthesis. Front Microbiol. 2021:12.
Spagnoletti FN, Kronberg F, Spedalieri C, Munarriz E, Giacometti R. Protein corona on biogenic silver nanoparticles provides higher stability and protects cells from toxicity in comparison to chemical nanoparticles. J Environ Manag. 2021;297:113434.
Esther Lydia D, Khusro A, Immanuel P, Esmail GA, Al-Dhabi NA, Arasu MV. Photo-activated synthesis and characterization of gold nanoparticles from Punica granatum L. seed oil: an assessment on antioxidant and anticancer properties for functional yoghurt nutraceuticals. J Photochem Photobiol B Biol. 2020;206:111868.
Pannico M, Calarco A, Peluso G, Musto P. Functionalized gold nanoparticles as biosensors for monitoring cellular uptake and localization in normal and tumor prostatic cells. Biosensors. 2018;8(4):87.
Lotha R, Sundaramoorthy NS, Shamprasad BR, Nagarajan S, Sivasubramanian A. Plant nutraceuticals (Quercetrin and afzelin) capped silver nanoparticles exert potent antibiofilm effect against food borne pathogen salmonella enterica serovar Typhi and curtail planktonic growth in zebrafish infection model. Microb Pathog. 2018;120:109–18.
Cholula-Díaz JL, Lomelí-Marroquín D, Pramanick B, Nieto-Argüello A, Cantú-Castillo LA, Hwang H. Synthesis of colloidal silver nanoparticle clusters and their application in ascorbic acid detection by SERS. Colloids Surf B: Biointerfaces. 2018;163:329–35.
Kim D, Kim J, Park Y, il, Lee N, Hyeon T. Recent development of inorganic nanoparticles for biomedical imaging. ACS Central Sci. 2018;4(3):324–36.
Yu JH, Kwon S-H, Petrášek Z, Park OK, Jun SW, Shin K, et al. High-resolution three-photon biomedical imaging using doped ZnS nanocrystals. Nat Mater. 2013;12(4):359–66.
Swain PS, Rao SBN, Rajendran D, Dominic G, Selvaraju S. Nano zinc, an alternative to conventional zinc as animal feed supplement: a review. Animal Nutr. 2016;2(3):134–41.
Jiang J, Pi J, Cai J. The advancing of zinc oxide nanoparticles for biomedical applications. Bioinorg Chem Appl. 2018;2018:1062562.
Alirezaie Alavijeh A, Barati M, Barati M, Abbasi DH. The potential of magnetic nanoparticles for diagnosis and treatment of cancer based on body magnetic field and organ-on-the-chip. Adv Pharm Bullet. 2019;9(3):360–73.
McClements DJ, Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci Food. 2017;1(1):6.
Yan Z-Y, Yao C-X, Wan D-Y, Wang L-L, Du Q-Q, Li Z-Q, Wu S-M. A sensitive and simple method for detecting Cu 2+ in plasma using fluorescent Bacillus amyloliquefaciens containing intracellularly biosynthesized CdSe quantum dots. Enzyme and microbial technology [Internet]. 2018 [cited 2021 Aug 21];119:37–44. Available from: https://pubmed.ncbi.nlm.nih.gov/30243385/
Meng H-M, Zhao D, Li N, Chang J. A graphene quantum dot-based multifunctional two-photon nanoprobe for the detection and imaging of intracellular glutathione and enhanced photodynamic therapy. Anal [Internet]. 2018 [cited 2021 Aug 21];143(20):4967–73. Available from: https://pubmed.ncbi.nlm.nih.gov/30225468/
Fan H, Yu X, Wang K, Yin Y, Tang Y, Tang Y, et al. Graphene quantum dots (GQDs)-based nanomaterials for improving photodynamic therapy in cancer treatment. Eur J Med Chem. 2019;182:111620.
Chen F, Hableel G, Zhao ER, Jokerst J, v. Multifunctional nanomedicine with silica: role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J Colloid Interface Sci. 2018;521:261–79.
Levingstone TJ, Herbaj S, Dunne NJ. Calcium phosphate nanoparticles for therapeutic applications in bone regeneration. Nano. 2019;9(11):1570.
Ahmed W, Elhissi A, Dhanak V, Subramani K. Carbon nanotubes. In: Emerging nanotechnologies in dentistry. Elsevier; 2018.
Simon J, Flahaut E, Golzio M. Overview of carbon nanotubes for biomedical applications. Materials. 2019;12(4):624.
Nile SH, Baskar V, Selvaraj D, Nile A, Xiao J, Kai G. Nanotechnologies in food science: applications, recent trends, and future perspectives. Nano Micro Lett. 2020;12(1):45.
Huang H, Feng W, Chen Y, Shi J. Inorganic nanoparticles in clinical trials and translations. Nano Today. 2020;35:100972.
Lin Z, Monteiro-Riviere NA, Riviere JE. Pharmacokinetics of metallic nanoparticles. WIREs Nanomed Nanobiotechnol. 2015;7(2):189–217.
Hoshyar N, Gray S, Han H, Bao G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine. 2016;11(6):673–92.
Wang Z, Malik AB. Nanoparticles squeezing across the blood–endothelial barrier via caveolae. Ther Deliv. 2013;4(2):131–3.
de Matteis V. Exposure to inorganic nanoparticles: routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics. 2017;5(4)
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–24.
Huang X, Zhang F, Zhu L, Choi KY, Guo N, Guo J, et al. Effect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots. ACS Nano. 2013;7(7):5684–93.
Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7(5):3926–38.
Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin F-H, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res. 2019;23(1):20.
