Abstract
The incidence of thyroid cancer in the United States is on the rise with an appreciably high disease recurrence rate of 20–30%. Anaplastic thyroid cancer (ATC), although rare in occurrence, is an aggressive form of cancer with limited treatment options and bleak cure rates. This chapter uses discussions of in vitro models that are representative of papillary, anaplastic, and follicular thyroid cancer to evaluate the crosstalk between specific cells of the tumor microenvironment (TME), which serves as a highly heterogeneous realm of signaling cascades and metabolism that are associated with tumorigenesis. The cellular constituents of the TME carry out varying characteristic immunomodulatory functions that are discussed throughout this chapter. The aforementioned cell types include cancer-associated fibroblasts (CAFs), endothelial cells (ECs), and cancer stem cells (CSCs), as well as specific immune cells, including natural killer (NK) cells, dendritic cells (DCs), mast cells, T regulatory (Treg) cells, CD8+ T cells, and tumor-associated macrophages (TAMs). TAM-mediated inflammation is associated with a poor prognosis of thyroid cancer, and the molecular basis of the cellular crosstalk between macrophages and thyroid cancer cells with respect to inducing a metastatic phenotype is not yet known. The dynamic nature of the physiological transition to pathological metastatic phenotypes when establishing the TME encompasses a wide range of characteristics that are further explored within this chapter, including the roles of somatic mutations and epigenetic alterations that drive the genetic heterogeneity of cancer cells, allowing for selective advantages that aid in their proliferation. Induction of these proliferating cells is typically accomplished through inflammatory induction, whereby chronic inflammation sets up a constant physiological state of inflammatory cell recruitment. The secretions of these inflammatory cells can alter the genetic makeup of proliferating cells, which can in turn, promote tumor growth.
This chapter also presents an in-depth analysis of molecular interactions within the TME, including secretory cytokines and exosomes. Since the exosomal cargo of a cell is a reflection and fingerprint of the originating parental cells, the profiling of exosomal miRNA derived from thyroid cancer cells and macrophages in the TME may serve as an important step in biomarker discovery. Identification of a distinct set of tumor suppressive miRNAs downregulated in ATC-secreted exosomes indicates their role in the regulation of tumor suppressive genes that may increase the metastatic propensity of ATC. Additionally, the high expression of pro-inflammatory cytokines in studies looking at thyroid cancer and activated macrophage conditioned media suggests the existence of an inflammatory TME in thyroid cancer. New findings are suggestive of the presence of a metastatic niche in ATC tissues that is influenced by thyroid tumor microenvironment secretome-induced epithelial to mesenchymal transition (EMT), mediated by a reciprocal interaction between the pro-inflammatory M1 macrophages and the thyroid cancer cells. Thus, targeting the metastatic thyroid carcinoma microenvironment could offer potential therapeutic benefits and should be explored further in preclinical and translational models of human metastatic thyroid cancer.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chronic inflammation
- Thyroid cancer
- Cells of TME
- Exosomes
- Cell-cell communication
- Epithelial to mesenchymal interaction
- Metastases
1 Tumor Microenvironment of Solid Tumors
1.1 Defining the Tumor Microenvironment
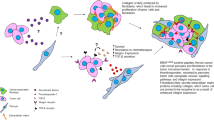
Solid tumors consist of two interdependent compartments – the carcinoma cells and the stroma (Fig. 1). Unlike the normal interstitial connective tissue, the tumor stroma is involved in malignant growth. The cellular constituents of the tumor stroma surrounding and embedded in the tumor make up the tumor microenvironment (TME). The tumor stroma consists of various stromal cells and a structural component known as the extracellular matrix (ECM) . The stromal cells secrete macromolecules that make up the ECM. These macromolecules are made of proteoglycans and glycoproteins, such as laminin, fibronectin, and structural proteins, including collagen and elastin. The stromal cells also secrete proteolytic enzymes leading to ECM degradation. This phenomenon along with disruption of the basement membrane becomes a prerequisite for the invasion process. During invasion, the matrix of the stroma is degraded by active proteases secreted into the tissue microenvironment leading to migration of tumor cells along the various components of the ECM [1, 2]. The ECM is responsible for the generation of signals that influence cellular proliferation, growth, migration, invasion, angiogenesis, and differentiation of cancer cells. Also, normal cells undergo apoptosis in the absence of contact with the ECM, proving it to be an important factor for cell survival. The composition of the ECM is modified and remodeled as the tumor progresses. As such, the malleable ECM microenvironment does not just provide structural support, but also has a profound influence on tumorigenesis [3]. The cellular components of the tumor stroma consist of cancer-associated fibroblasts (CAFs), pericytes, vascular endothelial cells, cancer stem cells and immune cells. The immune cells include dendritic cells, tumor-infiltrating lymphocytes, monocytes, and tumor-associated macrophages (TAMs). These cells, as well as the secretory molecules released by these cells and the tumor cells making up the TME including cytokines, chemokines, growth factors, and exosomes carrying cargo that all remodel the composition of the TME (Fig. 1), will be described in greater detail throughout this chapter. The interactions of these cells and their cellular constituents lead to consistent alterations in the TME network showcasing the dynamic nature of the TME.
1.2 Cellular Constituents of the Tumor Microenvironment
1.2.1 Cancer-Associated Fibroblasts (CAFs)
The main connective tissue cells that reshape the tumor stroma are fibroblasts and myofibroblasts. During the early stages of tumorigenesis, the fibroblasts undergo a change in both activity and phenotype, transforming into cancer-associated fibroblasts (CAFs). These cells produce macromolecules of the ECM, aid in angiogenesis, and synthesize various growth factors and cytokines. The basic fibroblast growth factor is a mitogenic factor for smooth muscle and is involved in various intracellular signaling pathways [4,5,6]. The interaction between fibroblasts and activated myofibroblasts occurs via direct cell-cell contact or by means of paracrine signaling. Presence of these cells is correlated with increased tumor aggressiveness and poor prognosis [7].
1.2.2 Endothelial Cells (ECs)
Endothelial cells (ECs) form the inner lining of the blood vessels. The associated vasculature is responsible for the delivery of nutrients and oxygen to tissues, organs, as well as developing tumors. Therefore, the formation of new blood vessels (neovascularization) and sprouting of new blood vessels from preexisting ones (angiogenesis) are both essential for the growth and the metastasis of tumors. Numerous pro-angiogenic factors , such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), and angiopoietins, are secreted into the TME to stimulate angiogenesis; this phenomenon is observed in the thyroid cancer TME. Among these growth factors, VEGF is the key pro-angiogenic factor, and activation of its receptor, VEGF receptor-2 (VEGFR-2), results in endothelial cell survival, proliferation, and vessel tubule formation. The degree and intensity of angiogenesis depend on the balance between the pro- and anti-angiogenic factors and their regulation [8].
1.2.3 Cancer Stem Cells (CSCs)
This subpopulation of tumor cells actively participates in the initiation and promotion of tumor formation. Cancer stem cells (CSCs) can self-renew, differentiate to diverse cell lineages, and seed new tumors. In vitro and in vivo experiments have shown that CSCs can differentiate into vascular endothelial cells. There is also speculation that CSCs can also differentiate into immune cells, such as tumor-associated macrophages (TAMs), in the TME, furthering the process of tumorigenesis and metastasis [9]. An interesting study done in mammary cancer cells by Mani et al. suggests that epithelial to mesenchymal transition (EMT) generates CSCs from mammary epithelial cancer cells [10].
1.2.4 Immune Cells
Pathological reports have shown that solid tumors are surrounded by abundant immune cell infiltrates. These cells belong to both arms of the immune system – innate and adaptive. These cells include lymphocytes, tumor-associated macrophages (TAMs), and various antigen-presenting cells (APCs). These cellular types are explained in further detail in Sect. 1.3.
1.3 Immune Cells of the Tumor Microenvironment
1.3.1 Natural Killer Cells (NK Cells)
Natural killer (NK) cells belong to the innate immune system and actively take part in initial tumor immune surveillance. The two types of NK cells, immunoregulatory and cytotoxic, are distinguished based on the expression of specific surface molecules – cluster of differentiation 16 (CD16) or 56 (CD56). The relative levels of immunoregulatory versus cytotoxic NK cells impact whether or not the TME has a pro-tumor phenotype, with a desire to polarize these NK cells in the cytotoxic direction to improve prognosis of disease, as described in the context of papillary and anaplastic thyroid cancer (ATC) in Sect. 2.6 [11].
1.3.2 Dendritic Cells
Dendritic cells (DCs) are key immune regulatory components that facilitate a critical connection between the innate and adaptive immune system, and arise from a hematopoietic stem cell lineage. Their main role lies in their antigen presentation ability, characterizing them as antigen-presenting cells (APCs). DCs are classified as one of the more “professional” APCs, a conclusion made on the basis of their efficient migration and T-cell activation. Upon foreign antigen detection, DCs will internalize, process, and project the antigen on its periphery, resulting in naive T-cell recognition and thus activation of an adaptive, and specifically tailored, immune response. DCs will present antigens in the context of major histocompatibility complex (MHC) class II and I in secondary lymphoid organs, a phenomenon termed as “cross-presentation.” In the event of pathologic environment establishment, DCs have the ability to recognize certain molecular patterns (pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs)) and elicit an immune response through migration and upregulation of costimulatory molecules. Examples of these costimulatory molecules include CD40, CD80, and CD86, which support the generation of a second set of signals that will initiate the adaptive immune response.
Within the tumor microenvironment (TME), there is a plethora of DC infiltrates that represent a variety of maturation stages and DC subsets. These subsets include plasmacytoid DCs (pDC), conventional DCs (cDCs) 1 and 2, and monocyte-derived DCs (mo-DCs). Tumors are said to contain seldom mature DCs, due to the fact that the TME is highly immunosuppressive, a characteristic that favors tumor proliferation [12]. With this being said, there is a correlation between increased levels of mature DCs and a positive prognosis. However, it has been seen that in certain TMEs, DCs have the potential to switch from serving as an immunostimulatory cell that drives potent anti-tumor activity to becoming an immunosuppressive accomplice. Cancer cells secrete immunosuppressive factors that are said to facilitate this pathologic “switch” in DC activity, leading to the formation of tumor-associated DCs (TADCs). TADCs characteristically promote neovascularization, which greatly favors tumor growth and establishment. This population of TADCs exerts their tumor-promoting effects through hindering antigen uptake and presentation, which greatly reduces the generation of an immune response; therefore, subsets of TADCs can serve as a target for the facilitation of therapeutic intervention [13].
1.3.3 Mast Cells
Mast cells are immune cells that elicit their effector function through either one of two processes: piecemeal degranulation and anaphylactic degranulation. The granules of mast cells are loaded with histamine, a potent inflammatory mediator that is released when an immune response is generated. Mast cell activation is typically triggered via IgE Fc region interaction with their FcεRI, making them key players in allergic responses. Mast cells are also prominent factors within the tumor microenvironment (TME) due to their extensive role in inflammation and their ability to induce neovascularization and support angiogenesis. Mast cells are said to support the proliferation of the TME and can contribute to the pathogenic nature and aggression of certain cancer types [14]. Depending on the anatomical location and type of tumor, mast cell involvement contributes to either a good or poor prognosis [15]. Within the TME, mast cells interact with other cellular residents, either through direct contact or through the release of characteristic mediators that have the ability to lead to TME remodeling. Studies have shown a plausible correlation between mast cells and the development of thyroid cancer. This correlation was made on the basis of significant mast cell infiltration within thyroid cancers that were more aggressive and invasive. Mast cell recruitment to the tumor site occurs via chemoattractant responses to vascular endothelial growth factor-A (VEGF-A), a protein released by thyroid cancer cells [16]. Thyroid carcinoma cells were also shown to alter the mast cell transcriptome, which was demonstrated through an IL-6, tumor necrosis factor-α (TNF-α), and colony-stimulating factor mRNA upregulation, which greatly supports the proliferative and inflammatory nature of the TME. Similar to thyroid cancer cells, pancreatic cancer cells also lead to the induction of mast cell migration. Upon mast cell infiltration into the TME, release of their associated cytokines, IL-13 and tryptase, leads to a proliferation of pancreatic cancer cells. Since the presence of mast cells favors tumor proliferation, blocking mast cell migration has led to the suppression of pancreatic cancer cell growth, and has contributed to a prognostic improvement [14].
1.3.4 T Regulatory Cells (Tregs)
CD4+ T helper (Th) cells can be polarized to express two different types of immune responses: the anti-tumorigenic Th1 response and the pro-tumorigenic Th2 response. T regulatory cells (Tregs) are associated with the pro-tumorigenic Th2 response. The polarization of the CD4+ T cells is influenced by the factors present in the TME.
Interestingly, double negative T cells have been more recently observed as previously undiscovered type of lymphocyte infiltration, which was showcased in a study by Imam et al. The double negative T cells do not express either CD8 or CD4 cell surface markers, and their secreted cytokines, particularly interferon-gamma (IFN-γ) and interleukin-17 (IL-17), repress the activation of CD8+ and CD4+ T cells. Thus, these double negative T cells make the TME favorable for persistent chronic inflammation, encouraging the tumor progression [17].
1.3.5 CD8+ Cytotoxic T Cells (CTLs)
Cluster of differentiation 8 (CD8) is a well-defined glycoprotein that spans the membrane of T lymphocytes and is further defined as a co-receptor for the T-cell receptor (TCR). Collectively, these cells are specifically denoted as CD8+ T lymphocytes and are key components of the adaptive immune system. Upon activation through presentation of tumor-associated antigens by antigen-presenting cells (APCs), these cells will differentiate into cytotoxic T lymphocytes (CTLs) and are responsible for eliciting their cytotoxic effects upon stimulation via interaction with major histocompatibility complex I (MHC I) and cognate antigen. This primes the CTLs against tumor cells expressing those specific antigens. Antigen presentation is an immunogenic phenomenon that describes the internalization of antigen followed by presentation on the cell periphery to elicit an immune-specific response through immune cell activation and differentiation. These cells will receive their antigen-specific signal, followed by subsequent activation via costimulatory signals and cytokines delivered by the APC to promote their targeted and specific effector functions. Activated CD8+ T cells will secrete two key cytokines that contain potent anti-tumor effects: tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ). These cells can also release two protein classes stored within their cytoplasmic granules, known as perforins and granzymes, which are pore-forming and pro-apoptotic proteins, respectively. These proteins work in concert to elicit an immune response on a foreign target. In addition to their released cytotoxic mediators, CTLs also will express chemokine receptors that will allow the cell to gain access to peripheral tissues [18]. Activation through tumor antigen recognition and presentation leads to the generation of an adaptive immune response, involving the activation of CTLs, and their subsequent recruitment and infiltration (tumor-infiltrating lymphocytes, TILs) into the area in which the tumor environment is being established. CTLs are a key component of discussion when referring to the tumor microenvironment (TME). TILs and inflammation together serve as a key feature of cancer [19]. Despite the high degree of heterogeneity of the TME, a major portion of the cellular composition is attributed to T-cell residents. CD8+ T-cell analysis within cancer patients has led to a better understanding of tumor immunology and antigen-specific immunotherapy. Activated T cells express a programmed cell death-1 (PD-1) receptor on its periphery, whereas its corresponding ligands, PD-L1 and PD-L2, are expressed by dendritic cells and macrophages. When activated CTLs bearing PD-1 receptors migrate to the TME, the receptor/ligand interactions promote resistance to endogenous anti-tumor activity that is typically exemplified by CTLs. Within the TME, PD-L1 is said to be overexpressed on the resident tumor cells, which greatly favors their further establishment and immune evasion through inhibition of CTLs. For example, in breast cancer (BC), instances of poorer prognosis have been shown in patients that express high levels of PD-1+ TILs, which coincides with their inhibition. Furthermore, in BC patients, their sites of malignant tissue had significantly less IL-2 and IFN-γ, which corresponds to the progressive loss/inhibition of cytotoxic activity, a phenomenon termed “T-cell exhaustion” [20].
