Abstract
The most common form of congenital adrenal hyperplasia (CAH) is due to 21-hydroxylase deficiency (21-OHd), a disease that is inherited in an autosomal recessive pattern due to the presence of pathogenic genetic variants in CYP21A2 gene. Close to this gene, there is a pseudogene CYP21A1P containing many pathogenic variants that render it inactive. During meiosis, one or more of these genetic variants can be transferred to the active gene and cause total or partial 21-hydroxylase deficiency. In general, the disease is considered as having three major clinical presentations. The classical forms, salt-wasting, and simple virilizing forms are the ones with the most severe reduction of enzymatic activity. The rest of the cases have milder genetic alterations that allow the production of 21-hydroxylase enzyme with only some reduction of its activity (40–80%) in a condition known as the non-classical CAH (NCAH). There is a close correlation between genetic alterations and the resulting phenotypes (>90%). Due to the gene and the pseudogene localization and the genetic complexity of the region, intergenic recombination events are generally the cause of CYP21A2 pathogenic modifications (micro-conversions, gene deletions, gene duplications, and the formation of chimeric genes).
Sequencing of the entire gene by PCR mutation-detection methods together with multiplex ligation-dependent probe amplification (MLPA) that allows the detection of variations in the copy numbers of genes has become the golden standard for studying the CYP21A2 gene. Familial segregation studies in which both parents are studied together with the proband should always be done. This complete study procedure allows the confirmation of the diagnosis, establishment of correlations between the genotype and phenotype predicting the severity status of the patients, and especially a correct genetic counseling for any couple wishing to conceive.
Until now, more than 250 pathogenic genetic variants capable of causing 21-hydroxylase deficiency have been identified. The majority of patients (~80%) are compound heterozygotes. Two thirds of NCAH patients carry a non-classical (mild) mutation together with a classical, more severe mutation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adrenal Hyperplasia, Congenital
- 21-Hydroxylase deficiency
- Genetic association studies
- Disorders of sex development (DSD)
- Adrenal cortex diseases
Introduction
Congenital adrenal hyperplasia (CAH) results from the insufficient synthesis of cortisol occurring as a consequence of a deficiency of one of the steroidogenic enzymes being inherited as an autosomal recessive disorder. Approximately 95% of the cases are due to 21-hydroxylase deficiency (21-OHd), an enzyme that is expressed mostly in the adrenal cortex [1]. It is one of the most common autosomal recessive diseases, but in spite of the progress in its genetic diagnosis, this is a complex process with significant risk of attaining incomplete results and consequently misunderstanding of the real situation [2].
Congenital adrenal hyperplasia due to 21-hydroxylase deficiency (21-OHd) encompasses very important disorders with high morbidity (the classical forms) or high prevalence (the non-classical forms). They affect patients’ life in many ways, ranging from salt-wasting and life-threatening crises to virilization with variable degrees of genital ambiguity and with all its consequences of gender determination and reconstructive surgeries, ultimately affecting normal sexuality and reproduction. Some researchers believe that this division of the classical forms is artificial as the simple virilizing forms may also have variable degrees of salt wasting [3,4,5]. Finally, there are non-classical forms (NCAH) in which the enzymatic activity is significantly retained, between 20% and 60%, and in consequence, there is normal production of mineralocorticoids and cortisol. The resulting phenotype is characterized mainly by androgen excess that is less severe or occurring later in life; nevertheless, it may have an impact on sexuality and reproduction or simply affect the patients’ self-esteem and quality of life. One of the important features of NCAH forms is their much higher prevalence in comparison to the classical forms.
Genetic alterations of classical salt-wasting forms lead to complete absence of 21-OH enzyme and consequently to impairment of cortisol and aldosterone biosynthesis. In classical forms, there is also highly increased secretion of androgens, clinically translated in female virilization and precocious puberty in males. This is the result of increase in ACTH production (or action) due to enzymatic blockage causing loss of feedback control upon hypothalamus and pituitary, leading to diversion of steroidogenic precursors to the androgenic pathways.
Other pathogenic genetic variants cause less severe alterations that are compatible with the synthesis of 21-hydroxylase protein with some activity, some cortisol and aldosterone synthesis, in amounts that are compatible with life, without the need for their replacement. In those cases, the disease manifests itself later in life, mostly by excessive production of androgens. In these “non-classical,” less severe forms, hyperandrogenemia also results from diversion of 17-hydroxyprogesterone and other precursors to alternative androgenic pathways in spite of the fact that cortisol is not significantly reduced, and ACTH not significantly elevated (altered enzymatic kinetics).
The Structure of the CYP21A2 Gene
CYP21A2 is located in chromosome 6, in the area of the human histocompatibility complex (HLA), a region with a complex organization of genes with a great variability in their size and copy numbers [6,7,8,9].
At a distance of 30 kb from CYP21A2, there is a pseudogene (e.g., a nonfunctional gene) named CYP21A1P. Both the functional gene and the pseudogene have ten exons and share a high level of genetic homology (98% in their exons and 96% in their introns) [10, 11]. The pseudogene is inactive because it accumulates a great number of mutations along human development.