Dölen Y, Valente M, Tagit O, Jäger E, van Dinther EAW, van Riessen NK, et al. Nanovaccine administration route is critical to obtain pertinent iNKt cell help for robust anti-tumor T and B cell responses. OncoImmunology. 2020;9(1):1738813.
Wu L, Rodríguez-Rodríguez C, Cun D, Yang M, Saatchi K, Häfeli UO. Quantitative comparison of three widely-used pulmonary administration methods in vivo with radiolabeled inhalable nanoparticles. Eur J Pharm Biopharm. 2020;152:108–15.
Kang H, Mintri S, Menon AV, Lee HY, Choi HS, Kim J. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale. 2015;7(45):18848–62.
Lasagna-Reeves C, Gonzalez-Romero D, Barria MA, Olmedo I, Clos A, Sadagopa Ramanujam VM, et al. Bioaccumulation and toxicity of gold nanoparticles after repeated administration in mice. Biochem Biophys Res Commun. 2010;393(4):649–55.
Kim YS, Kim JS, Cho HS, Rha DS, Kim JM, Park JD, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008;20(6):575–83.
von Goetz BG, Hungerbühler K. A physiologically based pharmacokinetic model for ionic silver and silver nanoparticles. Int J Nanomed. 2013;8:3365–82.
Moss DM, Siccardi M. Optimizing nanomedicine pharmacokinetics using physiologically based pharmacokinetics modelling. Br J Pharmacol. 2014;171(17):3963–79.
Yang G, Phua SZF, Bindra AK, Zhao Y. Degradability and clearance of inorganic nanoparticles for biomedical applications. Adv Mater. 2019;31(10):e1805730.
Casals E, Casals G, Puntes V, Rosenholm JM. Biodistribution, excretion, and toxicity of inorganic nanoparticles. In: Theranostic bionanomaterials; 2019. p. 3–26.
Hamidi M, Azadi A, Rafiei P, Ashrafi H. A pharmacokinetic overview of nanotechnology-based drug delivery systems: an ADME-oriented approach. Crit Rev Ther Drug Carrier Syst. 2013;30(5):435–67.
Choi S-J, Lee JK, Jeong J, Choy J-H. Toxicity evaluation of inorganic nanoparticles: considerations and challenges. Mol Cell Toxicol. 2013;9(3):205–10.
Saptarshi SR, Duschl A, Lopata AL. Interaction of nanoparticles with proteins: relation to bio-reactivity of the nanoparticle. J Nanobiotechnol. 2013;11(1):26.
Tenzer S, Docter D, Kuharev J, Musyanovych A, Fetz V, Hecht R, et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat Nanotechnol. 2013;8(10):772–81.
Balfourier A, Luciani N, Wang G, Lelong G, Ersen O, Khelfa A, et al. Unexpected intracellular biodegradation and recrystallization of gold nanoparticles. Proc Natl Acad Sci. 2020;117(1):103–13.
Bailly A-L, Correard F, Popov A, Tselikov G, Chaspoul F, Appay R, et al. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci Rep. 2019;9(1):12890.
Pandey S, Mahtab A, Kumar V, Jalees Ahmad F, Kamra Verma A, Talegaonkar S. Design and development of bioinspired calcium phosphate nanoparticles of MTX: pharmacodynamic and pharmacokinetic evaluation. Drug Dev Ind Pharm. 2019;45(7):1181–92.
Soenen SJ, Parak WJ, Rejman J, Manshian B. (intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications. Chem Rev. 2015;115(5):2109–35.
de Matteis V, Rojas M, Cascione M, Mazzotta S, di Sansebastiano G, pietro, Rinaldi R. Physico-chemical properties of inorganic NPs influence the absorption rate of aquatic mosses reducing cytotoxicity on intestinal epithelial barrier model. Molecules. 2021;26(10)
Ahmad MZ, Abdel-Wahab BA, Alam A, Zafar S, Ahmad J, Ahmad FJ, et al. Toxicity of inorganic nanoparticles used in targeted drug delivery and other biomedical application: an updated account on concern of biomedical nanotoxicology. J Nanosci Nanotechnol. 2016;16(8):7873–97.
Carnovale C, Bryant G, Shukla R, Bansal V. Identifying trends in gold nanoparticle toxicity and uptake: size, shape, capping ligand, and biological Corona. ACS Omega. 2019;4(1):242–56.
Sani A, Cao C, Cui D. Toxicity of gold nanoparticles (AuNPs): a review. Biochem Biophys Rep. 2021;26:100991.
Yaqoob SB, Adnan R, Rameez Khan RM, Rashid M. Gold, silver, and palladium nanoparticles: a chemical tool for biomedical applications. Front Chem. 2020;3:8.
Mao B-H, Chen Z-Y, Wang Y-J, Yan S-J. Silver nanoparticles have lethal and sublethal adverse effects on development and longevity by inducing ROS-mediated stress responses. Sci Rep. 2018;8(1):2445.
Sambale F, Wagner S, Stahl F, Khaydarov RR, Scheper T, Bahnemann D. Investigations of the toxic effect of silver nanoparticles on mammalian cell lines. J Nanomater. 2015;2015:1–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gautam, N., Kulkarni, A., Dutta, D., Talegaonkar, S. (2022). Pharmacokinetics of Long Circulating Inorganic Nanoparticulate Drug Delivery Systems. In: Patel, J.K., Pathak, Y.V. (eds) Pharmacokinetics and Pharmacodynamics of Nanoparticulate Drug Delivery Systems . Springer, Cham. https://doi.org/10.1007/978-3-030-83395-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-030-83395-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83394-7
Online ISBN: 978-3-030-83395-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)