Abstract
Hydrocephalus is a pathological state affecting all age groups, which is characterised by the accumulation of intracranial CSF under pressure, with or without distension of the cerebral ventricles. There are a multitude of underlying aetiologies. The development of CSF shunt surgery 70 years ago revolutionised management and converted a very morbid and frequently fatal condition to an eminently manageable one, albeit management is frequently complicated. Given its prevalence and association with other childhood conditions, it is important for all paediatric specialists to have at least a basic working knowledge of its management.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Hydrocephalus
- Ventriculomegaly
- Cerebrospinal fluid
- Ventriculoperitoneal shunt
- Endoscopic third ventriculostomy
1 Introduction
The central dogma of hydrocephalus is a simple one: cerebrospinal fluid (CSF) is constantly produced and reabsorbed. Disturbances in CSF flow and absorption lead to an accumulation within the ventricular system. Untreated, this leads to rising intracranial pressure (ICP) and active distension of ventricles in the majority of cases (Rekate 2011). The management of hydrocephalus is far from simple, however, as causes are numerous and heterogeneous. There are many nuances, and expert management is essential. Patients will often have multi-system disorders and complex needs, and thus there are some fundamental principles all clinicians involved in their care should know. Hydrocephalus is really a catch-all term encompassing many disparate aetiologies, each with their own vagaries of treatment. Defining hydrocephalus is thus problematic; however, the vital concern is whether there is associated raised ICP. Ventricular volume, while a useful adjunct especially in the radiologically surveillance of patients or in the acute setting, is secondary.
Radiological definitions, therefore, such as Evans ratio (ventriculomegaly=(maximal frontal horn diameter)/(transverse inner skull diameter) ≥ 0.3), the diameter (>2 mm) of the temporal horns, rounded third ventricle walls, etc. are crude guides and not universally applicable.
The aim of this chapter is not to provide an exhaustive guide to hydrocephalus in all its guises, but rather to provide the non-specialist with a practical approach to the management of patients with hydrocephalus, both treated and untreated.
2 Historical Overview
Hydrocephalus has been recognised as a pathological entity and studied since the time of Hippocrates in the fifth century BC. All the key figures of the history of medicine, including, but not limited to, Galen, Vesalius, Sylvius and Abulcasis, have at one point turned their enquiring minds to its study. Early writers sought to understand its origin, believing the soul to reside within the ventricles (Aschoff et al. 1999).
Progress in understanding the pathology did not occur until the physiology of CSF production was explored, firstly by Thomas Willis in the seventeenth century who identified the choroid plexus as the organ of origin. The CSF circulation was defined between the nineteenth and into the twentieth century with two of the contributors immortalised in anatomical nomenclature: Magendie and Luschka. In the early twentieth century, the practice of neurosurgery as we know it today was born due to pioneers such as Harvey Cushing, Sir Victor Horsley and Walter Dandy who all had a hand in developing hydrocephalus surgery (Lifshutz and Johnson 2001).
The first documented shunt surgery was performed in 1905, but the technique subsequently fell into disrepute due to high mortality. There was a resurgence in the 1950s, thanks to Frank Nulsen and Eugene Spitz ,who pioneered ventriculoatrial shunting and subsequently ventriculoperitoneal shunting (Rachel 1999). Spitz also developed the first working valve alongside hydraulics engineer, John Holter. Numerous subsequent valve designs have been developed including the Wade-Dahl-Till valve named after its designer’s hydraulics engineer, Stanley Wade, world-famous children’s author Roald Dahl and neurosurgeon Kenneth Till. The development of CSF shunting revolutionised the management of hydrocephalus, transforming it from a disabling and often fatal condition to an eminently treatable one.
3 Incidence
The prevalence of hydrocephalus in paediatric populations is 88/100,000 globally (Dewan et al. 2018a). Hydrocephalus is less prevalent in younger adults (11/100,000), but peaks in the elderly (175/100,000) in the over 65s and is >400/100,000 in those over 80, due to the occurrence of normal pressure hydrocephalus (NPH). It is also associated with congenital malformations of the brain and spinal cord in a significant proportion of cases in infants. Timely and effective surgical treatment is essential, as complications related to delay in treatment can be life-threatening. Once treated, the majority of patients will require lifelong support and follow-up, with up to 85% requiring further surgical intervention in their lifetime (Stone et al. 2013).
4 Aetiopathogenesis
Doctors love good taxonomy, and those interested in hydrocephalus are no different; thus, numerous classifications have been devised to varying success. Classically, hydrocephalus was either ‘obstructive’ or ‘communicating’, terms coined by one of the fathers of modern neurosurgery Walter Dandy in 1914. Subsequent grouping by aetiology into ‘congenital’ or ‘acquired’ causes has been used, but has little clinical relevance (Dandy and Blackfan 1914).
The ‘obstruction’ in obstructive hydrocephalus relates to a mass lesion (tumour, swelling) or some physical impediment (pus, blood) to the CSF circulation pathway prior to the point of reabsorption. Communicating hydrocephalus occurs due to obstruction also, though the obstruction is more functional, existing at the point of reabsorption, the prime example of communicating hydrocephalus being blood and blood breakdown products occluding the fine filtration substrate of the arachnoid granulations in cases of intraventricular haemorrhage. The terms obstructive and communicating are increasingly outdated and, in fact, give a gross over-simplification of a hugely complex and still poorly understood physiology (Tomycz et al. 2017). They do, however, provide a workable scheme around which we can make clinical decisions. The exception to this two-classification rule is where CSF production outstrips reabsorption. This is very rare, however, limited to choroid plexus tumours and in some circumstances of infection, such as ventriculitis, due to inflammation and increased ependymal blood flow.
In practical terms, whether ‘obstructive’ or ‘communicating’, CSF accumulates within the cranial CSF compartment, and pressure rises. The normal CSF pressure is 10–15 cm/H2O, as measured in the lumbar theca in the lateral position (7–12 cm/H2O in infants). Unchecked elevations in ICP can lead to coma and eventually death.
Hydrocephalic aetiology and age are inextricably linked resulting in the three peaks in incidence. The vast majority of neonates and infants will develop hydrocephalus either secondary to intraventricular haemorrhage (IVH) of prematurity or in association with congenital malformations, chief amongst which is spina bifida. The rest of the cases (at least in the developed world) are due to tumours, with a small number occurring following episodes of meningoencephalitis (the second leading cause globally) (Dewan et al. 2018b). During adulthood and middle age, the incidence is low and typically limited to cases of subarachnoid haemorrhage and as a consequence of obstructive tumours. With advancing years, the neurodegenerative condition, idiopathic normal pressure hydrocephalus (NPH), results in a third and final peak of hydrocephalus cases, summarised in Table 40.1. This division into different age groups and how this impacts on management neatly demonstrates the importance of understanding the pathoanatomical basis of a patient’s hydrocephalus. Careful consideration and understanding of this is crucial to making rational management decisions.
5 Pathophysiology
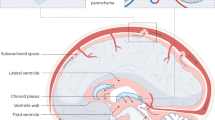
Understanding the pathophysiology of hydrocephalus requires some understanding of the physiology of CSF production and circulation. Approximately 80% of CSF is produced by the choroid plexus; tufts of capillaries enclosed in modified ependyma (membranous lining of the ventricles). This active process is supplemented (20%) by passive egress of fluid from the brain substance into the interstitial space, perivascular ‘Virchow-Robin’ spaces and onwards then to the body of CSF within the ventricular system.
CSF is produced at around 0.33 ml/min, resulting in a total of 20 ml/h and about 500 ml/day in an adult (Kimelberg 2004). At any point, there is about 150 ml of CSF within the neuroaxis, half of which is in the intracranial compartment. Volumes are obviously less in infants, proportional to body weight, but approximate adult values are reached by 5 years of age. Choroid plexus is found predominantly within the lateral ventricles but also within the third and fourth ventricles. From the point of secretion, CSF normally circulates from the lateral ventricles through the foramina of Monro into the third ventricle and then onwards through the aqueduct of Sylvius into the fourth ventricle in the posterior fossa. From the fourth ventricle, the CSF exits into the spinal subarachnoid space either via the midline foramen of Magendie or via the lateral foramina of Luschka into the basal cisterns and around the cerebral cortex in the subarachnoid space (Sakka et al. 2011). CSF is then reabsorbed into the cerebral venous system, via outpouchings of arachnoid mater known as arachnoid granulations. The arachnoid granulations bulge into the dural venous sinuses, where CSF is resorbed depending on a hydrostatic pressure differential (Khasawneh et al. 2018).
