Abstract
There are familial forms of both neurohypophyseal and nephrogenic diabetes insipidus (Table 13.1). Familial neurohypophyseal DI is most often due to autosomal dominant arginine vasopressin (AVP) mutations, with gradual onset in childhood due to progressive neuronal destruction that make early diagnosis challenging. Recessive mutations in AVP have rarely been described. Wolfram’s syndrome or DIDMOAD (diabetes insipidus, diabetes mellitus, obesity and optic atrophy), caused by mutations in WSF, is also autosomal recessive in inheritance; 70% develop DI in the 2nd or 3rd decade of life. Familial nephrogenic DI is most commonly due to X-linked mutations in AVPR2 or autosomal recessive mutations in AQP2 and presents in early infancy. In all familial forms, phenotype can be variable, even within a family. Treatment of familial DI is similar to that of other forms of DI, with desmopressin for nephrogenic DI and thiazide diuretics or indomethacin in nephrogenic disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Case Presentation
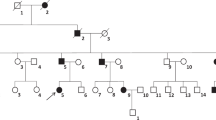
The patient is a 14-month old male, initially evaluated for poor weight gain. He was born full term with birthweight 7 pounds 2 ounces and had no issues with neonatal hypoglycemia. He nursed well as an infant, had no illnesses during this time, and was growing appropriately until around 1 year of age. Per the parents, he began drinking excessively (greater than 40 ounces per day), including drinking bath water, flower pots, puddles, and pet water bowls. He is requiring diapers changes every 1–1.5 hours, leaking with urine. He sometimes wakes at night and cries for water, and will quickly drink 8–10 ounces if offered. He prefers to drink water over milk or juice, and has a poor appetite for solid foods.
Of note, mother was diagnosed with central diabetes insipidus at age four and has been on desmopressin. She recalls that on imaging, her pituitary was “missing something”. She had no issues with growth as a child, and no fertility concerns. Father has no pertinent medical history. 3-year-old sister does not have similar symptoms, and has normal growth and development.
Physical exam is unremarkable. Normal genitalia and descended testes bilaterally. Random and first morning labs have shown normal glucose of 75–96 mg/dl and sodium 138–142 mg/dl. Creatinine was normal, and urinalysis showed no blood, protein, leukocytes, glucose, or ketones.
The patient was admitted for a water deprivation test, and had a sodium of 146 mmol/L after 18 hours without food or liquid intake. Urine output was calculated at 2.5 mL/kg/hour. Plasma osmolality was 298 mOsms/kg and urine osmolality was 300 mOsms/kg with urine specific gravity of 1.005. A pituitary MRI showed an absent posterior pituitary bright spot. Remainder of pituitary screening tests (thyroid hormone, cortisol, growth factors) were normal.
Assessment and Diagnosis
There are familial forms of both neurohypophyseal and nephrogenic diabetes insipidus (Table 13.1). Familial neurohypophyseal DI is most often due to autosomal dominant arginine vasopressin (AVP) mutations. 72 mutations have been identified, the majority in the NPII moiety or the signal peptide, though a few mutations in the AVP moiety have been described (Fig. 13.1). Most are missense or nonsense mutations, though a few deletions, indels, and splice site mutations have been found. Mutations lead to disordered processing or folding of AVP precursors; in most cases, the mutant is expressed, but retained in the endoplasmic reticulum, leading to cell death [1,2,3]. This is supported by autopsy studies showing absence of vasopressinergic neurons in affected subjects [4, 5]. There is also evidence to suggest that the mutant prohormones heterodimerize with wild-type AVP, preventing processing in a dominant negative manner [6]. The only evidence of genotype-phenotype correlation is with the c.55G > A (p.Ala19Thr) mutation, which causes abnormal cleavage of the signal peptide and is associated with later onset [7].
Onset is gradual due to progressive destruction of vasopressinergic neurons; thus, in early phases of the disease, AVP may be released during water deprivation, falsely confirming a normal response [8, 9]. Patients can present from a few months to a few years of age with polyuria, polydipsia, and failure to thrive. Presentation can be variable, even within the same family. There have been 3 autosomal recessive mutations in the AVP gene identified. All have presented with onset of DI in infancy, and good response to desmopressin.
