Abstract
Cardiac complications are a major cause for perioperative morbidity and mortality among patients undergoing elective noncardiac procedures. Such complications can also lead to prolonged hospitalization. However, there is paucity of adequate literature or guidelines dedicated towards the management of a cardiac patient undergoing low-risk outpatient surgical procedures. This chapter focuses on identifying a patient with increased risk for cardiac complications, optimizing preoperative testing, and mitigating any cardiac risks resulting from outpatient oral and maxillofacial surgery. The material presented in this chapter is based on the guidelines issued by the American College of Cardiology and the American Heart Association. These guidelines have consistently recommended against excessive testing and that interventions are rarely necessary to lower the surgical risk in itself unless also indicated for the long-term benefit of the patient. They have also reinforced that the purpose of the preoperative evaluation is not just to provide “clearance” but to perform a comprehensive cardiac evaluation for intermediate perioperative and long-term benefit, and preoperative tests should be performed only if results will influence treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiac patient
- Medically compromised patient
- Cardiac risks
- Ischemic heart disease
- Cardiac arrythmias
- Hypertension and dentistry
Cardiovascular disease represents about 30% of global mortality [1]. A thorough history and physical exam of the cardiac patient should involve appropriate questioning of symptoms and obvious signs of undiagnosed cardiac disease. The physical exam must also include auscultation of the heart for any murmurs, blood pressure in both arms when indicated, and carotid/jugular distention with bruits. In addition, examination of extremities for edema and hepatomegaly would indicate underlying cardiac disease. This chapter will discuss only the most common cardiac issues encountered in daily practice listed under the following subtitles:
-
1.
Hypertension
-
2.
Ischemic heart disease
-
3.
Cardiac arrythmias, pacemakers, and anticoagulation
-
4.
Valvular heart disease
-
5.
Congenital heart disease
-
6.
Anticoagulation
-
7.
Perioperative cardiac risk assessment
2.1 Hypertension
Hypertension is known to affect over a billion people worldwide with far-reaching consequences of heart disease and stroke in poorly controlled patients [2]. Due to its circadian pattern, blood pressure (BP) can be variable. For morning appointments, the reading can be high and be a normal pattern. It also increases with age and with anxiety. Primary hypertension is defined as a condition without identifiable causative factors. Secondary hypertension, on the other hand, has an identifiable cause. Endocrine and vascular conditions are the predominant causative factors for secondary hypertension. Others include alcoholism and obstructive sleep apnea [3].
Many patients with hypertension remain undiagnosed and poorly controlled in nearly half of them on treatment [4]. Untreated hypertension is one of the most important preventable causes of morbidity and mortality, since it is a major risk factor for stroke, myocardial infarction, heart failure, chronic kidney disease, cognitive decline, and premature death. It is one of the leading causes of death with more than seven million fatalities [5]. According to the Joint National Committee (JNC 8), hypertension is defined as a blood pressure reading >140/90 in otherwise healthy individuals. The committee further defines various stages of hypertension [6] (Table 2.1).
Proper technique for obtaining accurate blood pressure measurements mandates that a patient be seated quietly for at least 5 min in a chair, feet on the floor and arms supported at heart level. An appropriate-sized cuff, a cuff bladder that encircles at least 80% of the arm, should be used to ensure accuracy, and at least two measurements should be taken during the visit [7]. Oral procedures are inherently associated with elevated levels of anxiety and a potential for increased blood pressure. Such readings should be interpreted with caution since patient’s with elevated blood pressure tend to normalize upon administration of anxiolytics [8]. Several antihypertensive medications are available and may have interactions with common drugs used in oral surgical procedures (Table 2.2).
The induction of anesthesia, extubation, surgical pain, hypoxia, hypothermia, and volume overload are the events that could lead to blood pressure elevations and tachycardia [9]. In addition, the use of local anesthesia with epinephrine may potentiate hypertensive state. However, some patients may experience hypotension, cardiac arrythmias, angina pectoris, and myocardial infarction [10]. Injection of local anesthetics with greater concentration of epinephrine can increase blood pressure [11]. However it is open to debate whether such elevations have clinical significance. In addition, maximum dosage of epinephrine for patients with cardiovascular disease has been reported, but these dosage recommendations are based on poorly designed scientific studies and extrapolated data, and the recommendations should be weighed against the likelihood of significant endogenous catecholamine release from inadequate local anesthesia.