6 Pathology
One of the key factors to consider, certainly when timing surgical intervention in young children, is whether the skull plates are fused at the cranial sutures. A child with a closed fontanelle and fused sutures will not be able to accommodate for a relative increase in CSF volume without incurring dangerous elevations in ICP, for example, as their ICP dynamics conform to the Monro-Kellie doctrine (Fig. 40.1). Conversely, a premature neonate may accommodate, usually with a rapidly increasing head circumference. This can be temporised relatively safely with episodic ventricular taps.
The cranial vault is a fixed volume (approx. 1700 ml). The contents are the brain (1400 ml), CSF (150 ml) and blood (150 ml).
An increase in one of these components requires displacement, and a reduction in one or both of the other two components or the intracranial pressure will rise.
7 Diagnosis
7.1 Clinical Presentation
Clinically speaking, the vast majority of hydrocephalus will present one of five ways:
-
1.
On serial imaging of the premature neonate at risk for germinal matrix haemorrhage and intraventricular haemorrhage
-
2.
Following investigations for expanding head circumference—usually instigated in the community by a GP or health visitor
-
3.
Following imaging and investigations performed for symptoms of raised ICP (Table 40.2)
-
4.
Subsequent to routine fundoscopic examination by the child’s optometrist and identification of papilloedema
-
5.
Incidentally discovered when investigated for an unrelated cause, e.g. trauma
Radiological imaging forms the foundation of neurosurgical diagnosis. Cranial imaging is essential to the diagnosis of hydrocephalus, typically based on the presence of ventriculomegaly. It bears repeating, however, that normal-sized ventricles do not necessarily mean there is no problem with CSF flow and/or raised intracranial pressure (Dinçer and Özek 2011).
7.2 Plain Radiography
Plain radiographs of the skull do not have a role in the routine workup of hydrocephalus, although they can give clues to the underlying pathology. Signs of raised pressure include a ‘copper beaten’ or ‘thumbprinted’ appearance of the calvarium and splaying of the cranial sutures (Fig. 40.2). In addition, the aetiology of the raised pressure may be noted, e.g. traumatic fractures or abnormal calcification associated with a pineal region mass obstructing the aqueduct. Plain radiographs are, however, vital in the longer-term management of the hydrocephalus patient. Shunt series XR (skull, neck soft tissues, chest and/or abdomen) allow clinicians to follow the tract of the shunt (radio-opaque) to ensure all components are in continuity and exclude a shunt ‘fracture’ or disconnection, which could compromise shunt function (Fig. 40.3). Furthermore, a skull XR permits accurate evaluation of the setting in programmable shunt valves (see Sect. 40.9.1).
Plain radiograph of infant skull demonstrating the ‘copper beaten’ or ‘thumbprinted’ appearance of the calvarium due to moulding of the inner table over the cerebral cortex reflecting high ICP. Note the prominent coronal suture. Also note the discontinuity in the shunt (arrow) where the distal catheter has disconnected from the valve
7.3 Ultrasonography
Transcranial ultrasound is a very useful screening tool in neonates. It requires an open anterior fontanelle, which provides a window to assess intracranial structures. With minimal training and experience, operators (typically neonatologists) can achieve a diagnosis of intracerebral/intraventricular haemorrhage, with or without ventriculomegaly. It is vital to record the ventricular index (VI) to communicate effectively with colleagues. VI is defined as the width (in mm) from the falx to the lateral wall of the lateral ventricle at the level of the foramen of Monro, when viewed in the coronal plane (Fig. 40.4). As there are both left and right ventricles, a value is given for each as these are frequently subtly different. Blood within the ventricles, for example, in cases of IVH of prematurity, will be echo-bright and should be obvious to all (Fig. 40.4). Skilled and experienced operators can identify other underlying aetiologies, such as tumours or anatomical abnormalities; however, ultrasound is not ideally suited to this, and confirmatory cross-sectional imaging would be required (Dorner et al. 2018).
7.4 Computed Tomography (CT)
Computed tomography (CT) is a ubiquitous, rapid and easy-to-interpret tool in the diagnosis of hydrocephalus. A plain unenhanced CT will successfully diagnose hydrocephalus and the underlying aetiology in the vast majority of cases. CT diagnosis of hydrocephalus is based primarily on the presence or absence of ventriculomegaly (Fig. 40.5). Features suggestive of ventriculomegaly include ballooning of the frontal horns of the lateral ventricles, the presence of dilated temporal horns of the lateral ventricles (not typically visible in health) and periventricular low density suggesting transependymal passage of CSF under pressure. Other features include enlarged and rounded third ventricle, effacement of the cortical sulcal pattern and surface subarachnoid spaces especially at the vertex of the skull and Sylvian fissure and upward bowing of the corpus callosum. Radiological criteria quoted in the literature, such as Evan’s ratio, are not routinely used in current clinical practice, and imaging must be interpreted in the clinical context (history and examination) and with reference to previous imaging (Dinçer and Özek 2011).
Axial unenhanced CT demonstrating gross acute hydrocephalus. Note ballooning of the frontal horns of the lateral ventricles, dilated rounded third ventricle and prominent occipital horns. Note the periventricular lucency around the frontal horns (white arrows), which represents transependymal flow of CSF under pressure
CT is also frequently used to assess patients after insertion or revision of ventricular catheters for CSF diversion. The scan is useful to confirm satisfactory positioning as well as the state and size of the ventricles and can also reassure surgeons of the absence of complications, e.g. post-operative haematoma or IVH, in the event of difficult shunt revision surgery. Many neurosurgeons will perform a baseline or ‘well’ CT several weeks after recovery from shunt insertion or revision to capture the ventricular configuration at baseline. This can be used as a comparator should the patient present with suspicion of shunt dysfunction in the future (Pople 2002).
Despite the many advantages, CT is unfortunately less good at defining underlying causes than is magnetic resonance imaging (MRI). In addition, it exposes the child to radiation, the cumulative doses of which can be significant across a lifetime of treatment.
7.5 Magnetic Resonance Imaging (MRI)
MRI is the investigation of choice for delineating the ventricular size and the pathoanatomical substrate of hydrocephalus. It has many advantages over CT, not least of which is the avoidance ionising radiation exposure. As with CT scanning, multiplanar imaging allows the assessment of anatomical structures but with greater tissue determination. Fine membranous structures, such as the floor of the third ventricle, can be identified; useful as this is typically bowed down and backwards into the pre-pontine space, particularly in cases of obstructive hydrocephalus where the obstruction is at the level of the aqueduct of Sylvius or fourth ventricle. Modern scanning technology permits high-resolution and accurate image representation of CSF spaces and the ability to perform assessments of CSF flow. This can allow the identification of septations or cysts within the ventricles, common following intraventricular haemorrhage and infection. These would need to be traversed and fenestrated in order to successfully treat that patient’s hydrocephalus (Fig. 40.6). Pathological lesions, such as tumours, vascular malformations (e.g. vein of Galen malformation), cysts and even anatomical variants, are identified with an extremely high degree of accuracy by MRI; thus, for the assessment of de novo hydrocephalus, MRI is mandated.
(a) Axial T2-weighted MRI demonstrating multi-loculated hydrocephalus due to multiple septations (red arrows). (b) Axial T2 FLAIR demonstrating hydrocephalus with periventricular oedema due to transependymal flow. (c) Coronal T2-weighted MRI showing marked chronic hydrocephalus with expanded temporal horns (yellow arrows). (d) Sagittal T2-weighted MRI. Note expanded third ventricle with thinned corpus callosum and downward bowing of the third ventricle floor (blue arrows)
Like CT, MRI has a role in the post-operative imaging of patients with hydrocephalus; it is particularly useful in the assessment of internal CSF diversion procedures, such as endoscopic third ventriculostomy, where particular imaging paradigms (e.g. time-resolved 2D phase contrast) can be applied directly to confirm and even quantify CSF flow, for example, across a newly formed stoma or through a pre-existing CSF pathway, e.g. across the craniocervical junction, in cases of Chiari malformation (Fig. 40.13) (Dinçer et al. 2011).
8 Differential Diagnosis
The differential diagnosis for a child presenting with symptoms and signs of raised intracranial pressure is wide. Hydrocephalus is rarely the primary disease entity but rather a consequence of other underlying pathology. Accurate diagnosis obtained from imaging, blood tests, clinical history and examination will identify an underlying cause in the vast majority of cases.