To determine the impact that blocking PD-1 has on the establishment of CTLs within the TME, there is a stage-specific requirement that will determine whether or not the abrogated expression of the receptor will lead to a rise in CTL cytotoxicity or favor immune evasion. Results have shown that anti-PD-1 monoclonal antibody therapy can serve as a revitalization tool for exhausted T cells and has led to an increased production of IFN-γ, thus confirming successful activation of CTL activity [21]. This PD-1 blockade, however, is only successful when it occurs in a stage-specific manner. Blocking of PD-1 must occur after the CD8 T lymphocyte is exposed to the presented antigen. If anti-PD-1 is administered prior to antigen exposure, the CD8 T lymphocyte will become anergic, and linker for activation of T cells (LAT) and Akt will lack phosphorylation: an implication of failed cellular activation [21]. CTLs that express high levels of PD-1 have been correlated with an increased expression of T-cell immunoglobulin and mucin domain-3 (TIM-3) and lymphocyte activation gene 3 (LAG3), two inhibitory checkpoint molecules [22]. TIM-3 has specifically been identified as a marker for anti-PD-1 resistance, which makes this inhibitory molecule a key target for future study regarding cancer immunotherapy [22].
1.3.6 Tumor-Associated Macrophages (TAMs)
Macrophages are key components of the innate immune system ; they serve as the first responders to inflammation or pathogens and foreign antigens. Common myeloid progenitor cells give rise to blood monocytes that eventually differentiate into macrophages [23]. These cells of monocyte-macrophage lineage are very important for the maintenance of homeostasis in body tissues. Macrophages have multiple subtypes and possess the plasticity to switch between these subtypes. Based on the signals within the tissue microenvironment, macrophages can either be activated classically to an M1 phenotype or alternatively to an M2 phenotype. Cytokines secreted by Th1 cells, such as IFN-γ and tumor necrosis factor-alpha (TNF-α), or the bacterial moiety lipopolysaccharide (LPS), have the ability to activate macrophages to the M1 phenotype. On the other hand, M2 macrophages are subdivided into M2a, M2b, and M2c subtypes based on their activation stimulus. Th2 cytokines , such as IL-13 and IL-4, activate monocytes to the M2a macrophage phenotype. M2b macrophages are stimulated by LPS, toll-like receptors (TLRs), and IL-1 receptor antagonists. Lastly, M2c macrophages are induced by transforming growth factor beta (TGF-β), IL-10, or glucocorticoids [24]. The diversified phenotype of macrophages, based on their polarization, exhibits differential expression of cytokines, chemokines, and surface proteins. M1 macrophages are pro-inflammatory and produce inflammatory cytokines such as IL-6, IL-1, and TNF-α, which aid in the generation of an anti-tumor immune response. In contrast, M2 macrophages exert pro-tumorigenic, anti-inflammatory, and pro-vasculogenic actions. These actions are exerted via immunosuppressive cytokines, including IL-4, IL-13, and IL-10, as well as immune complexes and apoptotic cells.
Solid tumors , such as thyroid cancer, are composed of a highly heterogeneous mass of mutant cells embedded in the stroma, with these macrophages being a vital and prominent component. Hence, these macrophages are termed as tumor-associated macrophages or TAMs. A vast number of studies have shown that these TAMs exhibit the same characteristics of the immunosuppressive M2 macrophages in the TME and aid in tumor development and promotion, not just by cytokine secretion, but also by angiogenesis, increased survival, and metastasis of tumor cells [25, 26].
Studies done in mouse mammary carcinoma, murine fibrosarcoma , and B16 melanoma reveal high expression of immunosuppressive cytokines by TAMs isolated from such cancers. This is fortified by the presence of certain M2 markers, which include, but are not limited to, arginase-1, FIZZ1, and YM1 [27]. However, more studies are presenting newer evidence suggesting that the polarization of macrophages depends on the stage of the tumor. Tumorigenic M1 macrophages at sites of chronic inflammation contribute in the early stages of tumor progression [28], whereas M2 macrophages support angiogenesis, tumor growth, and tissue repairs in established tumors [26, 29, 30]. Epidemiological and clinical studies have shown the correlation between various infections causing chronic inflammation and an increased risk of cancer. Detailed investigation of this link between inflammation and cancer suggests that macrophages play a vital role in tumor onset at sites of chronic inflammation [31, 32].
Studies conducted in breast cancer suggest that the density of TAMs positively correlates with the angiogenic potential of the tumor, where increased density is associated with poor prognosis [33]. A meta-analysis study done on more than a thousand patients with solid tumors suggested that the plasticity and duplicity of TAMs can serve as a critical indicator for prognosis. The presence of immunosuppressive M2 macrophages correlates with poor prognosis of disease, in contrast to the anti-tumor M1 macrophages which improve the prognosis of the patients, therefore suggesting an anti-tumor role. Thus, there are varying and contradictory reports on the role of TAMs in cancer prognosis. A macrophage balance based on the TME signals will indicate the prognosis of the solid tumor [34].
The most important question remaining is that if these M1 macrophages are pro-inflammatory, how are they involved in tumorigenesis? It is believed that these pro-inflammatory M1 macrophages, through their persistent secretion of cytokines and reactive oxygen species (ROS), cause extensive surrounding tissue and DNA damage, generating mutations and altered p53 activity. This event predisposes the tissue cells to undergo premalignant, neoplastic transformation and tumor initiation [28, 29, 35]. Moreover, in vivo studies support this M1 macrophage activity by demonstrating how inflammatory cytokines like TNF-α, IL-1β, and IL-6 enhance tumorigenesis by sending out pro-survival signals to the proliferating neoplastic cells [36, 37]. Thus, it is the M1 phenotype that is present in tumor initiation and causes neoplastic transformation, a phenotypic transition, whereas M2 macrophages reside in the established tumors [27, 38]. “Mixed phenotype” macrophages also exist in established tumors . In vivo studies conducted to ascertain the TAM population in tumors suggest the presence of TAMs expressing M1 and M2 markers. Pro-inflammatory M1 macrophages express inducible nitric oxide synthase (iNOS), which is utilized to metabolize arginine to nitric oxide (NO) or reactive nitrogen species (RNS). In contrast, M2 immunosuppressive macrophages express arginase and metabolize arginine to urea and L-ornithine. This difference in arginine metabolism is one of the major indicators of the M1 vs M2 phenotype. However, TAMs of certain solid tumors express both arginase and iNOS, suggesting the presence of both phenotypes in the TME.
A study by Auffray et al. shows that macrophage phenotype can switch from M1 to M2, and vice versa, based on tissue environment [39]. This heterogeneity and plasticity of the macrophages modulates with the signals present in the TME. As the cancer progresses, the phenotype of the macrophage changes in accordance with the secretory factors of the tumor environment. At any given time point during tumorigenesis and advancement, there will be diversity of macrophages present at various stages of M1, M2, or intermediate transition.
2 Specificity of the Thyroid Tumor Microenvironment
2.1 Anatomy of the Thyroid Gland
The thyroid is a highly vascular, butterfly-shaped gland, located in the anterior neck, overlaying the trachea. The thyroid gland weighs approximately 15–25 grams in adults. It is the largest endocrine gland, consisting of two pear-shaped lateral lobes connected by the isthmus. The thyroid gland is surrounded by a dense fibrous capsule of connective tissue. This capsule also encloses four small parathyroid glands which are located posterior to the thyroid gland. Externally, the capsule is enveloped by pretracheal fascia (false capsule of deep cervical fascia) encompassing the vessels entering and leaving the gland. Overall, the thyroid gland has a rich blood supply of 5 mL/g/min, which includes the dense network of connecting vessels. The lymphatic vessels drain into the lymph nodes as well as directly into the veins. The gland receives its vasomotor innervations from cervical sympathetic ganglia [40,41,42,43]. The adult thyroid consists of about three million spherical-shaped follicles. These follicles are lined by a single epithelial cell layer and serve as the major functional units [41, 44]. Each follicle is composed of a colloid filled central cavity containing thyroglobulin (Tg) glycoprotein and is surrounded by a single layer of thyroid follicular cells. In addition, there are small numbers of parafollicular cells (C cells, parenchymatous cells) in the space surrounding the follicles. The primary function of C cells is to secrete calcitonin, a hormone that reduces blood calcium [45].
2.2 Thyroid Cancer
Thyroid cancer is the most prevalent endocrine malignancy, comprising more than 95% of all such malignancies in the United States [46,47,48]. It is the most rapidly rising cancer in the United States, with its incidence having tripled in the last 30 years [49]. Pathologically, thyroid cancer can be classified into four morphological types – papillary, follicular, anaplastic (undifferentiated), and medullary thyroid cancer. Papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC) are the differentiated thyroid cancer (DTC) types and are derived from thyroid epithelial cells. PTC and FTC represent 90–95% of all thyroid cancers. Interestingly, PTC alone makes up 75–85% of all thyroid cancers [50]. Medullary thyroid cancer (MTC) is derived from parafollicular C cells and makes up 5–10% of cases. The rarest and most fatal thyroid cancer is anaplastic (undifferentiated) thyroid carcinoma .
2.3 Heterogeneity of Thyroid Cancer Cells
The dynamic heterogeneity of tumors encompasses the transient presence of various cell populations, signaling cascades, and metabolism associated with tumorigenesis, which eventually becomes an established feature of the tumor microenvironment (TME). Clonal evolution occurs as a result of somatic mutations in a population of cells which gradually accumulate over a period of time, leading to tumor initiation. These clonal cells further propagate, gaining branch mutations, which give rise to subclonal populations of cells under the influence of various factors present in the TME. Such clonal and subclonal alterations lead to the formation of intratumoral genetic heterogeneity with genetically distinct clones existing within the same tumor. Such a phenomenon of evolutionary divergence of cellular variants is present in all solid tumors, including advanced thyroid cancer [51]. The heterogeneous genetic and epigenetic alterations in cancer cells offer a selective advantage to the cells in the TME for the promotion of growth and metastasis. Studies of breast cancer cells have denoted that only a few cancer cells are needed with such accrued genetic variants to drive the progression of cancers with individualized genotypes [52].
Thyroid cancer consists of a heterogeneous group of neoplasms as well, which are classified histologically based on the cells of origin. The exact etiology of thyroid cancer remains unclear to date, but it is considered to have a multifactorial etiopathogenesis. External environment factors such as radiation and dietary iodine can influence the incidence of thyroid cancer. Case studies have shown that patients with preexisting thyroid diseases such as goiter or autoimmune disorders – Hashimoto’s thyroiditis and Graves’ disease – have a constitutional predisposition to thyroid cancer in the future. Most thyroid cancers are sporadic in nature, with genetic and epigenetic modifications that are promoted by external factors as mentioned above [53]. Only about 5% of cases have familial cancer incidence, elucidating the role of molecular pathogenesis in thyroid cancer. Based on the tumorigenesis model of a number of other cancers, it has been proposed that thyroid cancers arise from the sequential accretion of genetic and epigenetic alterations.
The multistep tumorigenesis model suggests that the accumulation of multiple genetic alterations in the genome of thyrocytes leads to the generation of thyroid cancer. The damage in the genome can occur in the oncogenes or tumor suppressor genes, promoting neoplastic conversion. A complete analysis of the papillary thyroid cancer (PTC) genomic landscape was done by the TCGA Network (The Cancer Genome Atlas Network) recently, which suggested a low occurrence of overall somatic gene alterations in well-differentiated thyroid cancer. Unlike other cancers, only a handful of recurrent mutations are present in PTC. This means that PTC and follicular thyroid cancer (FTC) can be derived from activating mutations in RAS (13%) and/or BRAF (60%) genes or rearrangements of fusion proteins associated with receptor tyrosine kinases such as RET/PTC, NTRK1/3, and ALK [54]. Although the abovementioned genetic alterations are found in PTC, thyrocytes give rise to FTC due to point mutations in RAS gene or a rearrangement of PAX8/PPARγ genes. Interestingly, as with breast, ovarian, and pancreatic cancers, it was observed that the original somatic mutations were present in the metastases. Moreover, the secondary metastatic lesions contained additional genetic lesions, offering a greater genetic instability in tumorigenesis [55]. This multistep tumorigenesis model also suggests that poorly differentiated and undifferentiated thyroid cancer, such as anaplastic thyroid cancer (ATC), arises due to progression in acquisition of these small numbers of genetic mutations during dedifferentiation.
This provides two options for ATC formation; it arises either de novo or by dedifferentiation from preexisting well-differentiated thyroid cancer (WDTC), including PTC and FTC. The evidence points toward the latter with the existence of WDTC in ATC specimens, as well as the presence of BRAF and RAS gene mutations in differentiated and undifferentiated thyroid cancer. Cellular heterogeneity is also consequential to tumor progression. It incorporates both different kinds of cells and similar cells that possess different metabolic phenotype – aiding in cancer proliferation. Breast and ovarian cancers have a large population of associated fibroblasts in the TME, which not only are key for cancer progression but can also be used to predict the prognosis. Cellular heterogeneity is also important in thyroid cancer; however, the infiltration of the stromal and immune cells varies based on the aggressiveness of the tumor. Among the well-differentiated thyroid cancers, FTC is mostly considered homogeneous, whereas PTC has a large number of cancer-associated fibroblasts. In contrast, ATC has a higher infiltration of TAMs in the TME. The immune cell infiltrates are associated with poor outcomes in patients; however, the mechanism behind it is still being explored [52].
This heterogeneity of thyroid cancer with an accumulation of very few mutations offers a uniqueness to thyroid cancer. Standard treatment modalities and targeted therapies have made WDTC, especially PTC, curable. However, the same cannot be said about malignant/metastatic PTC, PDTC, or undifferentiated ATC. This propensity of ATC to develop metastasis still needs to be explored.
2.4 Anaplastic Thyroid Cancer
Anaplastic thyroid carcinoma (ATC) is an aggressive, undifferentiated cancer responsible for less than 1.7% of all thyroid cancer cases in the United States. Although rare, it remains one of the most fatal forms of the disease, representing an end stage of thyroid tumor progression. The median survival of patients with ATC is 5 months and the 1-year survival rate is less than 20% [56]. ATC is categorized as stage IV cancer with subgroups based on the involvement of adjacent neck structures or distant sites. Patients with intrathyroidal undifferentiated tumors are stage IVA, extrathyroidal extensions are stage IVB, whereas distant metastases are stage IVC. At the time of diagnosis, almost 90% of patients are in stage IVB and about 20% have distant metastases. ATC has a rapid onset; hence, patients usually present with symptoms suggestive of tracheal, esophageal, and nerve compression due to the rapidly growing neck mass [56, 57].
The undifferentiated phenotype of ATC may arise due to dedifferentiation of preexisting well-differentiated thyroid carcinomas, such as incompletely treated papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC). There is also evidence that about 80% of ATC patients have a long-standing goiter. Histologically, ATC completely loses the thyroid differentiation features and the normal thyroid cellular architecture. Instead, tumors are highly invasive with mitotic figures, multinucleated giant cells, large atypical nuclei, and widespread necrosis present [57,58,59].