Autosomal recessive mutations in the WSF1 gene are associated with Wolfram’s syndrome which presents with central diabetes insipidus, diabetes mellitus, optic atrophy, and deafness, (DIDMOAD) [10, 11]. Central DI is seen in 70% of patients; there is one reported case of isolated familial DI with WSF1 mutation. DI usually presents later in Wolfram’s, in the 2nd to 3rd decade of life; the mechanism of neuronal destruction has not been identified but mutant wolframin is known to cause ER aggregates and ER stress in other cell types, suggesting this may also be causal in hypophyseal cells [12]. Case reports have also described central DI due to mutations in PCSK1 and FGF8. There is also a report of X-linked transmission with no mutation yet identified [13,14,15].
Familial nephrogenic diabetes insipidus is caused by inactivating mutations of AVPR2 (AVP receptor) in 90% and AQP2 (aquaporin 2) in 10% of cases. Both lead to insensitivity of the distal nephron to AVP. Mutations in AVPR2 are associated with X-linked inheritance, and thus the majority of cases are in males with onset of complete diabetes insipidus in early infancy [16, 17]. Some female patients have been reported, with milder phenotype, thought to be related to skewed X-inactivation [18]. Over 200 mutations have been reported, mostly missense mutations causing misfolded protein and ER retention.
AQP2 mutations are autosomal recessive homozygous or compound heterozygous in the majority of cases, though some heterozygous autosomal dominant mutations are described [19]. 52 mutations have been identified. Missense mutations throughout the AQP2 gene result in misfolding and ER retention which ultimately causes cell death and complete diabetes insipidus. A few mutations do result in some cell surface AQP2 expression, and partial DI phenotype that may respond to desmopressin [20].
11 mutations in AQP2 are associated with autosomal dominant inheritance. These tend to be in the c-terminal, and impede cell trafficking and localization [21]. These mutations exert a dominant negative influence when mutant protein forms heterotetramers with wild-type protein, leading to misrouting, and reduced expression at cell surface. Depending on the mutation, there may still be functional wild-type homotetramers; thus onset of AD disease is later in onset and milder than other forms [22].
Management
Patients with genetic forms of diabetes insipidus should be treated similarly to those with non-familial forms, using 1-desamino-8-D-arginine vasopressin (desmopressin) for neurohypophyseal disease and thiazide diuretics or indomethacin for nephrogenic cases. There are novel therapeutics in development for X-linked nephrogenic DI, including cell-permeable AVPR2 agonists that act as molecular chaperones and prevent misfolding of protein, leading to cell surface expression [23]. Secretin receptor agonists and phosphodiesterase inhibitors are also being targeted to activate alternate pathways (bypassing AVPR2 activity) to AQP2 surface expression [24, 25].
Outcome
After the water deprivation test, desmopressin was given and led to decreased urine output. He continued treatment with desmopressin and showed improved appetite and weight gain, decreased polyuria and polydipsia. Genetic testing revealed mutation in AVP gene in patient and mother, and no mutation in father or older sister.
Clinical Pearls and Pitfalls
-
Familial forms of DI include AR, AD, or X-linked inheritance and are primary caused by mutations in AVP in central DI and AVPR2 and AQP2 in nephrogenic DI.
-
Mutations in WSF1 cause Wolfram (DIDMOAD) syndrome which is associated with DI in 70% of cases, in addition to diabetes mellitus and optic atrophy.
-
Phenotypes can vary, even within a family with the same mutation.
-
Diagnostic testing early in disease process may be misleading, so if family history is suggestive, the diagnosis should not be excluded.
-
Treatment of familial DI is similar to non-familial forms.
References
Russell TA, Ito M, Ito M, Yu RN, Martinson FA, Weiss J, et al. A murine model of autosomal dominant neurohypophyseal diabetes insipidus reveals progressive loss of vasopressin-producing neurons. J Clin Invest. 2003;112(11):1697–706.
Hagiwara D, Arima H, Morishita Y, Wenjun L, Azuma Y, Ito Y, et al. Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus. Cell Death Dis. 2014;5:e1148.
Ito M, Jameson JL, Ito M. Molecular basis of autosomal dominant neurohypophyseal diabetes insipidus. Cellular toxicity caused by the accumulation of mutant vasopressin precursors within the endoplasmic reticulum. J Clin Invest. 1997;99(8):1897–905.
Bergeron C, Kovacs K, Ezrin C, Mizzen C. Hereditary diabetes insipidus: an immunohistochemical study of the hypothalamus and pituitary gland. Acta Neuropathol. 1991;81(3):345–8.
Braverman LE, Mancini JP, Mcgoldrick DM. Hereditary idiopathic diabetes insipidus. A Case report with autopsy findings. Ann Intern Med. 1965;63:503–8.
Ito M, Yu RN, Jameson JL. Mutant vasopressin precursors that cause autosomal dominant neurohypophyseal diabetes insipidus retain dimerization and impair the secretion of wild-type proteins. J Biol Chem. 1999;274(13):9029–37.