9 Management
Hydrocephalus is an eminently manageable condition surgically speaking, albeit this may be complicated in a small proportion of cases. The treatment involves CSF diversion or ‘shunting’ from the intraventricular compartment of the brain to another compartment either intra- or extracranial. These shunts may be physically implanted or internally formed by creating an ostomy between cavities. The absolute indication for the treatment of hydrocephalus is the presence of signs and symptoms of raised intracranial pressure (Table 40.2). Caution should be applied and consideration of alternate management strategies made where available, especially in the presence of active/proven CNS/CSF infection, low body weight or other remediable causes. The mode of treatment is dependent on a multitude of factors outlined below.
Lesional hydrocephalus, i.e. secondary to a focal mass lesion causing CSF pathway obstruction, is usually best treated by the removal of the causative lesion. Examples of these might include an enlarging colloid cyst obstructing both foramina of Monro or a tumour within the posterior fossa compromising CSF egress via the fourth ventricle. In the case of acute symptomatic hydrocephalus, secondary to an operable or potentially operable lesion, a short-term CSF diversion may be employed to gain control of the immediate situation allowing time for further assessment. Insertion of an external ventricular drain (EVD) into the frontal horn of the lateral ventricle (by convention the right side as this is non-dominant in the majority of individuals) allows control of raised ICP and more detailed investigations and definitive treatment. Occasionally, despite resection of the offending lesion, the hydrocephalus persists, and in these cases, diversion of CSF to an alternative site of reabsorption is required.
9.1 Implantable CSF Shunts
Implantation of a CSF shunt is one of the most commonly performed procedures in neurosurgery worldwide. While one of the core procedures a neurosurgeon masters at the very beginning of their training and, thus, often disdained as ‘just a shunt’, one needs to be wary of creeping complacency. The consequences and potential long-term sequelae of a poorly thought-out or carelessly performed operation are serious and potentially dangerous. Complications of shunt surgery are detailed later in this chapter.
CSF flow is most frequently diverted into the peritoneal cavity via a ventriculoperitoneal or ‘VP’ shunt (VPS). Alternatives include direct drainage into the central venous circulation, a ventriculoatrial or ‘VA’ shunt (VAS) and much less frequently the pleural cavity (ventriculopleural shunt).
Shunts consist of a proximal (ventricular) catheter, a distal catheter and an interposed one-way valve. A valveless system may be implanted, though outside of the developing world these are limited to complex cases, usually following multiple failed revision surgeries. Shunt catheters are manufactured from hydrophobic silicone which is biologically inert, resists bacterial colonisation and retains its flexibility over decades, all crucial to longevity. Over the last two decades, manufacturers have devised numerous ways to improve the durability of shunts and avoid the two main complications, blockage and infection. There is level I (double-blind RCT) evidence to support the preference of antibiotic (rifampicin and clindamycin)-impregnated tubing to reduce the rate of infection-related failure and revision surgery (Mallucci et al. 2019).
The major differences between shunt systems are in the valve, and there is a wealth of choice available on the market. It should be stated at the outset that all evidence to date, including one RCT published in 1998, has failed to categorically establish superiority of one valve design over another (Drake et al. 1998; Kestle et al. 2000). More research in this area is needed.
The most frequent valves encountered are differential pressure valves (DPVs). These rely on a hydrostatic pressure gradient across the valve from inlet to outlet to drive valve opening and CSF flow. The alternative, flow regulating valves, are more rarely used. DPVs may have a fixed opening pressure (most common); manufacturers supply valves with a variety of pressure settings or that alternatively have a programmable function whereby the opening pressure can be adjusted manually by the use of a transcutaneous handheld device (Fig. 40.7). It is important to realise that although valves may be of equivalent opening pressures, they may have different internal resistances and behave quite differently in vivo. Numerous other adjustments and technical features, including gravity-assisted valves and anti-syphon devices, are available, but their discussion alone could fill a book and is thus outside of the scope of this chapter.
Pictorial illustration of the process of shunt valve reprogramming using the MIETHKE proGAV 2.0 system, one of the numerous programmable shunts on the market. (a) The setting is first confirmed. (b) A strong magnetic ring is used to reprogram the valve to the desired setting. (c) Post-reprogramming setting confirmation
Selection of valves and shunt types is ultimately a matter of preference and tailoring to patient and clinician requirements. To the untrained observer, these choices may seem random but are often based on individual or departmental collective experience (sometimes painful). Programmable valves, for example, may be inserted to allow adjustment in demand as young children (especially neonates) grow or in the elderly with NPH to prevent CSF overdrainage and complications thereafter (Serarslan et al. 2017). Gravity-assisted valves might be preferred again in younger patients to accommodate for rapid growth and change from predominantly recumbent to upright posture and the effect these have on the ‘syphoning’ effect (Schatlo et al. 2013). Finally, pragmatic concerns are also valid; considerations such as the size of the valve and the risk of tissue breakdown over it (particularly in neonates), or favouring one programmable interface over another, are taken into account and weighted appropriately.
For general surgeons who may come across intraperitoneal catheters, it is important to be aware that a significant proportion of resistance to CSF flow comes the distal catheter and is very much related to its length. Shortening a catheter in the peritoneum can have a marked impact on the function of shunt system so care should be taken to preserve shunt catheters when encountered.
9.2 Insertion of VP Shunt: The Technique
After induction of general anaesthesia and endotracheal intubation, the patient receives prophylactic intravenous antibiotics and is positioned on the operating table. The positioning should allow access to the insertion site on the cranium (usually parieto-occipital or frontal) and the site of distal implantation (neck, chest or abdomen). The ideal insertion point permits a catheter trajectory which accesses a body of CSF (typically the lateral ventricle) but which traverses the least amount of brain, avoiding eloquent cortex and crucial deep brain structures (basal ganglia). The tip of the catheter bears multiple perforations, and these should be lying free in CSF away from the ventricle wall and choroid plexus to prevent ingrowth of tissue and thereby catheter blockage. In the circumstance of small or ‘slit’ ventricles, this may not be possible. A sub-optimally placed shunt may still work but will be more prone to failure. A number of ‘classical’ cranial insertion points have been described based on surface anatomy; however, these pre-date the CT scan and image guidance era and so are now used to approximate insertion only. The commonly used reference points include:
-
In the parietal position: Keen’s point, three finger’s breadths above and behind the pinna and Frazier’s point, 6 cm cephalad from the inion and 3 cm from the midline.
-
Frontally: Kocher’s point dictates that burr holes are placed in the midpupillary line just anterior to the coronal suture.
As noted above, the standard of care now is to insert ventricular catheters using image guidance. There are a number of systems available, but broadly speaking, they utilise either real-time ultrasound guidance or cross-sectional (CT or MRI) data. Ultrasound guidance permits accurate localisation of the fluid-filled ventricles, and operators can first adjust to a suitable trajectory and then guide the echo-bright catheter to the desired target. Neuronavigation systems require volume cross-sectional imaging to be performed pre-operatively. This is then uploaded to the image guidance console where entry point, target and resulting trajectory can be planned. Avoidance of blood vessels and vital structures is thus ensured. These systems may rely on optical or electromagnetic (EM) technology. Optical systems use an infrared camera which ‘sees’ reflective markers attached to working instruments and a reference array which remains fixed relative to the patient’s head. EM technology uses an EM emitter, which creates a magnetic field around the target. The relative positions of a reference probe (attached to the patient) and working instruments are then computed. The results are equivocal; a virtual, real-time representation of your instrument’s position in 3D space relayed on a multiplanar (axial/coronal/sagittal or even 3D) reconstruction of the patient’s scan with sub-millimetric accuracy.
Shunt insertion is best done by two surgeons, with the opening of the cranium and the peritoneal cavity done simultaneously to shorten operative time. The cranial opening should be done with a small burr hole and a minimal dural opening, just wide enough to admit the catheter to minimise CSF bypass. In neonates, a drill is often not required to open the soft calvarium. The abdominal exposure is done carefully in layers to avoid visceral injury. Leaflets of the posterior rectus sheath and peritoneum are reflected and held in haemostats to identify and preserve the opening. The catheter is then tunnelled in the subcutaneous layer with care paid to avoiding vascular structures in the neck and inadvertent entry into the thoracic compartment. The distal catheter is placed into the peritoneal compartment under direct vision ensuring free passage and the wounds are closed in layers with absorbable sutures.