Compared to other types of thyroid cancer, ATC frequently metastasizes, is more aggressive, and is largely incurable, which emphasizes the importance in understanding the molecular and cellular mechanisms contributing toward the disease progression.
2.5 Amplified Metastatic Propensity of Anaplastic Thyroid Cancer
The genetic burden carried by papillary thyroid cancer (PTC) is lower than that of aggressive thyroid cancers, such as anaplastic thyroid cancer (ATC). Despite the presence of a number of mutually exclusive genetic alterations, clinically, PTC is indolent in nature, therefore making it an easy target for available drugs. In contrast, ATC has unfavorable clinical outcomes accompanied by fast growing tumors, metastasis, and invasion of distant sites. In addition to these pathological manifestations, ATC also exhibits resistance to current therapies, thus leading to poor prognosis of the disease. The fundamentals behind the aggressive nature of ATC have not been elucidated completely.
The metastatic ability of ATC is a fascinating property that is worth investigating to understand the pathogenesis of the disease. The metastatic cascade is said to be associated with the multistep tumorigenesis process. The gain of mutations, aside from the initial driver mutations, aids in progressive dedifferentiation of the cancer cells. The dedifferentiated cells have the ability to undergo epithelial to mesenchymal transition and invade the local structure at the primary site and travel to distant secondary sites.
Very limited information is available regarding the genomic basis of ATC . Aside from the mutations present in PTC, there is an additional accumulation of diverse mutations in TP53, PIK3CA, and the β-catenin encoding gene, specifically during the dedifferentiation process, thus contributing toward the aggressive nature of ATC. TP53 is the most common mutation found in ATC, followed by RAS, BRAF, β-catenin, and PIK3CA. A number of studies have suggested this claim concerning the gain of additional epigenetic and genetic alterations rather than a single genetic event in transforming the differentiated cancer cells into undifferentiated cells. This information is made possible due to the availability of whole exome sequencing and ultradeep sequencing of ATC specimens [54]. Moreover, next-generation sequencing (NGS) of PDTC and ATC has revealed that these tumors may have a unique genetic background that is distinct from the DTCs they originate from [60, 61]. However, it is important to note that most of the abovementioned mutations are pan-mutations commonly found in a number of other cancers.
Although a number of driver mutations (such as BRAF and RAS, as well as additional secondary mutations) that cause nuclear instability and dedifferentiation have been assessed with respect to ATC , it is quite evident that genetic lesions alone cannot define the ATC phenotype. The fact that ATC is still unresponsive to the current targeted therapies against driver mutations and possesses a very high metastatic propensity suggests that there are other factors influencing its pathogenesis. Multiple cancer studies have shown that targeting a single driver mutation will ultimately result in evolution of clones that propagate using alternate pathways. The signals for such evolution can be intrinsically derived from the cancer cells, or can come from other host factors such as the tumor microenvironment (TME), leading to thyroid cancer progression.
2.6 Thyroid Cancer and Inflammation
Several genetic and epigenetic factors affect the irreversible initiation of carcinoma. However, the advancement of tumorigenesis requires a promoting agent that induces proliferation of cancer cells. Chronic inflammation is regarded as a promoting agent for several types of cancer. Persistent infection causing chronic inflammation leads to recruitment of immune cells that secrete pro-inflammatory factors in the tissue microenvironment, causing DNA damage in the proliferating cells. These can permanently alter the genetic makeup of the proliferating cells, by way of point mutations, deletions, or rearrangements, triggering tumor promotion. The axiom of chronic inflammation and cancer is illustrated by bacterial or viral infections leading to associated malignancies, such as H. pylori, causing gastric ulcers, and hepatitis B virus, causing hepatocellular carcinoma [29]. Inflammation is an important process linked to tumor development and progression in thyroid cancer as well. It enhances cell proliferation by providing an environment rich in growth factors. It is suggested that inflammation is to be referred to as the seventh hallmark of solid tumors [62].
2.7 Dynamic Nature of the Thyroid Tumor Microenvironment
In the last two decades, several studies have demonstrated the importance of stromal cells in thyroid cancer progression [63, 64]. Papillary thyroid cancer (PTC) stroma consists of the ECM, along with a variety of stromal cells, namely, fibroblasts (and myofibroblasts), inflammatory cells, and blood vessels [65]. The thyroid cancer cells interact with the stromal cells, changing the behavior and coevolving with these stromal cells, whereby the tumor cells build a supportive environment for their own proliferation and propagation. Hence, there is a well-defined reciprocal relationship between the cancer cells and stromal cells in the thyroid cancer tumor microenvironment (TME).
Studies have shown that angiogenesis in the thyroid TME is in fact initiated due to paracrine signaling from the secretory factors of thyroid cancer cells and endothelial cells. This interaction may be influenced by the role of estrogen in promotion of metastatic thyroid cancer. It was observed that estrogen-stimulated VEGF secretion in turn promotes angiogenesis and tumor growth [66, 67]. Moreover, the new blood vessels formed in the tumor are branched and leaky, encouraging a more metastatic environment for thyroid cancer [68].
There is a heterogeneous population of cancer cells in the thyroid TME. This heterogeneity may contribute to the presence of cancer stem cells (CSCs) in the thyroid TME. Thyroid cancer follows the dynamic cancer stem cell (CSC) model, where the cells interconvert between CSCs and non-CSC cells. Such interconversion can be spontaneous or induced by certain processes such as EMT [69,70,71]. Most interestingly, thyroid CSCs are invasive and highly resistant to conventional treatment modalities, resulting in relapse [72].
Evidence has shown the existence of a mixed population of lymphocytes and macrophages in and around primary thyroid tumors. Thus, there exists a crucial relationship between thyroid cancer cells and immune cells. An association between the differentiated thyroid carcinoma and inflammatory microenvironment has been strongly recommended over the last decade [73].
2.8 Immune Cell Remodeling of the Thyroid Tumor Microenvironment
How does the interplay between thyroid cancer cells and immune cells impact the composition of the thyroid tumor microenvironment (TME), specifically? In terms of natural killer cells, when comparing infiltration of NK cells in papillary thyroid cancer (PTC) patients versus patients with goiters or healthy individuals, greater infiltration of CD56high CD16low NK cells is observed in the PTC patients. The percentage of immunoregulatory NK cells present is inversely correlated to the stage of disease. Although not a major presence, NK cytotoxic cells CD56low CD16high are also present in PTC, which is positively correlated with disease stage. In accordance with PTC trends, anaplastic thyroid cancer is accompanied by a lesser extent of cytotoxic NK cell infiltration, supporting tumor promotion [74, 75]. Thus, the thyroid TME has a mixture of different NK cells, and based on the predominant cell type, the phenotype of thyroid cancer can undergo alteration.
Another immune cell that bridges the gap between the innate and adaptive arms of the immune system is a type of professional antigen-presenting DCs. There exists a mutual relationship between thyroid cancer cells and DCs. PTC cells recruit DCs toward the tumor, and in return, DCs engulf the tumor-associated antigen to prime the host immune response. DC infiltration is observed more in PTC compared to follicular thyroid cancer (FTC) or adenomas. In contrast, DC infiltration is almost absent in poorly differentiated thyroid cancer, such as anaplastic thyroid cancer (ATC) [76, 77]. This might be due to T cells eliminating PTC better than the aggressive thyroid carcinomas, or with a decreased ability for the DCs to find, package, and present tumor-associated antigens from poorly differentiated cancers.
Thyroid cancer cells interact with mast cells in an opposite manner to their interaction with DCs. PTC attracts the mast cells at the tumor cell axis, and in return mast cells secrete cytokines promoting tumor growth, vascularization, and proliferation. Hence, PTC and FTC had a higher density of mast cells compared to adenomas or healthy thyroids [16, 78]. Mast cells also secrete interleukin-8 (IL-8) into the thyroid TME, which induces EMT in thyroid cancer cells, promoting the invasiveness of the cancer [79].
Within the adaptive immune system, thyroid cancer research involving the polarization of CD4+ T cells into Th1, Th2, and Treg cells has gained interest in the last decade. Many studies conducted on PTC samples have shown higher loads of infiltration of FoxP3+ Tregs. There is a direct correlation between the percentage of Treg cell infiltration in PTC and the aggressiveness of the disease. The higher the infiltration, the poorer the prognosis [80,81,82].
CD8+ cytotoxic T lymphocytes are conventionally believed to be anti-tumorigenic when present in abundance in the TME. However, different studies offer contradictory roles of CTLs in thyroid cancers. An immune-histological study was conducted in differentiated thyroid cancer (DTC) patients with chronic lymphocytic thyroiditis that associated an increase in CD8+ T-cell infiltration with improved disease-free survival. On the other hand, DTC patients with CD8+ T cells and increased Cox-2 expression also had higher relapse rates. Moreover, BRAFV600E-mutated PTC tumors showed a low CD8+/Foxp3+ ratio signifying the presence of immunosuppressive environment in BRAF-mutated tumors, thereby promoting the PTC microenvironment. Hence, low CD8+ T-cell recruitment to the tumor site may be the cause of proliferating thyroid cancer [83, 84]. It can be ascertained that the relationship between T cells and thyroid TME is very different from that observed in thyroid autoimmune disease (Hashimoto’s thyroiditis) as the lymphocytes in the latter disease actually eliminate the target cells. The double negative T cells are considered the dominant T cells in thyroid cancer, and they downregulate the expression of CD8+ and CD4+ T cells. Such immunoediting in the thyroid cancer microenvironment may lead to better survival of the developing thyroid tumor.
In addition to the important immunomodulatory roles of NK cells, DCs, and T cells as described above, one of the largest players in immune remodeling in thyroid cancer is tumor-associated macrophages. With TAM infiltration being an important facet of thyroid cancer, many studies have investigated its role in clinicopathological aspects of the disease. Qing and colleagues provided documented evidence that high levels of TAMs are associated with papillary thyroid carcinoma lymph node metastasis [85]. In addition, the presence of TAMs in the thyroid cancer microenvironment is correlated with larger tumor size, increased dedifferentiation, and decreased survival rates. Poorly differentiated thyroid cancer had higher density of TAMs, which was correlated with capsular invasions and extrathyroidal extensions [86, 87]. Ryder et al. also showed that conditional activation of BRAF in adult mice thyroids induced PTC along with TAM infiltration. The thyroid cancer cells secrete chemokines and cytokines that act as chemoattractants for the TAMs. Most of these TAMs belong to the immunosuppressive M2 phenotype, where their depletion reduces tumor growth [88]. Thus, several in vivo and human tissue studies indicate the presence of TAMs positively leading to tumor progression. Interestingly, the role of macrophages in differentiated thyroid cancer (DTC) differs from poorly differentiated thyroid cancer (PDTC) [89]. There is a strong association between the density of TAMs present in thyroid TME and its advanced histological grade. More than 50% of ATC tissues consist of TAMs with a peculiar microglial-like morphology. There is a very dense network of TAMs interlinked with cancer cells in ATC [90]. Intrinsic and extrinsic signals from the TME modulate the functions of TAMs to support the metastatic processes. Poorly differentiated and undifferentiated thyroid cancers had higher density of TAMs infiltrating the tumor, resulting in an increased aggressiveness of the tumor.
Although tissue-associated macrophages with various functional states are found to coexist in the same tumor [91], the preponderance of macrophage polarization and their role in thyroid tumor progression is still understudied. In addition, it is believed that crosstalk between TAMs and epithelial cells (ECs) facilitates induction of epithelial to mesenchymal transition (EMT), which is directly associated with cancer progression. Thus, thyroid cancer represents a complex bionetwork where cell-cell and cell-matrix interactions provide mutual influences resulting in cancer promotion, invasion, and metastasis. Thyroid cancer cells express mutated proteins that are recognized as non-self, activating the host immune system for their elimination. One aspect of the immune system is to recruit inflammatory cells to the tumor site to protect the host tissues. However, there is another side to this tumor-immune cell interaction. Tumor cells have their own secretory profile that recruits and activates immune cells. The immune cell secretory mediators are in turn utilized by the tumor cells to promote their own proliferation, migration, and invasion [92]. This to-and-fro interaction between cancer and immune cells is mediated through several secretory cytokines, chemokines, and exosomes, which form the secretome for the thyroid TME. The secretome majorly influences thyroid cancer growth, promotion, and advancement. Thus, it becomes important to understand the functionality of the soluble mediators of thyroid cancer-immune network.
2.9 Immune Surveillance in the Thyroid Tumor Microenvironment
The foremost immune response that occurs in a tumor microenvironment (TME) is the elimination of the cancer cells by a process called cancer immune surveillance. This mechanism was also explored in thyroid cancer relating the presence of T cells, B cells, and macrophages, as well as the absence of dendritic cells (DCs) with a better or worse prognosis in DTC patients, respectively [89]. However, some aggressive cancer cells escape elimination and proliferate in a less immunogenic environment, maintaining an equilibrium stage, and then eventually escaping from the immune surveillance. To hide from the host immune response, cancer cells recruit immune suppressive cells such as regulatory T cells (Tregs) and MDSC which secrete anti-inflammatory cytokines [80]. This makes the TME conducive for the growth and proliferation of the cancer cells. Contrary to previously mentioned reports, some researchers demonstrated that leukocytic infiltration of thyroid TME as well as tumor-associated lymph nodes, in fact, leads to thyroid cancer progression [81, 85]. The tumor-associated lymphocytes in well-differentiated thyroid cancers majorly consist of a mixture of T cells and macrophages, either inside or surrounding the thyroid cancer, which are pro-tumorigenic. The extensive leukocytic infiltration also correlates with the increase in tumor invasion, lymph node metastasis, and decrease in patient survival rates [93, 94]. This suggests that aggressive form of papillary thyroid cancer (PTC) as well as anaplastic thyroid cancer (ATC) might take up several immune escape mechanisms including, but not limited to, concomitant recruitment of immune suppressive and pro-tumorigenic immune cells in the thyroid TME.
2.10 Evolution of the Metastatic Phenotype Via Macrophages
It is believed that triggering epithelial to mesenchymal transition (EMT ) in thyroid tumor cells depends on an assortment of external signals [95, 96]. These signals are present in the tumor microenvironment (TME) in the form of various secretory mediators such as cytokines, chemokines, or secretory molecules – exosomes. The inflammatory microenvironment plays an important role in thyroid cancer occurrence and advancement. Patients with preexisting chronic inflammatory conditions tend to get advanced thyroid cancer, denoting a link between the inflammatory microenvironment and increased migratory capacity of the thyroid cancer cells. Deciphering the secretome pattern of inflammatory cells in the thyroid TME aids in bettering understanding that link [97, 98], specifically the effect of macrophage secretory components on the thyroid cancer phenotype. Based on macrophage polarization, the secretory mediators in the TME change. To understand the initiation and progression of thyroid cancer, it is important to assess the role of major secretory players which induce EMT. Moreover, the regulators of EMT do not just initiate the tumor progression, but rather influence the increase in cell survival and resistance to apoptosis/senescence, deeming the current therapies inadequate to treat aggressive invasive thyroid cancer. In the future, innovative therapeutic strategies could be explored that will target EMT regulators to curb advanced thyroid cancer, especially anaplastic thyroid cancer (ATC).
2.11 Interacting Molecules of the Thyroid Tumor Microenvironment
2.11.1 Cytokines and Chemokines
Cytokines are immune molecules secreted by the cells of the innate and adaptive immune system in the presence of cellular stress. Apart from the immune cells, tissue cells, like thyroid follicular cells, also secrete these immune mediators. In thyroid cancer, cytokines and chemokines are released from cancer cells, which act as a chemoattractant for inflammatory cells at the tumor site. As discussed above, preexisting inflammation is an important predisposing factor for thyroid cancer. The presence of persistent inflammation leads to excessive production of cytokines and chemokines, which causes tissue destruction and DNA damage in proliferating cells, thus contributing to the pathogenesis of thyroid cancer.