Hypertensive emergencies (i.e., severe elevations in BP [>180/110 mmHg] complicated by evidence of impending or progressive end-organ damage) require immediate BP reduction to prevent or limit end-organ damage. Blood pressure should be reduced by 10–15% (maximum of 20%) in a controlled fashion within the first hour with a continued decrease towards 160/100 mmHg over the next 2–6 h as tolerated by the patient. There was no benefit to deferring long-term treatment of patients with hypertension with diastolic blood pressures between 110 and 130 mmHg and no previous cardiac conditions [12]. Strategies may include having the patient take their usual daily medications, if not already done, as well as anxiety and stress reduction breathing and/or mindfulness exercises. If intravenous (IV) sedation is part of the treatment plan, then often the IV medications will result in blood pressure reduction, but ketamine should be avoided in these cases due to its propensity to increase blood pressure.
2.1.1 Ischemic Heart Disease
Cardiovascular disease remains a major cause of morbidity and mortality, particularly ischemic heart disease (IHD), a common cardiac condition [13]. Angina and myocardial infarction are known diseases as a result of IHD.
2.2 Angina Pectoris
Angina pectoris is classically described as a substernal pressure or squeezing sensation due to increased oxygen demand on the myocardium. The pain can also radiate to the neck and arms and usually is relieved at rest. Sublingual nitroglycerin (NTG) quickly resolves those symptoms, and then it is safe to proceed with the procedure. Coronary atherosclerosis is the common cause for angina. Angina in the dental office is most often a result of anxiety and fear resulting in tachycardia and angina. These patients are usually on nitrates and are prone to the risk of hypotension. If the symptoms persist despite rest and treatment with nitrates, a concern for myocardial infarction should be suspected.
2.3 Myocardial Infarction
Myocardial infarction is a condition due to ischemic death of the myocardium which is often not relieved with nitrates. In addition, patients complain of chest pain, nausea, pallor, and diaphoresis. MI usually results from a ruptured atherosclerotic plaque which then obstructs the coronary vessels leading to obstruction and ischemia. This is a medical emergency, and appropriate emergency activation should commence for timely thrombolysis. Differences between MI and angina are listed in Table 2.3.
2.4 Perioperative Myocardial Infarction (PMI)
Acute coronary syndrome occurs when an unstable plaque ruptures leading to acute coronary thrombosis, ischemia, and infarction. Physiological and emotional stresses are known to predispose patients to PMI. Tachycardia and hypertension which are common in the perioperative period may lead to rupture of plaques. Anxiety and stress response to surgery leads to a surge in catecholamines that leads to increased ionotropic and chronotropic effects on the heart. This causes coronary oxygen supply-demand imbalance which can also lead to PMI. Perioperative tachycardia is the most common cause of oxygen supply-demand imbalance. Stress-induced coronary vasoconstriction can also impair coronary perfusion leading to PMI. Heart failure is another common condition in patients with coronary artery disease. This condition can be aggravated by ischemia and volume overload, leading to cardiac decompensation and subsequent PMI. Contrary to popular belief, these patients are in fact better served with anxiolytic techniques such as nitrous oxide or sedation protocols safely [14].
During the 1980s, the rule prevailed to wait 6 months after a myocardial infarction before embarking on noncardiac surgery [15]. This recommendation was based on risk of cardiac events for general surgical procedures under general anesthesia [16]. Studies have shown that the cardiac risk after a previous infarction is less related to the age of the infarction than to the functional status of the ventricles [17]. In that context, a small infarction without residual angina in the context of a good functional status allows essential noncardiac surgery as soon as 6 weeks after the ischemic episode [18]. Current practice guidelines consider the period within 6 weeks of infarction as a time of high risk for a perioperative cardiac event, because it is the mean healing time of the infarct-related lesion [19]. The period from 6 weeks to 3 months is considered as intermediate risk for cardiac complication. In patients with ischemic event-related complications such as arrhythmias, ventricular dysfunction, or continued medical therapy, this risk period is extended beyond 3 months after the ischemic event. In uncomplicated cases, there is no reason for delaying surgery more than 3 months after an ischemic attack.