Post-operative radiological evaluation of a shunt’s placement may be performed with CT or MRI. There is unlikely to be significant improvement in the degree of ventriculomegaly at this stage. In circumstances of VP shunt revision, post-operative cross-sectional imaging can reassure there is no haemorrhage associated with the removal of the existing shunt—a common cause of early failure of revised shunts (Fig. 40.8).
Post-operative axial CT scan in a patient with symptoms of early shunt failure following a ventricular catheter revision surgery. CT demonstrates intraventricular haemorrhage along the shunt trajectory most commonly due to bleeding from avulsed choroid plexus that was adherent to the revised catheter
9.3 Complications of CSF Shunts
Shunt surgery is the mainstay of hydrocephalus management. It has been hugely successful since its adoption some seven decades ago. It has, however, been plagued by a number of complications some of which persist despite numerous iterations of technique and improvements in implanted technology. Between 11% and 25% of all patients will require shunt revision surgery within the first year of implantation and 85% during their lifetime, the most common and important reasons being mechanical failure (obstruction or overdrainage) and infection (Khan et al. 2013; Wu et al. 2007; Reddy et al. 2014).
Mechanical Dysfunction and Blockage
Any shunt may become obstructed at any point along its length, and this is the most common type of shunt malfunction accounting for approximately three quarters of all failures (Kestle et al. 2000). The most common site is the proximal catheter, where it is often noted at the time of revision that fronds of choroid plexus have migrated into the perforations at the tip of the catheter (Paff et al. 2018). There are various theories as to why this may happen but none have been definitively proven (Harris and McAllister 2nd. 2012). Accurate placement with the distal tip floating free within the body of CSF and not in contact with ventricle walls is believed to reduce the risk of proximal obstruction (Hayhurst et al. 2010). For years, it was believed that frontally placed catheters were less prone to this complication; however, this has not been born out in the literature (Dickerman et al. 2005).
The valve is the next most common site of shunt failure. Valve failure can result in either over- or more typically underdrainage. Implantation of a valve with too low a fixed pressure will result in low-pressure symptoms and require revision. This still qualifies as a shunt failure even if the system is working as designed. One study has demonstrated superiority in terms of survival for programmable shunts over non-programmable shunts when corrected for age and hydrocephalus aetiology; this has not been replicated in other studies, however (McGirt et al. 2007).
A number of theories regarding valve occlusion and the reasons underlying it exist. In reality, a number of factors will contribute. In the acute phase, active bleeding within the ventricular system puts the valve at high risk of failure. The blood can coagulate under low flow conditions within the fine calibre valve mechanism. This is not uncommon following proximal revisions as friable adherent choroid plexus has a propensity to avulse and bleed when existing ventricular catheters are removed. CSF infections predispose shunts to fail, and commonly, this is at the level of the valve. The pathological process is not fully understood, but it is likely that the innate immune response and resulting inflammatory reaction to infection cause the build-up of cellular debris within the shunt.
Distal occlusions (beyond the valve) are uncommon but do occur, and distal patency must therefore always be confirmed at the time of shunt exploration surgery. Common causes for distal failure are coiling within the layers of the abdominal wall, due to either tube expulsion by bowel peristalsis or, more likely, poor implantation technique. Entrapment within an abdominal pseudocyst or coiling within an intra-abdominal compartment due to adhesion formation is also commonly noted at distal revision. Finally, raised intra-abdominal pressure, for example, due to chronic constipation or mass lesion, has also been reported as a cause of shunt failure.
Overdrainage
Overdrainage of the ventricular system can occur when an inappropriately low-pressure valve is inserted into a shunt system, such that the cerebral mantle is allowed to collapse away from the overlying dura. This can result in tearing of delicate traversing draining veins and the formation of subdural haematomas. Similarly, overdrainage can be a positional phenomenon due to the siphoning of fluid into the peritoneal cavity when upright. This can be limited by utilising shunt designs with incorporated anti-siphon systems or gravitational valves which mitigate overdrainage in the upright position. Occasionally, mechanical failure of shunt mechanisms can occur wherein the resistance to drainage fails and patients experience low-pressure symptoms.
Chronic overdrainage of the ventricular system, especially in the context of previous infection, can result in small, non-compliant ventricles that do not expand when the shunt is blocked even when the pressure is very high. This is a particularly difficult problem termed the ‘slit ventricle syndrome’ and is one reason that ventricular volume is not a sure determinant of shunt patency. Chronic low pressure may be manifest in a thickened skull vault as well as thickened, enhancing meninges visible on post-contrast MRI. Subdural fluid collection (hygromas) may also be visible.
Mechanical failure in children is notoriously difficult to accurately diagnose except in cases of acutely raised ICP where children may present vomiting, lethargic or even in coma. More subtle presentations with headache, behavioural change, abnormal posturing and change in appetite/feeding may be all that is evident. Remember that a large proportion of shunted patients have complex needs and often global developmental delay and so are unable to volunteer much information. Parents of shunted children are coached to have a low threshold for presentation to hospital if they are concerned. Parents are also very sensitive to changes in behaviour and are often very experienced in identifying subtle changes that may be due to shunt malfunction. The emergency clinician or junior neurosurgeon dismisses parental concerns at their peril!
Disconnection
Shunt components have a frustrating habit of disconnecting, even in cases where they were properly and diligently secured (with a non-absorbable braded tie). Shunt material can become tethered along its length by adjacent tissue reaction. Older materials had a susceptibility to become calcified and stiff also. Rapid growth with tethering of distal/proximal components or rapid/extreme neck movements, such as those witnessed in generalised tonic-clonic seizures, can test shunt connections or fracture old and brittle tubing. In these cases, the presentation is often more subacute with subtle symptoms or signs of over- or underdrainage. Patients may even complain of pain, redness or swelling at the site of disconnection or fracture.
Infection
Infection remains an important complication, though rates have significantly improved in the last four decades, from between 14% and 24% reported in the mid-1970s to between 2% and 10% quoted in contemporary literature. Inoculation of the shunt almost universally occurs at the time of surgery, and infections present within 30 days of the index operation in the vast majority of cases (Kulkarni et al. 2001). It is usually due to skin commensal organisms, most often Staphylococcus species (Bayston 2018). Risk factors for shunt infection include young age, in particular premature neonates, post-operative CSF leak, improper technique and excess handling of the shunt prior to insertion and previous shunt infection (Simon et al. 2009; McGirt et al. 2003).
Patients with infection may present with obvious symptoms and signs of CNS infection: pyrexia, nausea and vomiting, headache, meningism and confusion. In addition, anorexia and abdominal pain may reflect a peritoneal reaction to infected CSF. There may also be tenderness and erythema tracking along the shunt tract. More often, however, patients present with a less well-defined illness, and often the only symptoms of infection are due to partial or complete shunt obstruction and resulting raised ICP. Diagnosis can therefore be challenging, and a ‘shunt tap’ is often advocated (Fig. 40.9). CSF aspirated aseptically via the shunt reservoir is sent for microscopy, gram stain, cell count and culture. Unfortunately, due to low numbers of viable bacteria present in the CSF of infected systems, microbiological culture can be negative in up to 25% of ‘confirmed’ infected cases. The CSF cell count is most instructive in this scenario, and a pleocytosis with predominant neutrophilia is highly suggestive of shunt infection particular with concordant clinical details (recent surgery, unwell patient).
Staphylococci that colonise a shunt survive in a biofilm and in deeper layers downregulate their metabolic activities and cell division markedly (Bayston 2018). The result is a relative resistance to antibiotics, and surgical explantation is necessary to manage the infection. The shunt is replaced with an EVD to maintain CSF diversion, and systemic intravenous antibiotics, with or without intrathecal antibiotics, are administered for 10–14 days before reimplantation (Fig. 40.10 (EVD)).
Improvements in operative technique, standardisation of practices, antimicrobial stewardship and most recently the adoption of antibiotic impregnated catheters have all contributed to improvements in infection rates. The considerable morbidity associated with infection mandates continued vigilance however, and timely and effective treatment of shunt infections is essential.
Ventriculoatrial Shunts
VA shunts are indicated in patients with concomitant intra-abdominal pathologies precluding the use of the peritoneum as a drainage site. Common coexistent pathologies include necrotising enterocolitis, peritonitis and extensive abdominal surgery. Complications specific to VA shunts include the need for repeated lengthening of the short distal catheter, higher risk of bacteraemia and sepsis as well as the risks of specific vascular complications, such as thrombosis, microemboli with resultant pulmonary hypertension, macroemboli with pulmonary embolism and vascular perforation.