TAMs infiltrating thyroid cancer form a major component of the thyroid cancer immune stromal network. M2 TAMs are present in a significantly higher density in papillary thyroid cancer (PTC) patients. These M2 polarized macrophages are immunosuppressive in nature and release anti-inflammatory IL-4, IL-13, and IL-10. These cytokines have a dual role; they exert a stimulatory effect on the tumor cells while suppressing the activation of cytotoxic T cells, thus promoting thyroid cancer survival and progression [99]. Moreover, PTC patients with concomitant Graves’ disease exhibited higher levels of IL-10 and IL-4, suggesting contribution of anti-tumor immunity [100].
Pro-inflammatory cytokines produced in the thyroid tumor microenvironment (TME) consist of IL-1, IL-6, IL-8, TNF-α, TGFβ, and MCP-1 (monocyte chemotactic protein-1). In vitro studies done in WDTC and anaplastic thyroid cancer (ATC) cell lines denote that these pro-inflammatory cytokines are secreted by thyroid cancer cells. Numerous studies have been performed to understand the role of these cytokines in PTC proliferation. Oncogenes upregulate the expression of these cytokines in PTC. Studies have shown that PTC with RET/PTC, RAS, and/or BRAF mutations have higher expression of these cytokines signifying the correlation of inflammation, oncogene activation, and tumor invasion [101,102,103]. IL-1 induces thyroid tumor growth and proliferation by activating pro-metastatic genes, angiogenesis, and other pro-inflammatory cytokines. Higher levels of IL-1β are found in the serum of PTC patients when compared to thyroiditis patients, suggesting its contribution in tumor pathogenesis [104]. IL-6 is another important interleukin for thyroid cancer survival and proliferation. It increases the migration and invasive properties of thyroid cancer cells by inducing EMT and stemness [79, 99].
Like IL-1, TNF-α is an acute response pro-inflammatory cytokine that activates several immune cells. It has multiple roles in cancer progression; it can induce apoptosis or necrosis, or cause increased angiogenesis, migration, and invasion of cancer cells. TNF-α is secreted by the TAMs in thyroid tumor environment, and its action depends on the particular downstream signaling in addition to its interaction with other cytokines [99, 105]. TNF-α is an important inflammatory stimulus with higher serum concentrations in several cancers. However, its role for thyroid cancer progression needs further investigation. TGFβ is another cytokine with a plethora of functions in cancer promotion. TGFβ promotes Tregs and hence induces a pro-tumorigenic response by suppressing cytotoxic T cells. Murine studies have shown that TAMs as well as thyrocytes produce TGFβ, which induces EMT in thyrocytes. Moreover, there was a higher expression of TGFβ in PTC tissue samples, suggesting its role in enhancing the invasion of thyroid cancer. Hence, the presence of TGFβ is associated with higher aggressiveness of the cancer [106, 107].
Oncogenic activation of MAPK pathways leads to release of a number of chemokines, such as CXCL1, CXCL8, CXCL9, CXCL10, and CXCL11. In vitro studies have revealed that PTC and ATC cell lines normally release huge quantities of IL-8 into the TME, which is enhanced by IL-1 or TNF-α stimulus. This IL-8 can induce EMT and stemness in thyroid cancer cells. Activation of the RET/PTC1 oncogene exogenously induced expression of IL-8 in normal human thyrocytes. PTC human tissues also displayed higher expression of IL-8 and CCL20 when compared to thyroiditis or normal tissues. A study suggests that this expression pattern may be due to a higher number of TAMs secreting IL-8 in PTC, thereby responsible for invasion and metastasis [79, 93, 103, 108]. Among all the chemokines present in thyroid TME, CXCL8 and its role in cancer survival and metastasis has been explored the most.
Although various cytokines and chemokines might be present in the thyroid TME, the focus in this chapter will be on the pro-inflammatory cytokines. Since these soluble mediators of inflammation are secreted by cancer cells and the stromal cells, it is safe to accept that there exists a mutual link between these TME components.
2.11.2 Exosomes
The various factors secreted in the thyroid tumor microenvironment (TME) consist not only of the soluble mediators – cytokines and chemokines – but other secretory mediators from inflammatory cells. An example of these additional secretary mediators comes in the form of small vesicles known as exosomes. Exosomes secreted by the tumor cells and inflammatory stromal cells, especially TAMs, offer a physical means of communication and transfer of regulatory molecules in the thyroid TME. Recent research has uncovered the important role exosomes play in the TME. Exosomes, once considered as “garbage bags,” are “cup”-shaped nanovesicles 30–100 nm in diameter and 1.13–1.19 g/mL in density. Although earlier considered as a means to remove unwanted materials from the cell, recently exosomes have gained much spotlight due to their role in the immune response. Exosomes contain functional proteins and nucleic acids, including microRNAs (miRNAs), messenger RNAs (mRNA), and DNA fragments, as their cargo [109, 110]. Exosomes are secreted actively by normal, tumor, and stromal cells through exocytosis pathways. They act as a shuttle for intercellular communication and crosstalk [111]. Tumor cells secrete a large number of exosomes, which provide a physical means to transfer intracellular molecules into the tumor stroma, where the inflammatory cells reside [112]. Thus, exosomes represent a novel link between cancer and inflammatory cells in the TME, especially in thyroid cancer.
Based on the cargo of these vesicles, they can either be degraded by the lysosome or released in the extracellular environment as exosomes. Exactly how the cargo gets sorted into the exosomes is not yet fully understood, but endosomal sorting complex required for transport (ESCRT)-dependent and ESCRT-independent methods are involved. The outer surface of exosomes consists of a complex lipid bilayer with integral membrane proteins, whereas the interior consists of sorted cargo [113]. The secretion or release of exosomes into the extracellular compartment occurs through MVBs/exosomal fusion with the cellular plasma membrane. Some of the components of this endocytic and exocytic machinery consist of Rab GTPases, cytoskeleton regulatory proteins, annexin, myosin, and fusion proteins such as SNAREs (SNAP (soluble NSF attachment protein) receptor) [109, 114]. Once secreted, the exosomes can travel to distant sites where they fuse with the target cell releasing their contents into the recipient cells. The regulatory signals thus pass on from the parent cell to secondary cells by way of exosomes.
Over 4000 different proteins have been isolated and purified from exosomes of patient samples as well as in vitro cell lines. The protein cargo of the exosomes contains many endosomal network-associated proteins. Some of these proteins are as follows: proteins involved in exosome biogenesis, membrane trafficking, and fusion, such as Rab proteins, GTPases, tumor suppressor gene 101, and annexin; heat shock proteins (Hsp70, Hsp60, and Hsp90); cytoskeletal proteins such as myosin, actin, and tubulin; adhesion proteins such as tetraspanins (namely, CD9, CD63, CD81, and CD82); and certain signal transducers, lipid-related proteins, metabolic enzymes, and MHC [115].
The complex lipid bilayer surrounding the exosomal core is enriched in a number of lipids, such as phospholipids, including phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylinositol (PI), sphingolipids (sphingomyelin and ceramide), diglycerides, and cholesterol. The exosomes are stable in the biological fluids and cell culture media because of the rigidity offered by the lipid bilayer. The presence of PS on the outer membrane of exosomes aids in the recognition and internalization of exosomes by the recipient cell. Exosomes also function as lipid carriers, aiding in shuttling immunosuppressive lipids in the TME, and thus aiding cancer progression [115, 116]. The exosomal lipid content differs from the parent cells’ as the exosomes contain lipids not just from the plasma membrane of the origin cells, but also from the Golgi apparatus. Thus, exosomes undergo selective protein and lipid sorting [117].
The nucleic acid content of the exosomes consists of various RNAs, such as mRNA, ribosomal RNA, long noncoding RNA (lncRNA), miRNA, and some DNA. The microRNAs, which are small noncoding RNA, form the major composition of the nucleic acids and are very important in the cellular regulation at posttranscriptional levels. The miRNAs can bind to the complementary sequences in the 3′-untranslated regions of the mRNA resulting in translational regulation affecting the protein expression. As the exosome shuttles from the parent cell to the secondary recipient cell, the cargo, including the miRNAs, gets transported. These miRNAs are responsible for a number of regulatory functions related to cellular growth, differentiation, and apoptosis.
ExoCarta is an assimilation of data on exosomal cargo, identifying more than 1600 mRNA, around 800 miRNAs, and over 4000 proteins in exosomes from different species and tissues. The protein and nucleic acid contents within the exosomes represent the cell from which it originated. However, different cell types, physiological conditions, or pathological entities cause variation in the exosomal content, making the exosomal cargo specific for that particular disease state. The major function of exosomes is the transport of biological molecules containing genetic and epigenetic information to target cells. Thus, it becomes important to study exosomes with respect to the TME, since various cells in the tumor secrete and uptake exosomes, which eventually regulates the cancer development [109].
Recently, interest has been generated regarding the role of exosomes in the immune response, especially the tumor immune response. This line of thought was instigated decades ago when it was first observed that APCs utilize exosomes enriched with MHC immunomodulatory molecules for antigen presentation. Studies have shown that dendritic cell and B-cell exosomes generate a strong immunogenic response by direct or indirect T-cell activation. Immature dendritic cells secrete exosomes that are taken up by neighboring mature dendritic cells releasing the antigen-MHC complex. This effectively increases the DC response against the specific processed antigen by the DCs who have not yet encountered a pathogen [114, 118, 119]. A study by Skokos and colleagues displayed that the administration of mast cell-derived antigen-containing exosomes into naïve mice led to the maturation of immature dendritic cells against that particular antigen to elicit specific immune response [120]. One way the exosomes elicit an immune response is by carrying bioactive cytokines as cargo, along with certain inflammasome components and IL-1β. Dendritic cell exosomes contain high quantities of TNF-α, which suggests the role of exosomes in activating the innate and adaptive branches of the immune system. This effect is further amplified by the nucleic acid cargo of exosomes that are released at the site of the responsive recipient cell [121, 122]. Another important aspect of exosome functionality is their role as carriers of surface molecules and genetic information. Due to inter- and intracellular shuttling of exosomes, surface protein from one cell can be induced into the recipient transformed or untransformed cells. The proteins contained within the exosomes include oncoproteins such as MET and KRAS, which are important for tumor formation and proliferation. Horizontal transfer of these oncoproteins to neighboring and/or distant normal cells enhances the potential for tumor propagation by transforming normal cells to neoplastic cells. Exosomes, as a multimolecular messenger, mediate cell-cell communication in autocrine, paracrine, and endocrine manners. Exosomal cargo has the ability to initiate signaling responses in tumorigenic cells, thus aiding and encouraging tumor survival and advancement.
In thyroid cancer, tumor cells and the stromal inflammatory cells, together, command the composition of the extracellular milieu. The inter- and intracellular communications occur in an autocrine and paracrine manner, involving soluble mediators such as growth factors, cytokines, chemokines, and exosomes. These interactions of cancer cell mediators can regulate TME to further maintain EMT and continue dissemination of the tumor to secondary sites [123, 124]. Thus, exosomes play an important role in modulating the TME in favor of disease progression [125]. Although miRNAs secreted by tumor-derived exosomes have been explored in a number of diseases, including breast, colon, lung, pancreatic, as well as other cancers, the search for exosomes and miRNAs in thyroid cancer is in nascent stages.
Circulating miRNAs from the serum of papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC) have been studied in the past to understand their role in thyroid tumor progression. miR-146b-5p, miR-221-3p, and miR-222-3p are consistently found to be overexpressed in well-differentiated thyroid cancer as well as anaplastic thyroid cancer (ATC) [126]. Moreover, there is overexpression of circulating miRNAs, such as let-7e, miR-151, and miR-222 in the serum of papillary thyroid cancer (PTC) patients [127]. It was only recently shown that these miRNAs have been identified to be present in the PTC-derived exosomes [128]. Yu et al. further explored the role of miRNAs as biomarkers for the differential diagnosis of various thyroid cancers. They found that the expression of miR-124-3p, miR-9-3p, and miR-5691 was significantly upregulated in PTC patients, whereas there was a downregulation of miR-4701 and miR-196b-5p. When the expression patterns were compared between benign nodules and PTC patients, miR-124-3p and miR-9-3p were found to be overexpressed in PTC patients, suggesting a distinct potential signature of PTC. Contrastingly, miR-196b-5p was expressed higher in benign nodules compared to PTC [129]. Another study by Li et al. showed that serum of PTC patients had that higher expression of circulating miR-25-3p and miR-451a when compared to benign nodules, suggesting their role in PTC differential diagnosis [130]. In order to distinguish PTC and FTC patients based on the plasma exosomal content, Samsonov et al. carried out a study in 60 patients with different thyroid nodal pathologies. They demonstrated a higher expression of miR-126, miR-145, and miR-31 in PTC-derived exosomes and an upregulation of miR-21 in FTC-derived exosomes. Interestingly, reciprocal and inversely related expression patterns of miR-21-5p (miR-221-3p) and miR-181a-5p were observed in PTC and FTC, respectively [131]. Such comparative analysis provides us with factors that are useful for distinguishing PTC and FTC by noninvasive methods.
A number of in vitro and patient sample-derived studies have focused on the expression pattern of the miR-146 family, making it an important effector in thyroid cancer. The miR-146 family, consistently overexpressed in differentiated thyroid cancer and ATC, has been isolated from patient serum and tumor-derived exosomes. The family consists of two genes: miR-146a and miR-146b. Both are under the regulation of transcription factor NFκB which in turn is activated to increase the invasiveness of the tumor by inducing EMT. Overall concomitant activation of signaling pathways (NFκB and Wnt pathways) and transcription of miR-146 family genes promote the aggressiveness of thyroid cancer, making it an important target for future therapies [132, 133].
ATC is characterized by the aggressive nature of the undifferentiated cancer cells to grow rapidly and cause metastasis. Loss of tumor suppressor genes, accumulations of genetic alteration, and impairment of important signaling pathways (MAPK and PI3K signaling pathways) contribute toward the pathogenesis of the anaplastic phenotype. Recent studies attribute this dysregulation to expression of miRNAs secreted into the TME by the anaplastic cancer cells and surrounding stromal cells. MicroRNAs, such as miR-146b, miR-221, and miR-222, are upregulated in ATC as well as differentiated thyroid cancer such as PTC and FTC. However, certain distinct expressions of miRNAs are associated only with ATC. Investigation of ATC samples from ten patients by Visone et al. revealed downregulation of certain miRNAs with tumor suppressor properties by influencing p53 transcription. These miRNAs include miR-30d, miR-125b, miR-26a, and miR-30a-5p. Complementary to this study, downregulation of let-7 and miR-200 families, and an upregulation of miR-221, miR-222, miR-17-92, and miR-125a-3p, was observed. Out of these, miR-200 and miR-30 family miRNAs are known to be involved in EMT regulation. The miR-30 family also blocks autophagy by repressing Beclin1 protein and preventing formation of autophagosomes. This is important as autophagy is known to cause resistance in ATC to chemotherapy. Thus, overexpressing miR-30 in ATC is a way to increase the sensitivity of the cancer cells to chemotherapeutic drugs [132, 134,135,136]. miR-17-92 clusters consist of seven different miRNAs. This cluster is associated with the BRAFV600E mutation and is present in PTC as well as ATC. Since the presence of this cluster has been associated with the aggressiveness of ATC and various other cancers, blockage of miRNAs associated with this cluster inhibited the growth of ATC by inducing apoptosis [132, 137]. A meta-analysis of various studies conducted in 2015 suggested that there are variation and discrepancies in the miRNA expression pattern of ATC. One possible reason for such discrepancy is the drastic change in the miRNA expression profiles of the tumor cell as it transforms from differentiated to undifferentiated thyroid carcinomas [133].
