Abstract
In this chapter, we review the incidence and natural history of bladder cancer at all stages in the United States and worldwide. Next, we highlight disparities in the diagnosis, treatment, and prognosis of bladder cancer, with particular focus on gender, race, insurance, and geography as important determinants of outcome. We also discuss the burden of financial toxicity in the management of muscle- and non-muscle-invasive bladder cancer for young, underinsured, and low-income patients in the United States. Finally, we summarize health-related quality-of-life issues surrounding survivorship, with respect to urinary diversion and sexual function after radical cystectomy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Incidence and Natural History
Bladder cancer is most common cancer globally, with over 500,000 new cases per year [1]. In the United States, it is the sixth most common cancer overall and the fourth most common cancer in men in 2020 following prostate, lung, and colon cancers [2]. Bladder cancer accounts for 4.5% of all new cancer diagnoses, with 81,400 new cases estimated for 2020 in the United States [2]. Bladder cancer is three to four times more common in men than in women, and the median age at diagnosis is 65–70 years [3].
Using statistical models via SEER, age-adjusted rates for new bladder cancer diagnoses have been falling on average 1.2% each year over 2008–2017 [2]. Age-adjusted death rates have been falling modestly on average 0.6% each year over 2009–2018 [2]. Five-year relative survival trends since 2000 are shown in Fig. 1.1.
Five-year survival trend of bladder cancer (all stages, genders, ethnicities). (Source: SEER 18 cancer registries. Created by https://seer.cancer.gov/explorer)
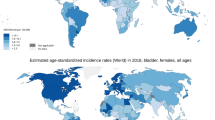
Bladder cancer incidence and mortality are variable worldwide, as shown on the heat maps in Figs. 1.2 and 1.3. While North America and Europe have the highest age-standardized incidence rates, the highest mortality rates appear to be concentrated in Northern Africa and parts of Europe. Regional differences in exposure to known risk factors such as cigarette smoking, occupational exposures, contaminated drinking water, and endemic chronic urinary infections by Schistosoma haematobium are all thought to be responsible for the observed variability in incidence, although access to care and the existence of robust registries are also key [4]. In contrast, variability in mortality rates is less drastic (Fig. 1.3). Reasons for this are less clear, perhaps related to less ambiguity in reporting deaths secondary to advanced bladder cancer related to muscle-invasive disease [5]. Currently, global efforts to reduce the burden of bladder cancer are centered around smoking cessation [6].
Age-standardized incidence rates of bladder cancer per 100,000 population globally. (Data source: GLOBOCAN 2020 Graph production: IARC (http://gco.iarc.fr/today) World Health Organization)
Age-standardized (world) mortality rates (per 100,000/year) of bladder cancer. (Data source: GLOBOCAN 2020 Graph production: IARC (http://gco.iarc.fr/today) World Health Organization)
Non-muscle Invasive Bladder Cancer
Malignant urothelial tumors that have not invaded the detrusor muscle layer are termed non-muscle invasive bladder cancer (NMIBC). About 70–80% of bladder tumors are NMIBC at presentation. Of these tumors, 70% are present as stage Ta, 20% as T1, and 10% as carcinoma in situ (CIS) [3].
NMIBC is associated with a high rate of recurrence, with increasing tumor grade predicting progression to muscle-invasive disease. For example, low-grade Ta tumors are associated with a high rate of tumor recurrence (15–70% at 1 year) but a low rate of progression to muscle-invasive disease (<5%). High-grade Ta tumors have a 13–40% chance of progressing to lamina propria invasion and a 6–25% chance of becoming muscle-invasive. T1 tumors have the worst malignant potential in terms of recurrence (80%) and progression (50% within 3 years). CIS is a noninvasive, high-grade tumor by definition and often coexists with other bladder tumors. CIS is associated with high rates of recurrence (82%) and progression (42–83%) especially if not treated with intravesical therapy.
In the absence of progression to muscle invasion , the long-term disease burden of NMIBC remains high. NMIBC is associated with a high symptom and health-related quality-of-life impact despite intravesical treatment. Disease management incurs high costs to healthcare systems , ranging from $2830 to $9554 per-patient Medicare expenditures per year [7].