9.4 Ventriculoperitoneal Shunts and Abdominal Surgery
Paediatric general surgeons will at times have to assess patients with VP shunts and potential abdominal pathology in both the acute and elective settings.
If, during an elective procedure without infection or contamination of the peritoneum, a peritoneal catheter is encountered, then it should merely be pushed aside gently and excluded from the field. Significant contamination of the field mandates externalisation of the shunt. The distal shunt tubing is assumed to be contaminated and is delivered externally through the skin to continue draining into a collection bag. Once the abdominal infection is treated, the external length of tubing is cut, and a new clean length of distal shunt tubing can be safely reimplanted.
Laparoscopic techniques are now commonplace, and experience has shown these to be entirely safe in the presence of a VP shunt (Rosenfeld et al. 2019; Walker and Langer 2000; Jackman et al. 2000). Early animal studies reported significant rises in ICP on induction of pneumoperitoneum as one would see in an ICP trace anytime a patient coughed or strained (Rosenthal et al. 1997). There was concern that prolonged raised intra-abdominal pressure could significantly impair valve or shunt function or potentially even lead to retrograde flow and significant pneumocephalus. Studies of shunted patients during laparoscopic surgery have not identified such risk or adverse events, and there are only scattered case reports of shunt failure associated with laparoscopy amongst the many thousands of cases performed in the last 20+ years. In vitro studies of shunt valves show them to be resistant to retrograde flow at pressures far in excess of that used for pneumoperitoneum (Neale and Falk 1999). While there is no good evidence to support safety concerns in this patient cohort, caution is still advised over high insufflation pressures (>16 mmHg) and prolonged surgery (>3 h) (Sankpal et al. 2011). Routine discussion with a neurosurgeon regarding the safety of laparoscopy in shunted patients is probably not necessary unless significant abdominal contamination is anticipated, in which circumstance externalising the shunt could be considered in advance.
Assessment of the acute abdomen with a shunt in situ is a difficult task and requires a holistic approach. Children may present with abdominal symptoms secondary to shunt malfunction, and it is important to define early if there are clinical or radiological signs of shunt dysfunction and not concentrate solely on the abdomen; similarly, it is vital that neurosurgeons seek an experienced opinion early in cases where the shunt seems to be functioning but the patient is symptomatic.
If there are abdominal symptoms and signs of peritonitis mandating laparotomy, then the shunt should be externalised, the CSF cultured, and antibiotics started. If the shunt is obviously infected in addition, i.e. signs of raised ICP, meningism and erythema tracking along the shunt, then the shunt is removed in its entirety and only reimplanted once effective antibiotic treatment of both the abdominal and CNS infection is completed.
Appendicitis is a common acute abdomen presentation, and children with VP shunts are not excepted from this. There is no evidence to suggest these patients have a worse outcome than non-shunted patients, and with caution, there should be no shunt-related complications (Ein et al. 2006; Barina et al. 2007). In an emergent appendicectomy, if the appendix is inflamed but not perforated, it is reasonable to leave the shunt in situ; if, however, there is any peritoneal soiling, then the shunt must be externalised.
Other common, but usually non-emergent, complications of peritoneal catheters include peritoneal pseudocysts and ascites. Pseudocysts are wall-less fluid collections accumulating between matted bowel loops; they may be complicated by infection. If there are no features of infection, then simple repositioning of the catheter in another portion of the peritoneum is all that is required.
9.5 Endoscopic Third Ventriculostomy (ETV)
Endoscopic exploration of the ventricular system with therapeutic intent had been explored in the early twentieth century (Demerdash et al. 2017). Early forays into neuroendoscopy were aimed at the management of hydrocephalus but had limited success. There were a handful of stumbling attempts to revive the technique in the subsequent decades, but these never translated to routine practice. These early failures were due largely to technological limitations, and so unsurprisingly, it wasn’t until the 1970s with the inception of the Hopkin’s rod endoscope that the technique really developed. Endoscopic applications have since exploded throughout the whole breadth of surgical practice, and neurosurgery is no exception.
The re-introduction of neuroendoscopy at this time was followed by a rapid broad adoption, and since then, a multitude of applications have been developed focused on, but not limited to, the management of hydrocephalus. The most ubiquitous of these is the endoscopic third ventriculostomy (ETV).
ETV is primarily indicated in cases of obstructive hydrocephalus, the aim being to provide a route whereby CSF can bypass the point of obstruction and enter the subarachnoid space, creating a functional shunt from the third ventricle into the basal cisterns, from where it may circulate and be reabsorbed. The major advantages of ETV over an implanted shunt are that it obviates the need for an implanted foreign body, thus avoiding risks of infection, material degradation, disconnection, misplacement, etc., and it maintains a more ‘physiological’ CSF dynamic, something that even modern shunt valves struggle to replicate.
9.6 ETV: The Technique
The technique of ETV is demonstrated in Fig. 40.11. A rigid endoscope is navigated into the frontal horn of the lateral ventricle through a frontal pre-coronal burr hole placed in the midpupillary line. After ventricular cannulation, the endoscope is introduced, and the operator orientates themselves noting the thalamic veins running toward the foramen of Monro. The foramen of Monro transmits CSF from the lateral to third ventricle and is bounded posteriorly by the anterior pole of the ipsilateral thalamus and anteriorly by the fornix—an important part of the limbic system that links the hippocampus to mammillary bodies. The fornices are integral to working memory function and thus must not be injured or put under excessive stretch. The endoscope is then navigated through the foramen of Monro into the third ventricle. On the third ventricle floor, the landmarks are reliably seen, the mammillary bodies posteriorly and the pituitary infundibulum (a red blush at the anterior apex of the third ventricle). Within a triangle drawn between these points is the tuber cinereum where the membranous floor of the third ventricle is thin and through which it is safe to pass. The tuber cinereum is punctured, and this ‘stoma’ then expanded sequentially with balloon or endoscopic forceps. Further membranes, in particular the membrane of Liliequist, should be identified and fenestrated, and a clear view to the pre-pontine cistern and the basilar artery is seen. The floor of the third ventricle is seen to billow as if, in the wind when the endoscope is withdrawn, a reassuring sign that the intervention has been successful (Fig. 40.11). Post-operatively, ventriculostomy patency can be investigated using phase contrast MRI or ‘CSF flow study’. On a midline sagittal cut, the flow of CSF across the stoma and turbulence within the pre-pontine cistern can be identified via a dark ‘flow void’ in this path (Fig. 40.12) (Dinçer et al. 2011).
Endoscopic images representing key stages and anatomy encountered at ETV. (a) View from the endoscope within the right lateral ventricle. Note the choroid plexus and posterior caudate vein (a), anterior caudate vein (b) and thalamostriate vein (c) converging towards the foramen of Monro (d). (b) Endoscopic image from within the third ventricle. Visible are the mammillary bodies (a) and pituitary infundibulum (b) bordering the thin membranous tuber cinereum (c). Just visible is the basilar artery (d) through the tuber cinereum. (c) A Fogarty catheter is used to perforate the floor of the third ventricle and the balloon (a) inflated to expand the ‘stoma’. (d) View with Fogarty catheter withdrawn demonstrating a perforation (a) through to underlying pre-pontine cistern. Note the fronds of arachnoid visible
CSF ‘flow’ studies employ various MRI sequences performed to produce a time-resolved 2D phase contrast with velocity encoding study. Images a–d show midline sagittal sections with typical post-operative appearances of a third ventriculostomy. The yellow arrows highlight the CSF flow void produced by a ‘jet’ of CSF moving from the third ventricle to the pre-pontine space
9.7 ETV with Choroid Plexus Coagulation
In a technique akin to that pioneered in the 1920s, choroid plexus coagulation sees the surgeon endoscopically cauterising the choroid plexus with the aim of reducing CSF production. This may be done in conjunction with an ETV (ETV-CPC), a sort of ‘belt and braces’ approach. Trials run in Uganda and subsequently in the USA in the 2000s showed advantage over ETV alone especially in younger children (<1 year) with certain hydrocephalic aetiologies (Stone and Warf 2014; Warf 2005). There remains controversy over the efficacy of this two-stage technique as opponents argue that CSF production and drive through the stoma help to maintain patency and thus reduce failure in the long term. In addition, the choroid plexus is a very vascular and friable structure, and interfering with it risks causing IVH. Furthermore, the choroid plexus is not merely a CSF ‘pump’ but generates intracranial pressure (ICP), maintains CSF homeostasis and provides micronutrients, proteins and hormones for neuronal and glial development, maintenance and function. Concerns regarding long-term neurocognitive outcome and the potential neurodevelopmental impairment exist (Spector et al. 2015).