Heart failure is an outcome of IHD and is associated with significant morbidity and mortality. Signs and symptoms include shortness of breath, rales, extremity edema, elevated jugular pulse, and fatigue. Heart failure is a major independent predictor of adverse perioperative outcome in noncardiac surgery. It carries a greater perioperative risk than ischemic heart disease. In the Framingham study, the overall mortality at 2 years was 25%. The overall prevalence in the general population is 1–2% [20]. This patient population must be expected to be taking multiple, long-term medications, including angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers, b-blockers, aldosterone antagonists, and diuretics, all with associated side effects (mostly electrolyte disturbances, renal insufficiency, and intraoperative therapy-resistant hypotension). The most important consideration when categorizing heart failure is whether left ventricular ejection fraction (LVEF) is preserved or reduced (less than 50%). A reduced LVEF in systolic heart failure is a powerful predictor of mortality. As many as 40–50% of patients with heart failure have diastolic heart failure with preserved left ventricular function [21, 22]. The New York Heart Association classification system is the simplest and most widely used method to gauge symptom severity [23].
2.5 Local Anesthesia in Cardiac Patients
When properly injected, vasopressors in local anesthesia can cause clinically insignificant arrythmias, and several systematic reviews have shown that the use of low concentration vasopressors in local anesthesia is safe for cardiac patients [24]. Despite the lack of absolute scientific evidence, various authors and guidelines recommend limiting the quantity or advise against the use of local anesthetics with vasopressors in these patients. However, there recommendations ignore the fundamental role that vasoconstrictors prolong the effects of local anesthetics. A patient who experiences pain and anxiety due to lack of appropriate local anesthesia can release endogenous catecholamines that could increase up to 10 times the base level and may reach significantly concentrations than the very low concentration of epinephrine used in local anesthesia [25]. The most frequent complications in cardiac patients after local anesthesia with a vasoconstrictor agent were identified on EKG as arrythmias. Most of these arrhythmias were clinically insignificant. The use of ≤4 carpules of lidocaine with epinephrine 1:100000 as a local anesthetic seems to be relatively safe for cardiovascular compromised patients [24].
2.6 Cardiac Arrythmias/Implantable Electronic Cardiac Devices
Cardiac arrythmias occur when there is an abnormality in impulse generation or conduction or both. Benign arrythmias sometimes occur in patients without cardiac disease and rarely pose a problem. Many episodes are asymptomatic, and extra beats are very common in normal people. However, tachyarrhythmias are often associated with a diminished cardiac output leading to symptoms like angina, dyspnea, palpitations, or syncope. These patients present with implanted electronic cardiac devices to manage arrythmias. Approximately 250,000 implantable cardiovascular electronic devices are placed each year in the United States [26]. Patient with implantable cardiac devices should provide the details of implantation date, device manufacturer, mode of the implant, model number, and serial number. Cardiac arrythmias are most commonly seen in surgical procedures under local anesthesia with or without vasoconstrictors [27]. Patients with preexisting rhythm disorders or heart failure are more prone to such arrythmias [28]. Insufficient local anesthesia and lighter plane of general anesthesia are the most common intraoperative factors leading to cardiac arrythmias. Effective pain and anxiety control are important in such patients.
2.6.1 Automated Implantable Cardioverter Defibrillators (AICD) and Pacemakers
AICD have been widely used in patients with a higher risk of sudden cardiac death due to ventricular fibrillation and tachyarrhythmias. These devices deliver shock immediately upon sensing such arrythmia providing defibrillation and cardioversion [29]. Symptomatic bradycardia is often managed by a pacemaker. Various types of pacemakers are available and implanted by the individual needs of the patients. Pacemakers have cardiac leads and the pulse generators. The leads may be single or dual lead depending on whether the atria or ventricles are paced. They can also be dual lead where both the chambers are stimulated. In addition, there are also biventricular pacing devices. In patients with implanted cardiac devices, caution should be exercised in the use of anesthetic adjunctive agents such as anticholinergics, beta-blockers, local anesthetics, and vasopressor agents [30]. Malfunction of pacemakers may lead to symptomatic bradycardias or tachycardias, although upper rate-limiting programming is done in recent devices to limit such tachyarrhythmias. Antiarrhythmic drugs may be considered to covert such rhythms [31].