Muscle-Invasive Bladder Cancer
Muscle-invasive bladder cancer (MIBC) is an aggressive disease associated with significant morbidity and mortality, ranging from T2 (invasion into muscularis propria) to T4 (local invasion of adjacent organs or musculature). Approximately 20–30% of bladder cancers are muscle-invasive at presentation. Unlike NMIBC, these cancers are biologically aggressive. 5-year overall survival, left untreated, approaches 5% [8]. In a population-based series of patients with newly diagnosed bladder cancer in Sweden, the untreated 5-year incidence of cancer-specific mortality was 86%, compared to 48% for treated patients. Untreated patients also had a higher risk of progression to metastatic disease (hazard ratio [HR] 2.40, 95% CI 1.28, 4.51), all-cause mortality (HR 2.63, 95% CI 1.65, 4.19), and cancer-specific mortality (HR 2.02, 95% CI 1.24, 3.30) [8]. Despite the introduction of neoadjuvant chemotherapy and innovations in extirpative and bladder preservation therapies, however, overall improvements in mortality for localized and regionalized disease have not been achieved in the past decade [9].
Metastatic Bladder Cancer
Approximately 4–5% of patients present with de novo metastatic disease and 50% progress after local therapy for muscle-invasive bladder cancer at 5 years [10]. An estimated 12,500 deaths per year in the United States are attributable to metastatic bladder cancer [11]. Via lymphatic and hematogenous channels, the spread of bladder cancer typically begins in the pelvic lymph nodes followed by the lungs, bones, liver, and brain. Prognosis is poor with cures rarely achieved, and the median survival of patients at diagnosis of metastatic urothelial cancer is 12 months though recently, the introduction of immune checkpoint inhibitors has demonstrated longer median survival compared to traditional chemotherapies.
Variant Histology
Variant histology comprises a heterogeneous group of tumors which include squamous, sarcomatoid, small cell/neuroendocrine , signet ring, micropapillary, and adenocarcinoma. Overall, variant histology is associated with worse overall survival, but these patients are often excluded from clinical trials. Furthermore, the individual characteristics and biology of each variant are not well understood. Interestingly, a study of 314,177 patients in NCDB found that younger patients (less than 40 years old) were more likely to have variant histology and that half of these cases were overrepresented by women who had worse overall survival [12].
National and Global Trends
In the United States, the overall incidence of bladder cancer is slowly increasing, likely due to improved diagnostic accuracy. Between 1973 and 2009, incidence increased from 21.0 to 25.5/100,000 person-years, driven largely by increase in localized and distant disease and paralleled by an equal decrease in unstaged disease [9]. Similarly, 5-year cancer-specific survival rates improved from 73.9% to 81.4% [9]. Population-based data show that while mortality rates for men with localized and regional disease have decreased over time, they have remained stable for women. Other contemporary studies have shown little change in overall or stage-specific relative survival, with underuse of neoadjuvant chemotherapy.
Disparities in the Diagnosis and Treatment of Bladder Cancer
Disparities exist in the diagnosis, treatment, and prognosis of bladder cancer nationally and worldwide. Screening rates can vary widely among patients which can lead to large differences in outcome, even among those with similar disease features. Currently, hematuria (gross or microscopic) remains the only indication for a bladder cancer workup, which consists of cystoscopy and upper tract imaging in the presence of risk factors [13]. A large proportion of patients are delayed in their referral for cystoscopy, and improvements in detection of earlier stages of disease have not occurred in the last three decades. In a recent study, only 42% of patients with documented hematuria with high-risk features were referred for further evaluation [14]. Access to care, lifestyle characteristics, such as smoking and obesity status, as well as education, referral patterns, and insurance status likely contribute to the observed disparities in diagnosis, treatment, and outcome. While differences in tumor biology may exist, nonbiological factors inevitably account for much of the variation, leading to health disparities linked to social, economic, and environmental disadvantage.