The neighbor localization and high homology of these two genes frequently lead to misalignments during meiosis that result in recombinations between the gene and the pseudogene (gene conversions) that are responsible for the majority of point mutations in the CYP21A2 gene.
In close proximity to these two genes, there are other genes in the same region of chromosome 6 forming genetic units called RCCX. These genes are RP1, C4, TNXB, and two truncated pseudogenes, RP2 and TNXA [12]. The genes C4B and C4A are translated into the fourth component of complement [13, 14], while TNXB encodes for an extracellular matrix protein called tenascin-X23 and the RP1 gene gives origin to a serine/threonine nuclear protein kinase [12]. The proximity of CYP21A2 and TNXB genes explains the existence of a syndrome with simultaneous characteristics of CAH and of the hypermobility type of Ehlers–Danlos syndrome, resulting from the simultaneous deletion of both genes the CAH-X syndrome [15,16,17].
The most common organization of this region has two RCCX modules, one with the CYP21A1P pseudogene and the other with the CYP21A2 active gene. The orientation of the genes from telomere to centromere is: RP1-C4A-CYP21A1P-TNXARP2-C4B-CYP21A2-TNXB. The haplotype, bearing two RCCX modules, is present in about 69% of the Caucasian population [18]. A mono-modular haplotype may also occur (in 17% of the population) while a “three modular” haplotype has been reported to be present in 14% [12, 19]. Most haplotypes with three modules have two copies of the CYP21A1P pseudogene and one copy of the CYP21A2 gene. Two copies of the CYP21A2 gene and only one of the CYP21A1P can also occur, and this situation has been described particularly in cases with the p.(Gln319∗) pathogenic variant and of chimeric CYP21A1P/CYP21A2 genes [19,20,21].
Another important aspect of the structure of CYP21A2 gene is the high prevalence of copy number variations, which in conjunction with the enormous amount of possible genetic variants makes the characterization of CYP21A2 alleles a difficult task. Genetic variants have been identified in both the coding and non-coding regions of the gene inclusively in the 5′UTR and the 3′UTR regions. Consequently, it is recognized that the characterization of the gene must include the sequencing of every exon, and the intron–exon boundaries [2].
The Origin of CYP21A2 Alterations
Due to gene and the pseudogene localization and genetic complexity of the region, recombination events are generally the cause of CYP21A2 pathogenic modifications.
In fact, intergenic recombinations between the inactive and active genes (gene conversions) are responsible for more than 95% of the pathogenic variants causing 21-OHd [22, 23].
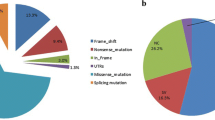
About 75% of the pathogenic variants are transferred by small conversions from the pseudogene during meiosis. These conversions can involve one or more pseudogene variants. They are called “microconversions.”
In the other 20–25% of the cases, 21-OHd CAH results from gross misalignment (unequal crossing over) during meiosis. Gene deletions, gene duplications, and deletions involving CYP21A2 and other contiguous genes usually ensue [24, 25]. Rarely, 21-OHd CAH can also be the result of uniparental isodisomy [26].
More than 250 genetic variants of CYP21A2 are capable of causing human disease. The majority of these will result in classical forms of 21-OHd [27]. One hundred and fifty-three genetic variants have been demonstrated to be missense mutations and have been shown to be able to give rise to all forms of the disease. On the contrary, nonsense and frameshift mutations always result in classical 21-OHd forms [27].
Genetic variants have also been observed in non-translated regions of CYP21A2 gene. Some of these affect the promoter, resulting in promoter conversion [28].
Sequencing of CYP21A2 gene in suspected cases should be considered essential in modern medical practice. Only a complete genetic analysis can accurately determine the genotype as pathogenic variants are frequently complex [2].
The effort to genotype a patient with 21-OHd used to be simplified by focusing on a group of 10 pathogenic variants that account for the majority of affected alleles. However, a recent study has demonstrated that this targeted CYP21A2 mutation analysis may fail to identify mutations on one allele in 10.4% of the cases [29].
In light of the present knowledge, familial segregation studies should always be done, as this is the only way to be sure if two detected pathogenic variants affect the two alleles (trans configuration) or are located in the same allele with the other one being normal (cis configuration). For this purpose, both parents have to be studied together with the proband.
CYP21A2 Genetic Modifications
There are large rearrangements and small conversions. Large recombinations result from unequal crossing over during meiosis. The other type of conversion occurs when a small segment of the functional CYP21A2 gene is replaced by a segment from the CYP21A1P pseudogene (microconversions). The altered CYP21A2 gene will carry a point mutation or a short sequence that may involve one or more exons [10, 22, 30, 31]. As these variants are pathologic, they will give rise to an inactive or at least significantly modified 21-hydroxylase protein.
Large Deletions and Conversions
Approximately 25% of alleles carry a deletion, a large gene conversion, or the formation of an inactive chimeric gene which is the result from a meiotic recombination in which the final product is a non-functional chimeric gene with its 5′ end belonging to CYP21A1P and the 3′ end to CYP21A2 [32,33,34].
Several different chimeric CYP21A1P/CYP21A2 genes have been identified as a result of variable length deletions [35,36,37,38].