Electromagnetic interference with these cardiac devices is a concern when cautery devices or lasers are used in these patients. The grounding pad of the cautery device should be placed as far away as possible from the pacemaker/ICD. In addition, use of bipolar cautery is recommended over monopolar cautery. To reduce the risk of interference, an external magnet placement may allow for asynchronous cardiac pacing. This depends on the type of pacemaker, and the manufacturing company should be contacted for guidance [32]. However, this approach is seldom employed nowadays.
Review of the EKG or consultation with the cardiology team can determine whether the patient is device dependent; information of the procedure, patient positioning, and anticipated sources of intraoperative electromagnetic interference should be discussed. Specific recommendations from a cardiologist regarding the cardiac device should be carefully documented [33]. Battery function of these devices is another concern. Usually, the lithium-ion batteries in the devices last over 10 years. Patients should be asked about the year of placement and if there has been any replacement of the devices for a changed battery.
2.7 Valvular Heart Disease
According to the American Heart Association, approximately five million people are diagnosed with valvular heart disease in the United States each year. Valvular heart disease is a common condition which can either be stenosis or regurgitation. Most commonly, the aortic and mitral valves are involved resulting in a heart murmur. It is not uncommon to detect murmurs during physical exam which have not been detected in the past. While most murmurs are benign and asymptomatic, a high-grade murmur warrants further evaluation by a cardiologist. Aortic valve stenosis is an independent risk factor for cardiac morbidity and mortality. Prosthetic heart valves require long-term anticoagulation depending whether they are mechanical or biological. The risk of thromboembolism is significantly higher in patients with mechanical prosthesis, particularly the mitral valve due to relatively low flow compared to aortic valve. These patients are anticoagulated, and any interruption of anticoagulation can predispose them to thrombosis. If there is appropriate indication to discontinue anticoagulants prior to extensive maxillofacial surgery, such a decision should be made in consultation with the patient’s cardiologist or primary care provider. Valvular heart disease poses a risk for developing bacterial endocarditis and exacerbating a preexisting congestive heart failure. According to the recent ACC/AHA guidelines, endocarditis prophylaxis before invasive oral procedures is recommended in patients with valvular heart disease [34].
2.7.1 Recommendations for Endocarditis Prophylaxis [34]
-
1.
Prosthetic cardiac valves, including transcatheter-implanted prostheses and homografts
-
2.
Prosthetic material used for cardiac valve repair, such as annuloplasty rings and chords
-
3.
Previous infective endocarditis (IE)
-
4.
Unrepaired cyanotic congenital heart disease or repaired congenital heart disease, with residual shunts or valvular regurgitation at the site of, or adjacent to the site of, a prosthetic patch or prosthetic device
-
5.
Cardiac transplant with valve regurgitation attributed to a structurally abnormal valve
2.8 Congenital Heart Disease
Congenital heart disease represents a common developmental anomaly. Atrial septal defect (ASD), ventricular septal defect (VSD), patent ductus arteriosus (PDA), congenital pulmonary stenosis, and aortic stenosis are some of the common conditions. A majority of these are repaired early in life upon detection, although some may go undiagnosed until later in life and some into adulthood. Saettele et al. developed a risk stratification chart (Table 2.4) of patients with congenital heart disease undergoing major surgery. The risk of conduction defects increases with the use of certain drugs (Table 2.5). However, currently no data exists on outpatient anesthesia for these patients. Until such evidence is available, these patients should undergo preoperative evaluation by their cardiologist, and their recommendations should guide the anesthesia plan. In addition, according to the current AHA guidelines, patients with unrepaired congenital heart diseases require endocarditis prophylaxis.
2.9 Anticoagulation
Various antiplatelet or anticoagulant drugs are available to reduce the risk of thrombus formation (Table 2.6). Cardiac conditions such as IHD, valvular heart disease, and cardiac arrythmias are indications for anticoagulant therapy. Surgical procedures of the oral cavity have an increased risk of bleeding. In the past interruption of anticoagulation therapy was commonly done to reduce the risk of oral bleeding. However, this inherently increased the risk of ischemic stroke or myocardial infarction.