Moreover, most of the abovementioned studies were performed with the circulating miRNAs. Since the composition of totally circulating miRNAs differs from the tumor-derived exosomal miRNAs, it is important to evaluate the miRNA content of ATC-secreted exosomes and compare it to other thyroid cancers.
3 Cellular Communication in the Tumor Microenvironment
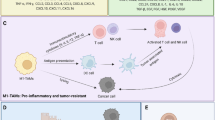
Although it is known that EMT is important for tumor cell progression and metastasis, and macrophages aid in tumor cell metastasis by inducing EMT in epithelial cells, the exact role of macrophages in thyroid cancer progression and induction of EMT in thyroid cancer cells remained understudied for quite some time. Tiwari et al. evaluated the crosstalk between macrophages and thyroid cancer cells using an in vitro model system and human thyroid cancer tissues, and reported that EMT is induced in thyroid cancer cells by pro-inflammatory macrophage secretory components. This was indicated by the enhanced expression of mesenchymal markers, and phenotypic changes, such as increased scattering and elongation of cancer cells. In addition, the migratory properties of the thyroid cancer cells under the influence of macrophages are also enhanced. They also analyzed the secretory components of macrophages, including cytokines and exosomes isolated from conditioned media, that cause phenotypic switching in thyroid cancer cells. These secretory elements activate macrophages at the tumor site, thus aiding thyroid cancer dissemination by induction of EMT in thyroid cancer cells, especially ATC. This establishes the EMT process as a basis for the metastatic propensity of ATC. Moreover, ATC cells secrete cytokines and exosomal miRNAs which aid in recruitment and activation of inflammatory cells, ripening the TME for EMT.
Hence, this mutual interaction between the inflammatory and cancer cells not only helps to decipher “EMT-associated tumor secretome” [97] but also specifies novel markers of thyroid cancer dissemination which can be targeted to suppress the metastatic potential. Overall, this indicates that the mutual interaction and crosstalk between cancer and inflammatory cells modulate the thyroid cancer phenotype – with crosstalk ultimately taking place between the tumor cells, the antigen-presenting cells, and the T cells.
M1 polarized pro-inflammatory macrophage secretory factors induce epithelial to mesenchymal transition in thyroid cancer cells as evidenced by repressed cell adhesion molecules, such as E-cadherin and β-catenin; increased expression of transcription factors such as NFκB, Twist, and Slug more prominently in ATC than PTC; a halt in proliferation; enhanced migration of thyroid cancer cells; and a change in morphology by acquiring mesenchymal phenotype as observed with cells becoming elongated and scattered indicating gain of mobility.
Reciprocal interaction between ATC cells and pro-inflammatory macrophages through chemotactic and secretory mediators (cytokines and exosomal miRNA) defines metastatic phenotype that is defined by pro-inflammatory cytokines/chemokines such as TNF-α, TGFβ, IL-6, IL-8 and IL-1, as well as chemotactic factors like MCP-1/2, MIP-1, and eotaxin-2, along with reactive oxygen species, present within the thyroid TME causing alteration in thyroid cancer cell phenotype; activated macrophage-secreted exosomes induce EMT in thyroid cancer cells – modulation of EM markers, change in morphology to mesenchymal phenotype, and decrease in proliferation; ATC cell-secreted exosomes activate tumor-associated macrophages; ATC cell-secreted exosomes contain a distinct group of tumor suppressive miRNAs that are downregulated.
Macrophage plasticity provides a conducive pro-inflammatory environment in thyroid cancer for phenotypic transition as observed in the human tissues by the presence of a mixed population of TAMs, present in ATC and malignant PTC, with a higher infiltration and greater interaction with tumor cells in ATC; pro-inflammatory M1 polarized macrophages infiltrate anaplastic as well as malignant PTC, which provides a niche for inducing epithelial to mesenchymal transition and promoting metastasis.
4 Future Directions
There are several other requisite features to any solid tumor including thyroid cancer. Hanahan and Weinberg have listed these unique traits of tumor cells which enable them to have sustained growth and metastasis. These well-known “hallmarks of cancer” include sustained proliferation, evasion of apoptosis and suppression, growth promotion, angiogenesis, invasion, and metastasis capabilities. Tumor formation is a multistep process resulting from the simultaneous occurrence of the above processes. In most cases, the initiation of the tumorigenesis is believed to be due to the acquisition of genetic mutations. The genetic alteration leads to transformation of a benign cell to malignant cells, leading to aggressive cancer formation (Hanahan & Weinberg, 2011). As such, looking at how these processes are impacted by alterations within the TME by its interacting components can shape future studies within this field.
4.1 Novel Targets of Therapeutic Intervention
The thyroid tumor microenvironment secretome offers early markers and putative targets for thyroid cancer metastasis and dissemination. The thyroid TME is composed of thyroid cancer cells in addition to stromal cells consisting of macrophages, fibroblast, mast cells, PMNs, and stem cells. Chronic inflammation is a key initiator of thyroid cancer. This was determined by a strong association found between the presence of preexisting inflammatory benign thyroid disease and the incidence of cancer in later years. Moreover, histopathological analysis of thyroid cancer has shown a dense infiltration of innate and adaptive immune cells surrounding, as well as within, thyroid cancer. This clearly indicates the presence of infiltrating lymphocytes and macrophages in thyroid tumors.
The spectrum of infiltrating stromal and immune cells in anaplastic thyroid cancer (ATC) differs from that in DTC. There is a higher number of T cells in papillary thyroid cancer (PTC) and a greater infiltration of TAMs in ATC. In either case, lymphocytic infiltration of thyroid cancer has been associated with poor disease outcomes. Studies conducted in breast, ovarian, and hepatocellular carcinoma have examined the role of TAMs in tumor proliferation and progression. TAMs release certain chemokines and cytokines which cause corresponding changes in the cell. These macrophages are usually in the form of inactive blood monocytes. They become activated in the presence of specific signals from the surrounding microenvironment. Under normal conditions, during the activation, monocytes are differentiated into either M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotype, depending on the type of signals. Macrophages in general have a complex transcriptome, and they are polarized to specific subtypes based on the specific function they have to perform. In the case of the TME, they are needed to accomplish the vital task of supporting the tumor [101, 138, 139]. Given the relationship between inflammation and thyroid cancer, further investigations into the interaction between M1 polarized pro-inflammatory macrophages and thyroid cancer cells, especially the more resilient ATC, and methodologies by which to remodel the macrophage polarization or the level of inflammation within the TME will have major treatment relevance.
TAMs infiltrate the tumor site and get activated by the secretory factors present in the TME. The activation and polarization of macrophages to a pro-inflammatory phenotype lends further support to thyroid cancer cells for proliferation and dissemination. The Tiwari lab has demonstrated that the secretory mediators of ATC cell lines, consisting of chemotactic factors and pro-inflammatory cytokines, recruit and activate the THP-1 monocytes to macrophages. Thus, cell-cell communication takes place between the anaplastic cancer cells and macrophages, which stimulates the macrophages to perform pro-inflammatory functions. However, such cellular interactions are not unidirectional. There exists reciprocal communication between the cancer and stromal cells in the TME as witnessed in a number of cancers [140, 141].
This crosstalk is facilitated by a number of secretory factors, including soluble mediators – cytokines/chemokines, growth factors, and exosomes with their miRNA cargo. Through examination of the secretome of the thyroid TME, and exploration of the mediators of cell-cell communication, the Tiwari lab found that M1 polarized macrophages expressed higher amount of IL-6 and iNOS. Additionally, the secretory profile of these activated macrophages was distinct from the un-activated monocytes. These activated M1 macrophages secreted chemotactic and pro-inflammatory cytokines such as IL-6, MIP-1α/β, TNF-α, IL-1β, ICAM-1, GMCSF, IL-8, IL-16, TGF-β1, RANTES, and MCP-1/2, further demonstrating M1 polarization. Similarly, cytokine profiles of thyroid cancer cells revealed certain distinct secretory profiles of each histological type of thyroid cancer. PTC cell lines secreted high amounts of IL-6, IL-8, and TIMP-2. IL-6 is secreted by the PTC in the range of 1530–1560 pg/ml. Follicular cancer cell lines secreted IL-6 (about 1240 pg/ml), IL-8, MIP-1α, RANTES, TIMP2, and very high quantities of TGFβ (about 800 pg/ml). Anaplastic cancer cell lines on the other hand secreted high levels of chemotactic and pro-inflammatory cytokines consisting of IL-1α, TGFβ (about 750 pg/ml), MIP-1α/β, eotaxin 2, MCP-1, and PDGF-BB. Most of these cytokines are usually in an undetectable range in healthy individuals. Only in cases of inflammation, injury, or disease does the cytokine levels modulate in accordance with the cellular responses. Hence, the detection of cytokines above normal physiological levels suggests some pathology offering clinicians and researchers the means to monitor disease occurrence and progression. The physiological level of IL-6 is less than 10 pg/ml, while TNF-α and TGFβ are less than 100 pg/ml, so if these pro-inflammatory mediators are extremely high in thyroid cancer patients, they could be important tools in diagnosis and prognosis.
The Tiwari lab also observed the secretion of a number of other pro-inflammatory cytokines by tumor cells to attract and recruit pro-tumorigenic immune cells (Table 1). The secretory profile we obtained from the thyroid cancer cells and the macrophages clearly suggests the presence of pro-inflammatory chemokines and cytokines in the TME. All of these cytokines have major implications for regulating the signaling cascade in cancer cells to promote tumorigenesis. The distinct cytokine profiles of papillary cancer and ATC denote the variation in the cellular makeup of each histological type of thyroid cancer. Thus, one way that the cells communicate with each other is through soluble mediators such as cytokines.
Other means of cellular crosstalk include reactive oxygen species and exosomes. Oxidative stress generates reactive oxygen species, which cause recruitment of infiltrating immune cells, as well as damage the cellular DNA. The latter results in initiation of a repair mechanism which leads to further accumulation of genetic mutations and activation of oncogenic pathways, promoting tumorigenesis.
Exosomes are being developed as cancer therapy targets and drug delivery systems. We have seen that exosomes and the cargo within play a critical role in tumor progression. Hence, to target this aspect, strategies are under development to control the release of exosomes from the tumor cells. This is thought to curb the signals that promote tumor formation. Moreover, dendritic cell exosomes, also known as dexosomes, are being developed as immunotherapeutic agents, whereby dexosomes are pulsed with tumor-derived peptides to activate the cytotoxic T-cell response against cancer. Lastly, due to good biodistribution, biocompatibility, and biostability, exosomes are being considered as drug carriers to deliver short interfering RNA (siRNA) and active drugs like paclitaxel to the target cells [109, 117, 154].
Thus, exosomes and their cargo regulating EMT have huge implications in finding putative therapeutic targets for cancer progression and metastasis. Exosomes are considered to be diagnostic and prognostic biomarkers due to the uniqueness of their cargo, particularly miRNA (depending on the pathology). Differences have been noted in the cargo profile of exosomes obtained from serum of healthy versus cancer patient, further emphasizing the use of exosomes as a prognostic marker. Higher levels of miR-195, miR-145, and let-7a in various cancers, followed by decrease in their expression postoperatively, suggest their role as prognostic marker. Additionally, the stability offered by the lipid bilayer makes the exosomes resistant to degradation under non-physiological conditions. This makes exosomes an easy tool for disease screening or using it as a noninvasive biomarker obtained from bodily fluids [155].
The Tiwari lab observed that anaplastic cancer cells under the influence of activated macrophage conditioned media generated higher levels of ROS. This suggests an unexplored role of reactive oxygen species generated from TAMs in enhancing the proliferative and malignant propensity of ATC cells. Conversely, THP-1 monocytes, as well as macrophages, treated with anaplastic cancer cell conditioned media generated higher levels of ROS. Such a reciprocal induction of ROS accumulation between ATC cells and TAMs, which has never been observed earlier, elucidates that ATC cells generate ROS which aids in tumor progression and recruitment of macrophages. This effect of ROS along with the secreted chemotactic cytokines by ATC indicates an additive effect in recruiting the macrophages at the tumor site. Previous work by our group has characterized the miRNA content of activated THP-1 cells using the immunopathology pathway miRNA PCR arrays. The Tiwari lab went one step further and characterized the miRNA content of the exosomes secreted by the thyroid cancer cells.
Researchers are now concentrating on the miRNA functionality for diagnosis and treatment modalities. However, most of the studies performed are focused on the circulating miRNA. The exploration of exosomal miRNA is still in its budding stage. Exosomes can be isolated from bodily fluids in a noninvasive way, and hence can become a very important diagnostic marker based on their content. The exosomal cargo predominantly consists of miRNAs, which are highly conserved noncoding RNA that regulate the posttranscriptional or translational protein expression. Distinct expression of miRNA from ATC-secreted exosomes when compared to PTC- or FTC-secreted exosomes can be observed. Comparative analysis indicated that eight miRNAs were downregulated in FTC-secreted exosomes compared to papillary. On the other hand, ten miRNAs were downregulated in anaplastic 8505C-secreted exosomes compared to papillary BCPAP, whereas three miRNAs were downregulated and four miRNAs upregulated in anaplastic 8505C-secreted exosomes in comparison to follicular CGTHW1. The specific miRNA, miR-30, inhibits the TGFβ1-induced EMT by downregulating transcription factor Snail [156], and interestingly an upregulation of this miRNA was observed in 8505C compared to CGTHW1-secreted exosomes. Another definite miRNA, miR-155, enhances cellular proliferation [128, 157], which was positively correlated in 8505C-secreted exosomes expressing higher levels of this miRNA compared to CGTHW1-secreted exosomes. Increased expression of miR-30c and miR-155 in 8505C-secreted exosomes suggests their imperative role in enhanced growth and dissemination of ATC.
An important miRNA that is considered a cancer biomarker is miR-21-5p. It is associated with an increase in cellular proliferation in aggressive cancer [158,159,160]. However, contrary to published studies, we observed a downregulation of exosomal miR-21-5p in CGTHW1 and 8505C in comparison to BCPAP. Another miRNA, miR-138, which is known to suppress cancer metastasis and invasion [161, 162], was downregulated in CGTHW1 and 8505C when compared to BCPAP-secreted exosomes. The specific microRNA, miR-26, is associated with different biological processes pertaining to gene regulation. miR-26 is supposed to possess tumor suppressive activity [163], and its downregulation is associated with resistance to chemotherapy drugs [158]. Downregulation of this miRNA as observed in 8505C opposes apoptosis and induces tumorigenesis in ATC.
Another such miR-125-5p functions as oncomiR in a few cancers and as a tumor suppressor in a number of solid tumors including thyroid cancer. An earlier study by Visone and group indicated that an overexpression of this miRNA led to inhibition of cellular growth [136]. Moreover, miR-125b is associated with repression of migration and invasion of ATC by targeting PIK3CD via PI3K/Akt/mTOR pathway [164]. This suggests a tumor suppressive action of miR-125b. The chemosensitivity to cisplatin is enhanced in osteosarcoma with overexpression of miR-125b, indicating that downregulation of this miRNA may be associated with resistance to chemotherapeutics, as observed in aggressive and malignant cancers. This tumor suppressive miRNA was downregulated in our ATC cell line 8505C-secreted exosomes in comparison to BCPAP.