Point Mutations and Small Deletions/Insertions
Most of the intergenic recombinations (75%) correspond to abnormal nucleotides normally present in the pseudogene that are transferred to the functional gene by microconversion [30] (Fig. 1). Other rearrangements have been reported including a deletion of 10 nucleotides in exon 8 or a duplication of 16 nucleotides in exon 9.
Other Pathogenic Variants
Many different pathogenic variants have been described, and these will certainly increase with the widespread utilization of molecular diagnosis. Most of these are rare, having been described in only one case or one family (see http://www.cypalleles.ki.se/cyp21.htm and http://www.hgmd.cf.ac.uk). Less than 5% of the pathogenic variants are not caused by gene conversions and so possibly not being presented in the pseudogene [39, 40].
Polymorphisms
Some alterations that are found in CYP21A2 gene do not affect 21-hydroxylase production and are considered normal polymorphisms [6]. It is possible that at least some of these variants are also present in the CYP21A1P gene and represent gene conversions not affecting the enzyme. However, others have been described only in the CYP21A2 gene.
Genotyping and Pregnancy
Genotyping of CYP21A2 gene as completely as possible is very important in couples who wish to conceive. It allows confirmation of the diagnosis, and it is the only way to do a correct genetic counseling.
The parents want to learn about the risk of having a child with salt-wasting form or with severe virilization in case of a female fetus.
It is well established that in a woman with CAH, the progeny will or will not have CAH according to the partner’s genotype. If he does not have any pathogenic variant in CYP21A2, the children will be carriers but will not have the disease. If the woman’s genotype consists of two mild pathogenic variants, for instance being homozygotic for V281L (p.(Val282Leu)) and her partner is a carrier of a CYP21A2 pathogenic variant, whatever it may be, 50% of her children will have NCAH (50% being merely a mathematical probability). However, in general population, the probability of being a carrier for a severe pathogenic variant is 1.7% (1 in 60) [41], and consequently the risk for having a child with classical 21-OHd CAH would be expected to be 1:600 since it has been reported that the probability of a patient with NCAH having a severe pathogenic variant together with a mild one is approximately 2/3 [29, 42]. The real frequency is however closer to 2.5% [43], and this made researchers believe that the carrier frequency is probably higher than is suspected, at least in some populations.
The actual recommendation is that both parents should be genotyped whenever possible as part of the prenatal study protocol if one potential progenitor has any form of 21-OHd CAH [43].
Genotype–Phenotype Correlations
There is a good correlation between genotype and the phenotype generally reported as reaching 90% or even 95% [33, 44,45,46,47]. However, it must be acknowledged that the clinical picture resulting from 21-hydroxylase deficiency is in fact a continuum of reductions in enzyme activity, and the three degrees of severity, in which the disease is generally classified, are only a simplification aiming to facilitate the clinical practice [48]. It is also recognized that the phenotypes can change with time and consequently a perfect correlation between genotype and phenotype is virtually impossible.
There are genetic variants that are considered to cause the most severe forms of the disease (100% enzymatic deficiency), resulting in salt-wasting forms of the classical 21-OHd CAH. These variants are called severe pathogenic variants (Fig. 2).
The missense pathogenic variant I172N (p.(Ile173Asn)) confers around 1–2% of the normal 21-hydroxylase activity, and even this small enzymatic activity is sufficient for a near normal aldosterone production, reducing almost completely the risk of salt-wasting crises. Thus, it is associated with the simple virilizing form of 21-OHd CAH. Pathogenic variants, as this one, are called the intermediate pathogenic variants (Fig. 2).
Other pathogenic variants including P30L, P453S, R339H, R369W, I230T [48], and V281L (clearly the most frequent pathogenic variant in NCAH cases in most series) result in a conservation of enzymatic activity of 20–60% and consequently in less severe symptoms which are associated with the non-classical form (mild pathogenic variants) (Fig. 2).
Importantly, most of the 21-hydroxylase-deficient patients are compound heterozygotes instead of homozygotes [3, 29], and this implies that the phenotype is generally affected by the residual 21-hydroxylase activity and consequently by the less severely mutated allele [33]:
-
1.
The most severe phenotypes (the classical forms) must have severe pathogenic variants in both alleles and none of the mild pathogenic variants.
-
2.
The NCAH patients may have two mild mutations (a situation that occurs in approximately 35% of the cases) or one mild and one severe ones (in the other 65%).
A mild pathogenic variant present in one of the alleles allows the synthesis of 21-hydroxylase up to 50–60% of the normal activity, in spite of the fact that the severe pathogenic variant, present in the other allele, does not contribute to any synthesis.
Although the correlation between genotype and phenotype is high, it has been observed that some of these pathogenic variants confer different phenotypes depending on if they are isolated or associated with another pathogenic variant.
In fact, there is some diversity of the phenotypes in patients with less severe pathogenic variants [5, 33, 49, 50]. It has been reported that in spite of being predictable that the phenotype will correspond to the less severely affected allele, the presence of a second allele with a more severe pathogenic variant can result in a more serious clinical phenotype [51,52,53,54,55] with higher degrees of hirsutism and also of higher 17OHP levels than cases with two mild genetic alterations [42, 56].