Atrial fibrillation is the one of the most common cardiac arrhythmic conditions that may require anticoagulation. Several risk factors were identified in these patients to reduce the risk of thromboembolism (Table 2.7). A score of greater than 2 requires managing these patients with anticoagulant therapy [37]. In addition, those with bioprosthetic valves and valve repair are considered increased risk and should be anticoagulated regardless of score.
The risk of perioperative stent thrombosis in cardiac patients is increased by noncardiac surgical procedure, when surgery is performed early after stent implantation and if dual antiplatelet therapy is discontinued [38]. Dual platelet therapy is known to cause more bleeding than single drug. Despite this, interruption of antiplatelet therapy is not indicated for routine oral surgical procedures [39]. In patients who are anticoagulated with warfarin, oral surgical procedures can be safely performed with therapeutic levels of anticoagulation up to INR 4.0 [40]. However, the type of surgery, including the number of extractions, and the technique, such as staged extractions or quadrant procedures, are critical to perioperative success. A meta-analysis study involving aspirin (ASA) and a control group showed longer bleeding time in the aspirin group. However, this increase is not shown to be statistically significant compared to the control group. Hence disruption of antiplatelets therapy was not indicated [41]. Several studies have overwhelmingly concluded against the interruption of antiplatelet therapies prior to oral and maxillofacial surgery although some authors have recommended the use of local hemostatic agents [42, 43]. Further, evidence suggests an approximately 5% stroke risk with cessation of anticoagulation medications in patients on such medications for atrial fibrillation [44].
In order to obtain local hemostasis, it is advisable to take into account all the hemostatic agents known. The local hemostatic measures include the use of hemostatic gauze with regenerated oxidized cellulose, gelfoam consisting of animal origin gelatin, topical thrombin, fibrin sealants, bone wax, sutures, electrocautery, and the use of tranexamic acid. In addition, recently, the US Food and Drug Administration approved idarucizumab, a monoclonal antibody fragment, for the treatment of patients taking dabigatran when reversal of the anticoagulant effects of dabigatran is needed for emergency surgery/urgent procedures or in life-threatening or uncontrolled bleeding.
2.10 Perioperative Cardiac Risk Assessment and Testing
The aims of cardiac risk stratification are to:
-
1.
Identify potentially life-threatening cardiac conditions
-
2.
Consider appropriate preoperative testing in high-risk patients
-
3.
Implement optimal perioperative management strategies to reduce the risk of cardiac morbidity and mortality
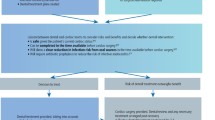
The risk of perioperative cardiac complications is the summation of the individual patient’s risk and cardiac stress related to the surgical procedure. The first step in cardiac risk stratification is to identify patients at risk for perioperative cardiac events. Historically, several indices have been described towards risk assessment in a surgical patient [45,46,47]. Recent studies have indicated the Goldman index may actually overestimate risk for today’s ambulatory surgical patient [48]. However, these indices are outdated due to significant advances in surgical and medical management of patients with cardiac disease. In addition, these indices were used to assess the cardiac risk in patients undergoing major noncardiac surgeries and do not provide optimal data to assess the risk in outpatient oral and maxillofacial surgery. Major noncardiac surgery patients who undergo general anesthesia may experience the risk of significant hemodynamic changes, renal dysfunction, pulmonary failure, and hypermetabolic states. However, such conditions are extremely rarely encountered in the oral surgical patient. Within the oral and maxillofacial surgical procedures, majority of the procedures are considered low risk for cardiac mortality and morbidity. Perhaps, the head and neck reconstructive procedures are considered as intermediate risk. Although not evaluated, patients with severe obstructive sleep apnea (pulmonary hypertension, uncontrolled HTN, diabetes, and renal failure) undergoing a combination of maxillomandibular advancement and soft tissue airway surgery could be classified as intermediate risk.