Gender
Although bladder cancer is more common among men, women with urothelial cancer are often diagnosed at higher tumor stages and tend to have a worse prognosis [15, 16]. These differences are even larger in squamous cell carcinoma, adenocarcinoma, and sarcoma of the bladder [17]. The stage migration may be due in part to delays in diagnosis, as women are more likely to undergo workup for cystitis and other benign causes before undergoing urology referral for cystoscopy. Women with bladder cancer are twice as likely to be diagnosed with a urinary tract infection than men prior to diagnostic workup [18]. Additionally, differences in referral patterns exist between men and women. At a Midwest managed care organization, 28% of women with hematuria were referred for urologic evaluation compared to 47% of men [19]. According to Medicare data, women experience greater geographic variation in cystoscopy rates than men when restricting to ICD-9 codes for hematuria only [20]. More recent investigations based on linked Surveillance, Epidemiology, and End Results (SEER)–Medicare data have corroborated that women are less promptly referred to a urologist and more likely to experience delays in hematuria evaluation [14].
Regarding overall prognosis, gender-specific differences are also known to exist in favor of men. White men of higher socioeconomic status have significantly longer survival times, compared to their non-White, female counterparts [21]. Even when controlling for stage, however, women appear to fare worse, with a higher risk of cancer-specific death within the first 3 years of follow-up [22]. In a recent study of 6809 patients with nonmetastatic MIBC, women were significantly more likely to receive a cystectomy compared to other bladder-preservation treatments yet found to have worse bladder cancer-specific survival than men, with no differences in overall survival [23].
Differences in hormone exposure, sex steroid receptor expression, social behaviors, environmental factors, and clinical management approach are thought to account for some of these differences. Importantly, hormonal pathways may drive subtype difference and thereby prognosis between men and women—in one study of 1000 bladder tumors, female tumors expressed higher levels of basal and immune-associated genes, while male tumors expressed higher levels of luminal markers and demonstrated higher androgen response activity [24]. A meta-analysis of 2049 patients from 13 retrospective studies found that the androgen receptor (AR) was downregulated in female tumors compared to male tumors, and in high-grade tumors compared to low-grade tumors [25]. Similarly, the estrogen receptor beta (ER) expression was higher in high-grade tumors compared to low grade and in muscle-invasive tumors compared to muscle-invasive. In NMIBC, ER was associated with worse recurrence-free survival [25].
In contrast, it appears that the use of neoadjuvant chemotherapy may equalize some of these differences. In a study of 1031 patients including 227 (22%) women, the female gender was associated with a higher rate of extravesical disease extension at diagnosis, but after administration of NAC, ypT stage was equally distributed between sexes. There were no independent associations between gender with regard to ypT0N0 or downstaging rates, overall survival, nor cancer-specific survival [26].
Race
In a large cancer registry-based study , black patients presented more frequently with advanced stage and high grade and experienced significantly worse outcomes. Multivariable analysis showed that black race, socioeconomic status, and health insurance status were all independently predictive of poorer survival when controlling for age, grade, stage, and gender [27]. Similar to female patients, black patients have lower cystectomy rates for muscle-invasive disease [28].
Black patients have the poorest cancer-specific survival when compared to whites and other minorities [9]. Even after accounting for age, tumor characteristics, gender, insurance, geography, and dates of diagnosis, black men and women with bladder cancer remain at significantly increased risk for death compared to whites , with black women faring consistently the worst [29]. In a study of over 22,000 patients in the Nationwide Inpatient Sample database, white patients who underwent cystectomy had a mortality rate of 2.8% compared with 4.2% for black patients and 3.9% for Hispanic patients. Black patients were also more likely to have prolonged hospitalization and in-hospital mortality [30]. Treatment differences may impact late-stage mortality more than early-stage mortality, which could explain the persistently higher mortality rates in black patients. With respect to non-muscle-invasive disease, black race, residence in an urban area, and a census area with low median income correlated with a lower-intensity surveillance regimen than recommended by guidelines [31].