The pathogenic variants IVS2-13 (c.293-13A/C>G) and I172N (p.(Ile173Asn)) can result in variable degrees of 21-hydroxylase activity (possibly through alternative splicing). Patients with these mutations are generally expected to be simple-virilizing cases but may sometimes present as salt-wasting forms, and the others may stand closer to NCAH [34, 57]. Similarly, the pathogenic variant P30L (p.(Pro31Leu)) which is considered to be a cause of NCAH has been reported to be responsible for cases of SV-CAH, as well [58, 59]. Considering about the residual 21-hydroxylase activity which is expected to present with P30L mutation, this is unexpected. The NCAH patients with P30L mutation can exhibit stronger virilization with clitoromegaly and advanced bone maturation [60]. Different mechanisms have been proposed for the increased androgenization of these patients, including the influence of other residues, accompanying promotor variability or mutations and finally variations in individual androgen sensitivity [59]. In fact, some concomitant factors capable of modifying the phenotype have been suggested such as the number of CAG repeats of the androgen receptor, other genes encoding proteins with 21-hydroxylase activity and alternative pathways of androgen biosynthesis capable of causing fetal virilization in females [59].
In a multi-national study of 1507 families with CAH, SV form of CAH was found in 17/74 patients having P30L mutation (23%) [46].
Even when it does not clearly result in a SV form, the clinical manifestations in patients with P30L will include stronger signs of virilization, earlier adrenarche, clitoromegaly, and some patients require treatment with glucocorticoids compared to other patients with NCAH form [39, 44, 61].
Moreover, genotypes P30L/I2 splice, P30L/Q318X, and P30L/8Δbp are especially associated with SV form of CAH [46]. If not treated with glucocorticoids, SV progresses steadily during childhood causing early puberty, short adult stature, and fertility problems in both genders including testicular adrenal rest tumors (TARTs) in men.
Incomplete correlation between genotype and phenotype may also result from not sequencing the whole gene and hence not having a full picture of the genetic alterations.
Genetic Sequencing
Sequencing of the entire gene by PCR mutation-detection methods together with multiplex ligation-dependent probe amplification are the golden standard for studying the CYP 21A2 gene.
General Considerations
Specific gene amplification by PCR has dramatically improved the sensitivity of different techniques to detect CYP21A2 pathogenic variants. Modernly, PCR conditions have been identified that allow the amplification of CYP21A2 without amplifying the very homologous CYP21A1P pseudogene. These conditions result from amplifying CYP21A2 in two segments (Fig. 3).
Proposed strategy to whole-gene sequence of the CYP21A2 gene. Numbered boxes represent CYP21A2 exons and arrows represent the primers. Adapted from [62]
PCR-based diagnosis may be complicated by the failure of amplifying one haplotype which may result in misdiagnosis. Examination of flanking microsatellite markers in all family members can minimize this problem.
Another important aspect is that, if only a DNA sample from the patient is analyzed, it is impossible to distinguish compound heterozygosity for different pathogenic variants occurring in trans and the presence of two pathogenic variants in the same allele (cis). Therefore, ideally both parents should also be analyzed, so as to most reliably determine the phase of different pathogenic variants (i.e., whether they lie on the same or opposite alleles). Analysis of parental alleles also permits differentiating homozygotes and hemizygotes (i.e., individuals who have a pathogenic variant on one chromosome and a deletion on the other).
DNA Sanger Sequencing
The whole-gene sequencing (together with MLPA) has become the standard procedure in cases of 21-OHd. It usually covers the coding regions and the flanking intron–exon regions of the gene. This method not only detects the more common genetic variants but can also detect the novel sequence variants helping to explain some cases in which there has not been a correlation between genotype and phenotype. Main difficulty results from the homology between the gene and the pseudogene. To avoid the co-amplification of pseudogene CYP21A2, whole-genomic sequencing may be performed selecting the functional CYP21A2 gene and amplifying it by PCR into two partially overlapping fragments, P1 and P2 respectively with one 517 and two 214 base-pairs (bp). After selective amplification of the targeted gene and subsequent purification, the PCR product is sequenced with internal primers that cover the entire CYP21A2 gene [62].
MLPA
After sequencing the entire CYP21A2 gene, one should also look for deletions and duplications [63, 64]. This is currently done using multiplex ligation-dependent probe amplification (MLPA) [65].
The MLPA assay is a technique that enables the detection of variations in the copy number of several human genes. Due to the large number of genes or genetic sequences that can be simultaneously analyzed, MLPA assay has become the gold standard for molecular analysis of all pathologies derived from the presence of gene copy number variation [66]. Besides, MLPA can be used to confirm the point mutations identified by sequencing analysis.
Detection of deletions and duplications of CYP21A2 gene and the CYP21A1P pseudogene by MLPA is currently performed, using the P050-CAH Kit (MRC-Holland). This high-resolution method uses only a single pair of PCR primers, and the specificity relies on the use of progressively longer oligonucleotide probes, in order to generate locus-specific amplicons of increasing size that can be resolved electrophoretically. Comparing the peak pattern obtained to that of the reference samples, it is possible to determine which probes/locus show abnormal copy numbers [67, 68].