The American College of Surgeons National Surgical Quality Improvement Program (NSQIP) risk calculator (https://riskcalculator.facs.org/RiskCalculator/PatientInfo.jsp) is a more comprehensive online tool and procedure specific [49]. Another simple yet valuable risk assessment tool is the myocardial infarction or cardiac arrest (MICA) calculator (https://www.mdcalc.com/gupta-perioperative-risk-myocardial-infarction-cardiac-arrest-mica) [50]. Recent studies have shown the MICA calculator outperformed several indicators. Preoperative functional status is another predictor of perioperative outcome. Studies in the past have shown that low exercise tolerance is associated with poor perioperative outcome [51, 52]. The Duke Activity Status Index (DASI) is based on a questionnaire and grades exercise ability related to physical activity such as:
-
Can take care of self, such as eat, dress, or use the toilet (1 MET)
-
Can walk up a flight of steps or a hill or walk on level ground at 3–4 mph (4 METs)
-
Can do heavy work around the house, such as scrubbing floors or lifting or moving heavy furniture, or climb two flights of stairs (between 4 and 10 METs)
-
Can participate in strenuous sports such as swimming, singles tennis, football, basketball, and skiing (>10 METs)
One metabolic equivalent of task (MET) is defined as 3.5 mL of oxygen consumed per kilogram body mass per minute. A cardiac patient with a functional capacity of more than 4 METs is considered low risk. However, the inability to climb a flight of stairs (4 METs) is significant because it is associated with cardiac events during major noncardiac surgery. At this time, it is unknown if this data can be extrapolated to oral surgical procedures considered as low risk. A recent study however concluded that subjectively assessed preoperative functional capacity did not accurately identify patients with poor cardiopulmonary status or predict postoperative morbidity or mortality in major noncardiac surgery [53]. A surgical classification system that identifies risk based on blood loss can be a valuable tool in risk stratification (Table 2.8).
Despite evidence that routine preoperative testing before elective, low-risk ambulatory surgery is not indicated, studies have shown that more than 60% of all patients underwent at least one laboratory test during their preoperative evaluation [55]. A systematic review of the current literature found that the incidence of abnormal test results that changed perioperative management ranged from less than 0.1% (CBC) to 2.6% (renal function tests) [56]. Inappropriate and unnecessary preoperative testing can lead to significant financial burden on the patient but may also lead to morbidity and mortality as a result of the testing [57]. According to the ACC/AHA perioperative guidelines, if the patient has had a cardiovascular evaluation in the previous 2 years and has not experienced new or worsening symptoms, further testing is usually unnecessary. If there has been no diagnostic workup, or if new or worsening cardiopulmonary symptoms are present, then additional testing may be indicated. Also, asymptomatic, functionally active patients with previous successful coronary revascularization within the last 6 years are in a low-risk category and should not be investigated further for a noncardiac operation [58].
In summary, perioperative cardiac management of the patient should be based on the best available scientific evidence, individual patient’s risk stratification, and cost-effectiveness. The focus should be on appropriate medical interview of the patient and determining their functional status, rather than burdening the already strained health care systems with unnecessary testing. Discussion with primary care providers or other consultants should focus less on preoperative “clearance” and more on determining if the patient is optimized. In addition, conditions like stress, anxiety, and fear of oral surgical procedures can cause endogenous release of catecholamines, particularly norepinephrine, which in turn can precipitate autonomic responses leading to conditions like hypertension and arrhythmias. Hence, control of anxiety and pain plays a major role in reducing complications related to cardiovascular disease.
References
World Health Organization. Global status report on noncommunicable disease. Geneva: WHO; 2014.
Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322.
Holm SW, Cunningham LL Jr, Bensadoun E, Madsen MJ. Hypertension: classification, pathophysiology, and management during outpatient sedation and local anesthesia. J Oral Maxillofac Surg. 2006;64(1):111–2.
Colhoun HM, Dong W, Poulter NR. Blood pressure screening, management and control in England 1994. J Hypertens. 1998;16:747–53.
Danaei G, Finucane MM, Lin JK, et al. Global burden of metabolic risk factors of chronic diseases collaborating group (blood pressure). Lancet. 2011;377(9765):568–77.
James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507–20.
Southerland JH, Gill DG, Gangula PR, Halpern LR, et al. Dental management in patients with hypertension: challenges and solutions. Clin Cosmet Investig Dent. 2016;8:111–20.