Another set of tumor suppressive miRNAs consist of miR-148a and miR-152-3p, belonging to the miR-148/152 family. Among the miRNAs from this family, miR-152 is known to act as tumor suppressor by targeting PIK3CA [165], whereas miR-148a is associated with inhibition of cancer stemness and self-renewal ability of the cancer cells [166, 167]. Both these miRNAs are downregulated in 8505C-secreted exosomes compared to other thyroid cancer cell-secreted exosomes. Downregulation of this family of miRNAs promotes tumor growth and proliferation in anaplastic cancer cells. Previous studies have shown that downregulation of miR-191 in medulloblastoma, acute myelogenous leukemia, and melanoma is associated with poor prognosis. Overexpression of miR-191 in thyroid follicular adenoma and carcinoma is associated with decreased cellular proliferation and migration by targeting CDK6. Earlier studies have shown a downregulation of this miRNA in FTC but not in PTC or ATC [168]. We observed a downregulation of miR-191 in anaplastic cancer cell line-secreted exosomes compared to papillary cancer cell line-secreted exosomes. Thus, our group has identified a distinct set of tumor suppressive miRNAs consisting of miR-125b, miR-138, miR-148a, miR-152, miR-191, and miR-26b, which are downregulated in ATC cell-secreted exosomes. This distinct miRNA profile of thyroid cancer is proposed to influence the metastatic proclivity/tendency of the cancer as observed here in ATC. A number of these miRNAs, such as miR-146a, miR-21-5p, and miR-138-5p, were upregulated in activated macrophage-derived exosomes. Moreover, miR-146a and miR-132-3p also promote the activation of monocytes [169].
Different biological functions are associated with the miRNAs. The cells in TME secrete exosomes that are shuttled from primary to secondary recipient cells. They carry within them these functional miRNAs, which are transcribed and translated to biologically relevant proteins that regulate cellular processes. This implies that cellular crosstalk is mediated by soluble mediators and exosomes, ultimately promoting tumorigenesis. Previous studies have focused on circulating or tissue miRNAs associated with thyroid cancers, generally in PTC. However, the exploration of miRNA cargo of exosomes secreted from ATC cells is still in its nascent stage. Previous studies have revealed the profile and functionality (to some extent) of the deregulated miRNAs in ATC tissues [132, 170]. The three major families of miRNAs downregulated in ATC are miR-200 family, miR-30 family, and let-7 family. In our comparative analysis between the various thyroid cancer-secreted exosomal miRNAs, we did not observe any variation in expression profile of miR-200 family or let-7 family. Common miRNAs upregulated in ATC tissues consist of miR-146, miR-221/222, and cluster miR-17-92 [132, 136, 170]. These miRNAs were not observed to be upregulated in ATC-secreted exosomes compared to other thyroid cancers. Thus, we can say that the profile of miRNAs obtained from ATC-secreted exosomes can be different from that obtained from the tissue.
The pro-tumorigenic functions of the exosome-derived miRNAs, through the regulation of genes in the recipient cells, have recently started to gain research interest. The role of exosomes as a drug delivery system, and for diagnostic purposes, has further added to their clinical relevance. However, only a handful of studies have examined the miRNA profile of circulating tumor-derived exosomes in FTC or PTC [131]. Exosomal miRNA cargo is understudied in ATC. In this study, we have come across a group of miRNA present in the exosomes secreted by ATC cells that have tumor suppressive effects. The fact that these miRNAs are downregulated suggests a profile of a huge set of genes that are regulated by these miRNAs, contributing to the metastatic phenotype in ATC.
The studies here are a clear indication that phenotype is regulated by epigenetic phenomenon of which miRNAs constitute a major cargo. We have defined the ATC phenotype based on miRNAs – miR-125b, miR-138, miR-148a, miR-152, miR-191, and miR-26b – that play a functional role in suppressing the ATC phenotype. Their downregulation paves the way for procurement of metastatic ATC phenotype, and as such, can be considered as “tumor suppressors.” Their biological function and specificity to ATC, however, remains to be determined. The other mode to establish whether PTC to ATC phenotype is linked is to examine the presence and gradual disappearance of these markers in the serum of patients. The steady disappearance of these miRNA from the serum designates them as a transition biomarker for early detection of ATC phenotype, and should be pursued further.
References
Egeblad, M., Rasch, M. G., & Weaver, V. M. (2010). Dynamic interplay between the collagen scaffold and tumor evolution. Current Opinion in Cell Biology, 22, 697–706.
Friedl, P., & Zallen, J. A. (2010). Dynamics of cell–cell and cell–matrix interactions in morphogenesis, regeneration and cancer. Current Opinion in Cell Biology, 22, 557–559.
Xing, M. (2013). Molecular pathogenesis and mechanisms of thyroid cancer. Nature Reviews Cancer, 13, 184–199.
Krizman, D. B., Pathak, S., Samaan, N. A., & Hickey, R. C. (1987). Genetic instability in fibroblasts of patients with thyroid cancers (TC). International Journal of Cancer, 39, 179–181.
Eggo, M. C., Hopkins, J. M., Franklyn, J. A., Johnson, G. D., Sanders, D. S., & Sheppard, M. C. (1995). Expression of fibroblast growth factors in thyroid cancer. The Journal of Clinical Endocrinology & Metabolism, 80, 1006–1011.
Jolly, L. A., Novitskiy, S., Owens, P., Massoll, N., Cheng, N., Fang, W., Moses, H. L., & Franco, A. T. (2016). Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by Braf V600E and Pten loss. Cancer Research, 76, 1804–1813.
Surowiak, P., Murawa, D., Materna, V., Maciejczyk, A., Pudelko, M., Ciesla, S., Breborowicz, J., Murawa, P., Zabel, M., Dietel, M., & Lage, H. (2007). Occurrence of stromal myofibroblasts in the invasive ductal breast cancer tissue is an unfavourable prognostic factor. Anticancer Research, 27, 2917–2924.
Hida, K., Ohga, N., Akiyama, K., Maishi, N., & Hida, Y. (2013). Heterogeneity of tumor endothelial cells. Cancer Science, 104, 1391–1395.
Huang, Z., Wu, T., Liu, A. Y., Ouyang, G., Huang, Z., Wu, T., Yi Liu, A., & Ouyang, G. (2015). Differentiation and transdifferentiation potentials of cancer stem cells. Oncotarget, 6, 39550–39563.
Mani, S. A., Guo, W., Liao, M.-J., Eaton, E. N., Ayyanan, A., Zhou, A. Y., Brooks, M., Reinhard, F., Zhang, C. C., Shipitsin, M., Campbell, L. L., Polyak, K., Brisken, C., Yang, J., & Weinberg, R. A. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell, 133, 704–715.
NK cells – need to find.
Hargadon, K. M., Bishop, J. D., Brandt, J. P., Hand, Z. C., Ararso, Y. T., & Forrest, O. A. (2015). Melanoma-derived factors alter the maturation and activation of differentiated tissue-resident dendritic cells. Immunology and Cell Biology, 94(1), 24–38.
Laoui, D., Keirsse, J., Morias, Y., et al. (2016). The tumor microenvironment harbours ontogenically distinct dendritic cell populations with opposing effects on tumor immunity. Nature Communications, 7, 13720.
Ma, Y., Hwang, R., Logsdon, C., & Ullrich, S. (2013). Dynamic mast cell-stromal cell interactions promote growth of pancreatic cancer. Microenvironment and Immunology, 73(13), 3927–3937.
Komi, D., & Redegeld, F. (2019). Role of mast cells in shaping the tumor microenvironment. Clinical Reviews in Allergy and Immunology, 58, 313–325.
Melillo, R. M., Guarino, V., Avilla, E., Galdiero, M. R., Liotti, F., Prevete, N., Rossi, F. W., Basolo, F., Ugolini, C., de Paulis, A., Santoro, M., & Marone, G. (2010). Mast cells have a protumorigenic role in human thyroid cancer. Oncogene, 29, 6203–6215.
Imam, S., Paparodis, R., Sharma, D., & Jaume, J. C. (2014). Lymphocytic profiling in thyroid cancer provides clues for failure of tumor immunity. Endocrine-Related Cancer, 21, 505–516.
Hashimoto, M., Kamphorst, A. O., Im, S. J., Kissick, H. T., Pillai, R. N., Ramalingam, S. S., Araki, K., & Ahmed, R. (2018). CD8 T cell exhaustion in chronic infection and cancer: Opportunities for interventions. Annual Review of Medicine, 69(1), 301–318.
Perica, K., Varela, J. C., Oelke, M., & Schneck, J. (2015). Adoptive T cell immunotherapy for cancer. Rambam Maimonides Medical Journal, 6(1), e0004.
Song, Q., Shi, F., Adair, M., Chang, H., Guan, X., Zhao, Y., Li, Y., Wu, G., & Wu, J. (2019). Cell counts, rather than proportion, of CD8/PD-1 tumor-infiltrating lymphocytes in a tumor microenvironment associated with pathological characteristics of chinese invasive ductal breast cancer. Journal of Immunology Research, 1–8.
Verma, V., Shrimali, R. K., Ahmad, S., et al. (2019). PD-1 blockade in subprime CD8 cells induces dysfunctional PD-1+-CD38hi cells and anti-PD-1 resistances. Nature Immunology, 20, 1231–1243.
He, Y., Cao, J., Zhao, C., Li, X., Zhou, C., & Hirsch, F. R. (2018). TIM-3, a promising target for cancer immunotherapy. OncoTargets and Therapy, 11, 7005–7009.
Hao, N.-B., Lü, M.-H., Fan, Y.-H., Cao, Y.-L., Zhang, Z.-R., & Yang, S.-M. (2012). Macrophages in tumor microenvironments and the progression of tumors. Clinical and Developmental Immunology, 2012, 1–11.
Sica, A., & Mantovani, A. (2012). Macrophage plasticity and polarization: In vivo veritas. The Journal of Clinical Investigation, 122, 787–795.
Rőszer, T., & szer T & s. (2015). Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators of Inflammation, 2015, 816460.
Mantovani, A., Sozzani, S., Locati, M., Allavena, P., & Sica, A. (2002). Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology, 23, 549–555.
Biswas, S. K., Sica, A., & Lewis, C. E. (2008). Plasticity of macrophage function during tumor progression: Regulation by distinct molecular mechanisms. The Journal of Immunology, 180, 2011–2017.
Balkwill, F., Charles, K. A., & Mantovani, A. (2005). Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell, 7, 211–217.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420, 860–867.
Lewis, C. E., & Pollard, J. W. (2006). Distinct role of macrophages in different tumor microenvironments. Cancer Research, 66, 605–612.
Gordon, S., & Taylor, P. R. (2005). Monocyte and macrophage heterogeneity. Nature Reviews Immunology, 5, 953–964.
Szebeni, G. J., Vizler, C., Kitajka, K., & Puskas, L. G. (2017). Inflammation and cancer: Extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Mediators of Inflammation, 2017, 9294018.
Leek, R. D., Lewis, C. E., Whitehouse, R., Greenall, M., Clarke, J., & Harris, A. L. (1996). Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Research, 56, 4625–4629.
Zhang, Q., Liu, L., Gong, C., Shi, H., Zeng, Y., Wang, X., Zhao, Y., & Wei, Y. (2012). Prognostic significance of tumor-associated macrophages in solid tumor: A meta-analysis of the literature M. O. Hoque, ed. PLoS One, 7, e50946.
Coussens, L. M., & Werb, Z. (2001). Inflammatory cells and cancer. Journal of Experimental Medicine, 193.
Pikarsky, E., Porat, R. M., Stein, I., Abramovitch, R., Amit, S., Kasem, S., Gutkovich-Pyest, E., Urieli-Shoval, S., Galun, E., & Ben-Neriah, Y. (2004). NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature, 431, 461–466.
Greten, F. R., Eckmann, L., Greten, T. F., Park, J. M., Li, Z.-W., Egan, L. J., Kagnoff, M. F., & Karin, M. (2004). IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell, 118, 285–296.
Chanmee, T., Ontong, P., Konno, K., & Itano, N. (2014). Tumor-associated macrophages as major players in the tumor microenvironment. Cancers, 6, 1670–1690.
Auffray, C., Fogg, D., Garfa, M., Elain, G., Join-Lambert, O., Kayal, S., Sarnacki, S., Cumano, A., Lauvau, G., & Geissmann, F. (2007). Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science, 317, 666–670.
Standring, S., & Berkovitz, B. (2005). Thyroid gland. In S. Standring, H. Ellis, J. C. Healey, D. Johnson, & A. Williams (Eds.), Gray’s anatomy (pp. 560–564). Churchill Livingstone.
Wondisford, F. E., & Radovick, S. (2009). Clinical management of thyroid disease. Saunders/Elsevier.
Bizhanova, A., & Kopp, P. (2009). The sodium-iodide symporter NIS and Pendrin in iodide homeostasis of the thyroid. Endocrinology, 150, 1084–1090.
Fancy, T., Gallagher, D., III, & Hornig, J. D. (2010). Surgical anatomy of the thyroid and parathyroid glands. Otolaryngologic Clinics of NA, 43, 221–227.
Khatawkar, A. V., & Awati, S. M. (2015). Thyroid gland – historical aspects, embryology, anatomy and physiology. International Archives of Integrated Medicine., 2, 165–171.
Hazard, J. B. (1977). The C cells (parafollicular cells) of the thyroid gland and medullary thyroid carcinoma. A review. The American Journal of Pathology, 88, 213–250.
Parkin, D. M., Bray, F., Ferlay, J., & Pisani, P. (2005). Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians, 55, 74–108.
Jemal, A., Siegel, R., Ward, E., Hao, Y., Xu, J., & Thun, M. J. (2009). Cancer statistics, 2009. CA: A Cancer Journal for Clinicians, 59, 225–249.
Aschebrook-Kilfoy, B., Schechter, R. B., Shih, Y.-C. T., Kaplan, E. L., Chiu, B. C.-H., Angelos, P., & Grogan, R. H. (2013). The clinical and economic burden of a sustained increase in thyroid cancer incidence. Cancer Epidemiology Biomarkers & Prevention, 22, 1252–1259.
American Cancer Society. (2020). Cancer facts and figures 2020, Atlanta.
Gimm, O., & Dralle, H. (2001). Differentiated thyroid carcinoma. In R. Holzheimer & J. Mannick (Eds.), Surgical treatment: Evidence-based and problem-oriented. Zuckschwerdt: W. Zuckschwerdt Verlag GmbH.
Morris, L. G. T., Riaz, N., Desrichard, A., Şenbabaoğlu, Y., Hakimi, A. A., Makarov, V., Reis-Filho, J. S., Chan, T. A., Morris, L. G. T., Riaz, N., Desrichard, A., Şenbabaoğlu, Y., Hakimi, A. A., Makarov, V., Reis-Filho, J. S., & Chan, T. A. (2016). Pan-cancer analysis of intratumor heterogeneity as a prognostic determinant of survival. Oncotarget, 7, 10051–10063.
Strickaert, A., Saiselet, M., Dom, G., De Deken, X., Dumont, J. E., Feron, O., Sonveaux, P., & Maenhaut, C. (2016). Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene, 1–6.
Makazlieva, T., Vaskova, O., & Majstorov, V. (2016). Etiopathogenesis of differentiated thyroid carcinomas. Open Access Macedonian Journal of Medical Sciences, 4, 517–522.