Final Considerations
Genotyping can also be used for disease prevention. Preimplantation genetic diagnosis is increasingly being performed to limit the transmission of several diseases, including CAH, being used in conjunction with in vitro fertilization.
Another aspect of prenatal diagnosis consists of early gender determination, by the detection of SRY in circulating fetal DNA that is present in maternal blood at very early stages of pregnancy. This allows the identification of male fetuses that do not need to be treated prenatally. In case of a female fetus, obstetricians and pediatricians may treat these cases during early stages of pregnancy to prevent genital ambiguity. It is also possible to perform sequencing methods of the CYP21A2 gene in circulating fetal DNA, but this is complex and still carries a significant possibility of false positives or false negatives. Chorionic villus sampling and amniocentesis can still be used for prenatal treatment to prevent the masculinization of external genitalia of the female fetus with classical 21-OHd; however, they are performed rather late [2, 29, 69].
References
Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005;365:2125–36. https://doi.org/10.1016/S0140-6736(05)66736-0.
Pignatelli D, Carvalho BL, Palmeiro A, Barros A, Guerreiro SG, Macut D. The complexities in genotyping of congenital adrenal hyperplasia: 21-hydroxylase deficiency. Front Endocrinol (Lausanne). 2019;10:432. https://doi.org/10.3389/fendo.2019.00432.
Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383:1248–61. https://doi.org/10.1056/NEJMra1909786.
Nimkarn S, Lin-Su K, Berglind N, Wilson RC, New MI. Aldosterone-to-renin ratio as a marker for disease severity in 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2007;92:137–42. https://doi.org/10.1210/jc.2006-0964.
Riedl S, Röhl F-W, Bonfig W, Brämswig J, Richter-Unruh A, Fricke-Otto S, et al. Genotype/phenotype correlations in 538 congenital adrenal hyperplasia patients from Germany and Austria: discordances in milder genotypes and in screened versus prescreening patients. Endocr Connect. 2019;8:86–94. https://doi.org/10.1530/EC-18-0281.
White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr Rev. 2000;21:245–91. https://doi.org/10.1210/edrv.21.3.0398.
Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003;349:776–88. https://doi.org/10.1056/NEJMra021561.
Parajes S, Quinterio C, Dominguez F, Loidi L. A simple and robust quantitative PCR assay to determine CYP21A2 gene dose in the diagnosis of 21-hydroxylase deficiency. Clin Chem. 2007;53:1577–84. https://doi.org/10.1373/clinchem.2007.087361.
Parajes S, Quinteiro C, Dominguez F, Loidi L. High frequency of copy number variations and sequence variants at CYP21A2 locus: implication for the genetic diagnosis of 21-hydroxylase deficiency. PLoS One. 2008;3:e2138. https://doi.org/10.1371/journal.pone.0002138.
Higashi Y, Yoshioka H, Yamane M, Gotoh O, Fujii-Kuriyama Y. Complete nucleotide sequence of two steroid 21-hydroxylase genes tandemly arranged in human chromosome: a pseudogene and a genuine gene. Proc Natl Acad Sci U S A. 1986;83:2841–5. https://doi.org/10.1073/pnas.83.9.2841.
White PC, New MI, Dupont B. Structure of human steroid 21-hydroxylase genes. Proc Natl Acad Sci U S A. 1986;83:5111–5. https://doi.org/10.1073/pnas.83.14.5111.
Yang Z, Mendoza AR, Welch TR, Zipf WB, Yu CY. Modular variations of the human major histocompatibility complex class III genes for serine/threonine kinase RP, complement component C4, steroid 21-hydroxylase CYP21, and tenascin TNX (the RCCX module). A mechanism for gene deletions and disease associations. J Biol Chem. 1999;274:12147–56. https://doi.org/10.1074/jbc.274.17.12147.
Carroll MC, Campbell RD, Porter RR. Mapping of steroid 21-hydroxylase genes adjacent to complement component C4 genes in HLA, the major histocompatibility complex in man. Proc Natl Acad Sci U S A. 1985;82:521–5. https://doi.org/10.1073/pnas.82.2.521.
White PC, New MI, Dupont B. Adrenal 21-hydroxylase cytochrome P-450 genes within the MHC class III region. Immunol Rev. 1985;87:123–50. https://doi.org/10.1111/j.1600-065x.1985.tb01148.x.
Merke DP, Chen W, Morissette R, Xu Z, Van Ryzin C, Sachdev V, et al. Tenascin-X haploinsufficiency associated with Ehlers-Danlos syndrome in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2013;98:E379–87. https://doi.org/10.1210/jc.2012-3148.
Miller WL, Merke DP. Tenascin-X, congenital adrenal hyperplasia, and the CAH-X syndrome. Horm Res Paediatr. 2018;89:352–61. https://doi.org/10.1159/000481911.
Kolli V, Kim H, Rao H, Lao Q, Gaynor A, Milner JD, et al. Measurement of serum tenascin-X in patients with congenital adrenal hyperplasia at risk for Ehlers-Danlos contiguous gene deletion syndrome CAH-X. BMC Res Notes. 2019;12:711. https://doi.org/10.1186/s13104-019-4753-7.