Lu DP, Lu GP. Hypnosis and pharmacological sedation for medically compromised patients. Compend Contin Educ Dent. 1996;17:32–40.
Varon J, Marik PE. Perioperative hypertension management. Vasc Health Risk Manag. 2008;4(3):615–27.
Bader JD, Bonito AJ, Shugars DA. Cardiovascular effects of epinephrine on hypertensive dental patients. Rockville: Agency for Healthcare Research and Quality; 2002. (Evidence Report/Technology Assessment Number 48. (Prepared by Research Triangle Institute under Contract No. 290-97-0011.) AHRQ Publication 02-E006.
Abu-Mostafa N, Aldawssary A, Assari A, et al. A prospective randomized clinical trial compared the effect of various types of local anesthetics cartridges on hypertensive patients during dental extraction. J Clin Exp Dent. 2015;7(1):e84–8.
Fleisher LA, Beckman JA, Brown KA, et al.; ACC/AHA Task Force Members. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 2002 guidelines on perioperative cardiovascular evaluation for noncardiac surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:1971–199.
Number of deaths for leading causes of death. National Center for Health Statistics. United States; 2015.
Mangano DT. Perioperative cardiac morbidity. Anesthesiology. 1990;72:153–84.
Rao TL, Jacobs KH, El-Etr AA. Reinfarction following anesthesia in patients with myocardial infarction. Anesthesiology. 1983;59:499–505.
Margaix Munoz M, Jimenez Soriano Y, Poveda Roda R, et al. Cardiovascular diseases in dental practice. Practical considerations. Med Oral Patol Oral Cir Bucal. 2008;13:E296–302.
Ryan TJ, Antman EM, Brooks NH. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction: executive summary and recommendations. Circulation. 1999;100:1016–30.
Tuman KJ. Perioperative cardiovascular risk: assessment and management. Anesth Analg. 2001;92:S106–12.
VanBelle E, Lablanche JM, Bauters C. Coronary angioscopic findings in the infarcted-related vessel within 1 month of acute myocardial infarction. Natural history and the effect of thrombolysis. Circulation. 1998;97:26–33.
Hammill BG, Curtis LH, Bennett-Guerrero E, et al. Impact of heart failure on patients undergoing major noncardiac surgery. Anesthesiology. 2008;108:559–67.
Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–9.
Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119(24):3070–7.
Dolgin M, Committee NYHAC. In: Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9. Dolgin M, editor. Boston: Little, Brown; 1994.
Godzieba A, Smektała T, Jędrzejewski M, et al. Clinical assessment of the safe use local anaesthesia with vasoconstrictor agents in cardiovascular compromised patients: a systematic review. Med Sci Monit. 2014;20:393–8.
Meyer FU. Haemodynamic changes under emotional stress following a minor surgical procedure under local anaesthesia. Int J Oral Maxillofac Surg. 1987;16:688–94.
Healy JS, Merchant R, Simpson C, et al. Society position statement: Canadian Cardiovascular Society/Canadian Anesthesiologists’ Society/Canadian Heart Rhythm Society joint position statement on the management of patients with implanted pacemakers, defibrillators, and neurostimulating devices. Can J Cardiol. 2012;28:141–51.
Campbell RL, Langston WG, Ross GA. A comparison of cardiac rate-pressure product and pressure-rate quotient with Holter monitoring in patients with hypertension and cardiovascular disease: a follow-up report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:125–8.
Blinder D, Shemesh J, Taicher S. Electrocardiographic changes in cardiac patients undergoing dental extractions under local anaesthesia. J Oral Maxillofac Surg. 1996;54:162–5.
Cappato R, Smith WM, Hood MA, et al. Subcutaneous chronic implantable defibrillation systems in humans. J Interv Card Electrophysiol. 2012;34(3):325–32.
Stone ME, Salter B, Fischer A. Perioperative management of patients with cardiac implantable electronic devices. Br J Anaesth. 2011;107 Suppl 1:i16–26.
Cismaru G, Gusetu G, Muresan L, et al. Recovery of ventriculo-atrial conduction after adrenaline in patients implanted with pacemakers. Pacing Clin Electrophysiol. 2015;38(7):857–63.