9.8 Indications for ETV
ETV is considered in cases of obstructive hydrocephalus including obstructions in the caudal portion of the third ventricle (pineal region), aqueduct, fourth ventricle and foramen magnum. It has an established role in the primary management of aqueduct stenosis (Kulkarni et al. 2018).
ETV enjoys an overall success rate of 60–90%, the significant variety due to age and aetiological factors. Rates improve with advancing age (children > 1 year) and with specific aetiologies, i.e. those with an anatomical obstruction such as aqueduct stenosis, posterior fossa tumours and Dandy-Walker malformation. Aetiologies with inferior rates of success are those classically termed ‘communicating’, i.e. post-haemorrhagic and post-infective.
The commonest causes seen in neonates are germinal matrix IVH and meningitis; thus, ETV is not a viable option in this cohort. The Hydrocephalus Research Network in the USA developed and in 2010 published the ETV Success Score (ETVSS), a reliable and practical predictive score based on age, aetiology and prior CSF shunt (Table 40.3) (Kulkarni et al. 2010).
9.9 Complications of ETV
ETV is a low-risk procedure, with major risks exceedingly rare (Bouras and Sgouros 2011). The headline potential immediate complication of ETV is the risk of damage to the basilar artery which lies in wait immediately below the site of stoma formation. The extremely rare but likely-immediately fatal complication of basilar artery injury is one which every neurosurgeon hopes to avoid. ETV, therefore, while a relatively simple procedure, should be performed by or under the supervision of an experienced operator.
Other potential complications include but are not limited to intraventricular haemorrhage with blood obscuring the operator’s view, injury to the thalamus or fornix usually as a consequence of poorly placed burr hole (due to inadequate or inaccurate planning) and misaligned trajectory, CSF leak and rarely infection.
In the long term, ventriculostomies may close over, although why this should occur is not well understood. Patients thus require follow-up just as a shunted patient would but probably not for as long. A patient who is asymptomatic 1 year on from ETV probably does not require routine follow-up, unless they have ongoing neurosurgical concerns. A high index of suspicion should be maintained, however, for late failure of ventriculostomy particularly in patients presenting with symptoms of raised ICP. For this reason, some surgeons opt to leave a ventricular access device (VAD) or ‘Ommaya reservoir’ in situ following ETV to permit ventricular access and evaluation of CSF pressure, as well as immediate decompression by therapeutic aspiration of CSF in the event of raised pressure.
9.10 Ventricular Access Devices and Ventriculosubgaleal Shunt
In the circumstances of a premature neonate with expanding head circumference, ventricular index and clinical evidence of raised intracranial pressure and bradycardic and apnoeic episodes without other identified cause (sepsis, reflux, etc.), consideration of CSF diversion is needed. In the first instance, a ventricular or subdural aspiration of CSF can be performed for both diagnostic and therapeutic purposes via the wide-open anterior fontanelle. A ventricular tap (up to 15 ml/kg or no more than 30 ml total) will inform the operator of opening pressure (often not high surprisingly) and CSF appearance/consistency (clear, straw coloured, blood stained, engine oil). Subsequent microbiological examination and culture is essential to diagnose or rule out CSF infection or ventriculitis. Furthermore, the extent and duration of the clinical response is most informative.
Repeated ventricular taps (twice weekly) were often recommended for neonates below the 2 kg threshold for shunt surgery, or in those not clinically stable, for transfer to a neurosurgical unit. Nowadays, repeated CSF access via this route is avoided, and placement of a ventricular access device or VAD is more commonly performed (Fig. 40.13). In those infants too unstable for transfer or surgery, serial ventricular tap is still a viable option however. The VAD provides a readily accessible silastic ‘bubble’ under the skin, which allows safer access to CSF, without the need for repeated traversing of the cerebral cortex. This can readily be converted to an external ventricular drain, by attaching a butterfly needle to a ‘Becker’ CSF collection and monitoring system. Furthermore, the reservoir can be connected to an internalised distal catheter at a later stage, thus converting it to a VP or VA shunt.
Unfortunately, even with diligent aseptic technique, there is a relatively high rate of infection (10%), the risk increasing with more extreme prematurity (Spader et al. 2015).
An attractive alternative to VAD then is the implantation of a ventriculosubgaleal shunt (VSGS) in which a ventricular catheter is connected to a valve and distal tubing implanted in a subgaleal pocket created at the time of insertion by blunt undermining of the galea aponeurotica. CSF drainage can then occur in a regulated fashion due to the inline valve. CSF accumulates in the pocket which then expands. The CSF is slowly resorbed via the lining of the pocket. The pocket can expand considerably, and this is a frequent concern for parents and inexperienced clinicians; however, as long as it is not under tension, no long-term sequelae are seen, and it is generally well tolerated (Eid et al. 2018) (Fig. 40.14). At such time as the infant is fit for distal implantation, the distal catheter can be simply tunnelled to an alternative and permanent location (cardiac atrium or peritoneal cavity). There is no demonstrable difference in the rates of conversion to VP shunt between VAD and VSGS; however, the average age and body weight at the time of conversion are greater in VSGS patients, and the rate of infection is lower (Wang et al. 2014).
9.11 Common Clinical Presentation
Three common presentations will now be discussed to give the non-specialist some insight into their management.
Managing Post-haemorrhagic Hydrocephalus of Prematurity (PHHP)
Premature neonates are at risk of intraventricular haemorrhage due to friable, immature and poorly supported vessels within the germinal matrix. Both neural and glial elements originate from this region found in the subependymal region (adjacent to the lateral ventricle) medial to the caudate nucleus. The germinal matrix involutes progressively during the third trimester and is limited to the caudothalamic groove by 32 weeks. It has essentially disappeared by 35 weeks; hence, the risk is inversely proportional to gestational age. Changes in cerebral blood flow and hypoxia predispose these vessels to loss of integrity resulting in intracerebral haemorrhage (ICH). ICH frequently ruptures into the ventricles causing intraventricular haemorrhage (IVH). Blood within the CSF as discussed impairs CSF resorption and induces ventriculomegaly and hydrocephalus.
Unfused sutures and wide-open anterior and posterior fontanelles allow these children to accommodate for a large increase in intracerebral CSF volume. The open fontanelle also permits transcranial ultrasound (see above) for diagnosis and surveillance. A number of grading systems have been devised to communicate the extent of IVH and correlate with outcome. The first broadly adopted was the Papile grade, first proposed in 1978 (Table 40.4) (Papile et al. 1978). There is some controversy over its reliability as progression from grades I to IV more likely represents a continuum rather than distinct haemorrhagic entities. In addition, the periventricular echodensities often seen and formerly classified as ICH and thus grade Papile grade IV are, in fact, more likely post-haemorrhagic venous infarctions (Parodi et al. 2020). Subsequent scales have been developed to account for these shortcomings which include the Volpe scale from 1986 which has largely superseded Papile (Table 40.4) (Guzzetta et al. 1986).
Higher IVH grading correlates with worse neurological outcome, higher mortality and progressive ventricular dilatation and as a result progression to CSF shunt requirement (Table 40.5).
The practicalities of surgical management of PHHP is covered earlier in this chapter. The complex and multifaceted non-surgical management of these difficult patients is obviously beyond the scope of this chapter. That said, serial head circumference measurement and cranial US with accurate VI trend recording constitute the cornerstone of monitoring and are the minimum dataset a consulting neurosurgeon will require. Typically, there will be numerous interactions between neonatologists and neurosurgeons over a period of weeks prior to intervention so accurate record keeping and communication within the teams is vital.
Approach to Newly Diagnosed Hydrocephalus in Childhood (Non-neonates)
The aetiologies underlying childhood hydrocephalus are closely age-associated. Management strategies and approaches will therefore be nuanced. Congenital causes of hydrocephalus, particularly aqueduct stenosis and mass lesions (tumours) obstructing CSF pathways, are the prime causes of symptomatic hydrocephalus in this age group.
In circumstances of acute presentation in a child ‘in extremis’, management should first follow an APLS algorithm with appropriate airway management if required being paramount. Subsequent management is to make a timely diagnosis, and in the context of a supportive history or neurologically impaired child, urgent cross-sectional brain imaging is mandated. CT scans are most appropriate in this circumstance as they are almost universally available and can be performed in a matter of minutes. If feasible and safe, any suggestion of a mass lesion requires further investigation with MRI. There is little role for post-contrast CT, unless MRI is contraindicated. In a life-threatening circumstance with a neurologically compromised child, definitive radiological assessment can be delayed until after management of the hydrocephalus is instituted.