Saxena K, Kitzmiller KJ, Wu YL, Zhou B, Esack N, Hiremath L, et al. Great genotypic and phenotypic diversities associated with copy-number variations of complement C4 and RP-C4-CYP21-TNX (RCCX) modules: a comparison of Asian-Indian and European American populations. Mol Immunol. 2009;46:1289–303. https://doi.org/10.1016/j.molimm.2008.11.018.
Blanchong CA, Zhou B, Rupert KL, Chung EK, Jones KN, Sotos JF, et al. Deficiencies of human complement component C4A and C4B and heterozygosity in length variants of RP-C4-CYP21-TNX (RCCX) modules in Caucasians. The load of RCCX genetic diversity on major histocompatibility complex-associated disease. J Exp Med. 2000;191:2183–96. https://doi.org/10.1084/jem.191.12.2183.
Haglund-Stengler B, Martin Ritzén E, Gustafsson J, Luthman H. Haplotypes of the steroid 21-hydroxylase gene region encoding mild steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1991;88:8352–6. https://doi.org/10.1073/pnas.88.19.8352.
Koppens PF, Hoogenboezem T, Degenhart HJ. Duplication of the CYP21A2 gene complicates mutation analysis of steroid 21-hydroxylase deficiency: characteristics of three unusual haplotypes. Hum Genet. 2002;111:405–10. https://doi.org/10.1007/s00439-002-0810-7.
Higashi Y, Tanae A, Inoue H, Fujii-Kuriyama Y. Evidence for frequent gene conversion in the steroid 21-hydroxylase P-450(C21) gene: implications for steroid 21-hydroxylase deficiency. Am J Hum Genet. 1988;42:17–25.
Donohoue PA, Jospe N, Migeon CJ, Van Dop C. Two distinct areas of unequal crossingover within the steroid 21-hydroxylase genes produce absence of CYP21B. Genomics. 1989;5:397–406. https://doi.org/10.1016/0888-7543(89)90002-5.
Miller WL. Clinical review 54: genetics, diagnosis, and management of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1994;78:241–6. https://doi.org/10.1210/jcem.78.2.8106606.
Speiser PW. Molecular diagnosis of CYP21 mutations in congenital adrenal hyperplasia: implications for genetic counseling. Am J Pharmacogenomics. 2001;1:101–10. https://doi.org/10.2165/00129785-200101020-00003.
Parker EA, Hovanes K, Germak J, Porter F, Merke DP. Maternal 21-hydroxylase deficiency and uniparental isodisomy of chromosome 6 and X results in a child with 21-hydroxylase deficiency and Klinefelter syndrome. Am J Med Genet A. 2006;140:2236–40. https://doi.org/10.1002/ajmg.a.31408.
Simonetti L, Bruque CD, Fernández CS, Benavides-Mori B, Delea M, Kolomenski JE, et al. CYP21A2 mutation update: comprehensive analysis of databases and published genetic variants. Hum Mutat. 2018;39:5–22. https://doi.org/10.1002/humu.23351.
Araújo RS, Mendonca BB, Barbosa AS, Lin CJ, Marcondes JA, Billerbeck AE, et al. Microconversion between CYP21A2 and CYP21A1P promoter regions causes the nonclassical form of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92:4028–34. https://doi.org/10.1210/jc.2006-2163.
Finkielstain GP, Chen W, Mehta SP, Fujimura FK, Hanna RM, Van Ryzin C, et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2011;96:E161–72. https://doi.org/10.1210/jc.2010-0319.
Tusié-Luna MT, White PC. Gene conversions and unequal crossovers between CYP21 (steroid 21-hydroxylase gene) and CYP21P involve different mechanisms. Proc Natl Acad Sci U S A. 1995;92:10796–800. https://doi.org/10.1073/pnas.92.23.10796.
Wedell A. Molecular genetics of congenital adrenal hyperplasia (21-hydroxylase deficiency): implications for diagnosis, prognosis and treatment. Acta Paediatr. 1998;87:159–64. https://doi.org/10.1080/08035259850157598.
White PC, Vitek A, Dupont B, New MI. Characterization of frequent deletions causing steroid 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 1988;85:4436–40. https://doi.org/10.1073/pnas.85.12.4436.
Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000;85:1059–65. https://doi.org/10.1210/jcem.85.3.6441.
Stikkelbroeck NM, Hoefsloot LH, de Wijs IJ, Otten BJ, Hermus AR, Sistermans EA. CYP21 gene mutation analysis in 198 patients with 21-hydroxylase deficiency in the Netherlands: six novel mutations and a specific cluster of four mutations. J Clin Endocrinol Metab. 2003;88:3852–9. https://doi.org/10.1210/jc.2002-021681.
White PC, New MI, Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984;81:7505–9. https://doi.org/10.1073/pnas.81.23.7505.
L’Allemand D, Tardy V, Grüters A, Schnabel D, Krude H, Morel Y. How a patient homozygous for a 30-kb deletion of the C4-CYP 21 genomic region can have a nonclassic form of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2000;85:4562–7. https://doi.org/10.1210/jcem.85.12.7018.