American society of anesthesiologist task force on perioperative management of patients with cardiac rhythm management devices. Practice advisory for the perioperative management of patients with cardiac implantable electronic devices: pacemakers and implantable cardioverter-defibrillators. An updated report by the American Society of Anesthesiologists Task Force on perioperative management of patients with cardiac implantable electronic devices. Anesthesiology. 2005;103:186–198.
Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) expert consensus statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management. Heart Rhythm. 2011;8:1114–5.
Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2017;135:e1159–95.
Saettele AK, Christensen JL, Chilson KL, Murray DJ. Children with heart disease: risk stratification for non-cardiac surgery. J Clin Anesth. 2016;35:479–84.
Olesen JB, Lip GY, Hansen ML, Hansen PR, Tolstrup JS. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:124.
Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery. Eur Heart J. 2010;31(19):2369–429.
Brilakis ES, Banerjee S, Berger PB. Perioperative management of patients with coronary stents. J Am Coll Cardiol. 2007;49:2145–50.
Lu SY, Tsai CY, Lin LH, Lu SN. Dental extraction without stopping single or dual antiplatelet therapy: results of a retrospective cohort study. Int J Oral Maxillofac Surg. 2016;45:1293–8.
Salam S, Yusuf H, Milosevic A. Bleeding after dental extractions in patients taking warfarin. Br J Oral Maxillofac Surg. 2007;45:463–6.
Zhao B, Wang P, Dong Y, Zhu Y, Zhao K. Should aspirin be stopped before tooth extraction? A meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:522–30.
Bajkin BV, Urosevic IM, Stankov KM, Petrovic BB, Bajkin IA. Dental extractions and risk of bleeding in patients taking single and dual antiplatelet treatment. Br J Oral Maxillofac Surg. 2015;53:39–43.
Dézsi BB, Koritsánszky L, Braunitzer G, Hangyási DB, Dézsi CA. Prasugrel versus clopidogrel: a comparative examination of local bleeding after dental extraction in patients receiving dual antiplatelet therapy. J Oral Maxillofac Surg. 2015;73(10):1894–900.
Broderick JP, Bonomo JB, Kissela BM, et al. Withdrawal of antithrombotic agents and its impact on ischemic stroke occurrence. Stroke. 2011;42:2509–14.
Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977;297:845–50.
Detsky AS, Abrams HB, Forbath N, Scott JG, Hilliard JR. Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index. Arch Intern Med. 1986;146:2131–4.
Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043.
Mingus ML. Patients with cardiac disease for ambulatory surgery. Anesth Clinics North Am. 1997;15:171–88.
Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–42. e1–3. Epub 2013 Sep 18.
Gupta PK, Gupta H, Sundaram A, et al. Development and validation of a risk calculator for prediction of cardiac risk after surgery. Circulation. 2011;124:381–7.
Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for peri-operative management of major surgery in the elderly. Chest. 1999;116:355–62.
Crawford RS, Cambria RP, Abularrage CJ, et al. Pre-operative functional status predicts peri-operative outcomes after infrainguinal bypass surgery. J Vasc Surg. 2010;51:351–9.
Wijeysundera DN, Pearse RM, Shulman MA, et al. Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet. 2018;391(10140):2631.
Abramowicz S, Roser SM. Medical management of patients undergoing dentoalveolar surgery. Oral Maxillofac Surg Clin. 27(3):345–52.
Benarroch-Gampel J, Sheffield KM, Duncan CB. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg. 2012;256(3):518–28.
Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am. 2003;87:7–40.
Priebe HJ. Preoperative cardiac management of the patient for non-cardiac surgery: an individualized and evidence-based approach. Br J Anaesthesia. 2011;107(1):83–96.
Eagle KA, Rihal CS, Mickel MC, et al. Cardiac risk of non-cardiac surgery: influence of coronary disease and type of surgery in 3,368 operations. Circulation. 1997;96:1882–7.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Gutta, R. (2022). Management of the Cardiac Patient. In: Meara, D.J., Gutta, R. (eds) Oral and Maxillofacial Surgery for the Medically Compromised Patient. Springer, Cham. https://doi.org/10.1007/978-3-030-82598-0_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-82598-0_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82597-3
Online ISBN: 978-3-030-82598-0
eBook Packages: MedicineMedicine (R0)