It is worth stating that such an urgent presentation seldom occurs. Hydrocephalus usually has a more subacute presentation with a prodrome of raised ICP features allowing earlier identification and diagnosis. A common route of diagnosis in fact involves the optician who notes papilloedema when examining a child referred for investigation of headaches by their GP or at routine eye test. Vomiting and subtle neurological features may also feature in the history as can multiple attendances to their GP. There may be features indicative of the underlying cause, i.e. ataxia from cerebellar tumours, cranial nerve palsies in brainstem or CP angle tumours, eye movement disorders from midbrain/pineal region tumours and visual failure from tumours near the optic apparatus. Hydrocephalus may also present secondary to infections such as meningitis or bacterial abscess although with these latter pathologies, there is usually a much more marked systemic illness. An MRI brain rather than CT is advised in these circumstances as a primary investigation as it yields much more information and permits prompt and appropriate decision-making without ionising radiation exposure to vulnerable tissues. This should ideally be performed at the referring unit, prior to referral to neurosurgery in a stable child with suspected hydrocephalus.
Approach to a Child with a Potentially Blocked Shunt
Children presenting with acute symptoms of shunt block or presenting in extremis with no history available can be difficult to diagnose. Children still die from unrecognised shunt failure, though this is thankfully very rare, and a high index of suspicion is vital to save lives.
The symptoms and signs of shunt failure are primarily those of raised ICP and have been covered at length but bear repetition. Any child with a VP shunt with a recent history of headaches, nausea and vomiting and/or progressive cognitive decline or neurological compromise should receive a CT head without delay. Children with prior episodes of shunt block are at higher risk of future blockages, and presentation with similar features, however apparently esoteric (behavioural change, not eating, odd-posturing), should raise suspicion of a further shunt block. Suffice it to say if you or the parents think ‘could the shunt be blocked?’ it needs investigating. These children should not be sent home from A&E without discussion with a neurosurgeon. The ‘query-blocked-shunt’ call is the most common contact a paediatric neurosurgeon on-call will receive and, while a cause of occasional frustration, is a call that should always be made.
9.12 Follow-Up of the Patient with Treated Hydrocephalus
Patients with treated hydrocephalus should remain under the care of a neurosurgeon. Previously, follow-up was variable, some clinicians following all patients and managing clinics full of well-shunt patients, while others discharged after a short follow-up period, relying on the fact that if the patient has a problem with their shunt, they would present to the hospital. Neither of these scenarios is ideal, and the growth in the clinical nurse specialist and nurse practitioner field has led to the ‘delegation’ of immediate responsibility for follow-up away from consultant-led clinics. Open- or rapid-access face-to-face or telephone clinics with experienced nurse specialists are a very effective method for triaging concerned shunt patients and keeping in touch with less engaged shunt patients. Baseline imaging several weeks after insertion of a VP shunt once recovered is useful to provide a ‘well scan’ against which future imaging can be contrasted. For young children, this should be updated every 1–2 years to keep pace with their changing head shape and size.
9.13 Outcome of Treated Paediatric Hydrocephalus
A number of longitudinal studies have reported long-term outcomes in children treated for hydrocephalus (Hoppe-Hirsch et al. 1998; Paulsen et al. 2015; Gmeiner et al. 2017). Overall mortality is reported between 5 and 39%, the large variability due to differences in how mortality was attributed and duration of follow-up. Shunt-related mortality is estimated at 4.6–17%, most due to infection (approximately 2/3) followed by mechanical failure (1/3). The majority (60–67%) of patients graduated from normal school; however, significant impairment in intelligence quotient has been noted compared with age-matched counterparts. Cognitive impairment has been related to the number of shunt failures a patient experiences in their lifetime. Self-reported quality-of-life metrics also show deficit compared with a healthy background population.
10 Conclusion
Hydrocephalus occurs as a consequence of a multitude of pathological processes and is incident at all stages of childhood. Once identified, it is eminently treatable, but this treatment can be complex and is not infrequently complicated. Treatment is surgical, but decisions regarding the timing and modality of intervention are nuanced. Follow-up is lifelong, for the most part, and presentation with a suspicion of shunt block will occur in virtually all patients at some point during this period. Up to 85% will require shunt revision surgery during their lifetime, and there should be a high index of suspicion for shunt failure when reviewing these patients acutely, especially in the context of a supportive history.
Further Reading
Cinalli G, Maixner WJ, Sainte-Rose C (eds) (2012) Pediatric hydrocephalus. Springer, New York
Ellenbogen JR, Waqar M, Pettorini B (2016) Management of post-haemorrhagic hydrocephalus in premature infants. J Clin Neurosci 31:30–34. https://doi.org/10.1016/j.jocn.2016.02.026
International Society of Paediatric Neurosurgery Guide: https://www.ispn.guide/. A freely available online educational resource providing up to date and evidence-based information on all paediatric neurosurgical conditions. Compiled by internationally renowned clinicians
Liebenberg WA, Johnson RB (2018) Neurosurgery for surgical trainees. Hippocrates Books, Carnforth
Mallucci C, Sgouros S (eds) (2016) Cerebrospinal fluid disorders. CRC, Boca Raton, FL
References
Aschoff A, Kremer P, Hashemi B, Kunze S (1999) The scientific history of hydrocephalus and its treatment. Neurosurg Rev 22(2–3):67–93. Discussion 4–5
Barina AR, Virgo KS, Mushi E, Bahadursingh AM, Johnson FE (2007) Appendectomy for appendicitis in patients with a prior ventriculoperitoneal shunt. J Surg Res 141(1):40–44
Bayston R (2018) Cerebrospinal fluid shunt infection: microbiological basis, pp 1–19
Bouras T, Sgouros S (2011) Complications of endoscopic third ventriculostomy. J Neurosurg Pediatr 7(6):643
Dandy WE, Blackfan KD (1914) An experimental, clinical and pathological study: Part 1.—Experimental studies. Am J Dis Child VIII(6):406–482
Demerdash A, Rocque BG, Johnston J, Rozzelle CJ, Yalcin B, Oskouian RJ et al (2017) Endoscopic third ventriculostomy: a historical review. Br J Neurosurg 31(1):28–32
Dewan MC, Rattani A, Mekary R, Glancz LJ, Yunusa I, Baticulon RE et al (2018a) Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg 1–15
Dewan MC, Rattani A, Fieggen G, Arraez MA, Servadei F, Boop FA et al (2018b) Global neurosurgery: the current capacity and deficit in the provision of essential neurosurgical care. Executive Summary of the Global Neurosurgery Initiative at the Program in Global Surgery and Social Change. J Neurosurg 1–10
Dickerman RD, McConathy WJ, Morgan J, Stevens QE, Jolley JT, Schneider S et al (2005) Failure rate of frontal versus parietal approaches for proximal catheter placement in ventriculoperitoneal shunts: revisited. J Clin Neurosci 12(7):781–783
Dinçer A, Özek MM (2011) Radiologic evaluation of pediatric hydrocephalus. Childs Nerv Syst 27(10):1543–1562
Dinçer A, Yildiz E, Kohan S, Memet ÖM (2011) Analysis of endoscopic third ventriculostomy patency by MRI: value of different pulse sequences, the sequence parameters, and the imaging planes for investigation of flow void. Childs Nerv Syst 27(1):127–135
Dorner RA, Burton VJ, Allen MC, Robinson S, Soares BP (2018) Preterm neuroimaging and neurodevelopmental outcome: a focus on intraventricular hemorrhage, post-hemorrhagic hydrocephalus, and associated brain injury. J Perinatol 38(11):1431–1443