Lee HH, Chang SF, Lee YJ, Raskin S, Lin SJ, Chao MC, et al. Deletion of the C4-CYP21 repeat module leading to the formation of a chimeric CYP21P/CYP21 gene in a 9.3-kb fragment as a cause of steroid 21-hydroxylase deficiency. Clin Chem. 2003;49:319–22. https://doi.org/10.1373/49.2.319.
Concolino P, Mello E, Minucci A, Giardina E, Zuppi C, Toscano V, et al. A new CYP21A1P/CYP21A2 chimeric gene identified in an Italian woman suffering from classical congenital adrenal hyperplasia form. BMC Med Genet. 2009;10:72. https://doi.org/10.1186/1471-2350-10-72.
de Carvalho DF, Miranda MC, Gomes LG, Madureira G, Marcondes JA, Billerbeck AE, et al. Molecular CYP21A2 diagnosis in 480 Brazilian patients with congenital adrenal hyperplasia before newborn screening introduction. Eur J Endocrinol. 2016;175:107–16. https://doi.org/10.1530/eje-16-0171.
Neocleous V, Fanis P, Phylactou LA, Skordis N. Genotype is associated to the degree of virilization in patients with classic congenital adrenal hyperplasia. Front Endocrinol (Lausanne). 2018;9:733. https://doi.org/10.3389/fendo.2018.00733.
Speiser PW, Dupont B, Rubinstein P, Piazza A, Kastelan A, New MI. High frequency of nonclassical steroid 21-hydroxylase deficiency. Am J Hum Genet. 1985;37:650–67.
Bidet M, Bellanné-Chantelot C, Galand-Portier MB, Tardy V, Billaud L, Laborde K, et al. Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. J Clin Endocrinol Metab. 2009;94:1570–8. https://doi.org/10.1210/jc.2008-1582.
Moran C, Azziz R, Weintrob N, Witchel SF, Rohmer V, Dewailly D, et al. Reproductive outcome of women with 21-hydroxylase-deficient nonclassic adrenal hyperplasia. J Clin Endocrinol Metab. 2006;91:3451–6. https://doi.org/10.1210/jc.2006-0062.
Zhang B, Lu L, Lu Z. Molecular diagnosis of Chinese patients with 21-hydroxylase deficiency and analysis of genotype-phenotype correlations. J Int Med Res. 2017;45:481–92. https://doi.org/10.1177/0300060516685204.
Haider S, Islam B, D’Atri V, Sgobba M, Poojari C, Sun L, et al. Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc Natl Acad Sci U S A. 2013;110:2605–10. https://doi.org/10.1073/pnas.1221133110.
New MI, Abraham M, Gonzalez B, Dumic M, Razzaghy-Azar M, Chitayat D, et al. Genotype-phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc Natl Acad Sci U S A. 2013;110:2611–6. https://doi.org/10.1073/pnas.1300057110.
Narasimhan ML, Khattab A. Genetics of congenital adrenal hyperplasia and genotype-phenotype correlation. Fertil Steril. 2019;111:24–9. https://doi.org/10.1016/j.fertnstert.2018.11.007.
Witchel SF, Azziz R. Nonclassic congenital adrenal hyperplasia. Int J Pediatr Endocrinol. 2010;2010:625105. https://doi.org/10.1155/2010/625105.
Speiser PW, Dupont J, Zhu D, Serrat J, Buegeleisen M, Tusie-Luna MT, et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Invest. 1992;90:584–95. https://doi.org/10.1172/jci115897.
Wedell A, Ritzén EM, Haglund-Stengler B, Luthman H. Steroid 21-hydroxylase deficiency: three additional mutated alleles and establishment of phenotype-genotype relationships of common mutations. Proc Natl Acad Sci U S A. 1992;89:7232–6. https://doi.org/10.1073/pnas.89.15.7232.
Nikoshkov A, Lajic S, Holst M, Wedell A, Luthman H. Synergistic effect of partially inactivating mutations in steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1997;82:194–9. https://doi.org/10.1210/jcem.82.1.3678.
Tardy V, Menassa R, Sulmont V, Lienhardt-Roussie A, Lecointre C, Brauner R, et al. Phenotype-genotype correlations of 13 rare CYP21A2 mutations detected in 46 patients affected with 21-hydroxylase deficiency and in one carrier. J Clin Endocrinol Metab. 2010;95:1288–300. https://doi.org/10.1210/jc.2009-1202.
Helmberg A, Tusie-Luna MT, Tabarelli M, Kofler R, White PC. R339H and P453S: CYP21 mutations associated with nonclassic steroid 21-hydroxylase deficiency that are not apparent gene conversions. Mol Endocrinol. 1992;6:1318–22. https://doi.org/10.1210/mend.6.8.1406709.
Dolzan V, Sólyom J, Fekete G, Kovács J, Rakosnikova V, Votava F, et al. Mutational spectrum of steroid 21-hydroxylase and the genotype-phenotype association in Middle European patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2005;153:99–106. https://doi.org/10.1530/eje.1.01944.