Insurance
Compared with those with private insurance , uninsured and Medicaid-insured patients are at least twice as likely to present with regional disease and 60% more likely to have locally advanced disease at diagnosis [32] while less likely to undergo radical cystectomy [28]. Large geographic variations also exist, with lower cystectomy rates in the south and northeast of the United States [28]. Bladder cancer patients who are either uninsured or Medicaid-insured exhibit 50% and 70% increased risks of death compared with privately insured patients, respectively [29]. In a large study of data from the National Cancer Data Base (NCDB) of nearly 29,000 patients, not only black patients were less likely to receive curative treatments than white patients (OR: 0.74; p < 0.001), but also patients without insurance, Medicaid beneficiaries, and young Medicare patients when compared to those with private insurance [33].
Geography
Physical access to a high-volume center plays a role in bladder cancer outcomes. In a study of nearly 4000 patients who had undergone radical cystectomy in the United States, distance to treatment facility was associated with delays in time to cystectomy (>3 months), but not cancer-specific or all-cause mortality after multivariable adjustment [34]. In contrast, other studies have shown that delays to cystectomy are associated with worse outcomes [35]. An NCDB study of over 18,000 patients who underwent surgical treatment for MIBC found lower use of neoadjuvant chemotherapy in settings of lower hospital cystectomy volume, treatment at a nonacademic facility, lower patient income , and receipt of partial cystectomy—interestingly, neither gender nor race was associated with the use of NAC [34].
Financial Toxicity
Financial toxicity , defined as the patient-level impact of the costs of care delivered, merits discussion in the management of both NMIBC and MIBC. Bladder cancer is the costliest cancer among the elderly, estimated at nearly $4 billion per year, and has the highest cost of any cancer when categorized on a per-patient basis [36]. NMIBC is particularly costly to treat in the United States , due to the frequency of surveillance, cost of intravesical chemotherapy, and need for repeat endoscopies and subsequent surgical treatment [37].
In a regional survey of 138 patients with bladder cancer, about a quarter of patients endorsed financial toxicity. Patients who were younger, were black, had NMIBC, and had less than a college degree were more likely to report financial toxicity including the inability to take time off work or afford general expenses, resulting in delaying care. Financial toxicity had deleterious effects on perceived physical and mental health, including cancer-specific health-related quality of life and functional well-being [38]. Similarly, a national survey of 226 patients demonstrated that people who were younger , with a household annual income less than $50,000, not retired, or with insurance that was neither Medicare nor employer-paid were significantly more likely to have worse financial toxicity. The majority of these patients would have wished to discuss cost in the context of treatment preferences [39].
Disparities in Survivorship
In recent years, there has been a growing awareness of not only the oncology but also the quality of life ramifications of a bladder cancer survivorship which can be prolonged. Significant declines in health-related quality-of-life (FR-QoL) scores related to physical health, vitality, and social functioning all decline after bladder cancer diagnosis. There is an unmet need for research, long-term support, and survivorship resources to address this gap. Within survivorship, continued disparities exist based on gender, race, and other factors [40].
Urinary Diversion
Long-term morbidity associated with radical cystectomy is often tied to urinary diversion, which remains the most studied HR-QoL domain for BC patients. Although studies are mixed, continent diversions (CD) such as ileal neobladders are generally linked to better HR-QoL outcomes, and this is largely affected by continence rates [41]. In a national study comparing CD with ileal conduit (IC) use in nearly 70,000 radical cystectomy cases for bladder cancer, white men were more likely to undergo CD compared to female and black counterparts. CD use was also regional, with the highest rates on the West coast, at teaching centers, and large hospitals [42]. Similarly, Hispanic patients were less likely to undergo CD at another high-volume center [43]. Possible causes for these disparities include clinician bias, patient preference , communication barriers, and proximity to high-volume centers, although these factors are not well studied.
Sexual Function
Sexual dysfunction is very common after radical cystectomy. Men after cystectomy have self-reported rates of erectile dysfunction as high as 80–90% with non-nerve sparing techniques and as low as 10–30% after nerve sparing techniques [44]. While much of the anatomical and surgical details of nerve sparing cystectomy in men is derived from decades of study in the radical prostatectomy literature, female sexual anatomy and function are much less defined and lack a standardized approach. Post-cystectomy sexual dysfunction in women can be grouped into female sexual pain disorders and disorders of orgasm due to damage to the clitoral branches of the internal pudendal artery [45]. Decline in sexual function also occurs in patients with NMIBC—in a study of over 200 patients, erectile dysfunction (60%), vaginal dryness (63%), and fear of contaminating sexual partner with intravesical therapy agents (23%) were most commonly reported , with over half of those interviewed endorsing sexual dysfunction [46].