Drake JM, Kestle JR, Milner R, Cinalli G, Boop F, Piatt J Jr et al (1998) Randomized trial of cerebrospinal fluid shunt valve design in pediatric hydrocephalus. Neurosurgery 43(2):294–303. Discussion 303–305
Eid S, Iwanaga J, Oskouian RJ, Loukas M, Jerry Oakes W, Shane TR (2018) Ventriculosubgaleal shunting—a comprehensive review and over two-decade surgical experience. Childs Nerv Syst 34(9):1639–1642
Ein SH, Miller S, Rutka JT (2006) Appendicitis in the child with a ventriculo-peritoneal shunt: a 30-year review. J Pediatr Surg 41(7):1255–1258
Gmeiner M, Wagner H, Zacherl C, Polanski P, Auer C, van Ouwerkerk WJ et al (2017) Long-term mortality rates in pediatric hydrocephalus—a retrospective single-center study. Childs Nerv Syst 33(1):101–109
Guzzetta F, Shackelford GD, Volpe S, Perlman JM, Volpe JJ (1986) Periventricular intraparenchymal echodensities in the premature newborn: critical determinant of neurologic outcome. Pediatrics 78(6):995–1006
Harris CA, McAllister JP 2nd. (2012) What we should know about the cellular and tissue response causing catheter obstruction in the treatment of hydrocephalus. Neurosurgery 70(6):1589–1601. Discussion 601–602
Hayhurst C, Beems T, Jenkinson MD, Byrne P, Clark S, Kandasamy J et al (2010) Effect of electromagnetic-navigated shunt placement on failure rates: a prospective multicenter study. J Neurosurg 113(6):1273–1278
Hoppe-Hirsch E, Laroussinie F, Brunet L, Sainte-Rose C, Renier D, Cinalli G et al (1998) Late outcome of the surgical treatment of hydrocephalus. Childs Nerv Syst 14(3):97–99
Jackman SV, Weingart JD, Kinsman SL, Docimo SG (2000) Laparoscopic surgery in patients with ventriculoperitoneal shunts: safety and monitoring. J Urol 164(4):1352–1354
Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F et al (2000) Long-term follow-up data from the Shunt Design Trial. Pediatr Neurosurg 33(5):230–236
Khan F, Shamim MS, Rehman A, Bari ME (2013) Analysis of factors affecting ventriculoperitoneal shunt survival in pediatric patients. Childs Nerv Syst 29(5):791–802
Khasawneh AH, Garling RJ, Harris CA (2018) Cerebrospinal fluid circulation: What do we know and how do we know it? Brain Circ 4:14–18
Kimelberg HK (2004) Water homeostasis in the brain: basic concepts. Neuroscience 129(4):851–860
Kulkarni AV, Drake JM, Lamberti-Pasculli M (2001) Cerebrospinal fluid shunt infection: a prospective study of risk factors. J Neurosurg 94(2):195–201
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S (2010) Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr 6(4):310–315
Kulkarni AV, Sgouros S, Leitner Y, Constantini S (2018) International Infant Hydrocephalus Study (IIHS): 5-year health outcome results of a prospective, multicenter comparison of endoscopic third ventriculostomy (ETV) and shunt for infant hydrocephalus. Childs Nerv Syst 34(12):2391–2397
Lifshutz JI, Johnson WD (2001) History of hydrocephalus and its treatments. Neurosurg Focus 11(2):E1
Mallucci CL, Jenkinson MD, Conroy EJ, Hartley JC, Brown M, Dalton J et al (2019) Antibiotic or silver versus standard ventriculoperitoneal shunts (BASICS): a multicentre, single-blinded, randomised trial and economic evaluation. Lancet 394(10208):1530–1539
McGirt MJ, Zaas A, Fuchs HE, George TM, Kaye K, Sexton DJ (2003) Risk factors for pediatric ventriculoperitoneal shunt infection and predictors of infectious pathogens. Clin Infect Dis 36(7):858–862
McGirt MJ, Buck DW 2nd, Sciubba D, Woodworth GF, Carson B, Weingart J et al (2007) Adjustable vs set-pressure valves decrease the risk of proximal shunt obstruction in the treatment of pediatric hydrocephalus. Childs Nerv Syst 23(3):289–295
Neale ML, Falk GL (1999) In vitro assessment of back pressure on ventriculoperitoneal shunt valves. Is laparoscopy safe? Surg Endosc 13(5):512–515
Paff M, Alexandru-Abrams D, Muhonen M, Loudon W (2018) Ventriculoperitoneal shunt complications: A review. Interdiscip Neurosurg 13:66–70
Papile LA, Burstein J, Burstein R, Koffler H (1978) Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 92(4):529–534
Parodi A, Govaert P, Horsch S, Bravo MC, Ramenghi LA (2020) Cranial ultrasound findings in preterm germinal matrix haemorrhage, sequelae and outcome. Pediatr Res 87(Suppl 1):13–24
Paulsen AH, Lundar T, Lindegaard KF (2015) Pediatric hydrocephalus: 40-year outcomes in 128 hydrocephalic patients treated with shunts during childhood. Assessment of surgical outcome, work participation, and health-related quality of life. J Neurosurg Pediatr 16(6):633–641
Pople IK (2002) Hydrocephalus and shunts: what the neurologist should know. J Neurol Neurosurg Psychiatry 73 Suppl 1(3):i22
Rachel RA (1999) Surgical treatment of hydrocephalus: a historical perspective. Pediatr Neurosurg 30(6):296–304
Reddy GK, Bollam P, Caldito G (2014) Long-term outcomes of ventriculoperitoneal shunt surgery in patients with hydrocephalus. World Neurosurg 81(2):404–410
Rekate HL (2011) A consensus on the classification of hydrocephalus: its utility in the assessment of abnormalities of cerebrospinal fluid dynamics. Childs Nerv Syst 27(10):1535–1541
Rosenfeld EH, Mazzolini K, DeMello AS, Yu YR, Karediya A, Nuchtern JG et al (2019) Do ventriculoperitoneal shunts increase complications after laparoscopic gastrostomy in children? J Surg Res 236:119–123
Rosenthal RJ, Hiatt JR, Phillips EH, Hewitt W, Demetriou AA, Grode M (1997) Intracranial pressure. Effects of pneumoperitoneum in a large-animal model. Surg Endosc 11(4):376–380
Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128(6):309–316
Sankpal R, Chandavarkar A, Chandavarkar M (2011) Safety of laparoscopy in ventriculoperitoneal shunt patients. J Gynecol Endosc Surg 2:91–93
Schatlo B, Hamed M, Grote A, Schramm J, Neuloh G (2013) Gravity assisted vs. medium pressure valves for communicating hydrocephalus show similar valve-revision rates. Acta Neurochir (Wien) 155(10):1987–1991
Serarslan Y, Yilmaz A, Çakır M, Güzel E, Akakin A, Güzel A et al (2017) Use of programmable versus nonprogrammable shunts in the management of normal pressure hydrocephalus: A multicenter retrospective study with cost–benefit analysis in Turkey. Medicine (Baltimore) 96(39)
Simon TD, Hall M, Riva-Cambrin J, Albert JE, Jeffries HE, LaFleur B et al (2009) Infection rates following initial cerebrospinal fluid shunt placement across pediatric hospitals in the United States. J Neurosurg Pediatr 4(2):156–165
Spader HS, Hertzler DA, Kestle JR, Riva-Cambrin J (2015) Risk factors for infection and the effect of an institutional shunt protocol on the incidence of ventricular access device infections in preterm infants. J Neurosurg Pediatr 15(2):156–160
Spector R, Keep RF, Robert Snodgrass S, Smith QR, Johanson CE (2015) A balanced view of choroid plexus structure and function: Focus on adult humans. Exp Neurol 267:78–86
Stone SS, Warf BC (2014) Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. J Neurosurg Pediatr 14(5):439–446
Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ (2013) Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr 11(1):15–19
Tomycz LD, Hale AT, George TM (2017) Emerging insights and new perspectives on the nature of hydrocephalus. Pediatr Neurosurg 52(6):361–368
Walker DH, Langer JC (2000) Laparoscopic surgery in children with ventriculoperitoneal shunts. J Pediatr Surg 35(7):1104–1105
Wang JY, Amin AG, Jallo GI, Ahn ES (2014) Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr 14(5):447–454
Warf BC (2005) Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg 103(6 Suppl):475–481
Wu Y, Green NL, Wrensch MR, Zhao S, Gupta N (2007) Ventriculoperitoneal shunt complications in California: 1990 to 2000. Neurosurgery 61(3):557–562. Discussion 62–3
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sunderland, G., Ellenbogen, J., Mallucci, C. (2023). Hydrocephalus. In: Puri, P., Höllwarth, M.E. (eds) Pediatric Surgery. Springer, Cham. https://doi.org/10.1007/978-3-030-81488-5_40
Download citation
DOI: https://doi.org/10.1007/978-3-030-81488-5_40
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-81487-8
Online ISBN: 978-3-030-81488-5
eBook Packages: MedicineMedicine (R0)