Menassa R, Tardy V, Despert F, Bouvattier-Morel C, Brossier JP, Cartigny M, et al. p.H62L, a rare mutation of the CYP21 gene identified in two forms of 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2008;93:1901–8. https://doi.org/10.1210/jc.2007-2701.
Speiser PW, Knochenhauer ES, Dewailly D, Fruzzetti F, Marcondes JA, Azziz R. A multicenter study of women with nonclassical congenital adrenal hyperplasia: relationship between genotype and phenotype. Mol Genet Metab. 2000;71:527–34. https://doi.org/10.1006/mgme.2000.3036.
Higashi Y, Tanae A, Inoue H, Hiromasa T, Fujii-Kuriyama Y. Aberrant splicing and missense mutations cause steroid 21-hydroxylase [P-450(C21)] deficiency in humans: possible gene conversion products. Proc Natl Acad Sci U S A. 1988;85:7486–90. https://doi.org/10.1073/pnas.85.20.7486.
Barbaro M, Soardi FC, Östberg LJ, Persson B, de Mello MP, Wedell A, et al. In vitro functional studies of rare CYP21A2 mutations and establishment of an activity gradient for nonclassic mutations improve phenotype predictions in congenital adrenal hyperplasia. Clin Endocrinol. 2015;82:37–44. https://doi.org/10.1111/cen.12526.
Kocova M, Anastasovska V, Falhammar H. Clinical outcomes and characteristics of P30L mutations in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocrine. 2020;69:262–77. https://doi.org/10.1007/s12020-020-02323-3.
Weintrob N, Dickerman Z, Sprecher E, Galatzer A, Pertzelan A. Non-classical 21-hydroxylase deficiency in infancy and childhood: the effect of time of initiation of therapy on puberty and final height. Eur J Endocrinol. 1997;136:188–95. https://doi.org/10.1530/eje.0.1360188.
Tankoska M, Anastasovska V, Krstevska-Konstantinova M, Naydenov M, Kocova M. Therapeutic challenges in a patient with the simple virilizing (SV) form of congenital adrenal hyperplasia (CAH) due to the P30L/I172N genotype. J Pediatr Endocrinol Metab. 2019;32:543–7. https://doi.org/10.1515/jpem-2018-0285.
Carvalho B, Pereira M, Marques CJ, Carvalho D, Leão M, Oliveira JP, et al. Comprehensive genetic analysis and structural characterization of CYP21A2 mutations in CAH patients. Exp Clin Endocrinol Diabetes. 2012;120:535–9. https://doi.org/10.1055/s-0032-1323805.
Kleinle S, Lang R, Fischer GF, Vierhapper H, Waldhauser F, Födinger M, et al. Duplications of the functional CYP21A2 gene are primarily restricted to Q318X alleles: evidence for a founder effect. J Clin Endocrinol Metab. 2009;94:3954–8. https://doi.org/10.1210/jc.2009-0487.
Parajes S, Quinteiro C, Domínguez F, Loidi L. High frequency of copy number variations and sequence variants at CYP21A2 locus: implication for the genetic diagnosis of 21-hydroxylase deficiency. PLoS One. 2008;3:e2138. https://doi.org/10.1371/journal.pone.0002138.
Concolino P, Mello E, Toscano V, Ameglio F, Zuppi C, Capoluongo E. Multiplex ligation-dependent probe amplification (MLPA) assay for the detection of CYP21A2 gene deletions/duplications in congenital adrenal hyperplasia: first technical report. Clin Chim Acta. 2009;402:164–70. https://doi.org/10.1016/j.cca.2009.01.008.
Krone N, Braun A, Weinert S, Peter M, Roscher AA, Partsch CJ, et al. Multiplex minisequencing of the 21-hydroxylase gene as a rapid strategy to confirm congenital adrenal hyperplasia. Clin Chem. 2002;48:818–25.
Schouten JP, McElgunn CJ, Waaijer R, Zwijnenburg D, Diepvens F, Pals G. Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification. Nucleic Acids Res. 2002;30:e57. https://doi.org/10.1093/nar/gnf056.
Krone N, Riepe FG, Partsch CJ, Vorhoff W, Brämswig J, Sippell WG. Three novel point mutations of the CYP21 gene detected in classical forms of congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Exp Clin Endocrinol Diabetes. 2006;114:111–7. https://doi.org/10.1055/s-2005-872841.
Witchel SF. Congenital adrenal hyperplasia. J Pediatr Adolesc Gynecol. 2017;30:520–34. https://doi.org/10.1016/j.jpag.2017.04.001.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Pignatelli, D., Pereira, S.S. (2021). Congenital Adrenal Hyperplasia Due to 21-Hydroxylase Deficiency: Genetic Characterization and the Genotype–Phenotype Correlation. In: Ertorer, M.E. (eds) Fertility and Reproductive Outcomes in Different Forms of Congenital Adrenal Hyperplasia. Springer, Cham. https://doi.org/10.1007/978-3-030-82591-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-82591-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82590-4
Online ISBN: 978-3-030-82591-1
eBook Packages: MedicineMedicine (R0)