Conclusion
Bladder cancer is associated with significant morbidity and only modest improvements in mortality in the past two decades despite the introduction of neoadjuvant chemotherapy and innovations in definitive local therapies. Progress in improving cancer survival has been hindered by existing disparities with respect to gender, race, and access to care, resulting in delays to diagnosis and leading to other adverse outcomes. The high financial cost of bladder cancer management and survivorship in the United States cannot be underestimated—a burden that is often shared with the patient. In the upcoming decade, with the emergence of novel therapeutics for bladder cancer, addressing disparities and health-related quality-of-life outcomes among bladder cancer patients will be of utmost importance.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. https://doi.org/10.3322/caac.21492.
National Cancer Institute. Bladder cancer — cancer stat facts n.d.. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed 15 Feb 2021.
Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66:4–34. https://doi.org/10.1016/J.UROLOGY.2005.07.062.
Cassell A, Yunusa B, Jalloh M, Mbodji MM, Diallo A, Ndoye M, et al. Non-muscle invasive bladder cancer: a review of the current trend in Africa. World J Oncol. 2019;10:123–31. https://doi.org/10.14740/wjon1210.
Richters A, Aben KKH, Kiemeney LALM. The global burden of urinary bladder cancer: an update. World J Urol. 2020;38:1895–904. https://doi.org/10.1007/s00345-019-02984-4.
Chung-Hall J, Craig L, Gravely S, Sansone N, Fong GT. Impact of the WHO FCTC over the first decade: a global evidence review prepared for the Impact Assessment Expert Group. Tob Control. 2019;28:s119–28. https://doi.org/10.1136/tobaccocontrol-2018-054389.
Lee LJ, Kwon CS, Forsythe A, Mamolo CM, Masters ET, Jacobs IA. Humanistic and economic burden of non-muscle invasive bladder cancer: results of two systematic literature reviews. Clinicoecon Outcomes Res. 2020;12:693–709. https://doi.org/10.2147/CEOR.S274951.
Martini A, Sfakianos JP, Renström-Koskela L, Mortezavi A, Falagario UG, Egevad L, et al. The natural history of untreated muscle-invasive bladder cancer. BJU Int. 2020;125:270–5. https://doi.org/10.1111/bju.14872.
Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 2013;37:219–25. https://doi.org/10.1016/J.CANEP.2013.02.002.
May M, Helke C, Nitzke T, Vogler H, Hoschke B. Survival rates after radical cystectomy according to tumor stage of bladder carcinoma at first presentation. Urol Int. 2004;72:103–11. https://doi.org/10.1159/000075962.
Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–49. https://doi.org/10.1016/S0140-6736(09)60491-8.
de la Calle C, Patil D, Wei JT, Scherr DS, Sokoll L, Chan DW, et al. Multicenter evaluation of the prostate health index to detect aggressive prostate cancer in biopsy Naïve men. J Urol. 2015;194:65–72. https://doi.org/10.1016/j.juro.2015.01.091.
Barocas DA, Boorjian SA, Alvarez RD, Downs TM, Gross CP, Hamilton BD, et al. Microhematuria: AUA/SUFU Guideline. J Urol. 2020;204:778–86. https://doi.org/10.1097/JU.0000000000001297.
Garg T, Pinheiro LC, Atoria CL, Donat SM, Weissman JS, Herr HW, et al. Gender disparities in hematuria evaluation and bladder cancer diagnosis: a population based analysis. J Urol. 2014;192:1072–7. https://doi.org/10.1016/j.juro.2014.04.101.
Lucca I, Klatte T, Fajkovic H, de Martino M, Shariat SF. Gender differences in incidence and outcomes of urothelial and kidney cancer. Nat Rev Urol. 2015;12:585–92. https://doi.org/10.1038/nrurol.2015.232.
Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The National Cancer Data Base report on bladder carcinoma. Cancer. 1996;78:1505–13. https://doi.org/10.1002/(SICI)1097-0142(19961001)78:7<1505::AID-CNCR19>3.0.CO;2-3.
Mungan NA, Kiemeney LAL, van Dijck JAA, van der Poel HG, Witjes JA. Gender differences in stage distribution of bladder cancer. Urology. 2000;55:368–71. https://doi.org/10.1016/S0090-4295(99)00481-1.
Cohn JA, Vekhter B, Lyttle C, Steinberg GD, Large MC. Sex disparities in diagnosis of bladder cancer after initial presentation with hematuria: a nationwide claims-based investigation. Cancer. 2014;120:555–61. https://doi.org/10.1002/cncr.28416.
Johnson EK, Daignault S, Zhang Y, Lee CT. Patterns of hematuria referral to urologists: does a gender disparity exist? Urology. 2008;72:498–502; discussion 502–3. https://doi.org/10.1016/j.urology.2008.01.086.
Han DS, Zhou W, Seigne JD, Lynch KE, Schroeck FR. Geographic variation in cystoscopy rates for suspected bladder cancer between female and male medicare beneficiaries. Urology. 2018;122:83–8. https://doi.org/10.1016/j.urology.2018.08.011.
Brookfield KF, Cheung MC, Gomez C, Yang R, Nieder AM, Lee DJ, et al. Survival disparities among African American women with invasive bladder cancer in Florida. Cancer. 2009;115:4196–209. https://doi.org/10.1002/cncr.24497.
Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. https://doi.org/10.1002/cncr.23986.
Grajales V, Bandari J, Hale NE, Yabes JG, Turner RM, Fam MM, et al. Associations between female sex and treatment patterns and outcomes for muscle-invasive bladder cancer. Urology. 2020. https://doi.org/10.1016/j.urology.2020.06.058.
de Jong JJ, Liu Y, Boorjian SA, Bivalacqua TJ, Porten SP, Wheeler T, et al. A genomic classifier for predicting clinically aggressive luminal bladder tumors with higher rates of pathological upstaging. J Urol. 2020. https://doi.org/10.1097/JU.0000000000000798.
Ide H, Inoue S, Miyamoto H. Histopathological and prognostic significance of the expression of sex hormone receptors in bladder cancer: a meta-analysis of immunohistochemical studies. PLoS One. 2017;12:e0174746. https://doi.org/10.1371/journal.pone.0174746.
D’Andrea D, Black PC, Zargar H, Zargar-Shoshtari K, Zehetmayer S, Fairey AS, et al. Impact of sex on response to neoadjuvant chemotherapy in patients with bladder cancer. Urol Oncol. 2020;38:639.e1–9. https://doi.org/10.1016/j.urolonc.2020.01.010.
Sung JM, Martin JW, Jefferson FA, Sidhom DA, Piranviseh K, Huang M, et al. Racial and socioeconomic disparities in bladder cancer survival: analysis of the California cancer registry. Clin Genitourin Cancer. 2019;17:e995–1002. https://doi.org/10.1016/j.clgc.2019.05.008.
Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185:72–8. https://doi.org/10.1016/J.JURO.2010.09.015.
Mallin K, David KA, Carroll PR, Milowsky MI, Nanus DM. Transitional cell carcinoma of the bladder: racial and gender disparities in survival (1993 to 2002), stage and grade (1993 to 2007). J Urol. 2011;185:1631–6. https://doi.org/10.1016/J.JURO.2010.12.049.
Taub DA, Hollenbeck BK, Cooper KL, Dunn RL, Miller DC, Taylor JMG, et al. Racial disparities in resource utilization for cystectomy. Urology. 2006;67:288–93. https://doi.org/10.1016/J.UROLOGY.2005.09.003.
Schrag D, Hsieh LJ, Rabbani F, Bach PB, Herr H, Begg CB. Adherence to surveillance among patients with superficial bladder cancer. JNCI J Natl Cancer Inst. 2003;95:588–97. https://doi.org/10.1093/jnci/95.8.588.
Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–31. https://doi.org/10.1016/S1470-2045(08)70032-9.