Landa, I., Ibrahimpasic, T., Boucai, L., Sinha, R., Knauf, J. A., Shah, R. H., Dogan, S., Ricarte-Filho, J. C., Krishnamoorthy, G. P., Xu, B., Schultz, N., Berger, M. F., Sander, C., Taylor, B. S., Ghossein, R., Ganly, I., & Fagin, J. A. (2016). Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. Journal of Clinical Investigation, 126, 1052–1066.
Le Pennec, S., Konopka, T., Gacquer, D., Fimereli, D., Tarabichi, M., Tomás, G., Savagner, F., Decaussin-Petrucci, M., Trésallet, C., Andry, G., Larsimont, D., Detours, V., & Maenhaut, C. (2015). Intratumor heterogeneity and clonal evolution in an aggressive papillary thyroid cancer and matched metastases. Endocrine-Related Cancer., 22, 205–216.
O’Neill, J. P., & Shaha, A. R. (2013). Anaplastic thyroid cancer. Oral Oncology., 49, 702–706.
Smallridge, R. C., & Copland, J. A. (2010). Anaplastic thyroid carcinoma: Pathogenesis and emerging therapies. Clinical Oncology (Royal College of Radiologists (Great Britain)), 22, 486–497.
Quiros, R. M., Ding, H. G., Gattuso, P., Prinz, R. A., & Xu, X. (2005). Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF andp53 mutations. Cancer, 103, 2261–2268.
Haghpanah, V., Fallah, P., Naderi, M., Tavakoli, R., Soleimani, M., & Larijani, B. (2016). Cancer stem-like cell behavior in anaplastic thyroid cancer: A challenging dilemma. Life Sciences, 146, 34–39.
Xu, B., & Ghossein, R. (2016). Genomic landscape of poorly differentiated and anaplastic thyroid carcinoma. Endocrine Pathology., 27, 205–212.
Sykorova, V., Dvorakova, S., Vcelak, J., Vaclavikova, E., Halkova, T., Kodetova, D., Lastuvka, P., Betka, J., Vlcek, P., Reboun, M., Katra, R., & Bendlova, B. (2015). Search for new genetic biomarkers in poorly differentiated and anaplastic thyroid carcinomas using next generation sequencing. Anticancer Research, 35, 2029–2036.
Colotta, F., Allavena, P., Sica, A., Garlanda, C., & Mantovani, A. (2009). Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis, 30, 1073–1081.
Daoud, S. A., Esmail, R. S. E. N., Hareedy, A. A., & Khalil, A. (2015). Stromal modulation and its role in the diagnosis of papillary patterned thyroid lesions. Asian Pacific Journal of Cancer Prevention: APJCP., 16, 3307–3312.
San Martin, R., Barron, D. A., Tuxhorn, J. A., Ressler, S. J., Hayward, S. W., Shen, X., Laucirica, R., Wheeler, T. M., Gutierrez, C., Ayala, G. E., Ittmann, M., & Rowley, D. R. (2014). Recruitment of CD34(+) fibroblasts in tumor-associated reactive stroma: The reactive microvasculature hypothesis. The American Journal of Pathology., 184, 1860–1870.
Radu, T. G., Ciurea, M. E., Mogoantă, S. Ş., Busuioc, C. J., Grosu, F., Ţenovici, M., Petrescu, I. O., & Vladu, I. M. (2016). Papillary thyroid cancer stroma – Histological and immunohistochemical study. Romanian Journal of Morphology and Embryology., 57, 801–809.
Rajoria, S., Suriano, R., Wilson, Y. L., George, A. L., Geliebter, J., Schantz, S. P., & Tiwari, R. K. (2011). Estradiol-mediated tumor neo-vascularization. Oncology Letters., 2, 453–457.
Kamat, A., Rajoria, S., George, A., Suriano, R., Shanmugam, A., Megwalu, U., Prakash, P. B., Tiwari, R., & Schantz, S. (2011). Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Archives of Otolaryngology–Head & Neck Surgery., 137, 1146.
Balkwill, F. R., Capasso, M., & Hagemann, T. (2012). The tumor microenvironment at a glance. Journal of Cell Science., 125, 5591–5596.
Ma, R., Bonnefond, S., Morshed, S. A., Latif, R., & Davies, T. F. (2014). Stemness is derived from thyroid cancer cells. Frontiers in Endocrinology., 5, 114.
Shimamura, M., Nagayama, Y., Matsuse, M., Yamashita, S., & Mitsutake, N. (2014). Analysis of multiple markers for cancer stem-like cells in human thyroid carcinoma cell lines. Endocrine Journal., 61, 481–490.
Nagayama, Y., Shimamura, M., & Mitsutake, N. (2016). Cancer stem cells in the thyroid. Frontiers in Endocrinology., 7, 20.
Vicari, L., Colarossi, C., Giuffrida, D., De Maria, R., & Memeo, L. (2016). Cancer stem cells as a potential therapeutic target in thyroid carcinoma. Oncology Letters., 12, 2254–2260.
Yano, Y., Shibuya, H., Kitagawa, W., Nagahama, M., Sugino, K., Ito, K., & Ito, K. (2007). Recent outcome of graves’ disease patients with papillary thyroid cancer. European Journal of Endocrinology., 157, 325–329.
Gogali, F., Paterakis, G., Rassidakis, G. Z., Liakou, C. I., & Liapi, C. (2013). CD3-CD16-CD56bright Immunoregulatory NK cells are increased in the tumor microenvironment and inversely correlate with advanced stages in patients with papillary thyroid cancer. Thyroid: Official Journal of the American Thyroid Association., 23, 7–9.
Wennerberg, E., Pfefferle, A., Ekblad, L., Yoshimoto, Y., Kremer, V., Kaminskyy, V. O., Juhlin, C. C., Höög, A., Bodin, I., Svjatoha, V., Larsson, C., Zedenius, J., Wennerberg, J., & Lundqvist, A. (2014). Human anaplastic thyroid carcinoma cells are sensitive to NK cell-mediated lysis via ULBP2/5/6 and chemoattract NK cells. Clinical Cancer Research., 20, 5733–5744.
Tsuge, K., Takeda, H., Kawada, S., Maeda, K., & Yamakawa, M. (2005). Characterization of dendritic cells in differentiated thyroid cancer. The Journal of Pathology., 205, 565–576.
Ugolini, C., Basolo, F., Proietti, A., Vitti, P., Elisei, R., Miccoli, P., & Toniolo, A. (2007). Lymphocyte and immature dendritic cell infiltrates in differentiated, poorly differentiated, and undifferentiated thyroid carcinoma. Thyroid, 17, 389–393.
Proietti, A., Ugolini, C., Melillo, R. M., Crisman, G., Elisei, R., Santoro, M., Minuto, M., Vitti, P., Miccoli, P., & Basolo, F. (2011). Higher Intratumoral expression of CD1a, Tryptase, and CD68 in a follicular variant of papillary thyroid carcinoma compared to adenomas: Correlation with clinical and pathological parameters. Thyroid, 21, 1209–1215.
Visciano, C., Liotti, F., Prevete, N., & Cali’ G, Franco R, Collina F, de Paulis A, Marone G, Santoro M & Melillo RM. (2015). Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8–Akt–slug pathway. Oncogene, 34, 5175–5186.
French, J. D., Weber, Z. J., Fretwell, D. L., Said, S., Klopper, J. P., & Haugen, B. R. (2010). Tumor- associated lymphocytes and increased FoxP3 + regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. The Journal of Clinical Endocrinology & Metabolism., 95, 2325–2333.
French, J. D., Kotnis, G. R., Said, S., Raeburn, C. D., McIntyre, R. C., Klopper, J. P., & Haugen, B. R. (2012). Programmed Death-1 + T cells and regulatory T cells are enriched in tumor- involved lymph nodes and associated with aggressive features in papillary thyroid cancer. The Journal of Clinical Endocrinology & Metabolism., 97, E934–E943.
Gogali, F., Paterakis, G., Rassidakis, G. Z., Kaltsas, G., Liakou, C. I., Gousis, P., Neonakis, E., Manoussakis, M. N., & Liapi, C. (2012). Phenotypical analysis of lymphocytes with suppressive and regulatory properties (Tregs) and NK cells in the papillary carcinoma of thyroid. The Journal of Clinical Endocrinology & Metabolism., 97, 1474–1482.
Angell, T. E., Lechner, M. G., Jang, J. K., Correa, A. J., LoPresti, J. S., & Epstein, A. L. (2014). BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid: Official Journal of the American Thyroid Association., 24, 1385–1393.
Cunha, L. L., Marcello, M. A., Nonogaki, S., Morari, E. C., Soares, F. A., Vassallo, J., & Ward, L. S. (2015). CD8+ tumour-infiltrating lymphocytes and COX2 expression may predict relapse in differentiated thyroid cancer. Clinical Endocrinology., 83, 246–253.
Qing, W., Fang, W.-Y., Ye, L., Shen, L.-Y., Zhang, X.-F., Fei, X.-C., Chen, X., Wang, W.-Q., Li, X.-Y., Xiao, J.-C., & Ning, G. (2012). Density of tumor-associated macrophages correlates with lymph node metastasis in papillary thyroid carcinoma. Thyroid, 22, 905–910.
Jung, K. Y., Cho, S. W., Kim, Y. A., Kim, D., Oh, B.-C., Park, D. J., & Park, Y. J. (2015). Cancers with higher density of tumor-associated macrophages were associated with poor survival rates. Journal of Pathology and Translational Medicine., 49, 318–324.
Ryder, M., Ghossein, R. A., Ricarte-Filho, J. C. M., Knauf, J. A., & Fagin, J. A. (2008). Increased density of tumor-associated macrophages is associated with decreased survival in advanced thyroid cancer. Endocrine-Related Cancer., 15, 1069–1074.
Ryder, M., Gild, M., Hohl, T. M., Pamer, E., Knauf, J., Ghossein, R., Joyce, J. A., & Fagin, J. A. (2013). Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression M. Ludgate, ed. PLoS One, 8, e54302.
Cunha, L. L., Morari, E. C., Guihen, A. C. T., Razolli, D., Gerhard, R., Nonogaki, S., Soares, F. A., Vassallo, J., & Ward, L. S. (2012). Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clinical Endocrinology, 77, 918–925.
Caillou, B., Talbot, M., Weyemi, U., Pioche-Durieu, C., Al Ghuzlan, A., Bidart, J. M., Chouaib, S., Schlumberger, M., & Dupuy, C. (2011). Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma I. Agoulnik, ed. PLoS One, 6, e22567.
Movahedi, K., Laoui, D., Gysemans, C., Baeten, M., Stangé, G., Van Bossche, J., Den Mack, M., Pipeleers, D., In’t Veld, P., De Baetselier, P., & Van Ginderachter, J. A. (2010). Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Research, 70, 5728–5739.
Wahl, L. M., & Kleinman, H. K. (1998). Tumor-associated macrophages as targets for cancer therapy. Journal of the National Cancer Institute., 90, 1583–1584.
Galdiero, M. R., Varricchi, G., & Marone, G. (2016). The immune network in thyroid cancer. OncoImmunology., 5, e1168556.
Modi, J., Patel, A., Terrell, R., Tuttle, R. M., & Francis, G. L. (2003). Papillary thyroid carcinomas from young adults and children contain a mixture of lymphocytes. The Journal of Clinical Endocrinology & Metabolism., 88, 4418–4425.
Sabbah, M., Emami, S., Redeuilh, G., Julien, S., Prévost, G., Zimber, A., Ouelaa, R., Bracke, M., De Wever, O., & Gespach, C. (2008). Molecular signature and therapeutic perspective of the epithelial-to-mesenchymal transitions in epithelial cancers. Drug Resistance Updates., 11, 123–151.
Moustakas, A., & Heldin, C.-H. (2007). Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Science., 98, 1512–1520.
Mathias, R. A., Wang, B., Ji, H., Kapp, E. A., Moritz, R. L., Zhu, H.-J., & Simpson, R. J. (2009). Secretome-based proteomic profiling of Ras-transformed MDCK cells reveals extracellular modulators of epithelial-mesenchymal transition. Journal of Proteome Research, 8, 2827–2837.
Palena, C., Hamilton, D. H., & Fernando, R. I. (2012). Influence of IL-8 on the epithelial-mesenchymal transition and the tumor microenvironment. Future Oncology (London, England), 8, 713–722.
Cunha, L. L., Marcello, M. A., & Ward, L. S. (2014). The role of the inflammatory microenvironment in thyroid carcinogenesis. Endocrine-Related Cancer., 21, R85–R103.
Vella, V., Mineo, R., Frasca, F., Mazzon, E., Pandini, G., Vigneri, R., & Belfiore, A. (2004). Interleukin-4 stimulates papillary thyroid cancer cell survival: Implications in patients with thyroid cancer and concomitant graves’ disease. The Journal of Clinical Endocrinology & Metabolism., 89, 2880–2889.
Guarino, V., Castellone, M. D., Avilla, E., & Melillo, R. M. (2010). Thyroid cancer and inflammation. Molecular and Cellular Endocrinology., 321, 94–102.
Muzza, M., Degl’Innocenti, D., Colombo, C., Perrino, M., Ravasi, E., Rossi, S., Cirello, V., Beck-Peccoz, P., Borrello, M. G., & Fugazzola, L. (2010). The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: Clinical and molecular studies. Clinical Endocrinology., 72, 702–708.
Borrello, M. G., Alberti, L., Fischer, A., Degl’innocenti, D., Ferrario, C., Gariboldi, M., Marchesi, F., Allavena, P., Greco, A., Collini, P., Pilotti, S., Cassinelli, G., Bressan, P., Fugazzola, L., Mantovani, A., & Pierotti, M. A. (2005). Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proceedings of the National Academy of Sciences of the United States of America., 102, 14825–14830.
Kammoun-Krichen, M., Bougacha-Elleuch, N., Mnif, M., Bougacha, F., Charffedine, I., Rebuffat, S., Rebai, A., Glasson, E., Abid, M., Ayadi, F., Péraldi-Roux, S., & Ayadi, H. (2012). Il-?? A potential factor for discriminating between thyroid carcinoma and atrophic thyroiditis. European Cytokine Network., 23, 101–106.
Lippitz, B. E. (2013). Cytokine patterns in patients with cancer: A systematic review. The Lancet Oncology., 14, e218–e228.
Eloy, C., Santos, J., Cameselle-Teijeiro, J., Soares, P., & Sobrinho-Simões, M. (2012). TGFbeta/Smad pathway and BRAF mutation play different roles in circumscribed and infiltrative papillary thyroid carcinoma. Virchows Archiv, 460, 587–600.
Flavell, R. A., Sanjabi, S., Wrzesinski, S. H., & Licona-Limón, P. (2010). The polarization of immune cells in the tumour environment by TGFβ. Nature Reviews Immunology., 10, 554–567.
Rotondi, M., Coperchini, F., & Chiovato, L. (2013). CXCL8 in thyroid disease: From basic notions to potential applications in clinical practice. Cytokine & Growth Factor Reviews., 24, 539–546.
Zhang, X., Yuan, X., Shi, H., Wu, L., Qian, H., & Xu, W. (2015). Exosomes in cancer: Small particle, big player. Journal of Hematology & Oncology., 8, 83.
Wang, Z., Chen, J.-Q., Liu, J.-L., & Tian, L. (2016). Exosomes in tumor microenvironment: Novel transporters and biomarkers. Journal of Translational Medicine, 14, 297.