McLeod JF, Kovács L, Gaskill MB, Rittig S, Bradley GS, Robertson GL. Familial neurohypophyseal diabetes insipidus associated with a signal peptide mutation. J Clin Endocrinol Metab. 1993;77(3):599A–G.
Elias PCL, Elias LLK, Torres N, Moreira AC, Antunes-Rodrigues J, Castro M. Progressive decline of vasopressin secretion in familial autosomal dominant neurohypophyseal diabetes insipidus presenting a novel mutation in the vasopressin-neurophysin II gene. Clin Endocrinol. 2003;59(4):511–8.
Babey M, Kopp P, Robertson GL. Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol. 2011;7(12):701–14.
Barrett TG, Bundey SE, Macleod AF. Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet. 1995;346(8988):1458–63.
Strom TM, Hörtnagel K, Hofmann S, Gekeler F, Scharfe C, Rabl W, et al. Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet. 1998;7(13):2021–8.
Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, et al. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem. 2005;280(47):39609–15.
Frank GR, Fox J, Candela N, Jovanovic Z, Bochukova E, Levine J, et al. Severe obesity and diabetes insipidus in a patient with PCSK1 deficiency. Mol Genet Metab. 2013;110(1–2):191–4.
McCabe MJ, Gaston-Massuet C, Tziaferi V, Gregory LC, Alatzoglou KS, Signore M, et al. Novel FGF8 mutations associated with recessive holoprosencephaly, craniofacial defects, and hypothalamo-pituitary dysfunction. J Clin Endocrinol Metab. 2011;96(10):E1709–18.
Habiby RL, Robertson GL, Kaplowitz PB, Rittig S. A novel X-linked form of familial neurohypophyseal diabetes insipidus. • 386. Pediatr Res. 1997;41:67.
Postina R, Ufer E, Pfeiffer R, Knoers NV, Fahrenholz F. Misfolded vasopressin V2 receptors caused by extracellular point mutations entail congential nephrogenic diabetes insipidus. Mol Cell Endocrinol. 2000;164(1–2):31–9.
Rosenthal W, Seibold A, Antaramian A, Lonergan M, Arthus MF, Hendy GN, et al. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature. 1992;359(6392):233–5.
Satoh M, Ogikubo S, Yoshizawa-Ogasawara A. Correlation between clinical phenotypes and X-inactivation patterns in six female carriers with heterozygote vasopressin type 2 receptor gene mutations. Endocr J. 2008;55(2):277–84.
Deen PM, Weghuis DO, Sinke RJ, Geurts van Kessel A, Wieringa B, van Os CH. Assignment of the human gene for the water channel of renal collecting duct aquaporin 2 (AQP2) to chromosome 12 region q12-->q13. Cytogenet Cell Genet. 1994;66(4):260–2.
Canfield MC, Tamarappoo BK, Moses AM, Verkman AS, Holtzman EJ. Identification and characterization of aquaporin-2 water channel mutations causing nephrogenic diabetes insipidus with partial vasopressin response. Hum Mol Genet. 1997;6(11):1865–71.
Kamsteeg EJ, Wormhoudt TA, Rijss JP, van Os CH, Deen PM. An impaired routing of wild-type aquaporin-2 after tetramerization with an aquaporin-2 mutant explains dominant nephrogenic diabetes insipidus. EMBO J. 1999;18(9):2394–400.
Marr N, Bichet DG, Lonergan M, Arthus M-F, Jeck N, Seyberth HW, et al. Heteroligomerization of an Aquaporin-2 mutant with wild-type Aquaporin-2 and their misrouting to late endosomes/lysosomes explains dominant nephrogenic diabetes insipidus. Hum Mol Genet. 2002;11(7):779–89.
Morello JP, Salahpour A, Laperrière A, Bernier V, Arthus MF, Lonergan M, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105(7):887–95.
Procino G, Milano S, Carmosino M, Barbieri C, Nicoletti MC, Li JH, et al. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int. 2014;86(1):127–38.
Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am J Physiol Renal Physiol. 2005;288(6):F1103–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Shah, R. (2021). Familial DI and Genetic Workup. In: Alter, C.A. (eds) Diabetes Insipidus in Children. Springer, Cham. https://doi.org/10.1007/978-3-030-83248-3_13
Download citation
DOI: https://doi.org/10.1007/978-3-030-83248-3_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-83247-6
Online ISBN: 978-3-030-83248-3
eBook Packages: MedicineMedicine (R0)