Gray PJ, Fedewa SA, Shipley WU, Efstathiou JA, Lin CC, Zietman AL, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol. 2013;63:823–9. https://doi.org/10.1016/J.EURURO.2012.11.015.
Duplisea JJ, Mason RJ, Reichard CA, Li R, Shen Y, Boorjian SA, et al. Trends and disparities in the use of neoadjuvant chemotherapy for muscle-invasive urothelial carcinoma. Can Urol Assoc J. 2019;13:24–8. https://doi.org/10.5489/cuaj.5405.
Russell B, Liedberg F, Khan MS, Nair R, Thurairaja R, Malde S, et al. A systematic review and meta-analysis of delay in radical cystectomy and the effect on survival in bladder cancer patients. Eur Urol Oncol. 2020;3:239–49. https://doi.org/10.1016/j.euo.2019.09.008.
Mossanen M, Gore JL. The burden of bladder cancer care: direct and indirect costs. Curr Opin Urol. 2014;24:487–91. https://doi.org/10.1097/MOU.0000000000000078.
Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. PharmacoEconomics. 2003;21:1315–30. https://doi.org/10.1007/BF03262330.
Casilla-Lennon MM, Choi SK, Deal AM, Bensen JT, Narang G, Filippou P, et al. Financial toxicity among patients with bladder cancer: reasons for delay in care and effect on quality of life. J Urol. 2018;199:1166–73. https://doi.org/10.1016/j.juro.2017.10.049.
Ehlers M, Bjurlin M, Gore J, Pruthi R, Narang G, Tan R, et al. A national cross-sectional survey of financial toxicity among bladder cancer patients. Urol Oncol. 2021;39:76.e1–7. https://doi.org/10.1016/j.urolonc.2020.09.030.
Bhanvadia SK. Bladder cancer survivorship. Curr Urol Rep. 2018;19:111. https://doi.org/10.1007/s11934-018-0860-6.
Ghosh A, Somani BK. Recent trends in postcystectomy health-related quality of life (QoL) favors neobladder diversion: systematic review of the literature. Urology. 2016;93:22–6. https://doi.org/10.1016/J.UROLOGY.2015.12.079.
Farber NJ, Faiena I, Dombrovskiy V, Tabakin AL, Shinder B, Patel R, et al. Disparities in the use of continent urinary diversions after radical cystectomy for bladder cancer. Bladder Cancer. 2018;4:113–20. https://doi.org/10.3233/BLC-170162.
Rios EM, Parma MA, Fernandez RA, Clinton TN, Reyes RM, Kaushik D, et al. Urinary diversion disparity following radical cystectomy for bladder cancer in the Hispanic population. Urology. 2020;137:66–71. https://doi.org/10.1016/j.urology.2019.12.017.
Modh RA, Mulhall JP, Gilbert SM. Sexual dysfunction after cystectomy and urinary diversion. Nat Rev Urol. 2014;11(8):445–53. https://doi.org/10.1038/nrurol.2014.151.
Pederzoli F, Campbell JD, Matsui H, Sopko NA, Bivalacqua TJ. Surgical factors associated with male and female sexual dysfunction after radical cystectomy: what do we know and how can we improve outcomes? Sex Med Rev. 2018;6:469–81. https://doi.org/10.1016/J.SXMR.2017.11.003.
Kowalkowski MA, Chandrashekar A, Amiel GE, Lerner SP, Wittmann DA, Latini DM, et al. Examining sexual dysfunction in non-muscle-invasive bladder cancer: results of cross-sectional mixed-methods research. Sex Med. 2014;2:141–51. https://doi.org/10.1002/SM2.24.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chu, C., Porten, S. (2021). Epidemiology of Bladder Cancer: Trends and Disparities. In: Bjurlin, M.A., Matulewicz, R.S. (eds) Comprehensive Diagnostic Approach to Bladder Cancer. Springer, Cham. https://doi.org/10.1007/978-3-030-82048-0_1
Download citation
DOI: https://doi.org/10.1007/978-3-030-82048-0_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-82047-3
Online ISBN: 978-3-030-82048-0
eBook Packages: MedicineMedicine (R0)