Théry, C., Zitvogel, L., & Amigorena, S. (2002). Exosomes: Composition, biogenesis and function. Nature Reviews Immunology, Published Online: 01 August 2002, 2, 569. https://doi.org/10.1038/nri855
Atretkhany, K. S. N., Drutskaya, M. S., Nedospasov, S. A., Grivennikov, S. I., & Kuprash, D. V. (2016). Chemokines, cytokines and exosomes help tumors to shape inflammatory microenvironment. Pharmacology and Therapeutics., 168, 98–112.
Tickner, J. A., Urquhart, A. J., Stephenson, S.-A., Richard, D. J., & O’Byrne, K. J. (2014). Functions and therapeutic roles of exosomes in cancer. Frontiers in Oncology., 4, 127.
Pant, S., Hilton, H., & Burczynski, M. E. (2012). The multifaceted exosome: Biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochemical Pharmacology, 83, 1484–1494.
Roma-Rodrigues, C., Fernandes, A. R., & Baptista, P. V. (2014). Exosome in tumour microenvironment: Overview of the crosstalk between normal and cancer cells. BioMed Research International, 2014, 179486.
Record, M., Carayon, K., Poirot, M., & Silvente-Poirot, S. (2014). Exosomes as new vesicular lipid transporters involved in cell–cell communication and various pathophysiologies. Biochimica et Biophysica Acta (BBA) – Molecular and Cell Biology of Lipids., 1841, 108–120.
Barile, L., & Vassalli, G. (2017). Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacology & Therapeutics.
VanNiel, G., Mallegol, J., Bevilacqua, C., Candalh, C., Brugière, S., Tomaskovic-Crook, E., Heath, J. K., Cerf-Bensussan, N., & Heyman, M. (2003). Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut, 52, 1690–1697.
Sprent, J. (2005). Direct stimulation of naïve T cells by antigen-presenting cell vesicles. Blood cells, Molecules & Diseases., 35, 17–20.
Skokos, D., Botros, H. G., Demeure, C., Morin, J., Peronet, R., Birkenmeier, G., Boudaly, S., & Mécheri, S. (2003). Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. Journal of Immunology (Baltimore, Md. : 1950), 170, 3037–3045.
Qu, Y., Franchi, L., Nunez, G., & Dubyak, G. R. (2007). Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. Journal of Immunology (Baltimore, Md.: 1950), 179, 1913–1925.
Obregon, C., Rothen-Rutishauser, B., Gerber, P., Gehr, P., & Nicod, L. P. (2009). Active uptake of dendritic cell-derived exovesicles by epithelial cells induces the release of inflammatory mediators through a TNF-α-mediated pathway. The American Journal of Pathology., 175, 696–705.
Atay, S., & Godwin, A. K. (2014). Tumor-derived exosomes: A message delivery system for tumor progression. Communicative and Integrative Biology., 7, e28231.
Bergmann, C., Strauss, L., Wieckowski, E., Czystowska, M., Albers, A., Wang, Y., Zeidler, R., Lang, S., & Whiteside, T. L. (2009). Tumor-derived microvesicles in sera of patients with head and neck cancer and their role in tumor progression. Head & Neck., 31, 371–380.
Huber, V., Filipazzi, P., Iero, M., Fais, S., & Rivoltini, L. (2008). More insights into the immunosuppressive potential of tumor exosomes. Journal of Translational Medicine, 6, 63.
Lee, J. C., Zhao, J. T., Clifton-Bligh, R. J., Gill, A., Gundara, J. S., Ip, J. C., Glover, A., Sywak, M. S., Delbridge, L. W., Robinson, B. G., & Sidhu, S. B. (2013). MicroRNA-222 and MicroRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer, 119, 4358–4365.
Yu, S., Liu, Y., Wang, J., Guo, Z., Zhang, Q., Yu, F., Zhang, Y., Huang, K., Li, Y., Song, E., Zheng, X., & Xiao, H. (2012). Circulating MicroRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. The Journal of Clinical Endocrinology & Metabolism., 97, 2084–2092.
Lee, Y. S., Lim, Y. S., Lee, J.-C., Wang, S.-G., Park, H.-Y., Kim, S. Y., & Lee, B.-J. (2015). Differential expression levels of plasma-derived miR-146b and miR-155 in papillary thyroid cancer. Oral Oncology., 51, 77–83.
Yu, S., Liu, X., Zhang, Y., Li, J., Chen, S., Zheng, H., Reng, R., Zhang, C., Chen, J., Chen, L., Yu, S., Liu, X., Zhang, Y., Li, J., Chen, S., Zheng, H., Reng, R., Zhang, C., Chen, J., et al. (2016). Circulating microRNA124-3p, microRNA9-3p and microRNA196b-5p may be potential signatures for differential diagnosis of thyroid nodules. Oncotarget, 7, 84165–84177.
Li, M., Song, Q., Li, H., Lou, Y., & Wang, L. (2015). Circulating miR-25-3p and miR-451a may be potential biomarkers for the diagnosis of papillary thyroid carcinoma R. B. Ray, ed. PLOS One, 10, e0132403.
Samsonov, R., Burdakov, V., Shtam, T., Radzhabovа, Z., Vasilyev, D., Tsyrlina, E., Titov, S., Ivanov, M., Berstein, L., Filatov, M., Kolesnikov, N., Gil-Henn, H., & Malek, A. (2016). Plasma exosomal miR-21 and miR-181a differentiates follicular from papillary thyroid cancer. Tumor Biology, 37, 12011–12021.
Fuziwara, C. S., & Kimura, E. T. (2014). MicroRNA deregulation in anaplastic thyroid cancer Biology. International Journal of Endocrinology, 2014, 743450.
Saiselet, M., Pita, J. M., Augenlicht, A., Dom, G., Tarabichi, M., Fimereli, D., Dumont, J. E., Detours, V., Maenhaut, C., Saiselet, M., Pita, J. M., Augenlicht, A., Dom, G., Tarabichi, M., Fimereli, D., Dumont, J. E., Detours, V., & Maenhaut, C. (2015). miRNA expression and function in thyroid carcinomas: A comparative and critical analysis and a model for other cancers. Oncotarget, 7, 52475–52492.
Boufraqech, M., Klubo-Gwiezdzinska, J., & Kebebew, E. (2016). MicroRNAs in the thyroid. Best Practice and Research: Clinical Endocrinology and Metabolism., 30, 603–619.
Braun, J., Hoang-Vu, C., Dralle, H., & Hutelmaier, S. (2010). Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene, 29, 4237–4244.
Visone, R., Pallante, P., Vecchione, A., Cirombella, R., Ferracin, M., Ferraro, A., Volinia, S., Coluzzi, S., Leone, V., Borbone, E., Liu, C.-G., Petrocca, F., Troncone, G., Calin, G. A., Scarpa, A., Colato, C., Tallini, G., Santoro, M., Croce, C. M., et al. (2007). Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene, 26, 7590–7595.
Gu, H., Liu, Z., & Zhou, L. (2017). Roles of miR-17-92 cluster in cardiovascular development and common diseases. BioMed Research International., 2017, 1–6.
Iriki, T., Ohnishi, K., Fujiwara, Y., Horlad, H., Saito, Y., Pan, C., Ikeda, K., Mori, T., Suzuki, M., Ichiyasu, H., Kohrogi, H., Takeya, M., & Komohara, Y. (2017). The cell-cell interaction between tumor-associated macrophages and small cell lung cancer cells is involved in tumor progression via STAT3 activation. Lung Cancer, 106, 22–32.
Qian, B.-Z., & Pollard, J. W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141, 39–51.
Vaňhara, P., & Souček, K. (2013). Mutual cytokine crosstalk between colon cancer cells and microenvironment initiates development of distant metastases. JAK-STAT., 2, e23810.
Wiseman, B. S., & Werb, Z. (2002). Stromal effects on mammary gland development and breast cancer. Science, 296, 1046–1049.
Azenshtein, E., Luboshits, G., Shina, S., Neumark, E., Shahbazian, D., Weil, M., Wigler, N., Keydar, I., & Ben-Baruch, A. (2002). The CC chemokine RANTES in breast carcinoma progression: Regulation of expression and potential mechanisms of promalignant activity. Cancer Research., 62, 1093–1102.
Gianoukakis, A. G., Khadavi, N., & Smith, T. J. (2008). Cytokines, graves’ disease, and thyroid associated ophthalmopathy. Thyroid: Official journal of the American Thyroid Association., 18, 953–958.
Lv, D., Zhang, Y., Kim, H.-J., Zhang, L., & Ma, X. (2013). CCL5 as a potential immunotherapeutic target in triple-negative breast cancer. Cellular and Molecular Immunology., 10, 303–310.
Aldinucci, D., & Colombatti, A. (2014). The inflammatory chemokine CCL5 and cancer progression. Mediators of Inflammation, 2014, 292376.
Wolpe, S. D., & Cerami, A. (1989). Macrophage inflammatory proteins 1 and 2: Members of a novel superfamily of cytokines. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology., 3, 2565–2573.
Driscoll, K. E. (1994). Macrophage inflammatory proteins: Biology and role in pulmonary inflammation. Experimental Lung Research., 20, 473–490.
Xu, J., Futakuchi, M., Iigo, M., Fukamachi, K., Alexander, D. B., Shimizu, H., Sakai, Y., Tamano, S., Furukawa, F., Uchino, T., Tokunaga, H., Nishimura, T., Hirose, A., Kanno, J., & Tsuda, H. (2010). Involvement of macrophage inflammatory protein 1a (MIP1a) in promotion of rat lung and mammary carcinogenic activity of nanoscale titanium dioxide particles administered by intra-pulmonary spraying. Carcinogenesis, 31, 927–935.
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454, 436–444.
Feghali, C. A., & Wright, T. M. (1997). Cytokines in acute and chronic inflammation. Frontiers in Bioscience: A Journal and Virtual Library., 2, d12–d26.
Hou, Z., Falcone, D. J., Subbaramaiah, K., & Dannenberg, A. J. (2011). Macrophages induce COX-2 expression in breast cancer cells: Role of IL-1β autoamplification. Carcinogenesis, 32, 695–702.
Kobawala, T. P., Patel, G. H., Gajjar, D. R., Patel, K. N., Thakor, P. B., Parekh, U. B., Patel, K. M., Shukla, S. N., & Shah, P. M. (2011). Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. Journal of Thyroid Research, 2011, 270149.
David, J., Dominguez, C., Hamilton, D., & Palena, C. (2016). The IL-8/IL-8R Axis: A double agent in tumor immune resistance. Vaccine, 4, 22.
Ren, J., He, W., Zheng, L., & Duan, H. (2016). From structures to functions: Insights into exosomes as promising drug delivery vehicles. Biomaterials Science, 4, 910–921.
Tomasetti, M., Lee, W., Santarelli, L., & Neuzil, J. (2017). Exosome-derived microRNAs in cancer metabolism: Possible implications in cancer diagnostics and therapy. Experimental & Molecular Medicine., 49, e285.
Zhang, J., Zhang, H., Liu, J., Tu, X., Zang, Y., Zhu, J., Chen, J., Dong, L., & Zhang, J. (2012). miR-30 inhibits TGF-β1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochemical and Biophysical Research Communications., 417, 1100–1105.
Kong, W., Yang, H., He, L., Zhao, J.-J., Coppola, D., Dalton, W. S., & Cheng, J. Q. (2008). MicroRNA-155 is regulated by the transforming growth factor β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Molecular and Cellular Biology., 28, 6773–6784.
Shi, J., & Jian. (2016). Considering Exosomal miR-21 as a biomarker for cancer. Journal of Clinical Medicine, 5, 42.
Lopes-Ramos, C. M., Habr-Gama, A., De, B., Quevedo, S., Felício, N. M., Cio, M., Bettoni, F., Koyama, F. C., Asprino, P. F., Galante, P. A., Gama-Rodrigues, J., Camargo, A. A., Oliva Perez, R., & Parmigiani, R. B. (2012). Overexpression of miR-21-5p as a predictive marker for complete tumor regression to neoadjuvant chemoradiotherapy in rectal cancer patients. BMC Medical Genomics, 7.
Yan, L., Cao, R., Liu, Y., Wang, L., Pan, B., Lv, X., Jiao, H., Zhuang, Q., Sun, X., & Xiao, R. (2016). MiR-21-5p links epithelial-mesenchymal transition phenotype with stem-like cell signatures via AKT signaling in keloid keratinocytes. Scientific Reports, 6, 28281.
Liu, X., Wang, C., Chen, Z., Jin, Y., Wang, Y., Kolokythas, A., Dai, Y., & Zhou, X. (2011). MicroRNA-138 suppresses epithelial-mesenchymal transition in squamous cell carcinoma cell lines. The Biochemical Journal., 440, 23–31.
Mitomo, S., Maesawa, C., Ogasawara, S., Iwaya, T., Shibazaki, M., Yashima-Abo, A., Kotani, K., Oikawa, H., Sakurai, E., Izutsu, N., Kato, K., Komatsu, H., Ikeda, K., Wakabayashi, G., & Masuda, T. (2008). Downregulation of miR-138 is associated with overexpression of human telomerase reverse transcriptase protein in human anaplastic thyroid carcinoma cell lines. Cancer Science., 99, 280–286.
Gao, J., & Liu, Q.-G. (2011). The role of miR-26 in tumors and normal tissues (review). Oncology Letters., 2, 1019–1023.
Bu, Q., You, F., Pan, G., Yuan, Q., Cui, T., Hao, L., & Zhang, J. (2017). MiR-125b inhibits anaplastic thyroid cancer cell migration and invasion by targeting PIK3CD. Biomedicine & Pharmacotherapy., 88, 443–448.
Ge, S., Wang, D., Kong, Q., Gao, W., & Sun, J. (2017). Function of MiR-152 as a tumor suppressor in human breast cancer by targeting PIK3CA. Oncology Research Featuring Preclinical and Clinical Cancer Therapeutics.
Liu, X., Li, J., Qin, F., & Dai, S. (2016). miR-152 as a tumor suppressor microRNA: Target recognition and regulation in cancer. Oncology Letters., 11, 3911–3939.
Sheng, W., Chen, Y., Gong, Y., Dong, T., Zhang, B., & Gao, W. (2016). miR-148a inhibits self-renewal of thyroid cancer stem cells via repressing INO80 expression. Oncology Reports., 36, 3387–3396.
Colamaio, M., Borbone, E., Russo, L., Bianco, M., Federico, A., Califano, D., Chiappetta, G., Pallante, P., Troncone, G., Battista, S., & Fusco, A. (2011). miR-191 Down-regulation plays a role in thyroid follicular tumors through CDK6 targeting. The Journal of Clinical Endocrinology & Metabolism., 96, E1915–E1924.
Bednarczyk, R. B. (2016). Crosstalk between macrophage secretory factors and breast cancer. New York Medical College.
Hébrant, A., Floor, S., Saiselet, M., Antoniou, A., Desbuleux, A., Snyers, B., La, C., de Saint, A. N., Leteurtre, E., Andry, G., & Maenhaut, C. (2014). miRNA expression in anaplastic thyroid carcinomas R. M. Luque, ed. PLoS One, 9, e103871.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jarboe, T. et al. (2021). Inflammatory Components of the Thyroid Cancer Microenvironment: An Avenue for Identification of Novel Biomarkers. In: Banerjee, D., Tiwari, R.K. (eds) Tumor Microenvironment: Cellular, Metabolic and Immunologic Interactions . Advances in Experimental Medicine and Biology, vol 1350. Springer, Cham. https://doi.org/10.1007/978-3-030-83282-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-83282-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83281-0
Online ISBN: 978-3-030-83282-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)